- Department of Cardiology, Xiangtan Central Hospital (The Affiliated Hospital of Hunan University), Xiangtan, China
Background: Takotsubo syndrome (TTS), characterized by transient wall motion aberrations and clinical manifestations akin to acute coronary syndrome, predominantly arises from significant physical or emotional stress, often throughout the perioperative period. The prevalence and mechanisms of this condition remain inadequately elucidated, particularly in the context of transcatheter valvular disease procedures. This knowledge gap may result in under-recognition and subsequent delays in diagnosis.
Case summary: A 76-year-old female was scheduled in our department for mitral transcatheter edge-to-edge repair (TEER). Despite the procedural success, multi-lead T-wave inversions and a 43% decrease in ejection fraction accompanied by new apical hypokinesis were noted postoperatively. Subsequent assessment revealed TTS. After receiving the optimal medical therapy, the patient was discharged after 10 days without experiencing acute chest pain or shortness of breath. The patient’s electrocardiogram (ECG) and function of the left ventricular function, particularly regional wall motion abnormalities, recovered on the 20th day after surgery.
Discussion: The limited literature reporting TTS post-TEER that we reviewed suggests that this rare complication must be anticipated in patients exhibiting an unexpected postoperative ECG and impaired myocardial contraction.
Conclusion: Researchers call for high-risk patient identification, adequate preoperative evaluation, and vigilant postoperative monitoring, and note the significance of early detection in optimizing therapeutic outcomes. Further research is imperative to further explore the management and prognosis of TTS following TEER.
1 Introduction
Takotsubo syndrome (TTS), also known as “Takotsubo cardiomyopathy,” “stress-induced cardiomyopathy,” or “broken heart syndrome,” was initially described in the Japanese literature in the early 1990s (1). It manifests as a transient left ventricle dysfunction, presenting with apical ballooning or mid-ventricular, basal, or focal wall motion abnormalities, and the clinical symptoms and signs usually simulate acute coronary syndromes (2, 3). This condition is frequently triggered by sudden emotional or physical stress, particularly during the perioperative period following cardiac and non-cardiac surgery (4). However, a comparable occurrence has yet to be thoroughly explained following mitral transcatheter edge-to-edge repair (TEER), which has reignited the debate regarding the publication mechanism and prognosis of TTS.
Herein, we present a distinctive instance of TTS following a successful TEER, analyze the potential etiology, and highlight early indicators of this situation. In addition, we searched PubMed with keywords related to “TEER” or “Mitral valve repair” along with “Takotsubo syndrome,” “Takotsubo cardiomyopathy,” or “left ventricular dysfunction” to conduct a brief review on TTS after the TEER procedure.
2 Case presentation
A 76-year-old woman (body mass index: 24.97 kg/m2) was admitted for symptomatic moderate to severe mitral regurgitation (MR). She was experiencing chest tightness and palpitations, her physical activity was mildly restricted, and she was classified as NYHA functional class II. The patient had no notable medical history and was not receiving treatment. Cardiac auscultation revealed a systolic murmur at the mitral focus assessed at 3/6. The electrocardiogram (ECG) on admission revealed an atrial fibrillation rhythm with an average ventricular rate of 90 bpm with no significant ST-T abnormalities. Transthoracic echocardiography (TTE) demonstrated a left ventricular (LV) ejection fraction of 71%, with moderate to severe MR (Figure 1A), moderate tricuspid regurgitation, and pulmonary arterial hypertension. Transesophageal echocardiography (TEE) confirmed a mitral valve area of 5.62 cm2, absent mitral stenosis, and a severe MR jet caused by a prolapse of the posterior mitral leaflet, measuring 16 mm in length (Figure 1B). The thyroid evaluation showed non-autoimmune subclinical hypothyroidism, characterized by elevated anti-thyroid stimulating hormone levels at 36.28 µIU/ml (NV: 0.34–5.6 µIU/ml), free T4 at 0.61 ng/dl (NV: 0.58–1.64 ng/dl), anti-thyroglobulin antibodies at 13.3 IU/ml (NV: <115 IU/ml), anti-thyroperoxidase antibodies at 10.9 IU/ml (NV: <34 IU/ml), and thyroid-stimulating receptor antibodies at 0.3 IU/ml (NV: <1.5 IU/L). The patient exhibited limited enthusiasm for surgical intervention. Consequently, we opted to discharge the patient with medication (rivaroxaban 10 mg/day, metoprolol 23.7 mg/day, thyroxine 50 μg/day, and furosemide 20 mg/temporary) and review her at a 3-month follow-up.
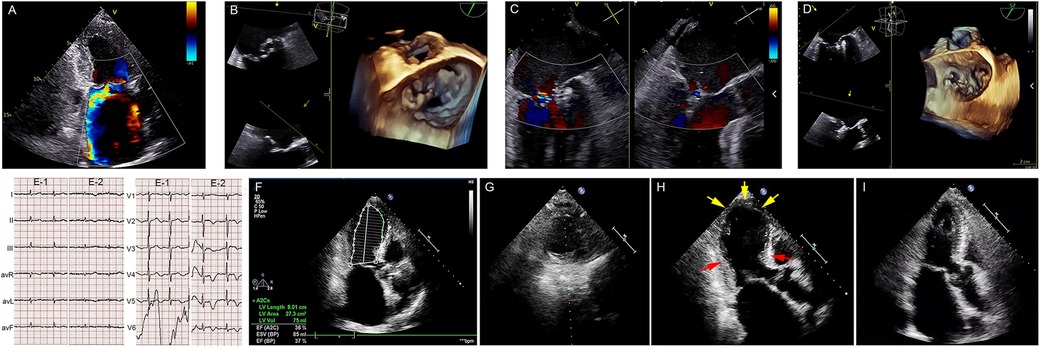
Figure 1. Takotsubo syndrome following mitral transcatheter edge-to-edge repair. (A) Preprocedural TTE. (B) Preprocedural 3D-TEE. (C,D) X-plane view with color flow and postprocedural TEE demonstrating mild MR after implantation of two clips. (E-1) ECG on admission and (E-2) postprocedural day 1. (F–G) Postprocedural TTE showing a significant reduction in LV function. (H) TTE showing apical akinesia (yellow arrowheads) and basal hyperkinesia (red arrows) on postoperative day 4. (I) Postoperative TTE on postoperative day 20 showing LV function and apical contraction recovery. TTE, transthoracic echocardiography; 3D-TEE, three-dimensional transesophageal echocardiograph; MR, mitral regurgitation; LV, left ventricle.
Over 2 months later, she presented to our department with progressively worsening dyspnea and decreased physical endurance. Her N-terminal pro-brain-type natriuretic peptide (NT-proBNP) level had increased from 1,623 pg/ml (NV: <1,800 pg/ml) to 2,928 pg/ml. TTE detected the progression of her mitral valve pathology with stable LV systolic function at 65%, and worsening MR due to a newly discovered chordae tendineae rupture (Supplementary Video 1). Subsequent coronary angiography detected no significant coronary lesions.
Given the high surgical risk and patient resistance, a TEER using the MitraClip device was carried out following a multidisciplinary heart team's deliberation (Figures 1C,D). At the beginning of the surgery, the patient's vitals were recorded as blood pressure of 100/74 mmHg, heart rate of 80 beats/min, and oxygen saturation of 98% on room air. Under general anesthesia, mild hypotension was managed with minimal doses of methoxamine, and transcatheter mitral valve repair was successfully performed using the MitraClip NT device manufactured by Abbott Vascular in Santa Clara, CA, USA (Supplementary Videos 2, 3). Continuous intraoperative monitoring was conducted using three-dimensional TEE and fluoroscopy. The MitraClip was deployed in the left atrium post-atrial septum puncture. The initial clip was placed at the A1-P1 coaptation site of the mitral valve, yielding moderate residual regurgitation. A second clip was successfully positioned at the A2-P2 coaptation site, resulting in minimal residual regurgitation and cessation of pulmonary venous reflux. The mean trans-mitral pressure gradient measured 2.5 mmHg. During the 215-min procedure, the patient's hemodynamics remained stable, without complications, and she was subsequently returned to the ward.
On postoperative day 1, the patient experienced no acute chest pain or dyspnea, and the invasive blood pressure monitoring showed fluctuations between 106-131/59-72 mmHg. The 12-lead ECG revealed significant, symmetric T-wave inversion across leads I, II, avL, and precordial leads V1–V6 (Figure 1E). A bedside cardiac ultrasound revealed no new changes. The patient received routine oral anti-heart failure medications and was moved back to the regular ward for recovery without incidents. By postoperative day 4, a follow-up TTE showed significantly reduced apical movement and a decreased left ventricular ejection fraction (LVEF) of 37%, although basal segment contraction had increased (Figures 1F–H, Supplementary Video 4). This unexpected finding prompted an immediate reevaluation, which disclosed a significant rise in NT-proBNP levels to 6,044 pg/ml and a modest rise in cardiac high-sensibility troponin T levels to 0.029 ng/ml (NV: 0.003–0.014 ng/ml).
The diagnosis of TTS was confirmed through the elimination process. The therapeutic regimen included rivaroxaban 15 mg/day, metoprolol 47.5 mg/day, sacubitril/valsartan 50 mg/day, spironolactone 20 mg/day, furosemide 20 mg/2 days, potassium chloride 500 mg/2 days, thyroxine 62.5 μg/day, and qili qiangxin capsules 3.6 g/day. The patient was discharged 10 days post-therapy, following stabilization of her LVEF and ongoing medication adherence. A 20-day follow-up confirmed her improved clinical status, as she was classified as NYHA class I and had restored ventricular kinesis showing a normalized ejection fraction (Figure 1I, Supplementary Video 4, and Supplementary Figure S1).
3 Discussion
Takotsubo syndrome has been described during various cardiac procedures, such as mitral valve replacement, due to factors such as surgical stress, cardiopulmonary bypass, and cardioplegic arrest (5). However, to our knowledge, this account of a TEER-related complication, which is both rare and potentially life-threatening, is unique. Following the TEER procedure, there was a notable decline in the patient’s LVEF and an occurrence of silent wall movement disorders. The diagnosis of TTS was delayed until 4 days post-operation, due to the absence of clinical signs. Fortunately, stable hemodynamics and strict adherence to prescribed medications contributed to a favorable outcome.
According to Mayo and InterTAK criteria, the clinical diagnosis of TTS is as follows: (1) transient hypokinesia, akinesia, or dyskinesia of the left ventricle, often presenting as apical ballooning or abnormalities in mid-ventricular, basal, or focal wall motions; (2) slight elevations in cardiac enzyme and biomarker levels; (3) absence of obstructive coronary disease; (4) new ECG abnormalities; and (5) excluding of other potential causes (2, 6). Our patient exhibited severe apical LV hypokinesis, T-wave inversions predominantly in the precordial leads, and a minor increase in troponin T levels, aligning with the typical TTS manifestations. Unfortunately, cardiac magnetic resonance (CMR) imaging was not performed due to concerns about the patient's ability to tolerate magnetic resonance imaging examinations and the mild nature of the symptoms. Undoubtedly, CMR can visualize the entire spectrum of functional and structural changes in those patients and provide additional value for differential diagnosis, pathophysiological insights, and complication detection (7).
The underlying pathophysiology of TTS remains intricate and is not yet fully elucidated. Previous studies have reported that the incidence of TTS more often affects postmenopausal women (>80%) with a significant burden of emotional stress and anxiety (8). In addition, hypothyroidism might also contribute to the onset of TTS. An observational study of 19,713 patients in the United States investigated the heightened connection between hypothyroidism and TTS (9). Although the nature of its exact pathophysiology is elusive, one hypothesis suggests that microvascular dysfunction may impair coronary flow reserve and myocardial perfusion (10). The patient in this report was diagnosed with subclinical hypothyroidism and treated with thyroxine and displayed persistently elevated preoperative anti-thyroid-stimulating hormone levels. Owing to the deterioration of clinical manifestations, performing surgery in patients before correcting thyroid function may have hidden dangers.
Aside from the patient's gender and age status, and the existing comorbidities mentioned above, any anesthetic or surgical intervention that significantly elevates catecholamine levels might exacerbate TTS. It is important to emphasize that the patient was administered modest doses of methoxamine to manage mild hypotension during surgery, following the induction with sevoflurane and remifentanil. It is conceivable that the development of TTS was triggered by both exogenous catecholamine administration and an endogenous catecholamine surge post-anesthetic (11). The emerging insight is that this increases the risk of experiencing TTS, especially when hypotension coincides with inadequate organ perfusion caused by peripheral vasodilation and decreased peripheral vascular resistance. A preferable approach involves pursuing fluid volume expansion.
Although it remains controversial, another possible explanation is that acute afterload mismatch following MR repair could have led to a reduction in LV systolic activity, potentially causing a low cardiac output (CO) state after the surgery. Patients undergoing mitral valve replacement surgery are known to experience a potential rise in left ventricular afterload due to the elimination of the low-resistance regurgitant flow into the left atrium (12). Early experience suggests a similar outcome with the MitraClip system appears rare. The proposed risk factors are an LVEF <40%, a dilated LV (end-diastolic dimension >6 cm, end-systolic dimension >4 cm), cardiac output <3.0 L/min or cardiac index (CI) <2.0 L/min, right ventricular dysfunction, and/or pulmonary artery systolic pressure (PASP) ≥60 mmHg (13–15). In this case, we observed the development of transient LV dysfunction shortly after TEER, which occurred in a patient with previously normal LV function, a mild dilated LV, and pulmonary hypertension, posing a unique challenge for clinical prediction.
Currently, there is a lack of consensus on the management of TTS post-TEER, with experimental methods including periprocedural volume management, dobutamine support, and mechanical assistance devices such as intra-aortic balloon pumps or Impella. Fortunately, the LV function of most of these patients recovers with no clinical sequelae. In a recent study that analyzed 317 individuals treated with successful TEER, 66.9% of patients displayed an LVEF reduction 1 month post-surgery compared to their baseline levels. This decrease was primarily due to reduced total stroke volume and widespread hypocontractility. However, surprisingly, an LVEF reduction post-TEER is associated with reduced mortality (13.3% vs. 5.7%, P = 0.019), and a lower readmission rate for heart failure (17.1% vs. 9.0%, P = 0.033), presumably indicating a more significant unloading effect of the procedure (16). This may elucidate the rapid recovery and absence of symptoms in our patient, highlighting the critical importance of the substantial reduction of valve regurgitation.
We summarized previous reports on TTS through Mitral TEER, which is presented in Table 1. The relevant information includes the epidemiological characteristics, symptoms and examination, postprocedural support, prognosis, and recovery status.
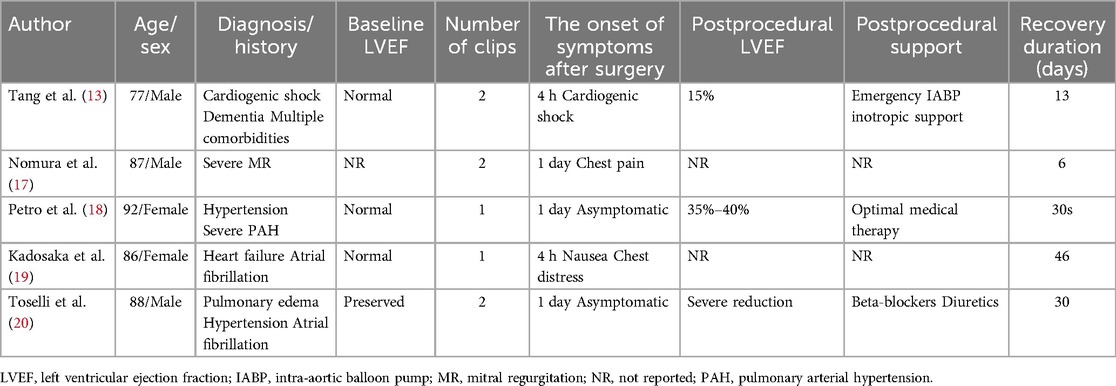
Table 1. Cases reported in the literature of Takotsubo syndrome after mitral transcatheter edge-to-edge repair.
4 Conclusion
This study represents the inaugural systematic brief review of TTS after the mitral TEER procedure. While the development of percutaneous mitral valve repair technology has enhanced cardiovascular symptom management, post-procedure LV dysfunction has become a challenging condition. Given the potential for unexpected clinical deterioration postoperatively, TEER should be performed at centers experienced in comprehensive preoperative preparation and diligent postoperative monitoring. Any subtle clinical symptoms and postoperative ECG changes in the patient may be the signal before adverse outcomes and necessitate a cardiac ultrasound evaluation as soon as possible. Further studies are imperative to elucidate the pathogenesis, prevention, and management of TTS following TEER.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SP: Conceptualization, Investigation, Writing – original draft. HH: Conceptualization, Investigation, Writing – original draft. YZ: Conceptualization, Investigation, Writing – original draft. RZ: Conceptualization, Investigation, Writing – original draft. DT: Conceptualization, Writing – review & editing. YZ: Conceptualization, Writing – review & editing. MW: Conceptualization, Investigation, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1516080/full#supplementary-material
Supplementary Video 1 | The preoperative triple chamber cardiac section image of the patient revealed significant mitral regurgitation and normal left ventricular apical contractility.
Supplementary Video 2 | The patient's esophageal echocardiography immediately after surgery showed a reduction in mitral regurgitation.
Supplementary Video 3 | The patient's esophageal echocardiography immediately after surgery showed a reduction in mitral regurgitation.
Supplementary Video 4 | On the fourth day post-surgery, the left ventricular short axis section indicated decreased contractility in the left ventricular apex.
Supplementary Video 5 | Triple chamber cardiac section view of the patient at the 20-day follow-up showed normalization of left ventricular ejection fraction and resolution of apical hypokinesia.
Supplementary Figure S1 | Mild to moderate mitral regurgitation on postoperative 20-day follow-up echocardiography.
References
1. Sato H, Uchida T, Dote K, Ishihara M. Takotsubo-type cardiomyopathy due to multivessel spasm. In: Kodama K, Haze K, Hon M, editors. Clinical Aspect of Myocardial Injury: From Ischemia to Heart Failure. Tokyo: Kagakuhyoronsha Publishing Co. (1990). p. 56–64.
2. Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, et al. International expert consensus document on Takotsubo syndrome (part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J. (2018) 39:2032–46. doi: 10.1093/eurheartj/ehy076
3. Lyon AR, Citro R, Schneider B, Morel O, Ghadri JR, Templin C, et al. Pathophysiology of Takotsubo syndrome: JACC state-of-the-art review. J Am Coll Cardiol. (2021) 77:902–21. doi: 10.1016/j.jacc.2020.10.060
4. Omerovic E, Citro R, Bossone E, Redfors B, Backs J, Bruns B, et al. Pathophysiology of Takotsubo syndrome—a joint scientific statement from the Heart Failure Association Takotsubo Syndrome Study Group and Myocardial Function Working Group of the European Society of Cardiology—part 1: overview and the central role for catecholamines and sympathetic nervous system. Eur J Heart Fail. (2022) 24:257–73. doi: 10.1002/ejhf.2400
5. Kim YS, Lim JY. Risk factors for Takotsubo syndrome following cardiac surgery: a case-control study. J Card Surg. (2021) 36:2767–73. doi: 10.1111/jocs.15626
6. Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. (2008) 155:408–17. doi: 10.1016/j.ahj.2007.11.008
7. Citro R, Okura H, Ghadri JR, Izumi C, Meimoun P, Izumo M, et al. Multimodality imaging in Takotsubo syndrome: a joint consensus document of the European Association of Cardiovascular Imaging (EACVI) and the Japanese Society of Echocardiography (JSE). Eur Heart J Cardiovasc Imaging. (2020) 21:1184–207. doi: 10.1093/ehjci/jeaa149
8. Gonuguntla K, Thyagaturu H, Shaik A, Roma N, Thangjui S, Alruwaili W, et al. Takotsubo cardiomyopathy and psychiatric illness—insight from National Inpatient Sample (NIS) and National Re-admission Database (NRD) 2016 to 2018. Curr Probl Cardiol. (2024) 49:102429. doi: 10.1016/j.cpcardiol.2024.102429
9. Zoltowska DM, Agrawal Y, Patria S, Aggarwal S, Reddy CS, Sareen N, et al. Association between hypothyroidism and Takotsubo cardiomyopathy: analysis of nationwide inpatient sample database. Rev Recent Clin Trials. (2018) 13:222–5. doi: 10.2174/1574887113666180402144600
10. Schweiger V, Gilhofer T, Fang R, Candreva A, Seifert B, Di Vece D, et al. Coronary microvascular dysfunction in Takotsubo syndrome: an analysis using angiography-derived index of microcirculatory resistance. Clin Res Cardiol. (2024) 113:1629–37. doi: 10.1007/s00392-023-02329-7
11. Akhtar MM, Cammann VL, Templin C, Ghadri JR, Lüscher TF. Takotsubo syndrome: getting closer to its causes. Cardiovasc Res. (2023) 119:1480–94. doi: 10.1093/cvr/cvad053
12. Siegel RJ, Biner S, Rafique AM, Rinaldi M, Lim S, Fail P, et al. The acute hemodynamic effects of MitraClip therapy. J Am Coll Cardiol. (2011) 57:1658–65. doi: 10.1016/j.jacc.2010.11.043
13. Tang G, Cohen M, Dutta T, Undemir C. Afterload mismatch after transcatheter mitral valve repair with MitraClip for degenerative mitral regurgitation in acute cardiogenic shock. Catheter Cardiovasc Interv. (2018) 92:E168–71. doi: 10.1002/ccd.27019
14. Hungerford SL, Dahle G, Duncan A, Hayward CS, Muller D. Peri-procedural management of transcatheter mitral valve replacement in patients with heart failure. Eur J Heart Fail. (2023) 25:890–901. doi: 10.1002/ejhf.2758
15. Biner S, Siegel RJ, Feldman T, Rafique AM, Trento A, Whitlow P, et al. Acute effect of percutaneous MitraClip therapy in patients with haemodynamic decompensation. Eur J Heart Fail. (2012) 14:939–45. doi: 10.1093/eurjhf/hfs069
16. Shechter A, Kaewkes D, Lee M, Makar M, Patel V, Koren O, et al. Correlates and prognostic implications of LVEF reduction after transcatheter edge-to-edge repair for primary mitral regurgitation. Eur Heart J Cardiovasc Imaging. (2023) 25:136–47. doi: 10.1093/ehjci/jead210
17. Nomura T, Munehisa Y, Nakashima M, Matsumoto T. Takotsubo syndrome following MitraClip procedure. Eur Heart J Case Rep. (2020) 4:1–2. doi: 10.1093/ehjcr/ytaa384
18. Petro J, Prol T, Nagai T, Marcoff L. Rare case of Takotsubo cardiomyopathy following mitraclip placement. Struct H. (2020) 4:518–9. doi: 10.1080/24748706.2020.1818906
19. Kadosaka T, Kamiya K, Nagai T, Anzai T. Takotsubo syndrome after transcatheter mitral valve repair. Circ J. (2021) 85:1100. doi: 10.1253/circj.CJ-21-0107
Keywords: mitral valve repair, Takotsubo syndrome, postoperative complications, mitral regurgitation, Takotsubo cardiomyopathy
Citation: Pang S, Huang H, Zhu Y, Zhou R, Tan D, Zhang Y and Wu M (2025) Takotsubo syndrome following mitral transcatheter edge-to-edge repair: a case report and literature review. Front. Cardiovasc. Med. 12:1516080. doi: 10.3389/fcvm.2025.1516080
Received: 23 October 2024; Accepted: 17 February 2025;
Published: 11 March 2025.
Edited by:
Roberto Valvo, Provincial Health Authority of Syracuse, ItalyReviewed by:
Alon Shechter, Rabin Medical Center, IsraelBeatrice Bacchi, Careggi University Hospital, Italy
Copyright: © 2025 Pang, Huang, Zhu, Zhou, Tan, Zhang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingxing Wu, d214OTE3MTIxQDEyNi5jb20=
†These authors have contributed equally to this work
 Si Pang
Si Pang Haobo Huang†
Haobo Huang† Mingxing Wu
Mingxing Wu