- 1Student Research Committee, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 2School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 3Department of Cardiology, Islamic Azad University, Tabriz, Iran
- 4Student Research Committee, Tehran University of Medical Sciences, Tehran, Iran
- 5School of Medicine, Hormozgan University of Medical Sciences, Bandar Abbas, Hormozgan, Iran
- 6Department of Occupational Health and Safety, School of Public Health, Shiraz University of Medical Sciences, Shiraz, Iran
- 7School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
- 8Department of Public Health, Faculty of Health and Paramedicine, Neyshabur University of Medical Sciences, Neyshabur, Iran
- 9Pharmaceutical Sciences Research Center, Tehran Medical Sciences, Islamic Azad University, Tehran, Iran
- 10School of Medicine, Golestan University of Medical Sciences, Gorgan, Golestan, Iran
Background and Aim: Hypertension (HTN) is a widespread global health challenge, and its increasing prevalence is attributed to individual and environmental risk factors. Persistent organic pollutants (POPs), especially polychlorinated biphenyls (PCBs), contribute to cardiovascular risk by accumulating in fatty tissues, which leads to oxidative stress and vascular inflammation. This review and meta-analysis aimed to investigate the association between PCB exposure and hypertension.
Methods: Adhering to the PRISMA 2020 guidelines, data sources such as PubMed, Scopus, Web of Science, and Google Scholar were systematically searched up to July 2024 to find observational studies on the link between PCBs and hypertension risk. Studies were reviewed and chosen according to established inclusion and exclusion criteria, focusing on observational studies examining PCB exposure and hypertension risk. Independent reviewers conducted data extraction, and the quality of studies was evaluated using the JBI critical appraisal tool. A meta-analysis with a random-effects model was conducted to determine combined odds ratios (ORs) for hypertension linked to total PCB exposure and specific PCB types.
Results: Of the 494 records identified, 21 studies met the inclusion criteria, comprising 5 cohort studies, 15 cross-sectional studies, and one case-control study, totaling 51,514 participants. Exposure to total PCBs correlated with an elevated risk of hypertension (OR = 1.78, 95% CI: 1.30–2.44). Dioxin-like PCBs were also associated with a heightened risk (OR = 1.54, 95% CI: 1.24–1.90), while non-dioxin-like PCBs were not significantly linked (OR = 1.16, 95% CI: 0.81–1.66). Among individual congeners, PCB-74, PCB-118, PCB-105, and PCB-153 were significantly related to higher hypertension risk.
Conclusion: These findings indicate a positive correlation between PCB exposure and hypertension, particularly with dioxin-like PCBs and certain PCB congeners. Additional research is necessary to clarify the mechanisms involved and to promote measures for reducing PCB exposure, particularly in high-risk populations.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42024595223, PROSPERO (CRD42024595223).
1 Introduction
Hypertension (HTN) is a crucial global health issue and one of the most significant risk factors for cardiovascular disease (1–3). It affects approximately 1.3 billion adults globally aged 30–79, contributing to a substantial health burden (1), particularly in countries with low and middle incomes (LMICs) (2, 3). Based on the 2023 WHO global report on HTN, this condition causes approximately 10.8 million deaths annually. Global prevalence of HTN is continuously rising, as WHO reports an increase from 24%–28% in the western Pacific region. From 144 million in 1990, the number rose to 346 million in 2019. In the same period, Southeast Asia experienced a rise in prevalence from 29%–32%. Over the past three decades, the HTN prevalence increased by 144% in Southeast Asia and the Western Pacific and 41% in Europe (1). This rising problem seems linked to well-established individual risk factors such as decreased physical activity, obesity, and increased salt consumption. Environmental factors, including contaminants like air pollution and heavy metals such as cadmium, lead, and pesticides, are likely to play an important role. Among environmental pollutants, polychlorinated biphenyls (PCBs), a type of persistent organic pollutant (POP), have gained particular attention, as they can lead to HTN by inducing oxidative stress, interfering with endocrine function, and causing vascular inflammation (4–9).
PCBs are persistent environmental contaminants detected across various ecosystems. They have been identified in the atmosphere (10, 11), in soils and water (12), and in marine sediments (13). PCBs bioaccumulate in human adipose tissue and follicular fluid, with potential implications for human health (14, 15). Wildlife exposure is also evident, with high concentrations found in avian species (16).
Despite regulatory bans since the 1970s, PCBs persist in outdated industrial equipment and continue to be released through waste incineration and other industrial processes (13). Their fat-soluble nature allows them to bioaccumulate in the fatty tissues of living organisms, leading to serious health issues (17, 18). An umbrella review reported that PCBs can cause different diseases, including type 2 DM, cardiovascular disease, non-Hodgkin lymphoma, breast cancer, liver disease in adults, low birth weight, and bronchitis in pediatric and infant populations (19). PCBs consist of 209 distinct congeners with varying chemical structures and physicochemical properties, potentially leading to different biological effects. Understanding the role of specific PCB congeners rather than total PCB concentrations alone is crucial, as these compounds may act through various biological mechanisms (20, 21). Some congeners exhibit estrogenic or anti-estrogenic activity that could affect blood vessel function. In contrast, others may activate different cellular pathways, like the aryl hydrocarbon receptor (AhR), potentially contributing to inflammatory and endothelial responses (17, 22). This diversity in biological mechanisms emphasizes the importance of evaluating PCB congeners individually in health risk assessments.
Prior research has shown heterogeneous findings regarding the connection between HTN and PCB congeners. Multiple studies have demonstrated a statistically significant connection between HTN development and the concentration of specific PCB congeners in serum (20, 23, 24). Other studies have failed to establish substantial correlations between specific other PCB congeners and hypertensive outcomes (25, 26). Given the complexity of PCB congeners, our study provides an updated synthesis of recent evidence to reflect the current understanding of this relationship. Additionally, unlike previous analyses that focused on a limited subset of PCB congeners, we aimed to examine a broader range of congeners, offering a comprehensive evaluation of their potential impact on hypertension. This systematic review and meta-analysis aimed to investigate the relationship of PCB exposure with hypertension.
2 Materials and methods
This study follows the 2020 PRISMA guidelines for systematic reviews and meta-analyses and applies the standard procedures outlined in the Cochrane Handbook to ensure a thorough and reliable review (27, 28). Before starting the review, we registered the study protocol in the International Prospective Register of Systematic Reviews (PROSPERO; registration ID: CRD42024595223), outlining the search strategy, inclusion criteria, and intended outcomes.
2.1 Search strategy and study selection
We searched PubMed, Scopus, Web of Science, and Google Scholar for research evaluating the effect of exposure to polychlorinated biphenyl on the risk of HTN for papers released up until July 21, 2024 (Supplementary Table S1). “Polychlorinated biphenyls” or “PCBs” and “hypertension” or “high blood pressure” are the medical MeSH terminology that we utilized. We manually looked for related studies using the included publications' citations to ensure we got all. We screened the titles and abstracts after utilizing EndNote to eliminate duplicate articles. To ensure articles were correctly included, we reviewed the deleted articles again. Two authors (F.N. and SF.H.) conducted the full-text screening process, and the reasons for excluding studies during the title, abstract, or full-text review were documented in detail. Each author reviewed and validated the other's screenings to ensure all relevant articles were included. In cases where both reviewers encountered uncertainty, the corresponding author (M.R.) conducted a further review to resolve any uncertainties. These methods allowed for an extensive and in-depth literature search, covering foundational and recent studies on our topic.
2.2 Inclusion and exclusion criteria
Only original articles were included. Studies involving laboratory animals, and non-original articles, including reviews and systematic reviews, were excluded. Individuals with systolic blood pressure (SBP) over 130 and diastolic blood pressure (DBP) over 80 were included. We first included those examining how PCBs affected metabolic disorders to ensure no publications were omitted. However, during full-text screening, we eliminated those that did not directly evaluate how PCBs affected HTN. We did not consider the kind of PCB when deciding which publications to include or exclude; however, studies that looked at all pollutants that were comparable to PCB without precisely evaluating the impact of PCB were not included. The investigation was carried out on all populations, and we did not use any age or gender-based inclusion or exclusion criteria in our article.
2.3 Data extraction
Two reviewers (I.E and A.S) developed a data extraction form and applied it to all studies that met the eligibility criteria. They extracted data independently and resolved any disagreements by consensus. The following details were recorded: first author, study population, country, publication year, detection methods for PCB exposure, PCB type, and any underlying metabolic conditions of participants. Individuals with a DBP > 80 and SBP > 130 were specifically examined. The PCBs extracted included DL-PCB, NDL-PCB, PCB-52, PCB-74, PCB-99, PCB-105, PCB-118, PCB-138, PCB-153, PCB-156, PCB-157, PCB-170, PCB-180, PCB-183, and PCB-187.
2.4 Quality assessment
Two blinded reviewers (Z.H and A.S) evaluated the studies' quality. The quality of the research was assessed using JBI's critical appraisal tools for studies on prevalence (29). When there were disagreements, a third reviewer was consulted. Essential items evaluated included the study population, exposure measurements, follow-up data, founding factors, outcome scope, and statistical analysis.
2.5 Statistical analysis
To determine the pooled odds ratio (OR) and associated 95% CI for the risk of HTN in patients in the highest quartile of PCB exposure as opposed to those in the lowest quartile, we performed numerous random-effects meta-analyses. The analyses included total PCBs, dioxin-like (DL), non-dioxin-like (NDL), and individual PCB congeners reported by at least four studies. Effect estimates varied from per-unit changes to comparisons across tertiles or quartiles. As explained in previous studies, we rescaled these estimates to quartiles to ensure a consistent approach for comparing study-specific estimates and interpreting findings (30). The restricted maximum likelihood (REML) model was applied to the analysis. Cochran's Q statistics and I2 tests were used to evaluate heterogeneity and inconsistency. Egger's regression test was also used to assess publication bias. Statistical significance was defined as P values below 0.05.
All analyses were conducted using R software (version 4.4.1, released on 2024-06-14) with the “meta” and “metafor” packages.
3 Results
3.1 Study selection
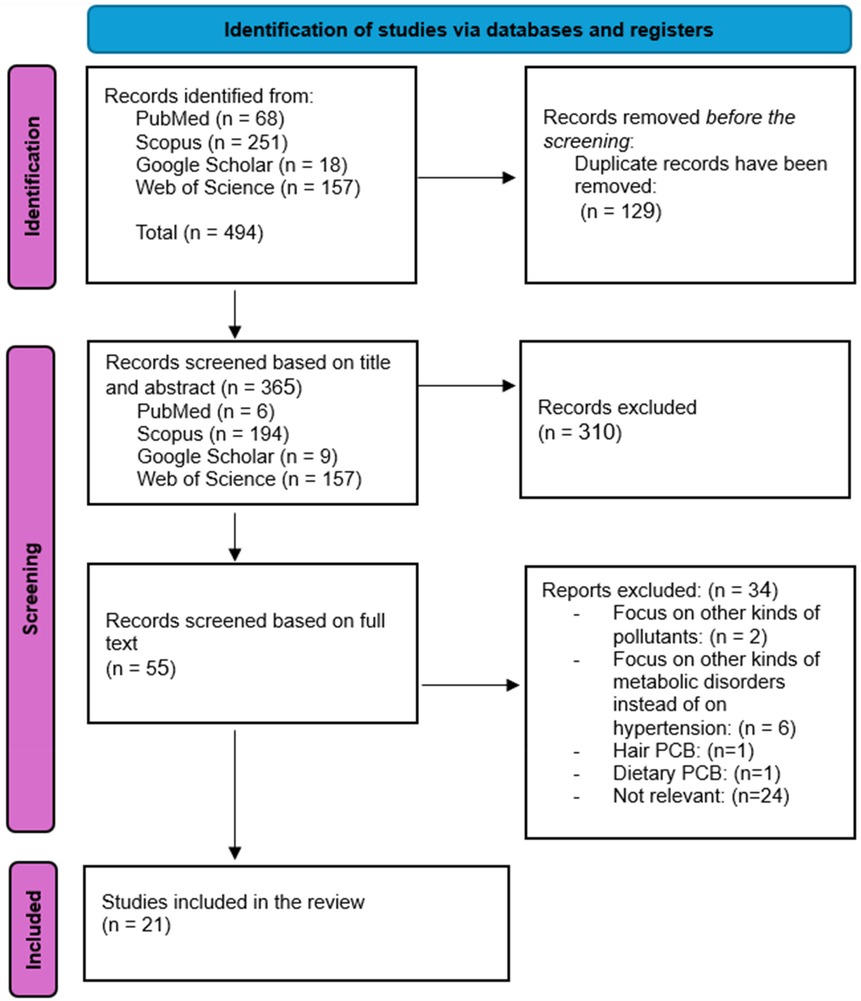
The process of study selection is illustrated in Figure 1. A total number of 494 records were detected through database searches, specifically from PubMed (n = 68), Web of Science (n = 157), Google Scholar (n = 18), and Scopus (n = 251). After removing 129 duplicate records, 365 were screened based on their titles and abstracts. Following this initial screening, 310 records were excluded due to irrelevance. Subsequently, 55 records underwent full-text screening. Of these, 34 records were excluded: 2 focused on pollutants other than polychlorinated biphenyls, 6 examined metabolic disorders unrelated to hypertension, 1 focused on hair PCBs, 1 focused on dietary PCBs, and 24 were irrelevant to our study topic. Ultimately, 21 studies fulfilled our inclusion criteria and were incorporated into this systematic review.
3.2 Study characteristics
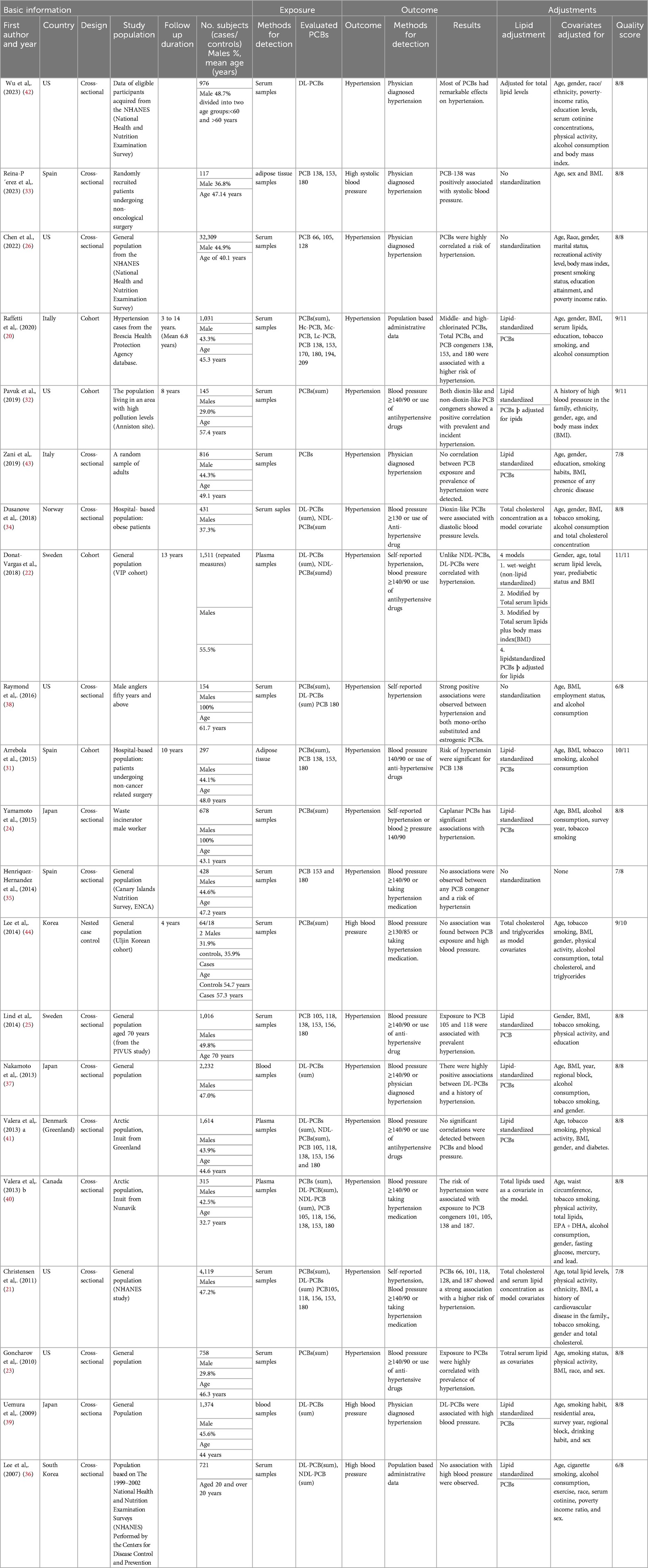
In total, 21 observational studies were included in this study, comprising 5 cohort studies (20, 22, 31–33), 15 cross-sectional studies (21, 23–26, 34–43), and a case-control study (44). Across these studies, 51,514 participants were involved, with 24,818 men and 25,975 women. The remaining 721 participants lack gender distinction, as one study did not specify male and female categories for these cases (36). The mean age across studies was 48.9 years, based on the 18 studies that provided age data. Age information was unavailable for three studies (36, 39, 42). The studies were carried out in several countries, including Canada (40), the USA (21, 23, 26, 32, 36, 38, 42), Norway (34), Japan (24, 37, 39), Spain (31, 33, 35), Italy (20, 43), Korea (44), Sweden (22, 25), and Greenland (41) (Table 1).
3.3 Quality of the studies
For quality assessment based on the JBI checklist, the included studies demonstrated generally high methodological quality across the different study designs. For the cross-sectional studies (Supplementary Table S2), a few studies were found to have minor limitations, such as Zani et al. (43) and Raymond et al. (38) studies, which had unclear reporting on the reliability of the outcome measures and the Henriquez-Hernandez et al. (35) study, which did not clearly describe strategies to address confounding factors. Regarding the cohort studies (Supplementary Table S3), the Pavuk et al. (32) and Arrebola et al. (31) studies did not report on the completeness of follow-up. For the single case-control study by Lee et al. (44) (Supplementary Table S4), all quality criteria were fulfilled, except for the exposure period, which was rated as “unclear” in terms of whether it was long enough to be meaningful.
3.4 Meta-analysis results
3.4.1 Total PCBs
The meta-analysis of nine studies examining total polychlorinated biphenyls (PCBs) demonstrated a significant positive connection with HTN, with an odds ratio (OR) of 1.78 (95% CI: 1.30–2.44) for the highest exposure category compared to the lowest. The random-effects model revealed moderate heterogeneity (I² = 53.18%, Q = 16.21, p = 0.0395) (Figure 2).
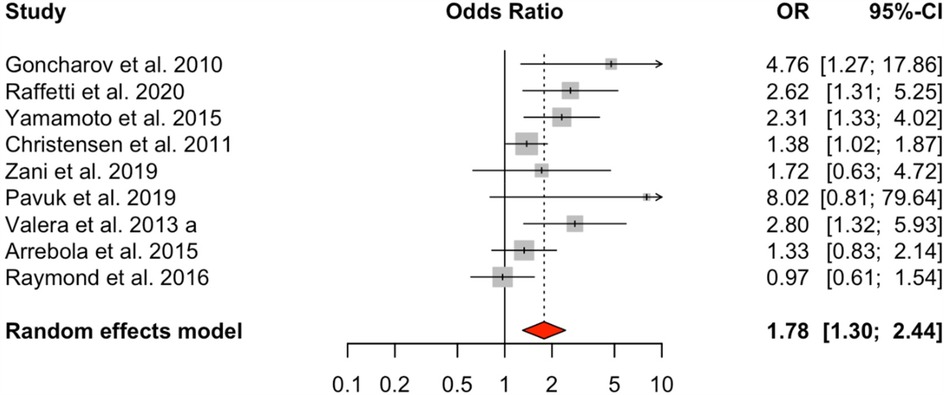
Figure 2. HTN risk based on total PCB exposure. Meta-analyses employing random-effects models. OR, odds ratio; CI, confidence interval.
3.4.2 PCB groups
Analysis of dioxin-like PCBs (DL-PCBs) across nine studies also revealed a significant positive association with HTN (OR = 1.54, 95% CI: 1.24–1.90), with a moderate level of heterogeneity (I² = 31.99%, Q = 10.02, p = 0.2634) (Figure 3). In contrast, non-dioxin-like PCBs (NDL-PCBs), examined in five studies, exhibited a non-significant association (OR = 1.16, 95% CI: 0.81–1.66) and showed moderate heterogeneity (I² = 48.34%, p = 0.0929) (Figure 4).
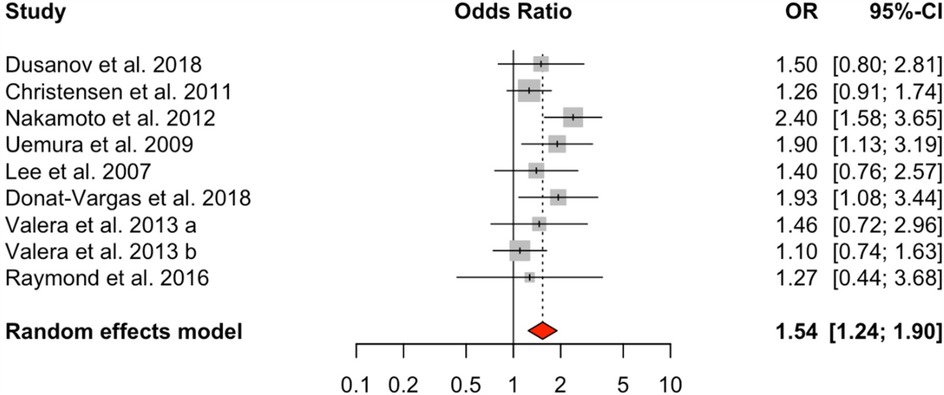
Figure 3. HTN risk based on DL-PCBs exposure. Meta-analyses employing random-effects models. OR, odds ratio; CI, confidence interval.
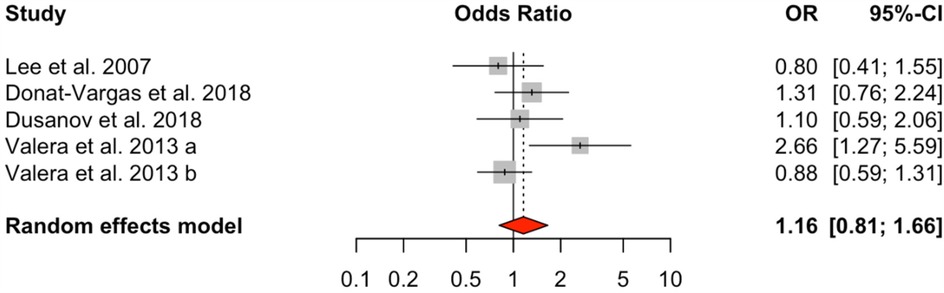
Figure 4. HTN risk based on NDL-PCBs exposure. Meta-analyses employing random-effects models. OR, odds ratio; CI, confidence interval.
3.4.3 Individual PCB congeners
Four of the individual PCB congeners analyzed demonstrated significant positive associations with hypertension. PCB-74 showed the strongest association (OR = 1.71, 95% CI: 1.37–2.14) showing no heterogeneity (I² = 0.00%, p = 0.5461) (Supplementary Figure S1), followed by PCB-118 (OR = 1.60, 95% CI: 1.31–1.96, I² = 15.71%) (Supplementary Figure S2), PCB-105 (OR = 1.45, 95% CI: 1.20–1.75, I² = 17.92%) (Supplementary Figure S3), and PCB-153 (OR = 1.27, 95% CI: 1.03–1.56, I² = 12.63%) (Supplementary Figure S4). These associations were characterized by low heterogeneity across studies.
Several PCB congeners showed nonsignificant associations with hypertension. PCB-187 (OR = 1.35, 95% CI: 0.83–2.19, I² = 73.22%, p = 0.0301) (Supplementary Figure S5) and PCB-138 (OR = 1.29, 95% CI: 0.98–1.68, I² = 29.89%, p = 0.2335) (Supplementary Figure S6) exhibited trending positive associations, though not reaching statistical significance. PCB-99 (OR = 1.10, 95% CI: 0.93–1.31, I² = 0.00%, p = 0.5478) (Supplementary Figure S7), PCB-180 (OR = 1.08, 95% CI: 0.88–1.34, I² = 16.99%, p = 0.5337) (Supplementary Figure S8), PCB-156 (OR = 1.06, 95% CI: 0.73–1.52, I² = 70.46%, p = 0.0108)(Supplementary Figure S9), PCB-157 (OR = 1.05, 95% CI: 0.77–1.44, I² = 54.62%, p = 0.0866) (Supplementary Figure S10), PCB-170 (OR = 1.02, 95% CI: 0.77–1.35, I² = 31.21%, p = 0.3644) (Supplementary Figure S11), and PCB-183 (OR = 1.02, 95% CI: 0.85–1.23, I² = 0.00%, p = 0.9124) (Supplementary Figure S12) all showed weak, non-significant associations. PCB-52 demonstrated a non-significant negative association (OR = 0.83, 95% CI: 0.68–1.02, I² = 0.01%, p = 0.3789) (Supplementary Figure S13). Heterogeneity varied considerably among these non-significant associations, ranging from none (I² = 0.00% for PCB-99 and PCB-183), low (I² = 16.99% for PCB-180), moderate (I² = 31.21% for PCB-170, I² = 54.62% for PCB-157), to high (I² = 73.22% for PCB-187 and I² = 70.46% for PCB-156).
The results of the Egger's test showed no significant publication bias in the analyzed studies (results not shown) (Supplementary Figure S14). Funnel plots for all analyses are provided in Supplementary Figures S14–S29.
5 Discussion
This systematic review and meta-analysis included 21 observational studies with 51,514 participants and focused on the association between PCBs and HTN. This analysis revealed a significant association between PCBs and HTN, with a stronger association for DL-PCBs, indicating their distinct impact on cardiovascular health, unlike NDL-PCBs, which showed no meaningful association.
PCBs contribute to HTN through mechanisms that impact vascular function, cellular signaling, and hormonal balance. These chemicals induce oxidative stress, impairing nitric oxide (NO) production, which promotes vasoconstriction and raises BP (45). Additionally, PCBs activate the poly (ADP-ribose) polymerase (PARP) pathway, depleting cellular energy and weakening vascular function (46). These compounds also disrupt calcium signaling, particularly by activating ryanodine receptors, which increase vascular tone and further contribute to HTN (47). Mitochondrial dysfunction from PCB exposure exacerbates oxidative stress and energy imbalance, reinforcing hypertensive effects (48). Lastly, PCBs disrupt thyroid and adrenal hormones, critical blood pressure regulators (49). This extensive interference with vascular function indicates the cardiovascular dangers associated with PCB exposure.
In line with other research that has connected elevated PCB levels to a greater risk of high blood pressure, our study showed a substantial correlation between total PCB exposure and HTN (20, 23, 24, 33, 50–52). However, Raymond et al. (38) and Arrebola et al. (2015) (31) reported weaker or non-significant associations (OR = 0.97, 95% CI: 0.61–1.54, and OR = 1.33, 95% CI: 0.83–2.14, respectively), possibly due to differences in age and body mass index (BMI), which could modify the PCB-HTN relationship. Age is a widely recognized risk factor for HTN, and total PCB serum levels tend to be higher in older individuals (20, 53). Crucially, it is shown that there is a substantially increased risk of HTN with rising serum PCB levels even after controlling for age, highlighting the separate relationship between PCBs and blood pressure (20). This indicates that PCBs may directly influence the risk of HTN rather than the correlation being attributable to age or other confounding variables.
Additionally, BMI modifies the PCB-HTN association, showing a more substantial effect in overweight individuals (20, 31). This is likely due to the lipophilic nature of PCBs (42, 54, 55) and slower elimination, which affects cardiovascular health. The slower elimination rate may heighten the toxic effects of PCBs on cardiovascular health, increasing HTN risk for those with higher BMI compared to individuals with lower BMI.
A positive association was identified in our analysis between DL-PCB exposure and HTN, consistent with findings from previous research (22, 33, 37–39, 56). This association suggests that the dioxin-like properties of certain PCB types may heighten cardiovascular risk by activating the aryl hydrocarbon receptor (AhR) (57–59), leading to blood vessel dysfunction and inflammation. In contrast to our study, Christensen et al. (21) and Valera et al. (40) observed weaker associations between DL-PCBs and HTN (OR = 1.26, 95% CI: 0.91–1.74 and OR = 1.10, 95% CI: 0.74–1.63, respectively) due to variations in the number of PCB congeners analyzed and differences in HTN definitions. We recommend that future studies include stratified analyses based on hypertension ranges to evaluate results using different blood pressure cut-off points.
Research investigating a broader spectrum of PCB varieties is more likely to identify associations with HTN. However, genetic variables may account for discrepancies in outcomes, especially in research, which includes a limited number of PCB types. Moreover, varying HTN criteria—such as excluding individuals with systolic blood pressure (SBP) < 140 mmHg—may impact prevalence estimates.
The relationship between NDL-PCBs and HTN appears insignificant (OR = 1.16, 95% CI: 0.81–1.66) in our analysis, consistent with findings from previous studies (22, 34, 36, 40). However, Valera et al. (41) found a stronger association (OR = 2.66, 95% Cl: 1.27–5.59), highlighting variability that may be due to population or methodological differences. The funnel plot suggests minimal publication bias, supporting the reliability of our results.
The analysis of individual NDL-PCB compounds revealed a positive but non-significant association for PCB-138 (OR = 1.29, 95% CI: 0.98–1.68) (26, 31, 40), and a positive and significant association for PCB-153 (OR = 1.27, 95% CI: 1.03–1.56) (21, 33). In contrast, NDL-PCB180 did not show a significant association similar to our study, indicating that it may not be related to HTN like the other two congeners (20, 21, 25, 31, 35, 41, 56). The differences in results could be due to the mechanism of action of these PCBs. PCB-153, PCB-180, and PCB-138 each contribute to HTN through distinct mechanisms. PCB-138 primarily induces inflammation and oxidative stress, affecting endothelial function (46). PCB-153 disrupts NO signaling and renal cell gene expression, impairing vasodilation and kidney function vital to blood pressure control (60, 61). PCB-180 uniquely impacts metabolic pathways, linking to metabolic syndrome, telomere shortening, and EGFR inhibition, promoting vascular stiffness and cellular aging (62, 63). These PCBs influence HTN via diverse pathways, highlighting their unique roles in vascular health. It's also essential to consider the combined effects of prolonged exposure to different PCB types, as their interactions might significantly impact overall cardiovascular risk.
PCB-74 demonstrated notable associations with HTN among specific congeners, likely due to its environmental persistence and bioaccumulation. We found a significant positive association between PCB-74 exposure and HTN (OR = 1.71, 95% Cl: 1.37–2.14), consistent with Wu et al. (42) and Christensen et al. (21), which also reported similar associations (OR = 2.46, 95% Cl: 1.44–4.21 and OR = 1.61, 95% Cl: 1.21–2.14, respectively). However, Chen et al. (26) observed a weaker association (OR = 1.52, 95% Cl: 0.85–2.71), potentially due to differences in participant age and gender distribution and other variables. Wu et al. (42) identified a stronger positive correlation between PCB-74 exposure and HTN in women. In Wu et al.'s study (42), men comprised 48.7% of participants; in Christensen et al.'s study (21), men comprised 47.2%. Conversely, Chen et al. (26) had a slightly lower male proportion at 44.9% and an average age of 40.1 years. While Wu et al.'s study (42) indicates that HTN linked to PCB-74 exposure is more common among younger people and women, Chen et al. (26) found a weaker link between HTN and PCB-74 exposure (OR = 1.52, 95% Cl: 0.85–2.71), even though their sample group was primarily young and female. The findings indicate that the relationship between PCB-74 exposure and HTN may be influenced more by factors other than age and gender. This points to the potential significance of additional biological or environmental elements in determining HTN risk. Prospective research is required to clarify these results.
5.1 Public health implications
These findings carry significant implications for public health. Because PCBs are widely found in the environment, especially in industrial areas (64, 65) their distribution patterns influenced by latitude, emission sources, and geography. In marine sediments, high-chlorinated congeners are more common in Antarctic regions, whereas low-chlorinated congeners dominate in the South China Sea, suggesting that climatic and environmental conditions play a key role in their accumulation (66, 67). Furthermore, Indoors, emissions from building materials such as floors and walls are significant sources of airborne PCBs, with surface films on these materials contributing to ongoing contamination (68). Additionally, PCB biomagnification varies across ecosystems, with freshwater systems often showing higher overall concentrations, while marine food webs, particularly in the Atlantic Ocean, exhibiting greater trophic magnification for certain congeners (69, 70). Moreover, unintentional sources, including pigments, paints, and combustion processes, also contribute to PCB levels in agricultural soils (71, 72). Therefore, regulations are urgently needed to reduce exposure. Strategies should include strict rules on PCB use, better waste management practices, and public awareness campaigns highlighting PCB exposure risks. Moreover, it is essential to set up programs that help people at higher risk. This includes those who already have health issues and anyone living near places where PCBs are found. These programs could educate people about the dangers of PCBs, provide regular health check-ups, and connect them with resources to help reduce their exposure. Targeting these groups can lower the health problems linked to PCBs.
Results from various studies can be challenging to interpret because of the complexity of PCB combinations and how they interact with biological systems. Future research should focus on examining different PCB types, individually and in combination, to understand their roles in the development of HTN. Prospective cohorts could help scientists learn which groups are most affected by PCBs and how their BP changes due to long-term exposure.
Our study provides several key strengths compared to previous meta-analyses, particularly Raffetti et al. (73).While Raffetti et al. (73) primarily focused on total PCBs, dioxin-like (DL-PCBs), non-dioxin-like (NDL-PCBs), and four individual congeners (DL-PCB 105, 118; NDL-PCB 138, 153), our study expanded the analysis to include 14 individual PCB congeners (e.g., PCB-52, PCB-74, PCB-99, PCB-105, PCB-118, PCB-138, PCB-153, PCB-156, PCB-157, PCB-170, PCB-180, PCB-183, PCB-187), offering a more comprehensive evaluation of the association between specific PCB congeners and hypertension risk. In addition, we included 21 studies with a total of 51,514 participants, surpassing the 17 studies and 29,153 participants in Raffetti et al. (2020).
This study has several limitations. First, varying definitions of HTN across studies may impact our results, as the American Heart Association (AHA) (74) lowered the threshold from 140/90 mmHg to 130/80 mmHg in 2017. Additionally, PCB levels have been decreasing at about 3.8% in Western populations per year (75), which could affect the relevance of exposure data over time. Finally, various studies applied different covariate adjustments, impacting their outcomes. Critical adjustments included gender, age, marital status, smoking, poverty income ratio (PIR), BMI, alcohol consumption, physical activity, and family history of HTN. For instance, some studies did not adjust for BMI (35, 36, 39) or smoking (22, 32, 33, 35, 38, 42), which could affect both HTN risk and the persistence of PCBs in the body. While most studies considered factors like age, gender, BMI, socioeconomic status, and lifestyle, they often overlooked diet and weight changes. In general, fatty fish is a significant source of PCBs for most people, yet it is also rich in polyunsaturated fats that can lower the risk of HTN. Similarly, losing weight may reduce HTN risk but can temporarily increase PCB levels in the blood as fat stores (where PCBs are concentrated) break down.
6 Conclusion
Our systematic review and meta-analysis reveal a notable association between PCBs and HTN. The analysis of 21 studies indicates that total PCB exposure is linked to HTN, particularly with DL-PCBs, which exhibited a strong relationship. Among individual congeners, PCB-74 had the most significant association with HTN. Among individual PCB congeners, PCB-74 demonstrated the strongest association with HTN, followed by PCB-118, PCB-105, and PCB-153, all showing substantial associations. Conversely, several other congeners exhibited non-significant trends, with some indicating positive associations and one (PCB-52) suggesting a non-significant negative trend. These findings underscore specific PCB congeners and types as potential risk factors for HTN, emphasizing the need for targeted environmental health policies and continued research into PCB exposure's cardiovascular impacts.
Data availability statement
Data of this study is available upon reasonable request from the corresponding author.
Author contributions
SH: Investigation, Writing – original draft. IE: Investigation, Formal analysis, Writing – original draft. AS: Investigation, Writing – original draft. FN: Investigation, Writing – original draft. BG: Data curation, Writing – original draft. SM: Validation, Writing – original draft. BS: Data curation, Writing – original draft. ZH: Validation, Writing – original draft. ZM: Validation, Writing – original draft. SA: Validation, Writing – original draft. AK: Validation, Writing – original draft. MR: Conceptualization, Project administration, Supervision, Methodology, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1529431/full#supplementary-material
References
1. Kario K, Okura A, Hoshide S, Mogi M. The WHO global report 2023 on hypertension warning the emerging hypertension burden in globe and its treatment strategy. Hypertens Res. (2024) 47(5):1099–102. doi: 10.1038/s41440-024-01622-w
2. Boateng EB, Ampofo AG. A glimpse into the future: modelling global prevalence of hypertension. BMC Public Health. (2023) 23(1):1906. doi: 10.1186/s12889-023-16662-z
3. Zhou B, Carrillo-Larco RM, Danaei G, Riley LM, Paciorek CJ, Stevens GA, et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. (2021) 398(10304):957–80. doi: 10.1016/S0140-6736(21)01330-1
4. Zhang Z, Zhao L, Zhou X, Meng X, Zhou X. Role of inflammation, immunity, and oxidative stress in hypertension: new insights and potential therapeutic targets. Front Immunol. (2022) 13:1098725. doi: 10.3389/fimmu.2022.1098725
5. Dinh QN, Drummond GR, Sobey CG, Chrissobolis S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed Res Int. (2014) 2014:406960. doi: 10.1155/2014/406960
6. Rodrigo R, González J, Paoletto F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens Res. (2011) 34(4):431–40. doi: 10.1038/hr.2010.264
7. Higashi Y. Roles of oxidative stress and inflammation in vascular endothelial dysfunction-related disease. Antioxidants (Basel). (2022) 11(10):1958. doi: 10.3390/antiox11101958
8. Steven S, Frenis K, Oelze M, Kalinovic S, Kuntic M, Bayo Jimenez MT, et al. Vascular inflammation and oxidative stress: major triggers for cardiovascular disease. Oxid Med Cell Longev. (2019) 2019:7092151. doi: 10.1155/2019/7092151
9. Touyz RM, Rios FJ, Alves-Lopes R, Neves KB, Camargo LL, Montezano AC. Oxidative stress: a unifying paradigm in hypertension. Can J Cardiol. (2020) 36(5):659–70. doi: 10.1016/j.cjca.2020.02.081
10. Carrera G, Fernández P, Grimalt J, Ventura M, Camarero L, Catalán J, et al. Atmospheric deposition of organochlorine compounds to remote high mountain lakes of Europe. Environ Sci Technol. (2002) 36:2581–8. doi: 10.1021/es0102585
11. Van Drooge B, Grimalt J, Camarero L, Catalán J, Stuchlík E, García CT. Atmospheric semivolatile organochlorine compounds in European high-mountain areas (central pyrenees and high Tatras). Environ Sci Technol. (2004) 38(13):3525–32. doi: 10.1021/es030108p
12. Galiulin R, Bashkin V. Organochlorinated compounds (PCBs and insecticides) in irrigated agrolandscapes of Russia and Uzbekistan. Water, Air, Soil Pollut. (1996) 89:247–66. doi: 10.1007/BF00171635
13. Fouial-Djebbar D, Ahmed A, Budzinski H. Determination of organochlorine compounds in coastal marine sediments from the southern west of the Mediterranean sea. Int J Environ Sci Technol. (2010) 7:271–80. doi: 10.1007/BF03326137
14. Biros F, Walker A, Medbery A. Polychlorinated biphenyls in human adipose tissue. Bull Environ Contam Toxicol. (1970) 5:317–23. doi: 10.1007/BF01539944
15. De Felip E, Di Domenico A, Miniero R, Silvestroni L. Polychlorobiphenyls and other organochlorine compounds in human follicular fluid. Chemosphere. (2004) 54(10):1445–9. doi: 10.1016/j.chemosphere.2003.10.040
16. Holmes D, Simmons J, Tatton J. Chlorinated hydrocarbons in British wildlife. Nature. (1967) 216:227–9. doi: 10.1038/216227a0
17. Hens B, Hens L. Persistent threats by persistent pollutants: chemical nature, concerns and future policy regarding PCBs-what are we heading for? Toxics. (2017) 6(1):1. doi: 10.3390/toxics6010001
18. Melymuk L, Blumenthal J, Sáňka O, Shu-Yin A, Singla V, Šebková K, et al. Persistent problem: global challenges to managing PCBs. Environ Sci Technol. (2022) 56(12):9029–40. doi: 10.1021/acs.est.2c01204
19. Symeonides C, Aromataris E, Mulders Y, Dizon J, Stern C, Barker TH, et al. An umbrella review of meta-analyses evaluating associations between human health and exposure to major classes of plastic-associated chemicals. Ann Glob Health. (2024) 90(1):52. doi: 10.5334/aogh.4459
20. Raffetti E, Donato F, De Palma G, Leonardi L, Sileo C, Magoni M. Polychlorinated biphenyls (PCBs) and risk of hypertension: a population-based cohort study in a North Italian highly polluted area. Sci Total Environ. (2020) 714:136660. doi: 10.1016/j.scitotenv.2020.136660
21. Yorita Christensen KL, White P. A methodological approach to assessing the health impact of environmental chemical mixtures: PCBs and hypertension in the national health and nutrition examination survey. Int J Environ Res Public Health. (2011) 8(11):4220–37. doi: 10.3390/ijerph8114220
22. Donat-Vargas C, Åkesson A, Tornevi A, Wennberg M, Sommar J, Kiviranta H, et al. Persistent organochlorine pollutants in plasma, blood pressure, and hypertension in a longitudinal study. Hypertension. (2018) 71(6):1258–68. doi: 10.1161/HYPERTENSIONAHA.117.10691
23. Goncharov A, Bloom M, Pavuk M, Birman I, Carpenter DO. Blood pressure and hypertension in relation to levels of serum polychlorinated biphenyls in residents of anniston, Alabama. J Hypertens. (2010) 28(10):2053–60. doi: 10.1097/HJH.0b013e32833c5f3e
24. Yamamoto K, Kudo M, Arito H, Ogawa Y, Takata T. A cross-sectional analysis of dioxins and health effects in municipal and private waste incinerator workers in Japan. Ind Health. (2015) 53(5):465–79. doi: 10.2486/indhealth.2015-0006
25. Lind PM, Penell J, Salihovic S, van Bavel B, Lind L. Circulating levels of p,p'-DDE are related to prevalent hypertension in the elderly. Environ Res. (2014) 129:27–31. doi: 10.1016/j.envres.2013.12.003
26. Chen H, Liang X, Chen L, Zuo L, Chen K, Wei Y, et al. Associations between household pesticide exposure, smoking and hypertension. Front Public Health. (2022) 10:754643. doi: 10.3389/fpubh.2022.754643
27. Chandler J, Cumpston M, Li T, Page MJ, Welch V. Cochrane Handbook for Systematic Reviews of Interventions. Hoboken: Wiley (2019).
28. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
29. Tools CA. JBI (2022). Available at: https://jbi global/critical-appraisal-tools (Accessed March 03, 2021).
30. Chêne G, Thompson SG. Methods for summarizing the risk associations of quantitative variables in epidemiologic studies in a consistent form. Am J Epidemiol. (1996) 144(6):610–21. doi: 10.1093/oxfordjournals.aje.a008971
31. Arrebola JP, Fernández MF, Martin-Olmedo P, Bonde JP, Martín-Rodriguez JL, Expósito J, et al. Historical exposure to persistent organic pollutants and risk of incident hypertension. Environ Res. (2015) 138:217–23. doi: 10.1016/j.envres.2015.02.018
32. Pavuk M, Serio Tara C, Cusack C, Cave M, Rosenbaum Paula F, Birnbaum Linda S. Hypertension in relation to dioxins and polychlorinated biphenyls from the anniston community health survey follow-up. Environ Health Perspect. (2019) 127(12):127007. doi: 10.1289/EHP5272
33. Reina-Pérez I, Artacho-Cordón F, Mustieles V, Castellano-Castillo D, Cardona F, Jiménez-Díaz I, et al. Cross-sectional associations of persistent organic pollutants measured in adipose tissue and metabolic syndrome in clinically diagnosed middle-aged adults. Environ Res. (2023) 222:115350. doi: 10.1016/j.envres.2023.115350
34. Dusanov S, Ruzzin J, Kiviranta H, Klemsdal TO, Retterstøl L, Rantakokko P, et al. Associations between persistent organic pollutants and metabolic syndrome in morbidly obese individuals. Nutr Metab Cardiovasc Dis. (2018) 28(7):735–42. doi: 10.1016/j.numecd.2018.03.004
35. Henríquez-Hernández LA, Luzardo OP, Zumbado M, Camacho M, Serra-Majem L, Álvarez-León EE, et al. Blood pressure in relation to contamination by polychlorobiphenyls and organochlorine pesticides: results from a population-based study in the Canary Islands (Spain). Environ Res. (2014) 135:48–54. doi: 10.1016/j.envres.2014.05.036
36. Lee DH, Lee IK, Porta M, Steffes M, Jacobs DR Jr. Relationship between serum concentrations of persistent organic pollutants and the prevalence of metabolic syndrome among non-diabetic adults: results from the national health and nutrition examination survey 1999–2002. Diabetologia. (2007) 50(9):1841–51. doi: 10.1007/s00125-007-0755-4
37. Nakamoto M, Arisawa K, Uemura H, Katsuura S, Takami H, Sawachika F, et al. Association between blood levels of PCDDs/PCDFs/dioxin-like PCBs and history of allergic and other diseases in the Japanese population. Int Arch Occup Environ Health. (2013) 86(8):849–59. doi: 10.1007/s00420-012-0819-8
38. Raymond MR, Christensen KY, Thompson BA, Anderson HA. Associations between fish consumption and contaminant biomarkers with cardiovascular conditions among older male anglers in Wisconsin. J Occup Environ Med. (2016) 58(7):676–82. doi: 10.1097/JOM.0000000000000757
39. Uemura H, Arisawa K, Hiyoshi M, Kitayama A, Takami H, Sawachika F, et al. Prevalence of metabolic syndrome associated with body burden levels of dioxin and related compounds among Japan’s general population. Environ Health Perspect. (2009) 117(4):568–73. doi: 10.1289/ehp.0800012
40. Valera B, Ayotte P, Poirier P, Dewailly É. Associations between plasma persistent organic pollutant levels and blood pressure in Inuit adults from Nunavik. Environ Int. (2013) 59:282–9. doi: 10.1016/j.envint.2013.06.019
41. Valera B, Jørgensen ME, Jeppesen C, Bjerregaard P. Exposure to persistent organic pollutants and risk of hypertension among Inuit from Greenland. Environ Res. (2013) 122:65–73. doi: 10.1016/j.envres.2012.12.006
42. Wu B, Guo X, Feng L, Gao J, Xia W, Xie P, et al. Combined exposure to multiple dioxins and dioxin-like polychlorinated biphenyls on hypertension among US adults in NHANES: a cross-sectional study under three statistical models. Environ Sci Pollut Res. (2023) 30(11):28730–44. doi: 10.1007/s11356-022-24271-3
43. Zani C, Magoni M, Speziani F, Leonardi L, Orizio G, Scarcella C, et al. Polychlorinated biphenyl serum levels, thyroid hormones and endocrine and metabolic diseases in people living in a highly polluted area in North Italy: a population-based study. Heliyon. (2019) 5(6):e01870. doi: 10.1016/j.heliyon.2019.e01870
44. Lee Y-M, Kim K-S, Kim S-A, Hong N-S, Lee S-J, Lee D-H. Prospective associations between persistent organic pollutants and metabolic syndrome: a nested case–control study. Sci Total Environ. (2014) 496:219–25. doi: 10.1016/j.scitotenv.2014.07.039
45. Helyar SG, Patel B, Headington K, El Assal M, Chatterjee PK, Pacher P, et al. PCB-induced endothelial cell dysfunction: role of poly (ADP-ribose) polymerase. Biochem Pharmacol. (2009) 78(8):959–65. doi: 10.1016/j.bcp.2009.06.019
46. Perkins JT, Petriello MC, Newsome BJ, Hennig B. Polychlorinated biphenyls and links to cardiovascular disease. Environ Sci Pollut Res. (2016) 23:2160–72. doi: 10.1007/s11356-015-4479-6
47. Pessah IN, Cherednichenko G, Lein PJ. Minding the calcium store: ryanodine receptor activation as a convergent mechanism of PCB toxicity. Pharmacol Ther. (2010) 125(2):260–85. doi: 10.1016/j.pharmthera.2009.10.009
48. Dutta SK, Mitra PS, Ghosh S, Zang S, Sonneborn D, Hertz-Picciotto I, et al. Differential gene expression and a functional analysis of PCB-exposed children: understanding disease and disorder development. Environ Int. (2012) 40:143–54. doi: 10.1016/j.envint.2011.07.008
49. Park SH, Lim J-e, Park H, Jee SH. Body burden of persistent organic pollutants on hypertension: a meta-analysis. Environ Sci Pollut Res. (2016) 23:14284–93. doi: 10.1007/s11356-016-6568-6
50. Everett CJ, Mainous AG, Frithsen IL, Player MS, Matheson EM. Association of polychlorinated biphenyls with hypertension in the 1999–2002 national health and nutrition examination survey. Environ Res. (2008) 108(1):94–7. doi: 10.1016/j.envres.2008.05.006
51. Goncharov A, Pavuk M, Foushee HR, Carpenter DO. Blood pressure in relation to concentrations of PCB congeners and chlorinated pesticides. Environ Health Perspect. (2011) 119(3):319–25. doi: 10.1289/ehp.1002830
52. Ha MH, Lee DH, Son HK, Park SK, Jacobs DR. Association between serum concentrations of persistent organic pollutants and prevalence of newly diagnosed hypertension: results from the national health and nutrition examination survey 1999–2002. J Hum Hypertens. (2009) 23(4):274–86. doi: 10.1038/jhh.2008.124
53. Mrema EJ. Exposure Assessment of Persistent Organic Pollutants and Associated Health Risks Among Italian Population. (2013).
54. Donat-Vargas C, Schillemans T, Kiviranta H, Rantakokko P, de Faire U, Arrebola JP, et al. Blood levels of organochlorine contaminants mixtures and cardiovascular disease. JAMA Network Open. (2023) 6(9):e2333347. doi: 10.1001/jamanetworkopen.2023.33347
55. Tornevi A, Sommar J, Rantakokko P, Åkesson A, Donat-Vargas C, Kiviranta H, et al. Chlorinated persistent organic pollutants and type 2 diabetes-a population-based study with pre-and post-diagnostic plasma samples. Environ Res. (2019) 174:35–45. doi: 10.1016/j.envres.2019.04.017
56. Valera B, Dewailly T, Poirier P. Association between methylmercury and cardiovascular risk factors in a native population of Quebec (Canada): a retrospective evaluation. Environ Res. (2013) 120:102–8. doi: 10.1016/j.envres.2012.08.002
57. Lind PM, Lind L. Are persistent organic pollutants linked to lipid abnormalities, atherosclerosis and cardiovascular disease? A review. J Lipid Atheroscler. (2020) 9(3):334. doi: 10.12997/jla.2020.9.3.334
58. Eske K, Newsome B, Han SG, Murphy M, Bhattacharyya D, Hennig B. PCB 77 dechlorination products modulate pro-inflammatory events in vascular endothelial cells. Environ Sci Pollut Res. (2014) 21:6354–64. doi: 10.1007/s11356-013-1591-3
59. Liu D, Perkins JT, Petriello MC, Hennig B. Exposure to coplanar PCBs induces endothelial cell inflammation through epigenetic regulation of NF-κB subunit p65. Toxicol Appl Pharmacol. (2015) 289(3):457–65. doi: 10.1016/j.taap.2015.10.015
60. Chen Y. Polychlorinated Biphenyls (PCBs)-induced gene Expression Profiling in Human Kidney Cells: Genomic Biomarkers. Washington, D.C.: Howard University (2006).
61. Curras-Collazo MC. Nitric oxide signaling as a common target of organohalogens and other neuroendocrine disruptors. J Toxicol Environ Health. (2011) 14(5-7):495–536. doi: 10.1080/10937404.2011.578564
62. Hardesty J. Epidermal Growth Factor Receptor (EGFR) Inhibition by Polychlorinated Biphenyls Contributes to Non-Alcoholic Fatty Liver Disease (NAFLD). (2018).
63. VanEtten S. Telomeres and Mitochondria as Potential Targets for the Toxicity of Polychlorinated Biphenyls (PCBs). (Doctoral dissertation). Buffalo, New York: State University of New York at Buffalo (2021).
64. Villalba A, Cecchetto F, Vazquez ND, Amarilla L, Ramirez CL, Galetto L, et al. Contaminant dynamics in honey bees and hive products of apiaries from environmentally contrasting Argentinean regions. Environ Res. (2024) 249:118306. doi: 10.1016/j.envres.2024.118306
65. Ogwu MC, Izah SC, Aigberua A, Ngun C. Implications on the one health triad of emerging and novel agro-aqua food contaminants. Front Sustain Food Syst. (2024) 8:1501930. doi: 10.3389/fsufs.2024.1501930
66. Deng Z, Li X, Chen C, Zhang N, Zhou H, Wang H, et al. Distribution characteristics and environmental fate of PCBs in marine sediments at different latitudinal regions: insights from congener profiles. Mar Pollut Bull. (2020) 161(Pt A):111710. doi: 10.1016/j.marpolbul.2020.111710
67. Deng Z, Han X, Chen C, Wang H, Bingbing M, Zhang D, et al. The distribution characteristics of polychlorinated biphenyls (PCBs) in the surface sediments of ross sea and drake passage, Antarctica: a 192 congeners analysis. Mar Pollut Bull. (2020) 154:111043. doi: 10.1016/j.marpolbul.2020.111043
68. Bannavti M, Marek R, Just C, Hornbuckle K. Congener-specific emissions from floors and walls characterize indoor airborne polychlorinated biphenyls. Environ Sci Technol Lett. (2023) 10:762–7. doi: 10.1021/acs.estlett.3c00360
69. Prince K, Taylor S, Angelini C. A global, cross-system meta-analysis of polychlorinated biphenyl biomagnification. Environ Sci Technol. (2020) 54(18):10989–1001. doi: 10.1021/acs.est.9b07693
70. Madgett A, Yates K, Webster L, McKenzie C, Brownlow A, Moffat C. The concentration and biomagnification of PCBs and PBDEs across four trophic levels in a marine food web. Environ Pollut. (2022) 309:119752. doi: 10.1016/j.envpol.2022.119752
71. Mao S, Liu S, Zhou Y, An Q, Zhou X, Mao Z, et al. The occurrence and sources of polychlorinated biphenyls (PCBs) in agricultural soils across China with an emphasis on unintentionally produced PCBs. Environ Pollut. (2020) 271:116171. doi: 10.1016/j.envpol.2020.116171
72. Hoang AQ, Aono D, Watanabe I, Tsugeki N, Kuwae M, Takahashi S. Historical record of polychlorinated biphenyls in a sediment core from Lake Biwa, Japan: significance of unintentional emission and weathering signals revealed by full congener-specific analysis. Sci Total Environ. (2021) 788:147913. doi: 10.1016/j.scitotenv.2021.147913
73. Raffetti E, Donat-Vargas C, Mentasti S, Chinotti A, Donato F. Association between exposure to polychlorinated biphenyls and risk of hypertension: a systematic review and meta-analysis. Chemosphere. (2020) 255:126984. doi: 10.1016/j.chemosphere.2020.126984
74. Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. (2018) 71(19):e127–248. doi: 10.1016/j.jacc.2017.11.006
75. Raffetti E, Speziani F, Donato F, Leonardi L, Orizio G, Scarcella C, et al. Temporal trends of polychlorinated biphenyls serum levels in subjects living in a highly polluted area from 2003 to 2015: a follow-up study. Int J Hyg Environ Health. (2017) 220(2):461–7. doi: 10.1016/j.ijheh.2017.01.002
Keywords: polychlorinated biphenyls, persistent organic pollutants, hypertension, high blood pressure, cardiovascular diseases
Citation: Hamzavi SF, Elahi Vahed I, Samadi Shams A, Nozari F, Gamzeh Latava B, Mardukhi S, Sabaghi B, Hosseini ZS, Masoumi Shahr-e Babak Z, Ahrari S, Keshavarzian A and Rahmanian M (2025) Association between polychlorinated biphenyls and hypertension risk: a systematic review and meta-analysis. Front. Cardiovasc. Med. 12:1529431. doi: 10.3389/fcvm.2025.1529431
Received: 16 November 2024; Accepted: 28 March 2025;
Published: 17 April 2025.
Edited by:
Alicia Del Saz lara, University of Castilla-La Mancha, SpainReviewed by:
Nerea Moreno-Herraiz, University of Castilla La Mancha, SpainIris Otero Luis, University of Castilla-La Mancha, Spain
Carla Geovanna Lever Megina, University of Castilla La Mancha, Spain
Copyright: © 2025 Hamzavi, Elahi Vahed, Samadi Shams, Nozari, Gamzeh Latava, Mardukhi, Sabaghi, Hosseini, Masoumi Shahr-e Babak, Ahrari, Keshavarzian and Rahmanian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Rahmanian, bW1kcmFobWFuaWFuQGdtYWlsLmNvbQ==
†These authors share first authorship
 Seyedeh Fatemeh Hamzavi
Seyedeh Fatemeh Hamzavi Iman Elahi Vahed2,†
Iman Elahi Vahed2,† Baroukh Gamzeh Latava
Baroukh Gamzeh Latava Mohammad Rahmanian
Mohammad Rahmanian