
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 12 February 2025
Sec. Sex and Gender in Cardiovascular Medicine
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1517288
This article is part of the Research Topic Sex-Specific Risk Factors and Cardiovascular Disease Risk in Women View all 9 articles
Introduction: Despite growing evidence for sex-specific differences in cardiovascular disease, sex is poorly considered in the management of infectious endocarditis (IE).
Purpose: This study aimed to assess sex-specific aspects in diagnosing IE.
Methods: All consecutive patients admitted at Ulm University Heart Center with suspected IE between 2009 and 2019 were included. IE was diagnosed using the Duke criteria. Risk factors, clinical presentation and in-hospital outcomes along with the impact of diagnostic delay were compared between male and female patients.
Results: IE was diagnosed in 96 of 118 men (81.4%) and 33 of 45 women (73.3%) (p = 0.121). Time to diagnosis was similar between the groups (p = 0.598). Regarding patient characteristics, men were younger (65.5 vs. 74.3 years, p = 0.006). Men exhibited a higher prevalence of predisposing cardiac conditions (p = 0.012) due to a higher frequency of a history of implantable cardioverter defibrillator implantation (p = 0.004), and were more likely to have poor dental status (p = 0.001), and coronary artery disease (p = 0.002). The incidence of the complications of heart failure with reduced ejection fraction (p = 0.007) and new-onset dialysis (p = 0.012) were higher, the time in the intensive care unit (p = 0.012) longer. Male sex was the only independent risk factor for in-hospital mortality [p = 0.036, HR 4.127 (95%-CI 1.096-15.538)]. Notably, only in the male cohort, a shorter time to diagnosis was associated with a lower mortality rate (p = 0.035, optimal cut-point 3.5 days). Men diagnosed within 3.5 days had a mortality rate of 13.5% compared to 31.8% for those diagnosed later (p = 0.028).
Conclusion: Men with suspected IE are younger, have more predisposing factors and experience a more complicated course of disease, while benefiting from early diagnosis. Therefore, recognizing the heightened risk profile specific to men during diagnosis can help to address their poorer prognosis.
Infective endocarditis (IE) is a life-threatening condition that poses several diagnostic and therapeutic challenges (1). It constitutes a major burden on the healthcare system with high morbidity and particularly elevated mortality rate of approximately 20% during the first hospitalization and 40% over five years (2). This burden is increasing over time, with the incidence of hospitalization for IE almost doubling between 2003 and 2016 (3). Notably, men are particularly affected (4, 5), with a 20% elevated incidence of IE (4) and a more pronounced increase in recent years (6). This development may be due to the differences in manifestation and progression of IE between men and women (7). Indeed, a higher proportion of culture-positive IE and Streptococcus viridans infection has been reported in men. While women are more prone to have infections of the left heart valves, aortic and mitral valve, men are more likely to develop vegetations of the tricuspid valve (5). Notwithstanding the substantial implications of these differences, sex is not among the criteria included in the Duke criteria, which are the global standard for the diagnosis of IE (8, 9). Additionally, demographic characteristics, risk factors and comorbidities, which influence the progression of the disease (10), differ between men and women (5). It has been documented that women with IE are more likely to have certain predisposing factors, including advanced age, prosthetic valve infection (10) and immunosuppression (5). Comorbidities associated with a poor prognosis are heterogeneously distributed between the sexes. Women are more likely to have chronic kidney disease (5), whereas men are more likely to have cardiovascular disease such as coronary artery disease (CAD) and heart failure (9–13). Moreover, quality of care appears to be inferior in women, as they tend to seek medical help later, are more likely to leave the hospital against medical advice (5, 10) and have lower rates of surgical intervention (5). Accordingly, women have been found to have higher mortality rates (5, 14). However, it is noteworthy, that the mortality is driven by the elderly (14). Another study only including patients aged 65 and older reported a higher mortality rate in men (6), thereby suggesting male sex to be a risk factor (6). Despite the prognostic relevance of these sex-based disparities, current guidelines for the diagnosis and management of IE do not account for sex (8, 9, 13). Overall, the data presented collectively illustrate that the role of sex in diagnosing IE remains poorly understood. Most importantly, they provide strong evidence that the implementation of sex-specific diagnostic management strategies could help to reduce the burden of IE (15). This study aimed to evaluate the sex-specific performance of the Duke criteria as standard for the diagnosis of IE and to identify possible sex-related differences, that can be incorporated to improve this algorithm.'
A monocentric, retrospective study was conducted, including all consecutive patients who presented with suspected IE at the Department of Medicine II at Ulm University Heart Center, Ulm, Germany, between December 2009 and November 2019. Patients who were hospitalized due to suspected IE were eligible for inclusion in the study. All patients who were suspected of having IE were included, regardless of whether a diagnosis of definitive IE was made at a later stage. All patients were subjected to recurrent assessment according to the Duke criteria as outlined by the European Society of Cardiology (ESC) in 2023 (9), within 24 h of admission and again throughout the duration of their initial hospitalization until discharge. The diagnosis provided at the time of discharge was considered the definitive diagnosis. All patients were treated in accordance with to the prevailing guidelines at that time (16, 17). Patients who were readmitted to the hospital for a second instance of suspected IE relapse were excluded from the study. Based on their sex, the patients were divided into the female and the male group. Sex was considered in accordance with the SAGER (Sex and Gender Equity in Research) Guidelines to ensure appropriate analysis and reporting of biological differences between male and female patients.
The study conforms to the guidelines of the Declaration of Helsinki, adheres to the STROBE statement and was approved by the local ethics committee (ethics application number University Ulm 90/20).
Demographic, clinical, imaging and laboratory data at the time of admission and during the hospital stay were extracted from our patient management system by two physicians and adjudicated by a third physician in case of any kind of disagreement. The positivity of the Duke criteria was assessed to evaluate the presence of IE resulting in the diagnosis of definitive IE, or no confirmation of the suspicion in case of possible or rejected IE (9, 17). For this purpose, clinical assessment and targeted cardiovascular examinations such as transthoracic echocardiography and, if appropriate, transesophageal echocardiography and Positron Emission Tomography-Computed Tomography (PET-CT) were performed according to the recommendations. In addition, 12-lead electrocardiography (ECG) and laboratory blood tests were performed. Left ventricular systolic function (left ventricular ejection fraction, LVEF) was measured either by echocardiography (EPIQ 7, Koninklijke Philips N.V., Eindhoven, The Netherlands) or cardiac ventriculography during cardiac catheterization and categorized as normal, mildly impaired, moderately impaired, or severely impaired, according to guideline-specific recommendations (18). Blood samples were taken for measurement of highly sensitive cardiac troponin T (hsTnT), N-Terminal pro-B-Type Natriuretic Peptide (NT-proBNP), creatinine and estimated glomerular filtration rate (eGFR), c-reactive protein (CRP), procalcitonin (PCT), leukocytes and neutrophils. Blood cultures and urine samples were collected for microbiological and virological pathogen testing and gathered retrospectively. Definitive IE was categorised into native valve, prosthetic valve and lead or device-related infections according to the cardiac structures involved. All data were collected as part of the clinical routine.
Endpoints were differences between male and female patients regarding diagnosis, baseline characteristics, disease course and outcomes, with a focus on the feasibility and impact of reducing diagnostic delay. First, demographic factors, comorbidities, predisposing factors and symptoms of IE were assessed and compared. In particular, the frequency of positive Duke criteria and diagnosis of IE were assessed, if appropriate recurrently, along with the time to diagnosis. Duke criteria were typical imaging and microbiological findings, fever > 38°C, the presence of a predisposing heart disease such as prosthetic valve, bicuspid aortic valve, congenital cardiac malformation, intracardial device such as implantable cardioverter-defibrillator (ICD) or pacemaker (PM), structural cardiomyopathy or intravenous drug abuse, vascular phenomena and immunological phenomena, according to the guidelines (9). Other predisposing factors assessed included poor dental status and immunosuppression. Secondary, the in-hospital course of disease and outcomes were assessed and compared between the groups. These included complications and hospitalization data. Complications included cardiac failure, acute kidney injury requiring new-onset of dialysis, and mortality. Length of total and intensive care unit (ICU)-stay and number of ICU-stays were assessed. Finally, the effect of delayed diagnosis on mortality was examined. For this purpose, the association of the time between admission and diagnosis and mortality was assessed. Time of diagnosis was defined as the point of time at which the final diagnosis in terms of IE was made, irrespective of the result. Where appropriate, the critical time period and its effect on mortality were assessed. In this term, the time to diagnosis was compared between the subgroups with and without mortality. The observation period was the time of the interventional hospital stay until discharge.
Continuous variables were presented as mean ± standard deviation or median together with the interquartile range (IQR) as appropriate. The Kolmogorov-Smirnov test was used to assess the normal distribution of continuous parameters. If a metric variable was not normally distributed at any measurement date, all values were presented as median together with the IQR. Categorial variables were described as numbers and percentages. Student's t-test, Mann-Whitney U-Test or chi-square test was used to compare variables between groups where appropriate. Multinominal cox regression analysis was used to calculate age-adjusted mortality. In addition, multivariate cox regression analysis was performed to assess the association between male sex and the risk of in-hospital mortality while adjusting for potentially confounding baseline characteristics. Sex was included as the primary independent variable. Baseline characteristics with a p-value < 0.05 in the univariate comparisons between the groups were included in the multivariate analysis. The enter method was applied for variable inclusion in the multivariate cox regression analysis, and the proportional hazards assumption was tested by creating interaction terms, with violations addressed by stratification or exclusion of problematic variables. The strength of the association was expressed as hazard ratio (HR) with 95%-confidence interval (CI). Binary regression, and additionally Pearson correlation analysis, were used to assess the effect of time to diagnosis on mortality and were performed separately for men and women. The performance of the model was assessed by receiver operating characteristic (ROC) analysis estimating sensitivity, specificity, and area under the curve (AUC). The optimal cut-off was calculated using the Youden index.
Parameters with a p-value < 0.05 were considered statistically significant. Statistical analysis was performed using SPSS Statistics 29 software (Version 2022, IBM, Armonk, NY, USA). Due to the exploratory nature of this study, all results of statistical tests have to be interpreted as generating hypotheses.
A total of 163 patients received inpatient treatment at our tertiary care center for suspected IE between December 2009 and November 2019. Of the total number of patients, 118 (72.4%) were men (male group) and 45 (27.6%) were female (female group). The baseline data for both groups are presented in Table 1. The median age was significantly lower in the male group compared to the female group [65.5 (56.3, 75.4) years vs. 74.3 (70.2, 82.7) years (p = 0.006)]. The male group exhibited a higher prevalence of CAD (50.8% vs. 24.4%, p = 0.002) (Figure 1) and a history of myocardial infarction (22.0% vs. 2.2%, p < 0.001). In the female group, the prevalence of thyroid dysfunction was higher (35.6% vs. 13.6%, p = 0.007), attributable to a greater proportion of women with hypothyroidism (31.1% vs. 11.9%, p = 0.014). The characteristics of IE are also shown in Table 1. In the male cohort, IE was more frequently observed in the tricuspid valve (9.3% vs. 2.2%, p = 0.044). Otherwise, there were no significant differences in the patients' baseline characteristics. Notably, the type of endocarditis, including native valve and prosthetic valve infections, as well as the proportion with lead infection, were comparable. Furthermore, there was no significant difference in the proportion of patients in whom vegetations or bacteria were detected. In both groups, bacteriemia was primarily attributable to Staphylococcus aureus, which was identified in approximately 30% of the patients (male group: 33.1% vs. female group: 28.9%, p = 0.613). Subsequently, infection with Enterococcus faecalis/faecium (male group 12.7% vs. female group 11.1%, p = 0.921) and Streptococcus viridans (male group 24.6% vs. female group 4.4%, p = 0.124) was observed. The proportion of men with infection with Streptococcus gallolyticus (bovis) was low (1.7% vs. female group 0.0%, p = 0.383). The HACEK group of bacteria (comprising Haemophilus aphrophilus and haemophilus, Aggregatobacter actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens and Kingella kingae) were not identified in the present cohort.
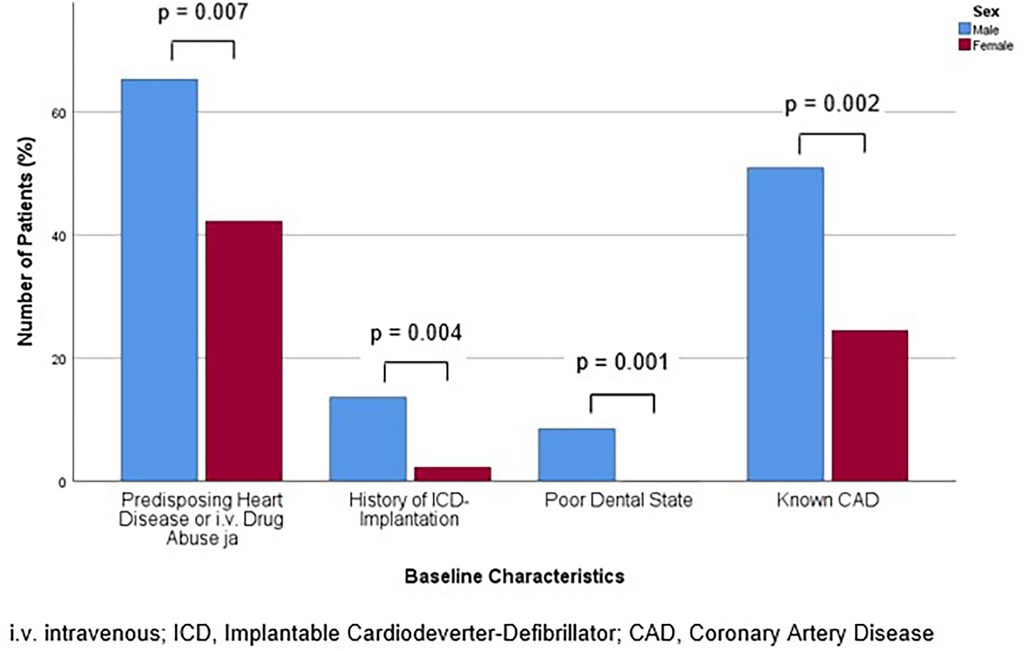
Figure 1. Bar chart for the percentage of patients with positive Duke criteria corresponding to the diagnosis of Infectious Endocarditis (IE) according to the Duke criteria overall, at admission and during the hospital stay, for males and females, compared with Mann-Whitney-U- or Students' t-test, as appropriate.
To evaluate the representation of any sex-specific differences in the diagnostic guidelines, a comparison was conducted between the presence of these criteria among males and females. In addition, the presence of other known risk factors and symptoms of IE were compared to assess their sex-specific expression.
A total of 129 of 163 patients (79.1%) were diagnosed with definitive IE until discharge. This affected 96 of 118 males (81.4%) and 33 of 45 females (73.3%), representing similar proportions of both groups (p = 0.121). At the time of admission, 31 (26.3%) males and 10 (22.2%) females were diagnosed with IE according to the Duke criteria. This was not a statistically significant difference between the sexes (p = 0.597). During the hospital stay, out of all 163 patients, 80 patients (67.8%) in the male group and 27 (60.0%) in the female group, were found to have positive Duke criteria, corresponding to the diagnosis of IE, which was also not a statistically significant difference (p = 0.352). The diagnostic delay, as determined by the time-to-diagnosis following admission (p = 0.598), was comparable between the two groups (Table 2). The proportion of patients with positive Duke criteria, corresponding to the diagnosis of IE, for the different time points assessed for both sexes is shown in Figure 2.
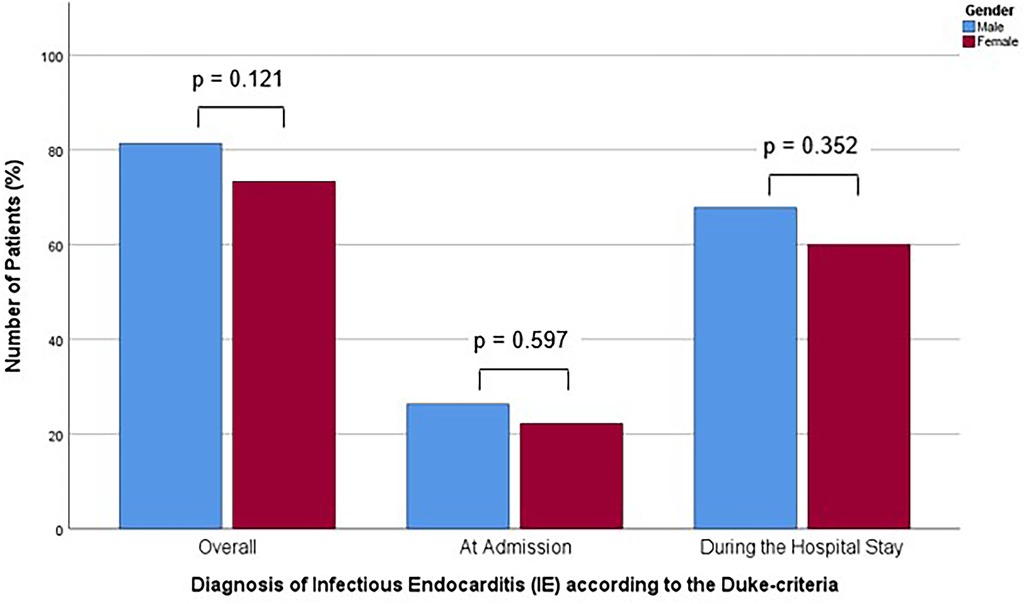
Figure 2. Bar chart for the percentage of patients with baseline characteristics with significant difference in prevalence between the sexes for males and females, compared with Mann-Whitney-U- or Students' t-test, as appropriate.
In line with the aforementioned results, at admission, the frequency of positivity for the major criteria, which are positive imaging or microbiological detection of bacteria typical for IE, was similar (p = 0.850). However, men exhibited a higher frequency of positive minor criteria, including fever, predisposing heart disease or intravenous drug abuse, vascular phenomena, immunologic phenomena, microbiological or imaging criteria suspicious for IE, but not fulfilling the corresponding major criterion (p = 0.024). An analysis of the individual criteria revealed that predisposing heart disease or intravenous drug abuse was more prevalent in males (65.3% vs. 42.2%, p = 0.007). This was primarily due to the high incidence of predisposing heart disease (61.9% vs. 40.0%, p = 0.012). In detail, a history of ICD implantation affected a greater proportion of males (13.6% vs. 2.2%, p = 0.004). Regarding the other risk factors, poor dental status was asserted exclusively and more frequently in males (8.5% vs. 0%, p = 0.001). Additionally, men were more likely to present with splenomegaly (33.1% vs. 13.3%, p = 0.004) (Table 3). The prevalence of the predisposing baseline characteristics of significant difference for men and women are displayed in Figure 1.
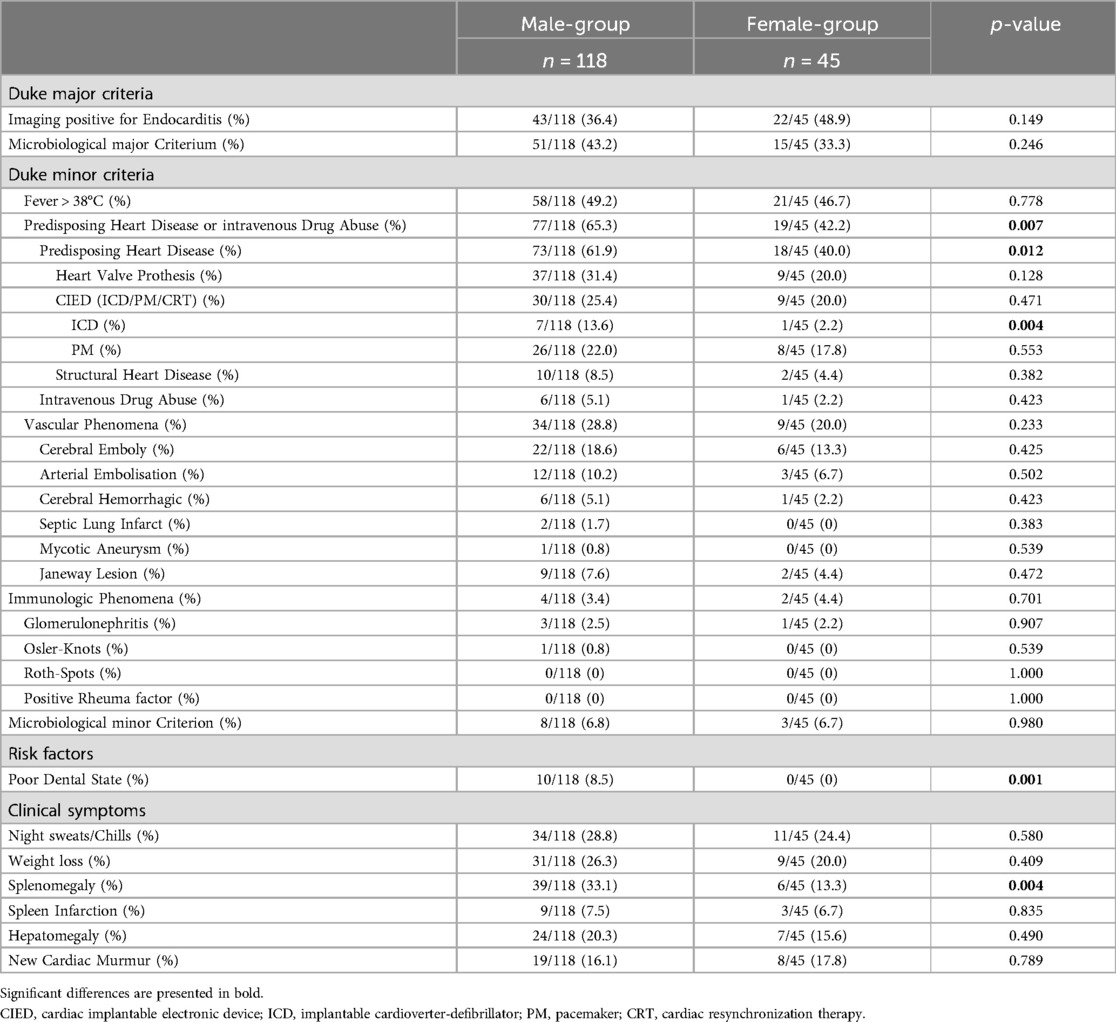
Table 3. Frequency of the Duke criteria, risk factors and clinical symptoms of IE for males and females.
In order to assess the impact of sex-specific factors on the course of disease, the complications and outcomes of males and females were compared.
A reduced cardiac function was observed more frequently in men (56.5% of male patients vs. 30.3% of female patients, p = 0.007) and their grade of LVEF was lower (p = 0.017). Furthermore, male patients exhibited elevated creatinine levels, both at admission [116.5 (83.8, 178.0) vs. 93.0 (70.0, 157.0) µmol/L, p = 0.008] and throughout the course of their hospitalization [peak 172.5 (103.5, 312.8) vs. 109.0 (88.0, 198.5) mmol/L, p = 0.006]. Consistently, the incidence of new-onset dialysis was higher in the male cohort (19.5% vs. 4.4%, p = 0.002). With regard to the treatment measures, the time until the initial administration of antibiotics was comparable (p = 0.276). Also, the overall length of hospitalization was comparable between the sexes (p = 0.065), but men spent a significantly longer time in the ICU [12.0 (6.0, 19.0) vs. 6.0 (3.0, 11.0), p = 0.012]. The proportion of patients, who required ICU-admission did not differ between the sexes (p = 0.183). Notably, the absolute mortality rate was comparable between the sexes (p = 0.272), while age-adjusted mortality was significantly higher in males (p = 0.013, HR 3.631, 95%-CI 1.308–10.075) (Table 4). The proportions of patients affected by the aforementioned complications for both sexes are shown in Figure 3. Multivariate analysis including all baseline characteristics of significant difference in the univariate comparisons between the groups (age, known CAD, history of myocardial infarction, predisposing heart disease or intravenous drug abuse, ICD, poor dental state, thyroid dysfunction, splenomegaly, location TV, hypothyroidism, predisposing heart disease) revealed male sex as (the only) independent risk factor for in-hospital mortality (p = 0.036, HR 4.127 (95%-CI 1.096–15.538). The covariates included are shown in Supplementary Table S1.
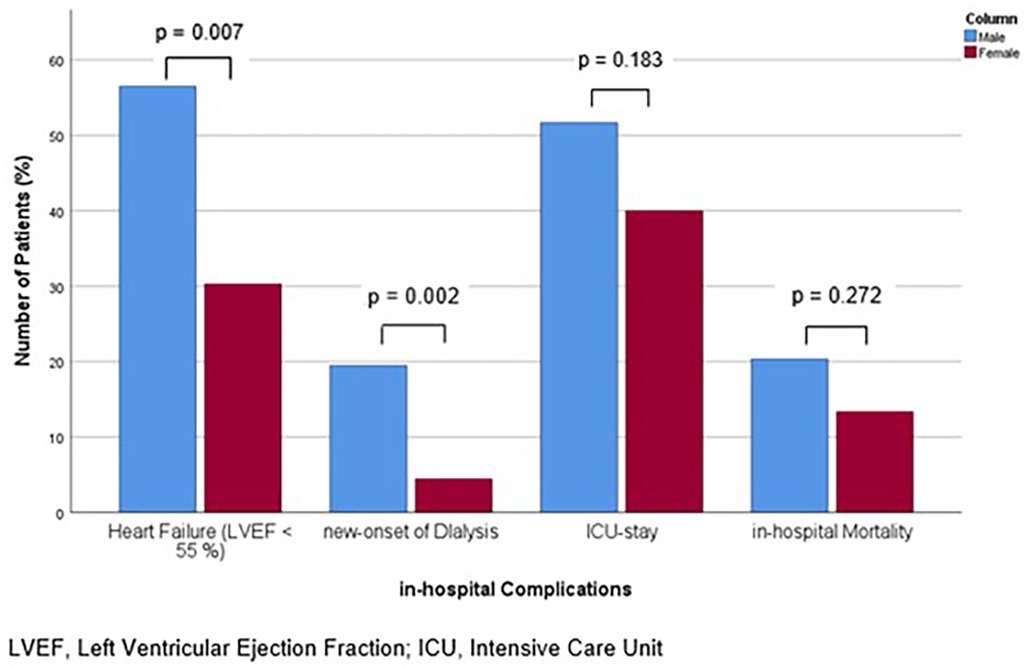
Figure 3. Bar chart for the percentage of patients with in-hospital complications of heart failure with reduced ejection fraction, new-onset of dialysis, ICU-stay and in-hospital mortality, for males and females, compared with Mann-Whitney-U- or Students' t-test, as appropriate.
To assess the comparability of patients who were definitively diagnosed with IE and who were not, we calculated and compared the complication rates of patients who were definitively diagnosed with IE and those who were not. Here, no differences were observed in the complication rates of new-onset of dialysis (15.5% vs. 14.7%, p = 0.909), heart failure 43.4% vs. 41.2%, (p = 0.967), length of ICU-stay [10.00 (5.00, 16.00) days vs. 15.00 (3.25, 19.00) days, p = 0.465] and in-hospital mortality (17.1% vs. 23.5%, p = 0.389) between the two groups.
In order to evaluate the potential benefits of reducing the time taken to reach a diagnosis, we examined the effect of diagnostic delay on mortality rates.
Deaths during the initial hospital stay occurred in 24 out of 118 patients (20.3%) in the male group and 6 out of 45 patients (13.3%) in the female group. In the male group, both binary regression analysis and Pearson correlation analysis revealed a statistically significant association between time to diagnosis and mortality (p = 0.035 and 0.017, respectively). ROC analysis for the prediction of mortality by time to diagnosis yielded an AUC of 0.627 (95%-CI 0.489–0.765, p = 0.078). The optimal cut-off point, as calculated by the Youden index, was 3.5 days, exhibiting a sensitivity of 58.3% and a specificity of 68.1%. Male patients diagnosed within this timeframe exhibited an event rate of 13.5% (10 of 74 patients), in comparison to 31.8% for those with a longer time to diagnosis (14 of 44 patients) (p = 0.028). Accordingly, there was a strong trend towards a longer time to diagnosis in patients who died compared to those who survived (8.8 ± 11.1 vs. 4.5 ± 6.8 days, p = 0.071). In contrast, no significant association between time to diagnosis and mortality was observed among women (p = 0.988 for both analyses). Furthermore, there time-to-diagnosis was comparable between female patients with and without mortality (p = 0.982). The time to diagnosis for patients with and without mortality, presented separately for each sex, is illustrated in Figure 4.
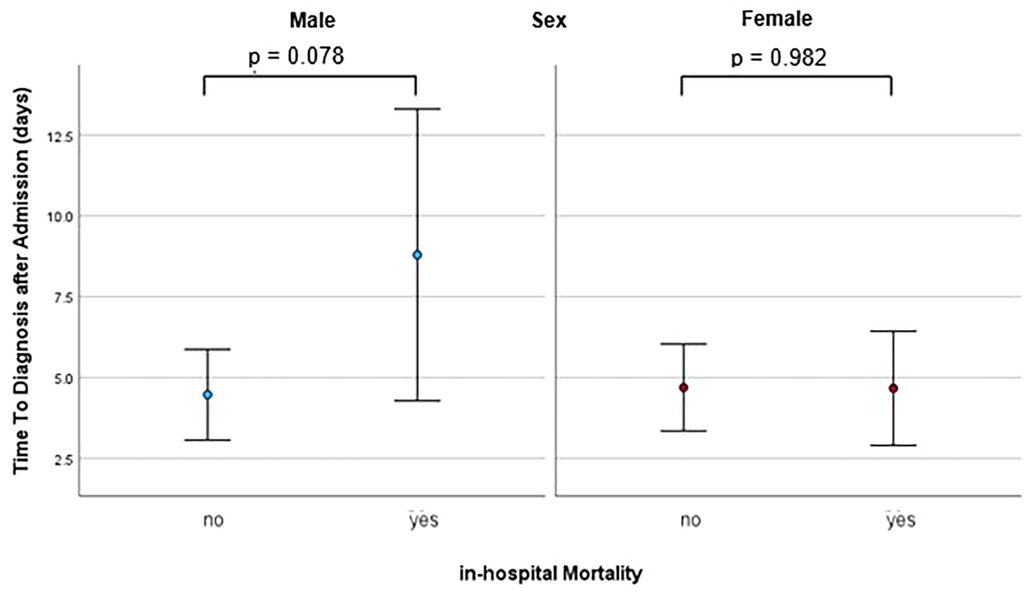
Figure 4. Dot plot with error bars showing the mean and standard deviation of time to diagnosis in patients with and without in-hospital mortality, seperately for males and females, calculated with binary regression analysis.
The present study analyzed the differences between men and women in IE in terms of risk factors and symptoms at baseline, outcomes, and the impact of diagnostic delay. Although the frequency of diagnosis in accordance with the current guidelines was comparable between the sexes, the prevalence of predisposing conditions was higher in men. In detail, predisposing heart disease, specifically a history of ICD-implantation, as well as poor dental state and known CAD were more prevalent in men. Furthermore, the course of disease in men was more severe in terms of organ failure. Importantly, males sex was the only independent risk factor for in-hospital mortality. Notably, a shorter time to diagnosis was found to be associated with lower in-hospital mortality only in male patients. The study design and the main findings are illustrated in the graphical abstract.
Diagnosing IE remains challenging nowadays and a definitive diagnosis is often elusive. Despite the transition from the old Israel Beth criteria to the Duke Criteria and subsequent updates, the latest iteration still exhibits a sensitivity of only 84.2%, while its specificity is better with 93.9% (19). This discrepancy is also reflected in the guidelines that differentiate between definitive IE (DIE), possible IE (PIE), and rejected IE (RIE) (10). In light of these data, indicating that IE is underdiagnosed, all patients diagnosed with suspicion and thus a certain probability of having IE were included in the present study to investigate potential new criteria. The high specificity of the Duke criteria of 93.9% (19) indicates, that this inclusion criterion of “suspected IE” ensures the capture of an accordingly high percentage of patients with IE. Our results of similar outcomes of patients with and without confirmation of the suspicion of IE reinforce this hypothesis that failure to definitively diagnose IE is not solely due to the absence of IE, but rather to the lack of diagnostic sensitivity of the Duke criteria. These results underscore the suitability of the present cohort of patients with “suspected IE” for investigating additional criteria to enhance the diagnostic performance of the Duke criteria, particularly in terms of sensitivity.
Diagnosis of IE is challenging due to its heterogeneous presentation, but decisive for treatment and thus prognosis (1). The Duke criteria represent the global standard for diagnosing IE as evidenced by their acceptance by the major cardiac societies of the ESC (8, 9) and the American College of Cardiology (ACC) (20) and its widespread utilization (21). Since there is compelling evidence that IE manifests differently between males and females (7), but sex is not considered in the Duke score as a criterion (9), we assessed and compared the sex-specific performance of these guidelines. In our study, out of the in total approximately 80% of patients who were diagnosed with IE using the Duke score, only approximately one quarter received this diagnosis even at the time of admission. Notably, while the number of positive minor criteria at admission was higher in men, this was not reflected in the overall positivity of the score. Conversely, the proportion of patients diagnosed with IE was comparable between men and women, indicating that sex does not significantly impact diagnosis by the Duke score. Furthermore, the time to positivity of the score was comparable, which serves to reinforce the absence of sex-based disparities. Thus, our results show a reflection of sex-specific aspects by the Duke criteria, but indicate that these disparities are underrepresented in the resulting score. In line with this, the suitability of the Duke criteria is demonstrated by the consistence of the core criteria throughout various stages of development, updates and validations (8, 9, 20, 22–24) and the high performance with a sensitivity of 84.2% and a specificity of 93.9% of the most recent iteration (19). On the other hand, in consistence with the shortcomings of the score found in our study, the WikiGuidelines Group Consensus Statement already pointed out limitations due to the absence of high-quality studies that definitely determine the most appropriate diagnostic schema (21). Thus, our findings underline the high performance of the Duke criteria, but emphasize the recently indicated limitations, as the implied elevated risk in men is not reflected in the total score and, about three-quarters of patients had a delayed diagnosis.
Given this higher frequency of minor criteria in men, it is essential to identify the underlying causes to enable appropriate assessment and care of IE. In detail, we observed a higher prevalence of a cardiac predisposition, affecting nearly two-thirds of the male patients, which was due to a higher frequency of patients with a history of ICD implantation. Additionally, a higher proportion of men exhibited poor dental status, with approximately 10% presenting with this condition. Furthermore, men were more likely to have the comorbidity CAD, which affected approximately half of them, and a history of myocardial infarction. These findings of the present study consistently indicate that men are at a higher risk of IE, as the association of these conditions with IE is well documented. Recent studies have reaffirmed congenital heart disease, a history of valve replacement, repair, degenerative valve disease, previous IE, a history of invasive procedures in the previous 60 days, intravenous drug use, and chronic intravenous access as risk factors (25–29). Congenital heart disease and intravenous illicit drug use have even been reported to be the most common predisposing conditions (21) and are already included in the Duke score as a minor criterion (9). In detail, approximately 10% of all IE cases have been found to be associated with drug use (30, 31). The implications of cardiac predisposition is demonstrated by the recommendation of the 2007 update of the American Heart Association, which suggests that IE prophylaxis for dental procedures should only be given to affected patients (32). Intravenous drug abuse has even been shown to be a complicating factor, as it is associated with high recurrence (33). The presence of a foreign intracardiac device, such as an ICD, is an obvious predisposing factor to IE, as it provides a potential portal of entry and medium for bacterial growth (9). The implication of this condition (9) is outlined by the high mortality rate associated with device-related IE of 10%–30% (34). The risk of developing IE associated with poor dental status is obvious, as this facilitates the spread of bacteria and their migration to the heart. This predisposition is even reinforced by the guidelines, which consider this condition for risk stratification in IE and as a reason for application of antibiotic prophylaxis (9). Accordingly, a recent study even identified poor oral hygiene as the primary factor contributing to the development of oral streptococcal IE (35). Although the in males more common comorbidity CAD with history of myocardial infarction not directly contributes to the risk factors mentioned in the guidelines (9), it may be a predisposing factor. Its effects, ischemia and myocardial damage with resulting structural cardiomyopathy, are among the predisposing cardiac conditions that form part of the Duke score (9). In addition, CAD may have an indirect negative impact on prognosis through its association with myocardial infarction and reduced LVEF, both of which are part of the Charlton comorbidity index, one of the predictors of poor outcome (9, 13). The importance of comorbidities is underlined by a recent study which showed that comorbidities are the main determinants of prognosis in IE in elderly patients (10). Considering these data on the impact of cardiac predisposition, specifically a history of ICD implantation, poor dental status and the comorbidity CAD, our finding of their increased prevalence in men indicates an increased risk of developing IE and even a high risk of complications and poor outcome. Accordingly, assessment of these characteristics at the time of admission could facilitate the implementation of appropriate care strategies.
In line with their increased risk profile, we observed a more severe clinical course of disease in male patients. Our study found an increased prevalence of symptoms and increased severity of disease in men, as evidenced by higher rates of complications and adverse outcomes. Specifically, men had high and compared to women increased rates of heart failure with reduced ejection fraction and kidney failure, and spent a longer average time in the ICU. heart failure with reduced ejection fraction was observed in more than half of male patients, and renal failure requiring new-onset of dialysis was observed in almost a quarter of male patients. Finally, despite the risk factor of older age of women, men had a comparable absolute mortality rate and a higher mortality rate after adjustment for baseline characteristics. About 20% of male patients died during their initial hospital stay. In line with the results of this study, numerous previous studies have documented a high incidence and negative prognostic implications of organ failure in IE and a less favorable prognosis in men. A recent study also reported very high rates of serious cardiac and systemic complications of IE, with nearly 80% of patients experiencing at least one such complication (36). Similar to our findings, congestive heart failure has been identified as the most common cardiac complication, occurring in approximately 50%–60% of IE episodes (1). The implications thereof have been demonstrated since the complications of heart failure and valvular dysfunction are among the most important factors influencing clinical outcome. In particular, the International Collaboration on Endocarditis-Prospective Cohort Study showed that the presence of heart failure in IE patients was associated with a significantly higher in-hospital mortality of 29.7% compared with 13.1% in patients without heart failure (37). Consistent with our findings, acute kidney injury has also been described as a common complication of IE with recently reported rates of 39.8% and 52.7% (38, 39). Its prognostic impact has been outlined as the risk of death increases by 23.1% for every 10 ml/min decrease in estimated endogenous creatinine clearance using the Cockcroft-Gault formula (40). Consequently, the increased complication rates observed in men in our study suggest a higher risk of mortality. However, it is important to note that men are more likely to be younger (4), whereas older age is associated with increased mortality (6, 10, 14). While several studies report lower absolute mortality rates in men (5, 14), a US-wide analysis including patients aged 65 years and older found higher mortality rates in men (6). It is also noteworthy, that in the study cited above, which found higher mortality rates in women, the sex-difference was time-dependent. While women had increased 30-day and 1-year mortality, in-hospital mortality did not significantly differ to that of men (5). Consistent with this, women have been reported to have a longer recovery time (7). Our findings on higher complication rates and consistently elevated early mortality rate after adjustment for baseline characteristics in men during the initial hospitalization emphasize men's increased vulnerability in the early stages of IE.
Given their more pronounced risk factors and increased early complication rates, it appears both feasible and necessary to reduce the time to diagnosis in men. The earlier onset of fatalities in men compared to women, combined with their more favorable long-term prognosis later on (5), underscores the potential of rapid diagnosis. In our study, a shorter time to diagnosis in men was associated with lower in-hospital mortality. In detail, men who were diagnosed within three and a half days had significantly lower in-hospital mortality rates, more than halved, compared to men with a longer delay in diagnosis. Notably, this finding was only seen in men, which may be due to a more rapid course of the disease in the early stage, as evidenced by their higher complication rates despite similar delays in diagnosis and initiation of antibiotic treatment. Consistent with our findings on the prognostic benefit of early diagnosis, it has been shown that patient outcomes in IE are highly variable and depend on prompt recognition and timely application of surgery when indicated (41), as targeted interventions are critical in reducing mortality (6). Thus, frequent delayed and missed diagnosis of IE affects patients' chances of recovery and survival (42). The recommendation to administer empirical antibiotics as soon as possible (43) and the reduction in mortality and embolic risk by early surgical intervention compared to medical therapy alone (44) highlight the critical role of prompt and targeted therapy. In line with our results, a recent study found a particularly pronounced benefit of early targeted therapy in the vulnerable elderly population, suggesting a particular benefit in high-risk patients (6). In consistence with this, our results show that early diagnosis is fundamental to improving outcomes in the particularly vulnerable male patient population. The more than 50% lower mortality rate in male patients diagnosed within 3.5 days impressively demonstrates the potential of early diagnosis in men to reduce the burden of IE.
The result of our study, that in the majority of patients diagnosed with definitive IE, this diagnosis was made later during the hospital stay, but not at the time of admission, underlines the feasibility and need for enhancing the diagnostic process. The findings of sex-specific disparities, with a higher prevalence of predisposing conditions, a higher risk of complications descriptive to male sex, as well as the benefit of rapid diagnosis in this cohort, provide valuable approaches to improve the diagnostic algorithm. As the Duke criteria aim to reflect the risk of the presence of IE, with each criterion being associated with a certain risk increase, male sex qualifies as an additional criterion according to our findings. Consequently, adding male sex to these criteria can help to improve this diagnostic score, particularly in terms of sensitivity, which currently stands at only 84.2% (19). Furthermore, as sex is immediately and easily assessable, incorporation of this criterion can shorten diagnostic delay. Given the particular criticality of diagnostic delay in men, such a revision can provide a targeted improvement in this aspect as well. Additionally, the establishment of sex-specific recommendations for the time for completing diagnostic work-up or sex-specific modification of the recommended timing for repetition, i.e., of TTE/TOE (5–7 days) (10), can address this point and reflect the urgency of diagnosing IE in men. Thus, we argue for the inclusion of male sex in the diagnostic algorithm for IE.'
This study evaluates sex-specific aspects in the management of IE that are not yet included in the guidelines. Considering our results, we can confirm the usefulness of the Duke criteria and other published risk factors for diagnosis and risk estimation in IE. As we found a higher prevalence of predisposing conditions and cardiac comorbidities in men, which may explain their worse, we would like to highlight the diagnostic potential of these conditions. In this course, we want to emphasize the criticality of diagnostic delay in men, particularly in light of the increased early complication rates and higher in-hospital mortality after adjustment for baseline characteristics. We therefore mitilate against a similar assessment of IE in men and women, as appreciation of the more pronounced risk profile associated with male sex could help to address the poorer prognosis of men. Moreover, we argue for considering the assessment of IE in men as an emergency.
The results of our study have to be interpreted with several confinements. As this is a retrospective, single-center study, it has several inherent limitations descriptive to this design. As the data collection was retrospective, some data were incomplete. As this was a single-center analysis, the number of patients included was relatively limited. Furthermore, the discrepancy in the number and the age of patients between the groups may be open to question. To address this issue, all patients in the defined time period were included without patient exclusion or pre-selection aiming to reduce selection bias as much as possible. Accordingly, these differences reflect the incidence and characteristics of the population, thereby providing an unbiased insight. However, we cannot exclude differences in certain baseline characteristics including socioeconomic state. Additionally, due to the explorative character of this study, all results must be interpreted as hypothesis-generating. Moreover, diagnostic assessment and treatment strategies may be a subject to debate due to their ongoing development. However, all diagnostic and therapeutic measures were indicated according to the then current guidelines and the Duke criteria remained stable over time (8, 9).
The datasets presented in this article are not readily available because the medical data of the patients is restricted in access and secured with 2- factor authentication. Requests to access these datasets should be directed tobG9yZGF5YXlpQHlhaG9vLmRl.
The studies involving humans were approved by Prof Dr. Florian Steger, PD Dr Orzechowski, Kathrin Stascheit, Ethics committee, University Ulm. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by- product of routine care or industry. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
SA: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. KR: Data curation, Formal Analysis, Validation, Writing – review & editing. SM: Data curation, Validation, Writing – review & editing. FR: Formal Analysis, Validation, Writing – review & editing. BH: Formal Analysis, Validation, Writing – review & editing. WR: Supervision, Writing – review & editing. DB: Formal Analysis, Supervision, Validation, Writing – review & editing. SD: Conceptualization, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1517288/full#supplementary-material
1. Kiefer TL, Bashore TM. Infective endocarditis: a comprehensive overview. Rev Cardiovasc Med. (2012) 13(2-3):105–20. doi: 10.3909/ricm0633
2. Bin Abdulhak AA, Baddour LM, Erwin PJ, Hoen B, Chu VH, Mensah GA, et al. Global and regional burden of infective endocarditis, 1990–2010: a systematic review of the literature. Glob Heart. (2014) 9(1):131–43. doi: 10.1016/j.gheart.2014.01.002
3. Alkhouli M, Alqahtani F, Alhajji M, Berzingi CO, Sohail MR. Clinical and economic burden of hospitalizations for infective endocarditis in the United States. Mayo Clin Proc. (2020) 95(5):858–66. doi: 10.1016/j.mayocp.2019.08.023
4. Khan MZ. Racial and gender trends in infective endocarditis related deaths in United States (2004–2017). Am J Cardiol. (2020) 129:125–6. doi: 10.1016/j.amjcard.2020.05.037
5. Slouha E, Al-Geizi H, Albalat BR, Burle VS, Clunes LA, Kollias TF. Sex differences in infective endocarditis: a systematic review. Cureus. (2023) 15(12):e49815. doi: 10.7759/cureus.49815
6. Ashraf H, Nadeem ZA, Ashfaq H, Ahmed S, Ashraf A, Nashwan AJ. Mortality patterns in older adults with infective endocarditis in the US: a retrospective analysis. Curr Probl Cardiol. (2024) 49(4):102455. doi: 10.1016/j.cpcardiol.2024.102455
7. Vlassoff C. Gender differences in determinants and consequences of health and illness. J Health Popul Nutr. (2007) 25(1):47–61.17615903
8. Frantz S, Buerke M, Horstkotte D, Levenson B, Mellert F, Naber CK, et al. Commentary on the 2015 European society of cardiology guidelines for infectious endocarditis. Der Kardiologe. (2016) 10:142–8. doi: 10.1007/s12181-016-0058-4
9. Delgado V, Ajmone Marsan N, de Waha S, Bonaros N, Brida M, Burri H, et al. 2023 ESC guidelines for the management of endocarditis: developed by the task force on the management of endocarditis of the European society of cardiology (ESC) endorsed by the European association for cardio-thoracic surgery (EACTS) and the European association of nuclear medicine (EANM). Eur Heart J. (2023) 44(39):3948–4042. doi: 10.1093/eurheartj/ehad193
10. Aksoy O, Meyer LT, Cabell CH, Kourany WM, Pappas PA, Sexton DJ. Gender differences in infective endocarditis: pre- and co-morbid conditions lead to different management and outcomes in female patients. Scand J Infect Dis. (2007) 39(2):101–7. doi: 10.1080/00365540600993285
11. Available online at: https://www.escardio.org/static-file/Escardio/Subspecialty/EACPR/Documents/score-charts.pdf (Accessed August 05, 2024).
12. Raberin A, Connes P, Barthélémy JC, Robert P, Celle S, Hupin D, et al. Role of gender and physical activity level on cardiovascular risk factors and biomarkers of oxidative stress in the elderly. Oxid Med Cell Longev. (2020) 2020:1315471. doi: 10.1155/2020/1315471
13. Von den Höfel N, Schulte T, Antwerpes F, Ostendorf N, Fink B. Charlson Comorbidity Index. Available online at: flexicon.doccheck.com. Charlson Comorbidity Index, (Accessed August 06, 2024).
14. Mettler SK, Alhariri H, Okoli U, Charoenngam N, Guillen RH, Jaroenlapnopparat A, et al. Gender, age, and regional disparities in the incidence and mortality trends of infective endocarditis in the United States between 1990 and 2019. Am J Cardiol. (2023) 203:128–35. doi: 10.1016/j.amjcard.2023.07.018
15. DeFilippis EM, Van Spall HGC. Is it time for sex-specific guidelines for cardiovascular disease? J Am Coll Cardiol. (2021) 78(2):189–92. doi: 10.1016/j.jacc.2021.05.012
16. Delgado V, Ajmone Marsan N, de Waha S, Bonaros N, Brida M, Burri H, et al. 2023 ESC guidelines for the management of endocarditis. Eur Heart J. (2023) 44(39):3948–4042. doi: 10.1093/eurheartj/ehad193 Erratum in: Eur Heart J. 2023 December 1;44(45):4780. doi: 10.1093/eurheartj/ehad625. Erratum in: Eur Heart J. 2024 January 1;45(1):56. doi: 10.1093/eurheartj/ehad776.37622656
17. Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, et al. 2015 ESC guidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the European society of cardiology (ESC). Endorsed by: European association for cardio-thoracic surgery (EACTS), the European association of nuclear medicine (EANM). Eur Heart J. (2015) 36(44):3075–128. doi: 10.1093/eurheartj/ehv319
18. Brugada J, Katritsis DG, Arbelo E, Arribas F, Bax JJ, Blomström-Lundqvist C, et al. 2019 ESC guidelines for the management of patients with supraventricular tachycardia. The task force for the management of patients with supraventricular tachycardia of the European society of cardiology (ESC). Eur Heart J. (2020) 41(5):655–720. doi: 10.1093/eurheartj/ehz467 Erratum in: Eur Heart J. 2020 November 21;41(44):4258. doi: 10.1093/eurheartj/ehz827.31504425
19. van der Vaart TW, Bossuyt PMM, Durack DT, Baddour LM, Bayer AS, Durante-Mangoni E, et al. External validation of the 2023 Duke-international society for cardiovascular infectious diseases diagnostic criteria for infective endocarditis. Clin Infect Dis. (2024) 78(4):922–9. doi: 10.1093/cid/ciae033
20. Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr., Tleyhej IM, Rybak MJ, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American heart association. Circulation. (2015) 132(15):1435–86. doi: 10.1161/CIR.0000000000000296
21. McDonald EG, Aggrey G, Tarik Aslan A, Casias M, Cortes-Penfield N, Dong MQD, et al. Guidelines for diagnosis and management of infective endocarditis in adults: a WikiGuidelines group consensus statement. JAMA Netw Open. (2023) 6(7):e2326366. doi: 10.1001/jamanetworkopen.2023.26366 Erratum in: JAMA Netw Open. 2023 August 1;6(8):e2332858. doi: 10.1001/jamanetworkopen.2023.32858. Erratum in: JAMA Netw Open. 2023 October 2;6(10):e2341784. doi: 10.1001/jamanetworkopen.2023.41784.37523190
22. Kuo YS, Tsai YM. Exploring the risk factors and possible role of calcium in infective endocarditis. Diagnostics. (2023) 13(14):2386. doi: 10.3390/diagnostics13142386
23. Philip M, Tessonier L, Mancini J, Mainardi J-L, Fernandez-Gerlinger M-P, Lussato D, et al. Comparison between ESC and Duke criteria for the diagnosis of prosthetic valve infective endocarditis. J Am Coll Cardiol Img. (2020) 13(12):2605–15. doi: 10.1016/j.jcmg.2020.04.011
24. Fowler VG Jr, Durack DT, Selton-Suty C, Athan E, Bayer AS, Chamis AL, et al. The 2023 Duke-international society for cardiovascular infectious diseases criteria for infective endocarditis: updating the modified Duke criteria. Clin Infect Dis. (2023) 77(4):518–26. doi: 10.1093/cid/ciad271 Erratum in: Clin Infect Dis. 2023 October 13;77(8):1222. doi: 10.1093/cid/ciad510.37138445
25. Murdoch DR, Corey GR, Hoen B, Miró JM, Fowler VG Jr, Bayer AS, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century. Arch Intern Med. (2009) 169(5):463–73. doi: 10.1001/archinternmed.2008.603
26. Toyoda N, Chikwe J, Itagaki S, Gelijns AC, Adams DH, Egorova NN. Trends in infective endocarditis in California and New York state, 1998–2013. JAMA. (2017) 317(16):1652–60. doi: 10.1001/jama.2017.4287
27. Havers-Borgersen E, Butt JH, Østergaard L, Petersen JK, Torp-Pedersen C, Køber L, et al. Long-term incidence of infective endocarditis among patients with congenital heart disease. Am Heart J. (2023) 259:9–20. doi: 10.1016/j.ahj.2023.01.012
28. Thornhill MH, Jones S, Prendergast B, Baddour LM, Chambers JB, Lockhart PB, et al. Quantifying infective endocarditis risk in patients with predisposing cardiac conditions. Eur Heart J. (2018) 39(7):586–95. doi: 10.1093/eurheartj/ehx655
29. Poorzand H, Hamidi F, Sheybani F, Ghaderi F, Fazlinezhad A, Alimi H, et al. Infective endocarditis: clinical characteristics and echocardiographic findings. Front Cardiovasc Med. (2022) 9:789624. doi: 10.3389/fcvm.2022.789624
30. Kadri AN, Wilner B, Hernandez AV, Nakhoul G, Chahine J, Griffin B, et al. Geographic trends, patient characteristics, and outcomes of infective endocarditis associated with drug abuse in the United States from 2002 to 2016. J Am Heart Assoc. (2019) 8(19):e012969. doi: 10.1161/JAHA.119.012969
31. Njoroge LW, Al-Kindi SG, Koromia GA, ElAmm CA, Oliveira GH. Changes in the association of rising infective endocarditis with mortality in people who inject drugs. JAMA Cardiol. (2018) 3(8):779–80. doi: 10.1001/jamacardio.2018.1602
32. Wilson W, Taubert KA, Gewitz M, Lockhart PB, Baddour LM, Levison M, et al. Prevention of infective endocarditis: guidelines from the American heart association: a guideline from the American heart association rheumatic fever, endocarditis, and kawasaki disease committee, council on cardiovascular disease in the young, and the council on clinical cardiology, council on cardiovascular surgery and anesthesia, and the quality of care and outcomes research interdisciplinary working group. Circulation. (2007) 116(15):1736–54. doi: 10.1161/CIRCULATIONAHA.106.183095 Erratum in: Circulation. 2007 October 9;116(15):e376-7.17446442
33. Rudasill SE, Sanaiha Y, Mardock AL, Khoury H, Xing H, Antonios JW, et al. Clinical outcomes of infective endocarditis in injection drug users. J Am Coll Cardiol. (2019) 73(5):559–70. doi: 10.1016/j.jacc.2018.10.082
34. Massoure PL, Reuter S, Lafitte S, Laborderie J, Bordachard P, Clementy J, et al. Pacemaker endocarditis: clinical features and management of 60 consecutive cases. Pacing Clin Electrophysiol. (2007) 30(1):12–9. doi: 10.1111/j.1540-8159.2007.00574.x
35. Duval X, Millot S, Chirouze C, Selton-Suty C, Moby V, Tattevin P, et al. Oral streptococcal endocarditis, oral hygiene habits, and recent dental procedures: a case-control study. Clin Infect Dis. (2017) 64(12):1678–85. doi: 10.1093/cid/cix237
36. Abegaz TM, Bhagavathula AS, Gebreyohannes EA, Mekonnen AB, Abebe TB. Short- and long-term outcomes in infective endocarditis patients: a systematic review and meta-analysis. BMC Cardiovasc Disord. (2017) 17(1):291. doi: 10.1186/s12872-017-0729-5 Erratum in: BMC Cardiovasc Disord. 2018 January 12;18(1):5. doi: 10.1186/s12872-018-0742-3.29233094
37. Lalani T, Chu VH, Park LP, Cecchi E, Corey GR, Durante-Mangoni E, et al. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern Med. (2013) 173(16):1495–504. doi: 10.1001/jamainternmed.2013.8203 Erratum in: JAMA Intern Med. 2013 October 28;173(19):1846.23857547
38. Ritchie BM, Hirning BA, Stevens CA, Cohen SA, DeGrado JR. Risk factors for acute kidney injury associated with the treatment of bacterial endocarditis at a tertiary academic medical center. J Chemother. (2017) 29(5):292–8. doi: 10.1080/1120009X.2017.1296916 Erratum in: J Chemother. 2017 Jun;29(3):195. doi: 10.1080/1120009X.2017.1305166.28245728
39. Von Tokarski F, Lemaignen A, Portais A, Fauchier L, Hennekinne F, Sautenet B, et al. Risk factors and outcomes of early acute kidney injury in infective endocarditis: a retrospective cohort study. Int J Infect Dis. (2020) 99:421–7. doi: 10.1016/j.ijid.2020.08.022
40. Buchholtz K, Larsen CT, Hassager C, Bruun NE. In infectious endocarditis patients mortality is highly related to kidney function at time of diagnosis: a prospective observational cohort study of 231 cases. Eur J Intern Med. (2009) 20(4):407–10. doi: 10.1016/j.ejim.2008.12.017
41. Cahill TJ, Baddour LM, Habib G, Hoen B, Salaun E, Pettersson GB, et al. Challenges in infective endocarditis. J Am Coll Cardiol. (2017) 69(3):325–44. doi: 10.1016/j.jacc.2016.10.066
42. Naderi HR, Sheybani F, Erfani SS. Errors in diagnosis of infective endocarditis. Epidemiol Infect. (2018) 146(3):394–400. doi: 10.1017/S0950268817002977
43. Gould FK, Denning DW, Elliott TS, Foweraker J, Perry JD, Prendergast BD, et al. Guidelines for the diagnosis and antibiotic treatment of endocarditis in adults: a report of the working party of the British society for antimicrobial chemotherapy. J Antimicrob Chemother. (2012) 67(2):269–89. doi: 10.1093/jac/dkr450 Erratum in: J Antimicrob Chemother. 2012 May;67(5):1304.22086858
Keywords: sex, infectious endocarditis, diagnostics, Duke criteria, risk factors, outcomes, diagnostic delay
Citation: Andreß S, Reischmann K, Markovic S, Rohlmann F, Hay B, Rottbauer W, Buckert D and d'Almeida S (2025) Men's more frequent predisposing factors in infectious endocarditis facilitate improvement of outcomes by shortening of diagnostic delay. Front. Cardiovasc. Med. 11:1517288. doi: 10.3389/fcvm.2024.1517288
Received: 25 October 2024; Accepted: 27 December 2024;
Published: 12 February 2025.
Edited by:
Anita Cote, Trinity Western University, CanadaReviewed by:
Alberto Albertini, Hesperia Hospital, ItalyCopyright: © 2025 Andreß, Reischmann, Markovic, Rohlmann, Hay, Rottbauer, Buckert and d'Almeida. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: S. d'Almeida, U2FzY2hhLmFsbWVpZGFAdW5pa2xpbmlrLXVsbS5kZQ==
†ORCID:
S. d'Almeida
orcid.org/0000-0002-6648-9318
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.