
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 10 February 2025
Sec. Heart Failure and Transplantation
Volume 12 - 2025 | https://doi.org/10.3389/fcvm.2025.1418914
 Dae-Young Kim1,†
Dae-Young Kim1,† Sang-Won Lee2,†
Sang-Won Lee2,† Dong-Ho Lee3
Dong-Ho Lee3 Sang-Chul Lee3,4
Sang-Chul Lee3,4 Ji-Hun Jang1
Ji-Hun Jang1 Sung-Hee Shin1
Sung-Hee Shin1 Dae-Hyeok Kim1,3
Dae-Hyeok Kim1,3 Wonik Choi3,5
Wonik Choi3,5 Yong-Soo Baek1,3,6*
Yong-Soo Baek1,3,6*
Background: Heart failure with mildly reduced ejection fraction (HFmrEF) has emerged as the predominant subtype of heart failure (HF). This study aimed to develop artificial intelligence (AI)-electrocardiography (ECG) to identify and predict the prognosis of patients with HFmrEF.
Methods: We collected 104,336 12-lead ECG datasets from April 2009 to December 2021 in a tertiary centre. The AI-ECG encompasses a novel model that combines an automatic labelling preprocessing method with a transformer architecture incorporating a triplet loss for HFmrEF analysis.
Results: The receiver operating characteristic analyses revealed that the area under the curve of AI-ECG for identifying all types of HF was acceptable [0.873, 95% confidence interval (CI): 0.864–0.893], while that for identifying patients with HFmrEF was relatively lower (0.824, 95% CI: 0.794–0.863) than that for those with HF with reduced ejection fraction (EF) (0.875, 95% CI: 0.844–0.912) and those with normal EF (0.870, 95% CI: 0.842–0.894). The analysis of ECG features showed significant increases in QRS duration (p = 0.001), QT interval (p = 0.045), and corrected QT interval (p = 0.041) with increasing “Severity by Euclidean distance”. Following the predictability analysis with another group of 953 patients for improvements of follow-up EF in HFmrEF, the patients were grouped into three clusters based on the AI-Euclidean distance; Cluster 1 had the most severe cases and poorer outcomes than Clusters 2 (p < 0.001) and 3 (p < 0.001).
Conclusions: AI-ECG presents an innovative approach for the prognostic stratification of cardiac contractility in patients with HFmrEF. In patients with HFmrEF, disease progression can be predicted using AI-ECG.
Heart failure (HF) with mildly reduced ejection fraction (HFmrEF) is defined as left ventricular (LV) ejection fraction (EF) between 41% and 49% (1); its prevalence is 10%–25% among patients with HF, and it is currently receiving attention for its clinical implications, including its complexity, heterogeneity, and dynamic features (2). Patients with HFmrEF have unique heterogeneous characteristics that may be enhanced with HF with improved EF (HFimpEF) or may be exacerbated by HF with reduced ejection fraction (HFrEF) (3, 4). Therefore, factors predicting LVEF improvement or deterioration in HFmrEF can be considered important variables (5).
Patients with HFmrEF had a relatively better LV systolic function than those with HFrEF. However, based on previous studies, it is difficult to conclude whether the prognosis of HFmrEF is absolutely better than that of HFrEF (6). Their EFs often change over time compared to those with other types of HF, and patients who progress from HFmrEF to HFrEF show a poorer prognosis than those who remain with the same type of HFmrEF or transition to HFimpEF (7). Therefore, predicting LV contractility before disease progression has significant implications for the clinical prognosis in these populations. The echocardiographic evaluation of LV EF is important in the serial follow-up of patients with HFmrEF. Because the clinical status is often not correlated with the current cardiac contractility, the diagnosis is sometimes delayed, and the regular examination with echocardiography is less effective in terms of time and cost in some patients. Thus, it would be helpful to identify the LV EF before performing echocardiography; however, to date, clinical or diagnostic devices to identify patients with HFmrEF other than echocardiography have not been developed.
Deep learning models have been introduced to detect HF using various electrocardiography (ECG) features (8–10). Several ECG signals have different characteristics according to HF types because their findings are associated with the process of cardiac remodelling and structural changes. It is possible to identify the types of HF using artificial intelligence (AI)-based ECG. AI-ECG has been validated for detecting HFrEF and HFpEF. However, there is a lack of data on identifying HFmrEF and predicting its prognosis using AI-ECG. Therefore, in this study, we aimed to develop AI-ECG to identify HFmrEF and to predict the prognosis of patients with HFmrEF.
The study design is shown in Figure 1. The study population included adult patients aged over 18 years who had performed ECGs within 3 months among those who underwent echocardiography on initial examination for clinical evaluation or health check-up. Among them, we excluded subjects with atrial fibrillation and other non-sinus rhythms such as junctional or cardiac pacing rhythm to simplify the analysis and concentrated on specific signal characteristics within a controlled group. From the 48,440 patients in the study cohort, 104,336 ECGs obtained at a single tertiary centre, Inha University Hospital in South Korea from April 2009 to December 2021 were retrospectively collected and used to develop AI-ECG. According to the current criteria, patients who experienced clinical symptoms of dyspnea that were accompanied by signs of pulmonary edema or effusion on chest x-ray and elevated N-terminal pro-brain natriuretic peptide were defined as HF (11). Among them, patients whose LVEF was 40% or less on initial echocardiography were classified by HFrEF, and those with LVEF between 41% and 49% were classified by HFmrEF. There were 6,819 ECGs for HFrEF, 9,077 for HFmrEF, and 88,440 for a population whose LVEF was ≥50%, regardless of HF symptoms. For all subjects, ECG data between April 2009 and December 2020 were used for training and validation, and those between January 2021 and December 2021 were used for holdout tests. This study was performed in accordance with the Declaration of Helsinki, and the study protocol was approved by the Institutional Review Board of Inha University Hospital (IRB number: 2023-03-024). The institutional review board waived the need for the informed consent of the patients because of the retrospective nature.
Our proposed method is schematically shown in Supplementary Figure 1 and has three novel features. First, we employed a technique for learning the R-peak positions through automatic labelling and machine learning. Second, our deep learning model based on transformer architecture (12) used in BERT (13) and GPT (14) incorporated both triplet (15) and cross-entropy losses to extract a representation vector of the input ECG and enabled the classification of patients with HFrEF (EF ≤40), HFmrEF (41 ≤EF ≤49), and normal EF (EF ≥50%). Third, we analysed the characteristics of HFmrEF ECG from the extracted vectors. The smaller the Euclidean distance between the representation vector of the ECGs of patients with HFmrEF and the average representation vector of the ECGs of those with normal EF, the higher the similarity between the ECGs of the respective patients with HFmrEF and ECGs of most patients with normal EF. Euclidean distance is a measure of the straight-line distance between two points in space, calculated by summing the squares of the differences in each coordinate and taking the square root of the result. Hence, we defined the difference between the Euclidean distance of the representation vector of the ECGs of patients with HFmrEF and that of the average representation vector of those with normal EF and HFrEF as “Severity by Euclidean distance”. Further details on the first and second distinctive features are provided in the Supplementary Material.
Furthermore, we performed an additional analysis to assess the potential of our model to predict whether LVEF improved at over 50% on follow-up echocardiography in patients with HFmrEF. For this analysis, we categorised patients into groups based on the severity of AI-ECG findings, referred to as “Clusters”. We included 953 patients with HFmrEF whose ECG and echocardiographic data were not used for the training, validation, or holdout tests. These patients underwent initial ECG and echocardiography between January 2010 and March 2022. On initial echocardiography, LVEF was 41%–49%, which was compatible with HFmrEF. Regardless of the etiology of HF, patients with HFmrEF had visited the hospital regularly and had been prescribed the optimal medication for HFmrEF. They performed follow-up echocardiography 3–18 months after the initial evaluation. Cluster groupings ranged from 1 to 3, indicating varying degrees of AI-ECG severity, with Cluster 1 representing the most severe.
Continuous variables are presented as the mean ± standard deviation, and categorical variables are presented as percentages and frequencies. The clinical and ECG components were compared using a one-way analysis of variance and χ2 test for continuous and categorical variables, respectively. The performance of the AI model was evaluated using receiver operating characteristic (ROC) curves to predict the accuracy, sensitivity, specificity, and F1 score of the dataset. The F1 score (balanced F-score) is the harmonic mean of precision and recall. For all variables, statistical significance was set at p < 0.05. All statistical analyses were performed using SPSS software (version 25.0; IBM Corp, Armonk, NY, USA).
The baseline clinical characteristics and ECG components of the patients in the training, validation, and test sets are presented in Table 1. The mean age of patients was 63.0 ± 15.9 years, and 55.8% were males. The proportions of patients with hypertension, diabetes mellitus, and stroke were 5.7%, 5.3%, and 5.4%, respectively. The mean LVEF was 59.7% ± 9.5%. On ECG, the ventricular rate per minute was 78.4 ± 19.9, and the PR interval, QRS duration, and corrected QT interval (QTc) were 164.1 ± 28.3, 93.8 ± 17.4, and 442.6 ± 38.5 ms, respectively.
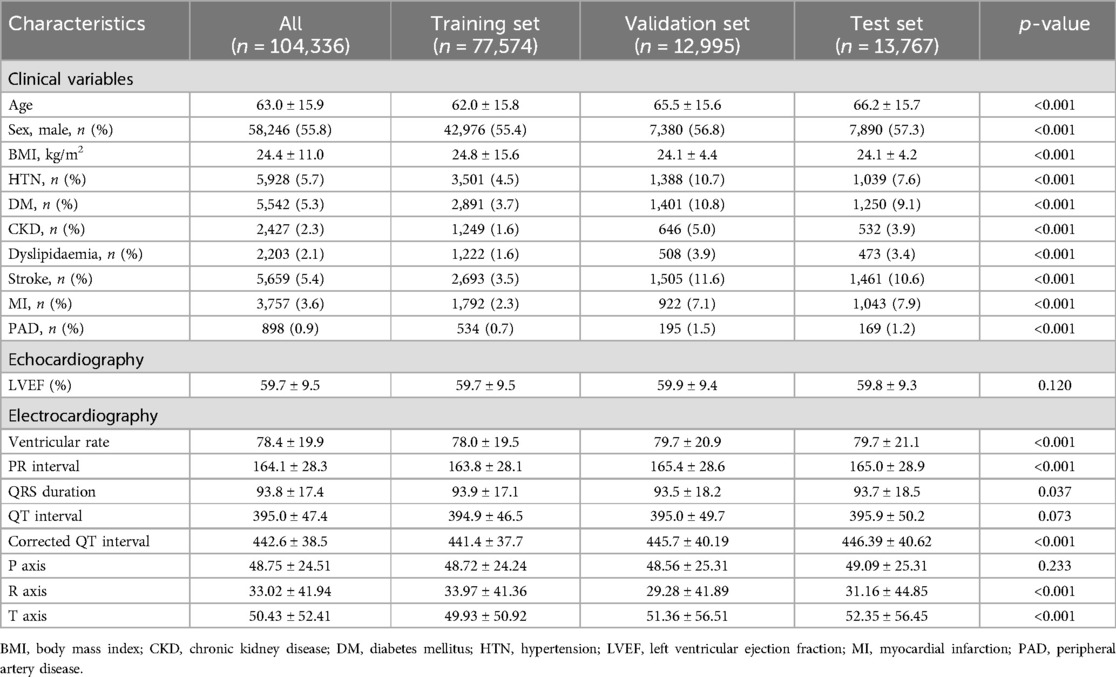
Table 1. Characteristics of the patients and laboratory results in the training, validation, and test sets.
We used the validated AI-ECG model to identify LVEF using ROC analyses. First, the predictability of the AI-ECG model was confirmed according to the three phenotypes of HF (Table 2; Figure 2). Predicting each HF phenotype of AI-ECG was acceptable. The overall accuracy was 87.3% (95% CI: 86.4–89.3) in identifying HF type: AI-ECG had the best performance in predicting patients with HFrEF [AUC 0.875, 95% confidence interval (CI): 0.844–0.912], followed by those with normal EF (AUC 0.870, 95% CI: 0.842–0.894) and HFmrEF patients (AUC 0.824, 95% CI: 0.794–0.863). Next, because we confirmed that the model had difficulty accurately classifying HFmrEF in the three-category classification, we also conducted binary classification of patients with LVEF of less than 40% (EF ≤40%) and those with more than 40% (EF >40%). The results showed that the model's areas under the curve (AUCs) for predicting these two groups were 0.86 (95% CI: 0.82–0.92) (Supplementary Figure 2).
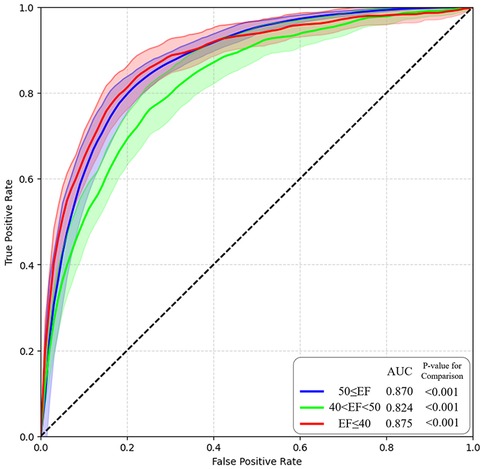
Figure 2. AI model performance and ROC curve. AI, artificial intelligence; ROC, receiver operating characteristic.
We extracted the representation vectors of the holdout test data using an AI-ECG. Figure 3 shows the transformation of the 256-dimensional representation vectors into two-dimensional data by applying T-distributed Stochastic Neighbor Embedding (T-SNE). The black, yellow, and purple stars in Figure 4 represent the mean vectors of patients with normal EF, HFmrEF, and HFrEF, respectively. Among the 13,767 holdout test dataset shown in Figure 3A, patients with normal EF occupied the largest area with 11,910 instances. With 984 instances, HFmrEF was more widely distributed because it shares characteristics with patients with normal EF and those with HFrEF. However, the HFmrEF is slightly skewed toward the HFrEF region. This indicates a higher similarity between the HFmrEF and HFrEF ECGs. The mean vector of the HFrEF of 873 patients was positioned furthest from the mean vector of the normal EF of patients and was predominantly distributed to the right. Figure 3B shows a visualisation of the representation vector extracted only from the ECGs corresponding to HFmrEF in the holdout test set. Figure 3C shows the partitioning of the HFmrEF representation vector into three clusters using k-means clustering with a parameter of k = 3. Notably, Cluster 1 resides in close proximity to the centre of the HFrEF vector, indicating the composition of patients with compromised health status. Conversely, Cluster 3, positioned closest to the centre of the normal EF vector, encompassed patients exhibiting relatively favourable health conditions. Cluster 2 was positioned between Clusters 1 and 3 but exhibited a skewed tendency toward Cluster 1. We applied the k-means clustering we learned here to follow up the external validation data and obtained intriguing results. An analysis of these findings is presented in Figure 4.
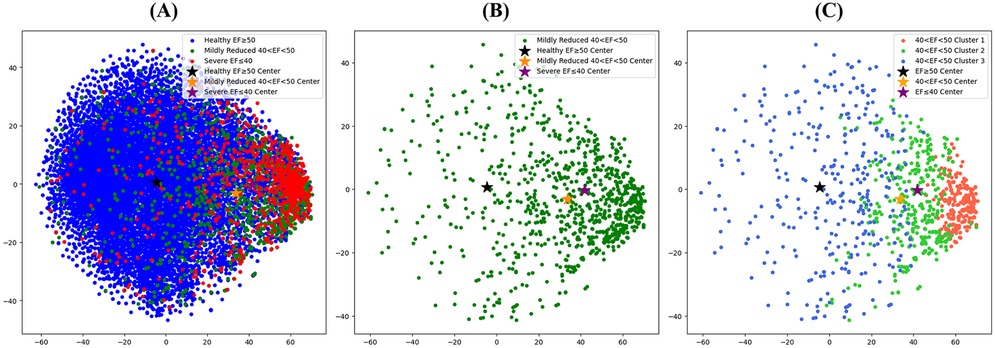
Figure 3. T-SNE visualization of the representation vectors extracted by the AI model. (A) T-SNE visualisation of the expression vectors extracted for all holdout test sets for patients with the AI-ECG model trained on the ECG dataset. (B) Visualization of representation vectors extracted from only the ECGs corresponding to HFmrEF in the holdout test set. (C) Visualization of the HFmrEF expression vector partitioned into three clusters by K-means clustering with a parameter of k = 3. AI, artificial intelligence; ECG, electrocardiography; EF, ejection fraction; HFmrEF, heart failure with mildly reduced ejection fraction; HFrEF, heart failure with reduced ejection fraction.

Figure 4. Kaplan–Meier analysis regarding improving LVEFs among the three “Cluster” groups by the AI-estimated Euclidean distance in patients with HFmrEF. AI, artificial intelligence; EF, ejection fraction; LV, left ventricle.
We analysed ECG features, such as PR, QT, QTc interval, QRS duration, and P, R, and T axes using the AI-estimated Euclidean distance to identify specific correlations with the “Severity by Euclidean distance” of the patients with HFmrEF. As a result, the QRS duration (p = 0.001), QT interval (p = 0.045), and QTc interval (p = 0.041) increased significantly with increasing “Severity by Euclidean distance” (Supplementary Figure 3).
We performed additional analysis of the three cluster groups to determine whether AI-ECG could predict the LVEF in patients with HFmrEF. In this cohort, the medication history of drugs proven to improve the prognosis in patients with HF was investigated, and the medications taken continuously for at least 3 months are presented in Supplementary Table 1. As a result, renin-angiotensin-aldosterone inhibitors were taken the most in Cluster 1. However, there was no significant difference in the proportion of beta-blockers, mineralo-receptor antagonists, and sodium-glucose cotransporter 2 inhibitors in each cluster. This meant that the medications did not show a consistently higher rate in either cluster. Kaplan–Meier analysis where the LVEF was improved by ≥50% on follow-up echocardiography revealed that Cluster 1 had poor results compared with Cluster 2 (p = 0.003) or Cluster 3 (p < 0.001) (Figure 4).
This study developed and validated AI-ECG to identify the types of HF in patients using 12-lead ECG; this demonstrated a reasonable performance in identifying patients with HFrEF and those with normal EF. Because the performance in identifying patients with HFmrEF was relatively low compared with that in identifying patients with other types of HF, we sought ECG characteristics using the AI-estimated Euclidean distance in these patients. It was revealed that the QRS duration, QT interval, and QTc interval showed significant changes according to severity. Based on these results, AI-ECG may be used to predict the prognosis of patients with HFmrEF.
Since the European Society of Cardiology Heart Failure Guidelines first recognised HFmrEF as an independent entity, different from HFrEF and HFpEF, in 2016, HFmrEF has been regarded as a major disease entity in all types of HF (16). It also poses a significant burden on global healthcare as its prevalence increases (17). As mentioned above, patients with HFmrEF have heterogeneous characteristics, and many previous studies on HFmrEF have not identified consistent similarities between the characteristics of HFmrEF and those of either HFrEF or HFpEF (18). Furthermore, patients with HFmrEF tend to exhibit a change in their phenotypes, which is represented by LVEFs over time, more predominantly than those with other types of HF (19). Therefore, developing a model that detects the degree of cardiac dysfunction using AI-ECG could help manage these patients (20).
Until now, several studies have been conducted to detect cardiac contractile dysfunction using AI (9, 10, 21, 22), Attia et al. (9) revealed an AI-based model for identifying patients with LV systolic dysfunction (defined as LVEF under 35%) using ECG and echocardiography. Its performance had an AUC of 0.93. After 3.4 years of follow-up, patients with baseline positive AI-ECG and negative echocardiography had significantly higher rates of LV systolic dysfunction. Another study by Zhang et al. (10) reported the AI model to detect patients with congestive HF, and the results showed good performance and accuracy, sensitivity, and specificity reached 94.97%, 89.38%, and 99.50%, respectively. A study by Yao et al. (21) designed an AI model for identifying patients with lower LVEF under 50% by randomized clinical trial. This model showed that the intervention group (the patients who used this AI model) had a higher rate of low EF (2.1% vs. 1.6% in control, odds ratio 1.32 (1.01–1.61) compared with the control group. Similarly, Vaid et al. (22) showed AI-ECG to predict cardiac dysfunction from ECG, and it demonstrated strong performance in detecting both right ventricular and LV systolic dysfunction (LVEF ≤40%), with AUCs of 0.84 and 0.94, respectively, across internal and external databases. All of those studies are consistent with our study in that they used DNN with numerous ECG data in a real-world population, predicted ventricular dysfunction defined by echocardiography, and showed good performance of AI model. However, no clinical trials have been conducted to detect HFmrEF. HFmrEF, unlike other HF phenotypes, has a narrow range with a LVEF of 41%–49%, and has complex and heterogeneous patient characteristics. Patients with HFmrEF have fewer complaints of dyspnoea or chest discomfort than those with HFrEF at the time of diagnosis. For this reason, predicting these patients and their prognosis could be a clinical burden. Therefore, it might be important to screen asymptomatic patients for HFmrEF and to prepare them for the possible manifestations of cardiac dysfunction. This AI-based trial could predict the prognosis of patients with HFmrEF, leading to appropriate medical strategies. Furthermore, its application in clinical practice would be utilized by longitudinal monitoring of patients, which would involve quick and precise detection of cardiac problems (23–26).
Given the increasing burden of HF on healthcare system, it is worth valuable to discuss the economic benefits of adoping AI-ECG in patients with HFmrEF. Most of the economic costs of HF patients are attributable to inpatient hospitalization costs (27). About 800,000 heart failure patients experienced hospitalization per year in the United States, and the cost per person was about $19,000. Considering the prevalence of HFmrEF (10%–25%), it is estimated that about 80,000–200,000 hospitalized patients were present. If the clinical effect of AI-ECG can reduce the hospitalization rate of HFmrEF patients by 10%, the expected economic savings per year can be estimated at about $19 million to $28 million.
AI-ECG in this study revealed an overall accuracy of 87.3% (95% CI: 86.4–89.3) and AUCs of >0.80 for each phenotype of HF. Based on these results, this model demonstrated a favourable performance. Other clinical trials predicting HF subtypes have shown comparable results. A previous study predicted LV systolic dysfunction as an LVEF of <35% in the normal population with an AUC of 0.93 (9). Another study achieved an AUC of 0.87 with a deep-learning model to detect HFpEF in the normal population (8). In our study, different detectability results were obtained for each type of HF. The AUCs for detecting an EF of ≤40% and ≥50% were 0.875 and 0.870, respectively. However, to detect an EF between 41% and 49%, the number of patients with HFmrEF was relatively low (0.824). We could not directly compare the performance of our model in detecting HFmrEF with that of the others, as there have been no other reference studies using AI involving patients with HFmrEF. However, it was postulated that since patients with HFmrEF have more heterogeneous, complex, and diverse aetiologies, it could be more difficult to detect patients with HFmrEF than those with other types of HF using AI-ECG (28). The results of the scarce pattern of T-SNE visualisation in patients with HFmrEF also reflect these features. Although detectability in the HFmrEF group was not effective, this model may provide important clues regarding the patient's clinical prognosis. For example, even in patients with HFmrEF with the same range of EF, AI-ECG could detect “those with the possibility of upcoming ‘HFrEF’ for one group and ‘those with normal EF’ for another group”. If patients are predicted to be in the HFrEF group and have more electrocardiographic characteristics of HFrEF than those not predicted, their clinical characteristics may be similar to those of patients with HFrEF. However, further studies with more detailed designs are required to verify this hypothesis.
In our study, the ability of AI-ECG to distinguish the ECG pattern in each HF phenotype was generally acceptable, except for HFmrEF. As a result of analysing the characteristics according to the AI-predicted EF Euclidean distance of patients with HFrEF and those with a normal range of EF, abnormal ECG findings such as a prolonged QRS duration, QT interval, and QTc interval, were closely correlated with those in patients predicted to have a condition closer to HFrEF. Conversely, a shorter QRS duration, QT interval, and QTc interval were related to a predicted ECG finding closer to that of the normal population. Previous studies correlate several ECG features with different types of HF. Purnasidha et al. created a scoring system to predict HFrEF based on ECG characteristics. A longer QRS duration (>100 ms) and QT interval are associated with HF with systolic dysfunction (29), and these findings support our observations, along with left atrial hypertrophy and right bundle branch block. Other studies also showed an association of a QRS duration of >100 ms with a reduced EF of <45% (30) and prolonged QTc interval with adverse outcomes, such as long-term mortality (31). Based on the ECG characteristics, our findings in the HFmrEF group (scattered pattern of T-SNE and lower performance in detecting the group) reflect the non-unified characteristics of patients with HFmrEF. Applying the AI-based model in patients with HFmrEF could have great clinical applicability as it can predict the prognosis of this patient cohort and the responses, such as drug response and cardiovascular outcomes.
This study had some limitations. First, this was a retrospective study, and there is a possibility of selection bias. To minimise this limitation, we collected data from approximately 100,000 ECGs from over 50,000 patients. Second, the study population was recruited from a single centre in South Korea. Further studies involving more institutions are required to validate this model. Third, given the inherent limitations of deep neural networks, they have structural limitations in creating causal relationships. To minimise these limitations, training, validation, and holdout testing were performed using large amounts of refined raw digital ECG data. Fourth, an echocardiographic assessment of the entire population was not performed using the same equipment, and not all analyses of LVEF were performed using Simpson's method, which might have produced inconsistencies in echocardiographic estimations. Fifth, in the analysis to verify the predictability of LVEF in clustering patients who underwent follow-up ECG, a consistent follow-up duration of ECG was not shown for each patient because of its retrospective nature. Future prospective studies should address these limitations. Additionally, to enhance the study's robustness, the training, validation, and holdout test sets were segmented based on different time periods. As a result, while the proportions of normal EF in the datasets are similar, the training set contains a larger number of normal EF, leading to clinical bias, as observed in Table 1.
AI-ECG could be used to identify patients with HFmrEF and predict future cardiac contractility based on ECG characteristics. Further prospective studies are required to examine the feasibility of its use in clinical practice.
The datasets presented in this article are not readily available due to privacy concerns. Requests to access the datasets should be directed to the corresponding author.
The studies involving humans were approved by Institutional Review Board of Inha University Hospital (IRB number: 2023-03-024). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because this study has the retrospective nature.
D-YK: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft. S-WL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Writing – review & editing. D-HL: Conceptualization, Data curation, Formal Analysis, Investigation, Funding acquisition, Resources, Software, Visualization, Writing – review & editing. S-CL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Resources, Visualization, Writing – review & editing, Methodology, Project administration, Supervision, Validation. J-HJ: Conceptualization, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Investigation, Software, Writing – original draft. S-HS: Methodology, Project administration, Software, Supervision, Validation, Visualization, Data curation, Writing – review & editing, Conceptualization, Investigation. D-HK: Data curation, Investigation, Formal Analysis, Writing – original draft. WC: Data curation, Formal Analysis, Investigation, Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Software, Writing – review & editing. Y-SB: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Writing – original draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by an Inha University Research Grant, a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: RS-2023-00265440), an Institute of Information & communications Technology Planning & Evaluation (IITP) grant funded by the Korea government (MSIT) [No. RS-2022-00155915, Artificial Intelligence Convergence Innovation Human Resources Development (Inha University)], and the Young Medical Scientist Research Grant through the Daewoong Foundation (DY20103C).
D-HL, S-CL, D-HK, WC, Y-SB were employed by DeepCardio Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1418914/full#supplementary-material
1. Lam C, Solomon SD. The middle child in heart failure: heart failure with mid-range ejection fraction (40–50%). Eur J Heart Fail. (2014) 16:1049–55. doi: 10.1002/ejhf.159
2. Savarese G, Stolfo D, Sinagra G, Lund LH. Heart failure with mid-range or mildly reduced ejection fraction. Nat Rev Cardiol. (2022) 19:100–16. doi: 10.1038/s41569-021-00605-5
3. Rastogi A, Novak E, Platts AE, Mann DL. Epidemiology, pathophysiology and clinical outcomes for heart failure patients with a mid-range ejection fraction. Eur J Heart Fail. (2017) 19:1597–605. doi: 10.1002/ejhf.879
4. Lund LH, Claggett B, Liu J, Lam CS, Jhund PS, Rosano GM, et al. Heart failure with mid-range ejection fraction in CHARM: characteristics, outcomes and effect of candesartan across the entire ejection fraction spectrum. Eur J Heart Fail. (2018) 20:1230–9. doi: 10.1002/ejhf.1149
5. Bhambhani V, Kizer JR, Lima JA, Harst P, Bahrami H, Nayor M, et al. Predictors and outcomes of heart failure with mid-range ejection fraction. Eur J Heart Fail. (2018) 20:651–9. doi: 10.1002/ejhf.1091
6. Nauta JF, Hummel YM, van Melle JP, Meer P, Lam CS, Ponikowski P, et al. What have we learned about heart failure with mid-range ejection fraction one year after its introduction? Eur J Heart Fail. (2017) 19:1569–73. doi: 10.1002/ejhf.1058
7. Gu J, Zf Y, Hl Z, Yq F, Jf Z, Cq W. Characteristics and outcomes of transitions among heart failure categories: a prospective observational cohort study. ESC Heart Failure. (2020) 7:616–25. doi: 10.1002/ehf2.12619
8. Kwon J-M, Kim K-H, Eisen HJ, Cho Y, Jeon K-H, Lee SY, et al. Artificial intelligence assessment for early detection of heart failure with preserved ejection fraction based on electrocardiographic features. Eur Heart J Digit Health. (2021) 2:106–16. doi: 10.1093/ehjdh/ztaa015
9. Attia ZI, Kapa S, Lopez-Jimenez F, McKie PM, Ladewig DJ, Satam G, et al. Screening for cardiac contractile dysfunction using an artificial intelligence–enabled electrocardiogram. Nat Med. (2019) 25:70–4. doi: 10.1038/s41591-018-0240-2
10. Zhang Y, Xia M. Application of deep neural network for congestive heart failure detection using ECG signals. J Phys Conf Ser. (2020) 1642:012021. doi: 10.1088/1742-6596/1642/1/012021
11. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. (2021) 42:3599–726. doi: 10.1093/eurheartj/ehab368
12. Vaswani A, Shazeer N, Parmar N, Uszkoreit J, Jones L, Gomez AN, et al. Attention is all you need. Adv Neural Inf Process Syst. (2017) 30:6000–10. doi: 10.48550/arXiv.1706.03762
13. Devlin J, Chang M-W, Lee K, Toutanova K. Bert: Pre-training of deep bidirectional transformers for language understanding. Prod NAACL-HLT (2019) 1(2):4171–86. doi: 10.48550/arXiv.1810.04805
14. Brown T, Mann B, Ryder N, Subbiah M, Kaplan JD, Dhariwal P, et al. Language models are few-shot learners. Adv Neural Inf Process Syst. (2020) 33:1877–901. doi: 10.48550/arXiv.2005.14165
15. Schroff F, Kalenichenko D, Philbin J. Facenet: a unified embedding for face recognition and clustering. Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (2015). p. 815–23.
16. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2016) 37:2129–200. doi: 10.1093/eurheartj/ehw128
17. Savarese G, Lund LH. Global public health burden of heart failure. Cardiac Failure Review. (2017) 3:7. doi: 10.15420/cfr.2016:25:2
18. Koh AS, Tay WT, Teng THK, Vedin O, Benson L, Dahlstrom U, et al. A comprehensive population-based characterization of heart failure with mid-range ejection fraction. Eur J Heart Fail. (2017) 19:1624–34. doi: 10.1002/ejhf.945
19. Dunlay SM, Roger VL, Weston SA, Jiang R, Redfield MM. Longitudinal changes in ejection fraction in heart failure patients with preserved and reduced ejection fraction. Circ Heart Fail. (2012) 5:720–6. doi: 10.1161/CIRCHEARTFAILURE.111.966366
20. Baek Y-S, Lee S-C, Choi W, Kim D-H. A new deep learning algorithm of 12-lead electrocardiogram for identifying atrial fibrillation during sinus rhythm. Sci Rep. (2021) 11:1–10. doi: 10.1038/s41598-020-79139-8
21. Yao X, Rushlow DR, Inselman JW, McCoy RG, Thacher TD, Behnken EM, et al. Artificial intelligence–enabled electrocardiograms for identification of patients with low ejection fraction: a pragmatic, randomized clinical trial. Nat Med. (2021) 27(5):815–9. doi: 10.1038/s41591-021-01335-4
22. Vaid A, Johnson KW, Badgeley MA, Somani SS, Bicak M, Landi I, et al. Using deep-learning algorithms to simultaneously identify right and left ventricular dysfunction from the electrocardiogram. Cardiovasc Imaging. (2022) 15(3):395–410. doi: 10.1016/j.jcmg.2021.08.004
23. Kulkarni P, Mahadevappa M, Chilakamarri S. The emergence of artificial intelligence in cardiology: current and future applications. Curr Cardiol Rev. (2022) 18(3):46–52. doi: 10.2174/1573403X17666211119102220
24. Sun X, Yin Y, Yang Q, Huo T. Artificial intelligence in cardiovascular diseases: diagnostic and therapeutic perspectives. Eur J Med Res. (2023) 28(1):242. doi: 10.1186/s40001-023-01065-y
25. Muzammil MA, Javid S, Afridi AK, Siddineni R, Shahabi M, Haseeb M, et al. Artificial intelligence-enhanced electrocardiography for accurate diagnosis and management of cardiovascular diseases. J Electrocardiol. (2024) 83:30–40. doi: 10.1016/j.jelectrocard.2024.01.006
26. Siontis KC, Noseworthy PA, Attia ZI, Friedman PA. Artificial intelligence-enhanced electrocardiography in cardiovascular disease management. Nat Rev Cardiol. (2021) 18(7):465–78. doi: 10.1038/s41569-020-00503-2
27. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. (2020) 141(9):e139–596. doi: 10.1161/CIR.0000000000000757
28. Kapoor JR, Kapoor R, Ju C, Heidenreich PA, Eapen ZJ, Hernandez AF, et al. Precipitating clinical factors, heart failure characterization, and outcomes in patients hospitalized with heart failure with reduced, borderline, and preserved ejection fraction. JACC Heart Fail. (2016) 4:464–72. doi: 10.1016/j.jchf.2016.02.017
29. Hendry PB, Krisdinarti L, Erika M. Scoring system based on electrocardiogram features to predict the type of heart failure in patients with chronic heart failure. Cardiol Res. (2016) 7:110. doi: 10.14740/cr473w
30. Murkofsky RL, Dangas G, Diamond JA, Mehta D, Schaffer A, Ambrose JA. A prolonged QRS duration on surface electrocardiogram is a specific indicator of left ventricular dysfunction. J Am Coll Cardiol. (1998) 32:476–82. doi: 10.1016/S0735-1097(98)00242-3
Keywords: artificial intelligence, electrocardiography, heart failure, predictability, ejection fraction
Citation: Kim D-Y, Lee S-W, Lee D-H, Lee S-C, Jang J-H, Shin S-H, Kim D-H, Choi W and Baek Y-S (2025) Electrocardiography-based artificial intelligence predicts the upcoming future of heart failure with mildly reduced ejection fraction. Front. Cardiovasc. Med. 12:1418914. doi: 10.3389/fcvm.2025.1418914
Received: 17 April 2024; Accepted: 20 January 2025;
Published: 10 February 2025.
Edited by:
Laura Fusini, Monzino Cardiology Center (IRCCS), ItalyReviewed by:
Abdulqadir J. Nashwan, Hamad Medical Corporation, QatarCopyright: © 2025 Kim, Lee, Lee, Lee, Jang, Shin, Kim, Choi and Baek. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong-Soo Baek, ZXhpc3Rzb29AaW5oYS5hYy5rcg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.