
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 07 February 2025
Sec. Intensive Care Cardiovascular Medicine
Volume 12 - 2025 | https://doi.org/10.3389/fcvm.2025.1454933
 Xin Huang1,2,3,4
Xin Huang1,2,3,4 Maobin Kuang1,2,3,4
Maobin Kuang1,2,3,4 Jiajun Qiu1,2,3,4
Jiajun Qiu1,2,3,4 Chao Wang1,2,3,4
Chao Wang1,2,3,4 Guotai Sheng3,4*
Guotai Sheng3,4* Yang Zou2*
Yang Zou2* Guobo Xie4*
Guobo Xie4*
Objective: Platelet-to-white blood cell ratio (PWR) as a comprehensive indicator of inflammatory response has been widely used to assess the prognosis of various diseases. However, the relationship between PWR and adverse outcomes in patients with acute decompensated heart failure (ADHF) remains unclear. This study aimed to evaluate the association between PWR and all-cause mortality within 30 days of hospitalization in ADHF patients from Jiangxi, China.
Methods: A total of 1,453 ADHF patients from the Jiangxi-ADHF study1 cohort were included. The primary outcome measure was all-cause mortality within 30 days of hospitalization. Multivariable Cox proportional hazards regression, restricted cubic spline regression, and receiver operating characteristic curve analysis were employed to explore the association between the inflammatory marker PWR and all-cause mortality in ADHF patients within 30 days of hospitalization.
Results: During the 30-day observation period, a total of 53 subjects experienced mortality events. Multivariable Cox regression showed a negative correlation between PWR and all-cause mortality within 30 days of hospitalization in ADHF patients. Restricted cubic spline regression demonstrated an L-shaped association between PWR and 30-day mortality risk (p for nonlinear = 0.038). Further threshold analysis revealed a threshold point for PWR at 15.88, where a decrease in PWR below this threshold was significantly associated with increased risk of all-cause mortality (p for log-likelihood ratio test = 0.046). Additionally, the results of receiver operating characteristic curve analysis indicated that PWR had high predictive accuracy for mortality events within 30 days of hospitalization in ADHF patients and is significantly better than the traditional HF marker N-Terminal Pro-Brain Natriuretic Peptide (AUC: NT-proBNP 0.69, PWR 0.76; Delong test P < 0.05). Subgroup analysis showed that compared to subjects with reduced or moderately reduced ejection fraction, ADHF patients with preserved ejection fraction had a lower risk of short-term mortality associated with PWR (HR:0.99 vs. 0.98 vs. 0.87, P for interaction = 0.0067).
Conclusion: This study reveals, for the first time, a negative correlation between the inflammatory marker PWR and all-cause mortality within 30 days of hospitalization in ADHF patients. Based on the threshold analysis findings, patients with ADHF and a PWR below 15.88 had a significantly higher risk of death within 30 days.
Acute decompensated heart failure (ADHF) is a life-threatening emergency characterized by symptoms and signs of pulmonary and systemic congestion, such as exertional dyspnea, orthopnea, and bilateral lower extremity edema (1, 2), often requiring hospitalization to improve prognosis (3, 4). Although a significant proportion of patients are discharged from the hospital due to symptom improvement, many still experience in-hospital mortality or readmission within a short period (5–8). Epidemiological studies have shown that the mortality rate within 30 days of hospitalization for ADHF patients is approximately 10%, and the readmission rate within 30 days is around 25%, significantly impacting the lives of ADHF patients (9–11). With advancements in medical technology, numerous biomarkers have been discovered for risk assessment in ADHF patients (12). However, considering the complex pathophysiology of ADHF, single markers may not accurately reflect the severity of the disease (12). Studies have found that the deterioration of ADHF may be associated with neurohormonal activation, cell apoptosis, and inflammatory cascade reactions (13). These changes can cause endothelial cell damage and fluid homeostasis imbalance, exacerbating systemic organ load and leading to adverse outcomes (14, 15). Therefore, it is necessary to incorporate indicators measuring these mechanisms into the risk assessment of ADHF patients upon admission.
Platelet-to-white blood cell ratio (PWR) is a systemic inflammation indicator that has garnered attention in recent years, first proposed by Toutouzas et al. It is calculated as the platelet (PLT) count divided by the white blood cell (WBC) count and is primarily used to assess the degree of inflammation in the body (16). Subsequently, further studies have shown that this parameter plays a key role in risk assessment and prognosis across a wide range of diseases. Specifically, current evidence supports that PWR can be used to assess the risk of common chronic diseases such as diabetes, chronic kidney disease, cerebral white matter lesions, sarcopenia, and stroke (17–22). Additionally, PWR can be used for risk prediction and prognosis assessment of a variety of critical diseases (23–35), which has the potential for a wide range of disciplinary applications. It is also worth noting that evidence from the German cohort study (MyoVasc) suggests that PWR plays an important role in assessing adverse clinical outcomes in HF patients (36). Considering the serious adverse short-term prognosis of ADHF patients, further clarification of the role and value of PWR in risk assessment of short-term prognosis in ADHF patients is of great significance.
This retrospective cohort study [Jiangxi-ADHF study1] included 1,790 ADHF patients who visited Jiangxi Provincial People's Hospital from January 2019 to December 2,022. The diagnosis of ADHF referred to the latest European Society of Cardiology (ESC) guidelines for the diagnosis and treatment of acute and chronic HF available in the year of hospitalization. Among the 1,790 patients, we excluded participants with the following characteristics: 23 participants with cirrhosis; 99 participants with stage 5 chronic kidney disease or a history of hemodialysis; 63 participants with pacemakers; 42 participants who underwent percutaneous coronary intervention within the last 3 months; 73 participants with malignancies; 1 participant with concomitant pregnancy; 12 participants under the age of 18; and 24 participants with missing PWR data. Ultimately, 1,453 subjects were included in this study. The detailed inclusion and exclusion process was shown in Figure 1.
The cohort study was conducted following the Helsinki Declaration. The use of research data was approved by the participants, and the study protocol was approved by the Ethics Committee of Jiangxi Provincial People's Hospital (IRB 2024−01).
Baseline data were collected and recorded by professional medical staff upon the patient's admission, including demographic information (age, gender), lifestyle habits (smoking, drinking), blood pressure (BP), left ventricular ejection fraction (LVEF), New York Heart Association (NYHA) functional classification, clinical comorbidities [hypertension, diabetes mellitus, coronary heart disease (CHD), cerebral infarction], etc.
Blood specimens were obtained within 24 h of the patient's admission to the hospital and measured using automated analyzers, including N-Terminal Pro-Brain Natriuretic Peptide (NT-proBNP), WBC, red blood cell count, hemoglobin, PLT, albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine, blood urea nitrogen, total cholesterol (TC), triglycerides, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol. Liver enzymes and lipid parameters were measured in the fasting state upon admission or on the morning of the second day after admission.
This study followed up participants from the time of admission, with the follow-up endpoint being the occurrence of outcome events or the 30th day after admission, whichever occurred first. The primary outcome event was all-cause mortality in ADHF patients within 30 days of hospitalization.
Participants were divided into three groups (low, moderate, and high) based on the tertiles of PWR. Normally distributed continuous data were expressed as mean (standard deviation), while non-normally distributed continuous data were expressed as median (interquartile range); categorical data were presented as frequency (percentage). Differences between groups were compared using Kruskal–Wallis H-test, one-way analysis of variance, or chi-square test depending on the data type.
First, Kaplan–Meier curves were plotted to assess the survival of ADHF patients in different PWR groups. Subsequently, several adjusted Cox regression models were constructed to evaluate the association between PWR and 30-day mortality in ADHF patients, with variance inflation factors less than 5 for covariates: Model 1 adjusted for gender, age, and comorbidities; Model 2 further adjusted for NYHA classification, LVEF, systolic BP, diastolic BP, and NT-proBNP based on Model 1; Model 3 adjusted for all non-collinear covariates. In addition, restricted cubic spline (RCS) regression was used to explore the dose-response relationship between PWR and 30-day mortality in ADHF patients. If a nonlinear association was detected, a recursive algorithm was applied to determine potential threshold points; Subsequently, a Cox regression model was created for each side of the threshold point, followed by a log-likelihood ratio test to determine whether a significant change occurred in the correlation between PWR and short-term mortality risk in ADHF patients before and after the threshold point.
We also conducted stratified analyses based on age, gender, NYHA classification, LVEF, and comorbidities such as hypertension, diabetes, cerebral infarction, and CHD, and analyzed potential interactions between these stratified variables and PWR using likelihood ratio tests. Finally, receiver operating characteristic curves were plotted to evaluate and compare the predictive value of PWR with the established ADHF biomarker NT-proBNP for all-cause mortality within 30 days of admission in ADHF patients, and the area under the curve (AUC) was calculated. Additionally, we evaluated the potential improvement in predictive performance by incorporating PWR into the NT-proBNP model, where AUC values were compared using the Delong test (37). All analyses in this study were performed using R language version 4.2.1 and Empower(R) version 2.20 statistical software, with a significance level set at P < 0.05 (two-sided).
This study included 1,453 participants, of whom 837 (57.6%) were male and 616 (42.4%) were female. During the 30-day observation period, 53 (3.65%) participants experienced mortality events.
Table 1 presents the baseline characteristics of ADHF patients at admission grouped according to the tertiles of PWR. Compared to participants with moderate to high PWR levels, those in the low PWR group exhibited significantly higher levels of age, WBC, hemoglobin, ALT, AST, creatinine, and NT-proBNP, while lower levels of PLT, ALB, TC, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol. Additionally, a higher proportion of male participants, those with diabetes, and those classified as NYHA grade IV were observed in the low PWR group.
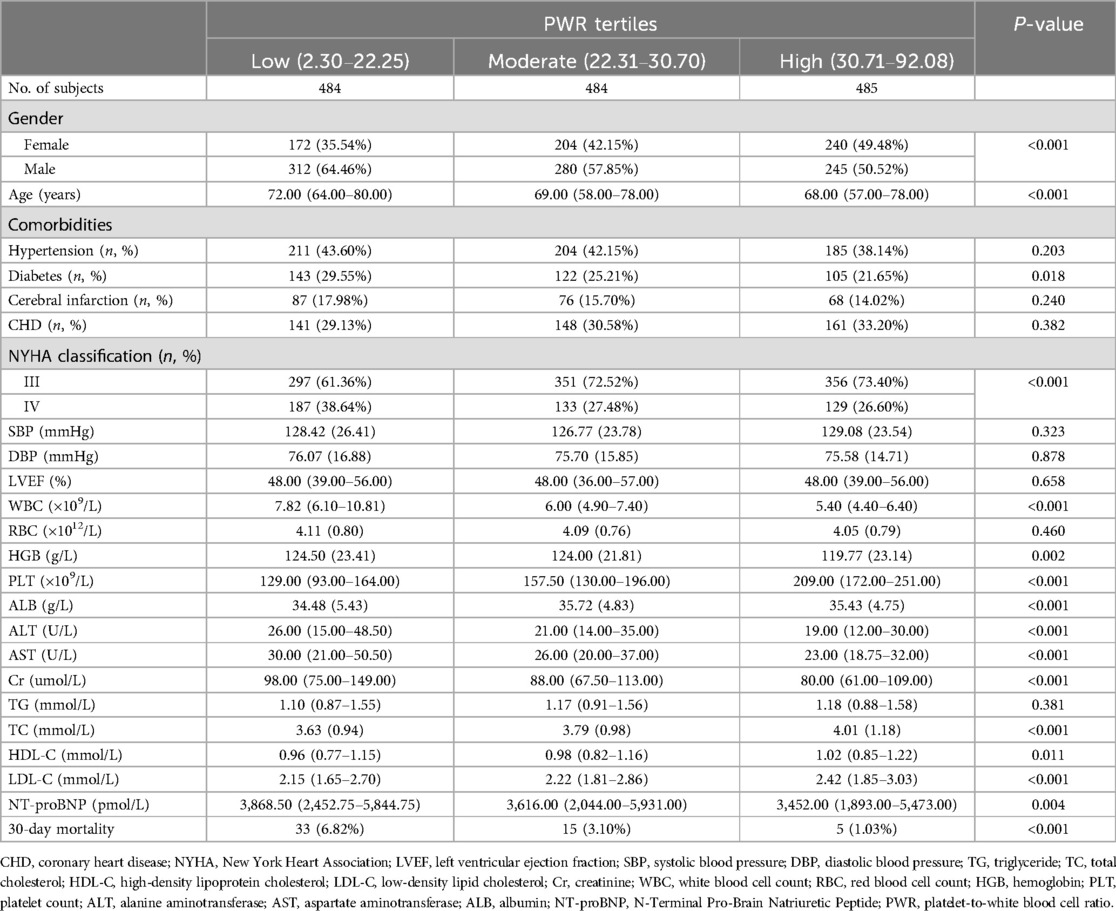
Table 1. Summary of baseline characteristics of the study population according to PWR tertiles group.
Figure 2 illustrates the 30-day cumulative survival curves for ADHF patients in the low, moderate, and high PWR groups. The results indicated that compared to ADHF patients with moderate to high PWR levels, those with low PWR had a significantly higher mortality rate within 30 days (log-rank P < 0.05).
We included PWR as both a continuous and categorical variable in four sequentially adjusted Cox regression models to explore its association with 30-day mortality in ADHF patients. Prior to establishing the multivariable Cox regression models, collinearity analysis was performed, with covariates having variance inflation factor values greater than 5 (ALT, TC) considered to have high collinearity and thus not included in subsequent models (Supplementary Table S1). When PWR was treated as a continuous variable, a negative correlation was observed between PWR and 30-day mortality after adjusting for all non-collinear covariates (Crude model HR: 0.90; Model 1 HR: 0.91; Model 2 HR: 0.93; Model 3 HR: 0.93). When PWR was treated as a categorical variable, compared to the low PWR group, the higher PWR groups exhibited stronger negative correlations with 30-day mortality risk of participants (Table 2). Specifically, the results of Model 3 showed that the 30-day mortality risk for ADHF patients in the high PWR group decreased by 76% compared to the low PWR group (HR 0.24, 95% CI: 0.06, 0.86). In summary, low PWR emerged as an independent risk factor for all-cause mortality during hospitalization in ADHF patients.
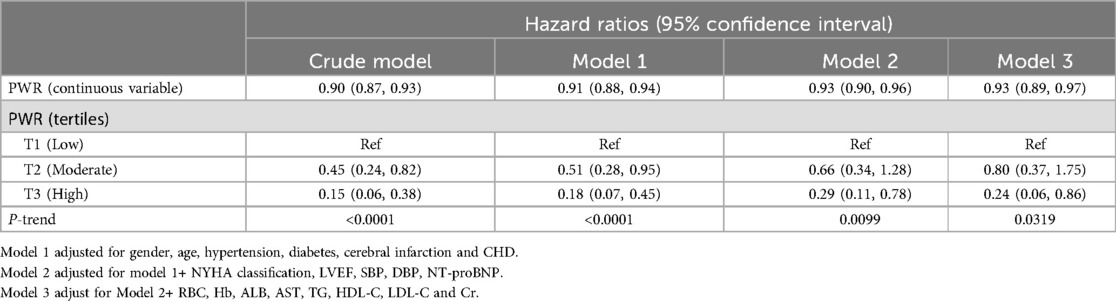
Table 2. Multivariable Cox regression analysis of the association between PWR and 30-day mortality in patients with ADHF.
We further assessed the dose-response relationship between PWR and the risk of 30-day mortality in ADHF patients using RCS regression models. As depicted in Figure 3, there was a nonlinear L-shaped association between PWR and 30-day mortality, with a potential threshold effect point (between 15 and 20). When PWR was below this threshold point, the risk of all-cause mortality in ADHF patients significantly decreased with increasing PWR, whereas the curve flattened when PWR exceeded this threshold point. We further employed segmented Cox regression analysis to calculate the optimal inflection point on the dose-response relationship curve between PWR and all-cause mortality risk, revealing the optimal inflection point to occur at a PWR of 15.88 (Table 3), where a decrease in PWR below this inflection point was significantly associated with increased risk of all-cause mortality.
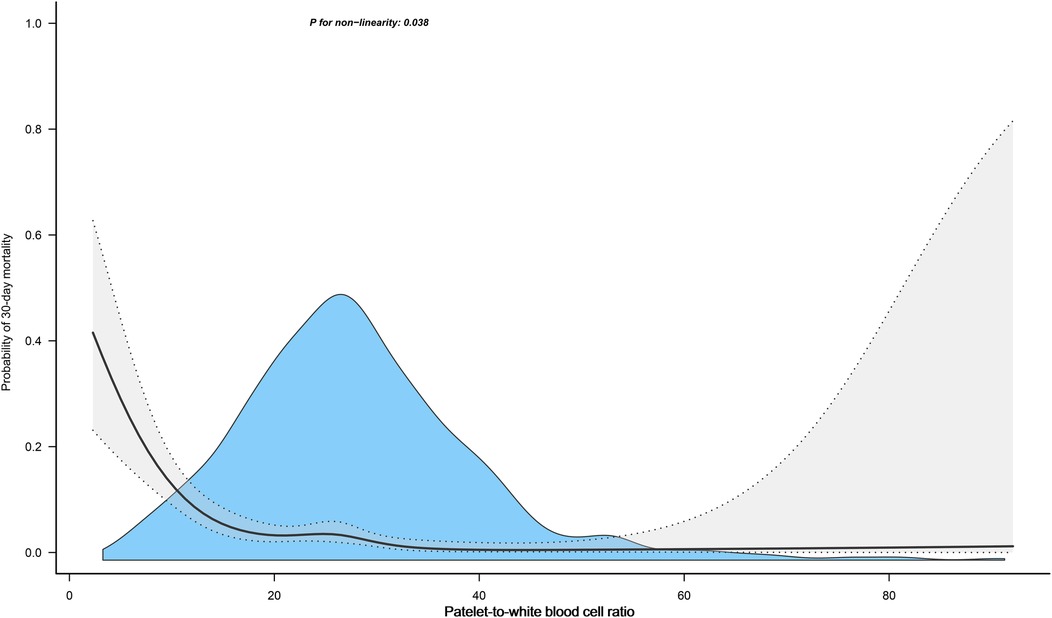
Figure 3. Fitting the dose-response relationship between PWR and 30-Day All-cause mortality in ADHF patients with 4 knots restricted cubic spline. Adjusted for gender, age, hypertension, diabetes, cerebral infarction, CHD, NYHA classification, LVEF, SBP, DBP, NT-proBNP, RBC, Hb, ALB, AST, TG, HDL-C, LDL-C and Cr.
We also evaluated and compared the predictive value of PWR and NT-proBNP for predicting mortality events within 30 days of admission in ADHF patients using receiver operating characteristic curves (Table 4). The study findings indicated that PWR exhibited a higher predictive value for 30-day mortality events in ADHF patients compared to NT-proBNP (AUC: NT-proBNP 0.69, PWR 0.76; Delong P < 0.05). Notably, when PWR was added to the NT-proBNP model for predicting 30-day mortality, we observed a significant enhancement in the model's predictive capability, with the AUC increasing from 0.69 to 0.80 (Delong test P < 0.01). These results underscored the importance of further evaluating the novel inflammatory marker PWR in predicting mortality risk among ADHF patients.
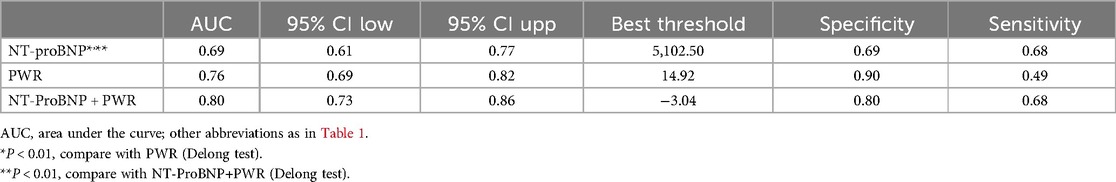
Table 4. ROC curves analyzing the predictive ability of NT-proBNP, PWR for 30-day mortality and the improvement of 30-day mortality prediction by adding PWR on top of NT-proBNP.
To explore whether the association between PWR and the risk of all-cause mortality differed among ADHF patients with different characteristics, we conducted subgroup analyses based on age, gender, NYHA classification, LVEF, and the presence of comorbidities such as hypertension, diabetes, cerebral infarction, and CHD. Significant differences of the association were observed only in the LVEF subgroup: compared to patients with HF with reduced ejection fraction (LVEF <40%) and those with HF with mid-range ejection fraction (LVEF 40%–49%), patients with HF with preserved ejection fraction (HFpEF: LVEF ≥50%) had a stronger negative correlation between PWR and 30-day mortality (HR: 0.99 vs. 0.98 vs. 0.87, P = 0.0067) (Table 5).
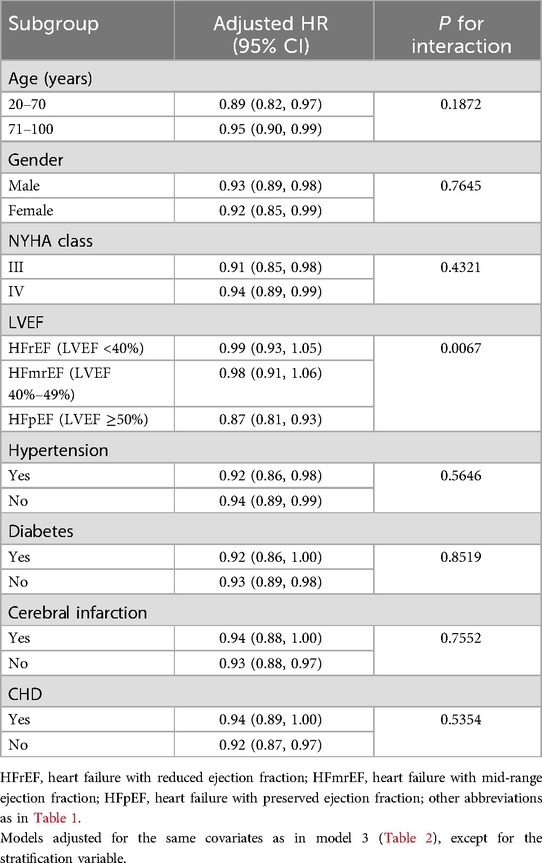
Table 5. Stratified analysis showed the relationship between PWR and 30-day mortality in patients with ADHF in different age, gender, NYHA class, LVEF and whether combined with hypertension/diabetes/cerebral infarction/CHD.
In this retrospective cohort study, we analyzed clinical data from 1,453 ADHF patients in Jiangxi, China, and revealed, for the first time, the association between PWR and the risk of all-cause mortality within 30 days of admission among Chinese ADHF patients. The results indicated that after controlling for confounding factors, PWR was negatively correlated with the risk of 30-day all-cause mortality, particularly when PWR was <15.88, where the risk of death in ADHF patients was significantly decreased with increasing PWR.
Hospitalization of ADHF patients is closely associated with high mortality and readmission rates (9–11, 38). Early identification of important risk factors during hospitalization for ADHF patients is crucial for preventing or reducing mortality rates. Previous studies have shown that systemic organ congestion is a significant factor contributing to adverse events in ADHF patients (39, 40). With further understanding of the disease, research has revealed that inflammatory reactions also contribute to the exacerbation of ADHF (13, 41). Inflammatory responses mediate the occurrence and development of diseases such as atherosclerosis (42), hypertension (43), and myocardial infarction (44), playing a crucial role in cardiovascular diseases. ADHF represents a common manifestation of advanced stages of various cardiovascular diseases (45), and numerous studies have demonstrated that inflammatory biomarkers can serve as indicators for evaluating the prognosis of ADHF patients. For example, Zhu X et al. constructed a new inflammation prognosis score system by incorporating different inflammatory indicators such as C-reactive protein, red cell distribution width, and neutrophil-to-lymphocyte ratio, which significantly improved the predictive ability of the model and can be used as a practical tool for individualized risk stratification of ADHF patients (46). Other studies have indicated that C-reactive protein and interleukin-6 are predictive factors for all-cause mortality in AHF patients (47, 48). Additionally, Ye GL et al. found in a cohort study of 443 AHF patients from northern China that the inflammatory marker PLT-to-lymphocyte ratio is independently correlated with all-cause mortality in ADHF patients; the higher the PLT-to-lymphocyte ratio, the greater the risk of mortality in ADHF patients (HR 2.437; 95% CI 1.302, 3.653) (49).
PWR is a novel biomarker reflecting systemic inflammation, with a decrease in this parameter indicating disruption of immune balance (50). As indispensable factors in the immune system, both PLT and WBC typically exhibit a sharp increase during acute infections (51, 52). However, in critically ill patients, PLT often remain at low levels, which may increase the risk of bleeding and mortality (53, 54). A cohort study from Japan found that a decrease in PLT level increases the risk of mortality and readmission in ADHF patients (55). Furthermore, compared to survivors, non-survivors of ADHF patients often have higher baseline levels of WBC upon admission, although this is not significantly correlated with the risk of death, which may be due to differences in the study population (56). Therefore, for acute HF populations, both decreased PLT and increased WBC levels increase the risk of mortality during hospitalization. As a composite index combining PLT and WBC, the PWR provides a more comprehensive assessment of disease severity. In the current study, we further identified PWR as an important biomarker for assessing short-term prognosis in patients with ADHF and significantly superior to the traditional HF marker NT-proBNP. Integrating previous studies (23–36) with current research findings, PWR appears to be a promising new marker in the field of critical illness. We suggest that healthcare workers in the emergency room as well as the intensive care unit need to focus on the PWR of their patients.
Systemic inflammatory responses are widely present in various human diseases, providing important protection against adverse factors (57). After establishing the association between PWR and short-term mortality prognosis in patients with ADHF, identifying critical PWR thresholds in different clinical scenarios may be important for clinical practice. In terms of chronic disease assessment, analyses of previous studies have shown an L-shaped association between PWR and diabetes, chronic kidney disease, and stroke similar to the current study, with an associated cutoff point of approximately 30 (17, 18, 22). In addition, in the assessment of critical illnesses, some studies have also calculated critical values for the prediction of poor prognosis by ROC analysis: A study from China analyzed the association between PWR and mortality in patients with subarachnoid hemorrhage and found that lower PWR was associated with increased risk of postoperative pneumonia and death (optimal cutoff point was 15.69) (25). Another study found that the risk of death significantly increased in patients with acute novel coronavirus infection when PWR was below 20.34 (26). A study following 269 pancreatic cancer patients found that a decrease in PWR provided a suitable environment for tumor cell growth, particularly when PWR was <6, where the risk of death was significantly elevated (29). Additionally, a study from Korea showed that PWR had evaluative significance for short-term adverse outcomes in patients with acute decompensated cirrhosis, with a threshold point of 12.1 (27). In our current analysis, through RCS analysis, we found that PWR has an L-shaped association with 30-day mortality risk in ADHF patients, with the PWR-related risk of death cutoff point being 15.88; in addition, by ROC analysis we also calculated the optimal threshold for predicting 30-day mortality in patients with ADHF to be 14.92; these findings are similar to several previous reports (25–27). Overall, PWR has a wide range of applications and has good potential for promotion. Relatively speaking, in the context of chronic disease risk assessment, the cutoff point for PWR is relatively lenient, with a recommended PWR value maintained below 30. However, for predicting adverse prognoses in acute and critical illnesses, a stricter criterion for the PWR cutoff point is required, with a recommended PWR value kept below 15.
In our subgroup analysis, we also identified a particular finding: among patients with HFpEF in ADHF, there existed a stronger negative correlation between PWR and 30-day mortality compared to those with reduced or intermediate ejection fractions. This suggests that, at equivalent PWR levels, HFpEF patients have a greater protective effect. Similarly, at equivalent PWR levels, ADHF patients with reduced and mid-range ejection fractions face a higher risk of mortality, indicating that the deterioration of cardiac systolic function further exacerbates the mortality risk associated with low PWR. It is well recognized that in the setting of inadequate effective circulating blood volume, myocardial cells mount complex inflammatory responses to danger signals, primarily involving: (1) the expression of pro-inflammatory and anti-inflammatory cytokines such as IL-6 and IL-10, initiating and modulating local inflammatory reactions (58); (2) the expression of chemotactic factors like KC and MIP-2, recruiting and activating appropriate subsets of inflammatory cells for response and repair (59, 60); and (3) the expression of cell surface adhesion molecules, particularly ICAM-1, facilitating interactions between inflammatory cells and the extracellular matrix as well as signaling cascades from the extracellular milieu to the intracellular environment (61–63). In the scenario of decreased cardiac contractility, these cytokines, chemotactic factors, subsequently recruited leukocytes, and cell surface adhesion molecules may instigate a cascade of inflammatory reactions, influencing the cardiac repair processes, ultimately leading to mortality (58–63). Considering the stronger negative correlation between PWR and mortality in HFpEF patients, which provides more protective information by comparison, close monitoring of inflammatory biomarkers such as PWR changes may be deemed more imperative, particularly for patients with HFpEF.
The current research findings have significant reference value for patients with ADHF. ADHF is known to have severe symptoms, rapid onset of seizures, and poor short-term prognosis, making it one of the most difficult inpatient diseases to properly manage in the clinic (3–8). In the current study, we tested the association of a facile novel inflammatory marker, PWR, with the 30-day prognosis of death in patients with AHDF and determined that PWR has an important application in the short-term prognostic assessment of patients with ADHF and is significantly superior to the traditional HF marker NT-proBNP. It should be noted that PWR is simple to obtain and requires only routine blood analysis (16), which is a very routine measurement in community clinics as well as in hospitals of different levels. Therefore, we believe that PWR can be a promising tool for risk stratification and prognostic assessment of patients with ADHF. Of course, we also hope that the results of the current study will be further validated in the future in other regions and races and will inform the development of future HF management guidelines.
(1) This study is the first to discover a negative correlation between PWR and all-cause mortality in ADHF patients within 30 days of hospital admission among the Chinese population. (2) PWR, composed of PLT and WBC, can be easily obtained through routine blood tests. (3) This study revealed an L-shaped correlation between PWR and 30-day mortality rate, identifying a PWR threshold of 15.88.
(1) The observational nature of the study limits further assessment of the impact of treatments targeting PWR on prognosis, necessitating further prospective research. (2) The study population mainly consists of individuals from various regions in Jiangxi, China, caution should be exercised when extrapolating the study findings to populations from other countries or provinces in China. (3) Despite extensive adjustment for covariates in the current analysis, some unmeasured factors may not have been included in the study. (4) Due to the lack of repeated measurement data for PWR, it is not possible to further explore the risk assessment value of PWR for short-term mortality in ADHF patients.
This study demonstrates a negative correlation between the inflammatory marker PWR and short-term mortality in ADHF patients, and its predictive performance for short-term death events is significantly better than the traditional HF marker NT-proBNP. Based on the findings of this study, we recommended using PWR to assess the risk of mortality in ADHF patients during hospitalization, providing valuable guidance for clinical management.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Ethics Committee of Jiangxi Provincial People's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
XH: Formal Analysis, Investigation, Software, Validation, Writing – original draft. MK: Investigation, Software, Writing – original draft. JQ: Investigation, Writing – review & editing. CW: Investigation, Writing – review & editing. GS: Conceptualization, Data curation, Methodology, Project administration, Supervision, Writing – review & editing. YZ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. GX: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Natural Science Foundation of Jiangxi Province [20232BAB216004], Scientific Research Fund of Jiangxi Cardiovascular Research Institute, and the Jiangxi Province Traditional Chinese Medicine Science and Technology Plan Project [2023B1218].
We would like to thank Jiangxi Provincial People's Hospital for its strong support to the research project and the members of the JX-ADHF1 research team for their great efforts in the data collection process.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1454933/full#supplementary-material
1. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the heart failure association (HFA) of the ESC. Eur J Heart Fail. (2022) 24:4–131. doi: 10.1002/ejhf.2333
2. Kurmani S, Squire I. Acute heart failure: definition, classification and epidemiology. Curr Heart Fail Rep. (2017) 14:385–92. doi: 10.1007/s11897-017-0351-y
3. Filippatos G, Angermann CE, Cleland JGF, Lam CSP, Dahlström U, Dickstein K, et al. Global differences in characteristics, precipitants, and initial management of patients presenting with acute heart failure. JAMA Cardiol. (2020) 5:401–10. doi: 10.1001/jamacardio.2019.5108
4. Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, et al. Heart disease and stroke statistics-2023 update: a report from the American Heart Association. Circulation. (2023) 147:e93–621. doi: 10.1161/CIR.0000000000001123
5. Spinar J, Parenica J, Vitovec J, Widimsky P, Linhart A, Fedorco M, et al. Baseline characteristics and hospital mortality in the acute heart failure database (AHEAD) main registry. Crit Care. (2011) 15:R291. doi: 10.1186/cc10584
6. Lepage S. Acute decompensated heart failure. Can J Cardiol. (2008) 24 Suppl B:6B–8B. doi: 10.1016/S0828-282X(08)71022-5
7. Lagu T, Pekow PS, Shieh MS, Stefan M, Pack QR, Kashef MA, et al. Validation and comparison of seven mortality prediction models for hospitalized patients with acute decompensated heart failure. Circ Heart Fail. (2016) 9:e002912. doi: 10.1161/CIRCHEARTFAILURE.115.002912
8. Adams KF Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the acute decompensated heart failure national registry (ADHERE). Am Heart J. (2005) 149:209–16. doi: 10.1016/j.ahj.2004.08.005
9. Suter LG, Li SX, Grady JN, Lin Z, Wang Y, Bhat KR, et al. National patterns of risk-standardized mortality and readmission after hospitalization for acute myocardial infarction, heart failure, and pneumonia: update on publicly reported outcomes measures based on the 2013 release. J Gen Intern Med. (2014) 29:1333–40. doi: 10.1007/s11606-014-2862-5
10. Krumholz HM, Merrill AR, Schone EM, Schreiner GC, Chen J, Bradley EH, et al. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circ Cardiovasc Qual Outcomes. (2009) 2:407–13. doi: 10.1161/CIRCOUTCOMES.109.883256
11. Bernheim SM, Grady JN, Lin Z, Wang Y, Wang Y, Savage SV, et al. National patterns of risk-standardized mortality and readmission for acute myocardial infarction and heart failure. Update on publicly reported outcomes measures based on the 2010 release. Circ Cardiovasc Qual Outcomes. (2010) 3:459–67. doi: 10.1161/CIRCOUTCOMES.110.957613
12. de la Espriella R, Núñez-Marín G, Codina P, Núñez J, Bayés-Genís A. Biomarkers to improve decision-making in acute heart failure. Card Fail Rev. (2023) 9:e13. doi: 10.15420/cfr.2023.08
13. Chen D, Assad-Kottner C, Orrego C, Torre-Amione G. Cytokines and acute heart failure. Crit Care Med. (2008) 36:S9–16. doi: 10.1097/01.CCM.0000297160.48694.90
14. Mentz RJ, O'Connor CM. Pathophysiology and clinical evaluation of acute heart failure. Nat Rev Cardiol. (2016) 13:28–35. doi: 10.1038/nrcardio.2015.134
15. Harjola VP, Mullens W, Banaszewski M, Bauersachs J, Brunner-La Rocca HP, Chioncel O, et al. Organ dysfunction, injury and failure in acute heart failure: from pathophysiology to diagnosis and management. A review on behalf of the acute heart failure committee of the heart failure association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail. (2017) 19:821–36. doi: 10.1002/ejhf.872
16. Toutouzas KG, Velmahos GC, Kaminski A, Chan L, Demetriades D. Leukocytosis after posttraumatic splenectomy: a physiologic event or sign of sepsis? Arch Surg. (2002) 137:924–8; discussion 928–9. doi: 10.1001/archsurg.137.8.924
17. Xiong Y, Zhong Q, Zhang Y, Qin F, Yuan J. The association between the platelet to white blood cell ratio and chronic kidney disease in an aging population: a four-year follow-up study. J Clin Med. (2023) 12:7073. doi: 10.3390/jcm12227073
18. Liu F, Wang T, Wang S, Zhao X, Hua Y. The association of platelet to white blood cell ratio with diabetes: a nationwide survey in China. Front Endocrinol (Lausanne). (2024) 15:1418583. doi: 10.3389/fendo.2024.1418583
19. Tang HH, Wang YJ, Wang Z, Yan GL, Qiao Y, Li X, et al. Predicting cerebral white matter lesions based on the platelet-to-white blood cell ratio in hypertensive patients. Brain Res. (2023) 1808:148340. doi: 10.1016/j.brainres.2023.148340
20. Gholizade M, Farhadi A, Marzban M, Mahmudpour M, Nabipour I, Kalantarhormozi M, et al. Association between platelet, white blood cell count, platelet to white blood cell ratio and sarcopenia in community-dwelling older adults: focus on Bushehr elderly health (BEH) program. BMC Geriatr. (2022) 22:300. doi: 10.1186/s12877-022-02954-3
21. Amalia L, Dalimonthe NZ. Clinical significance of platelet-to-white blood cell ratio (PWR) and national institute of health stroke scale (NIHSS) in acute ischemic stroke. Heliyon. (2020) 6:e05033. doi: 10.1016/j.heliyon.2020.e05033
22. Hu ZB, Zhong QQ, Lu ZX, Zhu F. Association of platelet-to-white blood cell ratio and platelet-to-neutrophil ratio with the risk of fatal stroke occurrence in middle-aged to older Chinese. BMC Geriatr. (2022) 22:430. doi: 10.1186/s12877-022-03134-z
23. Ko DG, Park JW, Kim JH, Jung JH, Kim HS, Suk KT, et al. Platelet-to-white blood cell ratio: a feasible biomarker for pyogenic liver abscess. Diagnostics (Basel). (2022) 12:2556. doi: 10.3390/diagnostics12102556
24. Xu J, Wang X, Chen W, Tian M, You C. Incorporating platelet-to-white blood cell ratio into survival prediction models for intracerebral hemorrhage: a nomogram approach. Front Neurol. (2024) 15:1464216. doi: 10.3389/fneur.2024.1464216
25. Wang K, Li R, Chen X, Zhao Y, Hao Q. Platelet-to-white blood cell ratio: a feasible predictor for unfavorable functional outcome in patients with aneurysmal subarachnoid hemorrhage. J Clin Neurosci. (2023) 115:108–13. doi: 10.1016/j.jocn.2023.07.019
26. Thungthienthong M, Vattanavanit V. Platelet-to-white blood cell ratio as a predictor of mortality in patients with severe COVID-19 pneumonia: a retrospective cohort study. Infect Drug Resist. (2023) 16:445–55. doi: 10.2147/IDR.S398731
27. Kim JH, Kim SE, Song DS, Kim HY, Yoon EL, Kim TH, et al. Platelet-to-white blood cell ratio is associated with adverse outcomes in cirrhotic patients with acute deterioration. J Clin Med. (2022) 11:2463. doi: 10.3390/jcm11092463
28. Zhao S, Pan H, Guo Q, Xie W, Wang J. Platelet to white blood cell ratio was an independent prognostic predictor in acute myeloid leukemia. Hematology. (2022) 27:426–30. doi: 10.1080/16078454.2022.2055857
29. Tang F, Dai P, Wei Q, Gan K, Wang Z, Chen H, et al. The neutrophil-to-monocyte ratio and platelet-to-white blood cell ratio represent novel prognostic markers in patients with pancreatic cancer. Gastroenterol Res Pract. (2021) 2021:6693028. doi: 10.1155/2021/6693028
30. Zhang J, Qiu Y, He X, Mao W, Han Z. Platelet-to-white blood cell ratio: a novel and promising prognostic marker for HBV-associated decompensated cirrhosis. J Clin Lab Anal. (2020) 34:e23556. doi: 10.1002/jcla.23556
31. Sabour S. Post-operative platelet-to-white blood cell ratio after splenectomy in patients with advanced ovarian cancer: methodological issues on diagnostic value and prediction to avoid misinterpretation. Int J Gynecol Cancer. (2020) 30:280. doi: 10.1136/ijgc-2019-000931
32. Lathouras K, Panagakis G, Bowden SJ, Saliaris K, Saso S, Haidopoulos D, et al. Diagnostic value of post-operative platelet-to-white blood cell ratio after splenectomy in patients with advanced ovarian cancer. Int J Gynecol Cancer. (2019) 29:1292–7. doi: 10.1136/ijgc-2019-000712
33. Qiu L, Pan M, Zhang R, Ren K. Maternal peripheral blood platelet-to-white blood cell ratio and platelet count as potential diagnostic markers of histological chorioamnionitis-related spontaneous preterm birth. J Clin Lab Anal. (2019) 33:e22840. doi: 10.1002/jcla.22840
34. Jie Y, Gong J, Xiao C, Zhu S, Zhou W, Luo J, et al. Low platelet to white blood cell ratio indicates poor prognosis for acute-on-chronic liver failure. Biomed Res Int. (2018) 2018:7394904. doi: 10.1155/2018/7394904
35. Garbens A, Wallis CJD, Bjarnason G, Kulkarni GS, Nathens AB, Nam RK, et al. Platelet to white blood cell ratio predicts 30-day postoperative infectious complications in patients undergoing radical nephrectomy for renal malignancy. Can Urol Assoc J. (2017) 11:E414–20. doi: 10.5489/cuaj.4478
36. Dahlen B, Schulz A, Göbel S, Tröbs SO, Schwuchow-Thonke S, Spronk HM, et al. The impact of platelet indices on clinical outcome in heart failure: results from the MyoVasc study. ESC Heart Fail. (2021) 8:2991–3001. doi: 10.1002/ehf2.13390
37. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. (1988) 44:837–45. doi: 10.2307/2531595
38. Chioncel O, Ambrosy AP, Maggioni AP. Temporal trends in the outcomes of acute heart failure: between consolatory evidences and real progress. Eur J Heart Fail. (2021) 23:432–5. doi: 10.1002/ejhf.2130
39. Arrigo M, Jessup M, Mullens W, Reza N, Shah AM, Sliwa K, et al. Acute heart failure. Nat Rev Dis Primers. (2020) 6:16. doi: 10.1038/s41572-020-0151-7
40. Metra M, Adamo M, Tomasoni D, Mebazaa A, Bayes-Genis A, Abdelhamid M, et al. Pre-discharge and early post-discharge management of patients hospitalized for acute heart failure: a scientific statement by the heart failure association of the ESC. Eur J Heart Fail. (2023) 25:1115–31. doi: 10.1002/ejhf.2888
41. Mann DL. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res. (2002) 91:988–98. doi: 10.1161/01.RES.0000043825.01705.1B
42. Libby P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr. (2006) 83:456S–60. doi: 10.1093/ajcn/83.2.456S
43. McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circ Res. (2015) 116:1022–33. doi: 10.1161/CIRCRESAHA.116.303697
44. Ong SB, Hernández-Reséndiz S, Crespo-Avilan GE, Mukhametshina RT, Kwek XY, Cabrera-Fuentes HA, et al. Inflammation following acute myocardial infarction: multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol Ther. (2018) 186:73–87. doi: 10.1016/j.pharmthera.2018.01.001
45. Wang ZV, Li DL, Hill JA. Heart failure and loss of metabolic control. J Cardiovasc Pharmacol. (2014) 63:302–13. doi: 10.1097/FJC.0000000000000054
46. Zhu X, Cheang I, Xu F, Gao R, Liao S, Yao W, et al. Long-term prognostic value of inflammatory biomarkers for patients with acute heart failure: construction of an inflammatory prognostic scoring system. Front Immunol. (2022) 13:1005697. doi: 10.3389/fimmu.2022.1005697
47. Michou E, Wussler D, Belkin M, Simmen C, Strebel I, Nowak A, et al. Quantifying inflammation using interleukin-6 for improved phenotyping and risk stratification in acute heart failure. Eur J Heart Fail. (2023) 25:174–84. doi: 10.1002/ejhf.2767
48. Zhang L, He G, Huo X, Tian A, Ji R, Pu B, et al. Long-term cumulative high-sensitivity C-reactive protein and mortality among patients with acute heart failure. J Am Heart Assoc. (2023) 12:e029386. doi: 10.1161/JAHA.123.029386
49. Ye GL, Chen Q, Chen X, Liu YY, Yin TT, Meng QH, et al. The prognostic role of platelet-to-lymphocyte ratio in patients with acute heart failure: a cohort study. Sci Rep. (2019) 9:10639. doi: 10.1038/s41598-019-47143-2
50. Griesshammer M, Bangerter M, Sauer T, Wennauer R, Bergmann L, Heimpel H. Aetiology and clinical significance of thrombocytosis: analysis of 732 patients with an elevated platelet count. J Intern Med. (1999) 245:295–300. doi: 10.1046/j.1365-2796.1999.00452.x
51. Medzhitov R. Origin and physiological roles of inflammation. Nature. (2008) 454:428–35. doi: 10.1038/nature07201
52. Strauss R, Wehler M, Mehler K, Kreutzer D, Koebnick C, Hahn EG. Thrombocytopenia in patients in the medical intensive care unit: bleeding prevalence, transfusion requirements, and outcome. Crit Care Med. (2002) 30:1765–71. doi: 10.1097/00003246-200208000-00015
53. Nijsten MW, ten Duis HJ, Zijlstra JG, Porte RJ, Zwaveling JH, Paling JC, et al. Blunted rise in platelet count in critically ill patients is associated with worse outcome. Crit Care Med. (2000) 28:3843–6. doi: 10.1097/00003246-200012000-00017
54. Venkata C, Kashyap R, Farmer JC, Afessa B. Thrombocytopenia in adult patients with sepsis: incidence, risk factors, and its association with clinical outcome. J Intensive Care. (2013) 1:9. doi: 10.1186/2052-0492-1-9
55. Yamaguchi S, Abe M, Arakaki T, Arasaki O, Shimabukuro M. Incremental prognostic value of platelet count in patients with acute heart failure—a retrospective observational study. Circ J. (2019) 83:576–83. doi: 10.1253/circj.CJ-18-0961
56. Milo-Cotter O, Felker GM, Uriel N, Kaluski E, Edwards C, Rund MM, et al. Patterns of leukocyte counts on admissions for acute heart failure–presentation and outcome–results from a community based registry. Int J Cardiol. (2011) 148:17–22. doi: 10.1016/j.ijcard.2009.10.009
57. Medzhitov R. The spectrum of inflammatory responses. Science. (2021) 374:1070–5. doi: 10.1126/science.abi5200
58. Brown MA, Jones WK. NF-kappaB action in sepsis: the innate immune system and the heart. Front Biosci. (2004) 9:1201–17. doi: 10.2741/1304
59. Madorin WS, Rui T, Sugimoto N, Handa O, Cepinskas G, Kvietys PR. Cardiac myocytes activated by septic plasma promote neutrophil transendothelial migration: role of platelet-activating factor and the chemokines LIX and KC. Circ Res. (2004) 94:944–51. doi: 10.1161/01.RES.0000124395.20249.AE
60. Massey KD, Strieter RM, Kunkel SL, Danforth JM, Standiford TJ. Cardiac myocytes release leukocyte-stimulating factors. Am J Physiol. (1995) 269:H980–7. doi: 10.1152/ajpheart.1995.269.3.H980
61. Davani EY, Dorscheid DR, Lee CH, van Breemen C, Walley KR. Novel regulatory mechanism of cardiomyocyte contractility involving ICAM-1 and the cytoskeleton. Am J Physiol Heart Circ Physiol. (2004) 287:H1013–22. doi: 10.1152/ajpheart.01177.2003
62. Hattori Y, Kasai K. Induction of mRNAs for ICAM-1, VCAM-1, and ELAM-1 in cultured rat cardiac myocytes and myocardium in vivo. Biochem Mol Biol Int. (1997) 41:979–86. doi: 10.1080/15216549700202041
Keywords: platelet-to-white blood cell ratio, short-term prognostic, PWR, acute decompensated heart failure, ADHF
Citation: Huang X, Kuang M, Qiu J, Wang C, Sheng G, Zou Y and Xie G (2025) Assessment of platelet-to-white blood cell ratio on short-term mortality events in patients hospitalized with acute decompensated heart failure: evidence from a cohort study from Jiangxi, China. Front. Cardiovasc. Med. 12:1454933. doi: 10.3389/fcvm.2025.1454933
Received: 27 June 2024; Accepted: 28 January 2025;
Published: 7 February 2025.
Edited by:
Alessandro Belletti, IRCCS San Raffaele Scientific Institute, ItalyReviewed by:
Flavia Bittar Britto Arantes, Universidade Federal de Uberlândia, BrazilCopyright: © 2025 Huang, Kuang, Qiu, Wang, Sheng, Zou and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guotai Sheng, dGdzMjAwNTA5QDE2My5jb20=; Yang Zou, anh5eHl6eUAxNjMuY29t; Guobo Xie, eGdiMTk4MTA4MzBAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.