
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 03 March 2025
Sec. Atherosclerosis and Vascular Medicine
Volume 12 - 2025 | https://doi.org/10.3389/fcvm.2025.1433107
Objective: Uric acid (UA), fibrinogen (FIB), and homocysteine (Hcy) are the main contributors to cardiovascular and cerebrovascular diseases, and are related to hypertension. Hypertension plays a role in atherosclerosis (CAS). We hence explored the correlations of UA, FIB, and Hcy levels with CAS in hypertensive patients.
Methods: Totally 170 hypertensive patients were retrospectively included and assigned into the Non-sclerosis, Thickened, and Plaque groups based on carotid intima-media thickness (cIMT), with serum UA, FIB, and Hcy compared. Correlations of UA, FIB, and Hcy with cIMT and carotid atherosclerotic plaque (CAP) were assessed using Spearman's correlation analysis. The risk factors of CAS were evaluated by logistic multivariate regression analysis. The predictive value of UA, FIB, and Hcy for CAS was estimated by the receiver operating characteristic (ROC) curve.
Results: UA, FIB, and Hcy were up-regulated in the Plaque group vs. other two groups. Serum UA, FIB, and Hcy were positively linked to cIMT and CAP, and were independent risk factors for CAS. The area under ROC curve of UA, FIB, Hcy levels and their combination for predicting CAS were 0.889, 0.855, 0.902, and 0.958, respectively. Hypertensive patients with high levels of UA, FIB, or Hcy were more likely to develop CAS.
Conclusion: Serum UA, FIB, and Hcy are positively correlated with cIMT and CAP, and are independent risk factors for CAS in hypertensive patients. High UA, FIB and Hcy expression could assist in predicting CAS in patients with hypertension, and the combination of the three was more valuable than all three alone.
Hypertension, which is defined as a persistent elevation in blood pressure levels equal to or exceeding 140/90 mmHg, is tied up with the development of cardiovascular disease and is widely prevalent in the general population (1). Hypertension is a condition that can be influenced by various factors, including lifestyle choices, dietary habits, and nutrient intake (2). Furthermore, hypertension is a predisposing factor for numerous cardiovascular diseases, thereby amplifying its importance as a risk factor (3, 4). Hypertension has now been acknowledged as the most significant risk factor for atherosclerosis. Carotid atherosclerosis (CAS) is a chronic, systemic condition and the primary cause of mortality in developed Western nations. With the development of China's economy and the alterations in lifestyle and dietary patterns, there has been a notable increase in the incidence of CAS, which has emerged as a critical contributor to mortality among middle-aged and elderly populations in China (4). CAS mainly refers to the gradual formation of carotid intima under the influence of various factors, and it initially presents as intima-media thickness, and gradually progresses to the formation of atherosclerotic plaques, which can rupture and become dislodged, leading to thrombosis and subsequent stenosis of the blood vessels, thereby triggering abnormal carotid artery blood supply (5).
Uric Acid (UA), fibrinogen (FIB) and homocysteine (Hcy) have been identified as significant contributors to the onset of cardiovascular diseases and may be linked with metabolic-related, such as hypertension (6–9). Serum UA has been extensively studied as a potential marker of inflammation and a risk factor for atherosclerosis, and UA metabolism plays a significant part in carotid plaque biology (10). UA has a direct impact on vascular function. The vascular endothelial cells express various UA transporters and incorporated UA impairs the production of nitric oxide, resulting in endothelial dysfunction (11). FIB is an essential coagulation factor that exerts a substantial influence on the progression of atherosclerosis via its ability to promote the proliferation of smooth muscle cells (12). FIB has been positively related with the prevalence and incidence of cardiovascular disease, primarily through mechanisms involving hyperviscosity and thrombosis in response to inflammatory processes. FIB has been reported as an independent predictor for early atherosclerotic disease, such as increased carotid intima–media wall thickness (cIMT) (13). Hcy, a byproduct of methionine methylation, possesses the capacity to disturb the regular functioning of vascular endothelial cells when there is an imbalance in its secretion, and this disruption can result in inflammatory reactions at the site of vascular injury, promoting the proliferation of smooth muscle cells, and ultimately contributing to the development of atherosclerosis (14). Furthermore, the serum UA level has been suggested to correlate with FIB and Hcy levels (15). It has been documented that the occurrence of CAS in patients with epilepsy has something to do with UA, FIB and Hcy (16). Nevertheless, the correlations between serum UA, FIB and Hcy levels with CAS in hypertensive patients has been scarcely investigated and require further investigation. Hence, the present study aimed to examine the correlations between serum UA, FIB and Hcy levels with CAS in individuals with hypertension to establish a reference range that could be utilized for predicting the severity of CAS in hypertensive patients. The primary finding of our study is that high expression of UA, FIB, and Hcy could assist in predicting CAS in hypertensive patients, with the combination of the three more valuable than all three alone. Our secondary findings indicated that serum UA, FIB, and Hcy were positively correlated with cIMT and CAP, and were independent risk factors for CAS. We propose that these findings may offer novel insights for the clinical diagnosis of CAS in hypertensive patients.
The study was conducted in compliance with the recommendations set forth by the Academic Ethics Committee of Heping hospital affiliated to Changzhi Medical College [No. (2024)011]. All procedures were performed abided by the Declaration of Helsinki strictly.
A minimum of 159 samples were required for this study, as calculated using G*Power 3.1.9.7 software (Supplementary Figure S1). A total of 170 hypertensive patients visited the geriatric outpatient clinic of Heping hospital affiliated to Changzhi Medical College from January 2020 to December 2022 were retrospectively included in this study based on the inclusion and exclusion criteria (Supplementary Figure S2). The study participants were then assigned into the Non-sclerosis group (n = 53) (patients whose carotid arteries were not yet sclerosed), the Thickened group (n = 57) (patients whose carotid arteries were thickened but no carotid atherosclerotic plaques were produced) and the Plaque group (n = 60) (patients who developed carotid atherosclerotic plaques) according to cIMT. The diagnosis and grouping of hypertension were conducted in accordance with the 2018 Chinese Guidelines for the Prevention and Treatment of Hypertension.
The inclusion criteria encompassed the hypertension patients who were with systolic blood pressure (SBP) ≥ 140 mmHg or diastolic blood pressure (DBP) ≥ 90 mmHg, underwent carotid artery ultrasonography and biochemical examination, and with complete clinical information.
The exclusion criteria included patients with pulmonary hypertension or autoimmune disease or complicated with other carotid artery diseases, vegetarians, patients who had recently consumed medications or foods that affected homocysteine level, such as folic acid, vitamins, and betaine, or patients who received proton pump inhibitor treatment in the past six months.
Hypertensive patients were grouped as per the following criteria: the Non-sclerosis group (cIMT < 1.0 mm); the Thickened group (1.0 mm ≤ cIMT ≤ 1.50 mm); the Plaque group (cIMT > 1.50 mm).
In the light of the Echocardiography Guidelines and Standards of the American Society (17), CAS was diagnosed as that carotid arteries exhibited limited or diffuse intima-media thickening, generally greater than 1.5 mm, along with additional atherosclerotic plaques (18–20).
Clinical data such as age, sex, body mass index (BMI), disease course, smoking history, drinking history, diabetes history, SBP, DBP, carotid atherosclerotic plaque (CAP) and cIMT, as well as biochemical data such as triacylglycerols (TG), total cholesterol (TC), UA, FIB and Hcy were acquired via the integrated hospital information system such as the outpatient medical record system and inspection system. All patients were instructed to fast for at least 12 h before venous blood collection. Elbow vein blood (9 mL) was collected in EDTA-K2 anticoagulated vacuum tubes and placed for 30 min. After centrifugation, the samples were preserved at −80°C for measurement. Serum levels of TG, TC, UA, FIB and Hcy were measured using a fully automatic biochemical analyzer (PUZS-300, TIPS, Shanghai, China).
cIMT was determined by Doppler color ultrasound (Vivid E95, GE, Boston, MA, USA) using a b-mode 12 MHz linear probe. All subjects were examined by a dedicated full-time ultrasonographer. The patients were positioned supine, with the neck fully extended by elevating the shoulders and slightly tilting the head backward. For the examination of one side, the head was tilted to the contralateral side. The ultrasound probe was positioned at the sternoclavicular joint, medial to the sternocleidomastoid muscle, with the sound beam oriented posteriorly. Both the long axis and short axis of the carotid artery were scanned. The detection range extended 1 cm above the initial site of the internal carotid artery. The thickness of the arterial wall, the presence or absence of plaque formation, the morphology and dimensions of the plaque, as well as the presence or absence of stenosis were observed. cIMT was defined as the distance between the intima-lumen interface and the medial-adventitia interface. The test was performed at the thickest point 1 cm away from the proximal segment of the bifurcation of the common carotid artery. Measurements were taken three times for both the left and right sides, with the left and right cIMT values calculated as the average of these three measurements. The final cIMT value was subsequently determined based on the average of the left and right cIMT values.
Logistic multivariate regression was utilized to analyze the independent risk factors for CAS. BMI, age, sex, disease course, drinking history, smoking history, diabetes history, SBP, DBP, and TG, TC, UA, FIB, and Hcy levels were included as covariates, and the development of CAS as the dependent variable. The values of “Plaque group” = 1, “Non-sclerosis group” and “Thickened group” = 0 were assigned. Based on the results of factor analysis, the regression outcomes were further examined using stepwise regression analysis, which controlled for the range of independent variables, to ensure the robustness of the regression findings.
SPSS 25.0 (IBM, Armonk, New York, USA) and GraphPad Prism 6.0 (GraphPad, San Diego, CA, USA) were applied for data analysis. The normality of measurement data was evaluated by Shapiro–Wilk test. Continuous variables that conformed to normal distribution were presented as mean ± standard deviation, with one-way analysis of variance (ANOVA) for comparisons among groups and Tukey's multiple comparison test for post hoc analysis. For data that did not conform to a normal distribution, results were expressed as median (minimum, maximum), and comparisons among groups were conducted using the Kruskal–Wallis test. Categorical variables were reported as number of cases and percentage (%), with the χ2 test for inter-group comparisons. Spearman correlation analysis was implemented to assess the correlations of UA, FIB and Hcy with cIMT and plaque score. The predictive value of UA, FIB, and Hcy for CAS was assessed using receiver operating characteristic (ROC) curve. P value was calculated using a two-sided test, and differences were considered statistically significant at P < 0.05.
No prominent differences in age, sex, BMI, and drinking history were observed among the Non-sclerosis, Thickened, and Plaque groups (all P > 0.05), while there were significant differences in smoking, SBP, DBP, TG, cIMT, and CAP in the three groups (Non-sclerosis group < Thickened group < Plaque group) (all P < 0.05). Disease course and TC did not exhibit significant differences between the Non-sclerosis group and the Thickened group (all P > 0.05), whereas significant differences in disease course and TC were observed in the Plaque group when compared to both the Non-sclerosis and Thickened groups (all P < 0.05, Table 1).
We compared serum UA, FIB and Hcy levels in three groups, with the results showing that serum UA, FIB and Hcy levels in the Non-sclerosis group were (385.74 ± 17.10) μmol/L, [5.64 (3.80–8.36)]g/L, [13.72 (6.06–23.70)] μmol/L, respectively, those in the Thickened group were (434.62 ± 27.56) μmol/L, [6.48 (4.23–13.80)] g/L, and [16.00 (6.79–23.64)] μmol/L, and those in the Plaque group were (478.78 ± 48.23) μmol/L, [8.05 (4.16–14.82)] g/L, and [26.43 (10.96–44.24)] μmol/L, respectively. These results demonstrated that UA, FIB and Hcy levels were distinctly elevated in the Plaque group relative to the Non-sclerosis and Thickened groups (Figures 1A–C, all P < 0.001).
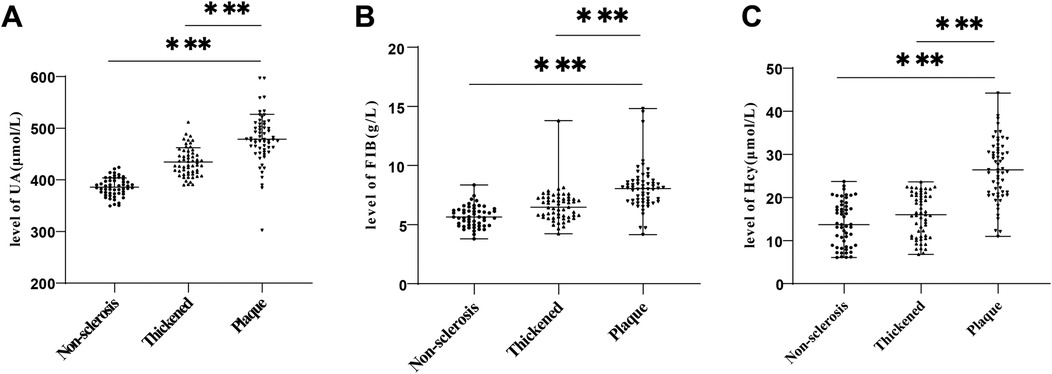
Figure 1. Comparisons of UA, FIB and Hcy levels in the three groups of hypertensive patients. (A) Comparison of UA levels; (B) Comparison of FIB levels; (C) Comparison of Hcy levels. Inter-group comparisons of normally distributed measurement data were conducted using one-way ANOVA. Inter-group comparisons of measurement data with non-normal distribution were performed using Kruskal–Wallis test. *** P < 0.001. UA, Uric acid; FIB, fibrinogen; Hcy, homocysteine.
There were significant differences in serum UA, FIB and Hcy levels among the three groups of hypertensive patients. Spearman's correlation coefficient was conducted to assess the correlations of UA, FIB and Hcy with CAS markers (cIMT and CAP), with the results showing that cIMT was favorably lined with UA (r = 0.757, P < 0.001), FIB (r = 0.598, P < 0.001) and Hcy (r = 0.620, P < 0.001) levels (Figures 2A–C), and CAP was positively interrelated with UA (r = 0.718, P < 0.001), FIB (r = 0.532, P < 0.001) and Hcy (r = 0.614, P < 0.001) levels (Figures 2D–F).
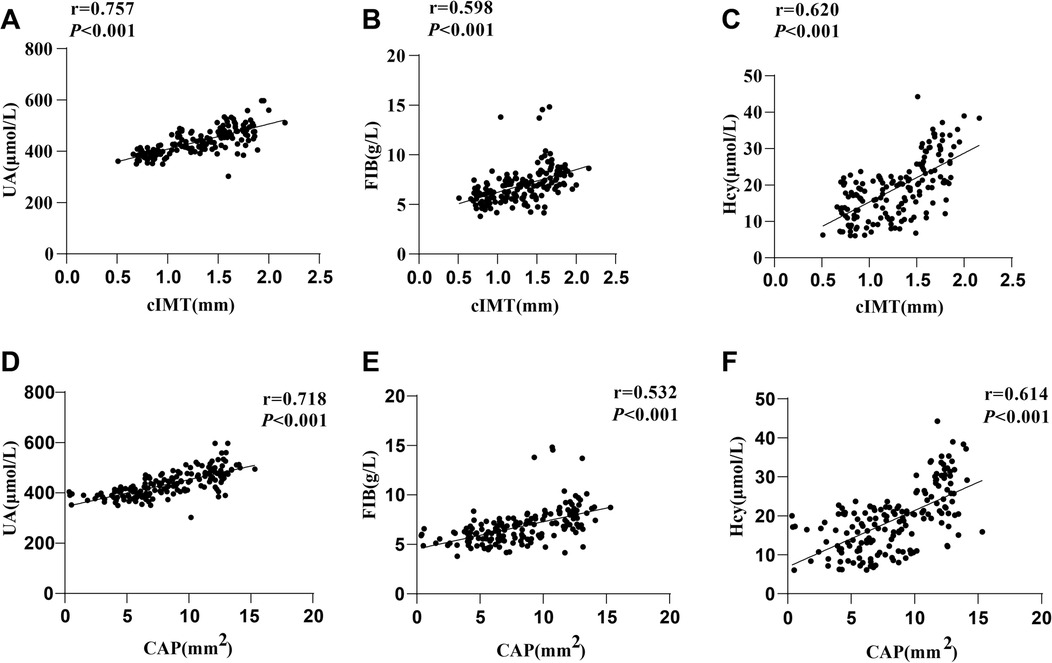
Figure 2. Correlation analyses. Spearman's correlation coefficient was used to analyze the correlations of cIMT with (A) UA, (B) FIB, and (C) Hcy, as well as the correlations of CAP with (D) UA, (E) FIB, and (F) Hcy. UA, Uric acid; FIB, fibrinogen; Hcy, homocysteine.
To probe whether UA, FIB and Hcy were risk factors for CAS, CAS was included as the dependent variable, and the indicators with P < 0.05 in Table 1 and Figure 1 were included as independent variables in the logistic univariate regression analysis model one by one. The results unveiled that disease course, smoking, diabetes, SBP, DBP, TG, TC, UA, FIB and Hcy levels were independent risk factors for CAS (all P < 0.05, Table 2). After adjusting for confounding variables and performing multicollinearity analysis (Table 3), variables with P < 0.05 and variance inflation factor (VIF) values <10 were incorporated in the logistic multivariate regression analysis model. As reflected by the results, SBP, DBP, TG, TC, UA, FIB, and Hcy levels were independent risk factors for CAS (all P < 0.05, Table 2).
ROC curves were plotted to assess the predictive value of UA, FIB and Hcy levels for CAS. As listed in Table 4 and Figure 3, area under the curve (AUC) for the UA level to predict CAS was 0.889 (sensitivity of 83.33%, specificity 86.36%, and cut-off value of 448.04 μmol/L), that for the FIB level was 0.855 (sensitivity 91.67%, specificity 66.36%, cut-off value 6.52 g/L), and that for the Hcy level was 0.902, with the sensitivity 66.67%, specificity 98.18%, and cut-off value 22.95 μmol/L. Subsequently, ROC curves were plotted to analyze the predictive value of combination of serum UA, FIB, and Hcy levels in hypertensive patients (a diagnostic panel model for UA, FIB, and Hcy was established, referred to as Combination) for CAS. It was found that the combination of UA, FIB and Hcy levels had an AUC of 0.958 (optimal sensitivity 90.00%, specificity 92.73%), indicating that the predictive value of the combination of the three factors was higher than that of each factor alone (all P < 0.01). Additionally, the patients were arranged into the low/high expression groups based on the cutoff values of UA, FIB and Hcy levels to compare the incidence of CAS. As shown in Table 5, highly-expressed UA, FIB or Hcy was more likely to lead to CAS.

Figure 3. ROC curve. ROC curves were plotted to assess the predictive value of UA, FIB, and Hcy levels for CAS. UA, Uric acid; FIB, fibrinogen; Hcy, homocysteine.
Hypertension is not solely a risk factor for cerebral, heart, and kidney diseases, but also serves as an autonomous risk factor for atherosclerosis (21, 22). Hypertension and atherosclerosis are widely recognized as the leading causes of mortality in both rich and poor nations (23). CAS is a significant manifestation of atherosclerosis, which is primarily bound up with the onset of stroke, and there are no specific preventive measures for CAS, apart from the implementation of established cardiovascular risk reduction strategies (24). Therefore, it is imperative to identify and implement efficacious strategies for CAS. Our findings highlighted that elevated levels of UA, FIB and Hcy might serve as predictive markers for CAS in hypertensive patients. Furthermore, the combined assessment of all these three biomarkers demonstrated greater predicative value compared to their individual evaluation.
Serum UA has been found to be correlated with various cardiovascular risk factors, including subclinical atherosclerosis and metabolic syndrome (25–27). Elevated level of UA have been recognized as a significant risk factor for conditions such as hypertension, and it has been observed that fluctuating UA level can not only trigger inflammation in the coronary arteries, but also plays a role in the progression of atherosclerosis (28). UA level exhibits positive correlations with BMI, TC, TG, SBP, DBP, and is independent risk factors for CAS in patients diagnosed with T2DM (29). FIB, as an inflammatory marker, plays a direct part in the development of atherosclerosis (30). It has been discovered that interaction between FIB and P-selectin pitches in the progression of atherosclerosis, and enhanced FIB level is a potential risk factor for atherosclerotic biomarkers (31). Another key focus of our study, Hcy is also recognized as an autonomous risk factor for atherosclerosis, a condition featured by the buildup of lipids within atherosclerotic plaques (32). It has been documented to expedite the progression of atherosclerosis and elicit vascular inflammation through the upregulation of inflammatory genes (33). A recent study has also unraveled that elevated serum Hcy level is tightly tied up with the severity of CAS (34). In this study, comparisons were made in terms of the levels of serum UA, FIB and Hcy among the three distinct groups of hypertensive patients, with the results showing that the levels of UA, FIB, and Hcy were markedly elevated in the Plaque group when compared to the Non-sclerosis and Thickened groups. Moreover, the multivariate regression model was used to identify independent risk factors for CAS. After adjusting for possible confounders such as age, sex, obesity, and diabetes history (35–37) and eliminating the effects of multicollinearity, logistic multivariate regression analysis results revealed that SBP, DBP, TG, TC, UA, FIB and Hcy levels were independent risk factors for CAS.
Ultrasound examination of the carotid arteries to determine cIMT and CAP predicts the risk of cardiovascular disease, and as a proxy for atherosclerotic disease, CAP contributes to approximately 20% of the risk associated with stroke and coronary artery diseases (38, 39). A recent study has demonstrated a pronounced correlation between serum UA level and cIMT (40). FIB level exhibits a favorable correlation with maximal cIMT, and there is an increased prevalence of carotid plaque in patients with raised FIB level (41). Hcy level is found to be significantly elevated in hypertensive patients with CAP compared to those without plaque, and elevated plasma Hcy level may play a role in the development of CAS (42). We conducted a Spearman correlation analysis to assess the relationships between UA, FIB and Hcy levels with the markers of CAS (cIMT and CAP). The findings indicated positive correlations between cIMT and the levels of UA, FIB and Hcy. Additionally, there were positive correlations between CAP and the levels of UA, FIB and Hcy. Elevated levels of UA, FIB, or Hcy were found to be associated with an increased risk of CAS. Subsequently, the predictive value of serum UA, FIB, or Hcy levels for CAS was assessed by plotting a ROC curve. The results revealed that the combined measurement of UA, FIB, and Hcy levels exhibited a greater predictive value for CAS compared to the individual measurements of these three biomarkers alone.
Although our findings indicated that UA, FIB, and Hcy levels in hypertensive patients were predictive of CAS, the dynamic changes in these biomarkers warranted further investigation. Many studies have explored the effect of antihypertensive medications on UA, FIB, and Hcy levels. For example, long-term use of diuretics has been linked to the development of hyperuricemia (43). Antihypertensive therapy utilizing calcium channel blockers has been associated with a reduction in the serum UA level (44). Angiotensin II receptor blockers have demonstrated efficacy in lowering the serum UA level in hypertensive patients (45). Diuretics, beta-blockers and alpha-1 blockers decrease glomerular filtration rate, while simultaneously elevating the serum UA level. In contrast, calcium channel blockers, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers, including losartan, do not appear to increase serum UA levels (46). However, it has been documented that among the five angiotensin II receptor blockers, only losartan significantly reduces serum UA levels within one year (47). Angiotensin II receptor blockers, including losartan and candesartan, have been shown to down-regulate FIB levels in hypertensive patients (48). A prior study has reported that the combination of angiotensin converting enzyme inhibitor delapril and the calcium channel blocker manidipine results in a reduction in the FIB level in hypertensive patients (49). Diuretics up-regulate the Hcy level, whereas beta-blockers and angiotensin converting enzyme inhibitors down-regulate Hcy in hypertensive patients (50). Additionally, long-term administration of calcium channel blockers has been found to remarkably diminish Hcy levels in hypertensive patients (51). The alterations in UA, FIB, and Hcy levels resulting from antihypertensive medications may introduce potential confounding effects on our findings.
Taken together, our study highlighted that elevated levels of UA, FIB and Hcy might serve as predictive markers for CAS in hypertensive patients. Furthermore, the combined assessment of all these three biomarkers demonstrated greater prognostic value compared to their individual evaluation. However, this article also had several important limitations to consider. First, the limited number of cases and events, as well as its single-center design, constrained the cross-sectional design. Both female sex and menopausal status have been shown to influence levels of UA, FIB and Hcy in hypertensive patients. Specifically, premenopausal estrogen level in women may enhance enhance renal clearance of urate, while serum UA concentrations are, on average, approximately 1 mg/dl higher in adult men compared to women (52). Additionally, FIB level is consistently elevated in women relative to men and tend to increase following menopause, which is associated with hormone deficiency in menopause elderly women (53, 54). Furthermore, plasma Hcy level is lower in women of childbearing age relative to their male counterparts and postmenopausal women, with the heightened risk of cardiovascular disease in postmenopausal women being linked with elevated Hcy levels (55). However, due to the limitations of single-centre and small sample size of this study, it was not possible to conduct separate analyses based on sex or menopausal status. Secondly, this study did not analyze the comorbidity of hyperhomocysteinemia in hypertensive patients different sexes nor did it explore the correlation between different Hcy subgroups and CAS, such as in patients with mild to moderate hyperhomocysteinemia. Thirdly, a related study examining patients with moderate or mild hyperhomocysteinemia found that the risk factor for CAS in patients with moderate hyperhomocysteinemia could be elevated to 1.4 (56), suggesting that varying degrees of hyperhomocysteinemia exert distinct influences on the onset and progression of CAS. Fourthly, our analysis on potential confounders, including the administration of antihypertensive medication, dietary habits, socioeconomic status, and genetic predisposition, was not comprehensive enough. As indicated in the previous studies, vegetarians display a lower level of serum high-density lipoprotein than individuals adhering to conventional diets. Moreover, vegetarians show an improved ability to combat atherosclerosis through the supplementation of vitamin B or folic acid (9, 57). Untreated hypertensive men residing in deprived neighborhoods are more likely to develop preclinical CAS compared to their counterparts living in affluent areas (58). Genetic predispositions also play a significant role in the incidence of CAS. For instance, The variant associated with coronary heart disease located at the 10q11.21 locus has been linked to cIMT and atherosclerosis (59). Consequently, it is imperative to extend the analysis of CAS occurrence among hypertensive patients in different Hcy subgroups to gain deeper insights into the clinical implications of Hcy level on CAS in hypertensive patients. In future research, we intend to expand the sample size and conduct a multicenter study to improve the understanding of the predictive capacity of UA, FIB and Hcy for hypertensive patients regarding CAS. Additional patient data will be collected and include incorporated into the analyses to minimize the influence of confounding factors that may impact the study's results. Additionally, we will further refine the clinical data through telephone follow-ups and analyze the effects of lipid-lowering therapy in subsequent studies.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
The studies involving humans were approved by the Academic Ethics Committee of Heping hospital affiliated to Changzhi Medical College [No. (2024)011]. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because this study was a retrospective study, no informed consent was signed.
LZ: Methodology, Resources, Validation, Visualization, Writing – review & editing. SL: Data curation, Formal Analysis, Investigation, Software, Writing – review & editing. JG: Conceptualization, Project administration, Supervision, Writing – original draft.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1433107/full#supplementary-material
Supplementary Figure S1 | The minimum sample size was calculated using G*Power software.
Supplementary Figure S2 | Flowchart.
1. O’Shea PM, Griffin TP, Fitzgibbon M. Hypertension: the role of biochemistry in the diagnosis and management. Clin Chim Acta. (2017) 465:131–43. doi: 10.1016/j.cca.2016.12.014
2. Ortega Anta RM, Jimenez Ortega AI, Perea Sanchez JM, Cuadrado Soto E, Lopez Sobaler AM. Nutritional patterns on prevention and control of hypertension. Nutr Hosp. (2016) 33(Suppl 4):347. doi: 10.20960/nh.347
3. Stamler J. Blood pressure and high blood pressure. Aspects of risk. Hypertension. (1991) 18(3 Suppl):I95–107. doi: 10.1161/01.hyp.18.3_suppl.i95
4. Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. (2020) 75(2):285–92. doi: 10.1161/HYPERTENSIONAHA.119.14240
5. Walubembe J, Ssinabulya I, Mubuuke AG, Kagwa MM, Babirye D, Okot J, et al. Carotid Doppler findings among patients admitted with stroke in two tertiary care facilities in Uganda: a hospital-based cross-sectional study. Res Sq [Preprint]. (2023). doi: 10.21203/rs.3.rs-2800534/v1
6. Shankar A, Wang JJ, Rochtchina E, Mitchell P. Positive association between plasma fibrinogen level and incident hypertension among men: population-based cohort study. Hypertension. (2006) 48(6):1043–9. doi: 10.1161/01.HYP.0000245700.13817.3c
7. Rodrigo R, Passalacqua W, Araya J, Orellana M, Rivera G. Homocysteine and essential hypertension. J Clin Pharmacol. (2003) 43(12):1299–306. doi: 10.1177/0091270003258190
8. Copur S, Demiray A, Kanbay M. Uric acid in metabolic syndrome: does uric acid have a definitive role? Eur J Intern Med. (2022) 103:4–12. doi: 10.1016/j.ejim.2022.04.022
9. Carnagarin R, Nolde JM, Ward NC, Lugo-Gavidia LM, Chan J, Robinson S, et al. Homocysteine predicts vascular target organ damage in hypertension and may serve as guidance for first-line antihypertensive therapy. J Clin Hypertens. (2021) 23(7):1380–9. doi: 10.1111/jch.14265
10. Mastroiacovo D, Ettorre E, Mengozzi A, Virdis A, Camerota A, Muselli M, et al. Serum uric acid levels are associated with the echogenic features of carotid plaque vulnerability in elderly patients with atherosclerotic disease. Metabolites. (2023) 13(6):693. doi: 10.3390/metabo13060693
11. Kushiyama A, Nakatsu Y, Matsunaga Y, Yamamotoya T, Mori K, Ueda K, et al. Role of uric acid metabolism-related inflammation in the pathogenesis of metabolic syndrome components such as atherosclerosis and nonalcoholic steatohepatitis. Mediators Inflamm. (2016) 2016:1–15. doi: 10.1155/2016/8603164
12. Gao DL, Johal MS. LRP-1 binds fibrinogen in a sialylation-dependent manner: a quartz crystal microbalance study. Langmuir. (2023) 39(30):10375–82. doi: 10.1021/acs.langmuir.3c00629
13. Schreiner PJ, Appiah D, Folsom AR. Gamma prime (gamma’) fibrinogen and carotid intima-media thickness: the atherosclerosis risk in communities study. Blood Coagul Fibrinolysis. (2017) 28(8):665–9. doi: 10.1097/MBC.0000000000000659
14. Li Y, Zhao Q, Cao Y, Si J, Li J, Cao K, et al. Probucol decreases homocysteine-stimulated CRP production in rat aortic smooth muscle cells via regulating HO-1/NADPH oxidase/ROS/p38 pathway. Acta Biochim Biophys Sin. (2020) 53(2):212–9. doi: 10.1093/abbs/gmaa163
15. Sabio JM, Vargas-Hitos JA, Mediavilla JD, Navarrete-Navarrete N, Zamora-Posadas M, Perez-Vicente S, et al. Correlation of asymptomatic hyperuricaemia and serum uric acid levels with arterial stiffness in women with systemic lupus erythematosus without clinically evident atherosclerotic cardiovascular disease. Lupus. (2010) 19(5):591–8. doi: 10.1177/0961203309355301
16. Hamed SA, Hamed EA, Hamdy R, Nabeshima T. Vascular risk factors and oxidative stress as independent predictors of asymptomatic atherosclerosis in adult patients with epilepsy. Epilepsy Res. (2007) 74(2–3):183–92. doi: 10.1016/j.eplepsyres.2007.03.010
17. Wolk MJ, Bailey SR, Doherty JU, Douglas PS, Hendel RC, Kramer CM, et al. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease. J Am Coll Cardiol. (2014) 63(4):380–406. doi: 10.1016/j.jacc.2013.11.009
18. Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, et al. Mannheim carotid intima-media thickness and plaque consensus (2004–2006–2011). Cerebrovasc Dis. (2012) 34(4):290–6. doi: 10.1159/000343145
19. Luna-Marco C, de Maranon AM, Hermo-Argibay A, Rodriguez-Hernandez Y, Hermenejildo J, Fernandez-Reyes M, et al. Effects of GLP-1 receptor agonists on mitochondrial function, inflammatory markers and leukocyte-endothelium interactions in type 2 diabetes. Redox Biol. (2023) 66:102849. doi: 10.1016/j.redox.2023.102849
20. Ebrahim S, Papacosta O, Whincup P, Wannamethee G, Walker M, Nicolaides AN, et al. Carotid plaque, intima media thickness, cardiovascular risk factors, and prevalent cardiovascular disease in men and women: the British regional heart study. Stroke. (1999) 30(4):841–50. doi: 10.1161/01.STR.30.4.841
22. Liu K, Chen B, Zeng F, Wang G, Wu X, Liu Y, et al. Apoe/NOS3 knockout mice as a novel cardiovascular disease model of hypertension and atherosclerosis. Genes. (2022) 13(11):1998. doi: 10.3390/genes13111998
23. Leong XF, Ng CY, Jaarin K. Animal models in cardiovascular research: hypertension and atherosclerosis. Biomed Res Int. (2015) 2015:1–11. doi: 10.1155/2015/528757
24. Whayne TF Jr. Prevention of carotid artery atherosclerosis: what is the evidence? Angiology. (2017) 68(8):661–8. doi: 10.1177/0003319716669460
25. Lim DH, Lee Y, Park GM, Choi SW, Kim YG, Lee SW, et al. Serum uric acid level and subclinical coronary atherosclerosis in asymptomatic individuals: an observational cohort study. Atherosclerosis. (2019) 288:112–7. doi: 10.1016/j.atherosclerosis.2019.07.017
26. Li X, Meng X, He Y, Spiliopoulou A, Timofeeva M, Wei WQ, et al. Genetically determined serum urate levels and cardiovascular and other diseases in UK biobank cohort: a phenome-wide Mendelian randomization study. PLoS Med. (2019) 16(10):e1002937. doi: 10.1371/journal.pmed.1002937
27. Dehlin M, Jacobsson L, Roddy E. Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. Nat Rev Rheumatol. (2020) 16(7):380–90. doi: 10.1038/s41584-020-0441-1
28. Kimura Y, Tsukui D, Kono H. Uric acid in inflammation and the pathogenesis of atherosclerosis. Int J Mol Sci. (2021) 22(22):12394. doi: 10.3390/ijms222212394
29. Li W, Wang Y, Ouyang S, Li M, Liu R, Zhang Y, et al. Association between serum uric acid level and carotid atherosclerosis and metabolic syndrome in patients with type 2 diabetes mellitus. Front Endocrinol. (2022) 13:890305. doi: 10.3389/fendo.2022.890305
30. Lu P, Liu J, Pang X. Pravastatin inhibits fibrinogen- and FDP-induced inflammatory response via reducing the production of IL-6, TNF-alpha and iNOS in vascular smooth muscle cells. Mol Med Rep. (2015) 12(4):6145–51. doi: 10.3892/mmr.2015.4149
31. Zhou BR, Pan Y, Zhai ZM. Fibrinogen and P-selectin expression in atherosclerosis model of Sprague Dawley rat. Chin Med J. (2011) 124(22):3768–72.22340239
32. Guo W, Zhang H, Yang A, Ma P, Sun L, Deng M, et al. Homocysteine accelerates atherosclerosis by inhibiting scavenger receptor class B member1 via DNMT3b/SP1 pathway. J Mol Cell Cardiol. (2020) 138:34–48. doi: 10.1016/j.yjmcc.2019.11.145
33. Elsherbiny NM, Sharma I, Kira D, Alhusban S, Samra YA, Jadeja R, et al. Homocysteine induces inflammation in retina and brain. Biomolecules. (2020) 10(3):393. doi: 10.3390/biom10030393
34. Jeon SB, Kang DW, Kim JS, Kwon SU. Homocysteine, small-vessel disease, and atherosclerosis: an MRI study of 825 stroke patients. Neurology. (2014) 83(8):695–701. doi: 10.1212/WNL.0000000000000720
35. Nurmohamed NS, Min JK, Anthopolos R, Reynolds HR, Earls JP, Crabtree T, et al. Atherosclerosis quantification and cardiovascular risk: the ISCHEMIA trial. Eur Heart J. (2024) 45(36):3735–47. doi: 10.1093/eurheartj/ehae471
36. Budoff MJ, Bhatt DL, Kinninger A, Lakshmanan S, Muhlestein JB, Le VT, et al. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: final results of the EVAPORATE trial. Eur Heart J. (2020) 41(40):3925–32. doi: 10.1093/eurheartj/ehaa652
37. Ambale-Venkatesh B, Yang X, Wu CO, Liu K, Hundley WG, McClelland R, et al. Cardiovascular event prediction by machine learning: the multi-ethnic study of atherosclerosis. Circ Res. (2017) 121(9):1092–101. doi: 10.1161/CIRCRESAHA.117.311312
38. Naqvi TZ, Lee MS. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging. (2014) 7(10):1025–38. doi: 10.1016/j.jcmg.2013.11.014
39. Kopczak A, Schindler A, Bayer-Karpinska A, Koch ML, Sepp D, Zeller J, et al. Complicated carotid artery plaques as a cause of cryptogenic stroke. J Am Coll Cardiol. (2020) 76(19):2212–22. doi: 10.1016/j.jacc.2020.09.532
40. Cicero AF, Salvi P, D'Addato S, Rosticci M, Borghi C. Brisighella heart study g. Association between serum uric acid, hypertension, vascular stiffness and subclinical atherosclerosis: data from the Brisighella heart study. J Hypertens. (2014) 32(1):57–64. doi: 10.1097/HJH.0b013e328365b916
41. De Luca G, Verdoia M, Cassetti E, Schaffer A, Cavallino C, Bolzani V, et al. High fibrinogen level is an independent predictor of presence and extent of coronary artery disease among Italian population. J Thromb Thrombolysis. (2011) 31(4):458–63. doi: 10.1007/s11239-010-0531-z
42. Catena C, Colussi G, Url-Michitsch M, Nait F, Sechi LA. Subclinical carotid artery disease and plasma homocysteine levels in patients with hypertension. J Am Soc Hypertens. (2015) 9(3):167–75. doi: 10.1016/j.jash.2014.12.020
43. Maloberti A, Dell'Oro R, Bombelli M, Quarti-Trevano F, Facchetti R, Mancia G, et al. Long-term increase in serum uric acid and its predictors over a 25 year follow-up: results of the PAMELA study. Nutr Metab Cardiovasc Dis. (2024) 34(1):223–9. doi: 10.1016/j.numecd.2023.10.009
44. Zhang D, Huang QF, Sheng CS, Li Y, Wang JG. Serum uric acid change in relation to antihypertensive therapy with the dihydropyridine calcium channel blockers. Blood Press. (2021) 30(6):395–402. doi: 10.1080/08037051.2021.1996220
45. Horio T, Iwashima Y, Yoshiyama M, Fukuda D, Hasegawa T, Fujimoto K. Serum uric acid-lowering effect of sacubitril/valsartan in hypertensive patients: evaluation by switching from angiotensin II receptor blockers. Blood Press Monit. (2024) 29(6):305–11. doi: 10.1097/MBP.0000000000000725
46. Ueno S, Hamada T, Taniguchi S, Ohtani N, Miyazaki S, Mizuta E, et al. Effect of antihypertensive drugs on uric acid metabolism in patients with hypertension: cross-sectional cohort study. Drug Res. (2016) 66(12):628–32. doi: 10.1055/s-0042-113183
47. Nishida Y, Takahashi Y, Susa N, Kanou N, Nakayama T, Asai S. Comparative effect of angiotensin II type I receptor blockers on serum uric acid in hypertensive patients with type 2 diabetes mellitus: a retrospective observational study. Cardiovasc Diabetol. (2013) 12:159. doi: 10.1186/1475-2840-12-159
48. Rayner BL, Trinder YA, Baines D, Isaacs S, Opie LH. Effect of losartan versus candesartan on uric acid, renal function, and fibrinogen in patients with hypertension and hyperuricemia associated with diuretics. Am J Hypertens. (2006) 19(2):208–13. doi: 10.1016/j.amjhyper.2005.08.005
49. Fogari R, Derosa G, Zoppi A, Lazzari P, Corradi L, Preti P, et al. Effect of delapril/manidipine vs olmesartan hydrochlorothiazide combination on insulin sensitivity and fibrinogen in obese hypertensive patients. Intern Med. (2008) 47(5):361–6. doi: 10.2169/internalmedicine.47.0449
50. Poduri A, Kaur J, Thakur JS, Kumari S, Jain S, Khullar M. Effect of ACE inhibitors and beta-blockers on homocysteine levels in essential hypertension. J Hum Hypertens. (2008) 22(4):289–94. doi: 10.1038/sj.jhh.1002325
51. Wang B, Wu H, Li Y, Ban Q, Huang X, Chen L, et al. Effect of long-term low-dose folic acid supplementation on degree of total homocysteine-lowering: major effect modifiers. Br J Nutr. (2018) 120(10):1122–30. doi: 10.1017/S0007114518002477
52. Nicholls A, Snaith ML, Scott JT. Effect of oestrogen therapy on plasma and urinary levels of uric acid. Br Med J. (1973) 1(5851):449–51. doi: 10.1136/bmj.1.5851.449
53. Folsom AR. Epidemiology of fibrinogen. Eur Heart J. (1995) 16(Suppl A):21–3. doi: 10.1093/eurheartj/16.suppl_A.21
54. Du W, Wang Z, Dong Y, Hu J, Chen X. Association between fibrinogen and bone mineral density in postmenopausal women. J Orthop Surg Res. (2023) 18(1):376. doi: 10.1186/s13018-023-03785-7
55. De Leo V, la Marca A, Morgante G, Musacchio MC, Luisi S, Petraglia F. Menopause, the cardiovascular risk factor homocysteine, and the effects of treatment. Treat Endocrinol. (2004) 3(6):393–400. doi: 10.2165/00024677-200403060-00007
56. Zhao J, Chen H, Liu N, Chen J, Gu Y, Chen J, et al. Role of hyperhomocysteinemia and hyperuricemia in pathogenesis of atherosclerosis. J Stroke Cerebrovasc Dis. (2017) 26(12):2695–9. doi: 10.1016/j.jstrokecerebrovasdis.2016.10.012
57. Dinu M, Abbate R, Gensini GF, Casini A, Sofi F. Vegetarian, vegan diets and multiple health outcomes: a systematic review with meta-analysis of observational studies. Crit Rev Food Sci Nutr. (2017) 57(17):3640–9. doi: 10.1080/10408398.2016.1138447
58. Petersen KL, Bleil ME, McCaffery J, Mackey RH, Sutton-Tyrrell K, Muldoon MF, et al. Community socioeconomic status is associated with carotid artery atherosclerosis in untreated, hypertensive men. Am J Hypertens. (2006) 19(6):560–6. doi: 10.1016/j.amjhyper.2005.12.008
59. Kiechl S, Laxton RC, Xiao Q, Hernesniemi JA, Raitakari OT, Kahonen M, et al. Coronary artery disease-related genetic variant on chromosome 10q11 is associated with carotid intima-media thickness and atherosclerosis. Arterioscler Thromb Vasc Biol. (2010) 30(12):2678–83. doi: 10.1161/ATVBAHA.110.213785
Keywords: serum uric acid, fibrinogen, homocysteine, hypertension, carotid atherosclerosis, carotid intima-media thickness, carotid atherosclerotic plaque area, receiver operating characteristic curve
Citation: Zhang L, Lu S and Guo J (2025) Correlations of serum uric acid, fibrinogen and homocysteine levels with carotid atherosclerosis in hypertensive patients. Front. Cardiovasc. Med. 12:1433107. doi: 10.3389/fcvm.2025.1433107
Received: 15 May 2024; Accepted: 23 January 2025;
Published: 3 March 2025.
Edited by:
Nhat Tu Le, Houston Methodist Research Institute, United StatesReviewed by:
Satyesh K. Sinha, University of California, Los Angeles, United StatesCopyright: © 2025 Zhang, Lu and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juanjuan Guo, anVhbl9kYjJfY3pAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.