- Department of Emergency Medicine, Hangzhou Third People’s Hospital, Hangzhou, China
Background: It's recognized that stress hyperglycemia ratio (SHR) is considered a significant indicator of poor prognosis in many diseases. However, its role in critically ill patients with acute heart failure (acute HF) remains underexplored.
Methods: We conducted a retrospective cohort study on patients with acute HF included in the Medical Information Mart for Intensive Care IV (MIMIC-IV) version 2.2 database. A restricted cubic spline (RCS) regression analysis was used to explore the relationship between SHR and the risk of all-cause mortality in these patients. Subsequently, a Cox regression model was used to evaluate the relationship between SHR and mortality in acute HF patients.
Results: A total of 1,644 acute HF patients were included in the study and divided into two groups: the low SHR group (SHR < 1.06, N = 823) and the high SHR group (SHR ≥ 1.06, N = 821). In our study, the 30-day, 90-day, 180-day, and 365-day mortality rates for acute HF were 7.0%, 12%, 15%, and 19%, respectively, with higher mortality rates observed in the high SHR group compared to the low SHR group. SHR levels showed a linear relationship with all-cause mortality. Furthermore, SHR as a continuous variable shows a significant positive correlation with 30-day (HR = 2.31, 95% CI: 1.58–3.39), 90-day (HR = 1.81, 95% CI: 1.31–2.52), 180-day (HR = 1.57, 95% CI: 1.16–2.12), and 365-day (HR = 1.41, 95% CI: 1.07–1.85) all-cause mortality. After categorization, high SHR remains associated with increased 30-day (HR = 2.4, 95% CI: 1.59–3.61), 90-day (HR = 1.76, 95% CI: 1.31–2.36), 180-day (HR = 1.51, 95% CI: 1.16–1.95), and 365-day (HR = 1.38, 95% CI: 1.09–1.73) all-cause mortality.
Conclusion: Our findings indicate that high SHR is an independent predictor of poor short- and long-term prognosis in acute HF patients. Understanding the impact of SHR on mortality in acute HF is crucial as it can assist clinicians in identifying high-risk patients and adjusting treatment strategies accordingly.
Introduction
Acute heart failure (acute HF) is a leading cause of hospitalization and mortality worldwide, imposing a significant burden on healthcare systems (1). In acute clinical settings, such as HF, stress hyperglycemia is commonly observed, characterized by transient elevations in blood glucose levels due to physiological stressors (2). It is typically reflective of the severity of the respective disease (3), induced through enhanced inflammation or activation of neurohormones (4). Research has shown that stress hyperglycemia is frequently observed in critically ill patients and is positively associated with mortality rates (4, 5). Moreover, it has been suggested as a potential indicator for predicting unfavorable outcomes in cerebrovascular disease (6–9), acute myocardial infarction (10–12), coronary artery disease (13), acute coronary syndrome (14), heart valvular disease (15), and sepsis (16, 17). In addition, recent studies have also highlighted the role of stress hyperglycemia in predicting adverse outcomes in patients with heart failure (18–22). Therefore, studying the impact of stress hyperglycemia on disease prognosis is crucial, as it can assist clinicians in identifying high-risk patients and adjusting their treatment strategies accordingly.
Previously, stress hyperglycemia was reflected by initial plasma glucose levels (23). However, plasma glucose levels are influenced by various factors (such as past glucose levels), which limits their ability to differentiate the state of stress hyperglycemia. To better assess the actual glycemic status of patients, the stress hyperglycemia ratio (SHR) has been proposed (24). The SHR is calculated as the ratio of an individual's acute glucose levels to their prior glucose levels, emphasizing the relative acute increase in glucose levels during stress responses or critical illness compared to their previous levels (24). Recent literature confirms that SHR reflects true stress hyperglycemia during hospitalization (11).
However, SHR in acute HF remains underexplored, particularly in critically ill patients, who have a worse prognosis. Therefore, we aim to elucidate the prognostic value of SHR by studying its impact on short-term and long-term mortality rates in this population.
Materials and methods
Data source
We conducted a retrospective observational study using the MIMIC-IV 2.2 database (25). It includes detailed medical records of patients hospitalized in the intensive care unit (ICU) at Beth Israel Deaconess Medical Center between 2008 and 2019. The institutional review boards of the Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center approved the establishment of the database. Author Hu Jingjing has been authorized to use the MIMIC-IV database (ID: 52583254) following the completion of an online education program by the National Institutes of Health.
Study population
From the MIMIC-IV database, patients meeting inclusion criteria for acute HF were identified based on ICD codes (42821, 42831, 42841, I5021, I5031, and I50811). The inclusion criteria comprised adult patients (≥18 years old) admitted to the ICU. For patients with multiple admissions, only data from their initial admission were included. Exclusion criteria included lack of plasma glucose at admission or HbA1c data, discharge or death within 48 h, and insufficient follow-up information. Figure 1 depicts the patient selection process.
Data extraction
A Structured Query Language (SQL) data extraction tool (pgAdmin 4) was utilized to retrieve the following data within the first 24 h of admission: (1) Demographic information including age, gender, and race; (2) Vital signs such as heart rate, respiratory rate (RR), systolic blood pressure (SBP), and diastolic blood pressure (DBP); (3) Pre-existing conditions including myocardial infarction (MI), congestive heart failure (CHF), chronic pulmonary disease, diabetes mellitus (DM), peripheral vascular disease, and cancer; (4) Laboratory tests conducted within the initial 24 h post-admission, encompassing hematocrit level, white blood cell count (WBC), platelet count, serum potassium level, serum sodium level, hemoglobin level, admission blood glucose, serum urea nitrogen level (BUN), serum creatinine level (SCR), anion gap, and other pertinent markers. The mean value was used for variables measured multiple times within the preceding 24 h; (5) Assessment of illness severity upon admission using the Simplified Acute Physiology Score II (SAPS II) and Sequential Organ Failure Assessment (SOFA) scores; (6) Administration of vasoactive medications, and mechanical ventilation (MV) during hospitalization; (7) Prognostic information includes ICU length of stay, total hospital length of stay, 30-day, 90-day, 180-day, and 365-day mortality.
SHR
SHR is calculated as the first blood glucose at admission (mg/dl)/[28.7 × HbA1c (%) - 46.7] (24).
Endpoints
The primary endpoint was 30-day all-cause mortality. Secondary endpoints included all-cause mortality rates within 90-day, 180-days, and 365-day.
Statistical analysis
We conducted a restricted cubic spline (RCS) regression analysis to explore the relationship between SHR and the risk of all-cause mortality in acute HF patients. Subsequently, the cut-off value (1.06) at which the hazard ratio (HR) for 30-day mortality risk equals 1 were used to categorize patients into high SHR (SHR ≥ 1.06) and low SHR groups (SHR < 1.06). The fundamental clinical characteristics of patients were assessed in accordance with the low and high SHR groups. We used the Shapiro-Wilk test to assess normality. Categorical variables were presented as numbers and percentages (%), and comparisons were made using the chi-square test. For normally distributed continuous variables, data were expressed as mean ± standard deviation (SD) and compared using Student's t-test. For non-normally distributed continuous variables, data were presented as median [interquartile range (IQR)] and compared using the Mann-Whitney U test. Subsequently, a Cox regression model was used to evaluate the relationship between SHR and mortality in patients with acute HF. In this study, variables with a P-value < 0.1 in the univariate Cox regression analysis, as well as those known to be associated with acute HF prognosis, were included in the multivariate regression analysis. Model I adjusted for nothing; Model II adjusted for age, gender, and race; Model III adjusted for SAPS II score, SOFA score, MI, CHF, stroke, chronic pulmonary disease, DM, cancer, vasopressin use, and mechanical ventilation; and Model IV adjusted based on Models II and III. Finally, a subgroup analysis was conducted based on age, gender, myocardial infarction, diabetes, and cancer. Except for the stratification variable, the adjustment technique was the same as in Model IV. Data analyses were conducted using R statistical software version 4.3.0. A two-tailed P-value of less than 0.05 defined statistical significance.
Results
Table 1 presents the baseline clinical characteristics of all participants. A total of 1,644 acute HF patients were included in the study and divided into two groups: the low SHR group (SHR < 1.06) and the high SHR group (SHR ≥ 1.06). In this study, the 30-day, 90-day, 180-day, and 365-day mortality rates for acute HF were 7.0%, 12%, 15%, and 19%, with mortality rates being higher in the high SHR group compared to the low SHR group. There were no significant differences between the two groups of patients in terms of age, gender, systolic blood pressure, platelet count, potassium level, SOFA score, SAPS II score, use of vasoactive drugs, mechanical ventilation, length of hospital or ICU stay, occurrence of CHF, peripheral vascular disease, cerebrovascular disease, chronic pulmonary disease, diabetes mellitus, and other diseases (all P > 0.05). Additionally, there were statistically significant differences between the two groups in terms of race, heart rate, respiratory rate, SpO2, WBC, hematocrit, hemoglobin, anion gap, BUN, creatinine, sodium level, and the occurrence rate of myocardial infarction (all P < 0.05).
RCS analysis between SHR level and mortality
SHR levels showed a linear relationship with 30-day, 90-day, 180-day, and 365-day mortality rates (all P > 0.05). All RCS results are presented in Figure 2. When SHR exceeded 1.06, the risk of death in acute HF markedly increased.
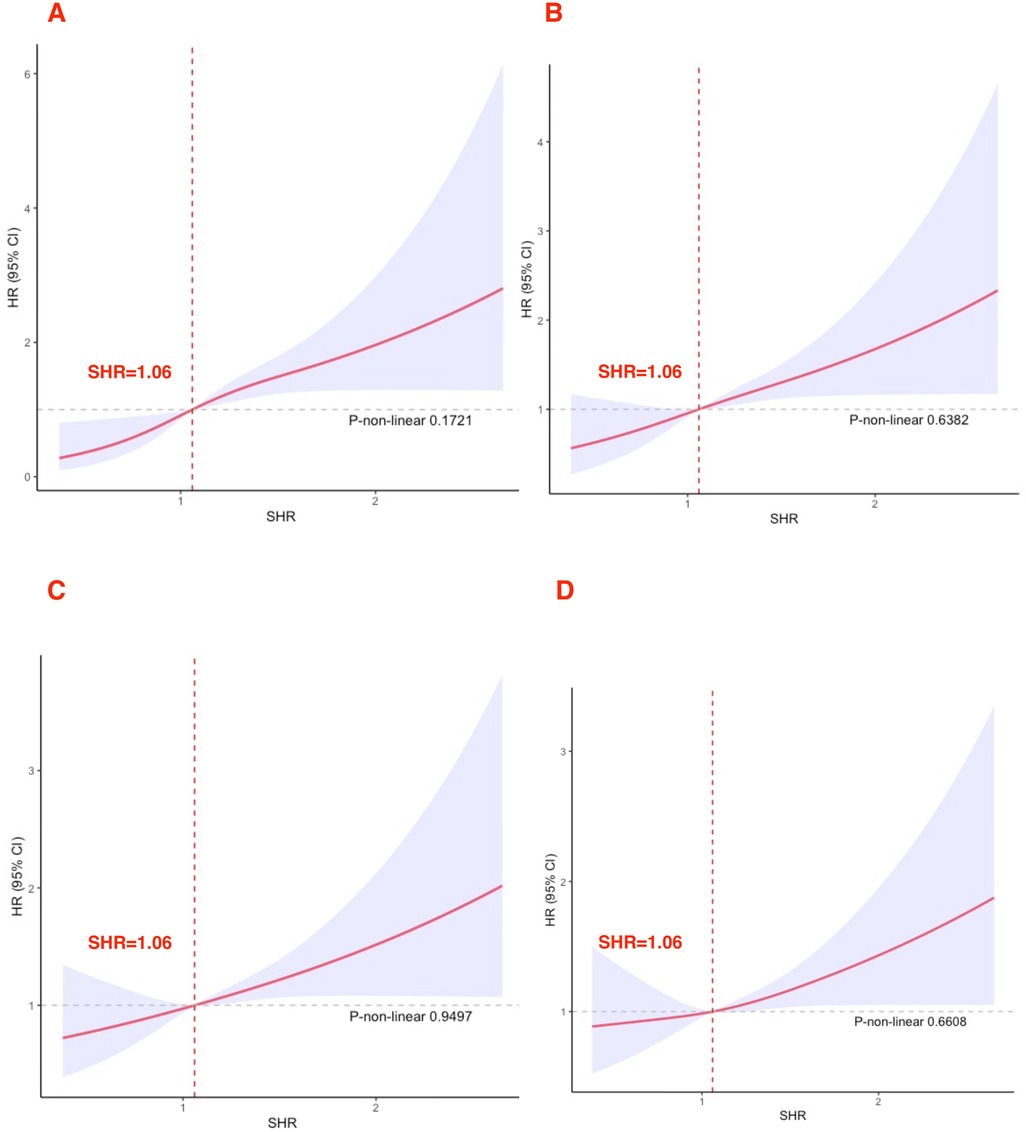
Figure 2. Cubic spline plot of the relation between high SHR and all-cause mortality (A) 30-day; (B) 90-day; (C) 180-day; (D) 365-day.
Relationship between SHR and all-cause mortality
Figure 3 displays the relationship between SHR and the risk of mortality in acute HF patients. In the unadjusted model (Model I), SHR as a continuous variable showed a significant positive correlation with 30-day (HR = 2.46, 95% CI: 1.74–3.48), 90-day (HR = 1.77, 95% CI: 1.31–2.39), 180-day (HR = 1.57, 95% CI: 1.19–2.07), and 365-day (HR = 1.42, 95% CI: 1.10–1.83) all-cause mortality rates. After stratification, high SHR remained associated with increased 30-day (HR = 2.47, 95% CI: 1.65–3.68), 90-day (HR = 1.74, 95% CI: 1.30–2.33), 180-day (HR = 1.50, 95% CI: 1.16–1.93), and 365-day (HR = 1.36, 95% CI: 1.09–1.71) all-cause mortality. In Model II, SHR as a continuous variable showed a significant positive correlation with 30-day (HR = 2.66, 95% CI: 1.86–3.80), 90-day (HR = 1.89, 95% CI: 1.39–2.57), 180-day (HR = 1.66, 95% CI: 1.24–2.21), and 365-day (HR = 1.49, 95% CI: 1.14–1.94) all-cause mortality. After categorization, high SHR remained associated with increased 30-day (HR = 2.48, 95% CI: 1.66–3.70), 90-day (HR = 1.77, 95% CI: 1.32–2.37), 180-day (HR = 1.53, 95% CI: 1.18–1.97), and 365-day (HR = 1.40, 95% CI: 1.12–1.75) all-cause mortality. In Model III, SHR as a continuous variable showed a significant positive correlation with 30-day (HR = 2.13, 95% CI: 1.47–3.09), 90-day (HR = 1.65, 95% CI: 1.20–2.27), 180-day (HR = 1.45, 95% CI: 1.08–1.94), and 365-day (HR = 1.31, 95% CI: 1.00–1.71) all-cause mortality. After categorization, high SHR was still linked to increased 30-day (HR = 2.30, 95% CI: 1.54–3.46), 90-day (HR = 1.65, 95% CI: 1.23–2.22), 180-day (HR = 1.43, 95% CI: 1.10–1.85), and 365-day (HR = 1.31, 95% CI: 1.04–1.64) all-cause mortality. In Model IV, SHR as a continuous variable showed a significant positive correlation with 30-day (HR = 2.31, 95% CI: 1.58–3.39), 90-day (HR = 1.81, 95% CI: 1.31–2.52), 180-day (HR = 1.57, 95% CI: 1.16–2.12), and 365-day (HR = 1.41, 95% CI: 1.07–1.85) all-cause mortality. After categorization, high SHR remained associated with increased 30-day (HR = 2.40, 95% CI: 1.59–3.61), 90-day (HR = 1.76, 95% CI: 1.31–2.36), 180-day (HR = 1.51, 95% CI: 1.16–1.95), and 365-day (HR = 1.38, 95% CI: 1.09–1.73) all-cause mortality.
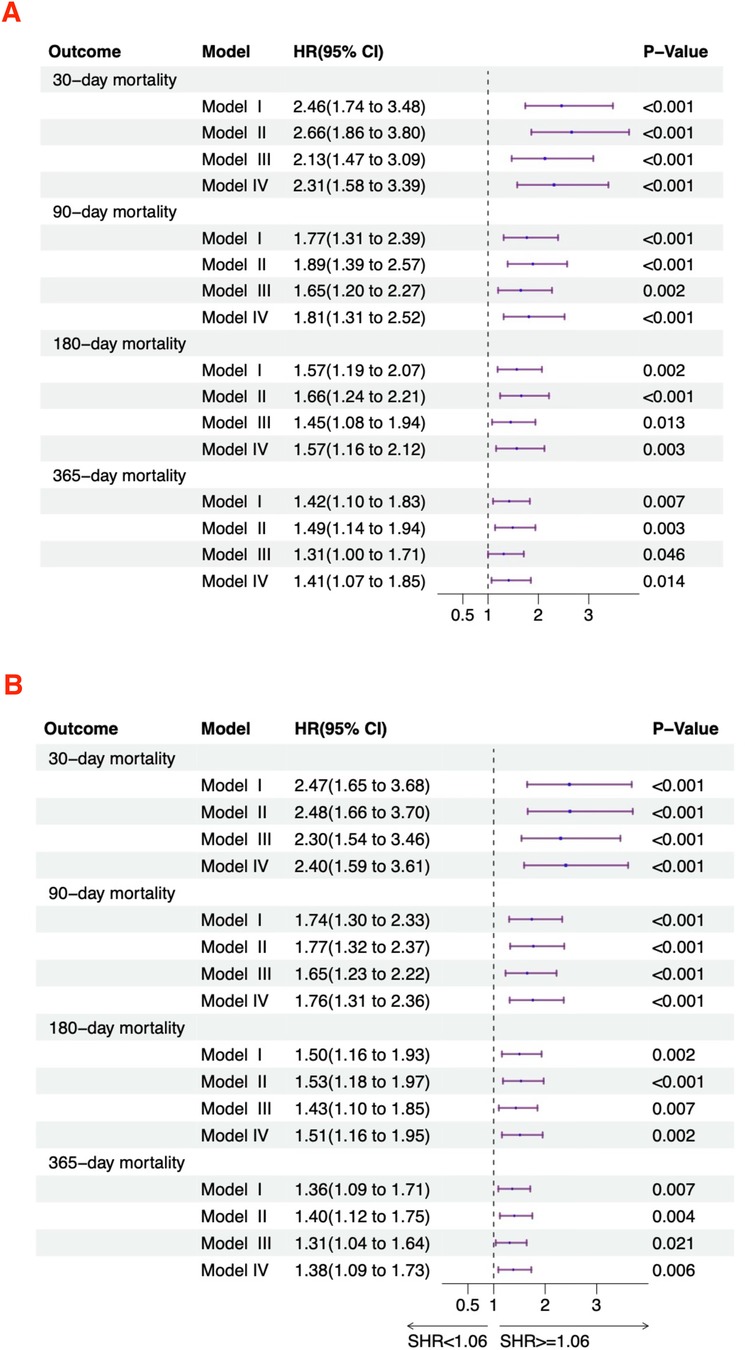
Figure 3. Hazard ratios for mortality based on SHR in acute HF patients. (A) Continuous variable; (B) Categorical variable.
Subgroup analyses
We further utilized subgroup analysis to evaluate the relationship between high SHR and all-cause mortality rates (Figures 4, 5). In the tumor subgroup, we did not observe an association between high SHR and mortality. However, high SHR was significantly correlated with the 30-day mortality in the remaining subgroups. For the 90-day mortality, we found that high SHR was associated with increased mortality in all subgroups except for those aged under 65 years with acute HF. Furthermore, we observed consistent results regarding SHR and mortality at 180 days and 365 days. Specifically, higher SHR was associated with mortality at both 180 days and 365 days in males, over 65 years of age, without diabetes, myocardial infarction, and in the non-tumor subgroup. In contrast, we found no association between high SHR and mortality at 180 days and 365 days in aged under 65 years, females, diabetes, or those without a history of myocardial infarction subgroup.
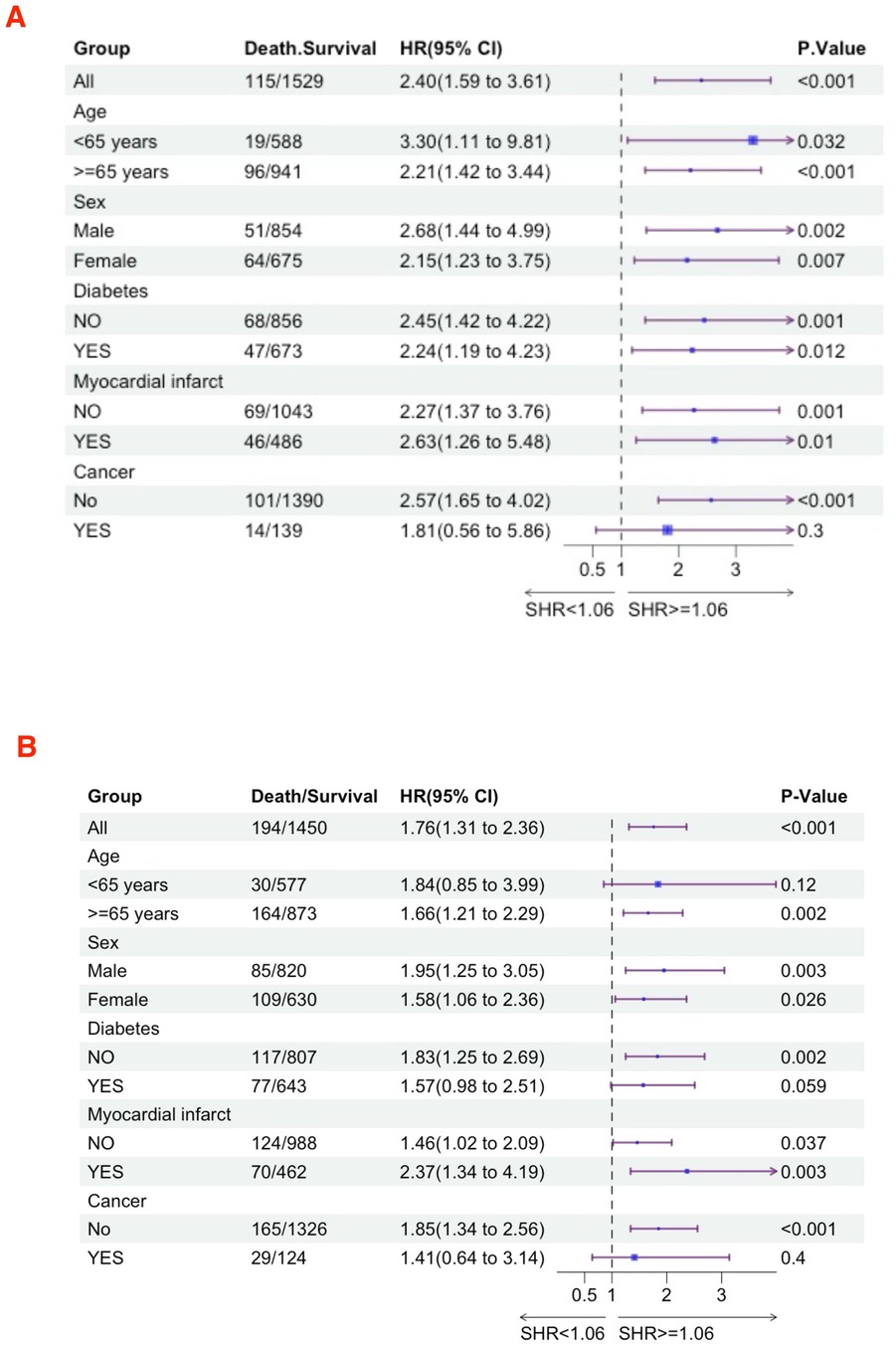
Figure 4. Subgroup analyses were performed to evaluate the association between high SHR and all-cause mortality (A) 30-day; (B) 90-day.
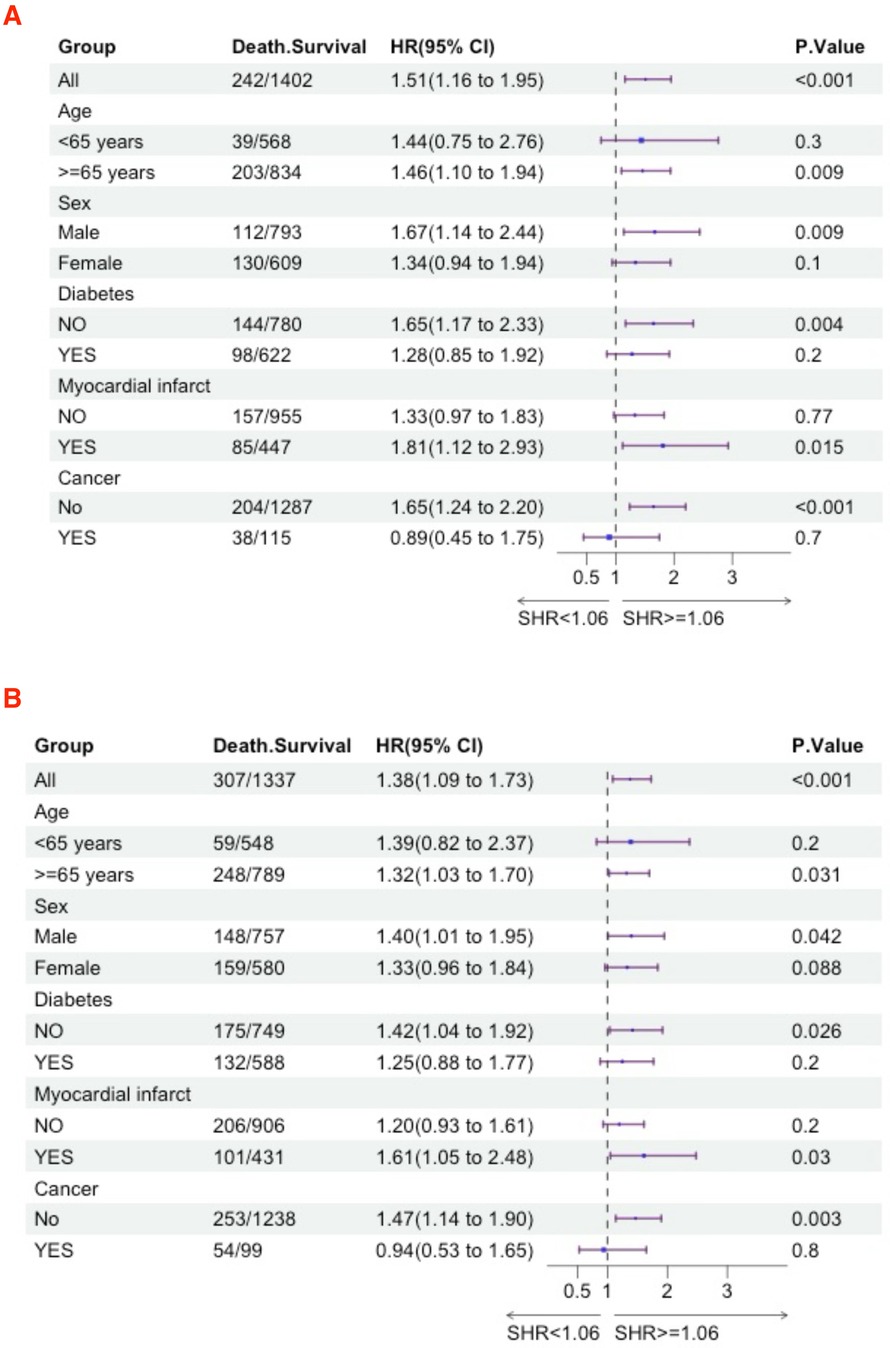
Figure 5. Subgroup analyses were performed to evaluate the association between high SHR and all-cause mortality (A) 180-day; (B) 365-day.
Discussion
In this study, we explored the relationship between SHR and prognosis in critically ill acute HF patients, finding that high SHR is associated with short-term and long-term all-cause mortality rates. We identified a linear relationship between SHR in critically ill acute HF patients and all-cause mortality rates. When SHR exceeded 1.06, there was a significant increase in the risk of mortality. In this study, acute HF patients with serum SHR ≥ 1.06 compared to those with SHR < 1.06 had a 2.4-fold higher risk of 30-day all-cause mortality, a 1.76-fold higher risk of 90-day all-cause mortality, a 1.51-fold higher risk of 180-day all-cause mortality, and a 1.38-fold higher risk of 365-day all-cause mortality, respectively. Our study results suggest that SHR holds promise as a tool for assessing adverse outcomes in acute HF patients.
Previous studies have indicated that SHR is a predictor of poor prognosis in HF patients. Zhou et al. conducted a study on 2,875 patients with HF and DM. They found that, compared to the lower SHR group, the adjusted odds ratio (OR) for composite cardiac events in the high SHR group was 1.89 (95% CI 1.26–2.87; P = 0.002) (20). Similarly, Mohammed et al. conducted a study on 400 patients with HF with preserved ejection fraction, followed for an average of 41 months. They found that elevated SHR was independently associated with an increased risk of all-cause composite events, cardiovascular death, and heart failure rehospitalization compared to patients with lower SHR (adjusted HR: 2.34, 95% CI 1.49–3.67; p < 0.001) (21). Khan also found similar conclusions. Furthermore, they demonstrated that patients over 65 years old had a 1.8 times higher likelihood of experiencing major adverse cardiovascular events compared to younger patients (95% CI: 1.1–2.9; P < 0.05), emphasizing age as a critical factor in risk stratification (22). Our study supports this conclusion as well. Apart from 30-day mortality, other outcomes indicate that high SHR is associated with a significantly higher risk of death in the population over 65 years old compared to younger individuals. Zhou et al. conducted a median 3.24-year follow-up study on 1,904 patients with acute HF. They found that acute HF patients in the highest SHR quintile (SHR > 1.14) had significantly higher risks of all-cause mortality (HR 2.76, 95% CI 1.63–4.68), cardiovascular (CV) mortality (HR 2.81, 95% CI 1.66–4.75), and HF rehospitalization (HR 1.54, 95% CI 1.03–2.32) compared to those in the lowest SHR quintile (0.64 < SHR ≤ 0.77). Additionally, they observed a U-shaped relationship between SHR and all-cause mortality (18). In our study, we found a linear relationship between SHR and both short-term and long-term mortality. This difference may be attributed to variations in the study population and follow-up duration. Our study exclusively included acute HF patients admitted to the ICU, who had higher comorbidities compared to those in previous studies. Therefore, they are at a higher risk of mortality. Our study shows that SHR has predictive value not only for long-term mortality but also for higher short-term mortality risk. In addition, contrary to our findings, Carrera et al. discovered a negative correlation between SHR and mortality. Compared to the first quintile, the HR for the second and third quintiles were 0.76 (95% CI: 0.58–0.99; P = 0.046) and 0.68 (95% CI: 0.52–0.89; P = 0.005), respectively (19). They found that patients with low SHR had hypoglycemia, which was negatively correlated with HF mortality rates.
The precise mechanism linking stress hyperglycemia in acute heart failure patients to adverse outcomes is not fully understood at present. First, stress hyperglycemia typically results from complex interactions of hormonal regulation (such as catecholamines, glucocorticoids, and cytokines) during stress or illness phases (26). It may be induced through activation of the sympathetic nervous system and enhanced activity of the hypothalamic-pituitary axis (27). Additionally, increased sympathetic nervous activity can promote the release of glucagon, which in turn stimulates glycogen breakdown in muscles and the liver. This leads to an increase in glucose entering circulation, ultimately resulting in elevated blood glucose levels (28). The mechanisms of heart failure involve various factors, including systemic inflammation, endothelial dysfunction, myocardial fibrosis, and diastolic dysfunction (29). Studies indicate that stress hyperglycemia can also lead to endothelial dysfunction, oxidative stress, and inflammation (30–32), as well as activate coagulation (33, 34). These changes can cause diminished cardiac function (35), facilitate fluid retention, and worsen HF symptoms. Secondly, stress hyperglycemia often indicates relative insulin deficiency, leading to increased fat breakdown and elevated circulating free fatty acid levels (27). The increase in circulating free fatty acids can be toxic to the myocardium, exacerbating myocardial cell damage, calcium overload, and arrhythmias. Insulin deficiency also reduces myocardial anaerobic glucose utilization capacity (36). Under these conditions, myocardial damage is further exacerbated. Finally, stress hyperglycemia can increase the risk of infection and exacerbate other comorbidities, potentially leading to non-cardiovascular-related mortality (37). Therefore, stress hyperglycemia occurs due to inflammation and neuroendocrine disturbances in HF. This, in turn, worsens the prognosis of heart failure patients by exacerbating oxidative stress, inflammatory states, and endothelial dysfunction. However, the mechanisms underlying the relationship between stress hyperglycemia and outcomes in HF patients require additional research.
We investigated the prognostic correlation between SHR and acute HF patients in the MIMIC database. However, our study has some limitations. Firstly, this is a retrospective study, and despite including numerous variables, there may still be potential confounding factors that were not accounted for. Additionally, due to limitations inherent in database studies, many factors related to HF (such as NT-proBNP, cardiac enzyme profiles, troponin, and echocardiography) were not included in this study. Finally, despite the large sample size in our study, the findings are only applicable to a specific subset of critically ill AHF patients in the United States. Further research is needed to determine its applicability to other populations. Furthermore, further studies should be performed to explore the relationship between SHR and the prognosis of acute HF patients.
Conclusion
Our findings indicate that high SHR is an independent predictor of poor short- and long-term prognosis in acute HF patients. Understanding the impact of SHR on mortality in acute HF is crucial as it can assist clinicians in identifying high-risk patients and adjusting treatment strategies accordingly.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The requirement of ethical approval was waived by boards of the Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center for the studies involving humans because The MIMIC database is anonymised and they were created in accordance with local policy. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin because The MIMIC database is anonymised and they were created in accordance with local policy.
Author contributions
TG: Data curation, Methodology, Writing – original draft. JH: Writing – original draft. YZ: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1463861/full#supplementary-material
References
1. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2023 focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the heart failure association (HFA) of the ESC. Eur J Heart Fail. (2024) 26(1):5–17. doi: 10.1002/ejhf.3024
2. Egi M, Bellomo R, Stachowski E, French CJ, Hart G. Variability of blood glucose concentration and short-term mortality in critically ill patients. Anesthesiology. (2006) 105(2):244–52. doi: 10.1097/00000542-200608000-00006
3. Chishiki T, Nagatomo Y, Saji M, Takei M, Goda A, Kohno T, et al. Divergent effect of blood glucose dysregulation on long-term clinical outcome in acute decompensated heart failure: a reappraisal in contemporary practice. Int J Cardiol. (2022) 365:91–9. doi: 10.1016/j.ijcard.2022.07.041
4. Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet. (2009) 373(9677):1798–807. doi: 10.1016/S0140-6736(09)60553-5
5. Ülger P, Yildiz E, Tyczynski B, Findeisen H, Kribben A, Janssen OE, et al. Effect of stress hyperglycaemia on acute kidney injury in non-diabetic critically ill patients? Int Urol Nephrol. (2023) 55(12):3253–9. doi: 10.1007/s11255-023-03612-2
6. Roberts G, Sires J, Chen A, Thynne T, Sullivan C, Quinn S, et al. A comparison of the stress hyperglycemia ratio, glycemic gap, and glucose to assess the impact of stress-induced hyperglycemia on ischemic stroke outcome. J Diabetes. (2021) 13(12):1034–42. doi: 10.1111/1753-0407.13223
7. Mi D, Li Z, Gu H, Jiang Y, Zhao X, Wang Y, et al. Stress hyperglycemia is associated with in-hospital mortality in patients with diabetes and acute ischemic stroke. CNS Neurosci Ther. (2022) 28(3):372–81. doi: 10.1111/cns.13764
8. Yuan C, Chen S, Ruan Y, Liu Y, Cheng H, Zeng Y, et al. The stress hyperglycemia ratio is associated with hemorrhagic transformation in patients with acute ischemic stroke. Clin Interv Aging. (2021) 16:431–42. doi: 10.2147/CIA.S280808
9. Deng Y, Wu S, Liu J, Liu M, Wang L, Wan J, et al. The stress hyperglycemia ratio is associated with the development of cerebral edema and poor functional outcome in patients with acute cerebral infarction. Front Aging Neurosci. (2022) 14:936862. doi: 10.3389/fnagi.2022.936862
10. Gao S, Liu Q, Ding X, Chen H, Zhao X, Li H. Predictive value of the acute-to-chronic glycemic ratio for in-hospital outcomes in patients with ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention. Angiology. (2020) 71(1):38–47. doi: 10.1177/0003319719875632
11. Sia CH, Chan MH, Zheng H, Ko J, Ho AF, Chong J, et al. Optimal glucose, HbA1c, glucose-HbA1c ratio and stress-hyperglycaemia ratio cut-off values for predicting 1-year mortality in diabetic and non-diabetic acute myocardial infarction patients. Cardiovasc Diabetol. (2021) 20(1):211. doi: 10.1186/s12933-021-01395-3
12. Cui K, Fu R, Yang J, Xu H, Yin D, Song W, et al. Stress hyperglycemia ratio and long-term mortality after acute myocardial infarction in patients with and without diabetes: a prospective, nationwide, and multicentre registry. Diabetes Metab Res Rev. (2022) 38(7):e3562. doi: 10.1002/dmrr.3562
13. Xu W, Song Q, Wang X, Zhao Z, Meng X, Xia C, et al. Association of stress hyperglycemia ratio and in-hospital mortality in patients with coronary artery disease: insights from a large cohort study. Cardiovasc Diabetol. (2022) 21(1):217. doi: 10.1186/s12933-022-01645-y
14. Yang J, Zheng Y, Li C, Gao J, Meng X, Zhang K, et al. The impact of the stress hyperglycemia ratio on short-term and long-term poor prognosis in patients with acute coronary syndrome: insight from a large cohort study in Asia. Diabetes Care. (2022) 45(4):947–56. doi: 10.2337/dc21-1526
15. Huang H, Liu J, Li Q, Qiao L, Chen S, Kang Y, et al. Relationship between stress hyperglycemia and worsening heart failure in patients with significant secondary mitral regurgitation. Atherosclerosis. (2024) 394:117306. doi: 10.1016/j.atherosclerosis.2023.117306
16. Scheen M, Giraud R, Bendjelid K. Stress hyperglycemia, cardiac glucotoxicity, and critically ill patient outcomes current clinical and pathophysiological evidence. Physiol Rep. (2021) 9(2):e14713. doi: 10.14814/phy2.14713
17. Fabbri A, Marchesini G, Benazzi B, Morelli A, Montesi D, Bini C, et al. Stress hyperglycemia and mortality in subjects with diabetes and sepsis. Crit Care Explor. (2020) 2(7):e0152. doi: 10.1097/CCE.0000000000000152
18. Zhou Q, Yang J, Wang W, Shao C, Hua X, Tang YD. The impact of the stress hyperglycemia ratio on mortality and rehospitalization rate in patients with acute decompensated heart failure and diabetes. Cardiovasc Diabetol. (2023) 22(1):189. doi: 10.1186/s12933-023-01908-2
19. Carrera MJ, Moliner P, Llauradó G, Enjuanes C, Conangla L, Chillarón JJ, et al. Prognostic value of the acute-to-chronic glycemic ratio at admission in heart failure: a prospective study. J Clin Med. (2021) 11(1):6. doi: 10.3390/jcm11010006
20. Zhou Y, Liu L, Huang H, Li N, He J, Yao H, et al. ’Stress hyperglycemia ratio and in-hospital prognosis in non-surgical patients with heart failure and type 2 diabetes. Cardiovasc Diabetol. (2022) 21(1):290. doi: 10.1186/s12933-022-01728-w
21. Mohammed AQ, Luo Y, Wang K, Su Y, Liu L, Yin G, et al. Stress hyperglycemia ratio as a prognostic indicator for long-term adverse outcomes in heart failure with preserved ejection fraction. Cardiovasc Diabetol. (2024) 23(1):67. doi: 10.1186/s12933-024-02157-7
22. Khan FR, Nawaz T, Sajjad W, Ali H, Hussain S, Amin M. Shifting the paradigm: how stress hyperglycemia alters the landscape of heart failure management. Cureus. (2024) 16(5):e59659. doi: 10.7759/cureus.59659
23. de Miguel-Yanes JM, Gonzalo-Hernando C, Muñoz-Rivas N, Méndez-Bailón M, Cava-Valenciano F, Torres-Macho J. First plasma glucose value after urgent admission and in-hospital mortality in acutely decompensated heart failure. Heart Lung. (2015) 44(2):137–40. doi: 10.1016/j.hrtlng.2014.11.006
24. Roberts GW, Quinn SJ, Valentine N, Alhawassi T, O'Dea H, Stranks SN, et al. Relative hyperglycemia, a marker of critical illness: introducing the stress hyperglycemia ratio. J Clin Endocrinol Metab. (2015) 100(12):4490–7. doi: 10.1210/jc.2015-2660
25. Johnson AEW, Bulgarelli L, Shen L, Gayles A, Shammout A, Horng S, et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci Data. (2023) 10(1):1. doi: 10.1038/s41597-022-01899-x
26. Barth E, Albuszies G, Baumgart K, Matejovic M, Wachter U, Vogt J, et al. Glucose metabolism and catecholamines. Crit Care Med. (2007) 35(9 Suppl):S508–18. doi: 10.1097/01.CCM.0000278047.06965.20
27. Angeli F, Reboldi G, Poltronieri C, Lazzari L, Sordi M, Garofoli M, et al. Hyperglycemia in acute coronary syndromes: from mechanisms to prognostic implications. Ther Adv Cardiovasc Dis. (2015) 9(6):412–24. doi: 10.1177/1753944715594528
28. Inzucchi SE. Hyperglycaemia and its therapy during acute coronary syndromes. Diab Vasc Dis Res. (2008) 5(4):259. doi: 10.3132/dvdr.2008.037
29. Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. (2013) 62(4):263–71. doi: 10.1016/j.jacc.2013.02.092
30. Krinock MJ, Singhal NS. Diabetes, stroke, and neuroresilience: looking beyond hyperglycemia. Ann N Y Acad Sci. (2021) 1495(1):78–98. doi: 10.1111/nyas.14583
31. Paolisso P, Foà A, Bergamaschi L, Donati F, Fabrizio M, Chiti C, et al. Hyperglycemia, inflammatory response and infarct size in obstructive acute myocardial infarction and MINOCA. Cardiovasc Diabetol. (2021) 20(1):33. doi: 10.1186/s12933-021-01222-9
32. Luc K, Schramm-Luc A, Guzik TJ, Mikolajczyk TP. Oxidative stress and inflammatory markers in prediabetes and diabetes. J Physiol Pharmacol. (2019) 70(6):809–24. doi: 10.26402/jpp.2019.6.01
33. McGovern KF, Lascola KM, Smith SA, Clark-Price SC, Wilkins PA, Schaeffer DJ, et al. The effects of hyperglycemia and endotoxemia on coagulation parameters in healthy adult horses. J Vet Intern Med. (2013) 27(2):347–53. doi: 10.1111/jvim.12052
34. Lemkes BA, Hermanides J, Devries JH, Holleman F, Meijers JC, Hoekstra JB. Hyperglycemia: a prothrombotic factor? J Thromb Haemost. (2010) 8(8):1663–9. doi: 10.1111/j.1538-7836.2010.03910.x
35. Timmer JR, Ottervanger JP, de Boer MJ, Dambrink JH, Hoorntje JC, Gosselink AT, et al. Hyperglycemia is an important predictor of impaired coronary flow before reperfusion therapy in ST-segment elevation myocardial infarction. J Am Coll Cardiol. (2005) 45(7):999–1002. doi: 10.1016/j.jacc.2004.12.050
36. Pres D, Gasior M, Strojek K, Gierlotka M, Hawranek M, Lekston A, et al. Blood glucose level on admission determines in-hospital and long-term mortality in patients with ST-segment elevation myocardial infarction complicated by cardiogenic shock treated with percutaneous coronary intervention. Kardiol Pol. (2010) 68(7):743–51.20648428
Keywords: glucose, glycosylated hemoglobin, stress hyperglycemia, acute heart failure, critically ill, mortality
Citation: Ge T, Hu J and Zhou Y (2024) The association between stress hyperglycemia ratio with mortality in critically ill patients with acute heart failure. Front. Cardiovasc. Med. 11:1463861. doi: 10.3389/fcvm.2024.1463861
Received: 4 September 2024; Accepted: 11 November 2024;
Published: 21 November 2024.
Edited by:
Cristiano Amarelli, Monaldi, Azienda dei Colli, ItalyReviewed by:
Francesco Cacciatore, University of Naples Federico II, ItalyMaria Simonenko, Almazov National Medical Research Centre, Russia
Copyright: © 2024 Ge, Hu and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingjing Hu, SHVqajk1MTAwOUAxNjMuY29t; Yidan Zhou, OTI5MzQ4NjkxQHFxLmNvbQ==
 Tingai Ge
Tingai Ge Jingjing Hu
Jingjing Hu
