
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
EDITORIAL article
Front. Cardiovasc. Med., 12 July 2024
Sec. Coronary Artery Disease
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1455983
This article is part of the Research TopicSpontaneous Coronary Artery Dissection: Current State of Diagnosis and TreatmentView all 10 articles
Editorial on the Research Topic
Spontaneous coronary artery dissection: current state of diagnosis and treatment
Spontaneous coronary artery dissection (SCAD) is relatively rare but potentially life-threatening condition characterized by the spontaneous separation of the layers of the coronary artery wall and the formation of intramural hematoma which compromises coronary blood flow (1, 2). Djokovic et al. discussed several mechanisms and theories to understand development of SCAD, including primarily structural weaknesses in the arterial wall with abnormalities in the connective tissue or smooth muscle cells predisposing spontaneous tearing or separation (“inside-out” or “outside in” hypothesis) (1, 2). Ultimately, the pathophysiology of SCAD likely involves a complex interplay of structural, hormonal, inflammatory, and genetic factors, highlighting the need for comprehensive research (3). Stanojević et al. discussed the most common predisposing factor like fibromuscular dysplasia, followed by inherited connective tissue disorders and systemic inflammatory diseases. Pregnancy and the use of sex hormones are common in younger females with SCAD. It was found that around 43% of acute coronary syndromes (ACS) cases among pregnant or postpartum women were caused by SCAD. It is also important to note that the presence of traditional risk factors for atherosclerosis does not exclude SCAD as a diagnosis in young patients with ACS.
Invasive coronary angiography remains the most important diagnostic tool in suspected SCAD, and Kovacevic et al. discussed the angiographic presentation of SCAD. According to Yip-Saw classification (4), there are three typical angiographic patterns of SCAD, but several potential pitfalls and essential differential diagnoses should be considered. Type 1 SCAD is characterized by a pathognomonic angiographic appearance and a recognizable radiolucent flap, usually affecting the proximal segments of coronary arteries. Type 2 SCAD is the most common type, presents as a smooth diffuse stenosis either with lumen restoration in the distal segment (Type 2a) or stenosis extending till the end of the artery (Type 2b). In addition to atherosclerosis, the most common mimic of SCAD type 2 is coronary vasospasm (focal or diffuse), which can be distinguished with intracoronary nitroglycerine injections. Type 3 SCAD is characterized by focal stenosis and underlying hematoma resembling a ruptured atherosclerotic plaque and is frequently missed by coronary angiography alone. Therefore, to distinguish features that mimic SCAD, high-resolution intracoronary imaging techniques such as intravascular ultrasound (IVUS) or optical coherence tomography (OCT), may be beneficial. Recently, additional type 4 SCAD has been proposed to describe total vessel occlusion, usually of a distal coronary artery (5). It is particularly challenging to diagnose, and is often misinterpreted as an atherosclerotic occlusion, thus being treated systematically by percutaneous coronary intervention (PCI). As type 4 SCAD often coexists with other SCAD types or occurs as a consequence of their progression, an intramural hematoma near the occlusion could be identified with intravascular imaging techniques. Krljanac et al. described the role of multimodality imaging, especially echocardiography and cardiac magnetic imaging (CMR), in the evaluation and follow-up of SCAD patients presenting with ST-elevation myocardial infarction (STEMI) (6). Previous studies showed that the majority of these patients have a mild myocardial infarctions and preserved or slightly impaired left ventricle (LV) systolic function (7). Therefore, the improvement in LV systolic function during follow-up is greater than that seen in patients with type 1 STEMI. These differences may be related to a higher prevalence of TIMI 3 flow at coronary angiography and an overall smaller ischemic burden in STEMI patients caused by SCAD than in those caused by erosion/rupture of the atherosclerotic plaque and subsequent thrombosis. However, there is no assurance that this applies to more complex types of SCAD, such as total vessel occlusion and multisegmental or multivessel engagement.
Mehmedbegovic et al. pointed out the importance of intravascular imaging techniques (IVUS and OCT) in the differential diagnosis between SCAD and other coronary lesions such as atherosclerotic plaque with or without intracoronary thrombus or myocardial bridging. The main disadvantage of invasive coronary angiography is that it is basically just a “luminography” that provides little information regarding artery wall integrity. Quite the opposite, IVUS and OCT would provide detailed phenomena typical of SCAD-like lesions such as the existence of an intimal flap, the presence and extent of intramural hematoma and/or thrombus, and the absence of atherosclerotic changes in the arterial wall. Intravascular imaging should therefore only be used if angiographic findings are unclear in large arteries (especially in SCAD types 3 and 4) and/or if further PCI is required (8–11). Current treatment strategies for SCAD patients were explained in detail in a comprehensive review by Ilic et al. (Figure 1). While no randomized clinical trials have been conducted on medical treatment for SCAD, treatment strategies generally emphasize a conservative approach since spontaneous healing of SCAD usually occurs in the first 30 days after the event (12, 13). Percutaneous coronary intervention is recommended for patients with ongoing ischemia and/or hemodynamic instability due to its high complication rates and low angiographic success rates (12, 14). However, the multicentre international “DIssezioni Spontanee COronariche (DISCO)” registry, which included 314 SCAD-patients, found that dual antiplatelet therapy (DAPT) was associated with a 2.6-fold higher risk for major adverse cardiovascular events (MACE) compared to single antiplatelet therapy (SAPT) with mainly aspirin at 1-year follow-up (15). These findings implicate that DAPT could be harmful in conservatively managed SCAD patients, especially those with intramural haematoma due to intramural bleeding aggravation, haematoma and dissection propagation and subsequent arterial lumen compression (15). Therefore, there is consensus that DAPT should be prescribed in SCAD patients, consisting of aspirin and clopidogrel, and should be limited to the first 30 days following hospital admission, except for those with stent implantation who should be treated in accordance with the current guidelines for ACS (1, 14). It is recommended to continue taking aspirin monotherapy after 1 month, but the duration of this therapy remains unknown. The current recommendations also support the use of beta-blockers as a first-line therapy for at least 1 year after the event since their use was associated with a significantly lower risk of SCAD recurrence (16). Other medications such as angiotensin-converting enzyme (ACE) inhibitors, angiotensin-receptor blockers (ARB), mineralocorticoid antagonists, loop diuretics and statins are recommended for patients with concomitant risk factors for atherosclerosis and coronary artery disease and/or heart failure with reduced or mild ejection fraction (EF<50%), in accordance with the current guidelines.
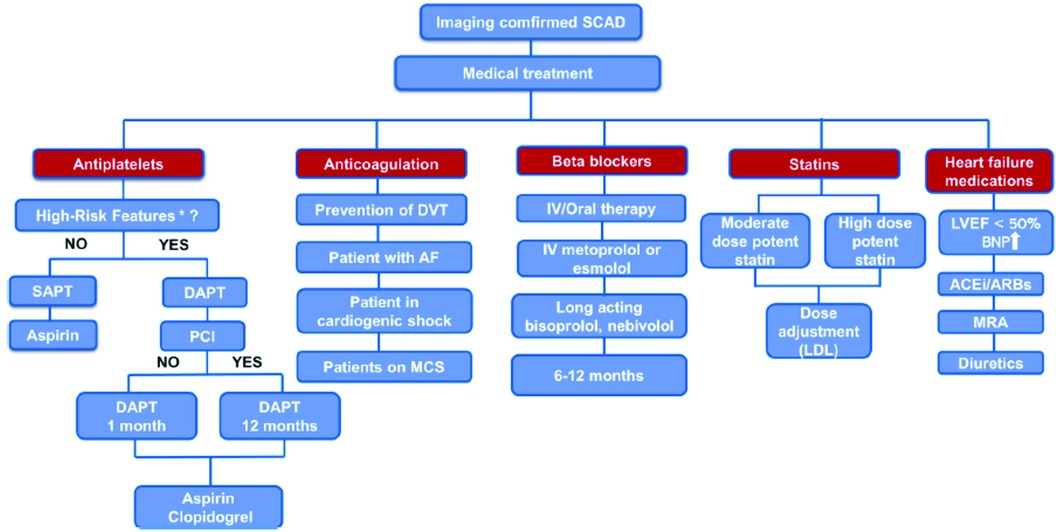
Figure 1 Proposed algorithm for medical treatment of imaging confirmed SCAD. *“high-risk” features - concomitant atherosclerosis, large thrombus burden, critical stenosis that was left untreated, and significant flow impairment in the affected coronary artery. AF, atrial fibrillation; ACEi, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; BNP, brain natriuretic peptide; DAPT, dual antiplatelet therapy; DVT, deep vein thrombosis; IV, intravenous; LVEF, left ventricle ejection fraction; MCS, mechanical circulatory support; MRA, mineralocorticoid receptor antagonist; PCI, percutaneous coronary intervention; SAPT, single antiplatelet therapy.
A percutaneous angioplasty using a cutting balloon as a novel interventional strategy for the treatment of SCAD was described by Maricic et al. This technique entails positioning of a cutting balloon inside the true lumen to cause controlled micro-incisions within the affected vessel, causing intimal fenestration and hematoma draining (1, 17). Consequently, the true arterial lumen is decompressed, and coronary blood flow is restored. According to current data, by using a smaller cutting balloon than the reference vessel diameter, the risk of vessel injury can be minimized, and the procedure can be more effective. The most often procedure complication is distal propagation of the subintimal hematoma with dissection extension, while coronary perforation and acute vessel closure are very rare. If such a situation arises, stenting may be the only option to stabilize the dissected coronary artery and provide additional support. Further research is needed to determine the long-term clinical implications and compare the efficacy and safety of cutting balloon angioplasty with other treatment options for SCAD.
A systematic review by Petrovic et al., which included 13 observational studies, examined clinical outcomes in 1,801 patients with SCAD treated conservatively (65%) or invasively (PCI 33%; coronary artery bypass grafting 1.3%). Percutaneous coronary intervention was associated with a higher rate of periprocedural complications, mostly hematoma extension and/or iatrogenic dissection, which frequently required the implantation of at least three stents with residual areas of dissection. The overall reported in-hospital and follow-up mortality rates were 1.2% and 1.3%, respectively. According to these results, conservative treatment is the preferred treatment option for patients with SCAD. A review by Apostolovic et al. focused on female patients in generative period (16–55 of age) with ACS caused by SCAD and compared clinical characteristics and outcomes between non-pregnant women with SCAD and pregnant women with SCAD. Compared to non-pregnant women, pregnant women have a greater chance of having SCAD in the left main and/or the left anterior descending artery (LAD); are more likely to have STEMI; and are more likely to undergo PCI. However, there were no differences regarding mortality rates or recurrent coronary dissection between these two study groups. Future research efforts with developing specialized SCAD registries will contribute to a better understanding of this condition and its outcomes.
SAp: Writing – review & editing, Writing – original draft, Investigation, Conceptualization. SAl: Writing – review & editing, Writing – original draft, Validation, Conceptualization. BB: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Adlam D, Alfonso F, Maas A, Vrints C Writing Committee. European Society of cardiology, acute cardiovascular care association, SCAD study group: a position paper on spontaneous coronary artery dissection. Eur Heart J. (2018) 39(36):3353–68. doi: 10.1093/eurheartj/ehy080
2. Hayes SN, Kim ESH, Saw J, Adlam D, Arslanian-Engoren C, Economy KE, et al. American Heart Association Council on peripheral vascular disease; council on clinical cardiology; council on cardiovascular and stroke nursing; council on genomic and precision medicine; and stroke council. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation. (2018) 137(19):e523–57. doi: 10.1161/CIR.0000000000000564
3. Adlam D, Berrandou TE, Georges A, Nelson CP, Giannoulatou E, Henry J, et al. Genome-wide association meta-analysis of spontaneous coronary artery dissection identifies risk variants and genes related to artery integrity and tissue-mediated coagulation. Nat Genet. (2023) 55(6):964–72. doi: 10.1038/s41588-023-01410-1
4. Yip A, Saw J. Spontaneous coronary artery dissection-A review. Cardiovasc Diagn Ther. (2015) 5(1):37–48. doi: 10.3978/j.issn.2223-3652.2015.01.08
5. Al-Hussaini A, Adlam D. Spontaneous coronary artery dissection. Heart Br Card Soc. (2017) 103(13):1043–51. doi: 10.1136/heartjnl-2016-310320
6. Krljanac G, Apostolovic S, Polovina M, Maksimovic R, Nedeljkovic Arsenovic O, Djordjevic N, et al. The follow-up of myocardial injury and left ventricular function after spontaneous coronary artery dissection. Front Cardiovasc Med. (2023) 10:1276347. doi: 10.3389/fcvm.2023.1276347
7. Díez-Villanueva P, García-Guimarães M, Sanz-Ruiz R, Sabaté M, Macaya F, Roura G, et al. Prognostic implications of left ventricular systolic dysfunction in patients with spontaneous coronary artery dissection. Eur Heart J. (2023) 12:299–305. doi: 10.1093/ehjacc/zuad035
8. Gudenkauf B, Hays AG, Tamis-Holland J, Trost J, Ambinder DI, Wu KC, et al. Role of multimodality imaging in the assessment of myocardial infarction with nonobstructive coronary arteries: beyond conventional coronary angiography. J Am Heart Assoc. (2022) 11(1):e022787. doi: 10.1161/JAHA.121.022787
9. Johnson TW, Räber L, di Mario C, Bourantas C, Jia H, Mattesini A, et al. Clinical use of intracoronary imaging. Part 2: acute coronary syndromes, ambiguous coronary angiography findings, and guiding interventional decision-making: an expert consensus document of the European association of percutaneous cardiovascular interventions. Eur Heart J. (2019) 40(31):2566–84. doi: 10.1093/eurheartj/ehz332
10. Räber L, Mintz GS, Koskinas KC, Johnson TW, Holm NR, Onuma Y, et al. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European association of percutaneous cardiovascular interventions. Eur Heart J. (2018) 39(35):3281–300. doi: 10.1093/eurheartj/ehy285
11. Prakash R, Starovoytov A, Heydari M, Mancini GB, Saw J. Catheter-induced iatrogenic coronary artery dissection in patients with spontaneous coronary artery dissection. JACC Cardiovasc Interv. (2016) 9(17):1851–3. doi: 10.1016/j.jcin.2016.06.026
12. Bocchino PP, Angelini F, Franchin L, D’Ascenzo F, Fortuni F, De Filippo O, et al. Invasive versus conservative management in spontaneous coronary artery dissection: a meta-analysis and meta-regression study. Hellenic J Cardiol. (2021) 62(4):297–303. doi: 10.1016/j.hjc.2021.02.013
13. Hassan S, Prakash R, Starovoytov A, Saw J. Natural history of spontaneous coronary artery dissection with spontaneous angiographic healing. JACC Cardiovasc Interv. (2019) 12(6):518–27. doi: 10.1016/j.jcin.2018.12.011
14. Byrne RA, Rossello X, Coughlan JJ, Barbato E, Berry C, Dan GA, et al. 2023 ESC guidelines for the management of acute coronary syndromes. Eur Heart J. (2023) 44(38):3720–826. doi: 10.1093/eurheartj/ehad191
15. Cerrato E, Giacobbe F, Quadri G, Macaya F, Bianco M, Mori R, et al. Antiplatelet therapy in patients with conservatively managed spontaneous coronary artery dissection from the multicentre DISCO registry. Eur Heart J. (2021) 42(33):3161–71. doi: 10.1093/eurheartj/ehab372
16. Saw J, Humphries K, Aymong E, Sedlak T, Prakash R, Starovoytov A, et al. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol. (2017) 70(9):1148–58. doi: 10.1016/j.jacc.2017.06.053
17. Alfonso F, Paulo M, Lennie V, Dutary J, Bernardo E, Jiménez-Quevedo P, et al. Spontaneous coronary artery dissection: long-term follow-up of a large series of patients prospectively managed with a “conservative” therapeutic strategy. JACC Cardiovasc Interv. (2012) 5(10):1062–70. doi: 10.1016/j.jcin.2012.06.014
Keywords: spontaneous coronary artery dissection (SCAD), acute coronary syndrome (ACS), intravascular imaging, intravascular ultrasound (IVUS), optical coherence tomography (OCT), percutaneous coronary intervention (PCI), cutting balloon (CB)
Citation: Apostolovic S, Aleksandric S and Beleslin B (2024) Editorial: Spontaneous coronary artery dissection: current state of diagnosis and treatment. Front. Cardiovasc. Med. 11:1455983. doi: 10.3389/fcvm.2024.1455983
Received: 27 June 2024; Accepted: 28 June 2024;
Published: 12 July 2024.
Edited and Reviewed by: Tommaso Gori, Johannes Gutenberg University Mainz, Germany
© 2024 Apostolovic, Aleksandric and Beleslin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Srdjan Aleksandric, c3JkamFuYWxla3NhbmRyaWNAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.