- 1Department of Cardiovascular Medicine, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Department of Neurology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 3Cardiac Electro-Physiology Laboratories, Regions Hospital, St. Paul, MN, United States
Background: There have been few instances of symptomatic bradycardia-arrhythmia in the context of area postrema syndrome (APS), and some of them have been implanted permanent pacemakers. Cardioneuroablation (CNA) has emerged as a viable therapy for the treatment of syncope induced by neutrally mediated bradycardia or atrioventricular block.
Methods: We report a young patient with recurrent cardiac asystole and syncope following persistent hiccups caused by neuromyelitis optica spectrum disorder (NMOSD), who successfully completed CNA treatment and avoided permanent pacemaker placement. We also summarized and analyzed 20 previously reported cases that were relevant to APS with bradyarrhythmia.
Results: In a patient with NMOSD, CNA can efficiently and safely eradicate symptomatic bradycardia-arrhythmia. A total of 21 cases were identified in the final analysis (including our case). The average age was 51 years old and female patients accounted for 38.1%. Brady-arrhythmia was presented in all patients, and 9 patients were implanted temporary or permanent pacemakers. 4 of the 9 patients were received permanent pacing therapy because they were not weaned off pacing support after etiological treatment.
Conclusions: Cardiac asystole and syncope after persistent hiccups may be the first signs of APS of medullary lesions, and CNA may be a useful therapy option for these patients in experienced centers. We believe that in this scenario, CNA may be a superior therapeutic option than permanent pacemaker placement. Additionally, the statement also serves as a cautionary reminder for health care professionals to establish an association between bradyarrhythmia and APS of medullary lesions in their clinical practice.
1 Introduction
The immune-mediated condition neuromyelitis optica spectrum disorder (NMOSD) is characterized by acute demyelinating and/or necrotizing lesions in the optic nerves and/or spinal cord. As a disease with medullary lesions, NMOSD manifests in a wide range of ways, from common ocular neuritis, acute myelitis, and area postrema syndrome (APS, such as hiccups, nausea, and vomiting) to more usual symptoms including syncope and cardiac arrhythmias. Some of those patients with bradyarrhythmias received temporary or permanent pacemaker implantation. Aquaporin 4 (AQP4) is a water channel expressed in “Astrocytes”, and anti-AQP4 antibody is a specific marker for NMOSD (1, 2).
Cardioneuroablation (CNA) targeting the cardiac parasympathetic ganglionated plexus (GP) has emerged as a viable treatment for syncope induced by neutrally mediated bradycardia or atrioventricular block in recent years (3). As a result, we thought this unique method may be effective in treating cardiac asystole induced by medullary-related neurologic disease such as NMOSD in this case. We present the case of a young woman who had persistent hiccups followed by repeated syncope owing to extended asystole pauses and was effectively treated with CNA. We also performed a systematic review of the literature on the management of symptomatic bradycardia-arrhythmia in patients with NMOSD or other Medullary diseases.
2 Case report
The patient was a 34-year-old Chinese female with a history of hypothyroidism and systemic lupus erythematosus (SLE) for 3 years. She took a minimal maintenance dose of steroids and 50 micrograms of levothyroxine sodium per day. Her SLE was in stable condition and thyroid function test kept normal during routine follow-up. 10 days before admission when she developed hiccups, nausea and vomiting without obvious causes. While visiting a nearby clinic, metoclopramide and intravenous fluids were ordered. However, the problems persisted. Five days earlier, she was admitted to a local hospital for repeated episodes of sudden onset dizziness and syncope following bouts of hiccups. She underwent a head computed tomography (CT) scan and without any positive findings. A 24-h Holter-monitor disclosed episodes of profound sinus bradycardia with slowest heart rate down to 14 bpm. There were 297 times of R-R intervals >2.0 s with the longest pause of 13.1 s (Figure 1). Permanent pacemaker therapy was offered but declined by the patient. After transferring to our cardiovascular department, the patient continued to have hiccups, nausea, and syncope without decreased vision and limb weakness. Routine laboratory examinations and markers of myocardial injury were in normal limitation. Thyroid function test was normal. Cardiac ultrasonography showed no abnormalities. Her consciousness was intact, and there was no abnormality in the cranial and peripheral nerves. A temporary pacemaker was implanted to maintain normal heart rate and prevent further syncope.
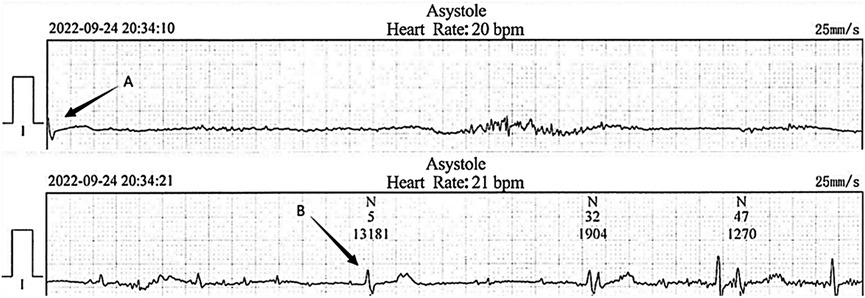
Figure 1. Electrocardiogram recorded at the local hospital. The cardiac arrest duration was 13.1 s following severe hiccups (between arrow A and B).
The patient was consulted by the neurology department at the very beginning and NMOSD was suspected. But considering that her symptoms and signs were not typical, we finally decided not to use methylprednisolone before her head MRI test done. However, head and neck MRI could not be performed because implanted temporary pacemaker and the lead were non-MR-conditional. After detailed discussion and counseling, patient and family agreed to proceed with CNA. The procedure was performed under Moderate sedation and routine electrophysiological testing was completed. The ganglionated plexus groups were mapped and ablated using a 3D electroanatomic mapping system, guided by anatomy and fragmented potentials. (CARTO3 Version 6; Biosense Webster, Diamond Bar, CA, USA) and a Thermocool® SmartTouch (Biosense Webster, Diamond Bar, CA, USA) irrigation catheter. The right atrium was initially reconstructed. Local fragmented intra-cardiac electrograms in the areas of aorto-superior vena cava GP and posteromedial left GP were eliminated (Figure 2). The left atrium was reconstructed and fragmented potentials were mapped. Ablation delivered at the targeted GPs (left superior GP, left inferior GP, right superior GP, and right inferior GP) (Figure 2). After ablation, the resting sinus rate increased from 54 bpm to 68 bpm, the long R-R interval > 2.0 s following hiccups was no longer present and temporary pacemaker was removed. There was no major complication related to the procedure. Two days after CNA procedure, persistent hiccup, vomit, and anorexia were still present and refractory to conventional medical therapy, but the episodes of dizziness and syncope had completely resolved. A 48-h Holter monitoring confirmed no R-R interval >2.0 s. MRI of the skull and spinal cord revealed a lesion at dorsal medulla oblongata (Figure 3). As the cerebrospinal fluid was tested positive for anti-AQP4 antibody, she was diagnosed as NMOSD. Considering the patient's low BMI (19.3) and the need for anti-coagulation therapy after CNA, methylprednisolone pulse therapy (800 mg per day instead of 1,000 mg) was given for five days and then changed to oral prednisolone with simultaneously ofatumumab for sequential immunosuppressive therapy. Her symptoms resolved and was discharged five days later with prednisolone (45 mg per day for the first 2 weeks, then gradually reducing dosage under the supervision of a physician), levothyroxine sodium 50 Ug per day and Edoxaban 30 mg per day for 2 months.
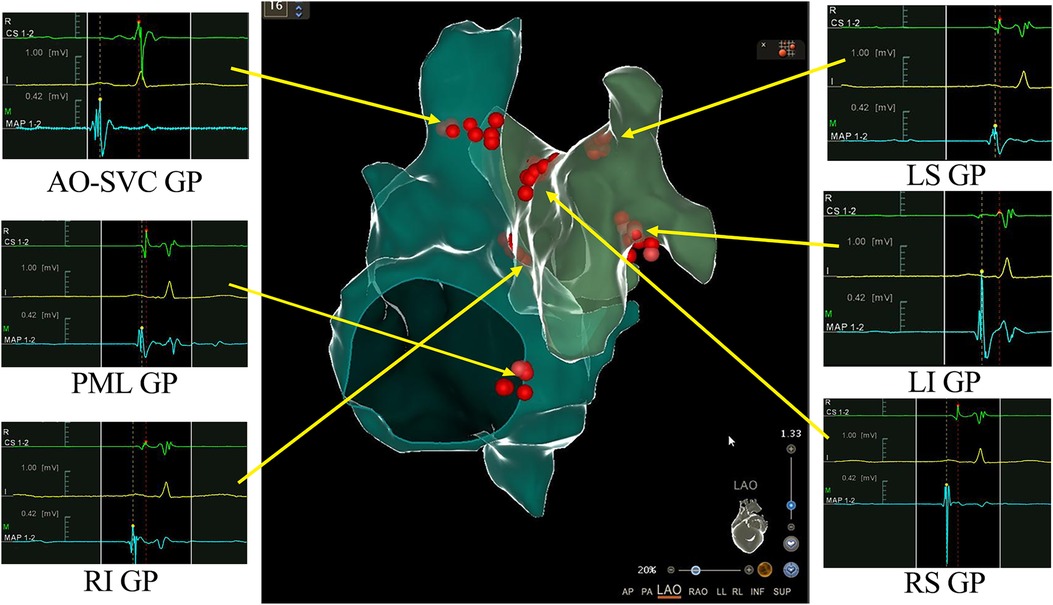
Figure 2. 3D view of ganglionated plexus (GPs) groups distribution and ablation in the right and left atrium. Combined 3-dimensional electroanatomic mapping and radiofrequency (RF) ablation based on presence of fragmented/fractionated potentials in sinus rhythm. Red dots indicate RF application on the GPs sites. AO-SVC GP, aorto-superior vena cava GP; PML GP, posteromedial left GP; LS GP, left superior GP; LI GP, left inferior GP; RS GP, right superior GP; RI GP, right inferior GP.
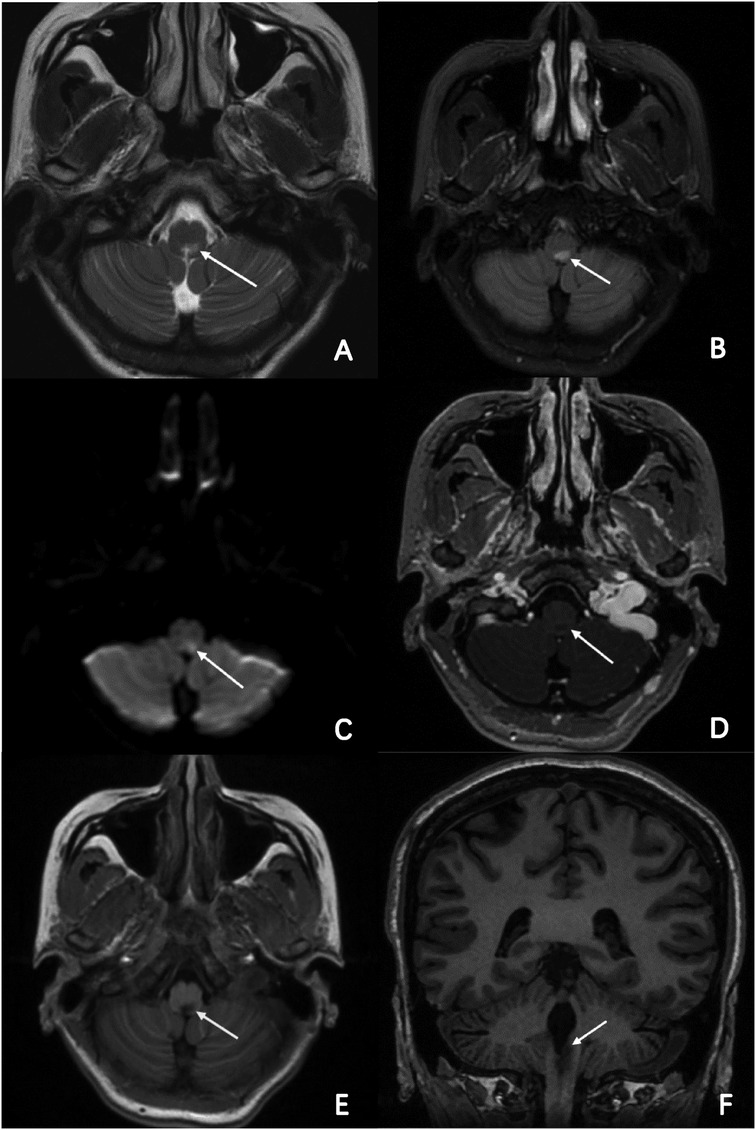
Figure 3. MR imaging of the brain and part of cervical cord. Axial T2-weighted, FLAIR and DWI brain MRI revealed a high-intensity lesion in the dorsal part of the medulla oblongata and cervical cord. (A–C; arrowheads) Axial enhanced T1-weighted and normal T1-weighted brain MRI revealed a low-intensity lesion in the same segment, (D,E; arrowheads) and clearer in coronal view. (F; arrowheads).
She remained asymptomatic and 24-h Holter monitor demonstrated normal sinus rhythm with appropriate chronotropic response at 3- and 6-months follow-up.
3 Discussion
Area Postrema Syndrome (APS) is rare among the symptoms of NMOSD, presenting as intractable nausea, vomiting, and hiccups, this condition is commonly misdiagnosed clinically. Herein, we described a NMOSD patient with dorsal medulla oblongata involvement who initially presented with persistent hiccups, nausea, vomiting, and episodes of syncope due to cardiac asystole. Neuromyelitis optica frequently affects the medullary tegmentum, leading to persistent hiccups and nausea in patients (4). The differential diagnosis for persistent hiccups includes central nervous system (CNS) diseases, gastrointestinal, thoracic, cardiac, toxic-metabolic and drug-related disorders (5) In most cases, the mechanism of hiccup is involved in the following 3 pathways: (1) the afferent pathway of the “vagal nerve, the phrenic nerve, or the thoracic-sympathetic nerve”, (2) the medulla oblongata, including the respiratory center, the “ambiguous nucleus”, and the “reticular formation”, and (3) the efferent pathway of the phrenic nerve or the motor nerves to respiratory muscles (6). Previous research revealed that parasympathetic stimulation and sympathetic inhibition caused by the lesion of the solitary nuclei in medulla oblongata can result in bradycardia and asystole (7). We assumed that lesions in medulla oblongata, specifically the solitary nucleus and medullary tegmentum triggered hiccups and bradycardia. Hiccups may further enhance parasympathetic outflow and result into severe bradycardia and long pauses.
When it comes to NMOSD with bradyarrhythmia, it is necessary to make a differential diagnosis with other neurological diseases such as multiple sclerosis and infarction, which can also give rise to cardio-inhibitory arrhythmia. The published studies are limited and the most of them are case reports or observation of small series. We summarized the published reports of bradycardia arrhythmia associated with the medullary lesion in Table 1 (7–26). A total of 21 cases were identified in the final analysis (including our case). The average age was 51 years old and female patients accounted for 38.1% (8/21). Brady-arrhythmia was presented in all patients, in addition, one case was combined with ventricle tachycardia and another one with paroxysmal atrial fibrillation, respectively reported by Berry and Koay (15, 21). Symptomatic bradycardia arrhythmia was secondary to NMOSD (9 cases), medullary infarction (8 cases) and multiple sclerosis (4 cases) in sequence. Notably, most of the patients with bradyarrhythmia had lesions in the medulla oblongata, except one patient with cervical cord lesions, one patient associated with midbrain and another case without MRI results.10-12 9 patients were received pacing therapy. 4 of the 9 patients were implanted permanent pacemaker because they were not weaned off pacing support after etiological treatment. Interestingly, none of MS patients had pacing treatment but three of four patients had noticeable improvement. Most of patients had better outcomes after pacing support and treatment of the underlying disease except one death (19).
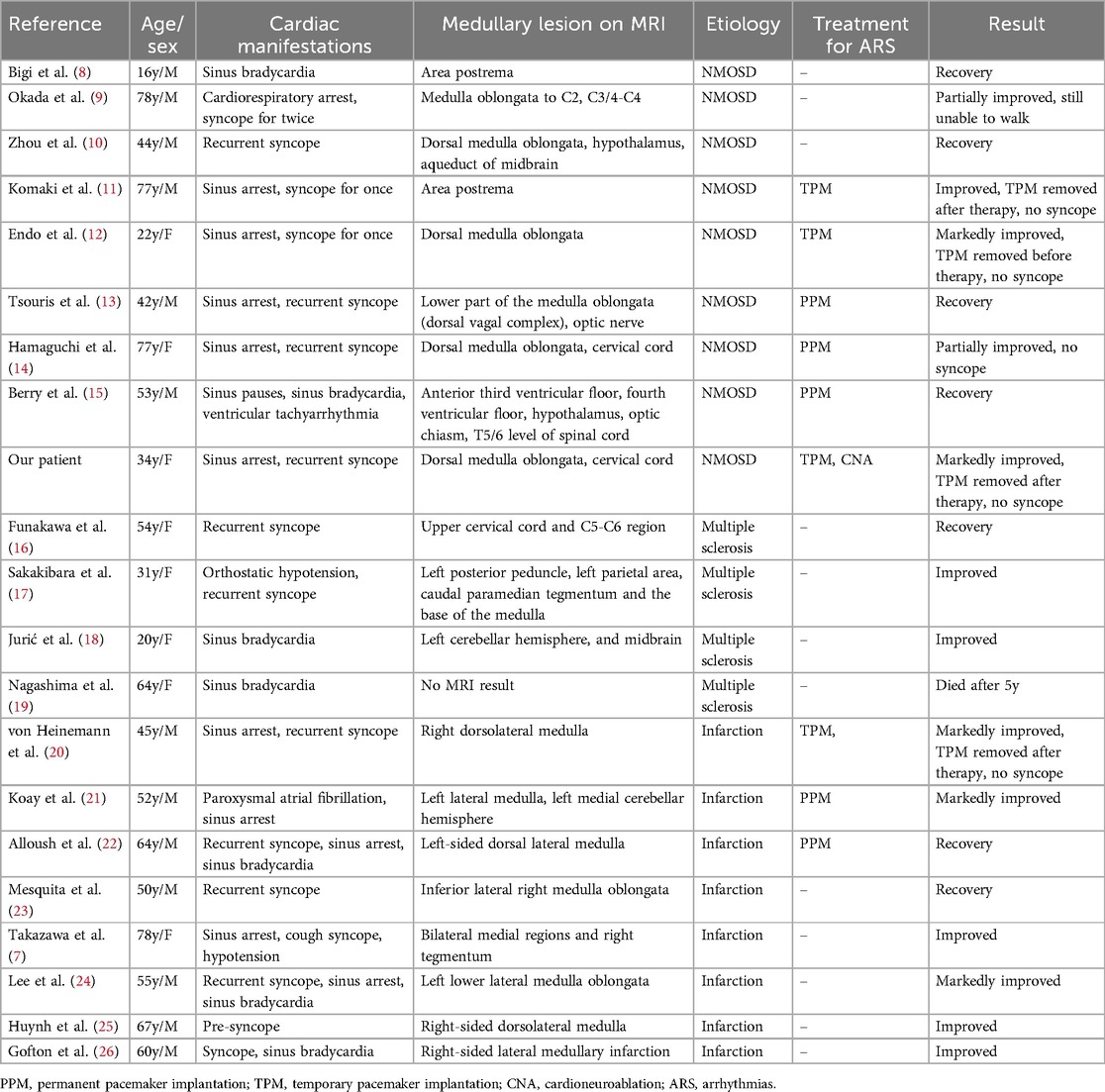
Table 1. Summary of published case reports of bradycardia-arrhythmia associated with the medullary lesion.
The anatomical lesion sites of these cases correlated with varied clinical presentations in neurological disorders that impact cardiovascular systems. Lesions in the medulla oblongata, particularly around the area postrema, could cause conducted disruptions manifesting as syncope and arrhythmias (8, 11, 13, 14). Lesions extended to cervical segments in certain cases,which would lead to a broader range of autonomic dysfunction, presenting as severe cardiac symptoms like sinus arrest (9). The spread of lesions to lateral or inferior parts of the brainstem or spinal cord could result in cardiogenic shock. This heterogeneity in lesion sites illustrates the intricate interactions between neuroanatomy and cardiovascular systems, necessitating tailored diagnostic and therapeutic approaches for optimal management of these conditions.
Although pacemaker is effective for treatment of symptomatic bradycardia-arrhythmia in patients with NMOSD, an implanted non-MR-conditional pacemaker may complicate the MR test which plays a key role in the earlier diagnosis of the disease. Pacemaker implantation itself is associated with complications such as infection, leads breakage and leads dislodgement (twiddler syndrome) (27). The bradycardia-arrhythmia in most patients with NMOSD is temporary and reversible when the primary disease is appropriately managed. CNA has been used to treat vasovagal syncope from functional atrioventricular block and/or sinus bradycardia in the recent years. Majority of the literature suggested the technique may be safe and effective in selected population (28). In our case, the profound cardiac asystole was likely mediated through the intrinsic cardiac autonomic nervous system. Catheter ablation of cardiac GPs may represent a better option than a permanent pacemaker insertion. To the best of our knowledge, this is the first instance of CNA successfully and safely removing symptomatic bradycardia arrhythmia in a patient with NMOSD. More research is needed to corroborate our findings.
Several limitations of our study should be acknowledged. There are limited studies in the literature on the treatment of bradycardia-arrhythmia in individuals with medullary disorders. The long-term efficacy of CNA in this specific disease needs to be confirmed in larger population and by registries. Although bi-atrial ablation procedure in this case achieved good outcome, right or left atrial ablation alone may be sufficient as ablating different GP sites may have different effects on sinoatrial and atrioventricular nodal function (29). Cost-effectiveness should be considered in the clinical practice.
4 Conclusions
This case demonstrated that medullary lesions may generate substantial bradycardia and asystole as the initial presentation of NMOSD before the onset of distinct neurological signs. The use of CNA may be more beneficial than implanting a pacemaker permanently. Additionally, the statement also serves as a cautionary reminder for health care professionals to establish an association between bradyarrhythmia and NMOSD in their clinical practice.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
EW: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. YL: Methodology, Writing – review & editing. GY: Resources, Writing – review & editing. GL: Validation, Writing – review & editing. JD: Data curation, Writing – review & editing. YW: Data curation, Writing – review & editing. WY: Data curation, Writing – review & editing. GC: Data curation, Writing – review & editing. DZ: Writing – review & editing. FJ: Conceptualization, Methodology, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. Igg marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. (2005) 202(4):473–7. doi: 10.1084/jem.20050304
2. Kremer L, Mealy M, Jacob A, Nakashima I, Cabre P, Bigi S, et al. Brainstem manifestations in neuromyelitis optica: a multicenter study of 258 patients. Mult Scler. (2014) 20(7):843–7. doi: 10.1177/1352458513507822
3. Pachon JC, Pachon EI, Pachon JC, Lobo TJ, Pachon MZ, Vargas RNA, et al. Cardioneuroablation"–new treatment for neurocardiogenic syncope, functional AV block and sinus dysfunction using catheter RF-ablation. Europace. (2005) 7(1):1–13. doi: 10.1016/j.eupc.2004.10.003
4. Misu T, Fujihara K, Nakashima I, Sato S, Itoyama Y. Intractable hiccup and nausea with periaqueductal lesions in neuromyelitis optica. Neurology. (2005) 65(9):1479–82. doi: 10.1212/01.wnl.0000183151.19351.82
5. Souadjian JV, Cain JC. Intractable hiccup. Etiologic factors in 220 cases. Postgrad Med. (1968) 43(2):72–7. doi: 10.1080/00325481.1968.11693139
6. Marsot-Dupuch K, Bousson V, Cabane J, Tubiana JM. Intractable hiccups: the role of cerebral MR in cases without systemic cause. AJNR Am J Neuroradiol. (1995) 16(10):2093–100.8585500
7. Takazawa T, Ikeda K, Kano O, Kabuki T, Kawabe K, Iwasaki Y. A case of sinus arrest and post-hiccup cough syncope in medullary infarction. J Stroke Cerebrovasc Dis. (2014) 23(3):566–71. doi: 10.1016/j.jstrokecerebrovasdis.2013.04.016
8. Bigi S, Makhani N, Banwell B. Hiccups, vomiting and the brain. Neuropediatrics. (2012) 43(02):PS16_09. doi: 10.1055/s-0032-1307127
9. Okada S, Takarabe S, Nogawa S, Abe T, Morishita T, Mori M, et al. Persistent hiccups followed by cardiorespiratory arrest. Lancet. (2012) 380(9851):1444. doi: 10.1016/S0140-6736(12)60900-3
10. Zhou JJ, Luo L. Hypersomnia and syncope as initial manifestations of neuromyelitis optica spectrum disorder: a case report. Zhonghua Nei Ke Za Zhi. (2019) 58(4):309–11. doi: 10.3760/cma.j.issn.0578-1426.2019.04.014
11. Komaki R, Chihara N, Hara A, Fujisawa S, Muramae N, Nakasone K, et al. A case of area postrema syndrome associated with sick sinus syndrome in an elderly patient with neuromyelitis optica spectrum disorder: case report. Neurol Clin Neurosci. (2020) 8(4):183–5. doi: 10.1111/ncn3.12380
12. Endo Y, Hayashi K, Ikawa M, Yamamura O, Ookura K, Hamano T. [A case of neuromyelitis optica spectrum disorder with persistent nausea and repeated syncope]. Rinsho Shinkeigaku. (2020) 60(2):142–5. doi: 10.5692/clinicalneurol.cn-001345
13. Tsouris Z, Dastamani M, Siokas V, Aloizou A, Zagouras A, Provatas A, et al. Recurrent episodes of syncope requiring pacemaker implantation as an initial presentation of neuromyelitis optica spectrum disorder. Mult Scler Relat Disord. (2020) 45:102423. doi: 10.1016/j.msard.2020.102423
14. Hamaguchi M, Fujita H, Suzuki T, Suzuki K. Sick sinus syndrome as the initial manifestation of neuromyelitis optica spectrum disorder: a case report. BMC Neurol. (2022) 22(1):56. doi: 10.1186/s12883-022-02580-x
15. Berry R, Panegyres PK. Peduncular hallucinosis and autonomic dysfunction in anti-aquaporin-4 antibody syndrome. Cogn Behav Neurol. (2017) 30(3):116–24. doi: 10.1097/WNN.0000000000000132
16. Funakawa I, Terao A. Intractable hiccups and syncope in multiple sclerosis. Acta Neurol Scand. (1998) 98(2):136–9. doi: 10.1111/j.1600-0404.1998.tb01734.x
17. Sakakibara R, Mori M, Fukutake T, Kita K, Hattori T. Orthostatic hypotension in a case with multiple sclerosis. Clin Auton Res. (1997) 7(3):163–5. doi: 10.1007/BF02308845
18. Jurić S, Mišmaš A, Mihić N, Barać AM, Habek M. Newly onset sinus bradycardia in the context of multiple sclerosis relapse. Intern Med. (2012) 51(9):1121–4. doi: 10.2169/internalmedicine.51.7139
19. Nagashima T, Yamada K, Uono M, Nagashima K. Multiple sclerosis co-existent with myxedema. An autopsy case report. J Neurol Sci. (1984) 66(2-3):217–21. doi: 10.1016/0022-510X(84)90010-8
20. von Heinemann P, Grauer O, Schuierer G, Ritzka M, Bogdahn U, Kaiser B, et al. Recurrent cardiac arrest caused by lateral medulla oblongata infarction. BMJ Case Rep. (2009) 2009:bcr02.2009.1625. doi: 10.1136/bcr.02.2009.1625
21. Koay S, Dewan B. An unexpected Holter monitor result: multiple sinus arrests in a patient with lateral medullary syndrome. BMJ Case Rep. (2013) 2013:bcr2012007783. doi: 10.1136/bcr-2012-007783
22. Alloush TK, Alloush AT, Sami M, Shokri HM. Sinus arrest following acute lateral medullary infarction. Neurol Sci. (2022) 43(11):6555–9. doi: 10.1007/s10072-022-06306-2
23. Mesquita A, Ferreira P, Carvalho M, Martins M. Acute stroke presenting as syncope: wallenberg syndrome. BMJ Case Rep. (2022) 15(7):e251203. doi: 10.1136/bcr-2022-251203
24. Lee SW, Lee H, Yoo J, Kim J, Lim KB. Sick sinus syndrome combined with wallenberg syndrome: a case report. Brain Neurorehabil. (2021) 14(3):e27. doi: 10.12786/bn.2021.14.e27
25. Huynh TR, Decker B, Fries TJ, Tunguturi A. Lateral medullary infarction with cardiovascular autonomic dysfunction: an unusual presentation with review of the literature. Clin Auton Res. (2018) 28(6):569–76. doi: 10.1007/s10286-018-0502-6
26. Gofton TE, Segal OR, Skanes AC, Fraser JA, Hyson HC. Lateral medullary syndrome presenting with ataxia and bradycardia. Can J Neurol Sci. (2009) 36(3):390–2. doi: 10.1017/S0317167100007216
27. Mulpuru SK, Madhavan M, McLeod CJ, Cha YM, Friedman PA. Cardiac pacemakers: function, troubleshooting, and management: part 1 of a 2-part series. J Am Coll Cardiol. (2017) 69(2):189–210. doi: 10.1016/j.jacc.2016.10.061
28. Vandenberk B, Lei LY, Ballantyne B, Vickers D, Liang Z, Sheldon RS, et al. Cardioneuroablation for vasovagal syncope: a systematic review and meta-analysis. Heart Rhythm. 19(11):1804–12. doi: 10.1016/j.hrthm.2022.06.017
29. Chen W, Liu Z, Xiao P, Xu Y, Li D, Xiong Q, et al. Extracardiac vagal stimulation-assisted cardioneuroablation: dynamically evaluating the impact of sequential ganglionated plexus ablation on vagal control of SAN and AVN in patients with sinoatrial node dysfunction. J Cardiovasc Dev Dis. (2022) 9(6):188. doi: 10.3390/jcdd9060188
Keywords: cardioneuroablation, asystole, NMOSD, medullary lesions, area postrema syndrome
Citation: Wang E, Li Y, Yu G, Liu G, Deng J, Wang Y, Yang W, Chen G, Zhu DW and Jia F (2024) Cardioneuroablation eliminating cardiac asystole associated with area postrema syndrome: a case report and literature review. Front. Cardiovasc. Med. 11:1453166. doi: 10.3389/fcvm.2024.1453166
Received: 24 June 2024; Accepted: 21 October 2024;
Published: 31 October 2024.
Edited by:
Rui Providencia, University College London, United KingdomReviewed by:
Nikhil Agrawal, University of Texas Health Science Center at Houston, United StatesHisanao Akiyama, St. Marianna University School of Medicine, Japan
Copyright: © 2024 Wang, Li, Yu, Liu, Deng, Wang, Yang, Chen, Zhu and Jia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: FengPeng Jia, amlhZmVuZ3Blbmc3MkAxMjYuY29t
 EnRun Wang
EnRun Wang YuanJing Li1
YuanJing Li1 FengPeng Jia
FengPeng Jia