- 1Karl Landsteiner University of Health Sciences, Krems, Austria
- 2Division of Internal Medicine 3, University Hospital St. Pölten, St. Pölten, Austria
- 3Karl Landsteiner Institute for Cardiometabolics, Karl Landsteiner Society, St. Pölten, Austria
- 4Klinik für Kardiologie, Universitätsspital Basel, Basel, Switzerland
- 5Division of Interventional Cardiology, Cardiovascular Diseases Department, University Hospital of Split (KBC Split), Split, Croatia
- 6Department of Cardiology, Royal Stoke University Hospital, University Hospitals of North Midlands NHS Trust, Stoke-on-Trent, United Kingdom
- 7Medical School, Sigmund-Freud University, Vienna, Austria
Background: The benefit of chronic total occlusion (CTO)-percutaneous coronary intervention (PCI) is controversial because of a lack of high-quality evidence. We aim to evaluate the impact of CTO-PCI on symptoms, quality of life and mortality.
Methods: We conducted a retrospective single center study of patients with CTO-PCI in a tertiary center in Austria. The study outcomes were Canadian Cardiovascular Society (CCS) angina score, quality of life measured by Seattle Angina Questionnaire (SAQ), and death at median follow up for patients with successful vs. failed CTO-PCI.
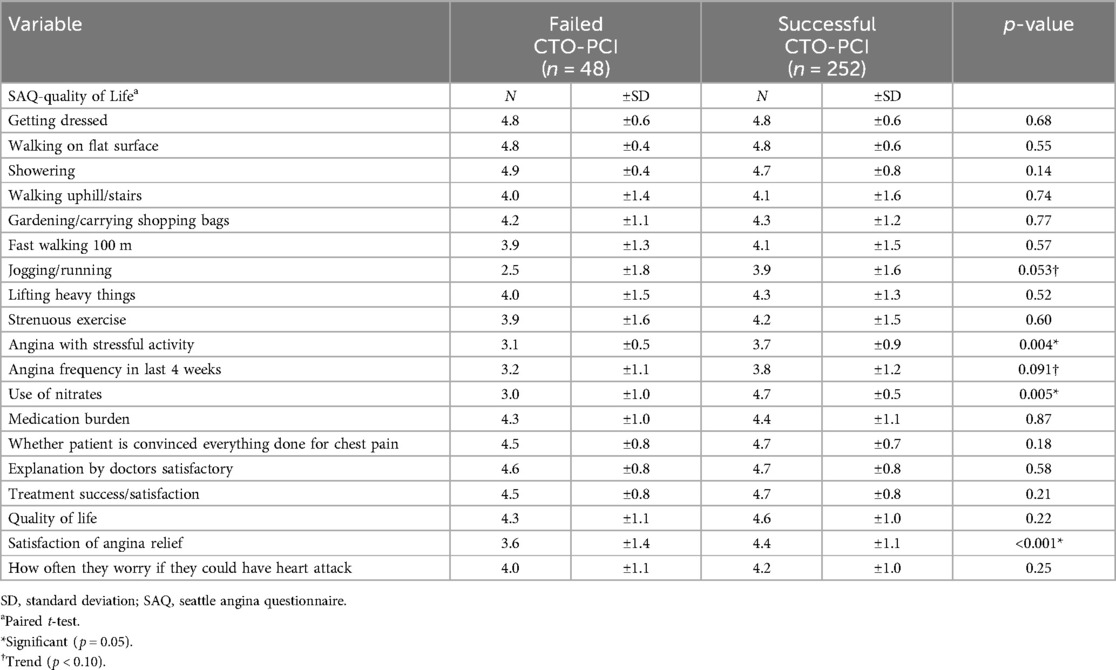
Results: A total of 300 patients underwent CTO-PCI for coronary artery disease, of which 252 (84%) were technically successful with median follow up of 3.4 years. There were no significant differences in in-hospital or all-cause mortality, major adverse cardiovascular event, or stent-related complications between the groups of failed and successful CTO-PCI. Among patients with successful CTO-PCI there was a significant improvement in CCS score, which was not found for the group with failed CTO-PCI. Successful reopening was associated with significant benefits of the SAQ domains of angina with stressful activity [3.7 ± 0.9 vs. 3.1 ± 0.5, p = 0.004, use of nitrates (4.7 ± 0.5 vs. 3.0 ± 1.0) p = 0.005] and satisfaction from angina relief (4.4 ± 1.1 vs. 3.6 ± 1.4 p < 0.001).
Conclusion: While there was no significant difference in mortality, successful CTO-PCI was associated with greater reduction in angina and the use of nitrates compared to unsuccessful CTO-PCI.
Introduction
Coronary angiography and percutaneous coronary intervention (PCI) are important cornerstones for the diagnosis and treatment of coronary artery disease (CAD) (1–3). The prevalence of coronary chronic total occlusions (CTO) in patients suffering from chronic coronary syndrome (CCS) is reported with 15%–25% (4, 5). Nevertheless, treatment of coronary CTOs remains uncommon in daily clinical practice (6, 7). Many cardiologists may hesitate to refer patients for revascularization due to the limited high-level evidence on its prognostic impact. Additionally, some operators may feel reluctant to attempt revascularization of CTO lesions because of procedural complexity, lack of specific skills, increased risks of complications, and the necessity for special equipment. However, recent strategical and technological advancements, improvement of equipment, better education, and training, as well as growing awareness of the high technical success (5, 8, 9) and low complication rates (8, 10) of CTO-PCI in the medical community, have lately resulted in the adoption of this treatment option in a larger population of patients (5, 11).
Despite the extensive research in the field of CTO-PCI over the last decades, whether chronic occlusions should be treated with optimal medical therapy (OMT) or PCI remains controversial. It is widely agreed that CTO-PCI has the potential to improve exercise limiting ischemic symptoms of angina or angina equivalents such as dyspnea in carefully selected patients (12–14). However, randomized controlled trials (RCT) have failed to demonstrate an effect of CTO-PCI on hard endpoints such as mortality (15). Widely, a major problem in this field of research is that CTO lesions are not well represented in large PCI trials. Generally, CTOs are excluded from RCTs. Specific RCTs on CTO-PCI often included less symptomatic patients since physicians may feel reluctant to randomize highly symptomatic patients to a conservative treatment arm. It is obvious that a difference in hard clinical endpoints in less symptomatic population at lower risk is difficult to demonstrate. Popular examples are the ORBITA (16) and ISCHEMIA (17) trials who failed to show a benefit for PCI over OMT in stable CAD. However, a recent, big observational study led by Park et al. (18) including 1,547 patients was able to show a long-term survival benefit of CTO-PCI. The primary endpoint, cardiac death at 10 years, was significantly reduced in the CTO-PCI cohort compared with the conservative treatment group. The findings of this study are consistent with previously reported observational data experiencing similar outcomes (19–21). However, to this day, no single large RCT on CTO-PCI vs. OMT has demonstrated a survival benefit of CTO recanalization.
The current state of CTO-PCI practice in Austria is largely unexplored. The aim of this project was to investigate if successful CTO-PCI leads to improvement in patient symptoms and prognosis in daily clinical practice.
Methods
We retrospectively evaluated all consecutive CTO-PCI cases performed at a single tertiary care academic medical center in Austria from January 2016 until December 2021 that had a complete clinical follow-up. We compared the clinical, technical, and procedural characteristics, as well as patient-reported change in Canadian Cardiovascular Society (CCS) angina grade and Seattle Angina Questionnaire (SAQ). Furthermore, we examined all-cause mortality and major adverse cardiovascular events (MACE) between patients with technically successful vs. failed CTO-PCI procedures.
Coronary CTOs were defined as coronary lesions with Thrombolysis in Myocardial Infarction (TIMI) grade 0 flow of at least 3-month duration. Estimation of the duration of occlusion was clinical, based on the first onset of angina, prior history of MI in the target vessel territory, or comparison with a prior angiogram. Antegrade wire escalation (AWE) was defined as antegrade PCI during which the guidewire crossed the lesion from “true to true” lumen. A procedure was defined as retrograde if an attempt was made to cross the lesion through a collateral vessel or bypass graft supplying the target vessel distal to the lesion. Antegrade dissection/re-entry (ADR) was defined as antegrade PCI during which a guidewire was intentionally introduced into the subintimal space proximal to the lesion, or re-entry into the distal true lumen was attempted following intentional or inadvertent subintimal guidewire or device crossing.
Success was defined as successful CTO revascularization with achievement of <30% residual diameter stenosis within the treated segment and restoration of TIMI grade 3 antegrade flow. In-hospital MACE included any of the following adverse events prior to hospital discharge: all-cause mortality, recurrent symptoms requiring urgent repeat target vessel revascularization (TVR) with PCI or emergent coronary artery bypass graft (CABG) surgery and stroke.
Follow-up started from the date of the CTO procedure and ended on the first occurrence of either of: date of death, emigration, or end of the study (December 31, 2021). All patients who underwent CTO-PCI in the observation period were contacted and interviewed via phone to examine symptom improvement, if written informed consent was obtained.
Systolic function was evaluated by left ventricular ejection fraction (LVEF,%) measured by the Simpson’s 2D biplane method from transthoracic echocardiography.
Statistical analysis
Statistical analysis was performed on Stata 13.0 (College Station, USA). Categorical variables were expressed as percentages and were compared using Pearson's Chi-square test or 2-tailed Fisher's exact test. Continuous variables were presented as mean ± standard deviation or median with interquartile range (IQR) and were compared using the t-test or Wilcoxon rank-sum test, as appropriate. For the comparison of the CCS class at baseline to follow up for successful and failed CTO-PCI, we used a paired t-test and changes in CCS class were presented in a figure. A Kaplan-Meier survival curve was constructed which was stratified by successful or failed CTO-PCI. Stepwise logistic regression with a p-value cutoff of 0.1 was used to identify factors associated with death and successful CTO-PCI. A two-sided P-value of 0.05 was considered statistically significant.
The Institutional Ethics Review Board of Karl Landsteiner University approved this study (Ethics Committee approval EK Nr: 1017/2021. The reporting of this study is in accordance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) recommendations (22). All procedures were undertaken in accord with Helsinki Declaration and postulates of good clinical practice.
Results
In this retrospective analysis, we examined the clinical characteristics and procedural outcomes of 300 patients who underwent coronary angiography and percutaneous coronary intervention (PCI) of chronic coronary syndrome. We report that 48 CTO-PCI procedures were not successful (16%) while CTO-PCI was successful in 252 of patients (84%) according to predefined procedural success criteria.
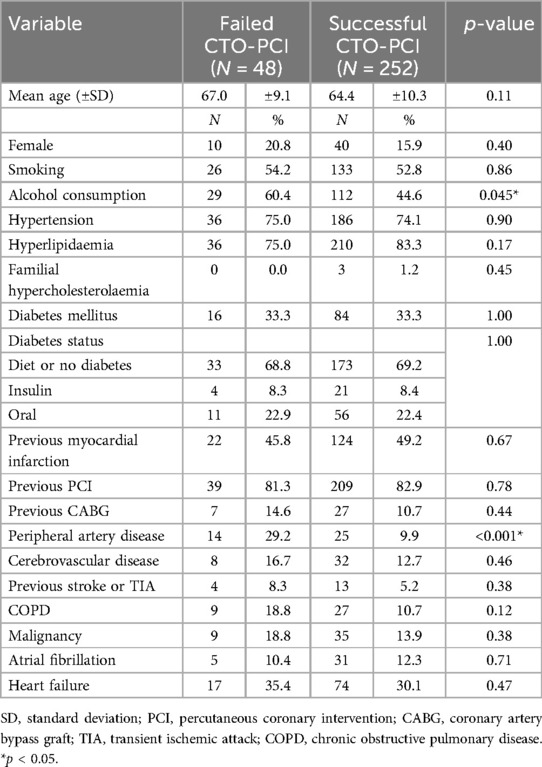
The demographics and comorbidities of patients with CTO-PCI are shown in Table 1. The mean age of the entire cohort was 66.2 ± 9.9 years, with no statistically significant difference regarding age observed between the failed and successful CTO-PCI (67.0 ± 9.1 vs. 64.4 ± 10.3 years respectively, p = 0.11). Gender distribution showed a slight predominance of males. In terms of comorbidities, there were significantly fewer patients with regular alcohol consumption (44.6% vs. 60.4%, p = 0.045) and peripheral artery disease (9.9% vs. 29.2%, p < 0.001) in the group with successful CTO-PCI.
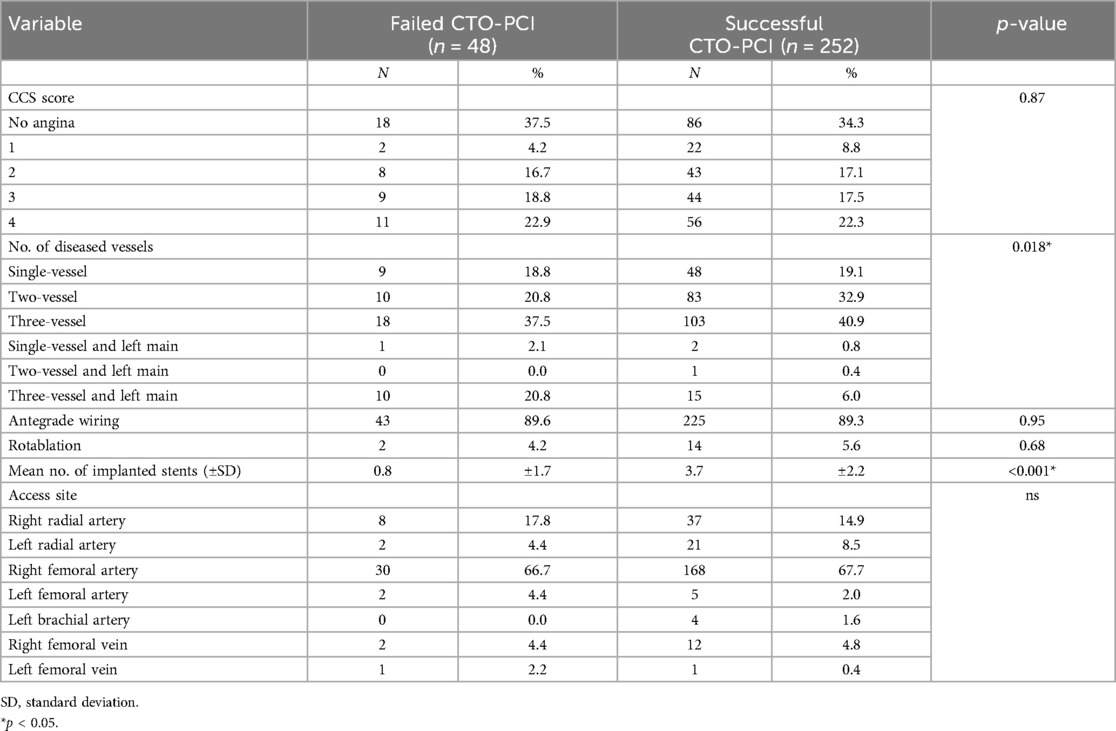
The angina class and coronary lesion characteristics are shown in Table 2. There was no significant difference in CCS score for patients with successful and failed CTO-PCI. The proportion of patients with three-vessel coronary disease was significantly higher in patients with failed CTO-PCI compared to those with successful CTO-PCI (58.3% vs. 46.8%, p = 0.018, respectively). Among patients with successful CTO-PCI the mean number of stents was significantly greater (3.7 vs. 0.8, p < 0.001). No statistically significant differences were observed between two groups concerning in the utilization of rotablation.
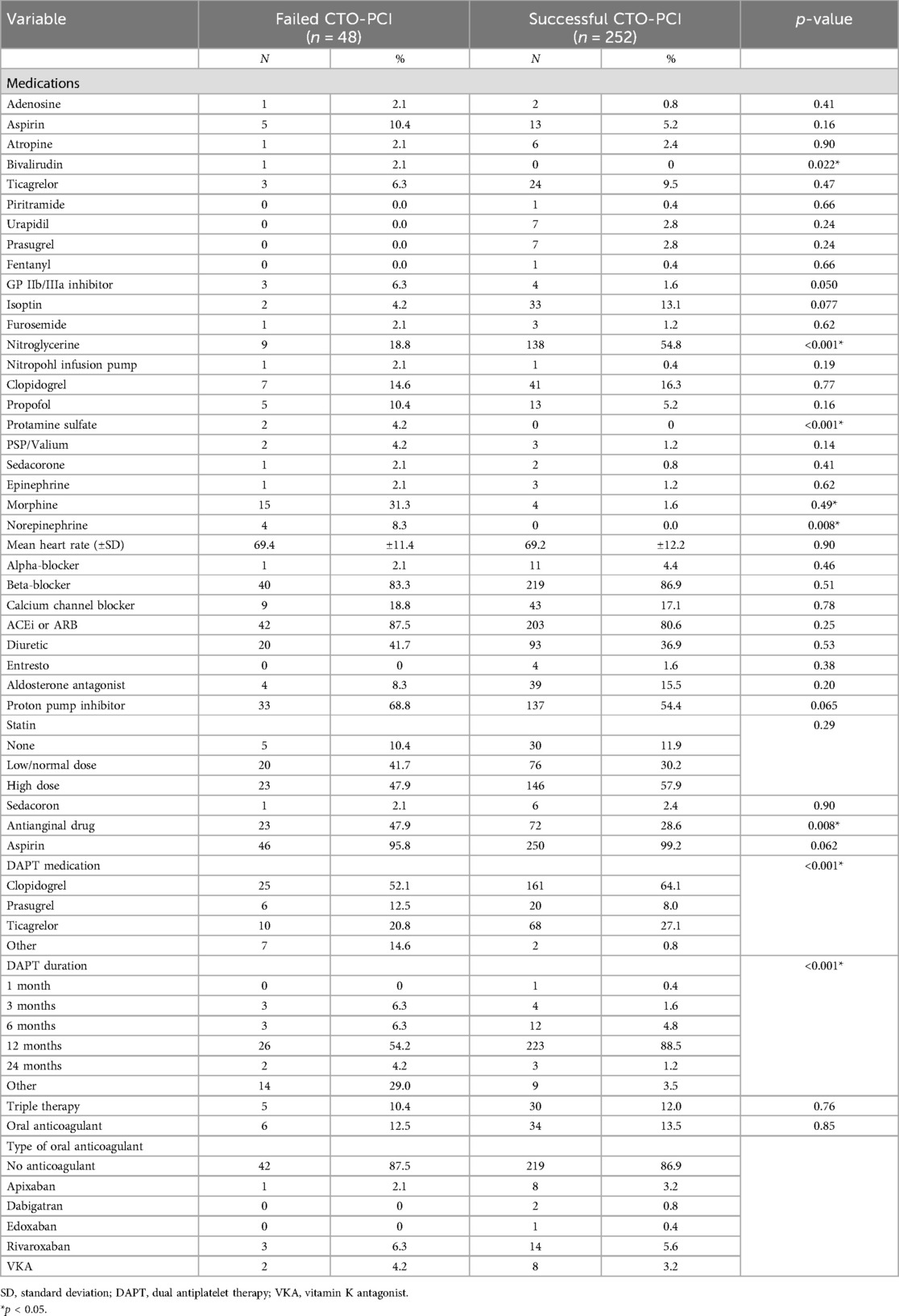
The medications that were administered during the procedure are reported in Table 3. Periprocedural administration of most medications did not significantly differ between the two groups. There were modest differences in use of bivalirudin (p = 0.022), heparin (p = 0.001), nitroglycerine (p < 0.001), protamine sulfate (p < 0.001) and norepinephrine (p = 0.008). We observed significant differences in the use of dual antiplatelet therapy (DAPT) and duration of DAPT comparing the groups with successful and failed CTO-PCI.
Echocardiographic and biochemical/laboratory characteristics of enrolled patients are shown in Supplementary Tables 1, 2, respectively. There were no significant differences in echocardiographic parameters between patients with successful and failed CTO-PCI. There were no differences in blood parameters for patients with successful vs. failed CTO-PCI aside from significantly greater pre-procedural hemoglobin count (13.9 vs. 13.3 g/dl, p = 0.031) and LDL cholesterol levels (93 vs. 80 mg/dl, p = 0.040).
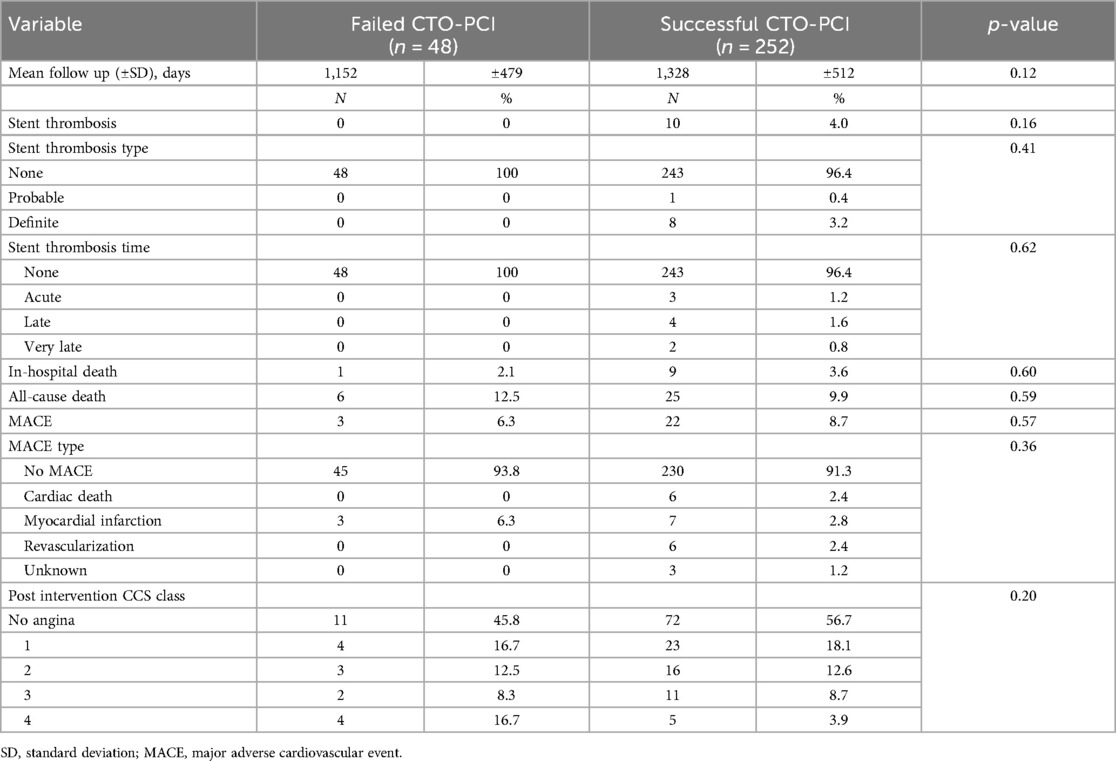
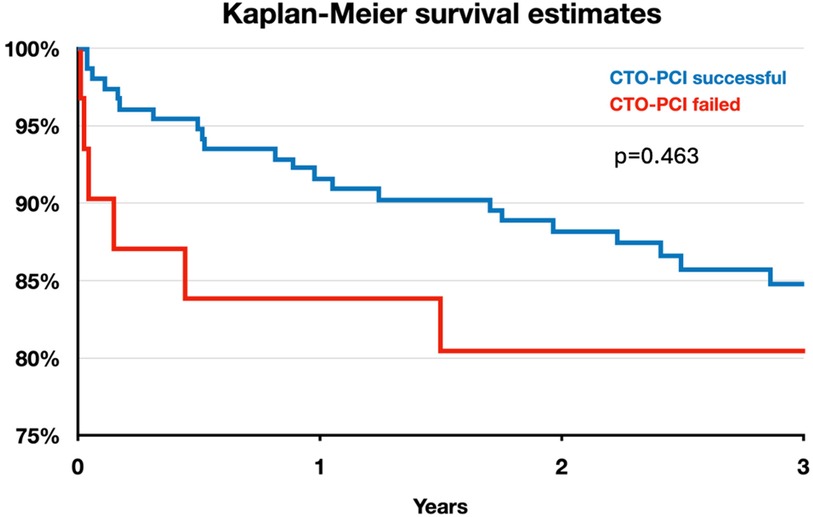
Clinical outcomes of interest are shown in Table 4. Figure 1 shows the Kaplan-Meier survival curve for patients with successful and failed CTO-PCI for the first 3-years of follow-up. Overall mortality was 9.9% and 12.5%, respectively (p = 0.59)). There were no significant differences observed with respect to in-hospital death, or MACE between the two groups (3.6% vs. 2.1%, p = 0.60, and 8.7% vs. 6.3%, p = 0.57, respectively). Additionally, the incidence of in-stent thrombosis, its subtypes, and associated complications did not differ significantly between the two groups.
Post-procedural assessments included a comprehensive evaluation of patient-reported outcomes and QoL measures (Table 5). A total of 177 patients provided informed consent and completed the questionnaire via telephone interview. The successful CTO-PCI group exhibited favorable outcomes in terms of angina relief and overall satisfaction. More specifically, CTO-PCI was associated with a significant improvement in several SAQ domains, as follows: angina with stressful activity (p = 0.005), angina frequency in the last 4 weeks (p = 0.004), use of nitrates (p = 0.008) and satisfaction of no anginal relief (p = 0.002).
The change in CCS class for patients with successful CTO-PCI and failed CTO-PCI is shown in Figure 2. Patients with successful CTO-PCI exhibited a significant improvement in follow-up vs. baseline self-reported CCS angina grade (p < 0.001). On the other hand, there was no statistical difference in CCS angina grade at follow-up vs. baseline in the group of patients that had failed CTO-PCI (p = 0.23).
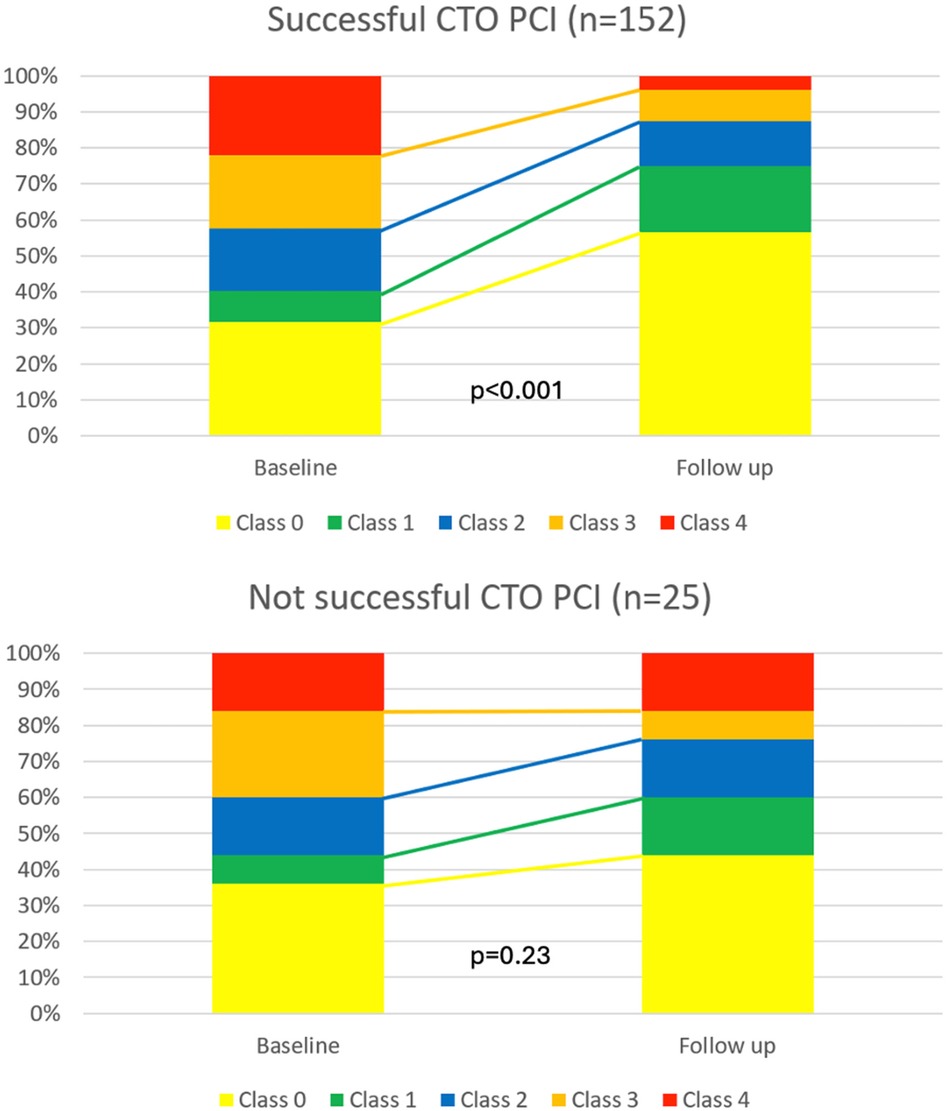
Figure 2. Change in CCS angina grade before and after follow-up for patients with chronic coronary syndrome and with successful vs. failed CTO-PCI.
Femoral access (OR 6.67, 95%-CI 1.69–26.38, p = 0.007), peripheral artery disease (PAD) (OR 2.95, 95%-CI 1.14–7.59, p = 0.025), heart failure (OR 2.37, 95%CI 1.06–5.31, p = 0.036), number of diseased vessels (OR 2.20, 95%-CI 1.15–4.21, p = 0.017) and heart rate (OR 1.05, 95%-CI 1.02–1.09, p = 0.002) were independently associated with higher mortality. The presence of PAD was the only independent predictor for failing CTO-PCI (OR 0.30, 95%-CI 0.14–0.66, p = 0.003). However, unsuccessful CTO-PCI was not independently associated with higher mortality.
Discussion
The key finding of our study was that successful CTO-PCI significantly improved symptoms and quality of life during follow-up, among patients with chronic coronary syndrome.
Notably, one third of the investigated collective did not experience typical angina prior to undergoing PCI. Consequently, these patients were scheduled for revascularization to reduce ischemia in CCS (3) or to facilitate complete revascularization after ACS (23–25). This observed proportion is similar to previously published data from the OPEN-CTO by Sapontis and coworkers (26), where 72% underwent PCI with the primary indication to relief ischemic symptoms. Nevertheless, the proportion of patients with no angina at baseline was below 10% (26). However, some CTO patients may underestimate their daily symptom burden and perceive a significant improvement in quality of life after successful PCI that they may not have anticipated previously.
Widely, observational research and RCTs have provided substantial evidence indicating successful CTO-PCI is associated with improved angina relief and quality of life (27–29). Likewise, our study could demonstrate significantly improved angina measured by CCS following successful PCI in contradiction to failed PCI. Moreover, all but one parameter of SAQ were numerically better in patients with successful PCI compared to those with failed PCI in our study, In case of four variables (angina with stressful activity (p = 0.005), angina frequency in the last 4 weeks (p = 0.004), use of nitrates (p = 0.008) and satisfaction of no anginal relief (p = 0.002)) this difference reached statistical significance.
To this date, two randomized trials were published which compared the benefit of CTO-PCI vs. OMT in patients with stable angina (15, 28). The DECISION-CTO trial (15) enrolled 834 patients with stable angina including a CTO as one of their lesions. The results showed no difference between PCI vs. OMT regarding the primary endpoint of death, MI, stroke, or re-revascularization. Moreover, this trial also failed to show a significant difference for the secondary endpoint of symptomatic improvement measured by changes in the Seattle Angina Questionnaire (SAQ). However, several limitations need to be considered when interpreting the results of this trial. Most importantly, the study design predetermined that non-CTO lesions were treated after the baseline assessment in both groups. Given the fact that 77% of patients in DECISION-CTO have multi-vessel disease, around 70% of patients in the conservative arm received PCI, which could possibly explain the improvement in SAQ in the conservative arm. Furthermore, there was a high cross-over rate of about 20% from the conservative arm to the CTO-PCI group. Hence, when the results were reported in as per-treated analyses (rather than as per-protocol), there was a significant decrease in MACE and spontaneous MI (both p = 0.01), a strong trend to reduced all-cause mortality (p = 0.06) and cardiac mortality (p = 0.08) in the CTO-PCI treated group when compared to conservative treatment. In the EURO-CTO trial (28) with 448 randomized patients, CTO-PCI outperformed OMT in terms of symptomatic benefit. Contrary to DECISION-CTO, all patients in this study were randomized after treatment of concomitant relevant non-CTO lesions. Consistently, confounding effects of non-CTO-PCI were negligible in this study since a rather low cross-over rate of 7% from OMT in the intention-to-treat analysis was reported. The results showed significant differences in improvement in angina frequency and quality of life in SAQ subscales favoring CTO-PCI. Regarding major adverse cardiovascular and cerebrovascular events, no significant differences between the two groups were observed. Although the results of both studies may seem contradictory at first glance, DECISION-CTO does not disprove the results of the EURO-CTO study, because of the major differences in study design and the given limitations. Whereas DECISION-CTO failed to proof the benefit of CTO-PCI on top of Non-CTO-PCI, EURO-CTO identified the isolated benefit of CTO-PCI vs. OMT. Recently, at 3 years follow-up of the EURO-CTO trial observed no difference in the incidence of cardiovascular mortality or myocardial infarction between PCI or OMT. However, a higher MACE rate was observed in the OMT group, which was primarily due to ischemia driven revascularization (9).
Several research on the population of patients with CAD have emphasized the significant influence of CTOs on outcomes (18, 21, 30–33). Several underlying mechanisms have been reported for this phenomenon. Patients with CTOs may be prone to experience fatal cardiac events in the future. Notably, patients with STEMI who receive primary PCI experience a threefold rise in 30-day mortality if there is a bystander CTO in a non-culprit artery (21).
Occasionally, the culprit vessel in acute STEMI serves as donor artery for a myocardial CTO territory by providing collateral supply. In these cases of “double jeopardy” mortality increases up to 52%, because of a large area of threatened myocardium and a higher risk for cardiogenic shock (21).
This is attributed to the “double jeopardy” phenomenon, which occurs when the sudden blockage of a donor vessel supplying collateral blood flow beyond the CTO poses a threat to a larger myocardial area (21, 34). Additionally, non-revascularized CTOs may be associated with impaired left ventricular ejection fraction (LVEF), a well-established prognostic marker for MACE (4). Malignant arrhythmias also appear to play a significant role in causing cardiovascular fatalities among patients with CTOs (30, 35, 36).
Despite all these pathophysiological considerations, our study did not find a benefit of successful CTO-PCI on hard clinical endpoints. There was an absolute decrease in mortality by 2.6%, favoring successful CTO-PCI (9.9% vs. 12.5%), but likely due to small sample size this numerical difference did not reach statistical significance. In the event of a subtle effect size a rather small study like ours might fail to discern it. Moreover, the mean follow-up time of 3.4 years of our study may be too short to detect a significant difference in all-cause death since data by Park et al. (18) suggest later benefits of CTO revascularization.
Comparable to our study, Lee et al. (37) and Yamamoto et al. (38) conducted studies that found no significant difference in the survival rates of patients who underwent successful CTO-PCI at 3-year and 4.6-year follow-ups, respectively. However, both trials reported a TVR rate of 20% after failed CTO-PCI, suggesting that a considerable number of patients initially labeled as having an “unsuccessful” procedure were successfully revascularized later. However, meta-analyses have shown that successful CTO-PCI was associated with a lower risk of MACE and death compared with failed procedures (39, 40). Christakopoulos et al. (40) analyzed 25 observational studies and found successful CTO-PCI was associated with lower risk of death [odds ratio (OR), 0.52; 95% CI, 0.43–0.63] and MACE (OR 0.59; 95% CI, 0.44–0.79), but not TVR (OR, 0.66; 95% CI, 0.36–1.23) or MI (OR, 0.73; 95% CI, 0.52–1.03) compared with failed CTO-PCI, during a median follow-up of 3 years. In the report by Megaly et al. (39), which represents a contemporary CTO-PCI population since 2010, the risk of MACE and death was significantly lower at 12 months in patients who had successful CTO-PCI.
In conclusion, our analysis demonstrated a symptomatic benefit of successful CTO-PCI among patients with chronic coronary syndrome in a nationally representative tertiary medical center in Austria. Further research, including larger observational studies and randomized controlled trials, are necessary to investigate the effects of CTO-PCI on symptom relief and mortality rates.
Limitations
Our study has some limitations worth discussing. First, this is a retrospective single center experience.
Second, this study represents information obtained from PCIs carried out from 2016 to 2021. However, this data may be considered outdated as it does not reflect the current state of the art in CTO-PCI due to technological advancements and improvements that occurred in this field over the last several years. Consequently, any impact on mortality and symptom improvements may have been underestimated, however, this notion should be investigated in future studies.
Another limitation is the extremely limited sample size of the unsuccessful group of only 25 probands, which is inadequate for making definitive conclusions. Notably, the proportion of patients with three-vessel coronary disease was significantly higher in patients with failed CTO-PCI compared to those with successful CTO-PCI which might be a major confounder in this setting. Furthermore, it cannot be excluded that the improvement of symptoms may have been attributable to the revascularization of non-chronically occluded coronary arteries rather than the chronic total occlusion lesion itself in these patients.
Moreover, one-third of the patients did not report typical angina, which was the primary endpoint of the study, prior to receiving PCI. However, this observation becomes even more intriguing considering that a significant difference was observed for this outcome. Regrettably, baseline values for the SAQ - scores pre-PCI were not available, limiting our ability to compare post PCI values and thus reducing the significance of this analyzed parameter.
One further constraint of our study is the absence of detailed information about ischemia testing for all patients, restricting a thorough analysis concerning this important aspect.
This analysis was limited to patients who underwent percutaneous coronary intervention, excluding those initially managed with optimal medical therapy alone. Consequently, the ability to compare outcomes between optimal medical management and successful PCI was constrained, potentially introducing selection bias and neglecting the placebo effect.
Conclusions
Successful CTO-PCI significantly improved the symptoms of angina and quality of life during follow-up, among patients with chronic coronary syndrome. CTO-PCI should be considered in symptomatic patients alongside OMT. Prospective studies with larger sample size become are warranted to further address this important research question.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Commission on Ethics and Scientific Integrity - Karl Landsteiner University Krems. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MW: Conceptualization, Funding acquisition, Investigation, Project administration, Writing – original draft, Writing – review & editing. KS: Supervision, Writing – review & editing. SA: Data curation, Investigation, Writing – review & editing. GLe: Conceptualization, Supervision, Writing – review & editing. ES: Data curation, Investigation, Writing – review & editing. DM: Data curation, Investigation, Writing – review & editing. PV: Writing – review & editing. JB: Writing – review & editing. CK: Data curation, Formal Analysis, Methodology, Writing – review & editing. GLa: Writing – review & editing. JM: Writing – review & editing. TW: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The authors want to appreciate the contribution of NÖ Landesgesundheitsagentur, legal entity of University Hospitals in Lower Austria, for providing the organizational framework to conduct this research. The authors also would like to acknowledge support by Open Access Publishing Fund of Karl Landsteiner University of Health Sciences, Krems, Austria
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1447829/full#supplementary-material
References
1. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. (2018) 39:119–77. doi: 10.1093/eurheartj/ehx393
2. Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. (2021) 42(14):1289–367. doi: 10.1093/eurheartj/ehaa575
3. Neumann FJ, Sechtem U, Banning AP, Bonaros N, Bueno H, Bugiardini R, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. (2020) 41:407–77. doi: 10.1093/eurheartj/ehz425
4. Fefer P, Knudtson ML, Cheema AN, Galbraith PD, Osherov AB, Yalonetsky S, et al. Current perspectives on coronary chronic total occlusions: the Canadian multicenter chronic total occlusions registry. J Am Coll Cardiol. (2012) 59(11):991–7. doi: 10.1016/j.jacc.2011.12.007
5. Konstantinidis NV, Werner GS, Deftereos S, Di Mario C, Galassi AR, Buettner JH, et al. Temporal trends in chronic total occlusion interventions in Europe. Circ Cardiovasc Interv. (2018) 11(10):e006229. doi: 10.1161/CIRCINTERVENTIONS.117.006229
6. Råmunddal T, Hoebers LP, Henriques JP, Dworeck C, Angerås O, Odenstedt J, et al. Chronic total occlusions in Sweden–a report from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). PLoS One. (2014) 9(8):e103850. doi: 10.1371/journal.pone.0103850. Erratum in: PLoS One. (2014) 9(10):e112370.
7. Grantham JA, Marso SP, Spertus J, House J, Holmes DR, Rutherford BD. Chronic total occlusion angioplasty in the United States. JACC Cardiovasc Interv. (2009) 2(6):479–86. doi: 10.1016/j.jcin.2009.02.008
8. Habara M, Tsuchikane E, Muramatsu T, Kashima Y, Okamura A, Mutoh M, et al. Comparison of percutaneous coronary intervention for chronic total occlusion outcome according to operator experience from the Japanese retrograde summit registry. Catheter Cardiovasc Interv. (2016) 87(6):1027–35. doi: 10.1002/ccd.26354
9. Werner GS, Hildick-Smith D, Yuste VM, Boudou N, Sianos G, Gelev V, et al. Three-year outcomes of A randomized multicentre trial comparing revascularization and optimal medical therapy for chronic total coronary occlusions (EuroCTO). EuroIntervention. (2023) 19(7):571–9. doi: 10.4244/EIJ-D-23-00312
10. Werner GS, Martin-Yuste V, Hildick-Smith D, Boudou N, Sianos G, Gelev V, et al. A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions interventional cardiology. Eur Heart J. (2018) 39:2484–93. doi: 10.1093/eurheartj/ehy220
11. Brilakis ES, Mashayekhi K, Tsuchikane E, Abi Rafeh N, Alaswad K, Araya M, et al. Guiding principles for chronic total occlusion percutaneous coronary intervention: a global expert consensus document. Circulation. (2019) 140:420–33. doi: 10.1161/CIRCULATIONAHA.119.039797
12. Joyal D, Afilalo J, Rinfret S. Effectiveness of recanalization of chronic total occlusions: a systematic review and meta-analysis. Am Heart J. (2010) 160(1):179–87. doi: 10.1016/j.ahj.2010.04.015
13. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. EuroIntervention. (2020) 14(14):1435–534. doi: 10.4244/EIJY19M01_01
14. Galassi AR, Vadalà G, Werner GS, Cosyns B, Sianos G, Hill J, et al. Evaluation and management of patients with coronary chronic total occlusions considered for revascularisation. A clinical consensus statement of the European association of percutaneous cardiovascular interventions (EAPCI) of the ESC, the European association of cardiovascular imaging (EACVI) of the ESC, and the ESC working group on cardiovascular surgery. EuroIntervention. (2024) 20(3):e174–84. doi: 10.4244/EIJ-D-23-00749
15. Lee SW, Lee PH, Ahn JM, Park DW, Yun SC, Han S, et al. Randomized trial evaluating percutaneous coronary intervention for the treatment of chronic total occlusion: the DECISION-CTO trial. Circulation. (2019) 139(14):1674–83. doi: 10.1161/CIRCULATIONAHA.118.031313
16. Al-Lamee R, Thompson D, Dehbi HM, Sen S, Tang K, Davies J, et al. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet. (2018) 391(10115):31–40. doi: 10.1016/S0140-6736(17)32714-9
17. Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O’Brien SM, Boden WE, et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. (2020) 382(15):1395–407. doi: 10.1056/NEJMoa1915922
18. Park TK, Lee SH, Choi KH, Lee JM, Yang JH, Bin SY, et al. Late survival benefit of percutaneous coronary intervention compared with medical therapy in patients with coronary chronic total occlusion: a 10-year follow-up study. J Am Heart Assoc. (2021) 10(6):19022. doi: 10.1161/JAHA.120.019022
19. Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. (2009) 360(10):961–72. doi: 10.1056/NEJMoa0804626
20. Farooq V, Serruys PW, Garcia-Garcia HM, Zhang Y, Bourantas C V, Holmes DR, et al. The negative impact of incomplete angiographic revascularization on clinical outcomes and its association with total occlusions: the SYNTAX (synergy between percutaneous coronary intervention with Taxus and cardiac surgery) trial. J Am Coll Cardiol. (2013) 61(3):282–94. doi: 10.1016/j.jacc.2012.10.017
21. van der Schaaf RJ, Vis MM, Sjauw KD, Koch KT, Baan J, Tijssen JGP, et al. Impact of multivessel coronary disease on long-term mortality in patients with ST-elevation myocardial infarction is due to the presence of a chronic total occlusion. Am J Cardiol. (2006) 98(9):1165–9. doi: 10.1016/j.amjcard.2006.06.010
22. Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. (2007) 4(10):1623–7. doi: 10.1371/journal.pmed.0040296
23. Henriques JPS, Hoebers LP, Råmunddal T, Laanmets P, Eriksen E, Bax M, et al. Percutaneous intervention for concurrent chronic total occlusions in patients with STEMI: the EXPLORE trial. J Am Coll Cardiol. (2016) 68(15):1622–32. doi: 10.1016/j.jacc.2016.07.744
24. Villablanca PA, Olmedo W, Weinreich M, Gupta T, Mohananey D, Albuquerque FN, et al. Staged percutaneous intervention for concurrent chronic total occlusions in patients with ST-segment-elevation myocardial infarction: a systematic review and meta-analysis. J Am Heart Assoc. (2018) 7(8):e008415. doi: 10.1161/JAHA.117.008415
25. Mehta SR, Wood DA, Storey RF, Mehran R, Bainey KR, Nguyen H, et al. Complete revascularization with multivessel PCI for myocardial infarction. N Engl J Med. (2019) 381(15):1411–21. doi: 10.1056/NEJMoa1907775
26. Sapontis J, Salisbury AC, Yeh RW, Cohen DJ, Hirai T, Lombardi W, et al. Early procedural and health Status outcomes after chronic total occlusion angioplasty: a report from the OPEN-CTO registry (outcomes, patient health Status, and efficiency in chronic total occlusion hybrid procedures). JACC Cardiovasc Interv. (2017) 10(15):1523–34. doi: 10.1016/j.jcin.2017.05.065
27. Kucukseymen S, Iannaccone M, Grantham JA, Sapontis J, Juricic S, Ciardetti N, et al. Association of successful percutaneous revascularization of chronic total occlusions with quality of life: a systematic review and meta-analysis. JAMA Netw Open. (2023) 6(7):E2324522. doi: 10.1001/jamanetworkopen.2023.24522
28. Werner GS, Martin-Yuste V, Hildick-Smith D, Boudou N, Sianos G, Gelev V, et al. A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur Heart J. (2018) 39(26):2484–93. doi: 10.1093/eurheartj/ehy220
29. Obedinskiy AA, Kretov EI, Boukhris M, Kurbatov VP, Osiev AG, Ibn Elhadj Z, et al. The IMPACTOR-CTO trial. JACC Cardiovasc Interv. (2018) 11(13):1309–11. doi: 10.1016/j.jcin.2018.04.017
30. Godino C, Giannattasio A, Scotti A, Baldetti L, Pivato CA, Munafò A, et al. Risk of cardiac and sudden death with and without revascularisation of a coronary chronic total occlusion. Heart. (2019) 105(14):1096–102. doi: 10.1136/heartjnl-2018-314076
31. Råmunddal T, Hoebers LP, Henriques JPS, Dworeck C, Angerås O, Odenstedt J, et al. Prognostic impact of chronic total occlusions: a report from SCAAR (Swedish coronary angiography and angioplasty registry). JACC Cardiovasc Interv. (2016) 9(15):1535–44. doi: 10.1016/j.jcin.2016.04.031
32. Claessen BE, Dangas GD, Weisz G, Witzenbichler B, Guagliumi G, Möckel M, et al. Prognostic impact of a chronic total occlusion in a non-infarct-related artery in patients with ST-segment elevation myocardial infarction: 3-year results from the HORIZONS-AMI trial. Eur Heart J. (2012) 33(6):768–75. doi: 10.1093/eurheartj/ehr471
33. George S, Cockburn J, Clayton TC, Ludman P, Cotton J, Spratt J, et al. Long-term follow-up of elective chronic total coronary occlusion angioplasty: analysis from the U.K. Central cardiac audit database. J Am Coll Cardiol. (2014) 64(3):235–43. doi: 10.1016/j.jacc.2014.04.040
34. Bataille Y, Déry JP, Larose É, Déry U, Costerousse O, Rodés-Cabau J, et al. Deadly association of cardiogenic shock and chronic total occlusion in acute ST-elevation myocardial infarction. Am Heart J. (2012) 164(4):509–15. doi: 10.1016/j.ahj.2012.07.008
35. Nombela-Franco L, Mitroi CD, Fernández-Lozano I, García-Touchard A, Toquero J, Castro-Urda V, et al. Ventricular arrhythmias among implantable cardioverter-defibrillator recipients for primary prevention: impact of chronic total coronary occlusion (VACTO primary study). Circ Arrhythm Electrophysiol. (2012) 5(1):147–54. doi: 10.1161/CIRCEP.111.968008
36. Nombela-Franco L, Iannaccone M, Anguera I, Amat-Santos IJ, Sanchez-Garcia M, Bautista D, et al. Impact of chronic total coronary occlusion on recurrence of ventricular arrhythmias in ischemic secondary prevention implantable cardioverter-defibrillator recipients (VACTO secondary study): insights from coronary angiogram and electrogram analysis. JACC Cardiovasc Interv. (2017) 10(9):879–88. doi: 10.1016/j.jcin.2017.02.008
37. Lee PH, Lee SW, Park HS, Kang SH, Bae BJ, Chang M, et al. Successful recanalization of native coronary chronic total occlusion is not associated with improved long-term survival. JACC Cardiovasc Interv. (2016) 9(6):530–8. doi: 10.1016/j.jcin.2015.11.016
38. Yamamoto E, Natsuaki M, Morimoto T, Furukawa Y, Nakagawa Y, Ono K, et al. Long-term outcomes after percutaneous coronary intervention for chronic total occlusion (from the CREDO-Kyoto registry cohort-2). Am J Cardiol. (2013) 112(6):767–74. doi: 10.1016/j.amjcard.2013.05.004
39. Megaly M, Khalil M, Basir MB, McEntegart MB, Spratt JC, Yamane M, et al. Outcomes of successful vs. Failed contemporary chronic total occlusion percutaneous coronary intervention. Cardiovasc Interv Ther. (2022) 37(3):483–9. doi: 10.1007/s12928-021-00819-x
Keywords: coronary artery disease (CAD), chronic total occlusion (CTO), percutaneous coronary intervention (PCI), quality of life, symptoms, mortality
Citation: Will M, Schwarz K, Aufhauser S, Leibundgut G, Schmidt E, Mayer D, Vock P, Borovac JA, Kwok CS, Lamm G, Mascherbauer J and Weiss T (2024) The impact of successful chronic total occlusion percutaneous coronary intervention on clinical outcomes: a tertiary single-center analysis. Front. Cardiovasc. Med. 11:1447829. doi: 10.3389/fcvm.2024.1447829
Received: 12 June 2024; Accepted: 10 September 2024;
Published: 27 September 2024.
Edited by:
Yao-Jun Zhang, Xuzhou Medical University, ChinaReviewed by:
Nino Cocco, Campus Bio-Medico University Hospital, ItalyVjekoslav Tomulic, Clinical Hospital Centre Rijeka, Croatia
Copyright: © 2024 Will, Schwarz, Aufhauser, Leibundgut, Schmidt, Mayer, Vock, Borovac, Kwok, Lamm, Mascherbauer and Weiss. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maximilian Will, bWF4aW1pbGlhbi53aWxsQHN0cG9lbHRlbi5sa25vZS5hdA==
†Present Address: Chun Shing Kwok, Department of Cardiology, Leighton Hospital, Mid Cheshire Hospitals NHS Foundation Trust, Crewe, United Kingdom
 Maximilian Will
Maximilian Will Konstantin Schwarz
Konstantin Schwarz Simone Aufhauser1,2,3
Simone Aufhauser1,2,3 Gregor Leibundgut
Gregor Leibundgut David Mayer
David Mayer Josip A. Borovac
Josip A. Borovac Chun Shing Kwok
Chun Shing Kwok Gudrun Lamm
Gudrun Lamm