- 1Faculty of Medicine, Transilvania University of Brasov, Brasov, Romania
- 2Department of Cardiology, Emergency County Hospital, Brasov, Romania
- 3Cardiovascular Rehabilitation Hospital Covasna, Covasna, Romania
- 4Clinical Laboratory, Emergency County Hospital, Brasov, Romania
- 5Department of Cardiology, ICCO Clinics, Brasov, Romania
Objective: The present study aimed at evaluating the association between sympathetic nervous system activation (SNS) and the severity of coronary artery disease (CAD). In addition, we tested the hypothesis that inflammation and oxidative stress influence the SNS activation.
Methods: Adult patients with severe CAD scheduled for coronary artery bypass graft (CABG) surgery were enrolled. SYNTAX I score was calculated based on coronary angiography. Systemic activation of the SNS was estimated through circulating levels of norepinephrine (NE). Plasma levels of pro-inflammatory cytokines (IL 1β, IL 6 and HIF 1α) and oxidative stress molecules (SOD-1 and LOX-1) were obtained prior to surgery.
Results: Circulating NE levels were significantly correlated with the severity of CAD, as assessed by the SYNTAX I score (p 0.002; r 0.329). Elevated levels of circulating pro-inflammatory markers were significantly correlated with increased NE concentrations (for IL-1β: p < 0.001, r = 0.49; for IL-6 and NE: p = 0.003, r = 0.32; for HIF-1α and NE: p = 0.049, r = 0.21). Additionally, oxidative stress molecules were associated with circulating NE levels (for SOD-1 and NE: p = 0.016, r = 0.26; for LOX-1 and NE: p = 0.004, r = 0.31).
Conclusion: In patients with CAD referred for CABG, SNS activation, indicated by plasma NE levels, was correlated with disease severity as assessed by the SYNTAX I score, as well as with markers of inflammation and oxidative stress. This suggests that inflammation, oxidative stress, and SNS activation form an interconnected network, with each component influencing the others. It might be of interest to develop a scoring system including inflammation and oxidative stress markers to identify patients that require a more aggressive approach to lower inflammation, oxidative stress and modulate the sympathetic nervous system. This could be of use especially in the setting of a scheduled intervention -such as CABG surgery.
1 Introduction
Inflammation plays a pivotal role in all stages of atherosclerosis, from subclinical endothelial dysfunction to severe atheroma burden and acute cardiovascular events (1). The interleukine-1 (IL-1) family is a major cytokine family (to date 11 members have been discovered; initially comprising only two forms, IL 1 α and IL 1 β) an associated with various cardiovascular diseases (2). Interleukine-6 (IL-6), also an important marker of inflammation, is independently associated with major adverse cardiovascular disease (2, 3). Previous studies have shown that injections of IL 1 and IL 6, pro inflammatory cytokines, cause an activation of the SNS, with release of norepinephrine (NE) from the central as well as the peripheral SNS (4). Hypoxic areas in atheroma plaques lead to the expression of hypoxia-inducible factor 1-alpha (HIF 1 α). HIF 1 α influences cellular functions and cytokine expression inmacrophages, vascular smooth muscle cells and endothelial cells, key elements of the atherosclerotic process (5). Oxidative stress contributes to the development of coronary artery disease (6), lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1), of the scavenger receptors for oxidized low-density lipoprotein cholesterol (ox-LDL)- and superoxide dismutase-1 (SOD 1) being crucial components of the process. Oxidative modifications of low-density lipoprotein to oxidized LDL play an important role in the initiation and progression of atherosclerosis. LOX-1 was identified as the major oxidized LDL receptor in endothelial cells (7). SOD 1 (along with other enzymes in the SOD family) protects cells by scavenging harmful superoxide radicals. SOD is an important marker of lipid peroxidation and of the progression of atherosclerosis correlated with oxidative stress (8).
In ischemic cardiomyopathy aberrant remodeling of the SNS occurs, with effects mainly modulated by NE. Histologic changes in stellate ganglia neurons have been observed resulting in inflammation, glial cell activation, and oxidative stress (9, 10).
Growing evidence suggests that the autonomic nervous system (ANS) plays a crucial role in regulating systemic inflammation, and an imbalance in the ANS may elevate the risk of acute cardiovascular events by promoting inflammation and damaging the endothelium (11).
Clinical studies have previously demonstrated an inverse correlation between SNS activation, assessed through heart rate variability, and chronic low-grade systemic inflammation in patients with stable CAD (12). However, it remains unclear whether SNS activation and inflammation are directly associated with the severity of CAD.
This study aimed to evaluate the association between norepinephrine (NE), as a surrogate marker of sympathetic nervous system (SNS) activation, and the severity of coronary artery disease (CAD), as determined by the SYNTAX score. Additionally, it sought to investigate potential correlations between inflammation, measured by IL-1β, IL-6, and HIF-1α, oxidative stress mediators, such as LOX-1 and SOD-1, and SNS activation, assessed through plasma NE levels, in patients with severe CAD who are candidates for surgical revascularization.
2 Methods
2.1 Design, study population
This study prospectively included 84 adult patients with severe CAD scheduled for CABG surgery, between January 2020 and June 2021. The study protocol obtained ethical clearance from the Ethical Committee of Transylvania University (registration number 1/2.03.2019) and adhered to the principles outlined in the Helsinki Declaration and the Code for Good Clinical Practice. A written Informed Consent was obtained from all patients.
Patients with acute coronary syndromes, significant valvular disease, severe hepatic or renal failure, recent or active bleeding, coagulation disorders, active malignancy, and inflammatory diseases (including infections and autoimmune disorders) were excluded from the study. Additionally, patients diagnosed with pheochromocytoma or those undergoing psychiatric treatment (such as with serotonin-norepinephrine reuptake inhibitors) were excluded due to the potential impact on norepinephrine (NE) levels. Patients taking alpha-2 blockers were also not included, and alpha agonists were not administered during anesthesia.
At admission, all patients underwent clinical, echocardiographic, and coronary angiography evaluations, and blood samples were collected in accordance with the clinic's protocol. Based on coronary angiogram, the SYNTAX I score was determined using the number of diseased arteries, the location, and the aspect of atherosclerotic plaques (https://syntaxscore.org/).
2.2 Measurement of biomarkers
Peripheral venous blood samples were drawn after a minimum of 8 h fasting, 3 days prior CABG. Samples were centrifuged and supernatant was frozen at −80°C until the final measurements. HIF 1 α, IL 1β, IL 6, LOX-1, and NE concentrations were determined by enzyme-linked immunosorbent assay (ELISA) and SOD-1 concentrations were measured using colorimetric determinations, with commercially available kits (Elabscience Biotechnology Inc., Houston, Texas, United States) in accordance with the manufacturer's instructions. All measurements were completed by the same technician who had no access to clinical information.
2.3 Statistical analysis
Categorical variables were expressed as n (%), normally distributed data and skewed data of continuous variables were expressed as mean ± standard deviation (SD), and median (minimum-maximum), respectively. The normality of continuous variables was tested by Shapiro-Wilk's test. To ascertain distinctions in the analyzed data, 2-tailed Pearson correlation (for variables with normal distribution) and Spearman's correlation (for variables with skewed distribution) were applied. Statistical significance was set at p < 0.05. Analysis was performed using Microsoft Excel 2007 and JASP 0.19 software.
3 Results
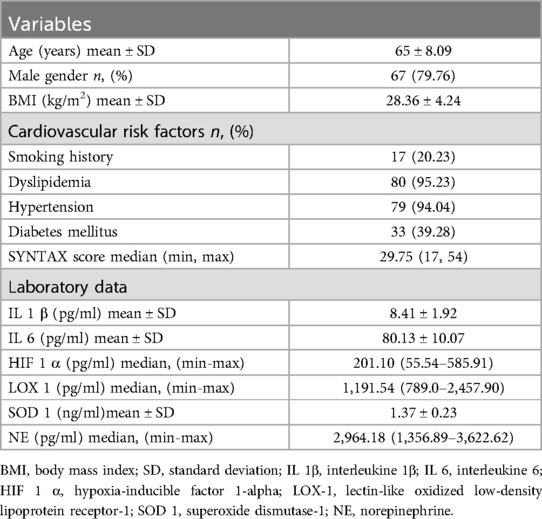
The study population consisted of 84 patients aged 46–88 (mean age: 65), of which 67 were males. The mean body mass index (BMI) was 28.36 kg/m2. The documented cardiovascular risk factors included smoking history (20.23%), dyslipidemia (95.23%), hypertension (94.04%), and diabetes mellitus (39.28%). Patients' characteristics and laboratory test findings are presented in Table 1.
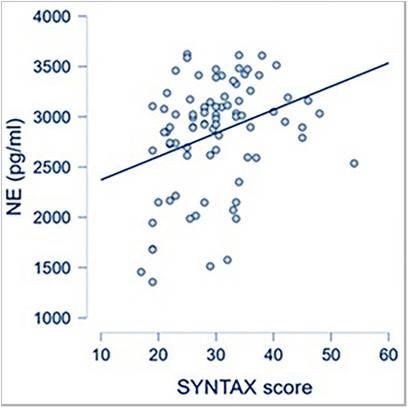
CAD severity, as reflected by a high SYNTAX score, was associated with increased systemic levels of NE, used as a surrogate for SNS activation (Figure 1).
The correlations between each inflammatory parameter and norepinephrine (NE) are shown in Figure 2. Statistically significant correlations were observed between IL-1β and NE, IL-6 and NE, as well as HIF-1α and NE.
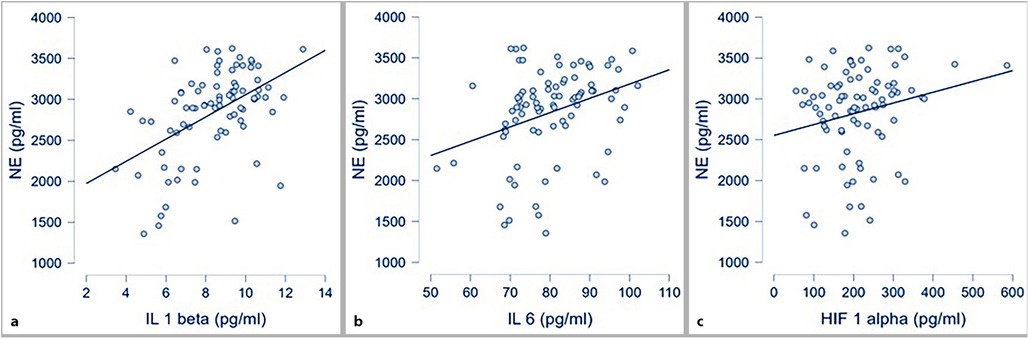
Figure 2. Correlations between inflammatory markers and NE; (a) IL 1 β and NE (p < .001; r 0.49); (b) IL 6 and NE (p = 0.003; r 0.32); (c) HIF 1α and NE (p 0.049; r 0.21).
Circulating levels of oxidative stress markers (SOD-1 and LOX-1) were also correlated with circulating NE at a statistically significant level, as presented in Figure 3.
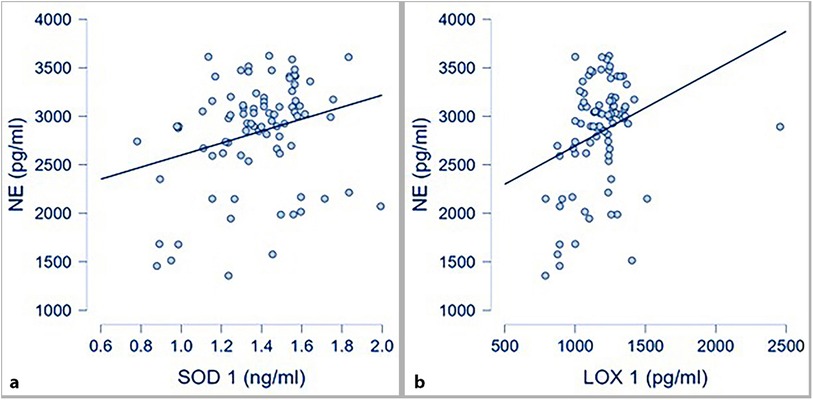
Figure 3. Oxidative stress markers and NE; (a) correlation between SOD-1 and NE (p 0.016; r 0.26); (b)* correlation between LOX-1 and NE (p .004; r 0.31; *spearman correlation for skewed distributed variables).
The analysis conducted to identify potential confounding factors found that none of the cardiovascular risk factors, including BMI, smoking, dyslipidemia, hypertension, and diabetes mellitus, was independently associated with NE levels, nor with the inflammatory markers (IL-1β, IL-6, HIF-1α) or oxidative stress indicators (SOD-1 and LOX-1) (see Supplementary Tables S1–S6 in the Supplementary Material).
4 Discussion
CAD is a disease state characterized by inflammation, increased oxidative stress and overstimulation of the SNS. Because these 3 aspects are interconnected, modulating any of them might prove useful for reducing the atherosclerotic burden and might, as well, have beneficial effects on the other components of this intricate network.
IL 1β plays a central role in local inflammation, being produced by monocytes/macrophages engulfed in the arterial wall in atherosclerosis. IL 1β mediates the production of IL 6, which in turn acts systemically activating more inflammatory cytokines and acute phase response proteins (13). Loss of viable myocardium in the setting of myocardial infarction is attributed to reperfusion injury and inflammatory response. Several recent trials have regarded pro-inflammatory cytokines as therapeutic targets, using specific antibodies in patients presenting with myocardial infarction. The largest of them, the CANTOS trial (Canakinumab Anti-inflammatory Thrombosis Outcome Study), which used Canakinumab, a monoclonal antibody binding selectively to IL 1β, enrolled patients with established CAD and residual inflammation. Its results proved a significant decrease of cardiovascular and cerebral events and cardiovascular death (14). However, the use of Tocilizumab, antibody targeting IL 6, did not show a significant clinical benefit in research conducted thus far (15, 16). An elevated pre-CABG surgery level of IL 6 has been associated with early graft occlusion and late cardiovascular events (17).
HIF 1 α is another molecule of interest, as it has links with both inflammation and oxidative stress. HIF 1 α stimulates macrophage activation, leading to lipid uptake and inflammation (including IL 1 β induction) and is also associated with reactive oxygen species (ROS) production by endothelial cells, activating NADPH oxidase genes (18). Also, HIF 1 α induces the expression of LOX 1 receptor at the cell surface, thus enhancing lipid uptake in macrophages (19). The HIF pathway remains of interest especially as its modulation could limit the effects of ischemia/reperfusion (for example in the of CABG surgery) or it could be used for ischemic pre-/post-conditioning, thus limiting the magnitude of an acute coronary event (20). However, recent research shows that maintaining an HIF 1 α intracellular homeostasis rather than totally inhibiting its effects could be the optimal approach to managing this important signaling pathway. HIF target-genes (such as adenosine receptors) could be activated directly. Some of these approaches may lead to novel pharmacologic strategies to prevent or treat organ injury in surgical patients (21). A recent trial showed that spironolactone is also involved in reducing HIF 1 α levels in patients undergoing CABG surgery (22).
SOD enzymes-SOD 1 being the major intracellular SOD- are part of the antioxidant defense system, implicated in lipid peroxidation and progression of atherosclerosis correlated with oxidative stress (23). However, in cases of increased SOD activity, such as in states of ischemia/reperfusion, the beneficial antioxidant effects are lost, increased damaged to the cell ensues (6, 8). CABG surgery induces increased levels of oxidative stress; hence the SOD system might be an efficient antioxidant (24).
Furthermore, increased ROS production also leads to the oxidation of native LDL to oxidized LDL (ox-LDL), which contributes to atherosclerotic plaque formation and progression by exerting its effects through its receptor-LOX-1 (25). LOX-1 expression is induced by several molecules: inflammatory cytokines (such as IL-1 and IL-6), angiotensin II, sheer stress, and glycation end-products. In turn, LOX-1 amplifies ROS formation by increasing NADPH oxidase activity. LOX-1 levels are upregulated in the heart after an ischemia/reperfusion event (CABG surgery), making it a potential target for therapy (26, 27).
Our study used a unique combination of biomarkers of inflammation (IL 1 beta, Il 6 and HIF 1 alpha) and oxidative stress (SOD 1 and LOX 1), each studied parameter proving to be correlated with increased levels of NE, neurohormone used to assess the systemic activity of the SNS.
To our knowledge, this is the first study to evaluate this combination of serum markers in patients with severe coronary artery disease (CAD) scheduled for surgical revascularization—a procedure that exposes patients to significant ischemia/reperfusion. The study demonstrates a statistical correlation between these markers and plasma norepinephrine (NE) levels, which serve as a surrogate for sympathetic nervous system (SNS) activation.
Recent research has proven that SNS is intimately associated with inflammation and oxidative stress (28, 29). Stimulation of central NE release also increases peripheral plasma NE: experimental studies conducted so far have shown that IL-1β and IL-6 injections determine short-lasting increases of NE (30, 31). In contrast, our study found high NE plasma concentrations correlated with high systemic levels of IL-1 β and IL-6, most likely a chronic state in patients with severe CAD.
Autonomic dysregulation is present throughout cardiovascular disease, with the interest in neuromodulation remaining increased. Previous research has proven that remodeling of the autonomic nervous system and the intrinsic cardiac nervous system occurs in various disease states, including the setting of myocardial infarction (32). NE exerts its effects on α and β adrenoceptors. α1 blockers exert anti-atherosclerotic effects by lowering blood pressure and modifying lipid profile (33). α2 adrenoceptors agonists also hold anti-atherosclerotic properties, possibly by reducing inflammation and stimulating oxidized LDL clearance, as recent animal studies show (34). Beta (β1, β2 and β3) blockers inhibit atherosclerosis progression by reducing circulating levels of proinflammatory cytokines, inhibiting oxidative stress, preventing LDL oxidation, decreasing monocyte adhesion to endothelium, and macrophage content in the atheroma plaque, conclusions drawn from experimental studies (35–37). However, the consequences of blunting the effect of SNS by renal denervation on atherosclerosis require further research, as present studies demonstrated that the procedure actually haslimiting adverse effects (38).
Recent research has proven that ischemic cardiomyopathy is accompanied by remodeling of the intrinsic cardiac nervous system, stellate ganglia, and higher nervous centers (39). Alterations of neurohumoral control and circulating catecholamines also occur (40). There is increasing evidence of the crosstalk between the immune system and the SNS (41). Cytokines stimulate the activation of SNS, which is further integrated at the hypothalamic level. In turn, NE can modulate the functions of the immune system.
Various markers of inflammation and oxidative stress work as a network and it is difficult to make associations between only one or few of them with cardiovascular risk and outcomes.
Moreover, excessive release of ROS can lead to cellular damage, negatively impacting not only the cardiovascular system, but also other bodily systems. Recent evidence suggests that oxidative stress plays a role in various neurodegenerative and neuropsychiatric disorders (42, 43).
5 Future directions
The prognostic value of a polyparametric approach was demonstrated in the setting of acute coronary syndromes, with the development of an Inflammatory Score, which unfortunately has not been further evaluated in other populations (44). It might be of interest to evaluate the prognostic value of a scoring system (including pro inflammatory markers, oxidative stress markers and NE) in the setting of ischemia/reperfusion injury (such as that induced by CABG). Furthermore, the use of targeted single cell treatments might be of interest in the management of selected patients (45).
6 Study limitations
Several limitations of the study must be mentioned. Firstly, the cytokines and oxidative stress markers that were tested are only a few of the molecules involved in the atherosclerotic process. Secondly, we only measured their circulating levels at one-point, serial determinations might be more suitable for establishing solid correlations.
7 Conclusion
In patients with CAD referred for CABG, SNS activation, as indicated by plasma NE levels, was correlated with disease severity, assessed by the SYNTAX I score. Additionally, NE levels were associated with markers of inflammation and oxidative stress, suggesting that inflammation, oxidative stress, and SNS activation form an interconnected network, where each component influences the others. It may be valuable to develop a scoring system that incorporates inflammation and oxidative stress markers to better identify patients who require a more aggressive strategy to reduce inflammation, oxidative stress, and modulate sympathetic nervous system (SNS) activity. Such a system could be particularly useful in the context of scheduled interventions, such as coronary artery bypass grafting (CABG) surgery, to tailor treatment and improve outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethical Board of the Transylvania University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AB: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. CDL: Writing – review & editing, Data curation, Visualization. CN: Writing – review & editing, Data curation, Formal analysis, Investigation. DŢ: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by Transilvania University of Brasov.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1480925/full#supplementary-material
Abbreviations
ANS, autonomic nervous system; BMI, body mass index; CABG, coronary artery bypass graft; CAD, coronary artery disease; ELISA, enzyme-linked immunosorbent assay; HIF 1 alpha, hypoxia-inducible factor 1-alpha; HRV, heart rate variability; IL-1, interleukine-1; IL-6, interleukine-6; LOX 1, lectin-like oxidized low-density lipoprotein receptor-1; NADPH, nicotinamide adenine dinucleotide phosphate; NE, norepinephrine; Ox-LDL, oxidized low-density lipoprotein cholesterol; ROS, reactive oxygen species; SD, standard deviation; SOD-1, superoxide dismutase-1; SNS, sympathetic nervous system; SYNTAX I, synergy between percutaneous coronary intervention with taxus and cardiac surgery.
References
1. Verma S, Buchanan MR, Anderson TJ. Endothelial function testing as a biomarker of vascular disease. Circulation. (2003) 108:2054–9. doi: 10.1161/01.CIR.0000089191.72957.ED
2. Liu Y, Zhang D, Yin D. Pathophysiological effects of various interleukins on primary cell types in common heart disease. Int J Mol Sci. (2023) 24:6497. doi: 10.3390/ijms24076497
3. Held C, White HD, Stewart RAH, Budaj A, Cannon CP, Hochman JS, et al. Inflammatory biomarkers interleukin-6 and C-reactive protein and outcomes in stable coronary heart disease: experiencesfrom the stability (stabilization of atherosclerotic plaque by initiation of darapladib therapy) trial. J Am Heart Assoc. (2017) 214:943–57. doi: 10.1161/JAHA.116.005077
4. Straub RH, Kees M, Janele D, Pongratz G, Schölmerich J, Härle P. Inflammatory mediators affect the autonomic nervous system. NeuroImmune Biol. (2007) 7:267–88. doi: 10.1016/S1567-7443(07)00215-3
5. Knutson AK, Williams AL, Boisvert WA, Shohet RV. HIF In the heart: development, metabolism, ischemia, and atherosclerosis. J Clin Invest. (2021) 131(17):e137557. doi: 10.1172/JCI137557
6. Brown DI, Griendling KK. Regulation of signal transduction by reactive oxygen species in the cardiovascular system. Circ Res. (2015) 116(3):531–49. doi: 10.1161/CIRCRESAHA.116.303584
7. Hofmann A, Brunssen C, Wolk S, Reeps C, Morawietz H. Soluble LOX-1: a novel biomarker in patients with coronary artery disease, stroke, and acute aortic dissection? J Am Heart Assoc. (2020) 9:e013803. doi: 10.1161/JAHA.119.013803
8. Peng JR, Lu TT, Chang HT, Ge X, Huang B, Li WM. Elevated levels of plasma superoxide dismutases 1 and 2 in patients with coronary artery disease. BioMed Res Int. (2016) 2016:3708905. doi: 10.1155/2016/3708905
9. Ajijola OA, Yagishita D, Reddy NK, Yamakawa K, Vaseghi M, Downs AM, et al. Remodeling of stellate ganglion neurons after spatially targeted myocardial infarction: neuropeptide and morphologic changes. Heart Rhythm. (2015) 12:1027–35. doi: 10.1016/j.hrthm.2015.01.045
10. Hadaya J, Ardell JL. Autonomic modulation for cardiovascular disease. Front Physiol. (2020) 11:617459. doi: 10.3389/fphys.2020.617459
11. Vaccarino V, Badimon L, Bremner JD, Cenko E, Cubedo J, Dorobantu M, et al. Depression and coronary heart disease: 2018 position paper of the ESC working group on coronary pathophysiology and microcirculation. Eur Heart J. (2020) 41:1687–96. doi: 10.1093/eurheartj/ehy913
12. Wang J, Liu W, Chen H, Liu C, Wang M, Chen H, et al. Novel insights into the interaction between the autonomic nervous system and inflammation on coronary physiology: a quantitative flow ratio study. Front Cardiovasc Med. (2021) 8:700943. doi: 10.3389/fcvm.2021.700943
13. Zhang H, Dhalla NS. The role of pro-inflammatory cytokines in the pathogenesis of cardiovascular disease. Int J Mol Sci. (2024) 25:1082. doi: 10.3390/ijms25021082
14. Ridker PM, Everett BM, Thuren T, Mac Fayden JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. (2017) 377:1119–31. doi: 10.1056/NEJMoa1707914
15. Holte E, Kleveland O, Ueland T, Kunszt G, Bratlie M, Broch K, et al. Effect of interleukin-6 inhibition on coronary microvascular and endothelial function in myocardial infaction. Heart. (2017) 103:1521–7. doi: 10.1136/heartjnl-2016-310875
16. Broch K, Anstensrud AK, Woxholt S, Sharma K, Tollefsen IM, Bendz B, et al. Randomized trial of interleukin-6 receptor inhibition in patients with acute ST-segment elevation myocardial infarction. J Am Coll Cardiol. (2021) 77:1845–55. doi: 10.1016/j.jacc.2021.02.049
17. Hedman A, Thomas Larsson P, Alam M, Håkan Wallen N, Nordlander R, Abdel Samad B. CRP, IL-6 and endothelin-1 levels in patients undergoing coronary artery bypass grafting. Do preoperative inflammatory parameters predict early graft occlusion and late cardiovascular events? Int J Cardiol. (2007) 120(1):108–14. doi: 10.1016/j.ijcard.2006.09.004
18. Thomas C, Leleu D, Masson D. Choesterol and HIF 1 alpha: dangerous liaisons in atherosclerosis. Front Immunol. (2022) 13:868958. doi: 10.3389/fimmu.2022.868958
19. Crucet M, Wüst SJA, Spielmann P, Lüscher TF, Wenger RH, Matter CM. Hypoxia enhances lipid uptake in macrophages: role of the scavenger receptors Lox1, SRA, and CD36. Atherosclerosis. (2013) 229:110–7. doi: 10.1016/j.atherosclerosis.2013.04.034
20. Heck-Swain KL, Koeppen M. The intriguing role of hypoxia-inducible factor in myocardial ischemia and reperfusion: a comprehensive review. J Cardiovasc Dev Dis. (2023) 10:215. doi: 10.3390/jcdd10050215
21. Yuan X, Lee JW, Bowser JL, Neudecker V, Sridhar S, Eltzschig HK. Targeting hypoxia signaling for perioperative organ injury. Anesth Analg. (2018) 126(1):308–21. doi: 10.1213/ANE.0000000000002288
22. Lou YM, Zheng ZL, Xie LY, Lian JF, Shen WJ, Zhou JQ, et al. Effects of spironolactone on hypoxia-inducible factor-1α in the patients receiving coronary artery bypass grafting. J Cardiovasc Pharmacol. (2021) 78(1):e101–4. doi: 10.1097/FJC.0000000000001040
23. Stocker R, Keaney JF Jr. Role of oxidative modifications in atherosclerosis. Physiol Rev. (2004) 84(4):1381–478. doi: 10.1152/physrev.00047.2003
24. Türker FS, Doğan A, Ozan G, Kıbar K, Erışır M. Change in free radical and antioxidant enzyme levels in the patients undergoing open heart surgery with cardiopulmonary bypass. Oxid Med Cell Longevity. (2016) 2016:1783728. doi: 10.1155/2016/1783728
25. Kattoor AJ, Goel A, Mehta JL. LOX-1: regulation, signaling and its role in atherosclerosis. Antioxidants. (2019) 8:218. doi: 10.3390/antiox8070218
26. Misaka T, Suzuki S, Sakamoto N, Yamaki T, Sugimoto K, Kunii H, et al. Significance of soluble lectin-like oxidized LDL receptor-1 levels in systemic and coronary circulation in acute coronary syndrome. BioMed Res Int. (2014) 2014:1–7. doi: 10.1155/2014/649185
27. Lu J, Wang X, Wang W, Muniyappa H, Hu C, Mitra S, et al. LOX-1 abrogation reduces cardiac hypertrophy and collagen accumulation following chronic ischemia in the mouse. Gen Ther. (2012) 19:522–31. doi: 10.1038/gt.2011.133
28. Black PH, Garbutt LD. Stress, inflammation and cardiovascular disease. J Psychosom Res. (2002) 52:1–23. doi: 10.1016/S0022-3999(01)00302-6
29. Bleeke T, Zhang H, Madamanchi N, Patterson C, Faber JE. Catecholamine-induced vascular wall growth is dependenton generation of reactive oxygen Species. Circ Res. (2004) 94:37–45. doi: 10.1161/01.RES.0000109412.80157.7D
30. Sweep CG, van der Meer MJ, Hermus AR, Smals AG, van der Meer JW, Pesman GJ, et al. Chronic stimulation of the pituitary-adrenal axis in rats by interleukin-1 beta infusion: in vivo and in vitro studies. Endocrinology. (1992) 130:1153–64. doi: 10.1210/endo.130.3.1311230
31. Späth-Schwalbe E, Born J, Schrezenmeier H, Bornstein SR, Stromeyer P, Drechsler S, et al. Interleukin-6 stimulates the hypothalamus-pituitary-adrenocortical axis in man. J Clin Endocrinol Metab. (1994) 79:1212–14. doi: 10.1210/jcem.79.4.7962296
32. Wang Y, Anesi J, Maier MC, Myers MA, Oqueli E, Sobey CG, et al. Sympathetic nervous system and atherosclerosis. Int J Mol Sci. (2023) 24:13132. doi: 10.3390/ijms241713132
33. Kinoshita M, Shimazu N, Fujita M, Fujimaki Y, Kojima K, Mikuni Y, et al. Doxazosin, an alpha 1-adrenergic antihypertensive agent, decreases serum oxidized LDL. Am J Hyperten. (2001) 14:267–70. doi: 10.1016/S0895-7061(00)01263-2
34. Wang Y, Nguyen DT, Anesi J, Alramahi A, Witting PK, Chai Z, et al. Moxonidine increases uptake of oxidized low-density lipoprotein in cultured vascular smooth muscle cells and inhibits atherosclerosis in apolipoprotein E-deficient mice. Int J Mol Sci. (2023) 24:3857. doi: 10.3390/ijms24043857
35. Ulleryd MA, Bernberg E, Yang LJ, Bergstrom GM, Johansson ME. Metoprolol reduces proinflammatory cytokines and atherosclerosis in ApoE-/- mice. Biomed Res Int. (2014) 2014:548783. doi: 10.1155/2014/548783
36. Chen SJ, Tsui PF, Chuang YP, Chiang DM, Chen LW, Liu ST. Carvedilol ameliorates experimental atherosclerosis by regulating cholesterol efflux and exosome functions. Int J Mol Sci. (2019) 20:5202. doi: 10.3390/ijms20205202
37. O’Mara AE, Johnson JW, Linderman JD, Brychta RJ, McGehee S, Fletcher LA, et al. Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity. J Clin Investig. (2020) 30:2209–19. doi: 10.1172/JCI131126
38. Wang Y. Does renal denervation inhibit atherosclerosis in humans? Austin J Cardiovasc Dis Atheroscler. (2015) 2:1013.
39. Nakamura K, Ajijola OA, Aliotta E, Armour JA, Ardell JL, Shivkumar K. Pathological effects of chronic myocardial infarction on peripheral neurons mediating cardiac neurotransmission. Auton Neurosci. (2016) 197:34–40. doi: 10.1016/j.autneu.2016.05.001
40. Goldberger JJ, Arora R, Buckley U, Shivkumar K. Autonomic nervous system dysfunction. J Am Coll Cardiol. (2019) 73(10):1189–206. doi: 10.1016/j.jacc.2018.12.064
41. Dusi V, Ghidoni A, Ravera A, De Ferrari GM, Calvillo L. Chemokines and heart disease: a network connecting cardiovascular biology to immune and autonomic nervous systems. Mediators Inflamm. (2016) 2016:5902947. doi: 10.1155/2016/5902947
42. Salim S. Oxidative stress and the central nervous system. J Pharmacol Exp Ther. (2017) 360(1):201–5. doi: 10.1124/jpet.116.237503
43. Sahab Negah S, Forouzanfar F. Oxidative stress is a new avenue for treatment of neuropsychiatric disorders: hype of hope? Curr Mol Med. (2023). doi: 10.2174/1566524023666230904150907
44. Correia LCL, Andrade BB, Borges VM. Prognostic value of cytokines and chemokines in addition to the GRACE score in non-ST-elevation acute coronary syndrome. Clin Chim Acta. (2010) 411(7–8):540–5. doi: 10.1016/j.cca.2010.01.011
Keywords: inflammation, coronary artery disease, oxidative stress, sympathetic nervous system, coronary artery bypass graft
Citation: Boieriu AM, Dumitrel Luca C, Neculoiu CD and Ţînţ D (2024) The impact of inflammatory and oxidative stress biomarkers on the sympathetic nervous system in severe coronary atherosclerosis. Front. Cardiovasc. Med. 11:1480925. doi: 10.3389/fcvm.2024.1480925
Received: 14 August 2024; Accepted: 27 September 2024;
Published: 14 October 2024.
Edited by:
Cristina Tudoran, Victor Babes University of Medicine and Pharmacy, RomaniaReviewed by:
Hamid Osman, Taif University, Saudi ArabiaAntoniu Octavian Petris, Grigore T. Popa University of Medicine and Pharmacy, Romania
Copyright: © 2024 Boieriu, Dumitrel Luca, Neculoiu and Ţînţ. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Diana Ţînţ, ZGlhbmF0aW50QGdtYWlsLmNvbQ==; ZGlhbmEudGludEB1bml0YnYucm8=
 Alexandra Maria Boieriu
Alexandra Maria Boieriu Cezar Dumitrel Luca
Cezar Dumitrel Luca Carmen Daniela Neculoiu4
Carmen Daniela Neculoiu4 Diana Ţînţ
Diana Ţînţ