- 1Department of Cardiac Pacing and Electrophysiology, Hopital Cardiologique du Haut-Leveque, Bordeaux University Hospital (CHU), Bordeaux, France
- 2IHU Liryc, Electrophysiology and Heart Modeling Institute, University Bordeaux, Bordeaux, France
Introduction: The precise pathophysiology of common atrial flutter remains imperfectly known. The mechanisms of arrhythmia initiation and the role of areas of slow conducting myocardium and functional block are still debated topics.
Methods: We conducted a detailed electrophysiological study of a patient to illustrate and refine these concepts. Prior to CTI ablation, electrophysiological study and electro-anatomical mapping were performed, focusing on initiation and maintenance mechanisms of the arrhythmia.
Results: The initiation of common atrial flutter takes place on the septal aspect of the cavo-tricuspid isthmus where functional unidirectional conduction block occurs. The direction of activation is therefore frequently counter-clockwise, and the arrhythmia stabilizes around the vena cavas and sinus venosus/crista terminalis region. No conduction slowing is present.
Conclusions: Common atrial flutter initiates when functional unidirectional conduction block occurs on the septal cavotricuspid isthmus. Its rotation is limited by anatomical and functional boundaries.
Introduction
Common atrial flutter refers to a macro-reentrant tachycardia around the tricuspid valve, in the clockwise or counter-clockwise direction (1). This arrhythmia is the most common organized atrial tachycardia and—as such—its initiation and maintenance have been thoroughly studied (2, 3). In terms of initiation, clinical studies have demonstrated the importance of premature atrial contractions (4) (PACs), and their role in determining the reentry direction (5). In terms of maintenance, the arrhythmia rotates around the tricuspid valve, and channels through the narrow region between this valve and the inferior vena cava called the cavo-tricuspid isthmus (CTI). Strategies targeting the initiation of the arrhythmia (pulmonary vein isolation to avoid premature atrial contractions) (6) or its maintenance (linear ablation at the CTI) have both demonstrated their efficiency, reinforcing our confidence in these two pathophysiological components.
Despite this, the precise pathophysiology of this arrhythmia remains imperfectly understood. More specifically, the precise mechanisms of arrhythmia initiation and the role of areas of slow conducting myocardium and functional block are still debated topics.
In this work, we examine existing literature describing the role of functional block in the initiation and maintenance of common atrial flutter, and a detailed electrophysiological study of a patient to illustrate and refine these concepts.
Methods
Mapping procedure
The initiation and maintenance mechanisms of common atrial flutter were studied in 10 patients in total. Prior to CTI ablation, electrophysiological study and electro-anatomical mapping were performed, focusing on initiation and maintenance mechanisms of the arrhythmia. Electro-anatomical mapping was performed using the Carto3 mapping system (Biosense Webster, Diamond Bar, CA). A steerable quadripolar catheter (Dynamic XT, Boston Scientific, MA) was positioned in the coronary sinus and used to perform atrial pacing. A multipolar mapping catheter (Octaray, Biosense Webster, Diamond Bar, CA) was used to perform the right atrial anatomical shell reconstruction and recording cardiac electrograms during pacing maneuvers. During pacing, it was positioned on the CTI, and the pacing protocol described hereafter was performed. In all but one patient, conduction block led to immediate initiation of CTI-dependent flutter, precluding thorough mapping of the arrhythmia onset.
The patient presented in this study is a 53-year old male patient with no comorbidities who was scheduled for atrial flutter ablation due to recurrent episodes of symptomatic counterclockwise common atrial flutter. The procedure was carried out under conscious sedation.
Results
Functional block identification and flutter induction protocol
Based on prior studies (5, 7, 8) functional block leading to atrial flutter initiation was presumed to occur in the “septal isthmus” region, and at the junction of the sinus venosus and crista terminalis. We therefore performed an atrial stimulation study to identify these functional blocks. Programmed atrial stimulation with S1–S2 was performed by pacing the proximal coronary sinus, starting at 600-400 ms intervals with 10 ms decrement up to the atrial effective refractory period (ERP). The multipolar mapping catheter was positioned on the CTI to identify abrupt changes in activation sequence or timing, reflecting functional block. Since S2 extrastimuli failed to produce functional block at atrial ERP (280 ms), S3 stimuli were added, starting at 300 ms, with 10 ms decrements with fixed S2 at 300 ms.
An abrupt change in the activation pattern on the CTI occurred after a 600-300-240 ms sequence. This pacing sequence was then repeated to construct a right atrial map of the S1, S2 and S3 activation patterns (see below).
When the map was complete, the S3 beat was further decremented. No additional abrupt changes were observed, until a sequence of 600-300-210 ms at which point CTI-dependent flutter was induced (Figure 1). A map of the flutter was created and compared to the previous maps.
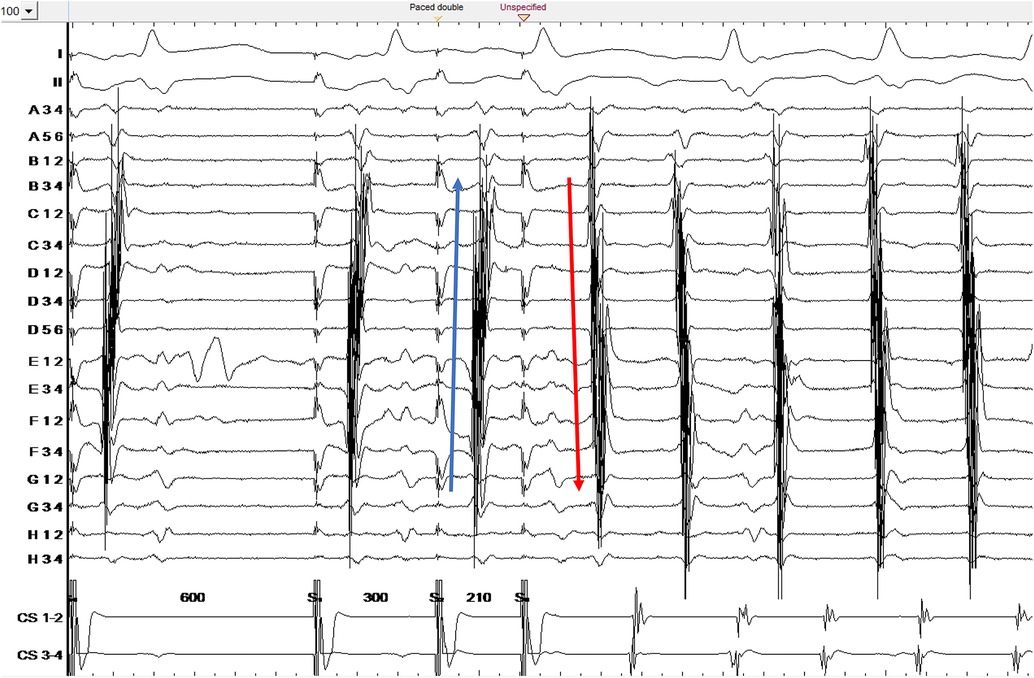
Figure 1. Counterclockwise common atrial flutter induction. Ortaray is positioned at the cavo-tricuspid isthmus level. Note the change in electrogram activation sequence for S3 due to functional block in the septal isthmus region. S3 depicts a unidirectional conduction block on the septal cavo-tricuspid isthmus.
S1, S2 and S3 mapping technique and interpretation
To map the S1, S2 and S3 activation patterns, Carto acquisition filters were set to acquire beats corresponding to cycle lengths in the 235–245 ms range on the coronary sinus, allowing us to construct an S3 activation map. All other filters were turned off except for mapping catheter position stability. The map was then copied, and the window of interest changed to visualize the S2 beat at the same points, and finally changed again to image the last S1 beat of the drive train (Figure 2).

Figure 2. Programmed atrial stimulation with S1-S2-S3 trains (from the proximal coronary sinus). In order to obtain S1, S2 and S3 maps during a single acquisition, the window of interest (WOI) was first set to register S3 potentials, then moved backwards to encompass S2 potentials and finally moved back again to bracket S1 potentials.
The main qualitative difference between the S1 and S2 maps is the appearance of a line block at the septal aspect of the SVC-RA junction (Figures 3A,B). This line of block is not relevant for CTI-dependent flutter induction.
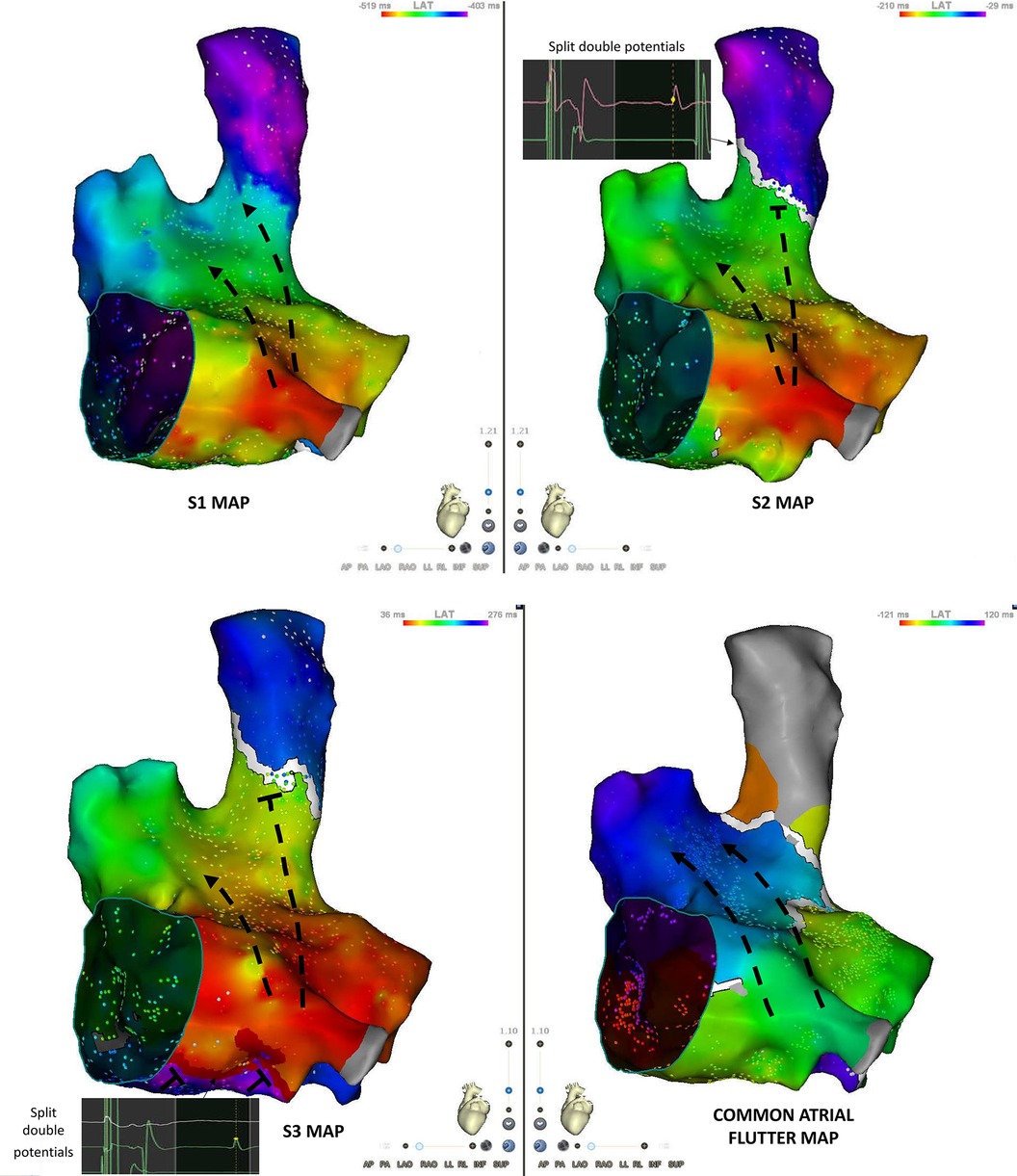
Figure 3. S1, S2, S3 and flutter maps of the right atrium in an anteroseptal view. S2 is associated with the occurrence of a conduction block at the junction between the superior vena cava and the right atrium. Double potentials are recorded along the line of block. S3 maps shows the appearance of a line of block in the “septal isthmus” region with double potentials. Atrial flutter map shows a counter-clockwise activation sequence.
The S3 map shows the additional appearance of a line of block at the septal aspect of the cavo-tricuspid isthmus (Figures 3B,C). The electrogram activation sequence inversion on the Octaray positioned at the CTI level clearly depicts this phenomenon (Figure 1).
The main qualitative difference between the atrial flutter and S3 maps was the line of block along the posterior aspect of the right atrium in the sinus venosus/crista terminalis region on which double potentials could be recorded (Figure 4). Isochronal mapping showed no significant conduction slowing in other areas.
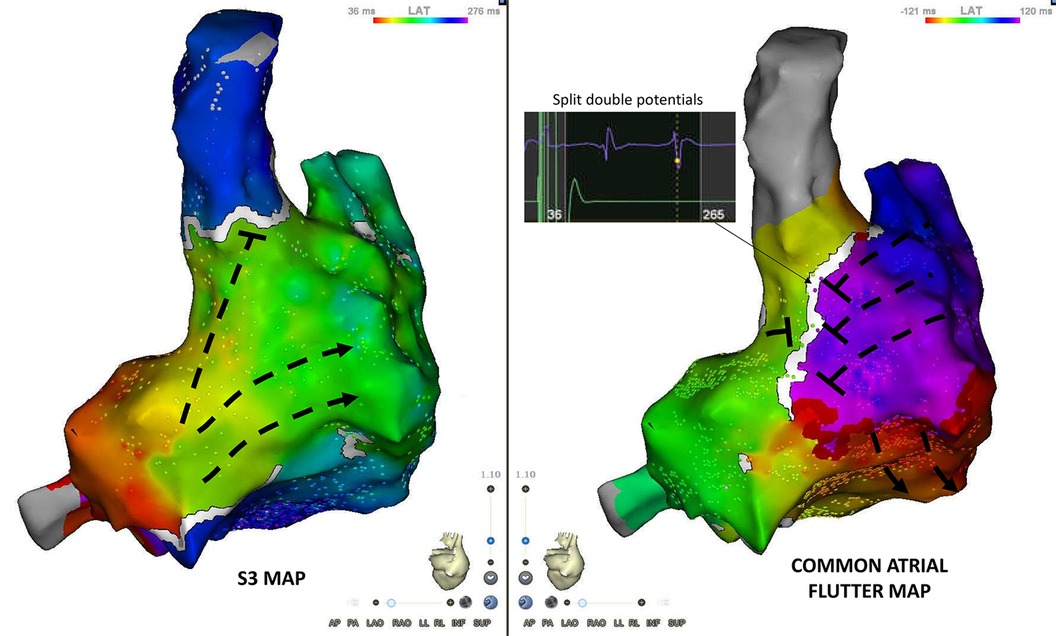
Figure 4. Posterior view of S3 and flutter maps. The main qualitative difference between both maps is the appearance of conduction block along the posterior aspect of the right atrium (sinus venosus/crista terminalis region). Double potentials are recorded along the line of block.
Discussion
Through this report, we illustrate the role of functional block in the initiation and maintenance of common atrial flutter.
Initiation: unidirectional conduction block on the septal cavo-tricuspid isthmus
In this patient, a double extra-stimulus provoked a unidirectional conduction block on the septal aspect of the cavo-tricuspid isthmus.
We confirm the observation made by Cosio et al. Indeed, the authors did not have electroanatomic maps at the time but based on electrogram analysis they described that typical flutter begins by low septal block (7). Olgin et al., using fluoroscopy and electrogram analysis described the site of unidirectional block during the initiation of clockwise and counterclockwise flutter was in the low right atrium isthmus (8).
Conduction block on the septal aspect of the cavo-tricuspid isthmus may be due to the occurrence of source-sink mismatch in this region characterized by a specific fiber arrangement. Atrial myocardium is very thin and fiber directions criss-cross in the approaches to the atrioventricular node (9), without the circumferential fibers that follow the tricuspid ring (10).
Moreover, the septal aspect of the cavotricuspid isthmus is consistently a site of wavefront collision in sinus rhythm (11). This fact may favor the occurrence of functional conduction block when a PAC from another location associated with a different wavefront occurs.
The direction of the circuit depends on the orientation of the conduction block. We describe that left sided activation (extrastimuli from the coronay sinus) induces counterclockwise flutter. Likewise, Olgin et al. showed that pacing from the smooth right atrium induced counterclockwise flutter, whereas pacing from the trabeculated right atrium induced clockwise flutter (8). PACs originating from the pulmonary veins are the main triggers for common flutter (6). Therefore, the conduction is directed from septal to lateral isthmus and unidirectional block induces counter-clockwise rotation. The latter type of flutter is thus the most prevalent.
Maintenance: circuit rotating around three anatomical and a functional boundary
After induction, the circuit stabilizes with a counter-clockwise rotation around three anatomical obstacles: the tricuspid annulus, the inferior and superior vena cava ostia.
Another conduction block is necessary for common atrial flutter maintenance: functional block along the posterior aspect of the right atrium, in the sinus venosus/crista terminalis region.
This phenomenon has been described by Friedman et al. in both clockwise and counterclockwise atrial flutter (12). It is probably due to craniocaudal alignment of myocardium at the level of the crista terminalis at the interface with the sinus venosus smooth myocardium. Myocardial anisotropy is also favored by a predominance of “end to end” gap junctions making transverse conduction up to 10 times slower than longitudinal conduction in that region (13).
Arenal et al. showed that transverse conduction block along the posterior sinus venosus is rate-dependent and the propensity to block depends on the location of the pacing site (sooner with posterior wall compared to lateral wall pacing) (5). This finding echoes the fact that pulmonary vein PACs frequently trigger atrial flutter (6), and therefore the sinus venosus will mostly be activated via the posterior wall of the right atrium in real life settings.
In our patient and with the pacing protocol we used, this conduction block occurred at shorter coupling intervals than the ones required for septal isthmus block. Nevertheless, this block appeared important for arrhythmia initiation and maintenance. By prolonging the conduction time required to go around the tricuspid valve, it allowed all cells along the circuit to exit their refractory period and transform unidirectional block into circular reentry.
Generalizability of these observations
Our ability to map this phenomenon (septal isthmus block without immediate flutter induction) implies that the conduction time of the unidirectionally blocked beat around the tricuspid annulus was shorter than the refractory period of the septal isthmus. This is likely a rare finding, explaining why it could not be observed in 9/10 of the patients in this study. The observations of Arenal et al. (5) suggest that—contrary to what was seen in the presented patient—block of the posterior sinus venosus generally occurs prior to the septal isthmus block (for longer extra stimulus intervals). This contributes to prolonging the peri-tricuspid conduction time and immediate initiation flutter after septal isthmus block.
Conclusions
The initiation of common atrial flutter takes place on the septal aspect of the cavo-tricuspid isthmus where functional unidirectional conduction block occurs. The direction of activation is therefore frequently counter-clockwise, and the arrhythmia stabilizes around anatomical and functional boundaries.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Comité éthique CHU de Bordeaux. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MA: Conceptualization, Data curation, Methodology, Writing – original draft. BS: Investigation, Methodology, Writing – review & editing. MeH: Writing – review & editing. PJ: Writing – review & editing. MiH: Writing – review & editing. JD: Conceptualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by a grant IHU LIRYC ANR-10-IAHU-04.
Acknowledgments
The authors thank the medical team, nurses and engineers without whom this work would not have been possible.
Conflict of interest
JD received modest consulting fees and speaking honoraia from Biosense Webster.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1494836/full#supplementary-material
References
1. Saoudi N, Cosío F, Waldo A, Chen SA, Iesaka Y, Lesh M, et al. A classification of atrial flutter and regular atrial tachycardia according to electrophysiological mechanisms and anatomical bases. A statement from a joint expert group from the working group of arrhythmias of the European society of cardiology and the North American society of pacing and electrophysiology. Eur Heart J. (2001) 22(14):1162–82. doi: 10.1053/euhj.2001.2658
2. Valeri Y, Bagliani G, Compagnucci P, Volpato G, Cipolletta L, Parisi Q, et al. Pathophysiology of typical atrial flutter. Card Electrophysiol Clin. (2022) 14(3):401–9. doi: 10.1016/j.ccep.2022.05.003
3. Compagnucci P, Casella M, Bagliani G, Capestro A, Volpato G, Valeri Y, et al. Atrial flutter in particular patient populations. Card Electrophysiol Clin. (2022) 14(3):517–32. doi: 10.1016/j.ccep.2022.05.002
4. Mines GR. On circulating excitations in heart muscles and their possible relation to tachycardia and fibrillation. Trans R Soc Can. (1914) 4:43–53.
5. Arenal A, Almendral J, Alday JM, Villacastín J, Ormaetxe JM, Sande JL, et al. Rate-dependent conduction block of the crista terminalis in patients with typical atrial flutter. Circulation. (1999) 99(21):2771–8. doi: 10.1161/01.CIR.99.21.2771
6. Schneider R, Lauschke J, Tischer T, Schneider C, Voss W, Moehlenkamp F, et al. Pulmonary vein triggers play an important role in the initiation of atrial flutter: initial results from the prospective randomized atrial fibrillation ablation in atrial flutter (triple A) trial. Heart Rhythm. (2015) 12(5):865–71. doi: 10.1016/j.hrthm.2015.01.040
7. Cosío FG, López-Gil M, Arribas F, González HD. Mechanisms of induction of typical and reversed atrial flutter. J Cardiovasc Electrophysiol. (1998) 9(3):281–91. doi: 10.1111/j.1540-8167.1998.tb00913.x
8. Olgin JE, Kalman JM, Saxon LA, Lee RJ, Lesh MD. Mechanism of initiation of atrial flutter in humans: site of unidirectional block and direction of rotation 11 all editorial decisions for this article, including selection of referees, were made by a guest editor. This policy applies to all articles with authors from the University of California San Francisco. J Am Coll Cardiol. (1997) 29(2):376–84. doi: 10.1016/S0735-1097(96)00480-9
9. Racker DK. Atrioventricular node and input pathways: a correlated gross anatomical and histological study of the canine atrioventricular junctional region. Anat Rec. (1989) 224:336–54. doi: 10.1002/ar.1092240303
10. Racker DK, Ursell PG, Hoffman BF. Anatomy of the tricuspid annulus. Circumferential myofibers as the structural basis for atrial flutter in a canine model. Circulation. (1991) 84:841–51. doi: 10.1161/01.CIR.84.2.841
11. Pambrun T, Derval N, Duchateau J, Ramirez FD, Chauvel R, Tixier R, et al. Sinus node exit, crista terminalis conduction, interatrial connection, and wavefront collision: key features of human atrial activation in sinus rhythm. Heart Rhythm. (2022) 19(5):701–9. doi: 10.1016/j.hrthm.2022.01.016
12. Friedman PA, Luria D, Fenton AM, Munger TM, Jahangir A, Shen WK, et al. Global right atrial mapping of human atrial flutter: the presence of posteromedial (sinus venosa region) functional block and double potentials. Circulation. (2000) 101(13):1568–77. doi: 10.1161/01.CIR.101.13.1568
Keywords: common flutter, initiation, maintenance, mechanisms, pathophysiology
Citation: Arnaud M, Sacristan B, Hocini M, Jais P, Haissaguerre M and Duchateau J (2024) Functional block in the initiation and maintenance of common flutter: detailed electrophysiological study and electro-anatomical mapping. Front. Cardiovasc. Med. 11:1494836. doi: 10.3389/fcvm.2024.1494836
Received: 11 September 2024; Accepted: 25 October 2024;
Published: 15 November 2024.
Edited by:
Rui Providencia, University College London, United KingdomReviewed by:
Paolo Compagnucci, Marche Polytechnic University, ItalyMark Gallagher, St George’s University Hospitals NHS Foundation Trust, United Kingdom
Giuseppe Stabile, Clinica Mediterranea, Italy
Copyright: © 2024 Arnaud, Sacristan, Hocini, Jais, Haissaguerre and Duchateau. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marine Arnaud, YXJuYXVkLm1hcmluZUB5YWhvby5mcg==
 Marine Arnaud
Marine Arnaud Benjamin Sacristan1,2
Benjamin Sacristan1,2 Meleze Hocini
Meleze Hocini Michel Haissaguerre
Michel Haissaguerre Josselin Duchateau
Josselin Duchateau