- 1Rani Therapeutics, San Jose, CA, United States
- 2Rani Therapeutics, San Antonio, TX, United States
Biotherapeutics such as peptides and antibodies are highly efficacious clinically but, unlike conventional medications, cannot be administered orally as they get digested and inactivated. Thus, biotherapeutics require parenteral routes for delivery, such as intravenous, intramuscular or subcutaneous administration. However, these delivery methods have limitations such as poor patient compliance or may require clinical supervision compared to oral therapies. We explored whether a novel, orally administered transenteric delivery system (Robotic Pill) could provide equivalent bioavailability to parenterally administered drugs. Utilizing an awake canine model, we demonstrated that orally administered Robotic Pills containing either human IgG or an anti-cytokine monoclonal antibody directed against either TNFα or interleukin-17A yielded bioavailability equivalent to parenterally administered controls. The ability to achieve clinically relevant blood levels of biotherapeutics via any orally administered preparation represents an important advance in drug delivery.
Introduction
Biotherapeutics represent an important and growing modality for the treatment of a range of diseases including cancer (Jin et al., 2022), endocrine disorders, viral infections (Li et al., 2022), and autoimmune disorders (Zeng et al., 2022). While biotherapeutic drugs are highly effective medications, delivery must be by injection (parenteral) to avoid destruction and inactivation in the harsh environment of the upper gastrointestinal tract. Thus, these medications can require painful and often repeated injections into skin or muscle or in some situations by intravenous administration, which requires a clinical setting with specialized care. The pain and inconvenience of chronic injections can lead to reduced patient compliance, poor quality of life and compromised disease management (Ho et al., 2006; Fu et al., 2009; Sequeira et al., 2019; Usach et al., 2019). Parenteral administration also may be complicated by direct adverse effects due to the injection such as infections, venous damage, or impaired mental state (Sequeira et al., 2019; Usach et al., 2019; Peyko et al., 2021).
Treatments for autoimmune disorders are particularly challenging as these diseases are typically chronic, requiring life-long treatments aimed at reducing inflammation. Estimates of the number of Americans suffering from autoimmune diseases range from 14–24 million and the number appears to be increasing (Dinse et al., 2020). These diseases are characterized by inflammation in joints (including the spine), skin, nervous system, digestive system, and even the eye. Common symptoms include fatigue, fever, muscle aches, joint pain and swelling, skin disorders, abdominal pain, altered digestion, and swollen glands. Thus, autoimmune disease expression can be diverse and extremely debilitating.
Generalized treatment for autoimmune diseases has relied heavily on medications designed to reduce inflammation or invoke systemic immunosuppression with corticosteroids. However, in the last decade or so, more targeted biotherapeutics, notably monoclonal antibodies directed at specific cytokine targets, have been developed and shown to be highly effective therapies for a range of autoimmune diseases (Lefebvre and McAuliffe, 2016; Jung and Kim, 2022). Some of the clinically available monoclonal antibodies are directed at tumor necrosis factor-alpha (TNFα) (Abramovits and Gupta, 2004; von Richter et al., 2019; Coghlan et al., 2021) and Interleukin-17 (IL-17) (Raychaudhuri, 2013; Kolbinger et al., 2022; Wohlrab et al., 2022; Xu et al., 2022; Yun et al., 2022) which underlie inflammatory responses in many autoimmune diseases. However, as with all biotherapeutics, the pain associated with frequently injecting these antibodies impacts patient compliance and disease management and several pharmaceutical companies have attempted to address this issue by developing infrequent dosing regimens with less painful and more convenient pen injectors for delivering these antibodies (Kivitz and Segurado, 2007; Karlsdottir et al., 2022).
As an alternative to parenteral drug delivery, we have developed an orally ingestible robotic pill capable of transenteric (intestinal) administration of biotherapeutics (Dhalla et al., 2022). The pill comprises a mechanical device that auto-injects the biotherapeutic agent into the wall of the small intestine from which it is rapidly absorbed. The transenteric injection is painless as the intestine is insensate to sharp stimuli (Gebhart and Bielefeldt, 2016). We previously demonstrated that the ingestible Robotic Pill reliably delivers biotherapeutics in porcine and canine models (Hashim et al., 2019a; Hashim et al., 2019b; Ruffy et al., 2019) and, in the first human clinical trial using this Robotic Pill delivery system, the transenterically delivered octreotide showed high bioavailability and no serious adverse events (Dhalla et al., 2022). The current report describes the pharmacokinetic profiles of three antibodies (polyclonal human IgG, monoclonal anti-TNFα and monoclonal anti-IL-17A) administered transenterically by the Robotic Pill in the awake canine model. These data indicate that biotherapeutic antibodies delivered via the transenteric route using the Robotic Pill achieve relative bioavailability comparable to parenterally administered (subcutaneous) biotherapeutics. Thus, the Robotic Pill represents an alternative, less invasive, non-painful mode for biotherapeutic drug delivery.
Materials and methods
Animals
All studies were conducted on awake female Beagle dogs weighing 7.5–13.0 kg in accordance with United States Department of Agriculture (USDA) regulations for animal care and use and following approval of the Institutional Animal Care and Use Committee of the respective test facilities.
Animals were briefly anesthetized and instrumented with an indwelling jugular access port for blood sampling 2–5 days prior to study. All animals were fasted overnight (> 10 h) prior to test drug delivery and monitored throughout the study for any signs of discomfort or drug reaction. Biotherapeutics were administered either intravenously (IV) via the cephalic vein, subcutaneously (SC) in the dorsal scapular area, or by oral administration via Robotic Pill (RP). A subset of dogs was instrumented with a duodenal access port through which the RP could be administered directly into the duodenum. A duodenal enterotomy was performed and an appropriately sized custom cannula inserted. The enterotomy incision was closed tightly around the cannula which was subsequently passed through an incision in the right lateral body wall and exteriorized. Animals were recovered from surgery prior to entering the study.
Robotic Pill
The Robotic Pill is a swallowable mechanical device enclosed in a 000-sized capsule designed to be orally ingested whole with water. An enteric coating protects the RP from dissolution in the acidic gastric environment (pH < 5.5). As the capsule enters the duodenum, the enteric coating and capsule shell begin to dissolve at the higher intestinal pH (> 6), thereby exposing the dissolvable reaction valve to the intestinal fluid. Dissolution of the reaction valve immediately leads to the mixing of two chemical reactants, citric acid and sodium bicarbonate, triggering a rapid chemical reaction to produce carbon dioxide (CO2). The CO2 gas inflates a balloon which aligns a microsyringe perpendicular to the long axis of the intestinal wall. The increasing balloon pressure provides the requisite force to actuate the microsyringe and inject the needle into the intestinal wall. In the moist tissue environment, the needle containing the drug dissolves within 10–15 min and the injected drug is absorbed into the bloodstream. Immediately upon needle delivery, the balloon deflates and is subsequently excreted through the GI tract with normal bowel movements. Additional information on device specifications have been described in a previous publication (Dhalla et al., 2022). Materials used in the RP are classified as food grade, food additive, active, or inactive food ingredient or GRAS (generally recognized as safe) by the United States Food and Drug Administration (FDA).
The RP contains two radiopaque markers: barium sulfate powder (Sigma-Aldrich) and a ring of bismuth (manufactured in house), which are used for tracking the transit and deployment of the RP within the GI tract using fluoroscopic imaging (Figure 1A). The barium sulfate powder is compacted in one end of the capsule shell. When the capsule shell dissolves, barium sulfate disperses indicating device deployment and drug delivery (Figure 1B) which is taken as T = 0 (± 5 min) for PK sampling. The second marker, a ring of bismuth is placed at the base of the microsyringe and stays within the balloon. The bismuth can be tracked through the GI tract to confirm the excretion of the device remnants.
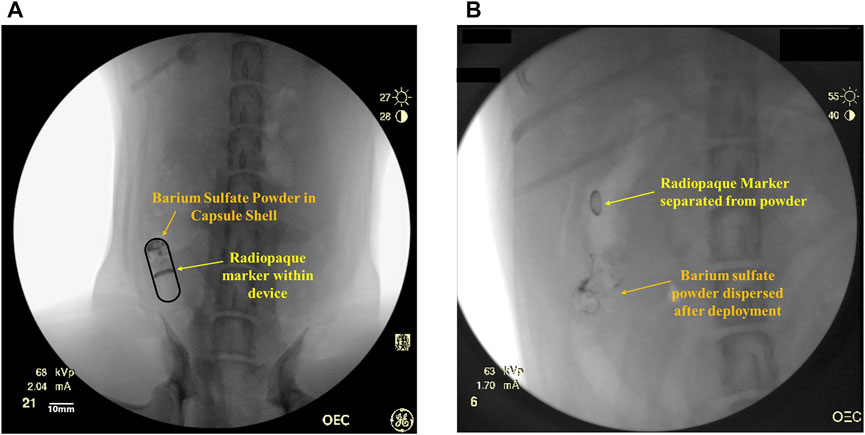
FIGURE 1. (A) Representative fluoroscopic image of an intact RP within the small intestine with a radiopaque marker (bismuth) in the device and barium sulfate powder within the capsule shell. (B) Representative fluoroscopic image of deployed RP in the small intestine. The barium sulfate in the capsule has dispersed inside the intestinal lumen and has been pushed away from the radiopaque bismuth marker within the device.
Procedures
The pharmacokinetics (PK) of three large molecular weight antibodies were assessed following drug administration via either the RP, intravenous or subcutaneous injection. RP doses were administered either orally or directly into a duodenal port. Animals receiving an RP orally underwent fluoroscopic imaging (OEC 9900 Elite Cardiac C-Arm Fluoroscope) to monitor the RP capsule’s transit through the gastrointestinal tract and confirm device deployment within the small intestine as described above.
The antibodies tested included purified human immunoglobulin G (IgG) (Alpha Diagnostic International, Inc., San Antonio, Texas, United States), human monoclonal anti-tumor necrosis factor α (anti-TNFα), and human monoclonal anti-interleukin-17A (anti-IL-17A). The anti-TNFα (GP2017, adalimumab biosimilar) and anti-IL-17A (CJM112) antibodies (Kaul et al., 2021) were supplied by the manufacturer (Sandoz/Novartis, Basel, Switzerland) and diluted with diluent provided by Sandoz/Novartis. Lyophilized powder (CJM112) or powder manufactured via an evaporation process (GP2017, IgG) was measured and diluted as a clinical formulation for IV or SC administration. Following the drug manufacturing process for the RP, protein content (and percent aggregation in the case of GP2017 and CJM112) in the drug batches was determined to not significantly change from the starting powder material as confirmed through validated ELISAs (and size exclusion chromatography for GP2017 and CJM112).
Serum drug levels were measured by selective ELISA assays at varying time points after dose administration. Blood samples were collected into red top tubes and allowed to clot for 30 min at room temperature. Samples were centrifuged (3,000 rpm/1096 g at 4°C) and serum collected and stored at −80°C until analysis. Serum antibody levels for IgG and anti-TNFα (GP2017) were quantified using indirect ELISA kits (Cat. No. 1750 and 200-310-AHG, respectively) developed by Alpha Diagnostics (Texas, United States), while the Sandwich ELISA method for anti-IL-17A (CJM112) was provided by Novartis/Sandoz (Basel, Switzerland).
Study 1: Human monoclonal anti-TNFα antibody (GP2017)
To evaluate the relative bioavailability of human monoclonal anti-TNFα (GP2017) delivered via RP, eleven awake Beagle dogs received GP2017 administered either IV (n = 3), SC (n = 3) or by RP capsules (n = 5) inserted directly into a duodenal port but were otherwise unchanged from the orally dosed RP described. GP2017 was administered by IV injection at a dose of 4.96 mg (0.46 ± 0.07 mg/kg). RP capsules (1 or 2 capsules) containing ∼2.3 mg of GP2017 each were administered to 5 dogs via the duodenal port (3 dogs received 1 capsule (0.24 ± 0.03 mg/kg) and 2 dogs received 2 capsules (4.66 mg dose, 0.48 ± 0.01 mg/kg). GP2017 was also administered at a dose of 4.96 mg to 3 dogs via SC administration using a clinically injectable formulation (0.57 ± 0.04 mg/kg). For each dose group, blood samples were collected at time 0, 4, 8, 12 h and thereafter once daily for 8 days and then every three days until day 28.
Study 2: Human immunoglobulin G (IgG)
The relative bioavailability and pharmacokinetics of human IgG was determined using 8 dogs and delivered either IV (n = 3), SC (n = 2) or orally via RP (n = 3) at a dose of ∼2.4 mg. The average IV dose was 0.27 ± 0.02 mg/kg, and the average SC dose was 0.28 ± 0.00 mg/kg. The RP contained ∼2.34 mg (0.28 ± 0.02 mg/kg). Human IgG and excipients were dissolved using 1 ml sterile water and administered IV and SC, respectively. Blood samples were collected for drug level analysis at time 0 and at various time intervals for 10–14 days (RP group; 0, time of fluoroscopic confirmation of deployment within small intestine, 4, 6 h and once daily for 10 days IV Group; 0, 2, 4, 6, 8 h and once daily for 14 days: SC Group; 0, 4, 6 h and once daily for 14 days).
Study 3: Human monoclonal anti-interleukin-17A antibody (CJM112)
The pharmacokinetics of human monoclonal anti-IL-17A antibody (CJM112) was evaluated in seventeen awake, female Beagle dogs. For dogs receiving CJM112 via IV (n = 3) or SC (n = 3) injection, the drug was dissolved in diluent supplied by Novartis to a final dose of 4.8 mg in 2 ml of solution (IV, 0.54 ± 0.03 mg/kg; SC, 0.54 ± 0.05 mg/kg). Dogs orally dosed with CJM112 via RP capsules received either a single RP capsule containing ∼2.4 mg of CJM112 (n = 7; 0.27 ± 0.01 mg/kg), or two RP capsules totaling ∼4.8 mg of CJM112 (n = 4; 0.55 ± 0.02 mg/kg). For all dose groups, blood samples were collected at time 0, 4, 8, 12 h, and once daily for 14 days and then at 17- and 21-day post-dose.
Bioanalytical and pharmacokinetic analyses
Non-Compartmental pharmacokinetic parameters and descriptive statistics were calculated using Phoenix® WinNonlin® 6.3 (Certara, Princeton, NJ, United States) and Microsoft Excel 365. Pharmacokinetic parameters and descriptive statistics for serum drug concentrations are presented using nominal sampling times and summarized by treatment group using descriptive statistics [mean and standard deviation (SD)]. For calculation of mean concentrations and generation of mean concentration-time profiles, all values below the limit of quantification (BLQ) were set to zero. For the non-compartmental analysis (NCA), a concentration that was BLQ was assigned a value of zero if it occurred in a profile before the first measurable concentration. The formation of Anti-Drug Antibodies (ADAs) was suspected based on the abrupt drops in serum drug concentrations across all molecules. Thus, bioavailability was calculated using only the timepoints prior to the observation of, or suspected development of ADAs. Pharmacokinetic parameters reported are listed in Table 1.
Results
Animal subjects
There were no significant findings from any of the clinical parameters measured (body weight, daily clinical observations, physical exams, clinical pathology). All RP group subjects tolerated administration of the RP and all capsule remnants were retrieved by natural enteral elimination within 96 h after administration. Animals remained healthy throughout the entirety of the study period, and those receiving the RP had no discernible signs of discomfort in relation to device deployment.
Study 1: Duodenal port administration of robotic pill containing human monoclonal anti-TNFα antibody (GP2017)
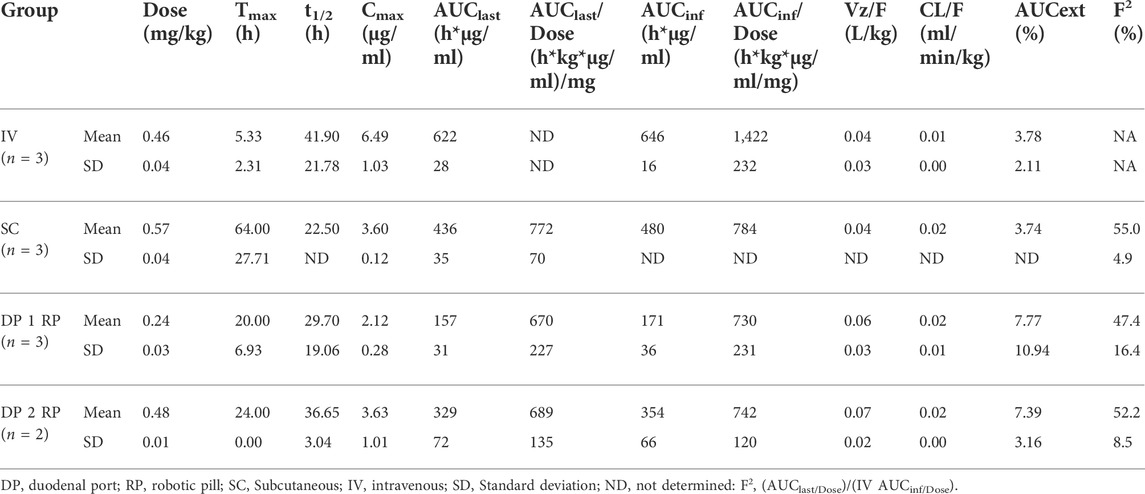
Pharmacokinetics, absolute and relative bioavailability of GP2017 was determined following delivery directly into the small intestine via RP and compared to intravenous and subcutaneous delivery. Following IV administration of 4.96 mg GP2017, mean serum concentrations reached peak levels at the first sampling time point (4 h post-dose) and declined steadily thereafter through the day 8 sampling time point. The median Tmax was 4 h (mean ± SD: 5.33 ± 2.31 h) and the mean Cmax was 6.49 ± 1.03 µg/ml (Table 2). Mean AUClast (622 ± 28 h*µg/ml) and AUCinf (646 ± 16 h*µg/ml) values were similar, indicating that the sample collections captured most of the drug exposure with less than 4% of the AUCinf value extrapolated. Both clearance (Cl) and apparent volume of distribution (Vz) were low and fit the non-compartmental analysis.
Administration of GP2017 via RP capsule through the duodenal port resulted in mean peak serum concentrations at 22 ± 5 h post-dose whether 1 or 2 RP capsules were given (Figure 2). The mean Cmax values were 2.12 ± 0.28 and 3.63 ± 1.01 µg/ml for 1 and 2 capsules, respectively (Table 2). Although Cmax values following administration of 2 RP capsules were less than 2-fold higher than following administration of 1 RP, the total systemic exposure was 2-fold higher (mean AUClast, 329 ± 72 vs. 157 ± 31 h*µg/ml; AUCinf, 354 ± 66 vs. 171 ± 36 h*µg/ml). Sample collections captured most of the drug exposure with less than 8% of the AUCinf value extrapolated. There were no differences in t1/2, Vz or Cl between the groups (Table 2). Absolute bioavailability for RP groups was 47% and 52% for one or two capsules, respectively. Relative bioavailability to the SC Group was 93.1% and 94.6%, for one or two RP, respectively. However mean bioavailability (F%) values were underestimated for SC group (discussed below). Based on these dose normalized values, duodenal administration of RP containing GP2017 resulted in virtually equivalent systemic exposure compared to that of subcutaneous administration.
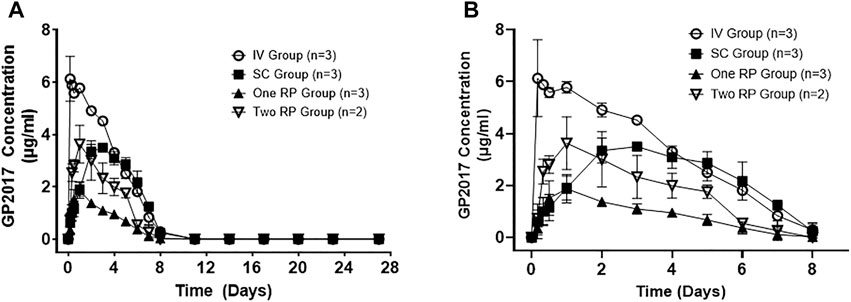
FIGURE 2. (A) Mean (± SD) serum concentrations of GP2017 (μg/mL) versus time (days) for Intravenous (IV; n = 3); Subcutaneous (SC; n = 3); One Robotic Pill (RP; n = 3) and Two RPs (n = 2). PK samples were analyzed via enzyme-linked immunosorbent assay (ELISA). (B) Enlarged view of serum GP2017 concentrations depicting Day 0–8 for all dose groups.
Subcutaneous administration of 4.96 mg in the SC Group (0.57 ± 0.02 mg/kg) resulted in longer median Tmax values of 48 h (mean 64 ± 27 h) (Table 2). Absolute bioavailability (based on AUClast) was 55.0% for the SC group. Note that AUClast was used to estimate %F because AUCinf could not be estimated due to abrupt drops in serum drug concentrations between days 5 to 8 suggesting the formation of ADAs.
Study 2: Oral administration of robotic pill containing human IgG
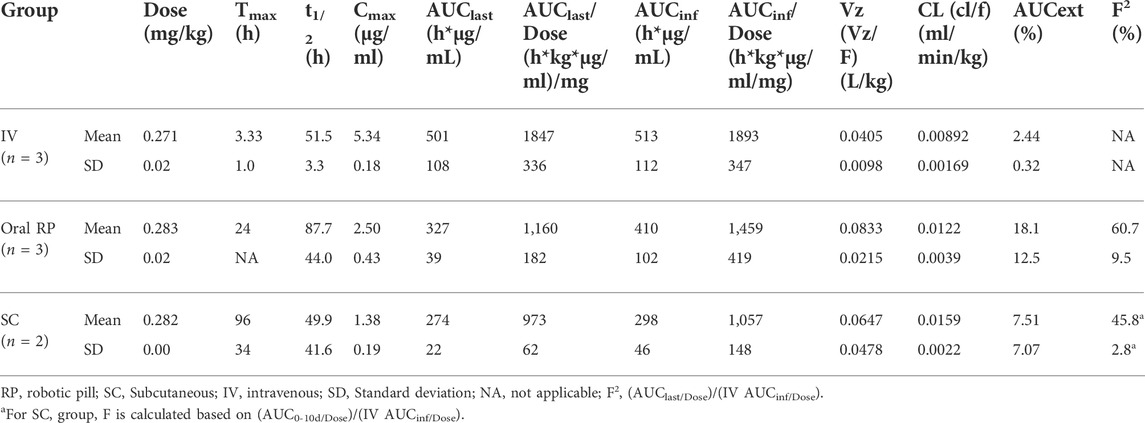
Pharmacokinetics of IgG obtained after administration via IV, RP and SC routes are shown in Figure 3. The RP, SC, and IV groups were conducted in sequence. Upon completion of the RP group, it was observed that serum drug concentrations had not yet reached baseline by day 10, therefore the blood collection timepoints were extended in the subsequent SC and IV groups.
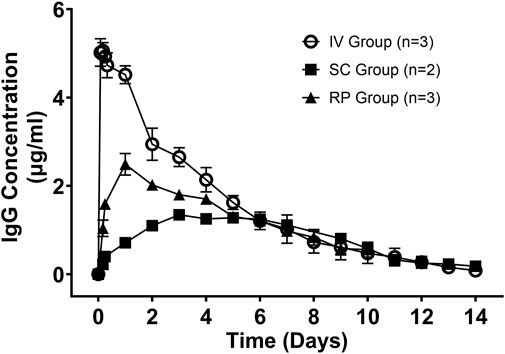
FIGURE 3. Mean (± SD) serum concentrations of Human IgG (µg/ml) versus time (days) for Intravenous (IV; n = 3); Subcutaneous (SC; n = 2); One Robotic Pill (RP; n = 3). PK samples were analyzed via enzyme-linked immunosorbent assay (ELISA).
Following IV administration, serum IgG concentrations were detected in the first sampling time point (2 h post-dose) and steadily declined through 14 days. The mean Cmax was 5.34 ± 0.18 µg/ml and mean Tmax was 3.33 ± 1.0 h. Mean AUClast and AUCinf values were similar at 501 ± 108 and 513 ± 112 h*µg/ml, respectively, indicating that the sample collections captured most of the PK exposure profile (less than 2.5% of AUCinf extrapolated). The mean clearance (CL) was relatively low at 0.00892 ± 0.00169 ml/min/kg and the mean volume of distribution (Vz) was also low at 0.0405 ± 0.0098 L/kg. The mean terminal elimination half-life was 51.5 ± 3.3 h. Complete pharmacokinetic parameters are shown in Table 3.
In animals receiving the oral RP, the mean total time from capsule ingestion to confirmed deployment within the small intestine was 144 ± 57 min, yielding peak serum IgG concentrations at 24 h in all three animals. The mean t1/2 following administration of the RP was 87.7 ± 44.0 h and ranged from 41 to 128 h. The variable terminal elimination half-life, with some values exceeding the half-life observed following IV administration, suggests that the true terminal elimination half-life was not observed in 2 of the 3 animals. The mean Cmax was 2.50 ± 0.43 µg/ml, and the mean AUClast and AUCinf values were 327 ± 39 h*µg/ml and 410 ± 102 h*µg/ml, respectively (Table 3). The percent extrapolated AUCinf values (AUCext) were 4.6%, 20.5% and 29.1%. For this reason, bioavailability was estimated by comparing dose-normalized AUClast values to the mean dose-normalized AUCinf value for IV administration. Bioavailability using AUClast for RP administration ranged from 50.0% to 68.3% (mean of 60.7%).
Following SC administration, serum levels of IgG increased but exhibited considerably longer Tmax values than either IV or RP administration (Figure 3). Each of two animals reached Cmax of 1.25 µg/ml by day 5 and Cmax of 1.51 µg/ml by day 3. Mean Cmax, AUC0-10d, AUClast and AUCinf values were 1.38 ± 0.19 µg/ml, 245 ± 11 h*µg/ml, 274 ± 22 h*µg/ml and 298 ± 46 h*µg/ml, respectively. Absolute bioavailability of IgG was calculated to be 43.9% and 47.8% following SC administration based on comparison of dose normalized AUC0-10d for SC and mean dose normalized AUCinf for IV. Thus, oral administration of IgG using the RP capsule attained bioavailability of 60.7%, which was comparable to SC administration (45.8%).
Study 3: Oral robotic pill administration of anti-interleukin-17A antibody (CJM112)
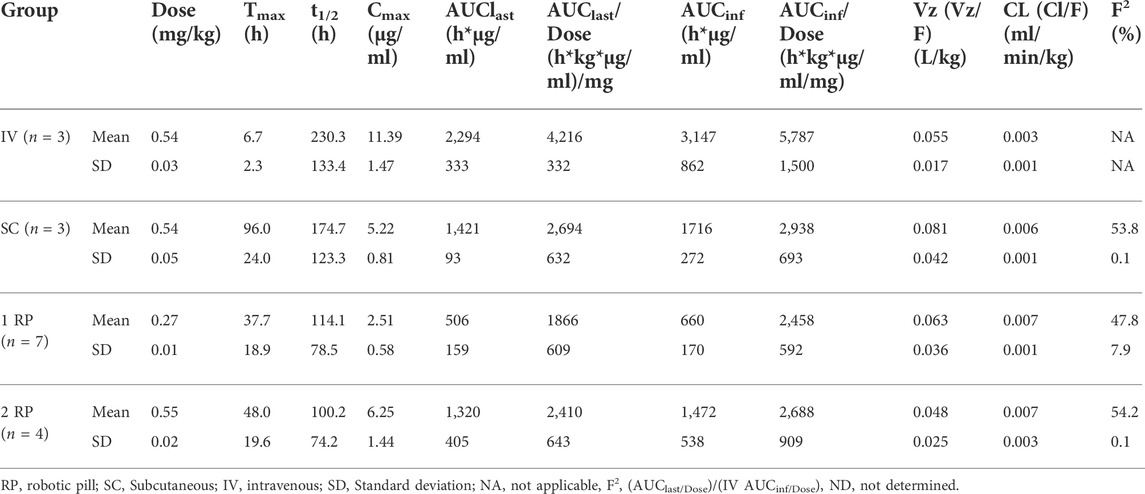
The objective of this study was to assess the bioavailability of a novel biotherapeutic administered orally via RPs. Pharmacokinetics of anti-interleukin-17A antibody delivered as one or two RP were compared to the pharmacokinetics of SC administration. The mean time from oral RP ingestion to confirmed deployment in the small intestine was 155 ± 89 min. The complete pharmacokinetic parameters for each group are shown in Table 4.
Mean serum concentrations of CJM112 over time for all groups are shown in Figure 4. The mean serum concentration of CJM112 following SC, one RP or two RP administration peaked at 72–120 h (96 ± 24 h), 24–72 h (37.7 ± 18.9 h), and 24–72 h (48 ± 19.6 h), respectively. Following SC administration, the mean ± SD for Cmax (5.22 ± 0.81 μg/ml), AUClast (1,421 ± 93 h*μg/ml), and the AUCinf (1,716 ± 272 h*μg/ml) were similar to values obtained after oral administration of two RP capsules [mean (Cmax 6.25 ± 1.44 μg/ml), (AUClast 1,320 ± 405 h*µg/ml), and (AUCinf 1,472 ± 538 h*μg/ml)]. Based on dose-normalized values, the systemic exposure of CJM112 was essentially equivalent following SC administration and the oral administration of two RPs. The mean bioavailability following SC administration was 53.8%, while the mean bioavailability following oral administration of two RPs was 54.2%.
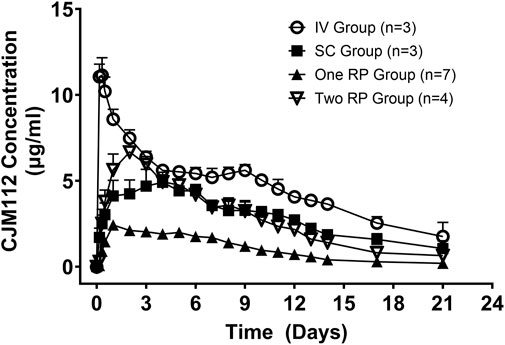
FIGURE 4. Mean (± SD) serum concentrations of CJM112 (μg/mL) versus time (days) for Intravenous (IV; n = 3); Subcutaneous (SC; n = 3); One Robotic Pill (RP; n = 7); Two RPs (n = 4). PK samples were analyzed via enzyme-linked immunosorbent assay (ELISA).
Discussion
This study demonstrates for the first time, the ability of a novel, orally administered, transenteric delivery system to administer therapeutic levels of monoclonal antibodies comparable to those yielded by standard subcutaneous administration (Table 5). The clinically relevant blood levels of biologic compounds or their biosimilars via an orally administered preparation represents an important advancement in drug delivery. The results of these preclinical studies in the canine model are complemented by the successful demonstration in humans of the same transenteric delivery mechanism to achieve systemic exposures of another biotherapeutic agent, octreotide, comparable to parenterally administered concentrations (Dhalla et al., 2022).
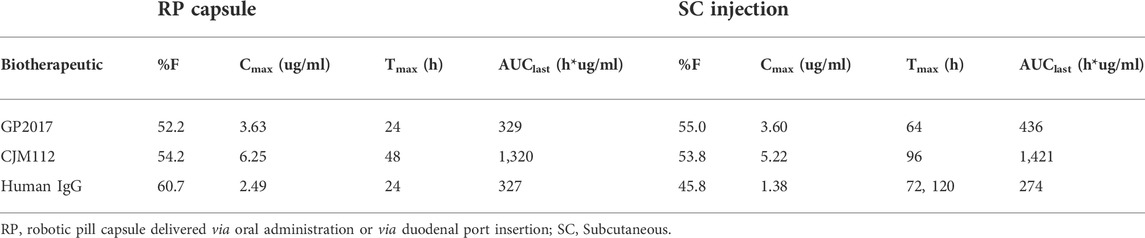
TABLE 5. Mean bioavailability, Cmax, Tmax and AUClast achieved after oral delivery of drugs via RP capsule vs. SC administration in the canine animal model.
Using this orally ingestible robotic pill platform, we evaluated the pharmacokinetics and bioavailability of polyclonal human IgG and two clinically important human monoclonal antibodies in the awake canine model. GP2017 (Sandoz/Novartis) is an anti-TNFα biosimilar of adalimumab (Heo, 2018; von Richter et al., 2019) and CJM112 (Novartis) is a novel anti-IL-17A antibody in the same class as secukinumab and ixekizumab, that has shown clinical efficacy (Kaul et al., 2021). These antibodies are representatives of the IgG class of monoclonal antibodies, the major class of therapeutic antibodies approved for clinical use today. The successful transenteric delivery and resultant bioavailability of these large, humanized proteins provides a framework for the oral delivery of a variety of therapeutic antibodies currently requiring parenteral administration.
TNFα is an inflammatory cytokine produced by macrophages/monocytes during acute inflammation such as seen with autoimmune disorders (Willrich et al., 2015). Adalimumab is a monoclonal antibody that inactivates TNFα thus reducing its inflammatory effects (Nuti et al., 2015; Willrich et al., 2015) and sold under the brand name Humira® (AbbVie, Lake Bluff, Illinois, United States), among others (Scheinfeld, 2003; Coghlan et al., 2021). This monoclonal antibody has been used to treat a variety of inflammatory diseases including rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, psoriasis, and uveitis, among others. Humira and biosimilars of Humira are delivered by SC injection of relatively high doses (40–80 mg) but at infrequent intervals of every other week. A single dose of 40 mg reportedly reaches 64% drug bioavailability and a Cmax of 4.7 ± 1.6 µg/ml of plasma within 5–6 days (Humira drug insert). Data presented here show that GP2017 delivered directly into the small intestine via RP exhibited 52% bioavailability, reached a Cmax of 2.12 ± 0.28 µg/ml within 24 h following administration of one RP capsule and 3.63 ± 1.01 µg/ml with two RPs, which is within reach of the clinical range.
To assess bioavailability of antibodies delivered orally, both human IgG and CJM112 were orally administered to awake canines via the RP. The pharmacokinetic data presented here demonstrates that oral administration of human IgG via the RP capsule resulted in similar systemic exposures compared to SC administration (mean bioavailability 60.7% vs. 45.8%, respectively). Importantly, oral administration reached peak serum IgG levels at 24 ± 0 h post-RP deployment, considerably faster than the SC group (96 ± 34 h).
CJM112 is a novel monoclonal antibody against IL-17A developed by Novartis Pharma AG (Basel, Switzerland) that has been evaluated in the early clinical stages. IL-17A is implicated in immune responses to infectious pathogens and in the pathogenesis of inflammatory autoimmune diseases. In human studies, Kaul et al. (2021) reported that CJM112 had clinical efficacy in moderate to severe psoriasis and was well tolerated in the doses tested. Examples of other drugs in this class include ustekinumab (Stelara®, Janssen Pharmaceuticals of Johnson and Johnson), bimekizumab [Bimzelx®, UCB (Union Chimique Belge)], secukinumab (Cosentyx®, Novartis) and ixekizumab (Taltz, Lilly). This class of drugs has shown effectiveness in treating a range of autoimmune diseases such as plaque psoriasis, psoriatic arthritis, axial spondylarthritis and ankylosing spondylitis (Glatt et al., 2017; Gordon et al., 2021; Loricera et al., 2021; Aparicio et al., 2022; Kolbinger et al., 2022; Oliver et al., 2022) representing an important new treatment tool. Like GP2017 however, these biotherapeutics must be delivered by injection.
In the current study, oral administration of CJM112 with the RP to awake canines resulted in virtually equivalent systemic exposure compared to that of subcutaneous administration. As in the case of GP2017, Cmax values occurred earlier following oral administration of CJM112 (48 ± 19.6 h from deployment) compared to SC injection (96 ± 24 h). These shorter Tmax values with the RP suggest delivery via the transenteric route enables more rapid access to the vascular compartment compared to SC administration. Cmax values were also similar for SC and RP administration (5.22 ± 0.81 vs. 6.25 ± 1.44 µg/ml for SC and 2RP, respectively). Mean bioavailability was essentially the same for SC administration (53.8%) and two robotic pills (54.2%). These data demonstrate that oral delivery of large molecular weight biotherapeutic antibodies is possible. Clinical dosing of IL-17A antibodies such as Cosentyx requires 150–300 mg monthly injections to achieve steady state values of 16.7–34.4 µg/ml, respectively (Cosentyx drug insert). Although it was not the intent of the current study to achieve clinical levels of CJM112, the data suggest it is conceivable that a daily oral dosing protocol could be developed to reach clinical values.
Oral drug treatments are preferred by patients and their prescribing physicians over injectables (Aletaha et al., 2020; Taylor et al., 2020). Unlike conventional medications, however, more than 350 biologic agents and their biosimilars cannot be administered orally as they are incapable of escaping digestion and thereby inactivation. The required parenteral administration of biologic drugs such as monoclonal antibodies has long been viewed as limiting. This, along with other drugs requiring parenteral administration, has prompted the development of a variety of novel orally administered alternatives. These approaches have utilized protective enteric coatings of drug capsules (Chuang et al., 2015), included protease inhibitors to enhance drug half-life (Bernkop-Schnurch, 1998), and added intestinal permeation enhancers to improve drug absorption across the intestinal mucosa (Aungst, 2012; Maher et al., 2016). While these strategies have shown some success for oral formulation of some biologics, the bioavailability using these strategies remains low (≤ 1%), limiting the approach to a few small peptides (Melmed et al., 2015; Brayden and Alonso, 2016; Granhall et al., 2019). Also, chronic use of permeation enhancers may damage the mucosal lining of the intestine potentially altering its protective barrier function which can lead to dose variability and potential safety concerns (McCartney et al., 2016).
More recent efforts have focused on co-administration of protease inhibitors or permeability modifiers with specialized carriers with only minor improvements in bioavailability (Bao et al., 2021). Other efforts have ignored the intestine altogether and explored protein and peptide delivery through transdermal patches containing microneedles (Aich et al., 2021) or inhalation of lyophilized therapeutic protein powders (Eedara et al., 2021). However, patients prefer the simplicity and painless delivery of oral medications and capsules are by far the most acceptable, and safest, form of oral drug administration to patients (Kaur et al., 2019).
Safety and tolerability of the robotic pill platform
All animals utilized for these studies were awake at time of drug administration. No animal showed signs of pain during or after capsule deployment or drug administration. Safety and tolerability of the RP delivery system was also demonstrated in humans (n = 72) with no adverse effects. In addition, data show that the RP was easily swallowed, withstood the acidic gastric environment, deployed painlessly in the small intestine, and was properly excreted with normal bowel movements (Dhalla et al., 2022). The hollow organs of the GI tract are insensate to typical noxious stimuli, such as puncturing and cutting while sensitive to stretch and distension (Gebhart and Bielefeldt, 2016). The absence of any reported pain or discomfort during deployment of the RP from human subjects suggests that the transient balloon inflation and deflation is insufficient to activate the intestinal stretch receptors. It is also noteworthy that, given the variability of capsule dissolution time in the small intestine coupled with the wide variation in intestinal motility rates, it is highly unlikely for the RP to inject the drug in the exact same location of the intestinal wall upon repeat administrations. This has been corroborated in non-clinical GLP safety studies in canines (unpublished) in which, following daily administration of the RP for 7 days, no evidence of any macro/microscopic tissue injury to the gastrointestinal tract was observed.
Evaluation of animal feces showed passage of non-digestible components of the RP capsules within 96 h. There was no evidence of large or small bowel obstruction. This is consistent with the subsequent human study in which no subject reported any perception during the transit of the RP through the GI tract and/or during the deployment of the device. Abdominal imaging conducted during a return visit of the human subjects 72–96 h after RP capsule ingestion confirmed that all device remnants had been excreted without sequelae in all subjects (Dhalla et al., 2022).
Limitations and challenges
All studies presented in this report utilized humanized antibodies in the canine model. While none of the canine subjects in this study exhibited signs of an allergic reaction, some animals produced levels of anti-drug antibodies during the period of drug measurements, as displayed by abrupt drops in serum drug levels indicative of immunogenicity. Data from these animals were not used to calculate bioavailability as they would have underestimated true bioavailability. Formation of anti-drug antibodies or immunogenicity was independent of mode of drug delivery. Formation of anti-drug antibodies does not compound the results of this study demonstrating equivalent bioavailability of oral and subcutaneous drug delivery but indicates the canine model may not be suitable for multi-day dosing studies with humanized biotherapeutics. In addition, the results of this study were focused on, and limited to, the pharmacokinetic parameters. Future studies will need to focus on pharmacodynamic (PD) studies in a suitable animal model to enable PK-PD analyses.
In general, we demonstrated that biotherapeutics reach the vascular space faster via the transenteric delivery than by standard subcutaneous injection. This finding mimics the rapid uptake of trans-gastric delivery reported by Abramson et al. (2022). However, this time-course does not take into account post-ingestion transit time from the stomach to the site of RP capsule drug delivery in the small intestine. From a clinical perspective, a limitation of the RP technology is the lack of predictability of the exact time of drug delivery following oral ingestion due to the inherent variability in gastric motility (Lee et al., 2014; Koziolek et al., 2015; Dhalla et al., 2022). The variability of gastric transit presumably impacts the time course of any orally ingested drug that is absorbed within the small intestine. In clinical trials, the time of gastric retention may be determined by fluoroscopically observing the location of radio-opaque markers incorporated into the RP capsule. Factors that influence gastric retention/passage of drug tablets/capsules into the small intestine include physicochemical factors and biological factors (Das et al., 2021). Physicochemical factors include size of the dosage form. For example, a large-sized tablet or capsule may encounter difficulty passing through the pyloric sphincter and thus be retained in the stomach for a long duration. The shape of the dosage form also affects the gastric retention time. Round or ring shape may facilitate passage (Streubel et al., 2006; Parmar et al., 2014; Das et al., 2021). Density of the system may also impact gastric passage. Biological factors such as the age of the subject affects the gastric retention time. Geriatric subjects show slower gastric transit (and pediatric subjects show faster gastric passage) than do normal adults (Parmar et al., 2014). In addition to subject age, male gender predisposes a subject to a faster gastric transit than females irrespective of their height, body surface, and weight. Fed and unfed states are also important determinants of the gastric emptying time (Singh and Kim, 2000) and the role of diet in the deployment of the RP capsule was evaluated and reported in a human trial (Dhalla et al., 2022).
Conclusion
We have shown in the awake canine model that transenteric delivery of monoclonal antibodies achieves bioavailability on par with that obtained with standard subcutaneous injections. Although the acute safety and reliability of the RP oral delivery system used here have been demonstrated in healthy human subjects in small clinical studies (Dhalla et al., 2022), it remains to be determined in larger, long-term studies with repeat administrations in patients and across broader demographics. Nevertheless, in these single administration studies, there was no observed incidence of pain or discomfort associated with the RP either during its deployment or during transit through the gastrointestinal tract, and all device remnants were safely and uneventfully excreted in all subjects. If confirmed in chronic studies in specific patient populations, this innovative drug delivery platform may offer an oral alternative for many patients with chronic diseases currently taking frequent and painful parenteral injections.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by the Institutional Animal Care and Use Committees of the respective Test Facilities.
Author contributions
AY managed all studies. AY, AD, and MH designed the studies and protocols. MI is the inventor of the RP and contributed to the development, planning and execution of the study. The manuscript was written by AY and JV and critically reviewed by all authors.
Funding
These studies received funding from Novartis Pharmaceuticals Corporation. The funders had the following involvement with the study: study design, interpretation of data, the writing of this article.
Conflict of interest
This study received funding from Novartis Pharmaceuticals Corporation. The funders had the following involvement with the study: study design, interpretation of data, critical review of this article. All authors are paid employees of Rani Therapeutics, LLC or its affiliate.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abramovits, W., and Gupta, A. K. (2004). Humira (adalimumab). Skinmed 3 (3), 157–159. doi:10.1111/j.1540-9740.2004.03466.x
Abramson, A., Frederiksen, M. R., Vegge, A., Jensen, B., Poulsen, M., Mouridsen, B., et al. (2022). Oral delivery of systemic monoclonal antibodies, peptides and small molecules using gastric auto-injectors. Nat. Biotechnol. 40 (1), 103–109. doi:10.1038/s41587-021-01024-0
Aich, K., Singh, T., and Dang, S. (2021). Advances in microneedle-based transdermal delivery for drugs and peptides. Drug Deliv. Transl. Res. 12, 1556–1568. doi:10.1007/s13346-021-01056-8
Aletaha, D., Husni, M. E., Merola, J. F., Ranza, R., Bertheussen, H., Lippe, R., et al. (2020). Treatment mode preferences in psoriatic arthritis: A qualitative multi-country study. Patient prefer. Adherence 14, 949–961. doi:10.2147/ppa.S242336
Aparicio, M., Guillen-Astete, C. A., Lopez-Medina, C., Sastre, C., and Rodriguez Martinez, F. J. (2022). Evidence for the use of secukinumab in patients with radiographic and non-radiographic axial spondyloarthritis in the last 5 years. Rheumatol. Ther. 9 (1), 73–94. doi:10.1007/s40744-021-00400-1
Aungst, B. J. (2012). Absorption enhancers: Applications and advances. AAPS J. 14 (1), 10–18. doi:10.1208/s12248-011-9307-4
Bao, X., Qian, K., and Yao, P. (2021). Insulin- and cholic acid-loaded zein/casein-dextran nanoparticles enhance the oral absorption and hypoglycemic effect of insulin. J. Mater. Chem. 9 (31), 6234–6245. doi:10.1039/d1tb00806d
Bernkop-Schnurch, A. (1998). The use of inhibitory agents to overcome the enzymatic barrier to perorally administered therapeutic peptides and proteins. J. Control. Release 52 (1-2), 1–16. doi:10.1016/s0168-3659(97)00204-6
Brayden, D. J., and Alonso, M.-J. (2016). Oral delivery of peptides: Opportunities and issues for translation. Adv. Drug Deliv. Rev. 106 (Pt B), 193–195. doi:10.1016/j.addr.2016.10.005
Chuang, E.-Y., Lin, K.-J., Lin, P.-Y., Chen, H.-L., Wey, S.-P., Mi, F.-L., et al. (2015). Self-assembling bubble carriers for oral protein delivery. Biomaterials 64, 115–124. doi:10.1016/j.biomaterials.2015.06.035
Coghlan, J., He, H., and Schwendeman, A. S. (2021). Overview of Humira biosimilars: Current European landscape and future implications. J. Pharm. Sci. 110 (4), 1572–1582. doi:10.1016/j.xphs.2021.02.003
Das, S., Kaur, S., and Rai, V. K. (2021). Gastro-retentive drug delivery systems: A recent update on clinical pertinence and drug delivery. Drug Deliv. Transl. Res. 11 (5), 1849–1877. doi:10.1007/s13346-020-00875-5
Dhalla, A. K., Al-Shamsie, Z., Beraki, S., Dasari, A., Fung, L. C., Fusaro, L., et al. (2022). A robotic pill for oral delivery of biotherapeutics: Safety, tolerability, and performance in healthy subjects. Drug Deliv. Transl. Res. 12 (1), 294–305. doi:10.1007/s13346-021-00938-1
Dinse, G. E., Parks, C. G., Weinberg, C. R., Co, C. A., Wilkerson, J., Zeldin, D. C., et al. (2020). Increasing prevalence of antinuclear antibodies in the United States. Arthritis Rheumatol. 72 (6), 1026–1035. doi:10.1002/art.41214
Eedara, B. B., Alabsi, W., Encinas-Basurto, D., Polt, R., and Mansour, H. M. (2021). Spray-dried inhalable powder formulations of therapeutic proteins and peptides. AAPS PharmSciTech 22 (5), 185. doi:10.1208/s12249-021-02043-5
Fu, A. Z., Qiu, Y., and Radican, L. (2009). Impact of fear of insulin or fear of injection on treatment outcomes of patients with diabetes. Curr. Med. Res. Opin. 25 (6), 1413–1420. doi:10.1185/03007990902905724
Gebhart, G. F., and Bielefeldt, K. (2016). Physiology of visceral pain. Compr. Physiol. 6 (4), 1609–1633. doi:10.1002/cphy.c150049
Glatt, S., Helmer, E., Haier, B., Strimenopoulou, F., Price, G., Vajjah, P., et al. (2017). First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br. J. Clin. Pharmacol. 83 (5), 991–1001. doi:10.1111/bcp.13185
Gordon, K. B., Foley, P., Krueger, J. G., Pinter, A., Reich, K., Vender, R., et al. (2021). Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): A multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet (London, Engl. 397 (10273), 475–486. doi:10.1016/s0140-6736(21)00126-4
Granhall, C., Donsmark, M., Blicher, T. M., Golor, G., Sondergaard, F. L., Thomsen, M., et al. (2019). Safety and pharmacokinetics of single and multiple ascending doses of the novel oral human GLP-1 analogue, oral semaglutide, in healthy subjects and subjects with type 2 diabetes. Clin. Pharmacokinet. 58 (6), 781–791. doi:10.1007/s40262-018-0728-4
Hashim, M., Beraki, S., Korupolu, R., Yamaguchi, A., Fusaro, L., Garapaty, A., et al. (2019a). Pharmacokinetics of an orally delivered antibody in awake dogs. Clin. Pharmacol. Ther. 105, S5. doi:10.1002/cpt.1344
Hashim, M., Korupolu, R., Syed, B., Horlen, K., Beraki, S., Karamchedu, P., et al. (2019b). Jejunal wall delivery of insulin via an ingestible capsule in anesthetized swine-A pharmacokinetic and pharmacodynamic study. Pharmacol. Res. Perspect. 7 (5), e00522. doi:10.1002/prp2.522
Heo, Y.-A. (2018). GP2017: An adalimumab biosimilar. BioDrugs 32 (6), 635–638. doi:10.1007/s40259-018-0318-x
Ho, P. M., Rumsfeld, J. S., Masoudi, F. A., McClure, D. L., Plomondon, M. E., Steiner, J. F., et al. (2006). Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch. Intern. Med. 166 (17), 1836–1841. doi:10.1001/archinte.166.17.1836
Jin, S., Sun, Y., Liang, X., Gu, X., Ning, J., Xu, Y., et al. (2022). Emerging new therapeutic antibody derivatives for cancer treatment. Signal Transduct. Target. Ther. 7 (1), 39. doi:10.1038/s41392-021-00868-x
Jung, S. M., and Kim, W.-U. (2022). Targeted immunotherapy for autoimmune disease. Immune Netw. 22 (1), e9. doi:10.4110/in.2022.22.e9
Karlsdottir, K., Gunnarsdottir, A. I., Grondal, G., Love, T. J., Stefansdottir, E., Davidsdottir, L. G., et al. (2022). A patients' perspective towards the injection devices for Humira and imraldi in a nationwide switching program. Front. Med. 9, 799494. doi:10.3389/fmed.2022.799494
Kaul, M., Jarvis, P., Rozenberg, I., Kolbinger, F., Di Padova, F., Calonder, C., et al. (2021). First-in-human study demonstrating the safety and clinical efficacy of novel anti-IL-17A monoclonal antibody CJM112 in moderate to severe plaque psoriasis. J. Eur. Acad. Dermatol. Venereol. 35 (5), 1143–1151. doi:10.1111/jdv.17071
Kaur, G., Arora, M., and Ravi Kumar, M. N. V. (2019). Oral drug delivery technologies-A decade of developments. J. Pharmacol. Exp. Ther. 370 (3), 529–543. doi:10.1124/jpet.118.255828
Kivitz, A., and Segurado, O. G. (2007). HUMIRA pen: A novel autoinjection device for subcutaneous injection of the fully human monoclonal antibody adalimumab. Expert Rev. Med. Devices 4 (2), 109–116. doi:10.1586/17434440.4.2.109
Kolbinger, F., Di Padova, F., Deodhar, A., Hawkes, J. E., Huppertz, C., Kuiper, T., et al. (2022). Secukinumab for the treatment of psoriasis, psoriatic arthritis, and axial spondyloarthritis: Physical and pharmacological properties underlie the observed clinical efficacy and safety. Pharmacol. Ther. 229, 107925. doi:10.1016/j.pharmthera.2021.107925
Koziolek, M., Grimm, M., Becker, D., Iordanov, V., Zou, H., Shimizu, J., et al. (2015). Investigation of pH and temperature profiles in the GI tract of fasted human subjects using the intellicap system. J. Pharm. Sci. 104 (9), 2855–2863. doi:10.1002/jps.24274
Lee, Y. Y., Erdogan, A., and Rao, S. S. C. (2014). How to assess regional and whole gut transit time with wireless motility capsule. J. Neurogastroenterol. Motil. 20 (2), 265–270. doi:10.5056/jnm.2014.20.2.265
Lefebvre, A. L., and McAuliffe, L. (2016). Targeted immunomodulatory therapy: An overview. R. I. Med. J. 99 (12), 19–22.
Li, D., Sempowski, G. D., Saunders, K. O., Acharya, P., and Haynes, B. F. (2022). SARS-CoV-2 neutralizing antibodies for COVID-19 prevention and treatment. Annu. Rev. Med. 73, 1–16. doi:10.1146/annurev-med-042420-113838
Loricera, J., Galindez-Aguirregoikoa, E., and Blanco, R. (2021). Safety of secukinumab for the treatment of active ankylosing spondylitis. Expert Opin. Drug Saf. 20 (6), 627–634. doi:10.1080/14740338.2021.1851363
Maher, S., Mrsny, R. J., and Brayden, D. J. (2016). Intestinal permeation enhancers for oral peptide delivery. Adv. Drug Deliv. Rev. 106 (Pt B), 277–319. doi:10.1016/j.addr.2016.06.005
McCartney, F., Gleeson, J. P., and Brayden, D. J. (2016). Safety concerns over the use of intestinal permeation enhancers: A mini-review. Tissue barriers. 4 (2), e1176822. doi:10.1080/21688370.2016.1176822
Melmed, S., Popovic, V., Bidlingmaier, M., Mercado, M., van der Lely, A. J., Biermasz, N., et al. (2015). Safety and efficacy of oral octreotide in acromegaly: Results of a multicenter phase III trial. J. Clin. Endocrinol. Metab. 100 (4), 1699–1708. doi:10.1210/jc.2014-4113
Nuti, F., Fiorino, G., and Danese, S. (2015). Adalimumab for the treatment of pediatric Crohn's disease. Expert Rev. Clin. Immunol. 11 (9), 963–972. doi:10.1586/1744666x.2015.1072048
Oliver, R., Krueger, J. G., Glatt, S., Vajjah, P., Mistry, C., Page, M., et al. (2022). Bimekizumab for the treatment of moderate-to-severe plaque psoriasis: Efficacy, safety, pharmacokinetics, pharmacodynamics and transcriptomics from a phase IIa, randomized, double-blind multicentre study. Br. J. Dermatol. 186 (4), 652–663. doi:10.1111/bjd.20827
Parmar, P., Pande, S., Shah, S., and Sonara, S. (2014). Floating drug delivery system: A novel approach to prolong gastric retention. World J. Pharm. Pharm. Sci. 3 (4), 418–444.
Peyko, V., Shams, D., and Lauver, A. R. (2021). Epinephrine auto-injection after allergic reaction leading to gas gangrene of the leg. Am. J. Case Rep. 22, e930889. doi:10.12659/ajcr.930889
Raychaudhuri, S. P. (2013). Role of IL-17 in psoriasis and psoriatic arthritis. Clin. Rev. Allergy Immunol. 44 (2), 183–193. doi:10.1007/s12016-012-8307-1
Ruffy, R., Hashim, M., Dhalla, A., Korupolu, R., Karamchedu, P., Syed, B., et al. (2019). 125-LB: An ingestible capsule to deliver parenteral pharmaceuticals into the jejunal wall. Diabetes 68 (Suppl. ment_1), 12. doi:10.2337/db19-125-LB
Sequeira, J. A. D., Santos, A. C., Serra, J., Estevens, C., Seica, R., Veiga, F., et al. (2019). Subcutaneous delivery of biotherapeutics: Challenges at the injection site. Expert Opin. Drug Deliv. 16 (2), 143–151. doi:10.1080/17425247.2019.1568408
Singh, B. N., and Kim, K. H. (2000). Floating drug delivery systems: An approach to oral controlled drug delivery via gastric retention. J. Control. Release 63 (3), 235–259. doi:10.1016/s0168-3659(99)00204-7
Streubel, A., Siepmann, J., and Bodmeier, R. (2006). Gastroretentive drug delivery systems. Expert Opin. Drug Deliv. 3 (2), 217–233. doi:10.1517/17425247.3.2.217
Taylor, P. C., Betteridge, N., Brown, T. M., Woolcott, J., Kivitz, A. J., Zerbini, C., et al. (2020). Treatment mode preferences in rheumatoid arthritis: Moving toward shared decision-making. Patient prefer. Adherence 14, 119–131. doi:10.2147/ppa.S220714
Usach, I., Martinez, R., Festini, T., and Peris, J.-E. (2019). Subcutaneous injection of drugs: Literature review of factors influencing pain sensation at the injection site. Adv. Ther. 36 (11), 2986–2996. doi:10.1007/s12325-019-01101-6
von Richter, O., Lemke, L., Haliduola, H., Fuhr, R., Koernicke, T., Schuck, E., et al. (2019). GP2017, an adalimumab biosimilar: Pharmacokinetic similarity to its reference medicine and pharmacokinetics comparison of different administration methods. Expert Opin. Biol. Ther. 19 (10), 1075–1083. doi:10.1080/14712598.2019.1571580
Willrich, M. A. V., Murray, D. L., and Snyder, M. R. (2015). Tumor necrosis factor inhibitors: Clinical utility in autoimmune diseases. Transl. Res. 165 (2), 270–282. doi:10.1016/j.trsl.2014.09.006
Wohlrab, J., Gerloff, D., and Gebhardt, K. (2022). Expression and activity of IL-17 receptor subunits in human cutaneous cells as targets for anti-IL-17 therapeutic antibodies. Biomed. Pharmacother. = Biomedecine Pharmacother. 146, 112569. doi:10.1016/j.biopha.2021.112569
Xu, J.-G., Jia, H., Chen, S., Xu, J., Zhan, Y., Yu, H., et al. (2022). Structural and functional insights into a novel pre-clinical-stage antibody targeting IL-17A for treatment of autoimmune diseases. Int. J. Biol. Macromol. 202, 529–538. doi:10.1016/j.ijbiomac.2022.01.119
Yun, J. S., Scardamaglia, L., Tan, C.-G., and McCormack, C. J. (2022). Successful secukinumab treatment of active bullous pemphigoid and chronic severe psoriasis: A case report. Australas. J. Dermatol. 63, e155–e158. doi:10.1111/ajd.13803
Keywords: oral delivery, biotherapeutic, robotic pill, small intestine, antibodies, canine
Citation: Yamaguchi A, Van Dam J, Dhalla AK, Horlen K, Imran M, Vo AT and Hashim MA (2022) Transenteric delivery of antibodies via an orally ingestible robotic pill yields high bioavailability comparable to parenteral administration in awake canines. Front. Drug. Deliv. 2:955569. doi: 10.3389/fddev.2022.955569
Received: 28 May 2022; Accepted: 04 July 2022;
Published: 09 August 2022.
Edited by:
Driton Vllasaliu, King’s College London, United KingdomReviewed by:
Gerard Cummins, University of Birmingham, United KingdomMaya Thanou, King’s College London, United Kingdom
Copyright © 2022 Yamaguchi, Van Dam, Dhalla, Horlen, Imran, Vo and Hashim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alyson Yamaguchi, YWx5c29uQHJhbml0aGVyYXBldXRpY3MuY29t
 Alyson Yamaguchi
Alyson Yamaguchi Jacques Van Dam1
Jacques Van Dam1