
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 13 February 2025
Sec. General Cardiovascular Medicine
Volume 12 - 2025 | https://doi.org/10.3389/fcvm.2025.1502205
Background: Current evidence suggests that cardiovascular disease (CVD) plays a role in the progression of chronic obstructive pulmonary disease (COPD). However, the relationship between CVD and the severity of COPD remains inadequately understood. Therefore, this study aims to elucidate the association between CVD and the severity of COPD.
Methods: In this cross-sectional study involving 7,152 individuals with COPD., Logistic regression, subgroup and sensitivity analyses were employed to evaluate the association between CVD, its subcategories, and the severity of COPD.
Results: Multivariable logistic regression analysis showed that CVD and hypertension remained independently associated with COPD severity (P < 0.001). Patients with CVD had a 1.701 times higher risk of developing severe or very severe COPD compared to those without CVD, while patients with hypertension had a 1.686 times higher risk of developing severe or very severe COPD compared to those without hypertension (P < 0.05). Subgroup analyses showed that the association between CVD and COPD severity remained stable among men, patients ≤ 70 years of age, patients > 70 years of age, BMI < 24 or ≥24 kg/m2, and never smokers, whereas coronary artery disease was significantly associated with COPD severity only among patients ≤ 70 years of age and never smokers (P < 0.05). In addition, hypertension was also stably associated with COPD severity among men, patients ≤ 70 years of age, patients > 70 years of age, BMI < 24 or ≥24 kg/m2, and never smokers. Sensitivity analyses reconfirmed the robustness of the associations of CVD and hypertension with COPD severity among patients who excluded bronchiectasis, tuberculosis, lung cancer, pulmonary hypertension, pulmonary heart disease, and diabetes (P < 0.05).
Conclusion: The strong association between CVD and its subcategories (mainly hypertension) and the severity of COPD suggests that the potential risk of exacerbation of CVD should also be addressed in the clinical management of patients with COPD. However, limitations of the cross-sectional design may limit the extrapolation of the results, and more large prospective clinical cohort studies are needed in the future to further validate the association.
In recent years, chronic obstructive pulmonary disease (COPD) has continued to maintain a high mortality rate and remains a significant public health concern worldwide. It is predicted that by 2030, COPD will become the third leading cause of death globally (1, 2). Clinically, patients with COPD often experience recurrent symptom exacerbations, a progressive decline in lung function, reduced quality of life, and decreased physical activity, which can ultimately lead to fatal outcomes (3, 4). Cardiovascular diseases (CVD) are primarily caused by atherosclerosis, which is characterized by the narrowing or blockage of arteries due to plaque buildup from fatty deposits on the artery walls. There is growing evidence suggesting a strong co-existence of COPD and CVD, with an intricate relationship between the two (5). In addition to common risk factors like smoking and age, inflammation plays a critical role in connecting these diseases. In the progression of COPD, the significance of chronic inflammation cannot be ignored. Chronic inflammation inflicts damage on the lung parenchyma and surrounding airways, leading to persistent respiratory symptoms and irreversible airflow limitation (6). In addition, low-grade chronic inflammation is associated with increased rates of atherosclerosis and insulin resistance, which play an important role in the occurrence and development of CVD (7). Fianlly, the progression of COPD is influenced by several factors, particularly its comorbidities (8–10). Research has shown that CVD are major contributors to the exacerbation of chronic lung conditions and significantly increase the risk of mortality in individuals with COPD (11–13).
However, much of the existing research has focused on individual CVD and has often involved small sample sizes, lacking representation from the Chinese population. Therefore, to further investigate the relationship between CVD and the severity of COPD, a large population-based cohort of 40,000 participants from northwestern and southeastern China between 2014 and 2021 was selected for a cross-sectional study. According to the current research background, in order to fill some knowledge gaps, this study innovatively evaluate the association of CVD and its subclassification with the severity of COPD patients through multiple statistical methods including multivariate logistic regression analysis, subgroup analysis and sensitivity analysis, aiming to provide new clues and theoretical basis for clinical management and prognosis assessment of COPD patients.
In this cross-sectional study, all participants were drawn from a population-based cohort in northwestern and southeastern China from 2014–2021. The cohort selected study participants from eight hospitals in five Chinese provinces with a total population of 40,000 through a multistage whole population sampling method. 7,152 individuals were included after excluding individuals with current psychiatric disorders, contraindications to pulmonary function testing, other conditions that affect pulmonary function test results, and those with significant missing information (Figure 1). The data for this study were derived from a multicenter cohort study, the protocols were reviewed and approved by all participating subcenters, all patients signed written informed consent, and the study complied with the basic principles of the Declaration of Helsinki.
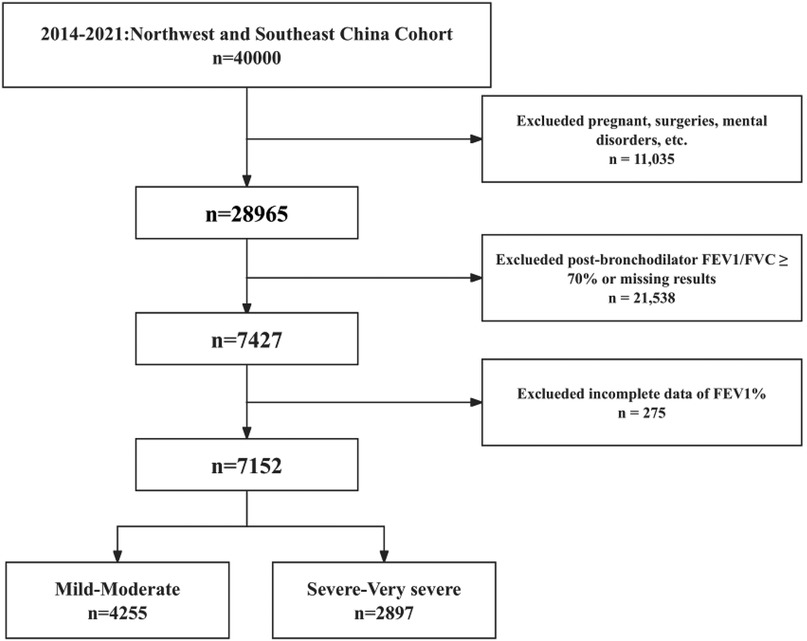
Figure 1. Study population inclusion flow chart. FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; FEV1%, percentage of predicted forced epiratory vlume in 1 s.
In this study, CVD and its subcategories [hypertension, arrhythmia, and coronary heart disease (CHD)] were considered as exposure factors. Hypertension was defined as a history of previous hypertension, current use of antihypertensive medication, or systolic blood pressure (SBP)/diastolic blood pressure (DBP) ≥ 140/90 mmHg (14). Arrhythmia was defined as a history of previous arrhythmia or current use of antiarrhythmic medication, including atrial, supraventricular, or ventricular arrhythmias. CHD was defined as a history of previous CHD or the presence of typical CHD symptoms, signs, and imaging. Two groups were divided according to the prevalence of CVD and its subclassifications, respectively.
In this study, both COPD and its severity were determined by lung function. Following the GOLD criteria, COPD was defined as forced expiratory volume in the first second (FEV1)/forced vital capacity (FVC) < 70%, and this ratio was used to assess the degree of airway obstruction after a bronchodilator test (after inhalation of 400 μg of salbutamol for at least 15 min) (15). A uniform spiral flow meter was used for all tests, and each subject underwent two pulmonary function tests, which were confirmed by repeating the test if the post-bronchodilator FEV1/FVC was less than 0.70 in both tests. Severity was graded primarily on the basis of FEV1 as a percentage of the predicted value: mild (GOLD 1): FEV1 ≥ 80% of predicted value. Moderate (GOLD 2): 50% ≤ FEV1 < 80% of predicted value. Severe (GOLD 3): 30% ≤ FEV1 < 50% expected. Very severe (GOLD 4): FEV1 < 30% predicted or FEV1 < 50% with chronic respiratory failure (16). In this study, participants were divided into two groups based on COPD severity: mild to moderate (n = 4,255) and severe to very severe (n = 2,897).
Data on participants' demographics, exposure factors, and medical conditions were gathered using questionnaires, spirometry, and blood pressure screenings. The main covariates used for this study included age, gender, body mass index (BMI), blood pressure, smoking, frequency of smoking, asthma, chronic bronchitis, bronchiectasis, emphysema, tuberculosis, lung cancer, pulmonary heart disease, chronic rhinitis, diabetes, BMI, SBP, DBP, and heart rate. BMI was defined as weight (kg)/height (m2), and SBP and DBP were the average of three consecutive measurements on different days. Smoking was defined as continuous smoking for more than 6 months, while smoking frequency was divided into three groups: never, occasional and daily. Chronic bronchitis was defined to include any of the following: (1) a previous diagnosis of chronic bronchitis; and (2) clinical symptoms of cough and sputum, with an onset lasting 3 months per year, for 2 or more consecutive years. Bronchiectasis was defined to include any of the following: (1) previous diagnosis of bronchiectasis; (2) imaging suggestive of bronchiectasis. Tuberculosis was defined to include any of the following: (1) previous diagnosis of tuberculosis; and (2) being on anti-tuberculosis medication. Lung cancer was defined as a history of physician-diagnosed lung cancer. Pulmonary heart disease was evaluated by a combination of self-reported past medical history and echocardiographic measurements during this hospitalization. Chronic rhinitis was diagnosed based on the patient's past medical history. Diabetes was defined as a history of a previous diagnosis of diabetes or being treated with glucose-lowering medication or having a fasting blood glucose ≥7.0 mmol/L or glycosylated haemoglobin ≥6.5% or a blood glucose ≥11.1 mmol/L after 2 h of an oral glucose tolerance test (17).
All statistical analyses in this study were performed using SPSS 26.0. Continuous variables that conformed to normal distribution were expressed as mean ± standard deviation, and differences between groups were tested by independent samples t-test. Continuous variables that did not conform to normal distribution were described as median (quartiles) and differences between groups were tested using non-parametric tests. Categorical variables were described as frequencies (percentages), and differences between groups were tested using the chi-square test. Univariate logistic regression analysis was used to assess the association of all variables with COPD severity, and then variables with P < 0.05 were selected for multifactorial logistic regression analysis to assess the independent association of CVD including CHD, arrhythmia, and hypertension with COPD severity. Then, stratified associations of CVD and its subcategories with COPD severity were further assessed by constructing 8 subgroups based on gender (male or female), age (≤70 or >70 years), BMI (<24 or ≥24 kg/m2), and smoking frequency (never, occasional or daily). Finally, the independent association of CVD and its subcategories with COPD severity was further evaluated in sensitivity analysis by excluding variables that might have an important impact on COPD severity, such as bronchiectasis, tuberculosis, lung cancer, pulmonary heart disease, and diabetes. All tests were two-sided and were considered statistically significant at P < 0.05.
As shown in Table 1, the study included 7,152 patients, of whom 5,846 (81.7%) were male, with a mean age of (68.36 ± 11.60) years. They were divided into two groups according to the severity of COPD: mild-moderate group and severe-very severe group. Compared with the mild-moderate group, the severe-very severe group was older, had a higher proportion of men and smokers, a higher proportion of occasional smokers, a higher prevalence of chronic bronchitis, emphysema, CVD, CHD and hypertension, but a lower prevalence of chronic rhinitis, and had higher levels of heart rate, but lower levels of BMI and FEV1/FVC (P < 0.05).
Univariate logistic regression analysis showed that age, male, smoking, smoking frequency, chronic bronchitis, chronic rhinitis, emphysema, CVD, CHD, hypertension, BMI, heart rate, and FEV1/FVC were all associated with COPD severity (P < 0.05) (Table 2). Multivariate logistic regression analysis showed (Table 3), both CVD and hypertension were significantly associated with COPD severity in model 1 (P < 0.05). In model 2, a association between CVD, CHD, and hypertension with COPD severity was observed (P < 0.05). In the fully adjusted model 3, CVD and hypertension remained independently associated with COPD severity (OR: 1.701, 95% CI: 1.320–2.191, P < 0.001; OR: 1.686, 95% CI: 1.282–2.218, P < 0.001). Specifically, patients with CVD had a 1.701 times higher risk of developing severe COPD compared to those without CVD, while patients with hypertension had a 1.686 times higher risk compared to those without hypertension (P < 0.05).
Subgroup analysis revealed an independent association between CVD and COPD severity in males, individuals aged ≤ 70 years, those aged > 70 years, individuals with a BMI < 24 or BMI ≥ 24 kg/m², and never smokers (P < 0.05). Additionally, CHD showed a significant association with COPD severity in individuals aged ≤ 70 years and never smokers, while hypertension was associated with COPD severity in males, individuals aged ≤ 70 years, those aged > 70 years, individuals with a BMI < 24 or BMI ≥ 24 kg/m², and never smokers (P < 0.05) (Table 4).
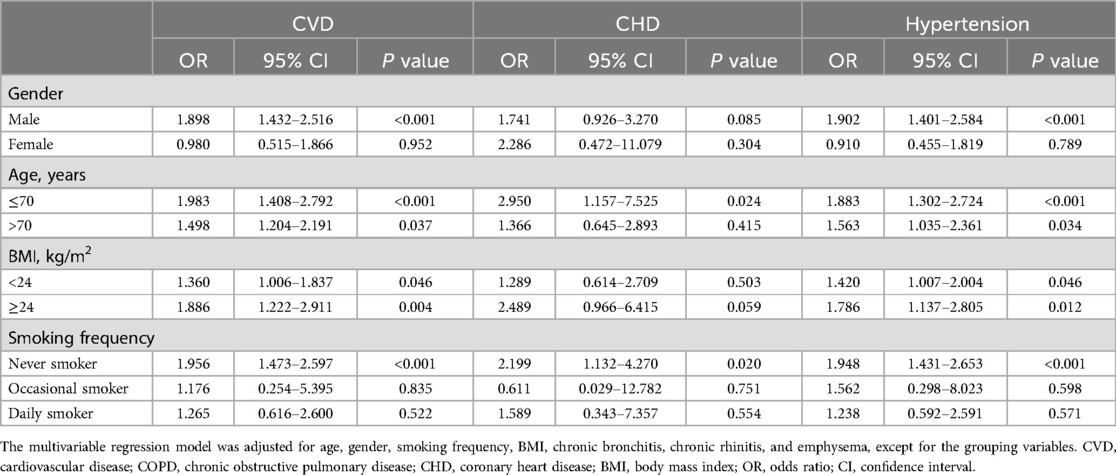
Table 4. Multivariable stratified analysis of the association between CVD, subtypes, and COPD severity.
Sensitivity analysis showed (Table 5), CVD and hypertension remained independently associated with COPD severity after excluding people with bronchiectasis, tuberculosis, lung cancer, pulmonary heart disease, and diabetes (P < 0.05). Specifically, the risk of severe COPD was 1.656 times higher in patients with CVD than in patients without CVD (95% CI: 1.254–2.186, P < 0.001), whereas the risk of severe COPD was 1.706 times higher in patients with hypertension than in patients without hypertension (95% CI: 1.266–2.229, P < 0.001).
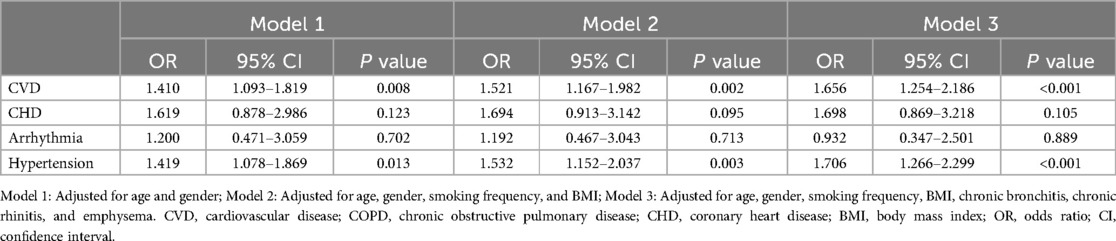
Table 5. Multivariate logistic regression analysis of the association between CVD, subtypes, and COPD severity: excluding bronchiectasis, tuberculosis, lung cancer, pulmonary heart disease, and diabetes.
In this study, we investigated the association between CVD and their subtypes with the severity of COPD. Our results demonstrated that CVD, particularly hypertension, was consistently and significantly associated with COPD severity, even after adjusting for multiple confounders. Although CHD did not show statistically significant differences between univariate and multivariate analyses, stratified analyses revealed that individuals with CHD had more severe COPD, particularly those aged ≤ 70 years and never smokers. These findings have important clinical implications for the early detection and prevention of severe COPD.
Current evidence suggests that CVD is associated with COPD. For example, Bai et al. found that patients at higher cardiovascular risk had a more rapid decline in lung function, especially for FEV1 and FVC, and that the rate of decline was further accelerated in those with CVD. High levels of physical and social activity can mitigate the negative effects of CVD, thus highlighting that we can intervene to reduce the impact of CVD on lung function (18). Secondly, Alter et al. found that CHD and hypertension were associated with all-cause mortality in COPD. Chronic coronary artery disease and hypertension are associated with increased mortality in patients with COPD, which underscores the need for extensive diagnostic testing in patients with COPD, especially to assess cardiovascular comorbidities (19). In contrast, Chen et al. found that patients with COPD had a higher prevalence of one or more CVD (including CHD, heart failure, heart attack and diabetes) compared to patients without COPD, which highlights the importance of the prevention and management of CVD in patients with COPD (5). In addition, Dalal et al. found that patients with COPD had a higher prevalence of CVD, which in turn led to an increased risk of mortality from cardiovascular-related causes. CVD as a co-morbidity may include several forms of disease such as angina, arrhythmia, cardiac hypertrophy and myocardial infarction. The presence of these CVD severely affects the survival expectancy of patients with COPD, significantly reducing their quality of life and the likelihood of long-term survival. which suggests a close association between COPD and CVD, and further studies are needed to explore the association (20). Almagro et al. discovered a close relationship between COPD and CVD, noting that the treatment strategies for these two conditions influence each other. This highlights the critical importance of appropriately managing both diseases (21). Furthermore, a Canadian COPD cohort study demonstrated a significant association between impaired lung function and the prevalence and incidence of CVD, particularly in patients with moderate to severe COPD. The study found that, compared with individuals with normal lung function, the prevalence of CVD was significantly higher in those with impaired lung function, with OR of 1.66 for the impaired lung function group and 1.55 for the COPD group. The hazard ratio for CVD incidence in both groups was approximately 2.09. Although adding impaired lung function to existing risk scores only slightly improved their predictive ability, the study emphasized the importance of early identification and intervention in individuals with impaired lung function to reduce CVD risk (22). In contrast, this study, based on a large cross-sectional cohort of COPD patients from Chinese hospitals, further explores the relationship between CVD and its subcategories (such as hypertension) with the severity of COPD. Both studies revealed a strong link between CVD and COPD; however, this study, through multivariable regression analysis, subgroup analysis, and sensitivity analysis, found that CVD and hypertension increased the risk of developing severe or very severe COPD by 1.701 times and 1.686 times, respectively. This association was particularly stable among men, individuals aged ≤ 70 years, those with different BMI categories, and never smokers. Sensitivity analyses, excluding potential confounding conditions such as bronchiectasis, tuberculosis, and pulmonary heart disease, confirmed the robustness of these findings. Additionally, unlike the longitudinal design of the Canadian cohort study, this study employed a cross-sectional design. While the cross-sectional approach revealed a strong association between CVD and COPD severity, it could not establish a causal relationship, highlighting the need for future prospective studies to validate these findings. In summary, both studies underscore the critical impact of CVD on COPD patients. The Canadian cohort study focuses on the risk of developing CVD, while this study emphasizes the relationship between CVD and the severity of COPD and its clinical management implications, reflecting the complementary focus of these investigations.
Despite the exciting findings of this study, the underlying biological mechanisms remain unknown. After conducting literature research, we identified several possible mechanisms between CVD and COPD. Firstly, traditional cardiovascular risk factors such as age and insulin resistance may also contribute to the accumulation of lipids between the ribs, and furthermore lead to a decrease in pulmonary static elastic recoil, which reduces lung function and ultimately exacerbates the condition of COPD patients (23, 24). Secondly, there may also be common pathogenic mechanisms between both CVD and COPD, such as inflammation, oxidative stress, and programmed cell death (25–28). Inflammation and oxidative stress play an important role in the occurrence and development of COPD. Inflammation is considered to be one of the causes of coronary atherosclerosis, especially in the case of coronary atherosclerosis, which includes blood-derived immune and inflammatory cells. Meanwhile, reactive oxygen species have also been shown to promote the formation of atherosclerotic plaques (29). Smoking, in particular, not only increases the level of inflammation in the body, but also leads to the formation and rupture of atherosclerotic plaques as well as an increased risk of CVD, which is more closely related to the development of COPD (30). These common associates may act on each other and exacerbate COPD in patients with CVD. Third, inflammation not only promotes the development of atherosclerosis by promoting the growth and migration of vascular smooth muscle cells, but also the damage of small airways and the loss of lung parenchyma. Since atherosclerosis and small airway lesions are the basis of CVD and COPD pathology, respectively, inflammation may be a potential biological mechanism linking the association of CVD and COPD (31). However, there are still more potential mechanisms that need to be further explored by more cellular and animal experiments.
In this study, we also provided some advantages for the clinical management of COPD. For example, this study was conducted using a standardised collection of subject information, which collected information from a representative sample of the Chinese population with a large and representative sample size. Secondly, in the baseline collection, we collected patients' lung function and grouped the subjects according to the standardised method, which explored the association between CVD and severity of COPD in a deeper and multidimensional way, and provided some clinical value and practical guidance for the clinical monitoring and management of COPD patients. However, this study also had certain flaws. For example, in this study, we only conducted a cross-sectional study, so we could only verify the association between CVD and COPD severity, but not the causal relationship, so we need to improve the relevant studies to continue to explore the relationship between the two. Secondly, the information of patients' CVD and its subclassification was collected from questionnaires without on-site monitoring and assessment by medical professionals, which had some recall bias, so more perfect prospective clinical trials are needed to further verify the relationship between the two. In addition, this study only focused on the Chinese population, which may limit the application of the findings to other countries and ethnicities. Finally, the current study lacked some common biomarkers (such as C-reactive protein and N-terminal pro-brain natriuretic peptide) to exclude more confounding factors, so more refined and systematic studies are needed to verify the association between the two. Despite these limitations, our study still provides some clues and theoretical basis for CVD in the clinical management of COPD patients and contributes to the promotion of the inclusion of CVD in the future clinical routine management of COPD.
Patients with severe-very severe COPD are associated with CVD, especially hypertension, compared with patients with mild-to-moderate COPD, which underscores the urgent need for targeted CVD prevention and management strategies in COPD patients. For future research, longitudinal studies are essential to explore the temporal relationship between COPD severity and the development of CVD, particularly hypertension. Interventional studies focusing on pharmacological or lifestyle-based interventions for hypertension in COPD patients should be prioritized. Additionally, more detailed mechanistic studies are needed to understand the pathways linking COPD with cardiovascular outcomes, to inform the development of more precise treatment protocols.
The datasets used in this study are not publicly available, as our research group is currently working on ongoing projects, and additional related publications are expected. However, the data can be provided upon reasonable request from the corresponding author. Please feel free to reach out if you would like to discuss further.
The studies involving humans were approved by Clinical Research Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
TL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. LC: Conceptualization, Data Curation, Formal Analysis, Investigation, Methodology, Software, Visualization, Writing – Original Draft, Writing – Review & Editing. HX: Conceptualization, Writing – review & editing. YZ: Conceptualization, Writing – review & editing. HY: Conceptualization, Writing – review & editing. HZ: Conceptualization, Funding acquisition, Project administration, Supervision, Validation, Writing – review & editing. CC: Conceptualization, Funding acquisition, Project administration, Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Ths study was supported by the following fundings: The National Key Research and Development Program of China grants (2016YFC1304000), the National Natural Scientiffc Foundation of China (82170017), the Zhejiang Provincial Key Research and Development Program (2020C03067), and the Health Industry Scientiffc Research Project of Gansu Province grants (GSWSKY2018-18).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conffict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. (2012) 379(9823):1341–51. doi: 10.1016/S0140-6736(11)60968-9
2. Ma L, Huo X, Yang A, Yu S, Ke H, Zhang M, et al. Metal exposure, smoking, and the risk of COPD: a nested case-control study in a Chinese occupational population. Int J Environ Res Public Health. (2022) 19(17):10896. doi: 10.3390/ijerph191710896
3. Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. International variation in the prevalence of COPD (the BOLD study): a population-based prevalence study [published correction appears in Lancet. 2012 Sep 1;380(9844):806]. Lancet. (2007) 370(9589):741–50. doi: 10.1016/S0140-6736(07)61377-4
4. Viniol C, Vogelmeier CF. Exacerbations of COPD. Eur Respir Rev. (2018) 27(147):170103. doi: 10.1183/16000617.0103-2017
5. Chen H, Luo X, Du Y, He C, Lu Y, Shi Z, et al. Association between chronic obstructive pulmonary disease and cardiovascular disease in adults aged 40 years and above: data from NHANES 2013–2018. BMC Pulm Med. (2023) 23(1):318. doi: 10.1186/s12890-023-02606-1
6. Brightling C, Greening N. Airway inflammation in COPD: progress to precision medicine. Eur Respir J. (2019) 54(2):1900651. doi: 10.1183/13993003.00651-2019
7. Soysal P, Arik F, Smith L, Jackson SE, Isik AT. Inflammation, frailty and cardiovascular disease. Adv Exp Med Biol. (2020) 1216:55–64. doi: 10.1007/978-3-030-33330-0_7
8. Vespasiani-Gentilucci U, Pedone C, Muley-Vilamu M, Antonelli-Incalzi R. The pharmacological treatment of chronic comorbidities in COPD: mind the gap!. Pulm Pharmacol Ther. (2018) 51:48–58. doi: 10.1016/j.pupt.2018.06.004
9. Berry MJ, Adair NE, Rejeski WJ. Use of peak oxygen consumption in predicting physical function and quality of life in COPD patients. Chest. (2006) 129(6):1516–22. doi: 10.1378/chest.129.6.1516
10. Drummond MB, Blackford AL, Benditt JO, Make BJ, Sciurba FC, McCormack MC, et al. Continuous oxygen use in nonhypoxemic emphysema patients identifies a high-risk subset of patients: retrospective analysis of the national emphysema treatment trial. Chest. (2008) 134(3):497–506. doi: 10.1378/chest.08-0117
11. Rabe KF, Hurst JR, Suissa S. Cardiovascular disease and COPD: dangerous liaisons? [published correction appears in Eur Respir Rev. 2018 Nov 21;27(150):185057. doi: 10.1183/16000617.5057-2018]. Eur Respir Rev. (2018) 27(149):180057. doi: 10.1183/16000617.0057-2018
12. Mannino DM, Doherty DE, Sonia Buist A. Global initiative on obstructive lung disease (GOLD) classification of lung disease and mortality: findings from the atherosclerosis risk in communities (ARIC) study. Respir Med. (2006) 100(1):115–22. doi: 10.1016/j.rmed.2005.03.035
13. Enriquez JR, de Lemos JA, Parikh SV, Peng SA, Spertus JA, Holper EM, et al. Association of chronic lung disease with treatments and outcomes patients with acute myocardial infarction. Am Heart J. (2013) 165(1):43–9. doi: 10.1016/j.ahj.2012.09.010
14. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of cardiology/American heart association task force on clinical practice guidelines [published correction appears in hypertension. 2018 Jun;71(6):e140–e144. doi: 10.1161/HYP.0000000000000076]. Hypertension. (2018) 71(6):e13–e115. doi: 10.1161/HYP.0000000000000065
15. Agustí A, Celli BR, Criner GJ, Halpin D, Anzueto A, Barnes P, et al. Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Eur Respir J. (2023) 61(4):2300239. doi: 10.1183/13993003.00239-2023
16. Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. (2013) 187(4):347–65. doi: 10.1164/rccm.201204-0596PP
17. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care. (2019) 42(Suppl 1):S13–28. doi: 10.2337/dc19-S002
18. Bai Y, Wang J, Song R, Wang Z, Qi X, Buchman AS, et al. Influence of cardiovascular risk burden on pulmonary function trajectory: role of physical and social activities. Aging. (2022) 14(15):6081–93. doi: 10.18632/aging.204201
19. Alter P, Lucke T, Watz H, Andreas S, Kahnert K, Trudzinski FC, et al. Cardiovascular predictors of mortality and exacerbations in patients with COPD. Sci Rep. (2022) 12(1):21882. doi: 10.1038/s41598-022-25938-0
20. Dalal AA, Shah M, Lunacsek O, Hanania NA. Clinical and economic burden of patients diagnosed with COPD with comorbid cardiovascular disease. Respir Med. (2011) 105(10):1516–22. doi: 10.1016/j.rmed.2011.04.005
21. Almagro P, Salvadó M, Garcia-Vidal C, Rodriguez-Carballeira M, Delgado M, Barreiro B, et al. Recent improvement in long-term survival after a COPD hospitalisation. Thorax. (2010) 65(4):298–302. doi: 10.1136/thx.2009.124818
22. Krishnan S, Tan WC, Farias R, Aaron SD, Benedetti A, Chapman KR, et al. Impaired spirometry and COPD increase the risk of cardiovascular disease: a Canadian cohort study. Chest. (2023) 164(3):637–49. doi: 10.1016/j.chest.2023.02.045
23. Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. (2008) 32(4):962–9. doi: 10.1183/09031936.00012408
24. Ren WY, Li L, Zhao RY, Zhu L. Age-associated changes in pulmonary function: a comparison of pulmonary function parameters in healthy young adults and the elderly living in Shanghai. Chin Med J. (2012) 125(17):3064–8.22932182
25. Divo M, Cote C, de Torres JP, Casanova C, Marin JM, Pinto-Plata V, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2012) 186(2):155–61. doi: 10.1164/rccm.201201-0034OC
26. Abdul Roda M, Fernstrand AM, Redegeld FA, Blalock JE, Gaggar A, Folkerts G. The matrikine PGP as a potential biomarker in COPD. Am J Physiol Lung Cell Mol Physiol. (2015) 308(11):L1095–101. doi: 10.1152/ajplung.00040.2015
27. Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. (2009) 33(5):1165–85. doi: 10.1183/09031936.00128008
28. Smith MC, Wrobel JP. Epidemiology and clinical impact of major comorbidities in patients with COPD. Int J Chron Obstruct Pulmon Dis. (2014) 9:871–88. doi: 10.2147/COPD.S49621
29. Senoner T, Dichtl W. Oxidative stress in cardiovascular diseases: still a therapeutic target? Nutrients. (2019) 11(9):2090. doi: 10.3390/nu11092090
30. Kong P, Cui ZY, Huang XF, Zhang DD, Guo RJ, Han M. Inflammation and atherosclerosis: signaling pathways and therapeutic intervention. Signal Transduct Target Ther. (2022) 7(1):131. doi: 10.1038/s41392-022-00955-7
31. Vogelmeier CF, Criner GJ, Martínez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary [published correction appears in arch bronconeumol. 2017 Jul;53(7):411–412. doi: 10.1016/j.arbres.2017.06.001]. Informe 2017 de la iniciativa global para el diagnóstico, tratamiento y prevención de la enfermedad pulmonar obstructiva crónica: resumen ejecutivo de GOLD [published correction appears in arch bronconeumol. 2017 Jul;53(7):411-412. doi: 10.1016/j.arbres.2017.06.001]. Arch Bronconeumol. (2017) 53(3):128–49. doi: 10.1016/j.arbres.2017.02.001
Keywords: chronic obstructive pulmonary disease, cardiovascular disease, hypertension, coronary heart disease, severity
Citation: Li T, Chen L, Xu H, Zheng Y, Yang H, Zhao H and Chen C (2025) The association between cardiovascular diseases and their subcategories with the severity of chronic obstructive pulmonary disease: a large cross-sectional study based on a Chinese hospital population cohort. Front. Cardiovasc. Med. 12:1502205. doi: 10.3389/fcvm.2025.1502205
Received: 26 September 2024; Accepted: 27 January 2025;
Published: 13 February 2025.
Edited by:
Nicola Mumoli, ASST Ovest Milanese, ItalyReviewed by:
Leonello Fuso, Catholic University of the Sacred Heart, ItalyCopyright: © 2025 Li, Chen, Xu, Zheng, Yang, Zhao and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongjun Zhao, emhhb2hvbmdqdW5Ad211LmVkdS5jbg==; Chengshui Chen, Y2hlbmNoZW5nc2h1aUB3bXUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.