
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 20 March 2025
Sec. General Cardiovascular Medicine
Volume 12 - 2025 | https://doi.org/10.3389/fcvm.2025.1465646
Background: MicroRNAs (miRNAs) add a new dimension to HD forecast, diagnosis, and therapy based on the potential applications. The miRNA-related research in the heart disease (HD) field has received close attention in the past two decades. However, there is a lack of studies that comprehensively and objectively analyze the current situation of miRNA application in the HD field using the bibliometrics method.
Objective: To comprehensively analyze the global scientific outputs of miRNAs in HD research from 2004 to 2023.
Methods: All the articles and reviews of miRNA-related research in the HD field were retrieved using the Web of Science core collection (WOSCC) title search, and bibliometric analysis was performed in Microsoft Excel 2019, CiteSpace, VOSviewer, and Bibliometrics (R-Tool of R-Studio).
Results: 3,874 publications were included in the bibliometric analysis. Collaborative network analysis indicates that China with the maximum number of publications (2,063) and the USA with the highest total citations (59,331) are influential countries in this field. Peking Union Medical College is the most prolific university with the maximum publications (134), and the University of California System is the most authoritative institution regarding betweenness centrality (0.27). PLOS ONE tops the journal list of publications, closely followed by the International Journal of Molecular Sciences and Scientific Reports with more than 100 articles. Considering the number of publications, citations, and total link strength overall, Olson. Eric N, Van Rooij Eva, Thum Thomas, Yang Baofeng, Wang Kun; and Lu Yanjie are authoritative authors in this field. The expression changes and regulatory mechanisms of specific miRNAs in various heart biological and pathophysiological processes have been the continuous research hotspots. “exosomes”, “extracellular vesicles”, “autophagy”, and “management” have been novel hot research topics since 2018, which focused on the diagnosis and treatment of HD. The current research development trend is how to translate the achievement of miRNA-related diagnosis and therapeutic drugs for HD into the clinic.
Conclusion: Our study revealed the intellectual structure of miRNA in HD research, which may help scholars understand this field comprehensively and find partners.
Heart diseases (HD) usually present as disorders of the coronary artery, myocardium, electrocardiogram pathway, etc. Cardiovascular disease (CVD) is still the leading cause of death throughout the world. However, approximately one-quarter reduction in mortality is expected by 2025, mostly related to the appearance of novel diagnostic techniques and therapies (1).
MicroRNAs (miRNAs) are small noncoding RNAs (∼23 nucleotides) that regulate gene expression by pairing to mRNAs at the post-transcriptional level in plants and animals (2). Functionally, miRNAs provide transcriptional control and play an important role in normal physiological development and pathological conditions.
Abundant studies on miRNAs in the development and progression of heart conditions implicate hope in disease diagnosis, prognosis, and treatment. Humans after birth have a limited capacity to regenerate heart tissue after injury (3). miRNA-related research brings the therapeutic potential for human heart injury by various diseases. The study discusses the current status and trends of miRNA-related research in various cardiac conditions, including ischemic HD, cardiac hypertrophy, arrhythmia, etc.
Bibliometric analysis involves analyzing published works objectively on an academic area over a specific period using mathematical and statistical methods, both qualitatively and quantitatively (4, 5). This method provides an objective view of the status, hotspots, trends, and frontiers by analyzing countries, institutions, journals, authors, and keywords of published works related to the specific research field (4, 6).
Despite the rapid universality of miRNA-related research in the last twenty years, there is still a lack of bibliometric analyses of “miRNAs in HD” research. Therefore, this study analyzed the overall situation of “miRNAs in HD” research in the past two decades with VOSviewer (7), CiteSpace (8) software programs, and R software. This may help scholars gain insight into the corresponding academic fields and find collaborations.
The raw data of this study were exported from the Web of Science Core Collection (WOSCC) database, a comprehensive, fundamental data source widely used for bibliometric analysis and information mapping in academia covering a large part of the medical literature (9).
In the WOSCC database, TS and TI stand for Topic and Title, respectively. For precise literature retrieval, we utilized title retrieval. The search format presented below: TI = (“miRNA*” OR “microRNA*” OR “miR*” OR “micro ribonucleic acid*” OR “micro RNA*” OR “RNA micro”) AND TI = (“heart” OR “cardi*” OR “coronary” OR “atrium*” OR “atrial” OR “ventricle*” OR “arrhythmia*”) AND PY = (2004–2023) AND LA = (English). The literature publication time was limited to December 31, 2023. The language was limited to English, “Article” was selected as the article or review type, and the retracted publication was excluded, resulting in 3,874 articles (Figure 1). The data was downloaded as plain text files in text formats, according to the above retrieval strategy. The search was completed on February 29, 2024. In the data-collecting process, the results were verified by two researchers separately. A third-party adjudication resolved discrepancies and was immediately followed by a three-way harmonization.
CiteSpace and VOSviewer are currently widely used software for bibliometric analysis and bibliometric network graph analysis (10), respectively. We used CiteSpace 6.2.R4 advanced visualization and VOSviewer 1.6.20 to visually analyze the overall network of journals, institutions, authors, references, and key nodes via the data files downloaded from the WOSCC database.
In addition, we used Bibliometrix (R-Tool of R-Studio) (11) to visually analyze the distribution of countries/regions, references, and keywords, and Microsoft Excel 2019 to show the publication and citation situation of the literature over the 20 years. All data in this study were obtained from public databases; therefore, ethical review was unnecessary.
Using VOSviewer, clustering analyses of nodes representing various countries/regions, journals, authors, and keywords were performed based on their occurrence in the download data. The circle's color, size, and connecting lines were used to describe the frequency and links of nodes. Moreover, analyses on countries/regions, journals, and keyword citation bursts were also performed by CiteSpace. The betweenness centrality refers to the core position of the nodes. The strength indicates the connection tightness between object data. The time distribution of keywords is described by beginning and end times.
There were 3,874 documents (3,381 articles and 493 reviews) on “miRNA in HD” in the WOSCC database from 2004 to 2023 (Figures 1, 2). The distribution of the top ten research areas according to WOS, the top 10 academic categories is reported in Table 1. It shows that 905 publications were included in the subject area “Cardiovascular Systems Cardiology” (23.361%), 778 in “Cell Biology” (20.083%), 704 in “Medicine Research Experimental” (18.172%), 580 in “Biochemistry Molecular Biology” (14.972%), and 388 in “Pharmacology Pharmacy” (10.015%), etc. The mean citation frequency per article is 40.36, with an H-index of 161. In-depth analysis indicates that the top 50 articles, in terms of citation frequency, accounted for 19.8% of the total citations, with an average of 613.5 citations per article.
Based on the data gathered from the WOSCC database, from 2004 January 1 to 2023 December 30, the enrolled 3,874 publications have received a cumulative total of 156,334 citations. The annual number of publications and citations for “miRNAs in HD” research is exhibited in Figure 2. Generally, the yearly volume of articles showed a rapid upward trend with a peak in 2019 (456) and a significant downward trend from then on. Similarly, the number of annual citations shows the same curve, reaching the inflection point of 21,329 in 2021.
Table 2 shows the top 20 countries/regions by publication quantity. Meanwhile, it shows the corresponding centrality, frequency of citations, and year when the first document was published. In miRNA-related studies on HD, China has the highest number of publications (2,063) and the highest number of total citations (61,164), followed by the USA (751, 59,331) and Germany (233, 15,957). The Netherlands has the highest average citation of all the countries/regions (84.6 times), followed by the USA (79 times) and Germany (68.5 times). The betweenness centrality of countries/regions is a critical indicator to identify the role of the countries/regions in the global network. Based on this indicator the USA has the highest centrality (0.46), England (0.24), and Germany (0.17) ensued. The USA has established a leading role regarding average citation and betweenness centrality in this field.
In review China and the USA were the most prominent countries, with the highest publications and citations. Currently, 76 countries/regions are involved in miRNA-related research of heart conditions, mainly located in East Asia, Oceania, North America, and Europe, with strong links among them (Figure 3A). As indicated in Figure 3A, the numbers of connecting lines indicate the mutual links between different countries/regions. Among lots of countries/regions, there is deep cooperation (Figure 3B). The closest relationship in terms of country-to-country cooperation is between China and the USA (Figure 3C). In addition, the USA also collaborates closely with Italy, the United Kingdom, Canada, Japan, Germany, Netherlands, Japan, Brazil, Australia, India, Sweden, Poland, Spain, and France, etc. It is the same situation between China and other countries/regions including Canada, the Netherlands, Japan, Singapore, Australia, and the United Kingdom. The visualization in VOSviewer of the countries/regions with miRNA-related literature of HD shows their cooperation similarly (Figure 3B). The network collaboration analysis shows that countries/regions are divided into 12 clusters in VOSviewer according to the cooperation closeness, marked with different colors. In Figure 3B, each node represents a country/region, and the node's radius expands with the increase of its document volume.
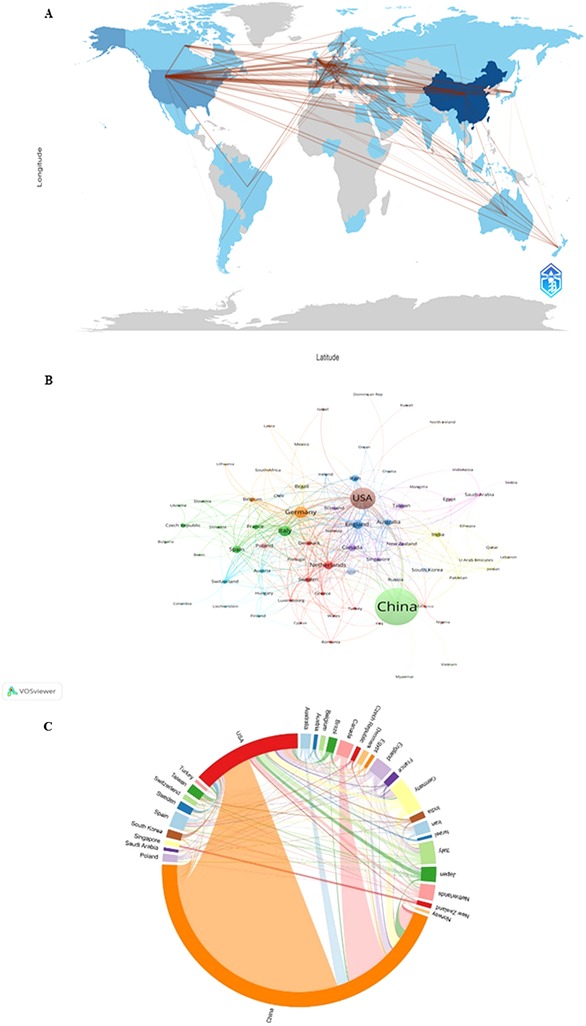
Figure 3. Analysis of country/region network in the "miRNAs in HD" research. (A) Countries/regions collaboration map in R software. (B) Collaborative network visualization of countries/regions in VOSviewer. The figure shows the countries/regions distribution of miRNA-related literature on heart disease. The size of the nodes indicates their literature quantity. The number of connecting lines is positively proportional to the closeness between different countries/regions. (C) Collaborative network visualization of countries/regions in VOSviewer.
Table 3 shows the top 20 institutions in terms of number of publications. Together their frequency of citations and the corresponding centrality are listed in the table. The institution with the highest number of publications is Peking Union Medical College (134), followed by Harbin Medical University (131) and Nanjing Medical University (100). This table shows fifteen institutions in China, followed by the USA with three and Germany with two institutions. Among the top 20 institutions, the University of California System (0.27), Harvard University (0.21), Harvard Medical School (0.1), and Hannover Medical School (0.1) show high centrality, which implies that these institutions have a significant effect on the development of the “miRNAs in HD” research.
The institution analysis aims to explore the global distribution of miRNA-related research of HD and help scholars seek cooperation. Using VOSviewer to plot institution collaboration network graphs, institutions with at least 25 documents cooperation are divided into 9 closely related blocks (Figure 4A). Harbin Medical University, Nanjing Medical University, and Shanghai Jiao Tong University are relatively highly productive institutions in the institutional cooperation network (Figure 3A), but their centralities are relatively low (Table 3).
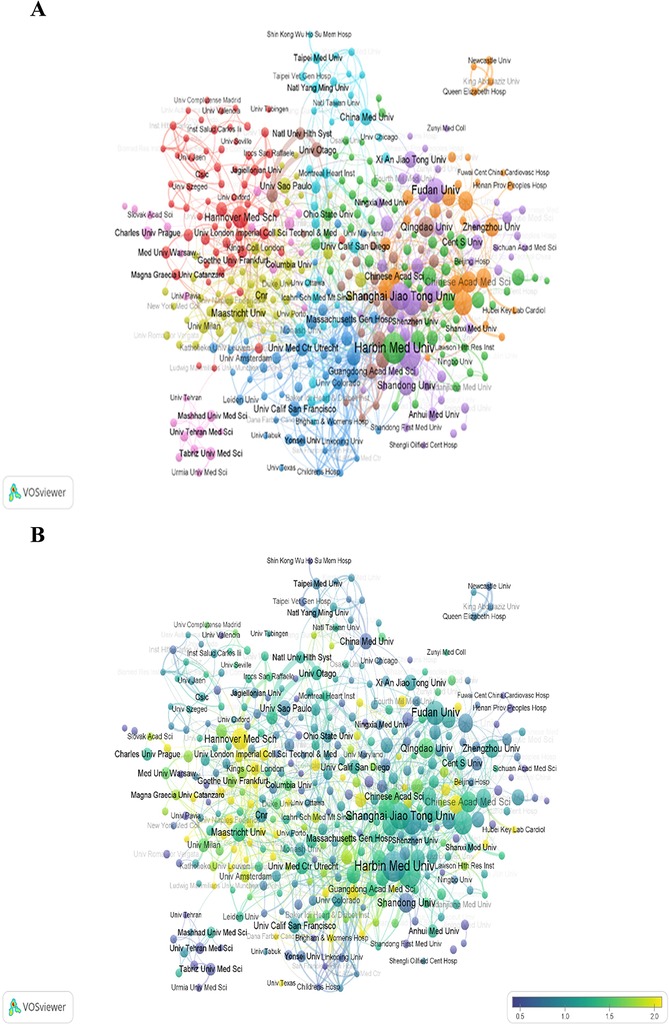
Figure 4. Analysis of institutions of the "miRNAs in HD" research in VOSviewer. (A) Collaborative network visualization of institutions with more than 25 documents. (B) Overlay visualization of institutions in VOSviewer. The number of articles published by institutions in 5 years from 2016 to 2020. The number of publications of the institution in the 5 years divided by the total publications from 2004 to 2003 is its heat value of the 5 years.
Figure 4B reveals the ratio of institutional publications to total publications from 2016 to 2020, a time when the studies of this field rapidly burst. The ratio numbers were achieved by dividing the publications amount of the five years in this field of each institution by their total amount of publications from 2004 to 2023. The closer the node color is to yellow, the higher the ratio. The high ratio indicates that the corresponding institution is an emerging force in this field in the five years. The figure shows that Hannover Medical School, the University of Texas, Fourth Military Medicine University, and some other institutions with yellow nodes have achieved rapid growth, having issued lots of papers much more than residual institutions during the five years. In contrast, the documents issued by Yonsei University, Shandong University, Tabriz University of Medical Sciences, China Medical University, Harbin Medical University, and University Texas Health Science Center of Houston represented by purple nodes are relatively few.
A total of 78 journals were involved in the “miRNAs in HD” research. The bibliometric online analysis platform identified journals with high publication volume and impact on the “miRNAs in HD” research.
The top 20 journals by publication volume with a total of 1,195 documents (30.85%) are listed in Table 4. Meanwhile, the table shows the corresponding total citations, average citations, the IF, the H-index, and the JCR category quartile of the above 20 journals in this field from 2004 to 2023. PLOS ONE (134, Q2, H-index 44) with the highest number of publications and H-index ranks first in this table. Two journals, International Journal of Molecular Sciences (119, Q2, H-index 16) and Scientific Reports (100, Q1, H-index 32) rank second and third in this table, respectively.
As Table 4 shows, five journals are distributed in quartile 1 (Q1), ten journals in quartile 2 (Q2), four journals in quartile 3 (Q3), and one journal in quartile 4 (Q4). Circulation Research has the highest journal IF (20.1). Cardiovascular Research comes second with IF 10.9. Other journals rank behind, including seven with IF between 5 and 10, and eleven with IF less than 5. Both Circulation Research (IF 20.1, H-index 52, Average Citations 210.25) and Cardiovascular Research (IF 10.9, H-index 30, Average Citations 102.76) are in quartile 1(Q1), with a relatively high IF value, H-index, and average citations, implying a strong impact of the two journals in this field.
The top 5 journals in terms of the number of publications were analyzed furtherly, and we plotted the cumulative publication number-time curve (Figure 5), including International Journal of Molecular Sciences, Journal of Molecular and Cellular Cardiology, Molecular Medicine Reports, PLOS ONE, and Scientific Reports. The result shows that the Journal of Molecular and Cellular Cardiology didn't publish any article in this field in the past two years. However, the number of articles in the other four journals, with an obvious upswing trend continuously during the last decade, suggests that the four journals prefer to publish relevant articles in this field currently. A total of twenty-six articles, the most publications among all the journals in the field in 2023, were published in International Journal of Molecular Sciences, indicating that it is the most popular journal for this academic field in the last year.
Figures 6A,B show the collaborative networks among the journals, in which the miRNA-related articles of HD were published. The clustering is based on the similarity of the journals and divided into 4 categories with different colors. The node size in the two figures indicates the number and the total citations of related articles in the corresponding journal, respectively. The journals of the blue cluster are devoted to clinical areas. The journals of the green cluster focus on physiology and biochemistry. The journals of the red cluster are mainly pharmacology and pharmacotherapy. The journals of the yellow cluster are centered on pathology.
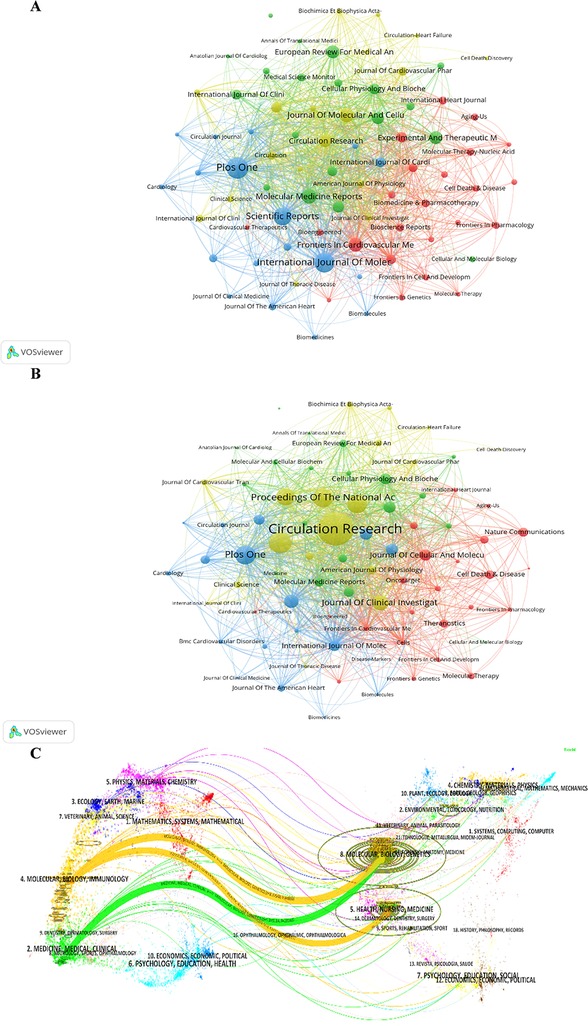
Figure 6. Journal analysis of the "miRNAs in HD" research. (A) Analysis of collaborative network visualization of journals in VOSviewer. The figure shows the journals with more than 10 documents. The colors of the nodes represent the journals in different clusters, and the size of the nodes indicates the frequency of their occurrence. (B) Analysis of collaborative network visualization of journals' citations in VOSviewer. (C) The dual-map overlay of journals on knowledge flow analysis in Citespace. Citing journals are on the left, cited journals are on the right, and colored paths indicate citation relationships.
The evolution of knowledge citations and co-citation between citing and cited journals was explored through the knowledge trajectory graph in Figure 6C. The dual-map overlay of journals shows the research topic distribution, citation trajectory, and research area changes in academic journals. Colored arcs connecting the citing journals on the left and cited journals on the other side indicate citation trajectory. The citing journals mainly include the issues of MATHMATICS, SYSTEMMS, MATHMATICAL, MOLECULAR, BIOLOGY, IMMUNOLOGY, MEDICINE, MEDICAL, and CLINICAL. The cited journals mainly include the issues of MOLECULAR, BIOLOGY, GENETICS, HEALTH, NURSING, MEDICINE, PSYCHOLOGY, EDUCATION, and SOCIAL. The cited topics have built the foundation, and the citing issues have formed the frontiers of the “miRNAs in HD” research.
In VOSviewer, co-cited authorship refers to the literature of two authors being cited by a third author simultaneously. The co-citation frequency reflects academic focus and research density. Table 5 shows the top 20 authors according to the number of publications, together with the corresponding institutions, citation times, and total link strength. The author with the highest number of publications is Thum Thomas (Cardior Pharmaceut GmbH, Germany) (47), followed by Yang Baofeng (Southeast University, China) (28), Shan Hongli (Royal Netherlands Academy of Arts & Sciences, Netherlands) (25), Lu Yanjie (Chengde Medical University, China) (24), and Xiao Junjie (Shanghai University, China) (22). Olson, Eric N (University of Texas, USA) (10,966) is the author who is most frequently cited, followed by Van Rooij Eva (University of Texas, USA) (7,779), Thum Thomas (Cardior Pharmaceut GmbH, Germany) (6,311), Condorelli, Gianluigi (Univ Milan, Italy) (3,852), and Yang, Baofeng (Southeast University, China) (3,630). It is worth noting that Olson Eric N, Van Rooij Eva, Thum Thomas, Yang Baofeng, Shan Hongli, and Lu Yanjie have a high volume of articles, a high number of citations, and strong total link strength at the same time, indicating their important effect in the “miRNAs in HD” field.
In VOSviewer, Figure 7A shows the collaborations of the authors in this field. A total of 110 authors with more than 10 published articles formed 8 clusters. Authors in various clusters are identified by the nodes in different colors, and their frequency of occurrence is determined by their size. Authors have deep cooperation between them inside a cluster. In addition, there are also one or two authors in each cluster connecting closely with other authors outside their clusters. Thum Thomas has a close relationship with Condorelli, Gianluigi, Doevendans, Pieter A, and, Devaux, Yvan, respectively. Yang, Baofeng has active connectivity with Zhang, Yan, Zhang, Li, and Li, Jing. While Chen Chen is in close collaboration with Zhang Yan, Wang Feng, and Wang Yan. Wang Kun is associated with Zhang, Yuan, and Zhang, Lei.
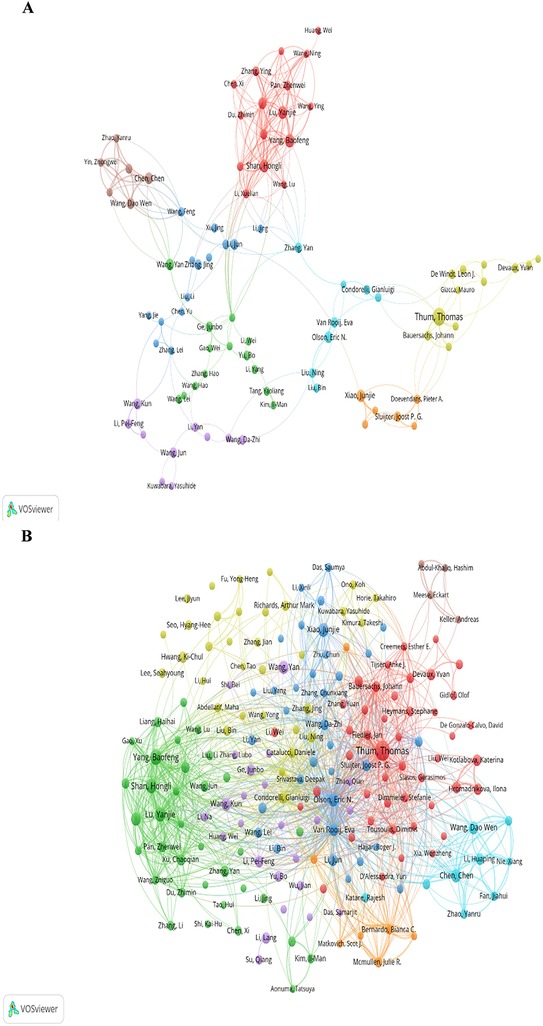
Figure 7. Authors analysis of the "miRNAs in HD" research in VOSviewer. (A) Authors collaborative network. (B) Authors citations collaborative network.
Figure 7B shows the co-cited authorship network of the “miRNA in HD” research. The authors with no less than 8 citations are mainly divided into 8 clusters: Thum, Thomas, Fiedler, Jan, Bauersachs, Johann, et al. (red); Olson, Eric N, Wang, Da-zhi, Van, Rooij E, et al. (dark blue); Wang Dao Wen, Chen Chen, Zhao Yanru, et al. (light blue); Yang, Baofeng, Lu, Yanjie, Shan, Hongli, et al. (green); Condorelli Gianluigi, Catalucci, Daniele, Liu, Ning, et al. (yellow); Ge, Junbo, Wang, Kun, Wang, Yan, et al. (purple); Bernardo, Bianca C, Mcmullen, Julie R, Matkovich, Scot J, et al. (orange); Keller, Andreas, Abdul-khaliq, Hashim, Meese, Eckart, et al. (brown).Olson Eric N, Van Rooij E, Thum Thomas, Lu, Yanjie, and Yang Baofeng are prominent nodes of the collaborative network and have plenty of contacts between them. Olson Eric N, Van Rooij E, and Thum Thomas are at the center of the whole network, with direct connections to the authors in other clusters. While Yang Baofeng and Lu, Yanjie have relatively less active contact with the authors outside their cluster.
A total of 9,681 keywords were obtained from the raw data, which could be divided into 5 clusters. Table 6 shows the top 20 keywords by their occurrences in the articles in the “miRNAs in HD” field, implying the popular research topics in this field. The keyword with the highest frequency is “micrornas” (2,075), followed by “expression” (1,286). Moreover, “heart failure” (759), “apoptosis” (654), “biomarker” (639), “heart” (544), and “inflammation” (478) are also frequent keywords, implying their corresponding researches are hot study topics in this field. After overviewing the corresponding articles, we found that the keywords usually connecting “miRNAs” with heart conditions, such as “heart failure”, “myocardial infarction”, “cardiovascular disease”, and “cardiac hypertrophy”, etc.) are “apoptosis”, “proliferation”, “cancer”, “inflammation”, “expression”, “biomarker”, “circulating microrna”, “gene expression”, “cell”, “mechanisms”, and “activation”, etc. Table 7 shows the top 20 keywords with the strongest citation bursts, ranked by beginning year. In the table, the “year” indicates the average time of keyword appearance. The “begin” and “end” indicate the beginning and ending time of the keyword citation burst occurrence, respectively. The keywords including “muscle specific microrna”, “in vivo”, and “gene expression” have a higher citation burst with a strength value above 15, indicating the keyword's high occurrence frequency during the specific period. The keywords including “autophagy” (from 2018), “diabetic cardiomyopathy” (from 2019), “cardiac dysfunction” (from 2019), “extracellular vesicles” (from 2019), “long noncoding rna” (from 2018), “exosomes” (from 2020), and “management” (from 2021), have strong citation bursts till 2023, suggesting the study novel hot frontiers in this field recently. The articles on keywords of “exosomes” and “extracellular vesicles” were focused on their carrier role of miRNAs as potential biomarkers or treatment of HD, and so does “management”.
A co-occurrence network visualization of keywords with more than 15 occurrences is conducted in VOSviewer (Figure 8A). The lines between different keywords represent the co-occurrence relationship between them. The thickness of lines is proportional to the closeness of the connection between them. There are 5 clusters in terms of the study areas after excluding a single keyword as a cluster. One cluster links to the others with lots of lines, suggesting that they are intersecting areas in research directions. The blue cluster is related to potential therapy of HD (heart failure, diseases, expression, differentiation, fibrosis, down-regulation, hypertrophy, signature, identification, target, etc.). The yellow cluster is related to cell repair and angiogenesis (stem cells, endothelial cells, progenitor cells, angiogenesis, myocardial infarction, exosomes, repair, etc.). The green cluster is related to the relationship between cardiovascular and miRNAs (inflammation, cardiovascular diseases, circulating miRNAs, biomarker, impact, association, risk factors, etc.). The red cluster is related to cell death (apoptosis, autophagy, pyroptosis, dysfunction, etc.). Different clusters are linked with lots of lines, indicating that they are cross-cutting areas in each research direction.
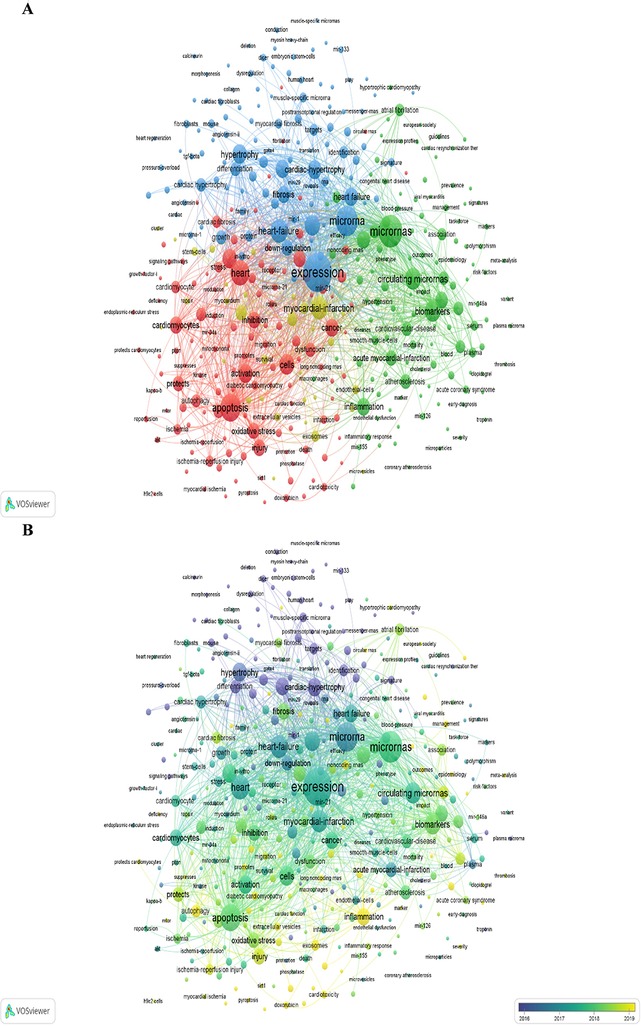
Figure 8. Keyword analysis of the "miRNAs in HD" research in VOSviewer. (A) Collaborative network visualization of keywords. (B) Keywords shift analysis over time. The color of a keyword node presents the average time of the keyword occurrence.
Figure 8B marks the keywords with different colors according to the average year of their occurrences. The yellow keywords occurred later than the blue and purple ones. The color changes from purple to blue, then blue to green, and green to yellow indicating the dynamic development of the keyword over time. The size of the node indicates the keyword frequency, and the color of it implies the research focal points during a certain time. The result shows that “injury”, “inflammation”, “exosomes”, “cardiotoxicity”, “doxorubicin”, “sepsis”, “autophagy”, “pyroptosis”, etc., are novel research topics lately, in line with Table 7.
Overall, the “miRNAs in HD” research focused on the potential mechanisms, regulatory pathways, and expression change of specific miRNAs in various heart biological/pathological processes including autophagy, inflammation, and programmed death such as pyroptosis and apoptosis, especially in cancer, sepsis, injury situation. Consequently, in the twenty years, most of the research in this field is still in the basic stage of exploration, and the research hotspots are not highly concentrated. However, “exosomes”, “extracellular vesicles”, “autophagy”, and “management” have been novel hot research topics since 2018, which focused on the diagnosis and treatment of HD.
Articles with high citation ranks have played a major role in the development of this research field. Hence, we analyzed the highly cited publications. The top 10 publications by citation frequency were ranked in Supplementary Table 1. The most frequently cited article was “Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis” (1,510) by Van Rooij, Eva, et al., which focused on the miR-29 family role as a regulator of cardiac fibrosis after myocardial infarction (12). The article titled “MicroRNA-133 controls cardiac hypertrophy” (1,464) by Care, Alessandra, et al, ranked second in the table, which focuses on the possible functional role of miRNAs in cardiac hypertrophy (13). The result showed that miR-133, and possibly miR-1 are key regulators of cardiac hypertrophy. The article titled “Control of stress-dependent cardiac growth and gene expression by a microRNA” (1,329) by Van Rooij, Eva, et al., focused on the miR-208 role in the cardiac stress response and ranked third (14). It is noting that another article by Van Rooij, Eva, et al., ranked fifth in the table, titled “A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure” (1,251), focusing on the miR-195 role as a stress-inducible miRNA in cardiac hypertrophy (15).
The top four articles found that some miRNAs were potential targets for HD by measuring miRNA expression in both mice or rats and human hearts in vivo. Three of the top ten articles by citation frequency by the same author, Van Rooij, Eva, indicated the important role of the author in the field. The almost equally noteworthy writer in the table was Zhao Y, from the Gladstone Institute of Cardiovascular Disease, having two articles, ranking fourth and sixth, respectively. His articles entitled “Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis” (2005), and “Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2” (2007), also introduced important basic research of miRNA regulation mechanism in this field (16, 17).
In addition, China, Italy, Germany, and the Netherlands have one article in the top 10 articles by citation frequency, respectively. Out of the ten references, six are published by the USA, indicating the pivotal role of the USA in miRNA-related basic research in HD again.
The incidence and mortality rates for HD have remained high in the past decades, which is one of the global medical issues (18). Current evidence proves that specific miRNAs can modulate cardiac-embryo development and cardiac remolding in various ways (3, 19). miRNA-related research has gradually flourished since the first miRNA (lin-4) was discovered in C. elegans in 1993 (20, 21). The role of miRNAs continues to become definite with the research development, although many unknowns remain. As critical regulatory mediators of various biological and pathophysiological processes, miRNAs play an important role in cardiac development and diseases. It has been a trend to transform “miRNAs in HD” research achievement into clinical application in the current prognostication, diagnosis, and treatment of HD.
The “miRNA in HD” research has discovered some miRNAs that possess tissue and disease specificity, such as miR302 (22), miR-411 (23), and MiR-181a (24), etc. in myocardial infarction, miR-150-5p (25), miR-132 (26), miR-122 (27) and miRNA-129-5p (28), etc. in heart failure (HF), and miR-222 (29), miR-133 (13), and miR-155 (30), etc. in cardiac hypertrophy. Scientists described a new mode of regulating heart conditions, pointing to the potential diagnostic, prognostic, and therapeutic application of miRNAs for HD. Hence, miRNAs have been implicated the hope in the treatment of HD.
Some of the novel therapeutic approach of targeting specific miRNA, known as miRNA-targeted therapeutic, was proven successful in many preclinical studies for HD, with miRNA mimics carrying miRNA sequences or miRNA inhibitors (mainly antisense oligonucleotides, miRNA sponges, and erasers (3, 22, 24, 31–36). miRNA mimics and miRNA inhibitors were respectively designed to achieve therapeutic goals by upregulating or downregulating miRNA expression and then altering the target gene silencing effect. The challenges that miRNA-targeted therapeutic confront are the lack of safety, efficacy, stability, targeting specificity, delivery efficiency, etc. The innovation of the novel delivery systems, including chemical modification, the structure of binding them to small molecular substances (such as cholesterol and vitamin E), and appropriate delivery carriers (such as liposomes and nanocarriers) can only improve the above defects of miRNA mimics or inhibitors to different extents. Therefore, perfectly transforming miRNAs-target therapeutics into clinical applications is still difficult.
Some new techniques have recently overcome the stability and target-gene escape by suitable delivery systems and chemical modification. Gu et al. utilized exosomes loaded with miR302 through the cardiomyocyte-specific peptide to reduce myocardial ischemia and reperfusion (I/R) injury (22). Zhi et al. utilized the effective delivery of hypertrophic miR-182 inhibitor by cholesterol-containing nanocarriers to prevent pressure overload-induced cardiac hypertrophy (33).
To date, miRNA-related research has advanced into the clinical trial phase or clinical application for various diseases (37, 38), with a few for HD (26, 39). MiR-132 can cause myocardial remodeling through its high level of expression and its effect on signaling pathways in heart tissue, resulting in HF (26, 40). CDR132l, a miR-132 inhibitor, known as a milestone breakthrough in HF treatment, can attenuate or reverse HF (41). Its phase 1b clinical trial has confirmed that CDR132l is a relatively safe and well-tolerated miRNA therapeutic in stable chronic HF patients for the first time, without obvious toxicity (41). Although this study is limited by the small sample, the result of miRNA therapeutic effect in humans for HD is encouraging. Besides, the trial named HF-REVERT (Phase 2, multicenter, randomized, parallel, 3-arm, placebo-controlled Study to Assess Efficacy and Safety of CDR132l in Patients with Reduced Left Ventricular Ejection Fraction after Myocardial Infarction) is in progress, which aims to further evaluate the efficacy and safety of CDR132l in HF patients after acute myocardial infarction (42).
Interestingly, observational studies have identified specific geographical patterns in the exploration of miRNAs as diagnostic and prognostic biomarkers, as well as therapeutic agents, for cardiovascular diseases. These patterns suggest that differences in disease prevalence and genetic heterogeneity among regions may influence the direction and outcomes of research efforts.
The United States emphasizes the close integration of basic research and clinical applications, taking a leading position in the research and development of therapeutic targets and drugs for cardiovascular diseases that use miRNA. Researchers conduct in-depth studies on the mechanism of miRNA in heart development, often utilizing advanced gene-editing technologies and animal models (43, 44). They engage in a cross-disciplinary integration of fields such as molecular biology, genetics, and cardiology. These researchers not only achieve continuous breakthroughs in basic theoretical research but also actively collaborate with pharmaceutical companies to advance drug development.
Europe focuses more on exploring the role of miRNA in the pathogenesis of common heart diseases such as coronary heart disease and cardiomyopathy, as well as the application of miRNA as biomarkers in disease diagnosis and prognosis assessment (45). For example, through large-scale clinical sample analysis, researchers seek specific miRNA combinations as indicators for diagnosing coronary heart disease or evaluating the prognosis of cardiomyopathy (3). In the field of cardiac regeneration, studies investigate how miRNA regulates the proliferation and differentiation of cardiomyocytes, offering new insights into treating diseases like myocardial infarction. Some universities, with research teams that prioritize international cooperation, jointly undertake large-scale research projects. In their research, they excel at using systems biology methods to integrate multi-omics data and comprehensively analyze the role of miRNA in heart diseases (46, 47).
China centers on an extensive association between miRNA and cardiovascular diseases, covering various conditions such as arrhythmia, myocardial ischemia, cardiac hypertrophy, and heart failure (17, 48, 49). At the same time, China is also actively exploring the development of miRNA-based diagnostic methods and treatment strategies. For example, researchers in China are studying the value of miRNA in early disease diagnosis and investigating the use of traditional Chinese medicine and other methods to regulate miRNA expression for the treatment of cardiovascular diseases (50–52). The miRNA-related research has created a new era for Chinese traditional medicine in global clinical practice based on combining it with new scientific techniques.
Other Asian Countries like Japan and South Korea also have certain strengths in miRNA research. They keep up with the international frontiers in terms of technical applications and basic research (53–55). At the same time, they carry out relevant research in combination with the disease characteristics and research advantages of their own countries, such as studying the mechanism of miRNA in hypertensive heart disease (56, 57).
Australia has also achieved certain results in miRNA research on cardiovascular diseases. Its research teams pay attention to international cooperation and have carried out in-depth study on the epigenetic regulation of miRNA in heart diseases (58, 59).
Our study visualized the “miRNAs in HD” research in the past twenty years. We provided a comprehensive overview of this field based on different levels. The analyses of countries/regions, journals, and authors provided the research distribution, and the keyword analysis offered important clues about the research hots, frontiers, and trends.
China and the USA are in dominant positions in this field, with the maximum number of publications and total citations, respectively. However, the USA has established a leading role regarding the higher average citation and betweenness centrality than China. Peking Union Medical College is the most prolific university with the maximum publications, and University of California System is the most authoritative institution regarding betweenness centrality. PLOS ONE, Circulation Research, Journal of Molecular and Cellular Cardiology, and Scientific Reports, are the popular journals in this academic field. The International Journal of Molecular Sciences is the most popular journal for this academic field in 2023 with 26 articles. Thomas with 47 articles is the most prolific author. Van Rooij, Eva has the most frequently cited article, titled “Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis”, which suggests his important influence in this field. Considering the number of publications, citations, and total link strength overall, Olson. Eric N, Van Rooij Eva, Thum Thomas; Yang Baofeng, Wang Kun; and Lu Yanjie are authoritative authors in this field.
The study shows that the researchers in this field have been continuously devoted to basic research, and the research hotspots have not been highly concentrated during the past twenty years. The expression changes and regulatory mechanisms of specific miRNAs in various heart biological and pathophysiological processes have always been this field's research direction. The development trend in this field is how to transform the current achievements into clinical applications. Although the researchers in this field have obtained preliminary success, there is a long way to clinical practice.
The investigation into miRNAs has introduced a groundbreaking approach to the regulation of gene expression following transcription. Moreover, circulating miRNAs hold immense promise as diagnostic and prognostic biomarkers. However, to confirm their dependability, a multitude of challenges must be tackled. The challenges encompassing accurate detection and analysis techniques, the standardization of biological samples, the validation of miRNA function, the transition to clinical applications, the sharing and integration of data, accounting for individual variations, and the appropriate selection of experimental models all demand urgent attention and resolution.
The promise of miRNA-based therapeutic approaches is indeed captivating, yet significant challenges remain to be conquered. Delving into the target genes of microRNAs and understanding their functions is pivotal to crafting miRNA-based interventions for a range of human diseases. The creation of miRNA mimetics and inhibitors stands out as a promising strategy for either restoring function or counteracting endogenous miRNAs. However, the administration of high doses of these synthetic miRNA analogs can inadvertently trigger an innate immune response, leading to an unwarranted surge in cytokine expression. Furthermore, extensive research is ongoing to enhance the stability of miRNA mimics and inhibitors during the delivery process. Addressing these issues is essential for the successful future implementation of miRNA-based treatments.
Multidisciplinary team collaboration, interdisciplinary studies and emerging technologies may provide hope for further development of miRNA research in heart diseases. The application of emerging technologies such as nanotechnology, proteomics, and metabolomics, artificial intelligence, and machine learning can help promote research on miRNA in the field of heart diseases, enhance our understanding of heart diseases, and provide possibilities for the development of new diagnostic and therapeutic strategies. For example, nanotechnology (including liposomes, nanocarriers, and exosomes) addresses the challenge of delivering miRNA mimics and inhibitors, which have help transform miRNA research into clinical application. In this article, researchers have the chance to identify potential collaborators and key hotspots that align with their interests, which is in line with my writing intention.
By using bibliometric analysis, our study provides better insight into research frontiers and focus, but several inherent limitations are unavoidable. First, the raw data downloaded only from the WOSCC database has selective bias. However, compared to other data resources, the WOSCC database can provide more comprehensive and adequate information (9). Second, our study only included English articles, and we may have deleted some important articles published in other languages.
However, our study has incorporated the majority of “miRNAs in HD” research from 2004 to 2023, and we believe that the results would not be different even inclusion of new articles. The interplay between miRNA and various HD is still under intense investigation. There are many questions not well understood and require further investigation. In addition, research on “miRNAs in HD” may exhibit heterogeneity, necessitating further studies to replicate the research findings in order to identify reliable results. The potential of miRNA as a diagnostic tool, prognostic indicator, and therapeutic target for HD still requires validation in independent, large-scale cohorts.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
YJ: Methodology, Project administration, Software, Visualization, Writing – original draft. JD: Data curation, Validation, Writing – original draft. QY: Formal analysis, Writing – original draft, Validation. YM: Data curation, Writing – original draft, Investigation. JL: Investigation, Writing – original draft. WZ: Conceptualization, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by a clinical research grant from Air Force Medical University (NO. 2022LC2202).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1465646/full#supplementary-material
1. Joseph P, Leong D, McKee M, Anand SS, Schwalm JD, Teo K, et al. Reducing the global burden of cardiovascular disease, part 1: the epidemiology and risk factors. Circ Res. (2017) 121(6):677–94. doi: 10.1161/circresaha.117.308903
2. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. (2009) 136(2):215–33. doi: 10.1016/j.cell.2009.01.002
3. Lock MC, Tellam RL, Botting KJ, Wang KCW, Selvanayagam JB, Brooks DA, et al. The role of miRNA regulation in fetal cardiomyocytes, cardiac maturation and the risk of heart disease in adults. J Physiol (Lond). (2018) 596(23):5625–40. doi: 10.1113/jp276072
4. Ninkov A, Frank JR, Maggio LA. Bibliometrics: methods for studying academic publishing. Perspect Med Educ. (2022) 11(3):173–6. doi: 10.1007/s40037-021-00695-4
5. Bertoglio R, Corbo C, Renga FM, Matteucci M. The digital agricultural revolution: a bibliometric analysis literature review. IEEE Access. (2021) 9:134762–82. doi: 10.1109/access.2021.3115258
6. Roldan-Valadez E, Salazar-Ruiz SY, Ibarra-Contreras R, Rios C. Current concepts on bibliometrics: a brief review about impact factor, eigenfactor score, CiteScore, SCImago journal rank, source-normalised impact per paper, H-index, and alternative metrics. Ir J Med Sci. (2019) 188(3):939–51. doi: 10.1007/s11845-018-1936-5
7. van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. (2010) 84(2):523–38. doi: 10.1007/s11192-009-0146-3
8. Chen C, Song M. Visualizing a field of research: a methodology of systematic scientometric reviews. PLoS One. (2019) 14(10):e0223994. doi: 10.1371/journal.pone.0223994
9. Tengilimoğlu D, Orhan F, Şenel Tekin P, Younis M. Analysis of publications on health information management using the science mapping method: a holistic perspective. Healthcare. (2024) 12(3):287. doi: 10.3390/healthcare12030287
10. Sharifi A. Urban resilience assessment: mapping knowledge structure and trends. Sustainability. (2020) 12(15):18. doi: 10.3390/su12155918
11. Aria M, Cuccurullo C. Bibliometrix: an R-tool for comprehensive science mapping analysis. J Informetr. (2017) 11(4):959–75. doi: 10.1016/j.joi.2017.08.007
12. van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. (2008) 105(35):13027–32. doi: 10.1073/pnas.0805038105
13. Carè A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, et al. MicroRNA-133 controls cardiac hypertrophy. Nat Med. (2007) 13(5):613–8. doi: 10.1038/nm1582
14. van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science (New York, NY). (2007) 316(5824):575–9. doi: 10.1126/science.1139089
15. van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, et al. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A. (2006) 103(48):18255–60. doi: 10.1073/pnas.0608791103
16. Zhao Y, Samal E, Srivastava D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature. (2005) 436(7048):214–20. doi: 10.1038/nature03817
17. Zhao Y, Ransom JF, Li A, Vedantham V, von Drehle M, Muth AN, et al. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell. (2007) 129(2):303–17. doi: 10.1016/j.cell.2007.03.030
18. Mensah GA, Fuster V, Murray CJL, Roth GA. Global burden of cardiovascular diseases and risks, 1990–2022. J Am Coll Cardiol. (2023) 82(25):2350–473. doi: 10.1016/j.jacc.2023.11.007
19. Kalayinia S, Arjmand F, Maleki M, Malakootian M, Singh CP. MicroRNAs: roles in cardiovascular development and disease. Cardiovasc Pathol. (2021) 50:107296. doi: 10.1016/j.carpath.2020.107296
20. Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. (1993) 75(5):843–54. doi: 10.1016/0092-8674(93)90529-y
21. Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. (1993) 75(5):855–62. doi: 10.1016/0092-8674(93)90530-4
22. Gu J, You J, Liang H, Zhan J, Gu X, Zhu Y. Engineered bone marrow mesenchymal stem cell-derived exosomes loaded with miR302 through the cardiomyocyte specific peptide can reduce myocardial ischemia and reperfusion (I/R) injury. J Transl Med. (2024) 22(1):168. doi: 10.1186/s12967-024-04981-7
23. Nugroho AB, Stafford N, Zi M, Prehar S, Potter R, Kwon D, et al. Micro RNA-411 expression improves cardiac phenotype following myocardial infarction in mice. JACC-Basic Transl Sci. (2022) 7(9):859–75. doi: 10.1016/j.jacbts.2022.05.008
24. Zhu J, Wang Q, Zheng Z, Ma L, Guo J, Shi H, et al. MiR-181a protects the heart against myocardial infarction by regulating mitochondrial fission via targeting programmed cell death protein 4. Sci Rep. (2024) 14(1):6638. doi: 10.1038/s41598-024-57206-8
25. Abu-Halima M, Meese E, Saleh MA, Keller A, Abdul-Khaliq H, Raedle-Hurst T. Micro-RNA 150-5p predicts overt heart failure in patients with univentricular hearts. PLoS One. (2019) 14(10):13. doi: 10.1371/journal.pone.0223606
26. Täubel J, Hauke W, Rump S, Viereck J, Batkai S, Poetzsch J, et al. Novel antisense therapy targeting microRNA-132 in patients with heart failure: results of a first-in-human phase 1b randomized, double-blind, placebo-controlled study. Eur Heart J. (2021) 42(2):178–88. doi: 10.1093/eurheartj/ehaa898
27. Shi YJ, Zhang Z, Yin QQ, Fu C, Barszczyk A, Zhang XF, et al. Cardiac-specific overexpression of miR-122 induces mitochondria-dependent cardiomyocyte apoptosis and promotes heart failure by inhibiting Hand2. J Cell Mol Med. (2021) 25(11):5326–34. doi: 10.1111/jcmm.16544
28. Mi S, Huang F, Jiao ML, Qian Z, Han MM, Miao Z, et al. Inhibition of MEG3 ameliorates cardiomyocyte apoptosis and autophagy by regulating the expression of miRNA-129-5p in a mouse model of heart failure. Redox Rep. (2023) 28(1):14. doi: 10.1080/13510002.2023.2224607
29. Liu XJ, Li HB, Hastings MH, Xiao CY, Damilano F, Platt C, et al. miR-222 inhibits pathological cardiac hypertrophy and heart failure. Cardiovasc Res. (2024) 120(3):262–72. doi: 10.1093/cvr/cvad184
30. Seok HY, Chen JH, Kataoka M, Huang ZP, Ding J, Yan JL, et al. Loss of MicroRNA-155 protects the heart from pathological cardiac hypertrophy. Circ Res. (2014) 114(10):1585–95. doi: 10.1161/circresaha.114.303784
31. Wang K, Jiang Z, Webster KA, Chen JH, Hu HX, Zhou Y, et al. Enhanced cardioprotection by human endometrium mesenchymal stem cells driven by exosomal MicroRNA-21. Stem Cells Transl Med. (2017) 6(1):209–22. doi: 10.5966/sctm.2015-0386
32. Duygu B, de Windt LJ, Martins PAD. Targeting microRNAs in heart failure. Trends Cardiovasc Med. (2016) 26(2):99–110. doi: 10.1016/j.tcm.2015.05.008
33. Zhi Y, Xu C, Sui D, Du J, Xu FJ, Li Y. Effective delivery of hypertrophic miRNA inhibitor by cholesterol-containing nanocarriers for preventing pressure overload induced cardiac hypertrophy. Adv Sci. (2019) 6(11):1900023. doi: 10.1002/advs.201900023
34. Verjans R, Derks WJA, Korn K, Sönnichsen B, van Leeuwen REW, Schroen B, et al. Functional screening identifies MicroRNAs as multi-cellular regulators of heart failure. Sci Rep. (2019) 9(1):6055. doi: 10.1038/s41598-019-41491-9
35. Zhou H, Tang W, Yang J, Peng J, Guo J, Fan C. MicroRNA-related strategies to improve cardiac function in heart failure. Front Cardiovasc Med. (2021) 8:773083. doi: 10.3389/fcvm.2021.773083
36. Guo R, Nair S. Role of microRNA in diabetic cardiomyopathy: from mechanism to intervention. Biochim Biophys Acta Mol Basis Dis. (2017) 1863(8):2070–7. doi: 10.1016/j.bbadis.2017.03.013
37. van der Ree MH, de Vree JM, Stelma F, Willemse S, van der Valk M, Rietdijk S, et al. Safety, tolerability, and antiviral effect of RG-101 in patients with chronic hepatitis C: a phase 1B, double-blind, randomised controlled trial. Lancet. (2017) 389(10070):709–17. doi: 10.1016/s0140-6736(16)31715-9
38. Beg MS, Brenner AJ, Sachdev J, Borad M, Kang YK, Stoudemire J, et al. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Invest New Drugs. (2017) 35(2):180–8. doi: 10.1007/s10637-016-0407-y
39. Hennessy EJ, Moore KJ. Using microRNA as an alternative treatment for hyperlipidemia and cardiovascular disease: cardio-miRs in the pipeline. J Cardiovasc Pharmacol. (2013) 62(3):247–54. doi: 10.1097/FJC.0b013e31829d48bf
40. Wang G, Wang R, Ruan Z, Liu L, Li Y, Zhu L. MicroRNA-132 attenuated cardiac fibrosis in myocardial infarction-induced heart failure rats. Biosci Rep. (2020) 40(9):BSR20201696. doi: 10.1042/bsr20201696
41. Batkai S, Genschel C, Viereck J, Rump S, Bär C, Borchert T, et al. CDR132l improves systolic and diastolic function in a large animal model of chronic heart failure. Eur Heart J. (2021) 42(2):192–201. doi: 10.1093/eurheartj/ehaa791
42. Bauersachs J, Solomon SD, Anker SD, Antorrena-Miranda I, Batkai S, Viereck J, et al. Efficacy and safety of CDR132l in patients with reduced left ventricular ejection fraction after myocardial infarction: rationale and design of the HF-REVERT trial. Eur J Heart Fail. (2024) 26(3):674–82. doi: 10.1002/ejhf.3139
43. Song R, Zhang L. MicroRNAs and therapeutic potentials in acute and chronic cardiac disease. Drug Discov Today. (2024) 29(11):104179. doi: 10.1016/j.drudis.2024.104179
44. Maynard LH, Humbert O, Peterson CW, Kiem HP. Genome editing in large animal models. Mol Ther. (2021) 29(11):3140–52. doi: 10.1016/j.ymthe.2021.09.026
45. Khidr EG, Abulsoud AI, Doghish AA, El-Mahdy HA, Ismail A, Elballal MS, et al. The potential role of miRNAs in the pathogenesis of cardiovascular diseases - a focus on signaling pathways interplay. Pathol Res Pract. (2023) 248:154624. doi: 10.1016/j.prp.2023.154624
46. Mens MMJ, Maas SCE, Klap J, Weverling GJ, Klatser P, Brakenhoff JPJ, et al. Multi-omics analysis reveals MicroRNAs associated with cardiometabolic traits. Front Genet. (2020) 11:110. doi: 10.3389/fgene.2020.00110
47. Fernandes M, Patel A, Husi H. C/VDdb: a multi-omics expression profiling database for a knowledge-driven approach in cardiovascular disease (CVD). PLoS One. (2018) 13(11):e0207371. doi: 10.1371/journal.pone.0207371
48. Chen Y, Liu S, Liang Y, He Y, Li Q, Zhan J, et al. Single dose of intravenous miR199a-5p delivery targeting ischemic heart for long-term repair of myocardial infarction. Nat Commun. (2024) 15(1):5565. doi: 10.1038/s41467-024-49901-x
49. Wei F, Ren W, Zhang X, Wu P, Fan J. miR-425-5p is negatively associated with atrial fibrosis and promotes atrial remodeling by targeting CREB1 in atrial fibrillation. J Cardiol. (2022) 79(2):202–10. doi: 10.1016/j.jjcc.2021.09.012
50. Peng Y, Liao B, Zhou Y, Zeng W. Ginsenoside Rb2 improves heart failure by down-regulating miR-216a-5p to promote autophagy and inhibit apoptosis and oxidative stress. J Appl Biomed. (2023) 21(4):180–92. doi: 10.32725/jab.2023.024
51. Liu G, Tan L, Zhao X, Wang M, Zhang Z, Zhang J, et al. Anti-atherosclerosis mechanisms associated with regulation of non-coding RNAs by active monomers of traditional Chinese medicine. Front Pharmacol. (2023) 14:1283494. doi: 10.3389/fphar.2023.1283494
52. Wang M, Yan M, Tan L, Zhao X, Liu G, Zhang Z, et al. Non-coding RNAs: targets for Chinese herbal medicine in treating myocardial fibrosis. Front Pharmacol. (2024) 15:1337623. doi: 10.3389/fphar.2024.1337623
53. Hayasaka T, Takehara N, Aonuma T, Kano K, Horiuchi K, Nakagawa N, et al. Sarcopenia-derived exosomal micro-RNA 16-5p disturbs cardio-repair via a pro-apoptotic mechanism in myocardial infarction in mice. Sci Rep. (2021) 11(1):19163. doi: 10.1038/s41598-021-98761-8
54. Natsume Y, Oaku K, Takahashi K, Nakamura W, Oono A, Hamada S, et al. Combined analysis of human and experimental murine samples identified novel circulating MicroRNAs as biomarkers for atrial fibrillation. Circ J. (2018) 82(4):965–73. doi: 10.1253/circj.CJ-17-1194
55. Kiyosawa N, Watanabe K, Morishima Y, Yamashita T, Yagi N, Arita T, et al. Exploratory analysis of circulating miRNA signatures in atrial fibrillation patients determining potential biomarkers to support decision-making in anticoagulation and catheter ablation. Int J Mol Sci. (2020) 21(7):2444. doi: 10.3390/ijms21072444
56. Lee C, Cho S, Jeong D. Inhibition of miR-25 ameliorates cardiac dysfunction and fibrosis by restoring krüppel-like factor 4 expression. Int J Mol Sci. (2023) 24(15):12434. doi: 10.3390/ijms241512434
57. Watanabe K, Narumi T, Watanabe T, Otaki Y, Takahashi T, Aono T, et al. The association between microRNA-21 and hypertension-induced cardiac remodeling. PLoS One. (2020) 15(2):e0226053. doi: 10.1371/journal.pone.0226053
58. Stølen TO, Høydal MA, Ahmed MS, Jørgensen K, Garten K, Hortigon-Vinagre MP, et al. Exercise training reveals micro-RNAs associated with improved cardiac function and electrophysiology in rats with heart failure after myocardial infarction. J Mol Cell Cardiol. (2020) 148:106–19. doi: 10.1016/j.yjmcc.2020.08.015
Keywords: miRNA, heart disease (HD), VOSviewer, CiteSpace, visual analysis
Citation: Jin Y, Duan J, Yin Q, Ma Y, Lou J and Zhang W (2025) Bibliometric and visual analysis of miRNAs in heart diseases from 2004 to 2023. Front. Cardiovasc. Med. 12:1465646. doi: 10.3389/fcvm.2025.1465646
Received: 16 July 2024; Accepted: 10 February 2025;
Published: 20 March 2025.
Edited by:
Arun Samidurai, Virginia Commonwealth University, United StatesReviewed by:
Tarun Pant, Medical College of Wisconsin, United StatesCopyright: © 2025 Jin, Duan, Yin, Ma, Lou and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Zhang, MTU2OTk5MTg1MTBAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.