- Department of Radiology, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, China
Objective: To compare the image quality, radiation dose, and examination time between non-electrocardiogram (ECG)-gated coronary CT angiography (ECG-less CCTA) and conventional ECG-gated CCTA using wide-detector CT, and validate its clinical applicability.
Methods: In this prospective study, 109 patients with suspected coronary artery disease were divided into ECG-less (Group A, n = 59) and ECG-gated (Group B, n = 50) groups. Objective metrics (CT attenuation, noise, SNR, CNR), subjective image quality (4-point scale), and examination time were analyzed. Diagnostic performance (sensitivity, specificity) was evaluated against invasive coronary angiography (ICA). A modified ECG-less protocol (Group A2, n = 30) was implemented to optimize radiation dose. Plaque characterization agreement was assessed using Cohen’s κ.
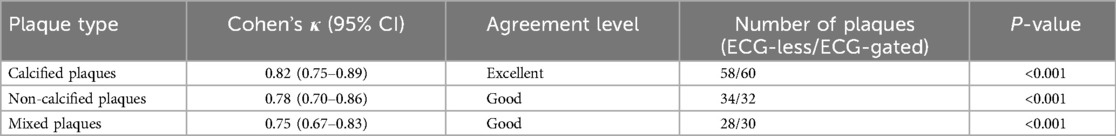
Results: The ECG-less group demonstrated higher radiation dose (2.83 ± 0.93 vs. 1.90 ± 1.41 mSv, p < 0.001) but significantly shorter examination time (225.03 ± 33.37 vs. 330.06 ± 56.35 s, p < 0.001). The modified ECG-less protocol reduced the effective dose by 28% (2.03 ± 0.75 mSv, p < 0.001 vs. Group A), achieving statistical comparability to the conventional group (p = 0.62). Subjective image scores (4-point scale) and SNR/CNR showed no significant differences between groups (p > 0.05). ECG-less CCTA achieved per-segment sensitivity/specificity of 93.3%/97.5% and per-patient 94.4%/50% for detecting ≥50% stenosis. Plaque characterization exhibited high agreement (calcified: κ = 0.82; non-calcified: κ = 0.78; mixed: κ = 0.75).
Conclusion: ECG-less CCTA provides comparable image quality and diagnostic accuracy to conventional ECG-gated CCTA while significantly reducing examination time. This technique is applicable in emergency scenarios where ECG lead placement is unfeasible (e.g., severe trauma, unreliable ECG signals).
1 Introduction
Coronary Artery Disease (CAD) remains a leading cause of morbidity and mortality worldwide (1, 2). Coronary Computed Tomography Angiography (CCTA), recognized for its non-invasive nature and high negative predictive value, has emerged as an essential tool for quantifying coronary artery stenosis and characterizing atherosclerotic plaques (3–5). Advances in technology have steadily enhanced the success rate and accuracy of CCTA (6). Moreover, there is an increasing demand to broaden its applicability to a wider patient population, address more complex scenarios, boost physicians’ confidence in cardiac diagnostics, and enhance the overall patient experience during examinations.
Nowadays, CCTA is no longer restricted by factors such as patient age, heart rate, and even breath-holding requirements. However, it still relies on electrocardiogram-gated (ECG) scanning (7, 8), which presents certain challenges. While ECG-gated CCTA remains the standard for most patients, ECG-less protocols may fill critical gaps in niche emergency scenarios where ECG lead placement is physically impossible (e.g., severe burns, trauma) or diagnostically unreliable (e.g., cardiac tamponade with attenuated QRS voltage). Additionally, certain patients may be unable to connect to the ECG due to the necessity of cardiac monitoring and various other life-saving measures during the scanning procedure. Furthermore, the need to apply ECG leads can result in patients exposing their chest skin, which negatively impacts the overall patient experience.
With the development of advanced technologies, the latest CCTA scans are equipped with wide detectors (16 cm), high rotation speeds (0.23 s/r), and effective coronary motion correction algorithms. Wide detectors enable single axial scans for coronary imaging, while high rotation speeds achieve superior temporal resolution. Additionally, coronary motion correction algorithms allow software-based compensation for coronary motion. These advancements have paved the way for ECG-less CCTA scanning. Consequently, this study aims to evaluate the differences in imaging performance between ECG-less CCTA and conventional ECG-gated CCTA.
2 Materials and methods
2.1 Patients
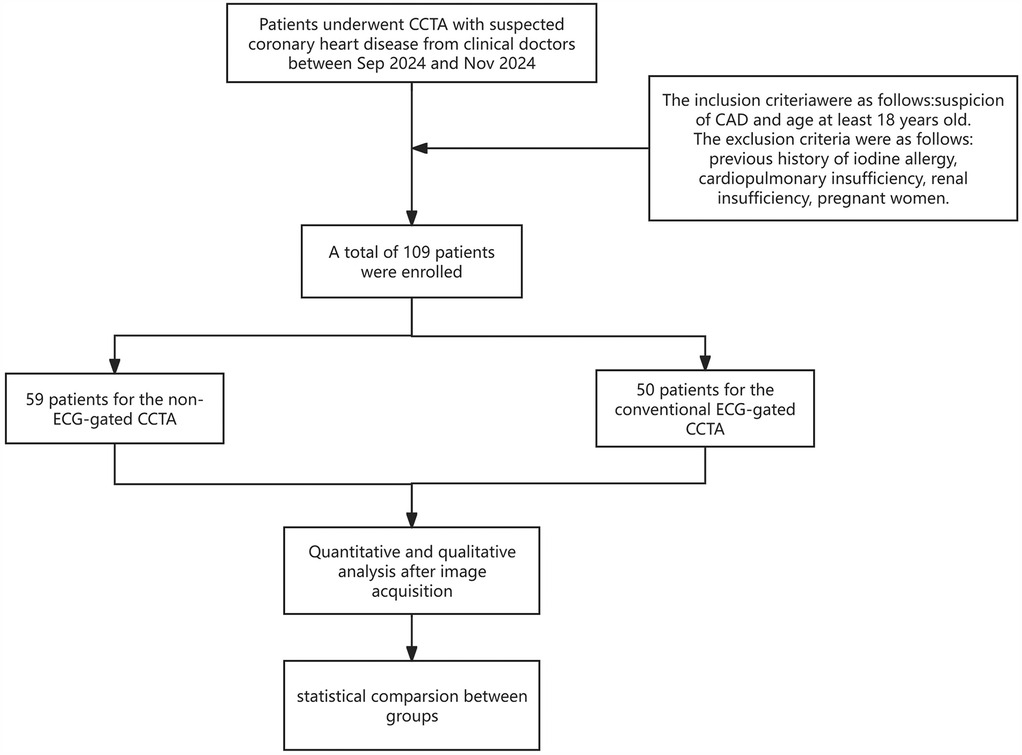
This prospective cohort trial was approved by our Institutional Review Board (K2024473) and was conducted following the Declaration of Helsinki (1964). Informed consent was obtained from all patients. We enrolled 109 consecutive patients between July 2024 and November 2024 with suspected CAD undergoing CCTA examination. The first 59 consecutive patients (group A, n = 59) were scanned without ECG gating, whereas the remaining consecutive patients (60–109; group B, n = 50) were scanned with ECG gating. The inclusion criteria were suspected CAD and age ≥ 18 years. Exclusion criteria were as follows: a history of iodine allergy, a coronary artery bypass graft, cardiopulmonary insufficiency, renal insufficiency and pregnancy. Details of the patient selection process are provided in Figure 1.
Following initial findings, a modified ECG-less protocol was tested in an additional cohort (Group A2, n = 30) to explore the feasibility of reducing radiation dose with ECG-less technology. Ethical approval and informed consent were obtained.
2.2 Image acquisition and reconstruction
All patients underwent CCTA using a 256-row CT scanner (Revolution Apex Expert, GE Healthcare, WI, USA). For both groups, tube voltage (80, 100, or 120 kV) was automatically selected according to the scout of patients, with automatic tube current modulation and a preset noise index of 20 HU. In group A, ECG-less CCTA was performed, and the data acquisition window was set to 200–750 ms. And in group A2, a narrower time window (300–600 ms) was applied to target diastolic phases (other parameters were kept consistent with group A). A simulated ECG signal was generated based on the estimated heart rate (HR) collected via pulse measurement prior to CCTA, without ECG leads placed on the patient. This simulated ECG signal was used to virtually gate the CT data acquired during a complete heart cycle (9). In group B, a conventional ECG-gated scanning mode was used. The data acquisition window was automatically gated based on the patient’s R-R interval, which was determined by their HR. Threshold monitoring was set at the aortic root, with an enhancement threshold of 180 HU and a delay time of 4 s.
Bolus tracking technique was employed for contrast injection, using Iopromide (370 mgI/100 ml, Bayer, Germany). The contrast volume was calculated as 0.6 ml/kg × body weight, with an injection time of 10 s. This was followed by a saline flush, injected at the same flow rate as the iodine contrast agent for 10 s. Beta-blockers and nitroglycerin were not administered to the patient before the CCTA examination.
Optimal reconstruction phases were automatically selected by the scanning system (Smart Phase technique, GE Healthcare), with a reconstruction slice thickness and interval of 0.625 mm. A coronary motion correction algorithm (SnapShot Freeze 2, SSF2, GE Healthcare) combined adjacent cardiac phases data within a single cardiac cycle to account for and compensate for coronary motion, improving image quality. This technology was specifically applied to vessel segments with significant motion artefacts. All images were reconstructed using deep learning image reconstruction algorithm (TrueFidelity™, GE Healthcare) combined with SSF2, ensuring minimal motion artifacts in the selected phases (10).
2.3 Image quality
2.3.1 Objective evaluation
CT attenuations and standard deviations (SD) at the aortic root (AO), proximal left main coronary (LMCA-P), middle left anterior descending artery (LAD-M), distal left anterior descending artery (LAD-D), middle left circumflex branch (LCX-M), distal left circumflex branch (LCX-D), proximal right coronary artery (RCA-P), middle right coronary artery (RCA-M), distal right coronary artery (RCA-D), and perivascular adipose tissue (PVAT) were measured by two cardiac radiologists on axial images on a post-processing workstation (AW4.7, GE Healthcare). The average value obtained from both radiologists was used for further analysis. The SD of the AO was considered image background noise (11). Regions of interest (ROI) were defined within a range of 20–400 mm2, carefully avoiding vascular walls and calcified plaque regions to ensure accurate measurements. The signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) were calculated using the following equations (12):
(1) SNR = CT attenuation of the ROI/image background noise.
(2) CNR = (CT attenuation of the ROI—CT attenuation of the PVAT)/image background noise.
A coronary artery enhancement threshold of 300 HU was considered satisfactory and sufficiently diagnostic (13).
2.3.2 Subjective evaluation
Image quality was independently evaluated by two radiologists, blinded to the imaging protocol, with 8 years and over 15 years of experience in cardiovascular imaging. The evaluation was based on the 18-segment classification system of the American Heart Association (14). A 4-point Likert scale was used to grade image quality as follows (15): 4 points, all major coronary segments are clearly visualized with adequate luminal enhancement, excellent contrast against surrounding tissues, and no artifacts; 3 points, all major coronary segments are well visualized, though with mild artifacts in the vessel wall or slightly blurred margins, and exhibit fair contrast with surrounding tissues; 2 points, all major coronary segments display moderate or stepped artifacts in the vessel wall and poor contrast but remain diagnostic; and 1 points, all major coronary segments exhibit severe motion artifacts, appear misaligned and discontinuous, and are non-diagnostic.
In addition to stenosis grading, atherosclerotic plaque characterization was performed in a subset of 40 patients (20 from each group) randomly selected from the cohort. Plaques were classified into three categories based on the SCCT guidelines: (1) calcified plaques, ≥130 HU density with no adjacent non-calcified components; (2) non-calcified plaques, <130 HU density without calcification; (3) mixed plaques, Containing both calcified (>130 HU) and non-calcified (<130 HU) components within the same lesion. Two blinded radiologists independently classified plaques on both ECG-less and ECG-gated CCTA images. Inter-observer agreement was calculated using Cohen’s kappa (κ) statistics.
2.4 ECG-less stenosis diagnosis performance
For the ECG-less group, diagnostic performance was evaluated using invasive coronary angiography (ICA) as the reference standard. Patients who underwent ICA were identified from the ECG-less group through an electronic medical records system. Two experienced observers, with 10 and 15 years of expertise in catheter angiography, independently assessed the degree of vascular stenosis based on ICA images. These observers were blinded to the CCTA results, and any disagreements were resolved through discussion. Similarly, two radiologists with 8 years and over 15 years of experience in cardiovascular imaging independently evaluated the vascular stenosis using CCTA images, with discrepancies also resolved through consensus discussion.
The evaluation of stenosis using ICA and CCTA was conducted at three levels: per-patient level, per-vessel level (four main coronary arteries), and per-segment level (18 segments). We defined significant coronary artery disease as luminal narrowing of ≥50% (16). Segments with a diameter of less than 1.5 mm were excluded from the analysis.
2.5 Radiation dose and examination time
The Dose Length Product (DLP) and volume CT dose index (CTDIvol) of CCTA imaging for each patient were recorded, and the effective radiation dose (ED) was calculated according to the formula ED = κ* DLP, where κ = 0.014 mSv/(mGy × cm) (17). The examination time from lying on the examination bed until the examination was completed was recorded. All examinations were performed by a single technician.
2.6 Statistical analysis
The Kolmogorov–Smirnov test was used to evaluate normally distributed data. Normally distributed continuous data are expressed as the mean ± SD, whereas non-normally distributed continuous data are expressed as the median with interquartile range. The count data are expressed as absolute values and corresponding frequencies/percentages. Statistical analysis between two independent samples was performed with the independent sample t-test or Mann–Whitney U-test. The chi-square test was used for statistical analysis of count data. The consistency of the subjective scores of the two physicians was tested using the kappa value, where 0.21–0.40 indicates poor consistency, 0.41–0.60 indicates moderate consistency, and 0.61–0.80 indicates good consistency. A probability (p) value of <0.05 was considered statistically significant. The diagnostic accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of CCTA to detect > 50% diameter stenosis on ICA were calculated from the chi-squared test of the contingency table on the per-segment, per-vessel, and per-patient level. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 26.0. (IBM Corporation, Armonk, NY, USA).
3 Results
3.1 Patient characteristics
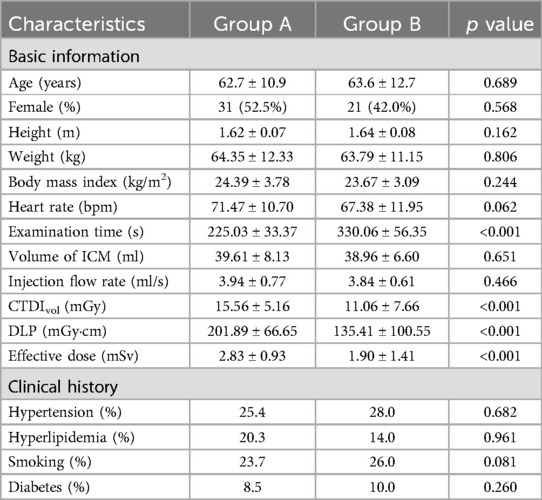
A total of 109 patients were included in the study, with 59 patients in group A and 50 patients in group B. No statistically significant differences were observed in age, height, weight, body mass index, heart rate, gender, clinical history and image noise between the two groups (all p > 0.05, Table 1).
In terms of radiation dose, the ED was significantly higher in group A than in group B (2.83 ± 0.93 vs. 1.90 ± 1.41 mSv, p < 0.001, Table 1). Additionally, there was a significant difference in the total examination time between group A and group B (225.03 ± 33.37 vs. 330.06 ± 56.35 s, p < 0.001, Table 1). The mean examination time in group A was reduced by approximately 32% compared to group B. At the same time, there were no significant differences in the amount and flow rate of iodine contrast agent between the two groups (both p > 0.05, Table 1).
3.2 Image quality
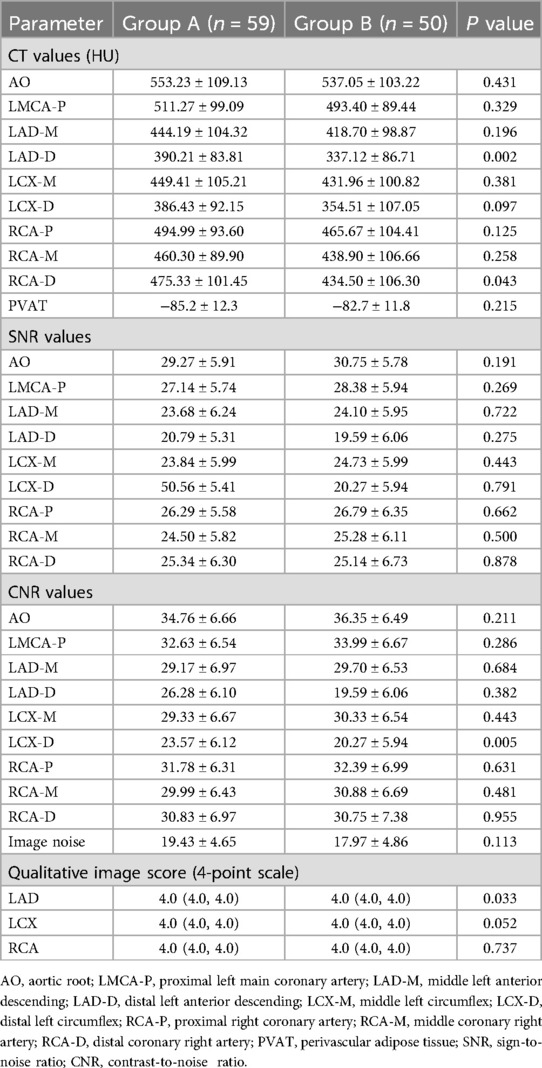
There was no significant difference in CT attenuation values between the two groups (p > 0.05; Table 2), but CT attenuation values for LAD-D and RCA-D were significantly higher in group A than in group B (p < 0.05, Table 2). The CT attenuation values of each vascular segment in group A were higher than those in group B (Table 2). In terms of SNR values, there was no significant difference between the two groups (p > 0.05, Table 2). There were no significant differences in the CNR values of all vessels between the two groups (p > 0.05, Table 2), but CNR for LCX-D were significantly higher in group A than in group B (p < 0.05, Table 2). The image quality scores were comparable between groups A and B for all vessels (p > 0.05, Table 2), however, the scores of LAD was significantly higher in group A than in B (p < 0.05, Table 2). Inter-rater reliability of the qualitative image score was good between the two radiologists (κ = 0.86).
3.3 ECG-less stenosis diagnosis performance
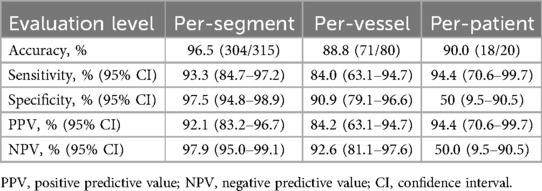
In group A, 20 patients underwent ICA examination after CCTA imaging, resulting in the analysis and comparison of 80 coronary arteries and 315 segments. The diagnostic accuracy of ECG-less CCTA for the detection of ≥50% stenosis on per-segment, per-vessel, and per-patient level were 96.5%, 88.8% and 90.0%, respectively (Table 3). The sensitivity of ECG-less CCTA for the detection of ≥50% stenosis on per-segment, per-vessel, and per-patient level were 93.3%, 84.0%, and 94.4%, respectively (Table 3). The weighted kappa value for agreement between two independent readers in CCTA was 0.84 and in ICA was 0.94.
The modified ECG-less protocol (Group A2) achieved a 28% reduction in effective dose (2.03 ± 0.75 mSv vs. 2.83 ± 0.93 mSv in Group A, p < 0.001) while maintaining diagnostic accuracy (per-segment sensitivity: 91.2% vs. 93.3%, p = 0.45; specificity: 96.8% vs. 97.5%, p = 0.68). Radiation dose in Group A2 was statistically comparable to conventional ECG-gated CCTA (1.90 ± 1.41 mSv, p = 0.62) (Supplementary Table S1).
In a post hoc analysis of 40 patients (20 per group), ECG-less CCTA demonstrated high agreement with ECG-gated CCTA in plaque characterization (calcified: κ = 0.82; non-calcified: κ = 0.78; mixed: κ = 0.75) (Table 4).
4 Discussion
This study demonstrated that the objective image quality and subjective scores of ECG-less CCTA were not inferior to those of conventional ECG-gated CCTA, and ECG-less CCTA reduced examination time by approximately 46.6%. However, the radiation dose of ECG-less CCTA was significantly higher than that of conventional. ECG-less CCTA is both feasible and broadly applicable, as it does not impose strict on heart rate, rhythm or respiratory state. These advantages are attributable to advancements in the hardware and software of modern CT technology. On the hardware side, wide-body CT detectors now provide a maximum coverage of up to 16 cm, enabling single axial scans for coronary artery imaging. Additionally, gantry rotation speeds have been increased to as fast as 0.23 s/r, significantly reducing motion artifacts during scanning. From the software perspective, algorithms specifically designed to correct for coronary motion have been developed. Notably, the SSF2 algorithm, which reconstructs coronary arteries using three-phase images, offers enhanced correction for coronary motion, further improving image quality. Moreover, the Smart Optimal Phase Selection Technology (Smartphase) can quickly and automatically select the phase with the least artefacts among multiple phases of the cardiac coronary arteries to reconstruct the CCTA with the best phase, which is an indispensable and critical key in achieving the success of ECG-less CCTA.
In this study, the CT values of all vascular segments in group A were higher than those in group B, and all exceeded 325 HU, meeting the requirements of coronary diagnosis guidelines (16). There were no significant differences between the two groups regarding SNR and image noise. In terms of CNR, the value for LCX-D in group A was significantly higher than that in group B, while no significant difference was observed in the other vascular segments. Regarding the subjective scores, all three main vessels received 4 points in group A, and the image quality fully met the diagnostic requirements. The diagnostic accuracy was 90.0% at the per-patient level in the 20 patients in the present study, which is consistent with these reports in terms of diagnostic accuracy (18, 19). However, only 20 patients (33.9%) in the ECG-less group underwent ICA, introducing potential verification bias. This reflects real-world clinical practice where ICA is typically reserved for high-risk cases. To address this, in future trial, enrolling only patients who have both CCTA and ICA.
Beyond stenosis grading, our study demonstrates that ECG-less CCTA achieves high accuracy in plaque characterization (κ = 0.82 for calcified plaques), comparable to ECG-gated protocols. This capability is critical for risk stratification, as vulnerable non-calcified plaques are associated with higher cardiovascular event rates (3, 5). The slightly lower agreement for mixed plaques (κ = 0.75) may reflect challenges in delineating heterogeneous components during rapid coronary motion, which could be mitigated by future motion correction algorithms.
In this study, the average effective radiation dose of ECG-less CCTA was 2.83 ± 0.93 mSv, which increased by about 48% compared with conventional prospectively ECG-gated CCTA. However, compared with other studies, the effective radiation dose of ECG-less CCTA is close to or even lower than that of the third-generation dual-source CT (20). The slightly higher effective radiation dose in this study was mainly due to the widened acquisition time window (200–750 ms). To address this issue, upcoming research could investigate these dose optimization strategies: (1) Dynamic Tube Current Modulation, which entails adjusting the tube current in real-time according to vascular motion patterns during the card.ac cycle, thereby minimizing unnecessary radiation exposure during high-motion phases (21); (2) AI-Driven Reconstruction Algorithms, where deep learning methods such as TrueFidelity™ effectively reduce image noise at lower radiation doses, improving the signal-to-noise ratio (11); (3) Personalized Acquisition Windows, where narrowing the time window to 300–500 ms for patients with stable heart rates helps capture essential diastolic phases while also decreasing radiation dose (7).
In a follow-up study, we found that our modified ECG-less protocol achieved a 28% reduction in effective dose (2.03 mSv) compared to the original protocol, approaching the dose level of conventional ECG-gated CCTA (1.90 mSv, p = 0.62, Supplementary Table S1). This was accomplished through a combination of narrowed acquisition windows (300–600 ms).
Two studies (21, 22) showed that prospectively ECG-triggered CCTA in a single cardiac cycle can achieve high image quality and accuracy with low radiation dose using a wide detector and single-source CT system, but it was limited to patients with HRs <75 beats/min. In this study, we found that ECG-less CCTA can obtain satisfactory image quality between 51 bpm and 112 bpm (Table 1, Figure 2). Therefore, we can assume that ECG-less CCTA can maintain image quality at arbitrary heart rates.
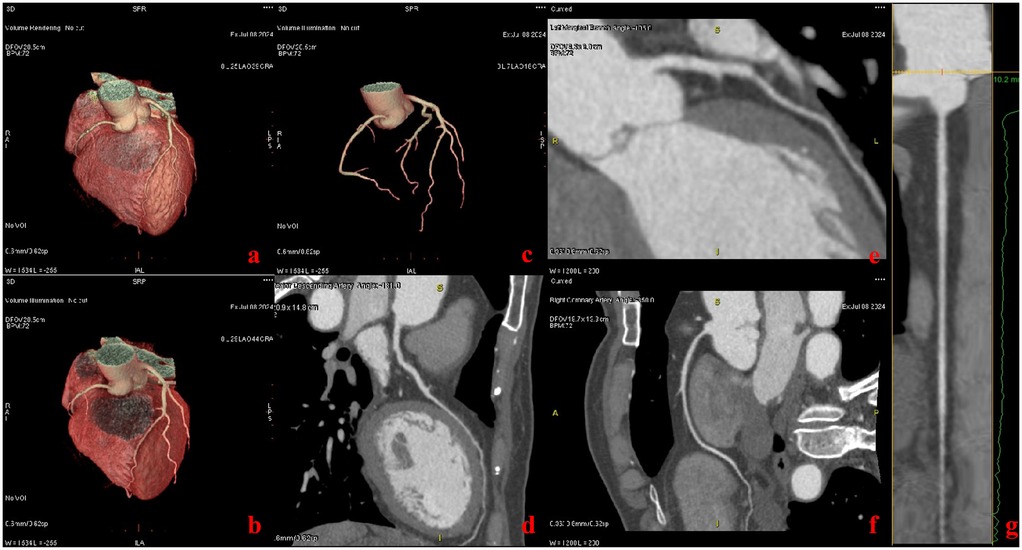
Figure 2. Patient with chief complaint of chest pain (heart rate: 68 bpm). Images were acquired with the ECG-less CCTA. Volume rendering reformat CT images show the heart (a–c). Curved multiplanar reformat CT images show the left anterior descending artery (d), left marginal branch (e), and the right coronary artery (f, g).
The average examination time for ECG-less CCTA is 225 s (Table 1), allowing for the completion a full CCTA in four minutes while maintaining satisfactory image quality. The mean time reduction of 1.7 min with ECG-less CCTA may appear modest in routine practice. Its value is most evident in scenarios where ECG lead placement is precluded, such as: (1) severe thoracic trauma or burns preventing lead adhesion; (2) cultural/religious objections to chest exposure; (3) Unreliable ECG signals (e.g., cardiac tamponade, hyperkalemia). In these cases, ECG-Less gating eliminates delays associated with lead application or signal optimization.
In this study, we have demonstrated that ECG-less CCTA can reduce examination time and improve the efficiency of CCTA while maintaining image quality. However, this study has the following limitations. First, the relatively small sample size, particularly the subgroup of patients undergoing ICA for validation (n = 20 in the ECG-less group), may have introduced statistical instability in specificity calculations. For instance, a single false-positive case disproportionately reduced per-patient specificity (50%), underscoring the need for larger validation cohorts. Second, subgroup analyses based on heart rate, BMI, or coronary calcification burden were not feasible due to imbalanced distributions (e.g., only 5 patients with BMI > 30 kg/m2 in the ECG-less group), which limits insights into performance variability across clinically relevant subpopulations. To address these limitations, we will conduct a multicenter trial with protocol- mandated ICA validation for all participants in the future. This initiative will enable robust subgroup stratification and refine the technique’s applicability in complex populations, including those with high calcium burden or arrhythmias. Third, the ECG-less CCTA radiation dose is higher than the conventional ECG-gated CCTA radiation dose because of the wide exposure interval (200–750 ms) we set. Therefore, future research should reduce the exposure time without affecting the conclusions of this study. Fourth, the single-center recruitment and subjective image quality assessments by institutional radiologists may introduce selection and observer bias. To enhance external validity, future studies will involve blinded image evaluations by independent readers from external centers. At last, emergency patients were excluded from this study, limiting direct extrapolation of our findings to time-sensitive scenarios. This design choice was made to ensure protocol standardization, but future trials must validate ECG-less CCTA in hemodynamically unstable cohorts.
5 Conclusion
In conclusion, ECG-less CCTA can obtain satisfactory image quality while shortening the examination time and improving the examination efficiency. Furture multicenter, large-scale studies are warranted to further validate its clinical value in complex lesions and acute/critical care scenarios.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Zhejiang University School of Medicine Sir Run Run Shaw Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
KW: Conceptualization, Data curation, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing, Resources, Visualization. YZ: Data curation, Investigation, Methodology, Software, Writing – original draft. BC: Data curation, Formal analysis, Methodology, Project administration, Writing – original draft. HR: Writing – original draft, Writing – review & editing, Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Software, Supervision, Validation, Visualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the Medical Engineering Research Program of the National Institute of Hospital Administration, NHC (2024MEB315).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1570743/full#supplementary-material
References
1. Bhatt DL, Lopes RD, Harrington RA. Diagnosis and treatment of acute coronary syndromes: a review. JAMA. (2022) 327(7):662–75. doi: 10.1001/jama.2022.0358
2. Wong CX, Brown A, Lau DH, Chugh SS, Albert CM, Kalman JM, et al. Epidemiology of sudden cardiac death: global and regional perspectives. Heart Lung Circ. (2019) 28(1):6–14. doi: 10.1016/j.hlc.2018.08.026
3. Williams MC, Moss AJ, Dweck M, Adamson PD, Alam S, Hunter A, et al. Coronary artery plaque characteristics associated with adverse outcomes in the SCOT-HEART study. J Am Coll Cardiol. (2019) 73(3):291–301. doi: 10.1016/j.jacc.2018.10.066
4. Ferencik M, Mayrhofer T, Bittner DO, Emami H, Puchner SB, Lu MT, et al. Use of high-risk coronary atherosclerotic plaque detection for risk stratification of patients with stable chest pain: a secondary analysis of the PROMISE randomized clinical trial. JAMA Cardiol. (2018) 3(2):144–52. doi: 10.1001/jamacardio.2017.4973
5. Williams MC, Kwiecinski J, Doris M, McElhinney P, D’Souza MS, Cadet S, et al. Low-attenuation noncalcified plaque on coronary computed tomography angiography predicts myocardial infarction: results from the multicenter SCOT-HEART trial (Scottish computed tomography of the HEART). Circulation. (2020) 141(18):1452–62. doi: 10.1161/CIRCULATIONAHA.119.044720
6. Hagar MT, Soschynski M, Saffar R, Rau A, Taron J, Weiss J, et al. Accuracy of ultrahigh-resolution photon-counting CT for detecting coronary artery disease in a high-risk population. Radiology. (2023) 307(5):e223305. doi: 10.1148/radiol.223305
7. Liu ZY, Ma ZP, Gao K, Ding W, Zhao YX. Coronary computed tomography angiography using an optimal acquisition time window based on heart rate determined during breath-holding following free breathing. J Comput Assist Tomogr. (2024) 49(2):265–70. doi: 10.1097/RCT.0000000000001666
8. Liu Z, Sun Y, Zhang Z, Chen L, Hong N. Feasibility of free-breathing CCTA using 256-MDCT. Medicine. (2016) 95(27):e4096. doi: 10.1097/MD.0000000000004096
9. Nabipoorashrafi A, Chakeri Z, Chalian H. Cardiac CT angiography (CCTA) without ECG gating. J Cardiovasc Comput Tomogr. (2024) 18(4):S64–5. doi: 10.1016/j.jcct.2024.05.152
10. Yamaguchi S, Ichikawa Y, Takafuji M, Sakuma H, Kitagawa K. Usefulness of second-generation motion correction algorithm in improving delineation and reducing motion artifact of coronary computed tomography angiography. J Cardiovasc Comput Tomogr. (2024) 18(3):281–90. doi: 10.1016/j.jcct.2024.02.008
11. Jin L, Gao P, Wang K, Li J, Li M. Intraindividual evaluation of effects of image filter function on image quality in coronary computed tomography angiography. Front Cardiovasc Med. (2022) 9:840735. doi: 10.3389/fcvm.2022.840735
12. Pontone G, Muscogiuri G, Baggiano A, Andreini D, Guaricci AI, Guglielmo M, et al. Image quality, overall evaluability, and effective radiation dose of coronary computed tomography angiography with prospective electrocardiographic triggering plus intracycle motion correction algorithm in patients with a heart rate over 65 beats per Minute. J Thorac Imaging. (2018) 33(4):225–31. doi: 10.1097/RTI.0000000000000320
13. Jin L, Wang K, Wang X, Li C, Sun Y, Gao P, et al. Bodyweight-adjusted contrast media with shortened injection duration for step-and-shoot coronary computed tomography angiography to acquire improved image quality. J Thorac Imaging. (2024) 39(3):146–56. doi: 10.1097/RTI.0000000000000696
14. Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, et al. A reporting system on patients evaluated for coronary artery disease. Report of the ad hoc committee for grading of coronary artery disease, council on cardiovascular surgery, American heart association. Circulation. (1975) 51(4):5–40. doi: 10.1161/01.CIR.51.4.5
15. Liang J, Wang H, Xu L, Yang L, Dong L, Fan Z, et al. Diagnostic performance of 256-row detector coronary CT angiography in patients with high heart rates within a single cardiac cycle: a preliminary study. Clin Radiol. (2017) 72(8):694.e7–694.e14. doi: 10.1016/j.crad.2017.03.004
16. Abbara S, Blanke P, Maroules CD, Cheezum M, Choi AD, Han BK, et al. SCCT Guidelines for the performance and acquisition of coronary computed tomographic angiography: a report of the society of cardiovascular computed tomography guidelines committee: endorsed by the North American society for cardiovascular imaging (NASCI). J Cardiovasc Comput Tomogr. (2016) 10(6):435–49. doi: 10.1016/j.jcct.2016.10.002
17. Jia CF, Zhong J, Meng XY, Sun XX, Yang ZQ, Zou YJ, et al. Image quality and diagnostic value of ultra low-voltage, ultra low-contrast coronary CT angiography. Eur Radiol. (2019) 29(7):3678–85. doi: 10.1007/s00330-019-06111-0
18. Koplay M, Erdogan H, Avci A, Sivri M, Demir K, Guler I, et al. Radiation dose and diagnostic accuracy of high-pitch dual-source coronary angiography in the evaluation of coronary artery stenoses. Diagn Interv Imaging. (2016) 97(4):461–9. doi: 10.1016/j.diii.2015.10.008
19. Li ZN, Yin WH, Lu B, Yan HB, Mu CW, Gao Y, et al. Improvement of image quality and diagnostic performance by an innovative motion-correction algorithm for prospectively ECG triggered coronary CT angiography. PLoS One. (2015) 10(11):e0142796. doi: 10.1371/journal.pone.0142796
20. Jin L, Gao Y, Shan Y, Sun Y, Li M, Wang Z. Qualitative and quantitative image analysis of 16 cm wide-coverage computed tomography compared to new-generation dual-source CT. J Xray Sci Technol. (2020) 28(3):527–39. doi: 10.3233/XST-190624
21. Chen MY, Shanbhag SM, Arai AE. Submillisievert median radiation dose for coronary angiography with a second-generation 320-detector row CT scanner in 107 consecutive patients. Radiology. (2013) 267(1):76–85. doi: 10.1148/radiol.13122621
22. de Graaf FR, Schuijf JD, van Velzen JE, Kroft LJ, de Roos A, Reiber JH, et al. Diagnostic accuracy of 320-row multidetector computed tomography coronary angiography in the non-invasive evaluation of significant coronary artery disease. Eur Heart J. (2010) 31(15):1908–15. doi: 10.1093/eurheartj/ehp571
Keywords: coronary computed tomography angiography, electrocardiogram, wide-detector CT, image quality, radiation dose
Citation: Wang K, Zhang Y, Chen B and Ren H (2025) Comparing image quality of coronary CT angiography with and without ECG-gating in wide-detector CT. Front. Cardiovasc. Med. 12:1570743. doi: 10.3389/fcvm.2025.1570743
Received: 4 February 2025; Accepted: 31 March 2025;
Published: 11 April 2025.
Edited by:
Marcello Chiocchi, University of Rome Tor Vergata, ItalyReviewed by:
Thomas Stocker, LMU Munich University Hospital, GermanyEleonora Melotti, IRCCS Ospedale Galeazzi Sant'Ambrogio, Italy
Copyright: © 2025 Wang, Zhang, Chen and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Ren, MzE5NDA3NkB6anUuZWR1LmNu
 Kun Wang
Kun Wang Yueqiao Zhang
Yueqiao Zhang