- 1Division of Medical Genetics, Department of Internal Medicine, The University of Texas Health Science Center at Houston, Houston, TX, United States
- 2Department of Anatomy and Cell Biology, McGill University, Montreal, QC, Canada
Objective: We sought to determine if hypertension in combination with a “variant of uncertain significance” that disrupts protein function, MYH11 p.Arg247Cys, would induce aortic dissections in a mouse model.
Approach and results: Administration of L-NAME via drinking water and a high salt diet increased blood pressure in WT and Myh11R247C/R247C mice and triggered type A dissections with cardiac tamponade in 20% of the Myh11R247C/R247C mice. Myh11R247C/R247C aortas have aberrant smooth muscle contractile unit-elastin connections by transmission electron microscopy, along with increased focal adhesion signaling at baseline, which further increases with hypertension.
Conclusion: Gene-environment interactions trigger aortic dissections in Myh11R247C/R247C mice.
Introduction
Acute ascending aortic dissections (ADs; Stanford classification type A) cause sudden death in up to half of afflicted individuals, and these sudden deaths are primarily due to retrograde dissection of the blood from the aortic intimal tear at the sinotubular junction into the pericardial sac, causing pericardial tamponade (1). Typically, enlargement of the ascending aorta or root precedes the AD, and prophylactic surgery of these aneurysms is recommended when the diameter reaches >5.0 cm to prevent AD (2). However, studies on patients presenting with acute ADs determined that approximately 50% of dissections occur with no enlargement or at diameters <5.0 cm in diameter (3).
The major risk factors for ADs are genetic variants and increased biomechanical force on the aorta, primarily due to hypertension (HTN) (4). Pathogenic variants in MYH11, which encodes the smooth muscle cell (SMC)-specific isoform of myosin heavy chain, predispose to ADs, and these rare variants are primarily in-frame deletions in the coiled-coil domain, which disrupt thick filament formation (5, 6). MYH11 and other mutant genes that confer a highly penetrant risk for AD disrupt proteins in a structural element in the aorta, termed the elastin-contractile unit (ECU), which links extensions from the elastin fibers to SMC contractile units through cell surface focal adhesions (FAs) (7). We have also identified a MYH11 rare variant of unknown significance (VUS), MYH11 p.Arg247Cys, in patients with thoracic aortic disease, and disruption of the corresponding amino acid in MYH7 causes familial hypertrophic cardiomyopathy (8, 9). We previously showed that Myh11R247C/R247C mice do not form aneurysms despite decreased aortic contraction, and explanted Myh11R247C/R247C SMCs have increased focal adhesion kinase (FAK) signaling (9). Increased FA signaling has also been shown in the Acta2−/− mouse model, which develops aortic aneurysms, and single cell transcriptomics of Fbn1mgr/mgr mice and angiotensin II-infused mice implicate FA signaling in ADs (10–12). When a second genetic hit, loss of one allele of Acta2, was introduced in Myh11R247C/R247C mice, the mice developed thoracic aortic enlargement, supporting that MYH11 p.Arg247Cys is a risk allele for thoracic aortic disease (13). Here, we report that HTN leads to death due to acute ADs in the absence of aortic enlargement in 20% of Myh11R247C/R247C mice, indicating that this is also a risk allele for acute ADs.
Methods
Hypertensive model in mice
Wild-type (WT) and Myh11R247C/R247C mice on 129S8/SvEv and C57BL/6 mixed background were produced as previously reported (9). Littermate controls were used for all experiments to ensure equal percentages of the two strains between treatment and control groups. All protocols were approved by the Animal Welfare Committee at the University of Texas Health Science Center at Houston.
Since incidence of thoracic aortic disease is substantially higher in men (4), only male mice were treated and assessed in this study. Myh11R247C/R247C and WT littermates were randomized into HTN or untreated groups. High salt diet (8% NaCl diet from Harlan Laboratories) and L-Nω-nitroarginine methyl ester (L-NAME, Cayman Chemical) were used to induce HTN in male mice [treatment initiated at 8–9 weeks of age except for the transmission electron microscopy (TEM) studies]. L-NAME was dissolved at 3.0 g/L in the drinking water, and fresh water was prepared and replaced daily.
Blood pressure was alternatively increased in mice through intraperitoneal injection of 0.05 mg norepinephrine (NE) on the evening of day 1, then injection with 0.025 mg norepinephrine in the morning and evening on day 2.
Additional methods are available in the supplement.
Results
Hypertension causes aortic root dissection in Myh11R247C/R247C mice
We previously demonstrated decreased contraction of aortic segments but no aortic enlargement or spontaneous death in the Myh11R247C/R247C mice (9). To increase biomechanical forces on the aorta, 8-week-old male WT and Myh11R247C/R247C mice were subjected to a high salt diet and the nitric oxide synthase inhibitor L-NAME. Littermates were randomized into HTN or untreated groups. Since incidence of thoracic aortic disease is substantially higher in males, only male mice were treated and assessed in this study (4). The treatment significantly increased blood pressure within two weeks to the same extent in Myh11R247C/R247Cand WT mice (Figure 1A). Over the sixteen weeks after HTN induction, sudden deaths occurred in 36% of the Myh11R247C/R247C mice (14 deaths/39 mice) while only five WT mice died (5 deaths/21 mice), and no untreated mice of either genotype died (p = 0.003 by Kaplan–Meier analysis) (Figure 1B). Nine Myh11R247C/R247C deaths compared with just one WT death occurred in the first two weeks after HTN induction. Necropsy of Myh11R247C/R247C mice identified blood in the pericardial sac by visual inspection and histology (Figure 1C; Supplementary Figure 1A). Serial sectioning from the aortic valve to the ascending aorta identified intimal tears at the sinotubular junction as the cause of the pericardial tamponade, thus mimicking ADs in patients. (Figure 1C; Supplementary Figure 1). No pericardial tamponade or intimal tears were observed in the dead WT mouse. Echocardiography identified no enlargement of the aortic root or ascending aorta in the surviving Myh11R247C/R247C mice out to six months of age, four months after HTN induction (Figure 1D; Supplementary Figure 2A). After echocardiography, aortas were fixed and paraffin embedded; hematoxylin and eosin (HE) staining confirmed increased thickness of the medial layer in the hypertensive compared with untreated Myh11R247C/R247C mice (Supplementary Figure 2B). To confirm that HTN was responsible for the deaths in the Myh11R247C/R247C mice, HTN was induced using intraperitoneal injections of NE, leading to a systolic blood pressure increase of 50 mmHg within 10 min of the injection. Two of nine WT mice compared with seven of fourteen mutant mice died suddenly on day two of NE injections, and pericardial tamponade was observed in the Myh11R247C/R247C mice without enlargement of the aorta (p value = 0.2, Figure 1E; Supplementary Figure 3). Thus, other methods to induce HTN also lead to aortic dissections in Myh11R247C/R247C mice.
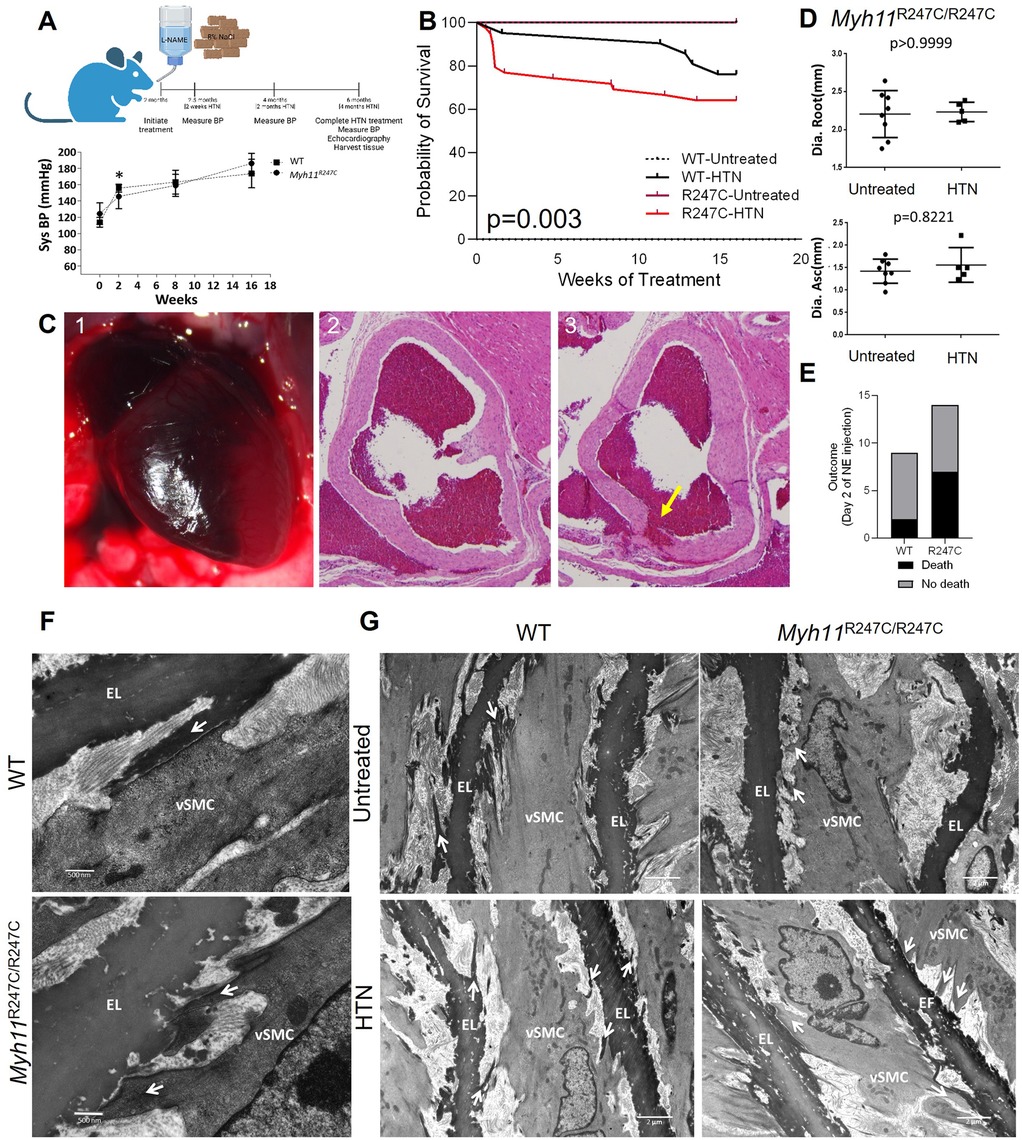
Figure 1. HTN in Myh11R247C/R247C mice induces aortic dissections. (A) Top panel shows a schematic of the experiment design: L-NAME and high salt diet were initiated in 2 month old mice. Bottom panel shows tail cuff blood pressure measurements indicating significantly increased systolic and diastolic blood pressures within two weeks after initiation of L-NAME and high salt diet treatment in both WT and Myh11R247C/R247C mice. (B) Kaplan–Meier survival analysis shows reduced survival over sixteen weeks of HTN induction in hypertensive Myh11R247C/R247C mice compared with hypertensive WT mice or normotensive mice (n = 16 untreated WT, n = 27 untreated Myh11R247C/R247C, n = 21 HTN WT, n = 39 HTN Myh11R247C/R247C). (C) Example necropsy on Myh11R247C/R247C mouse that did not survive shows pericardial tamponade (1). Sectioning through the aortic root shows a tear in the intimal layer, i.e., dissection (3); an adjacent section does not show the dissection (2). (D) Echocardiography after four months of HTN treatment reveals no increase in aortic diameter in either WT or Myh11R247C/R247C mice after HTN induction. Dots represent individual mice, lines represent the mean ± standard deviation. Statistical comparison was measured by non-parametric Mann–Whitney analysis. (E) Injection of norepinephrine (NE) induced sudden death within two days in 6/14 Myh11R247C/R247C mice compared with 2/9 WT mice. (F) Transmission electron microscopy (TEM) images of the four-week-old aorta (untreated) show aberrant structure of the elastin-contractile unit in Myh11R247C/R247C aortas with increased distortion of Myh11R247C/R247C SMCs after HTN. Arrows indicate projections linking smooth muscle cells (vSMC) with elastin fibers (EL); in WT tissue those projections initiate from the EL, while in Myh11R247C/R247C aortas those projections initiate from vSMC (n = 2 aortas per genotype, minimum of 3 visual fields per aorta). (G) TEM images of untreated four-week-old aortas and specimen at six weeks of age after two weeks of HTN confirm increased distortion (n = 2 aortas per genotype and treatment condition, minimum of 3 visual fields per aorta).
Aberrant structure and signaling of the SMC elastin-contractile unit in the hypertensive Myh11R247C/R247C mice
TEM of 4-week-old WT ascending aortas confirmed previous findings of the structure of the aortic media: specifically, oblique extensions from the elastic fibers linked to FAs on the surface of SMCs, indicating formation of intact ECUs (Figure 1F) (14). In contrast, ECUs were structurally abnormal in 4-week-old Myh11R247C/R247C mice; the oblique extensions from the elastin fiber were not present but rather cellular extensions from the SMCs attached to the elastin fiber (Figure 1F). Moreover, HTN treatment for two weeks induced distortion of the elastin extensions but little distortion of the SMCs in the WT aortas, whereas the SMCs are distorted with HTN in the Myh11R247C/R247C aortas (Figure 1G). It is important to note that the untreated mice were imaged at four weeks of age, while the HTN mice were imaged at six weeks of age, and therefore we cannot rule out that age-related changes are responsible for the differences observed in the HTN groups.
The distortion of the abnormally formed ECUs in aortic SMCs with HTN in the Myh11R247C/R247C mice suggests that mechanosensing pathways downstream of FAs may be activated. Global transcriptional changes in the Myh11R247C/R247C descending aortas with and without HTN induction for two weeks were assessed using RNA-sequencing; 644 upregulated and 817 downregulated genes were identified (Figure 2A). GSEA analysis using WikiPathways identified five upregulated and no downregulated pathways; of the five upregulated pathways, two involve FA signaling (Figure 2B; Supplementary Figure 2). We previously showed that Myh11R247C/R247C SMCs explanted from the ascending aorta had altered FAs and increased phosphorylation at tyrosine 397 of FAK (p-FAK) levels when compared to WT SMCs (9). Here, we found that in vivo immunoblot analyses confirm increased levels of p-FAK in the Myh11R247C/R247C aortas at baseline (Figure 2C), and levels of p-FAK increase further after two weeks of HTN induction in Myh11R247C/R247C aortas but not in WT aortas (Figure 2D). Since phosphorylation of the RLC cannot be accurately assessed in vivo due to its transient nature (15), these levels were assessed in explanted SMCs. Exposure to soluble fibronectin (FN) activates FAs in the SMCs, and the Myh11R247C/R247C SMCs have higher levels of p-FAK and p-RLC than WT SMCs after 30 min of FN exposure (Figure 2E). Importantly, a FAK inhibitor, PF-228, significantly attenuated phosphorylation of both FAK and RLC 2 h after FN treatment in Myh11R247C/R247C SMCs (Figure 2E).
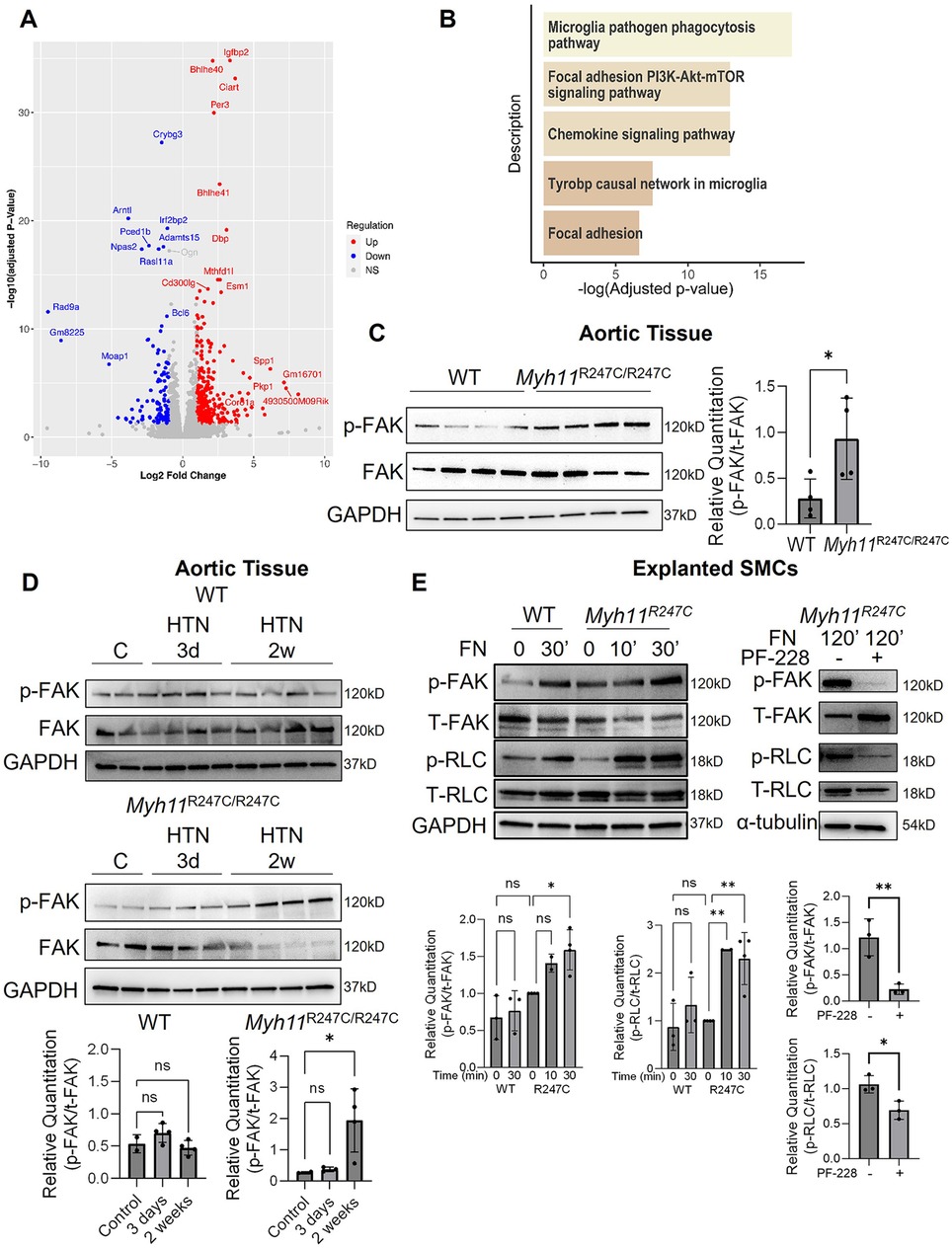
Figure 2. Aberrant ECU structure and FA signaling in Myh11R247C/R247C aortas. (A,B) Bulk RNA-sequencing analysis on descending aortic tissue revealed differentially expressed genes (A), and GSEA analysis of those genes revealed the top five upregulated pathways in the Myh11R247C/R247C mice after HTN include focal adhesion-related signaling (B). (C) Western blot analysis confirms increased FAK activation in Myh11R247C/R247C aortic tissue at baseline compared to WT tissue (without HTN). (D) Further analysis of WT or Myh11R247C/R247C tissue shows increased FAK activation within two weeks of HTN induction in Myh11R247C/R247C but not WT aortas. (E) Explanted SMCs from Myh11R247C/R247C have increased activation of FAK and increased phosphorylation of myosin regulatory light chain (RLC) after activation of the focal adhesions by short-term addition of fibronectin (FN) compared with SMCs explanted from littermate controls. The right panel shows Myh11R247C/R247C SMCs treated with FN for two hours with or without the FAK inhibitor PF-228. All blot quantitations are shown; each dot represents an individual mouse (C,D) or an independently-run experiment (E) Statistical analysis was performed via two-tailed student's t-test (C) or via one-way ANOVA with Dunn's post-test (D,E). *p < 0.05, **p < 0.005, HTN, hypertension; vSMC, smooth muscle cell; EF, elastin fiber; FAK, focal adhesion kinase; RLC, myosin regulatory light chain.
Discussion
Although genetic evidence supports that disruption of SMC force generation is a primary driver of ADs (16, 17), Myh11R247C/R247C mice have decreased aortic contraction but do not form aneurysms (9). However, the Myh11R247C allele does cause aortic enlargement in combination with a second genetic “hit”, loss of one allele of Acta2 (13). Here, increasing systolic blood pressure using L-NAME and high salt diet rapidly induced sudden death due to ADs and pericardial tamponade in 23% of Myh11R247C/R247C mice without evidence of aortic enlargement. HTN is the major risk factor for patients with thoracic ADs (4), and the results presented here indicate that sudden increases in blood pressure can induce ADs without aortic enlargement. Thus, this study is the first to demonstrate that sudden onset of HTN in conjunction with a VUS in MYH11 confers a low penetrant risk for acute ADs.
We further show that ECUs have an aberrant structure in Myh11R247C/R247C aortas, suggesting SMC contraction is required for the formation of extensions from elastin to SMCs. Additionally, transcriptomic data and immunostaining of aortic lysates indicate increased FAK signaling at baseline in the Myh11R247C/R247C aortas, which increases further with the induction of HTN in the Myh11R247C/R247C aortas. Importantly, these conclusions are based on analyses of mice that did not dissect, so although our data indicate that HTN increases FA-related signaling, other pathways driving dissection may have not been identified due to survival bias. Thus, these data suggest that the loss of SMC contractile function in Myh11R247C/R247C aortas increases signaling through FAs, and most likely through increases in downstream p-RLC levels and augmented actinomyosin motor function, which we hypothesize may compensate for the defective myosin function, thus preventing aortic disease. With the onset of HTN, the acute and excessive activation of FAK is predicted to further drive downstream actinomyosin motors but also activate additional pathways. Because few Myh11R247C/R247C mice die after the first two weeks of HTN treatment, we hypothesize that chronic HTN leads to adaptive changes that prevent further deaths. An important limitation of the current study is that only male mice were analyzed; further work is needed to establish whether these effects are sex-dependent. Thus, these data illustrate the interaction between a risk variant and HTN in triggering acute ADs and suggest a hypothesis that compensatory changes in the aorta occur in response to the Myh11 mutation that prevent both aneurysm formation at baseline and further AD deaths with chronic HTN.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Institutional Animal Care and Use Committee (IACUC) for the University of Texas Health Science Center at Houston (UTHealth). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
CK: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing. SW: Investigation, Validation, Writing – original draft. ZZ: Investigation, Validation, Visualization, Writing – review & editing. PG: Formal Analysis, Visualization, Writing – review & editing. YY: Investigation, Validation, Writing – review & editing. X-YD: Investigation, Validation, Writing – review & editing. TZ: Investigation, Validation, Writing – review & editing. JC: Investigation, Validation, Writing – review & editing. ED: Investigation, Resources, Writing – review & editing. DM: Conceptualization, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by a Leducq Foundation Network of Excellent, the National Heart, Lung and Blood Institute (R01HL109942 and 5R01HL146583 to DM), American Heart Association Merit Award, Remembrin' Benjamin Foundation, and the John Ritter Foundation.
Acknowledgments
We would like to acknowledge contributions to this work by Lea-Jeanne Ringuette, Gregory Aird, and Larry Zhu.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1492768/full#supplementary-material
Abbreviations
AD, aortic dissection; ECM, extracellular matrix; ECU, elastin-contractile unit; FA, focal adhesion; FAK, focal adhesion kinase; FN, fibronectin; HTN, hypertension; NE, norepinephrine; SMC, smooth muscle cell; L-NAME, L-Nω-nitroarginine methyl ester; RLC, myosin regulatory light chain; TEM, transmission electron microscopy; VUS, variant of unknown significance; WT, wild-type.
References
1. Prakash SK, Haden-Pinneri K, Milewicz DM. Susceptibility to acute thoracic aortic dissections in patients dying outside the hospital: an autopsy study. Am Heart J. (2011) 162:474–9. doi: 10.1016/j.ahj.2011.06.020
2. Hiratzka L, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: executive summary. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Catheter Cardiovasc Interv. (2010) 76:43. doi: 10.1002/ccd.22537
3. Pape LA, Tsai TT, Isselbacher EM, Oh JK, O'gara PT, Evangelista A, et al. Aortic diameter > or = 5.5 cm is not a good predictor of type A aortic dissection: observations from the international registry of acute aortic dissection (IRAD). Circulation. (2007) 116:1120–7. doi: 10.1161/CIRCULATIONAHA.107.702720
4. Zhou Z, Cecchi AC, Prakash SK, Milewicz DM. Risk factors for thoracic aortic dissection. Genes (Basel). (2022) 13:1814. doi: 10.3390/genes13101814
5. Pannu H, Tran-Fadulu V, Papke CL, Scherer S, Liu Y, Presley C, et al. MYH11 mutations result in a distinct vascular pathology driven by insulin-like growth factor 1 and angiotensin II. Hum Mol Genet. (2007) 16:2453–62. doi: 10.1093/hmg/ddm201
6. Zhu L, Vranckx R, Khau Van Kien P, Lalande A, Boisset N, Mathieu F, et al. Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat Genet. (2006) 38:343–9. doi: 10.1038/ng1721
7. Karimi A, Milewicz DM. Structure of the elastin-contractile units in the thoracic aorta and how genes that cause thoracic aortic aneurysms and dissections disrupt this structure. Can J Cardiol. (2016) 32:26–34. doi: 10.1016/j.cjca.2015.11.004
8. Roopnarine O, Leinwand LA. Functional analysis of myosin mutations that cause familial hypertrophic cardiomyopathy. Biophys J. (1998) 75:3023–30. doi: 10.1016/S0006-3495(98)77743-4
9. Kuang SQ, Kwartler CS, Byanova KL, Pham J, Gong L, Prakash SP, et al. Rare, nonsynonymous variant in the smooth muscle-specific isoform of myosin heavy chain, MYH11, R247C, alters force generation in the aorta and phenotype of smooth muscle cells. Circ Res. (2012) 110:1411–22. doi: 10.1161/CIRCRESAHA.111.261743
10. Pedroza AJ, Tashima Y, Shad R, Cheng P, Wirka R, Churovich S, et al. Single-cell transcriptomic profiling of vascular smooth muscle cell phenotype modulation in Marfan syndrome aortic aneurysm. Arterioscler Thromb Vasc Biol. (2020) 40:2195–211. doi: 10.1161/ATVBAHA.120.314670
11. Papke CL, Cao J, Kwartler CS, Villamizar C, Byanova KL, Lim S-M, et al. Smooth muscle hyperplasia due to loss of smooth muscle alpha-actin is driven by activation of focal adhesion kinase, altered p53 localization and increased levels of platelet-derived growth factor receptor-beta. Hum Mol Genet. (2013) 22:3123–37. doi: 10.1093/hmg/ddt167
12. Zhang C, Li Y, Chakraborty A, Li Y, Rebello KR, Ren P, et al. Aortic stress activates an adaptive program in thoracic aortic smooth muscle cells that maintains aortic strength and protects against aneurysm and dissection in mice. Arterioscler Thromb Vasc Biol. (2023) 43:234–52. doi: 10.1161/ATVBAHA.122.318135
13. Kwartler CS, Gong L, Chen J, Wang S, Kulmacz R, Duan X-Y, et al. Variants of unknown significance in genes associated with heritable thoracic aortic disease can be low penetrant “risk variants”. Am J Hum Genet. (2018) 103:138–43. doi: 10.1016/j.ajhg.2018.05.012
14. Davis EC. Smooth muscle cell to elastic lamina connections in developing mouse aorta. Role in aortic medial organization. Lab Invest. (1993) 68:89–99.8423679
15. Kamm KE, Stull JT. Myosin phosphorylation, force, and maximal shortening velocity in neurally stimulated tracheal smooth muscle. Am J Physiol. (1985) 249:238. doi: 10.1152/ajpcell.1985.249.3.C238
16. Milewicz DM, Guo D-C, Tran-Fadulu V, Lafont AL, Papke CL, Inamoto S, et al. Genetic basis of thoracic aortic aneurysms and dissections: focus on smooth muscle cell contractile dysfunction. Annu Rev Genomics Hum Genet. (2008) 9:283–302. doi: 10.1146/annurev.genom.8.080706.092303
Keywords: myosin heavy chain, focal adhesion, elastin-contractile unit, thoracic aortic dissection, vascular disease, aortic dissection, smooth muscle cell (SMC)
Citation: Kwartler CS, Wang S, Zhou Z, Guan P, Yu Y, Duan X-Y, Zhang T, Chen J, Davis EC and Milewicz DM (2025) Inducing hypertension in Myh11R247C/R247C mice triggers aortic dissections with increased focal adhesion kinase signaling. Front. Cardiovasc. Med. 12:1492768. doi: 10.3389/fcvm.2025.1492768
Received: 7 September 2024; Accepted: 31 January 2025;
Published: 14 February 2025.
Edited by:
Margreet R. De Vries, Leiden University Medical Center (LUMC), NetherlandsReviewed by:
Mabruka Alfaidi, University of Nebraska Medical Center, United StatesDunpeng Cai, University of Missouri, United States
Copyright: © 2025 Kwartler, Wang, Zhou, Guan, Yu, Duan, Zhang, Chen, Davis and Milewicz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dianna M. Milewicz, ZGlhbm5hLm0ubWlsZXdpY3pAdXRoLnRtYy5lZHU=
†These authors have contributed equally to this work
 Callie S. Kwartler
Callie S. Kwartler Shanzhi Wang1,†
Shanzhi Wang1,† Zhen Zhou
Zhen Zhou Pujun Guan
Pujun Guan Yang Yu
Yang Yu Dianna M. Milewicz
Dianna M. Milewicz