- 1Department of Cardiothoracic and Vascular Surgery, Deutsches Herzzentrum der Charité, Berlin, Germany
- 2Institute of Medical Informatics, Charité – Universitätsmedizin Berlin, Berlin, Germany
- 3Berlin Institute of Health, Charité – Universitätsmedizin Berlin, Berlin, Germany
- 4Berlin Institute for the Foundations of Learning and Data (BIFOLD), Technical University of Berlin, Berlin, Germany
- 5German Centre for Cardiovascular Research (DZHK), Partner Site Berlin, Berlin, Germany
- 6Centre for Cardiovascular Telemedicine, Deutsches Herzzentrum der Charité, Berlin, Germany
- 7Department of Cardiology, Angiology, and Intensive Care Medicine, Deutsches Herzzentrum der Charité, Berlin, Germany
- 8Institute for Bioinformatics and Medical Informatics, Eberhard-Karls-Universität Tübingen, Tübingen, Germany
- 9Department of Health Sciences and Technology, Translational Cardiovascular Technologies, Eidgenössische Technische Hochschule Zürich, Zürich, Switzerland
Background: Remote patient management may improve prognosis in heart failure. Daily review of transmitted data for early recognition of patients at risk requires substantial resources that represent a major barrier to wide implementation. An automated analysis of incoming data for detection of risk for imminent events would allow focusing on patients requiring prompt medical intervention.
Methods: We analysed data of the Telemedical Interventional Management in Heart Failure II (TIM-HF2) randomized trial that were collected during quarterly in-patient visits and daily transmissions from non-invasive monitoring devices. By application of machine learning, we developed and internally validated a risk score for heart failure hospitalisation within seven days following data transmission as estimate of short-term patient risk for adverse heart failure events. Score performance was assessed by the area under the receiver-operating characteristic (ROCAUC) and compared with a conventional algorithm, a heuristic rule set originally applied in the randomized trial.
Results: The machine learning model significantly outperformed the conventional algorithm (ROCAUC 0.855 vs. 0.727, p < 0.001). On average, the machine learning risk score increased continuously in the three weeks preceding heart failure hospitalisations, indicating potential for early detection of risk. In a simulated one-year scenario, daily review of only the one third of patients with the highest machine learning risk score would have led to detection of 95% of HF hospitalisations occurring within the following seven days.
Conclusions: A machine learning model allowed automated analysis of incoming remote monitoring data and reliable identification of patients at risk of heart failure hospitalisation requiring immediate medical intervention. This approach may significantly reduce the need for manual data review.
Introduction
Heart failure (HF) is a major cause of mortality and morbidity and poses a substantial burden for the health care system. After the first HF related hospitalisation, the median survival is only 2.4 years (1), and every subsequent hospital admission further worsens prognosis (1). Furthermore, HF related hospitalisations are a main driver of health care related costs (2). Due to the ageing of the population and the increasing rate of comorbidities, HF related hospitalisations are expected to increase further. Thus, prevention of HF exacerbations requiring in-patient or emergency care management is a crucial aspect in HF management.
Early detection of patients with worsening HF status allows timely initiation of medical interventions that may prevent hospitalisations. In particular, this has been demonstrated in the setting of invasive hemodynamic monitoring by means of pulmonary pressure sensors (3). However, the invasive character of this monitoring combined with the high device costs are barriers to wide implementation (3).
As demonstrated in several randomised trials, remote monitoring of easily obtainable clinical parameters may also trigger early medical interventions and reduce the number of HF related hospitalisations and adverse events (4, 5). One of the major obstacles of broad application of remote monitoring of HF patients in clinical practice is the resource-intensive need for manual review of the collected data by trained personnel (6, 7). An automated assessment of incoming patient data regarding the risk for imminent events would allow focussing on patients most likely to benefit from medical contact and intervention, thus reducing the burden for health care professionals and allowing a much higher number of patients under such surveillance.
While numerous studies have made use of parameters routinely recorded as part of usual HF care to automatically assess patients’ health status (8–16), the literature on automatic assessment of frequently transmitted sensor data is much scarcer (17–24), especially in the context of validated, operational remote monitoring programs using non-invasive devices. Further, how automatic assessments could be effectively utilized within such a program, and how this affects the caregiver's patient capacity are currently open questions. With this work, we seek to lessen the gap in this research area by developing and validating a machine learning (ML) model for automated patient risk assessment by analysis of data from the Telemedical Interventional Management in Heart Failure II (TIM-HF2) randomised trial, and quantifying the resulting effect on the patient capacity through a simulation approach.
Methods
TIM-HF2 study design and participants
This analysis was conducted on the data of the TIM-HF2 trial, a randomised, multi-centre trial assessing the benefits of a structured remote patient management (RPM) programme. The design and main results of the trial have been reported previously (25, 26). In brief, TIM-HF2 was conducted in Germany between 2013 and 2018 and included 1,571 patients with a history of HF, New York Heart Association (NYHA) class II or III and a HF hospitalisation not longer than one year prior to randomisation, regardless of the left ventricular ejection fraction (LVEF). Patients were randomized to either RPM + usual care or to usual care only, and followed for 12 months. All patients underwent quarterly out-patient visits consisting of medical history, physical examination, collection of blood samples for biomarkers and assessment of concomitant treatments. Patients assigned to RPM were equipped with and trained in the use of a home telemonitoring system, which transmitted body weight, blood pressure, heart rate, ECG recording, peripheral capillary oxygen saturation, and self-rated well-being on a scale from one through five to a telemedical centre (TMC) on a daily basis. In the TMC, physicians and HF-nurses performed reviews of all patients’ incoming data and initiated interventions, if needed. The priority order for data review was defined by a pre-specified, conventional algorithm based on a set of heuristic rules (Supplementary Table 3) (25, 26).
For model development and validation in this current study, we used all patients of the full analysis set as defined in the TIM-HF2 trial. The final dataset contained 773 patients assigned to usual care, and 765 patients assigned to RPM + usual care.
All required ethics committee approvals, covering also the work presented here, were obtained.
Outcome definition for the machine learning analysis
Primary outcome of the current analysis was unplanned HF hospitalisation occurring within seven days following data transmission.
Candidate predictors
To account for the widely differing underlying baseline risk of the patients, we first created a new candidate predictor variable estimating the likelihood of all-cause death within one year based on variables that were gathered during the baseline out-patient visit prior to randomisation. For this purpose, 84 variables were considered (Table 1). The resulting predictor expressing the underlying baseline patient risk is hitherto referred to as baseline risk variable. The methodology for creation of the baseline risk variable was similar to the method applied for development of the main ML risk model and is described below.
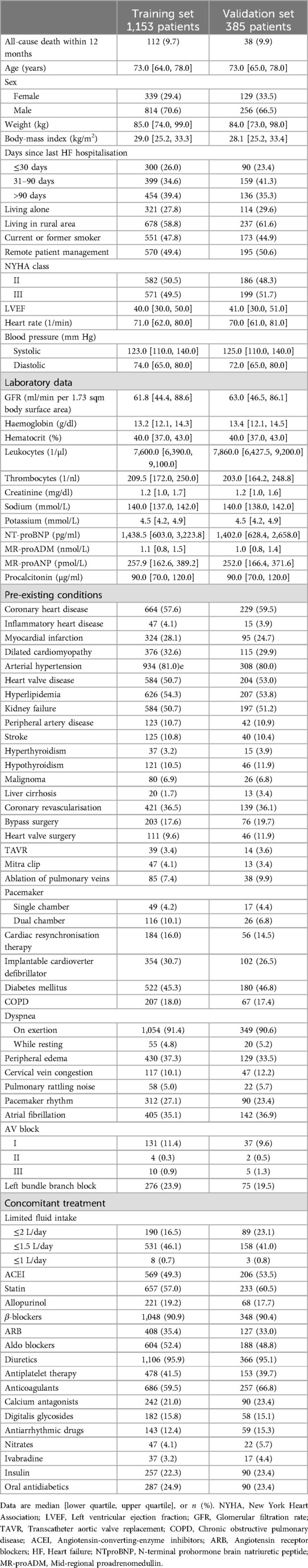
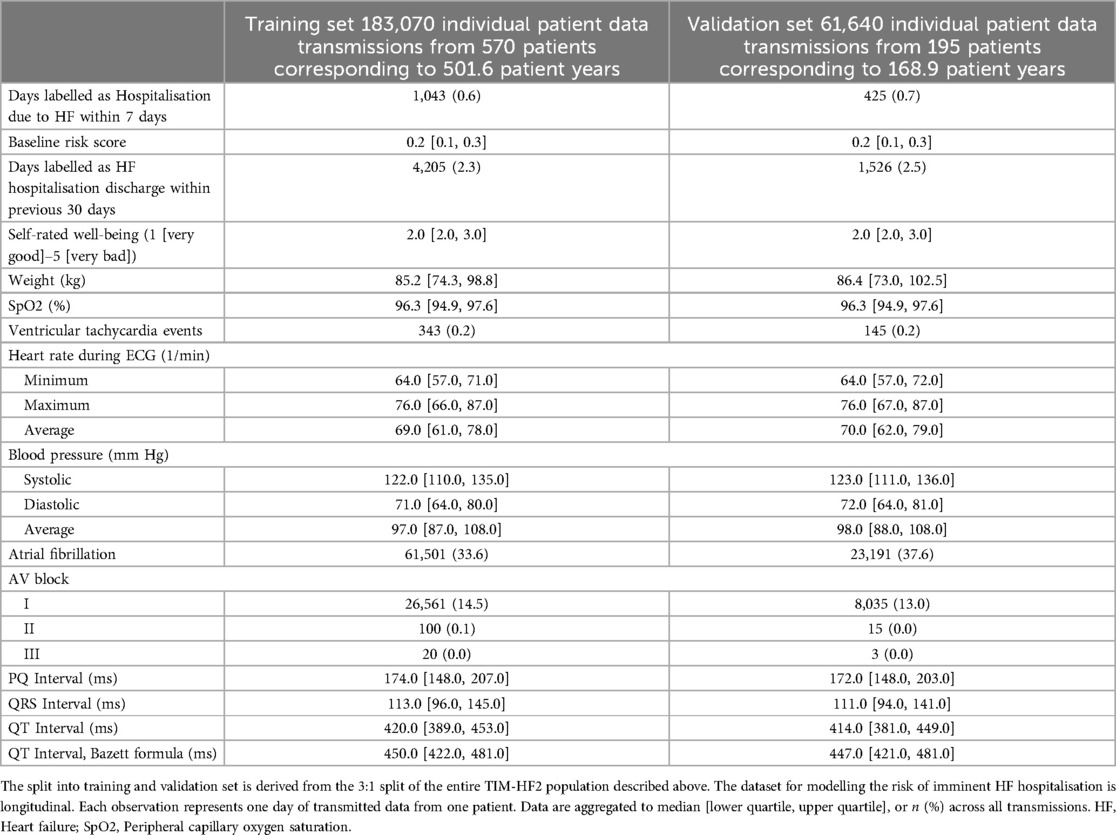
Table 1. Baseline characteristics of the TIM-HF2 trial population, split 3:1 into training and validation set.
For development of the main ML model for prediction of unplanned HF hospitalisation within 7 days following data transmission, we considered 18 variables (5 binary, 13 numerical) resulting from daily data transmissions, including ECG characteristics, blood pressure, oxygen saturation, weight, and self-rated well-being (Table 2). Additionally, we considered the baseline risk variable, and whether a previous hospitalisation due to HF occurred within 30 days prior to data transmission. This resulted in a total of 20 candidate predictors that were considered for the model.
Missing values
Missing values, or values set as missing because they exceeded plausible limits (Supplementary Tables 1 and 2) were scarce throughout the dataset. Biomarkers, which were collected quarterly during the study, contained up to 11.4% missing values. All other predictors contained less than 5% missing values. For daily transmitted variables, we used forward-filling to impute missing values where previous recordings were available, and backward-filling otherwise. For variables contained in the baseline risk model, we used linear regression imputation using up to four regressors chosen based on highest correlation.
Model development
Model development and validation were performed in accordance with the guidelines for transparent reporting of a multivariable prediction model for individual prediction or diagnosis (TRIPOD) (27).
We performed a randomised 3:1 split of patients into a training set for model development, and a hold-out validation set. The split was stratified for all-cause death within one year.
For development of the baseline risk variable, we used a Random Forest classifier (28) trained on 1,153 patients from both the RPM and the usual care group. The effect of RPM was captured in a binary predictor.
The risk for HF hospitalisation within the 7 days following data transmission was modelled via a Multilayer Perceptron (MLP) (29) using the daily data transmissions of 570 patients from the RPM group over a one-year study period. A summary of the model development and validation strategy is displayed in Figure 1.
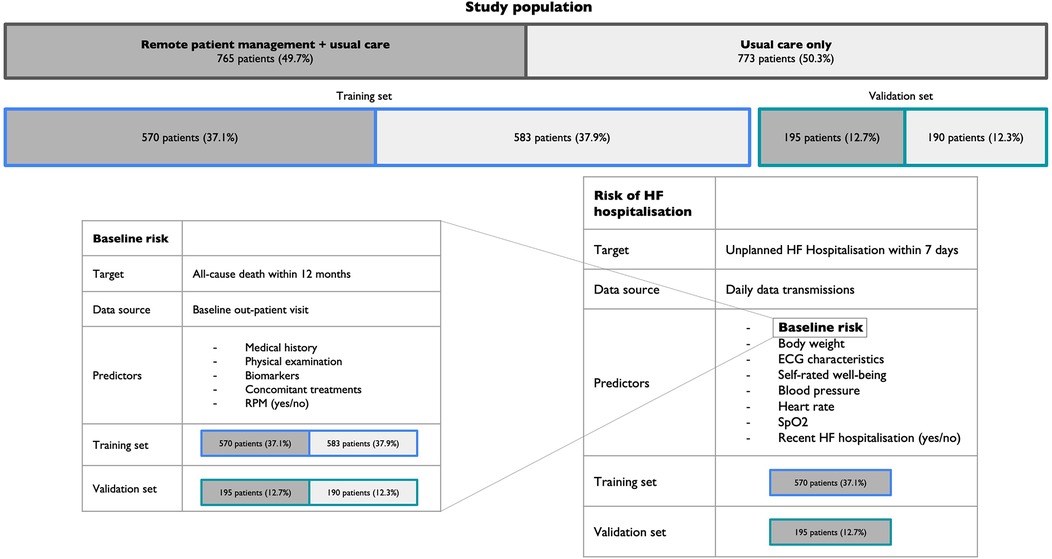
Figure 1. Model development and validation strategy. RPM, Remote patient management; HF, Heart failure; SpO2, Peripheral capillary oxygen saturation.
We defined a space of potential model hyperparameters, over which we performed a randomised search (30) and chose the optimal values based on the highest area under the receiver operating characteristic (ROCAUC) in fold-wise cross-validation using only patients from the training set. With the optimal set of hyperparameters, we trained the final model on the entire training population. Implementation details are given in the Supplementary Material.
We initially included all available predictors and ranked them via repeated permutation importance (28) according to their univariate impact on ROCAUC. For development of the baseline risk variable, we eliminated an increasing number of low-ranked predictors, performed hyperparameter tuning using only the remaining ones, and settled for the combination of predictors yielding the highest cross-validated ROCAUC. For the main model with 20 initial predictors, we also calculated the permutation importance of each feature and excluded those with a non-positive importance.
All analyses were performed using Python, version 3.7 (31), specifically (but not exclusively) the scikit-learn package, version 0.24.1 (32) for building the ML pipeline, and TensorFlow, version 2.4.1 (33) for deep learning.
Model validation
The model for the baseline risk variable was validated in the hold-out validation set of 385 patients. For reference, its performance was compared to the established Seattle Heart Failure Model (8).
The main ML model for prediction of unplanned HF hospitalisation within the following seven days was validated in the 195 RPM patients from the hold-out validation set. The performance of the model was compared with the performance of the conventional algorithm that was applied in the TIM-HF2 trial and that was based on a set of heuristic prioritisation rules (Supplementary Table 3) (26).
For visual inspection of discriminatory performance, we plotted the receiver operating characteristic (ROC) and precision recall (PR) curves. The PR curve displays the relationship between the sensitivity (also referred to as recall) and the precision (also referred to as positive predictive value), and can be informative in imbalanced classification problems, where the ROC can appear overly optimistic (34). For both ROC and PR, we additionally calculated the area under the curve (AUC). The 95% confidence intervals for the AUCs, and p-values for AUC comparisons were constructed using 10,000 bootstrap samples (35, 36).
We further visualized the trend of the score that was provided by the model in the 60 days preceding unplanned HF hospitalisations.
Simulation of daily ranking
We assessed the feasibility of a policy focusing on daily review of transmitted data from high-risk patients only. For this purpose, a one-year long telehealth setting containing all 195 RPM patients from the validation set was emulated. The start date of RPM for all patients was artificially shifted to the same day, and patients were then continuously ranked on a daily basis based on their estimated risk for unplanned HF hospitalisation within seven days. We could thus estimate the proportion of HF hospitalisations within seven days following data transmission that would have been detected on a given day if only a certain fixed fraction of top-ranked patients had been clinically evaluated by the TMC staff.
Results
Baseline risk variable
For the baseline risk variable, the predictor elimination process resulted in a final model of 27 predictors (Supplementary Table 4), of which N-terminal prohormone brain natriuretic peptide (NT-proBNP) stood out as the most impactful (Supplementary Figure 1). ROC and precision recall curves for the baseline risk variable and the SHFM are displayed in Figure 2. The baseline risk score had an overall satisfactory performance significantly outperforming the SHFM (AUC in ROC 0.793 vs. 0.677, p = 0.012; AUC in PR 0.298 vs. 0.165, p = 0.014).
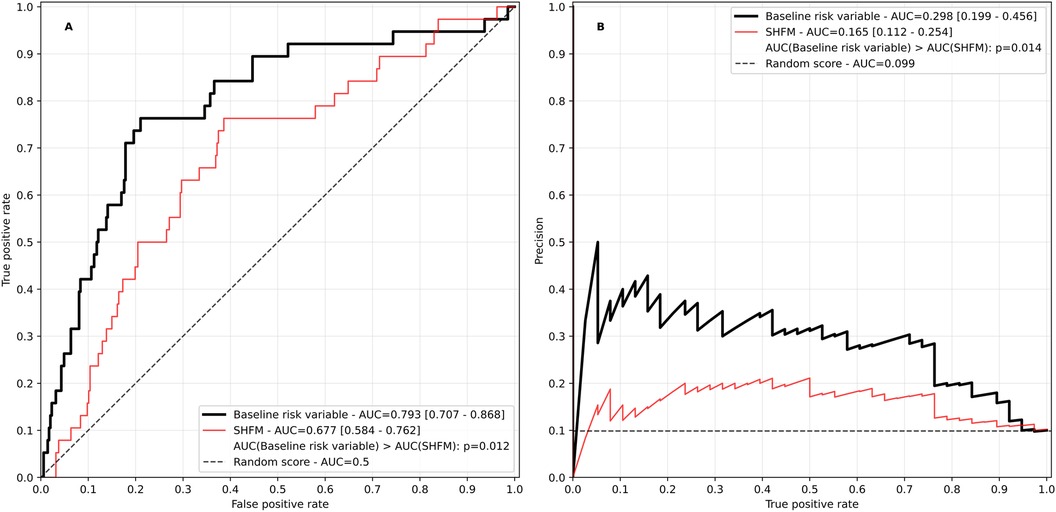
Figure 2. Receiver operating characteristic (A) and precision recall curve (B) of the baseline risk variable. The Seattle heart failure model (SHFM) score is plotted for reference. The SHFM score was calculated excluding information on uric acid, lymphocytes and cholesterol due to unavailability. AUC, area under the curve. Values in square brackets indicate the 95% confidence interval.
Prediction of unplanned HF hospitalisation within 7 days following data transmission
For the main ML model for prediction of unplanned HF hospitalisation occurring within the seven following days, the predictor elimination process resulted in a final model of 14 predictors (Supplementary Table 4). Among these, the most impactful was the baseline risk variable (Figure 6).
The model had a good performance with a ROCAUC of 0.855 and a PRAUC of 0.061, significantly outperforming the conventional algorithm based on a heuristic rule set that was used in the TIM-HF2 trial (ROCAUC 0.727, and PRAUC 0.018, p < 0.001 for both comparisons, Figure 3).
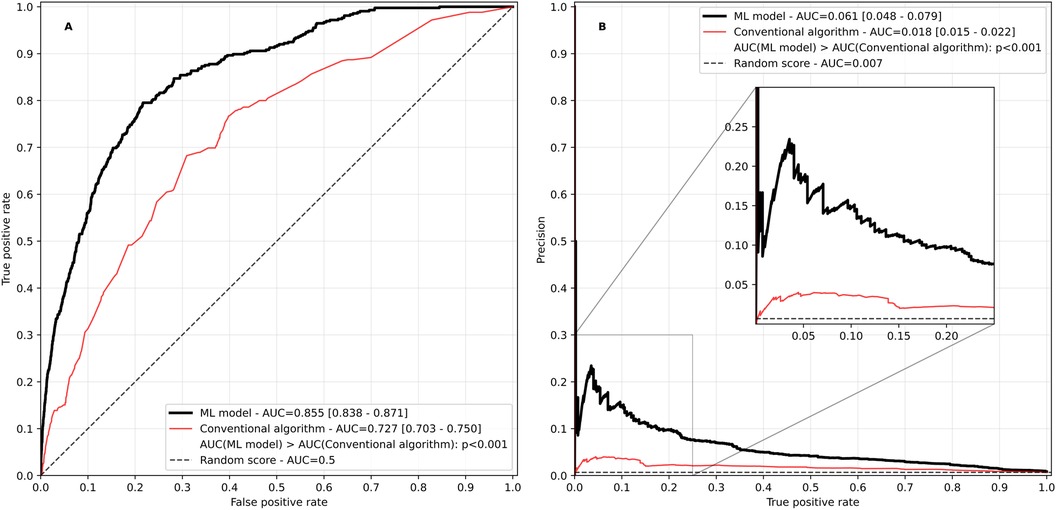
Figure 3. Receiver operating characteristic (A) and precision recall curve (B) of the ML-based risk score in comparison with the conventional algorithm based on heuristic rules used in the TIM-HF2 trial. AUC, area under the curve. Values in square brackets indicate the 95% confidence interval.
Figure 4 shows how the ML based model and the conventional algorithm evolved on average in the 60 days prior to an unplanned HF hospitalisation. The median score of patients in stable condition without HF hospitalisation approaching a randomly selected date within their follow-up period is shown for comparison. The median conventional algorithm score is highly volatile, and throughout the observed 60-day window, the median score of patients in stable condition is at times within the interquartile range of the score of patients approaching an unplanned HF hospitalisation. In contrast, the median ML-based score of patients approaching an unplanned HF hospitalisation is clearly separated from the score of patients in stable condition throughout the observed time window and exhibits a continuous upward trajectory starting approximately three weeks prior to a HF hospitalisation.
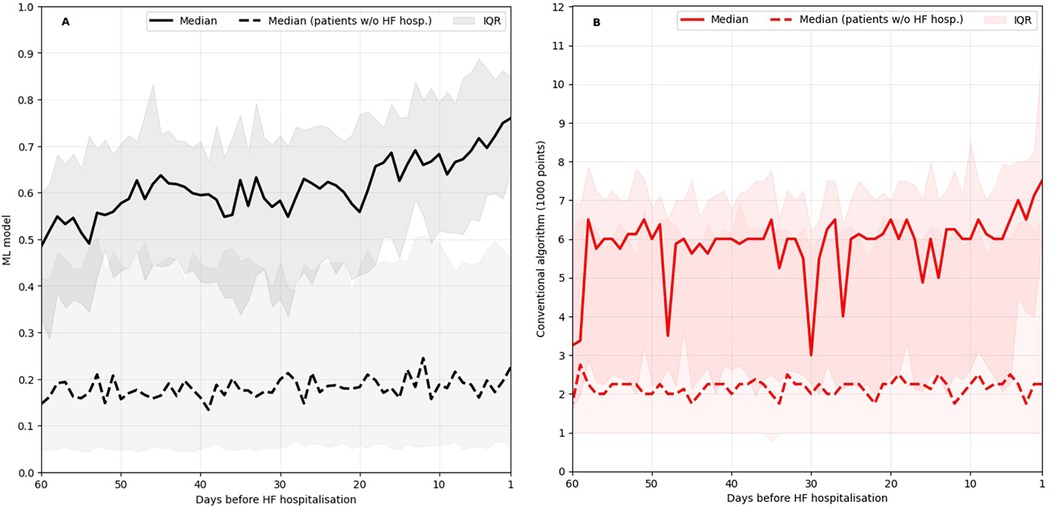
Figure 4. ML-based score evolution (A) and conventional algorithm evolution (B) leading up to HF hospitalisations in comparison to the evolution leading up to a random date for patients without HF hospitalisation. IQR, inter quartile range.
In an RPM scenario where on each day only a fraction of patients would have received medical attention based on highest estimated risk, the ML model performed better than the conventional algorithm with regard to detection of imminent unplanned HF hospitalisations (Figure 5). In this simulation, a case was considered as detected if a particular patient belonged to the fraction of top-ranked patients and would have thus received medical attention at least once in the seven days preceding the event.
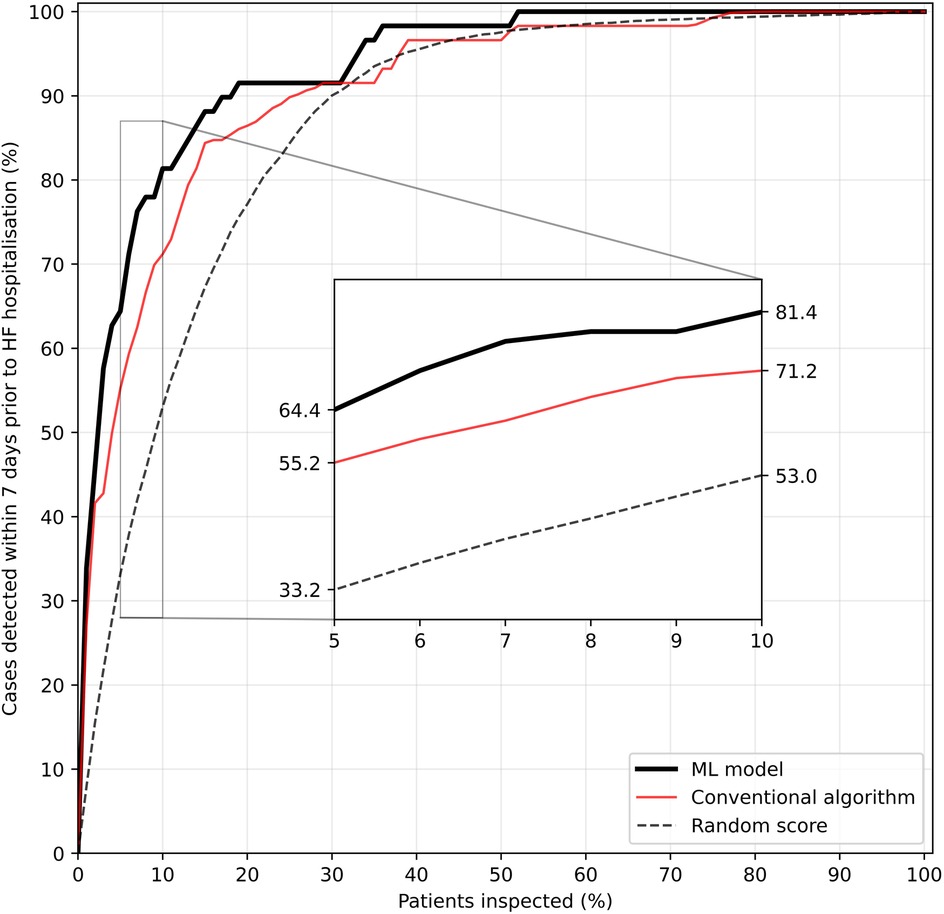
Figure 5. Fraction of HF hospitalisations detected within seven days prior to the event as a function of the fraction of top-ranked patients inspected per day during a one-year RPM setting. A case is considered detected if a patient is among the top-ranked patients inspected at least once in the seven days preceding hospitalisation. Repeated simulations (n = 100) were used for tie-breaking in the heuristic rules and for calculating a random score's (randomly allocated number between 0 and 1 per patient and day) performance.
The superiority of the ML-based risk score becomes especially obvious when the fraction of inspected top-ranked patients is low. If prioritisation would have been made on the basis on the ML model, evaluating only the 10% highest ranked patients would have led to detection of 81.4% of unplanned HF hospitalisations within the following seven days, an increase of 10 percentage points over the conventional algorithm. To detect 95% of all cases of imminent HF hospitalisations, only the top-ranked one third of all patients would have had to be clinically evaluated on a daily basis in this simulated scenario.
Discussion
Telemedical HF care has been transitioning from clinical trials to real-life settings. While retrospective studies on its implementation highlight the safety and benefits (37–40), its full potential is far from unleashed (41). For the ongoing implementation—driven by the ESC guidelines (42) and accelerated by the COVID-19 pandemic (41, 43)—the upscaling of capacities is a key issue for providers and patients. In this study, we demonstrated a path towards optimising the operational effectiveness of RPM in HF through artificial intelligence.
We developed and validated an ML-based risk model that considers both the patient's daily condition based on parameters transmitted through non-invasive monitoring devices, as well as the patient's baseline risk described through parameters collected during out-patient visits. The resulting model predicts unplanned HF hospitalisations within seven days in patients undergoing RPM, and out-performs a conventional rule-based algorithm that had been used during the TIM-HF2 trial for priority ranking (26).
During ML modelling on the daily transmitted data, we faced two key challenges: a severe imbalance between patients with and without HF hospitalisations in the next seven days (training prevalence 0.6%), and a lack of heterogeneity in the training set due to the ∼180,000 data transmissions stemming from only 570 individuals. We sought to alleviate these challenges by using the baseline risk variable as a-priori knowledge of the patient's health condition and passing it as a predictor into the ML model. In the development of the baseline risk variable, we were able to make use of both study arms of the TIM-HF2 trial by explicitly incorporating the information on RPM or usual care (the only structural difference between the cohorts due to the randomized design of the trial) in its development process, which consequently doubled the sample size and increased its robustness. Permutation importance computation confirms that the ML model of unplanned HF hospitalisations relies heavily on this information (Figure 6), and the model achieves high discriminatory performance. This is highlighted both by the ROCAUC of 0.855, and the clear separation between the median scores of patients with and without upcoming HF hospitalisations.
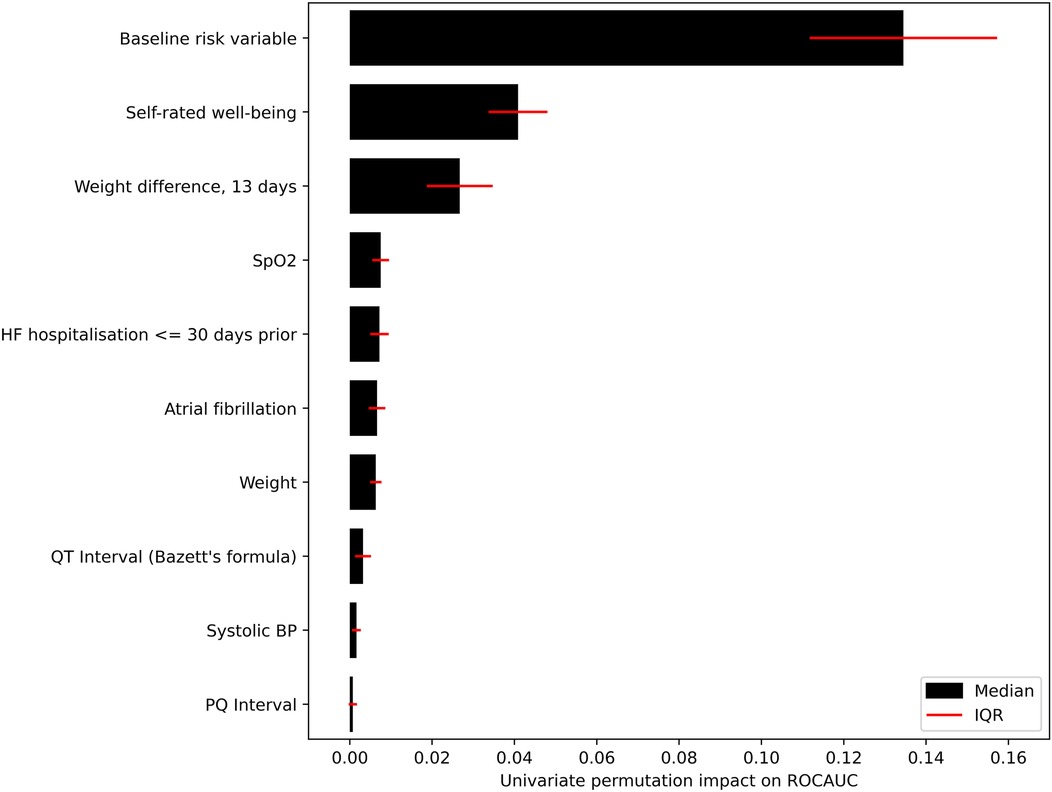
Figure 6. Impact of the 10 highest ranked predictors in the ML model. Impact measured by repeatedly permuting each predictor before making predictions and measuring the resulting drop-off in ROCAUC.
The first key result of this study is the steady upward trajectory of the median ML-based score as patients approach HF hospitalisation starting as early as three weeks prior to the event (Figure 4). This indicates that the model is sensitive to changes in the patients’ health status weeks before acute decompensation, much earlier than the typical onset of acute symptoms like dyspnoea or oedema (44). Sensitivity to changes this early in the HF deterioration process has thus far only been achieved through invasive hemodynamic monitoring (44). In contrast, the developed risk score relies on a single data transmission of multiple vital parameters per day through non-invasive sensors. Thus, through its implementation in the RPM care concept, our findings indicate the potential for timely intervention to reduce the risk of HF hospitalisation.
The second key result is the high detection rate of HF hospitalisation in a simulated one-year RPM scenario, where patients were ranked daily depending on their estimated risk. Ranking based on the ML-based score proved to be especially beneficial when the fraction of top-ranked patients undergoing review is small, and the theoretical gain in patient capacity therefore large. Daily evaluation of the top-ranked 10% of the patient population proved to be sufficient to review over 80% of all cases of HF hospitalisations at least once in the seven days preceding the event, and reviewing the top-ranked third pushes this number to 95% (Figure 5). Thus, the integration of our ML-based risk score in a decision support system (DSS) could fundamentally change the RPM caregiver's workflow by switching from a one-to-one correspondence between data transmission and review to a risk-adjusted review frequency. Reviewing per day a fixed fraction of top-ranked patients could amount to multiplying the patient capacity compared to the daily review of all patients, without additional staff.
In practice, ranking patients solely based on an ML-based score has downsides which need to be accounted for in the implementation of the DSS. No patient, even if classified as stable by the model, should exceed a pre-specified number of days without clinical evaluation. Patients newly added to the program, or recently released from the hospital require special attention to ease the care transition (45), and should be prioritised independent of risk score. Nevertheless, we were able to show that implementing a risk-adjusted review frequency might be a viable approach to increase the operational effectiveness of RPM providers.
This study exhibits three key limitations. First, despite the time series nature of the dataset, the implemented MLP makes little use of the development of predictors over time, except for the inclusion of weight differences. More complex models designed for sequential data could potentially uncover temporal effects like the influence of onsetting or retreating atrial fibrillation. Applying other types of models to this problem, possibly including additional data sources like raw ECG or voice recordings, remains a topic for future studies.
Second, although the TIM-HF2 trial included 765 patients undergoing remote patient management, the validation of our ML-based risk score was performed on a relatively small subset of only 195 patients who were held out from the model development process. This limited validation cohort was necessary to allocate sufficient data for robust training of the ML model. However, the small number of patients in the validation set may limit the generalizability and may increase the susceptibility to outliers. Third, our findings lack external validation. Our findings rely on a retrospective analysis of the TIM-HF2 dataset, including a retrospective simulation approach to estimate fractions of detected cases of HF hospitalisation using the ML-based risk score. A prospective study is needed to test how our proposed approach of reviewing from all daily data transmissions only a pre-selected fraction affects patients’ mortality and morbidity.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: the raw TIM-HF2 trial data cannot be made publicly available, as this is not covered by the written informed consent provided by the participating patients. Limited data access might be obtainable upon reasonable request by contacting the Charité Centre for Cardiovascular Telemedicine or the corresponding author. Requests to access these datasets should be directed toZnJpZWRyaWNoLmtvZWhsZXJAZGh6Yy1jaGFyaXRlLmRl.
Ethics statement
The studies involving humans were approved by Charité—Universitätsmedizin Berlin Ethikkommission. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
NH: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. AM: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. KK: Funding acquisition, Writing – review & editing. TK: Writing – review & editing. MH: Writing – review & editing. SS: Writing – review & editing. FB: Writing – review & editing. CE: Writing – review & editing. VF: Writing – review & editing. GH: Writing – review & editing. ND: Supervision, Writing – original draft, Writing – review & editing. FK: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The German Federal Ministry of Education and Research supported the TIM-HF2 trial with a research grant (number 13KQ0904B). This study is part of the Telemed5000 project funded by a research grant of the German Federal Ministry of Economic Affairs and Climate Action (grant number: 01MD19014A). Nils Hinrichs and Alexander Meyer are supported by the Berlin Institute for the Foundations of Learning and Data (BIFOLD).
Acknowledgments
We thank the physicians and nurses at the telemedical centre for their daily medical review of the patients and especially the patients involved in the TIM-HF2 trial. We thank Sheila Rieger for careful copy editing of the manuscript and Volker Moeller for data management.
Conflicts of interest
AM is co-founder of x-cardiac GmbH, advisory board member of Kenkou GmbH, and reports consulting and/or speaker fees from Pfizer, Medtronic and Edwards, all outside the submitted work. CE is co-founder of Codiag AG and x-cardiac GmbH, and reports consulting fees from Abacus Health and speaker fees from Merck, all outside the submitted work. GH serves as Abbott steering committee member, outside the submitted work. FB has received funding from Medtronic; has received grants from the German Federal Ministry of Education and Research, the German Federal Ministry of Health, the Berlin Institute of Health, Hans Böckler Foundation, Einstein Foundation, and the Berlin University Alliance outside the submitted work; he has received personal fees from Elsevier Publishing; and has received other funding from the Robert Koch Institute, all outside the submitted work. VF has received educational grants (including travel support), fees for lectures and speeches, fees for professional consultation, and research and study funds from Medtronic GmbH, Biotronik SE & Co, Abbott GmbH & Co KG, Boston Scientific, Edwards Lifesciences, Berlin Heart, Novartis Pharma GmbH, JOTEC GmbH, and Zurich Heart, all outside the submitted work. FK has received a research grant from the German Federal Ministry of Education and Research for the TIM-HF2 trial and a research grant from the German Federal Ministry of Economic Affairs and Climate Action for the AI-based analysis of the TIM-HF2 data. He reports personal fees from Abbott, Sanofi-Aventis, Novartis, Roche, AstraZeneca, SHL, Medtronic, Biotronik; support for attending meetings and/or travel from the German Society of Internal Medicine and the European Society of Cardiology; co-ownership of a patent in cooperation with BRAHMS GmbH, all outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1457995/full#supplementary-material
References
1. Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. (2007) 154(2):260–6. doi: 10.1016/j.ahj.2007.01.041
2. Osenenko KM, Kuti E, Deighton AM, Pimple P, Szabo SM. Burden of hospitalization for heart failure in the United States: a systematic literature review. J Manag Care Spec Pharm. (2022) 28(2):157–67. doi: 10.18553/jmcp.2022.28.2.157
3. Lander MM, Aldweib N, Abraham WT. Wireless hemodynamic monitoring in patients with heart failure. Curr Heart Fail Rep. (2021) 18(1):12–22. doi: 10.1007/s11897-020-00498-4
4. Faragli A, Abawi D, Quinn C, Cvetkovic M, Schlabs T, Tahirovic E, et al. The role of non-invasive devices for the telemonitoring of heart failure patients. Heart Fail Rev. (2021) 26(5):1063–80. doi: 10.1007/s10741-020-09963-7
5. Veenis JF, Radhoe SP, Hooijmans P, Brugts JJ. Remote monitoring in chronic heart failure patients: is non-invasive remote monitoring the way to go? Sensors. (2021) 21(3):887. doi: 10.3390/s21030887
6. Stevenson LW, Ross HJ, Rathman LD, Boehmer JP. Remote monitoring for heart failure management at home. J Am Coll Cardiol. (2023) 81(23):2272–91. doi: 10.1016/j.jacc.2023.04.010
7. Koehler F, Koehler K, Prescher S, Kirwan B-A, Wegscheider K, Vettorazzi E, et al. Mortality and morbidity 1 year after stopping a remote patient management intervention: extended follow-up results from the telemedical interventional management in patients with heart failure II (TIM-HF2) randomised trial. Lancet Digit Health. (2020) 2(1):e16–24. doi: 10.1016/S2589-7500(19)30195-5
8. Wayne C. L, Dariush M, David T. L, Santosh C. S, Stefan D. A, Anne B. C, et al. The seattle heart failure model. Circulation. (2006) 113(11):1424–33. doi: 10.1161/CIRCULATIONAHA.105.584102
9. Ouwerkerk W, Voors AA, Zwinderman AH. Factors influencing the predictive power of models for predicting mortality and/or heart failure hospitalization in patients with heart failure. JACC Heart Fail. (2014) 2(5):429–36. doi: 10.1016/j.jchf.2014.04.006
10. Angraal S, Mortazavi BJ, Gupta A, Khera R, Ahmad T, Desai NR, et al. Machine learning prediction of mortality and hospitalization in heart failure with preserved ejection fraction. JACC Heart Fail. (2020) 8(1):12–21. doi: 10.1016/j.jchf.2019.06.013
11. Chicco D, Jurman G. Machine learning can predict survival of patients with heart failure from serum creatinine and ejection fraction alone. BMC Med Inform Decis Mak. (2020) 20(1):1–16. doi: 10.1186/s12911-020-1023-5
12. Bradley J, Schelbert EB, Bonnett LJ, Lewis GA, Lagan J, Orsborne C, et al. Predicting hospitalisation for heart failure and death in patients with, or at risk of, heart failure before first hospitalisation: a retrospective model development and external validation study. Lancet Digit Health. (2022) 4(6):e445–54. doi: 10.1016/S2589-7500(22)00045-0
13. Sabouri M, Rajabi AB, Hajianfar G, Gharibi O, Mohebi M, Avval AH, et al. Machine learning based readmission and mortality prediction in heart failure patients. Sci Rep. (2023) 13(1):18671. doi: 10.1038/s41598-023-45925-3
14. Ru B, Tan X, Liu Y, Kannapur K, Ramanan D, Kessler G, et al. Comparison of machine learning algorithms for predicting hospital readmissions and worsening heart failure events in patients with heart failure with reduced ejection fraction: modeling study. JMIR Form Res. (2023) 7:e41775. doi: 10.2196/41775
15. Zheng B, Liang T, Mei J, Shi X, Liu X, Li S, et al. Prediction of 90 day readmission in heart failure with preserved ejection fraction by interpretable machine learning. ESC Heart Fail. (2024). doi: 10.1002/ehf2.15033
16. Averbuch T, Sullivan K, Sauer A, Mamas MA, Voors AA, Gale CP, et al. Applications of artificial intelligence and machine learning in heart failure. Eur Heart J Digit Health. (2022) 3(2):311–22. doi: 10.1093/ehjdh/ztac025
17. Chausiaux OE, Keyser M, Williams GP, Nieznański M, Downer PJ, Garnett RE, et al. Heart failure decompensation alerts in a patient’s home using an automated, AI-driven, point-of-care device. BMJ Case Rep. (2022) 15(4):e248682. doi: 10.1136/bcr-2021-248682
18. Josef S, Carsten S, Biykem B, Jose N-N, Peter W, Stephan W, et al. Continuous wearable monitoring analytics predict heart failure hospitalization. Circ Heart Fail. (2020) 13(3):e006513. doi: 10.1161/CIRCHEARTFAILURE.119.006513
19. Gontarska K, Wrazen W, Beilharz J, Schmid R, Thamsen L, Polze A. Predicting medical interventions from vital parameters: towards a decision support system for remote patient monitoring. In: Tucker A, Henriques Abreu P, Cardoso J, Pereira Rodrigues P, Riaño D, editors. Artificial Intelligence in Medicine. Basel, Switzerland: Springer International Publishing (2021). p. 293–7. Available online at: https://link.springer.com/chapter/10.1007/978-3-030-77211-6_33 (Accessed January 11, 2024).
20. D’Onofrio A, Solimene F, Calò L, Calvi V, Viscusi M, Melissano D, et al. Combining home monitoring temporal trends from implanted defibrillators and baseline patient risk profile to predict heart failure hospitalizations: results from the SELENE HF study. Europace. (2022) 24(2):234–44. doi: 10.1093/europace/euab170
21. Moazeni M, Numan L, Szymanski MK, Van der Kaaij NP, Asselbergs FW, van Laake LW, et al. Monitoring left ventricular assist device parameters to detect flow- and power-impacting complications: a proof of concept. Eur Heart J Digit Health. (2023) 4(6):488–95. doi: 10.1093/ehjdh/ztad062
22. Moazeni M, Numan L, Brons M, Houtgraaf J, Rutten FH, Oberski DL, et al. Developing a personalized remote patient monitoring algorithm: a proof-of-concept in heart failure. Eur Heart J Digit Health. (2023) 4(6):455–63. doi: 10.1093/ehjdh/ztad049
23. Boehmer JP, Hariharan R, Devecchi FG, Smith AL, Molon G, Capucci A, et al. A multisensor algorithm predicts heart failure events in patients with implanted devices: results from the MultiSENSE study. JACC Heart Fail. (2017) 5(3):216–25. doi: 10.1016/j.jchf.2016.12.011
24. Cardoso I, Cunha PS, Laranjo S, Grazina A, Viegas JM, Portugal G, et al. Validation of a heart failure risk score in a cohort of cardiac resynchronization therapy patients under remote monitoring: results from the TriageHFTM algorithm. J Innov Card Rhythm Manag. (2023) 14(9):5576–81. doi: 10.19102/icrm.2023.14093
25. Koehler F, Koehler K, Deckwart O, Prescher S, Wegscheider K, Kirwan B-A, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. (2018) 392(10152):1047–57. doi: 10.1016/S0140-6736(18)31880-4
26. Koehler F, Koehler K, Deckwart O, Prescher S, Wegscheider K, Winkler S, et al. Telemedical interventional management in heart failure II (TIM-HF2), a randomised, controlled trial investigating the impact of telemedicine on unplanned cardiovascular hospitalisations and mortality in heart failure patients: study design and description of the intervention. Eur J Heart Fail. (2018) 20(10):1485–93. doi: 10.1002/ejhf.1300
27. Moons KGM, Altman DG, Reitsma JB, Ioannidis JPA, Macaskill P, Steyerberg EW, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. (2015) 162(1):W1–73. doi: 10.7326/M14-0698
29. Goodfellow I, Bengio Y, Courville A. Deep Learning. Cambridge, Massachusetts, USA: MIT Press (2016). p. 800.
30. Bergstra J, Bengio Y. Random search for hyper-parameter optimization. J Mach Learn Res. (2012) 13(10):281–305.
31. Van Rossum G, Drake FL. Python Software Foundation. Available online at: www.python.org (cited July 1, 2021).
32. Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, et al. Scikit-learn: machine learning in python. J Mach Learn Res. (2011) 12(null):2825–30.
33. Abadi M, Barham P, Chen J, Chen Z, Davis A, Dean J, et al. Tensorflow: a system for large-scale machine learning. Proceedings of the 12th USENIX Conference on Operating Systems Design and Implementation. (OSDI’16), USA: USENIX Association (2016), p. 265–83.
34. Yu M, Tham Y-C, Rim TH, Ting DSW, Wong TY, Cheng C-Y. Reporting on deep learning algorithms in health care. Lancet Digit Health. (2019) 1(7):e328–9. doi: 10.1016/S2589-7500(19)30132-3
35. Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J-C, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. (2011) 12(1):77. doi: 10.1186/1471-2105-12-77
36. Boyd K, Eng KH, Page CD. Area under the precision-recall curve: point estimates and confidence intervals. In: Blockeel H, Kersting K, Nijssen S, Železný F, editors. Machine Learning and Knowledge Discovery in Databases. Berlin Heidelberg: Springer (2013), p. 451–66. Available online at: https://link.springer.com/chapter/10.1007/978-3-642-40994-3_29 (Accessed December 12, 2023).
37. Ploux S, Strik M, Ramirez FD, Buliard S, Chauvel R, Dos Santos P, et al. Remote management of worsening heart failure to avoid hospitalization in a real-world setting. ESC Heart Fail. (2023) 10(6):3637–45. doi: 10.1002/ehf2.14553
38. Auener SL, van Dulmen SA, Atsma F, van der Galiën O, Bellersen L, van Kimmenade R, et al. Characteristics associated with telemonitoring use among patients with chronic heart failure: retrospective cohort study. J Med Internet Res. (2023) 25:e43038. doi: 10.2196/43038
39. Knoll K, Rosner S, Gross S, Dittrich D, Lennerz C, Trenkwalder T, et al. Combined telemonitoring and telecoaching for heart failure improves outcome. NPJ Digit Med. (2023) 6(1):1–9. doi: 10.1038/s41746-023-00942-4
40. Severino P, D’Amato A, Prosperi S, Magnocavallo M, Maraone A, Notari C, et al. Clinical support through telemedicine in heart failure outpatients during the COVID-19 pandemic period: results of a 12-months follow up. J Clin Med Res. (2022) 11(10):2790. doi: 10.3390/jcm11102790
41. Lee KC-S, Breznen B, Ukhova A, Martin SS, Koehler F. Virtual healthcare solutions in heart failure: a literature review. Front Cardiovasc Med. (2023) 10:1231000. doi: 10.3389/fcvm.2023.1231000
42. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. (2021) 42(36):3599–726. doi: 10.1093/eurheartj/ehab368
43. Xu P, Hudnall M, Zhao S, Raja U, Parton J, Lewis D. Pandemic-triggered adoption of telehealth in underserved communities: descriptive study of Pre- and postshutdown trends. J Med Internet Res. (2022) 24(7):e38602. doi: 10.2196/38602
44. Angermann CE, Rosenkranz S. Telemonitoring und pulmonalisdruck-geführte therapie der herzinsuffizienz. Internist. (2018) 59(10):1041–53. doi: 10.1007/s00108-018-0495-1
Keywords: heart failure, decision support (DS), telemedicine, machine learning, remote patient care, risk stratification
Citation: Hinrichs N, Meyer A, Koehler K, Kaas T, Hiddemann M, Spethmann S, Balzer F, Eickhoff C, Falk V, Hindricks G, Dagres N and Koehler F (2024) Artificial intelligence based real-time prediction of imminent heart failure hospitalisation in patients undergoing non-invasive telemedicine. Front. Cardiovasc. Med. 11:1457995. doi: 10.3389/fcvm.2024.1457995
Received: 1 July 2024; Accepted: 9 September 2024;
Published: 20 September 2024.
Edited by:
Andre Rodrigues Duraes, Federal University of Bahia (UFBA), BrazilReviewed by:
Marcos Machado, Professor Edgard Santos University Hospital Complex, BrazilFederica Moscucci, Sapienza University of Rome, Italy
Copyright: © 2024 Hinrichs, Meyer, Koehler, Kaas, Hiddemann, Spethmann, Balzer, Eickhoff, Falk, Hindricks, Dagres and Koehler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexander Meyer, YWxleGFuZGVyLm1leWVyQGRoemMtY2hhcml0ZS5kZQ==
†These authors share senior authorship
 Nils Hinrichs
Nils Hinrichs Alexander Meyer1,2,3,4,5*
Alexander Meyer1,2,3,4,5* Sebastian Spethmann
Sebastian Spethmann Felix Balzer
Felix Balzer