- 1Department of Transfusion Medicine, Faculty of Medicine, RWTH University Hospital Aachen, RWTH Aachen University, Aachen, Germany
- 2Department of Cardiothoracic Surgery, Heart Centre Trier, Barmherzigen Brüder Hospital, Trier, Germany
- 3Department of Cardiac Surgery, Faculty of Medicine, RWTH University Hospital Aachen, RWTH Aachen University, Aachen, Germany
- 4Dr. Arab Cardiology Clinic, Jeddah, Saudi Arabia
- 5Department of Cardiac Surgery, Klinikum Links der Weser, Bremen, Germany
- 6Department of Thoracic Surgery, Faculty of Medicine, RWTH University Hospital Aachen, RWTH Aachen University, Aachen, Germany
Introduction: The impact of different degrees of hypothermia in patients undergoing type A aortic dissection (TAAD) repair remains controversial. The purpose of this study was to compare the clinical outcomes of patients who received deep hypothermic circulatory arrest (DHCA) (<20°C) and those of patients who received moderate hypothermic circulatory arrest (MHCA) (20–28°C).
Methods: Between January 2011 and December 2020, 143 patients underwent surgical treatment for TAAD with CA and unilateral antegrade selective cerebral perfusion (uSCP). In this retrospective analysis, we evaluated the clinical outcomes of 143 individuals (103 who received DHCA vs. 40 who received MHCA). The primary outcome was the composite of major events (CMEs) including delirium, acute kidney injury (AKI), and in-hospital mortality. The secondary outcomes were overall mortality, bleeding, rethoracotomy, and length of intensive care unit (ICU) stay, among other things.
Results: Compared with the MHCA group, the DHCA group presented a greater incidence of postoperative complications, as follows: AKI (26 (25.2%) vs. 3 (7.5%), p = 0.020), delirium (23 (22.3%) vs. 2 (5%), p = 0.014), re-exploration rate (21 (20.4%) vs. 2 (5.0%), p = 0.024), and prolonged intensive care unit (ICU) stay (7.8 (4.4, 14.1) vs. 5.7 (2.4, 10) days, p = 0.019). The median cardiopulmonary bypass time (255 (210, 280) vs. 210 (190, 251) min, p = 0.010) and median cross-clamp time (140 (110, 180) vs. 125 (100, 160) min, p = 0.023) were significantly longer in the DHCA group. The German Registry for Acute Aortic Dissection Type A (GERAADA) score was significantly higher in the MHCA group (22.7 ± 9.1 vs. 19 ± 7.2, p = 0.012). The adjusted odds ratio for CME in the MHCA group was 0.78 (95% CI: 0.52–1.17, p = 0.001). The use of MHCA demonstrated a protective effect on reducing postoperative delirium (OR: 0.28, 95% CI: 0.14–0.46, p < 0.01) and postoperative AKI (OR: 0.29, 95% CI: 0.14–0.49, p < 0.01). Overall survival after two years did not differ between the two groups (log-rank, p = 0.16).
Conclusion: The principal findings of our study indicate that DHCA elevates the risk of postoperative AKI and delirium. As a result, the duration of hospitalization and intensive care unit stay was markedly extended. Consequently, MHCA should be favored over DHCA when the clinical circumstances permit, since DHCA remains a secure alternative in intricate dissection instances.
Introduction
Aortic dissection is a rare but life-threatening medical condition with a significant mortality rate. Despite early detection, sophisticated surgery, and improved perioperative treatment, the in-hospital death rate remains high among patients suffering from acute type A aortic dissection (ATAAD) (1, 2). Nevertheless, compared with other therapeutic options, surgery results in the highest survival rate after ATAAD. However, compared with other cardiovascular procedures, treatment of ATAAD with surgery has the highest mortality rate. The primary causes of mortality are predominantly neurological, cardiac, ischemic, and hemorrhagic (3–5). The choice of appropriate surgical strategy has remained challenging in recent decades because of the substantial susceptibility of brain tissue to ischemia and the high risk of embolization during aortic repair. Hypothermia can sufficiently reduce cerebral metabolic demand to allow reasonable periods of circulatory arrest (5, 6). A lower internal temperature ensures reduced metabolic activity (7, 8). However, lower temperatures during hypothermic circulatory arrest (HCA) require longer rewarming times, require longer cardiopulmonary bypass (CPB) times, and are associated with increased risks of CPB-related complications (9–11). Our study aimed to investigate the perioperative morbidity and mortality rates associated with implementing deep hypothermic circulatory arrest (DHCA) vs. moderate hypothermic circulatory arrest (MHCA).
Materials and methods
Patient collection and study protocol
The study was conducted at the cardiothoracic surgery department of the University Hospital of RWTH Aachen in Germany. A review of our clinical electronic database from January 2011 to January 2020 revealed that 166 consecutive patients underwent surgical treatment for ATAAD. Twenty-three patients were excluded according to the following exclusion criteria: mild hypothermic circulatory arrest (n = 7), profound hypothermic circulatory arrest (n = 5), and death before establishing CPB due to major bleeding (n = 11). The patients were divided into two groups and received DHCA (n = 103) between 14.1–20°C or MHCA (n = 40) between 20.1–28°C (6). We conducted our study with retrospective patient data collected from our institutional database. In accordance with the STROBE criteria and the Declaration of Helsinki, approval was authorized by the Research Ethics Committee at RWTH University Aachen, Germany (EK 20-003). The ethics board waived the requirement for informed consent, considering the retrospective nature of the study.
Definitions of outcomes
The primary endpoint of our study was a composite of major events (CME), which included in-hospital mortality, postoperative delirium, and acute kidney injury. In-hospital mortality was defined as death that occurred during the same hospital stay when the operation was performed. Acute kidney injury (AKI) was defined by the Kidney Disease Improving Global Outcomes criteria (9). the secondary endpoints included the following: the incidence of newly developed permanent neurological deficit (PND), which was confirmed by a cranial CT scan; temporary neurological deficit (TND), which was defined as the occurrence of reversible delirium, agitation, disorientation, or motoric deficit that was resolved prior to discharge (as evidenced by a normal CT scan); postoperative sepsis, pneumonia, bleeding, rethoracotomy, length of ICU-stay and total length of hospital stay.
Surgical technique and perioperative standard
The ATAAD diagnosis was confirmed by contrast-enhanced CT. In accordance with our hospital standards, all procedures were performed emergently by an experienced surgical team. Our monitoring included a five-lead ECG, pulse oximetry, near-infrared spectroscopy (NIRS), transesophageal echocardiography (TEE), and urinary bladder and nasopharyngeal temperature monitoring. The patients were divided into the DHCA and MHCA groups according to nasopharyngeal temperature. Arterial lines were placed unilaterally in the femoral artery and in both radial arteries. General anesthesia was induced with a combination of propofol, sufentanil, and rocuronium. A midline sternotomy was performed immediately. Prior to the initiation of CPB, intravenous (i.v.) heparin was administered in a weight-adjusted dose until the activated coagulation time (ACT) reached at least 450 s Subsequently, cannulation was performed. The variations in arterial cannulation sites were arranged as follows: the right subclavian artery with an artificial vessel graft via an end-to-side anastomosis, the femoral artery, the left ventricle (LV), or the ascending aorta. Venous cannulation was performed in the femoral vein or the right atrium. After the initiation of CPB, the patients were cooled to establish targeted hypothermic circulatory arrest (HCA). After cross-clamping of the aorta, the heart was arrested with 4–6°C Bretschneider solution (CUSTODIOL®) following our clinical protocol. Moreover, 1,000 mg of methylprednisolone was intravenously administered during the anesthesia induction process, followed by 500 mg of sodium thiopental after the initiation of CPB for neuroprotection. Furthermore, ice packs were used to cool the head. Unilateral antegrade selective cerebral perfusion (uSCP) was established by directly cannulating the innominate artery with a 9F catheter. The cannula was used to initiate uSCP at a rate of 10–12 ml/kg/min. The target pressure ranged from 50 to 60 mmHg, and the perfusate temperature ranged from 20 to 28°C.
In the past ten years, there has been a shift from using DHCA to using MHCA during ATAAD repair at our institution. The choice between DHCA and MHCA, and the use of uSCP, was based on the surgeon's best performance and expertise and was influenced by various criteria such as age, preoperative renal function, aortic disease, and the level of complexity involved in the intended arch reconstruction. This is in accordance with the EACTS/STS guidelines (12), as there is a class IIa recommendation with the following statement: A target hypothermic circulatory arrest temperature should be determined based on the anticipated extent of repair, expected duration of lower body HCA and presence of preoperative malperfusion.
Statistical analysis
Means and standard deviations are used to present continuous variables, while percentages and absolute numbers are used to present categorical variables. The R Studio interface version 4.0.3 (RStudio Team, Boston, MA) was used for all analyses, alongside with the jamovi project (2020) (jamovi computer software, version 2.3.8; JAMovi.org), which is available at https://www.jamovi.org/. The Kolmogorov‒Smirnov test was used to assess the distribution of continuous data. The data that followed a normal distribution are presented as mean ± standard deviation (SD) and were analyzed using the Student's t-test. The median and interquartile range were utilized to characterize data that did not follow a normal distribution, while the Mann‒Whitney U-test was used for comparison. The categorical variables were analyzed using either the chi-square test or Fisher's exact test. Additionally, to calculate the adjusted odds ratio (OR) of postoperative complications after MHCA compared to those after DHCA, variables with a p < 0.05 in the univariable analysis were entered into a multivariable logistic regression model (backward stepwise logistic regression) with adjustments for confounders, including preoperative malpersion and the German Registry for Acute Aortic Dissection Type A (GERAADA) score (13). Given the limited sample size of the MHCA group and to mitigate potential confounding variables, we employed the propensity scores (PS) methodology. Logistic regression analysis was employed to determine the propensity score, and a closest neighbor matching approach was utilized at a 1:1 ratio. The matching caliber was established at 0.2 times the standard deviation of the logit score. Covariates comprised age, gender, GERAADA score, and malperfusion status. Given the limited sample size of the MHCA group and to mitigate potential confounding variables, we employed the propensity scores (PS) methodology. Logistic regression analysis was employed to determine the propensity score, and a closest neighbor matching approach was utilized at a 1:1 ratio. The matching caliber was established at 0.2 times the standard deviation of the logit score. Covariates comprised age, gender, GERAADA score, and malperfusion status. After matching we used the treatment effect analysis from STATA. eteffects estimates the average treatment effect (ATE), the average treatment effect on the treated (ATET), and the potential-outcome means (POMs) from observational data when treatment assignment is correlated with the potential outcomes. It allows for continuous, binary, count, fractional, and nonnegative outcomes and requires a binary treatment (14). Kaplan–Meier survival curves were generated, and the log-rank test was used to assess linear trends. All p values were rounded to three decimal places or are presented as a number including at least one nonzero digit. A p-value of 0.05 or lower indicates statistical significance.
Results
Pre- and perioperative comparisons
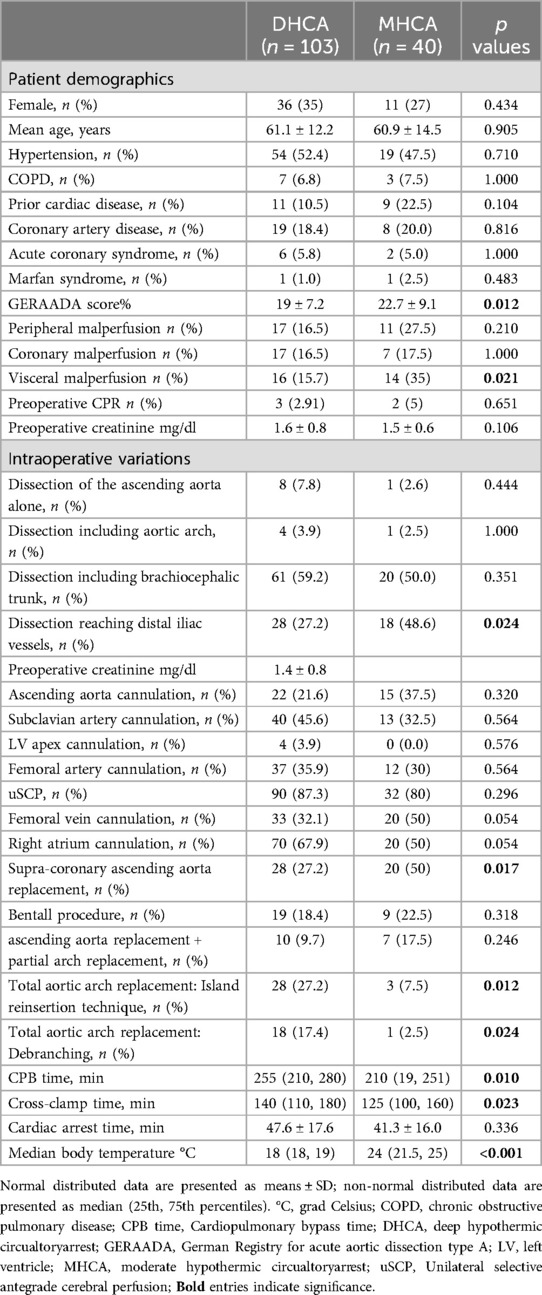
After screening a total of 166 patients, 23 were excluded. The final group consisted of 143 patients, including 103 (72%) who underwent aortic surgery with DHCA and 40 who underwent aortic surgery with MHCA (28%) (Table 1). The DHCA and MHCA groups had mean ages of 61.1 ± 12.2 and 60.9 ± 14.5 years, respectively, and included 36 (35%) and 11 (27%) females, respectively. The GERAADA score was significantly higher in the MHCA group than in the DHCA group (22.7 ± 9.1% vs. 19 ± 7.2%, p = 0.012). The preoperative occurrence of peripheral and coronary malperfusion did not differ between the groups, whereas the incidence of preoperative visceral malperfusion was significantly higher in the MHCA group than in the DHCA group (14 (35%) vs. 16 (15.7%), p = 0.021, respectively) (Table 1). The distribution of cannulation sites did not differ between the two groups (Table 1). The median body temperature was 18°C (18, 19) in the DHCA group and 24°C (21.5, 25) in the MHCA group (p < 0.001).
Patients in the MHCA group had significantly more isolated supracoronary ascending aorta replacements (20 (50%) vs. 28 (27.9%); p = 0.024). The number of patients who underwent Bentall procedures and ascending aorta replacement + partial aortic arch replacement was similar between the two groups (Table 1). Total arch replacement, either via reimplantation of an island of the aortic wall containing the ostia of the innominate artery (IA), left carotid artery (LCA), or left subclavian artery (LSA), was performed significantly more often under DHCA conditions (28 (27.2%) vs. 3 (7.5%); p = 0.012) or using the anatomic branch technique (18 (17.4%) vs. 1 (2.5%); p = 0.024) (Table 1).
Postoperative complications
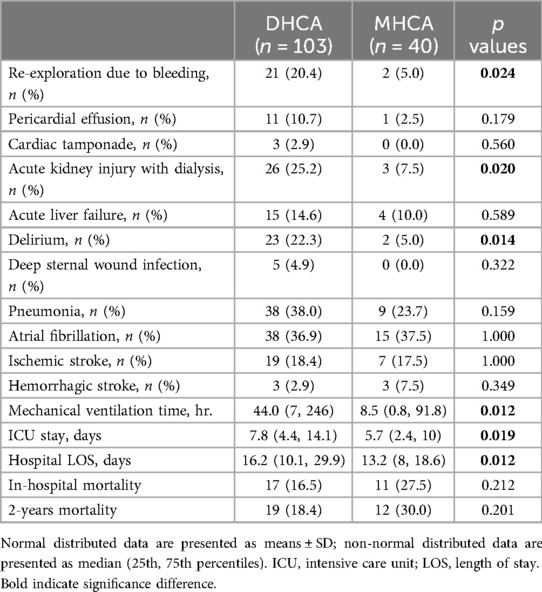
The CPB duration and cross-clamp time were significantly prolonged in the DHCA group compared to the MHCA group: CPB time (255 (210, 280) vs. 210 (190, 251) min, p = 0.010) and cross-clamp time (140 (110, 180) vs. 125 (100, 160) min, p = 0.023) (Table 2). The incidence of bleeding requiring rethoracotomy was significantly greater in the DHCA group than in the MHCA group (21 (20.4%) vs. 2 (5.0%); p = 0.024). The frequency of acute kidney injury (AKI) with the need for dialysis was also significantly higher in the DHCA group than in the MHCA group (26 (25.2%) vs. 3 (7.5%), p = 0.020), as well as the incidence of postoperative delirium (23 (22.3%) vs. 2 (5%), p = 0.014) (Table 2). Patients in the DHCA group had substantially longer mechanical ventilation times than patients in the MHCA group (44.0 (7, 246) vs. 8.5 (0.8, 91.8) hr., p = 0.012). Consequently, the DHCA group exhibited prolonged intensive care unit (ICU) stays (7.8 (4.4, 14.1) vs. 5.7 (2.4, 10) days, p = 0.019) as well as longer entire hospital lengths of stay compared with the MHCA group (16.2 (10.1, 29.9) vs. 13.2 (8, 18.6) days, p = 0.012). In terms of postoperative ischemic or hemorrhagic stroke, no significant difference was noted between the two groups, and in-hospital mortality did not differ between the two groups (17 (16.5%) vs. 11 (27.5%); p = 0.212). The Kaplan‒Meier curve after the 2-year follow-up revealed no significant difference in survival between patients who received DHCA and those who received MHCA (Figure 1). We did not find any correlation between the HCA time and postoperative outcomes.
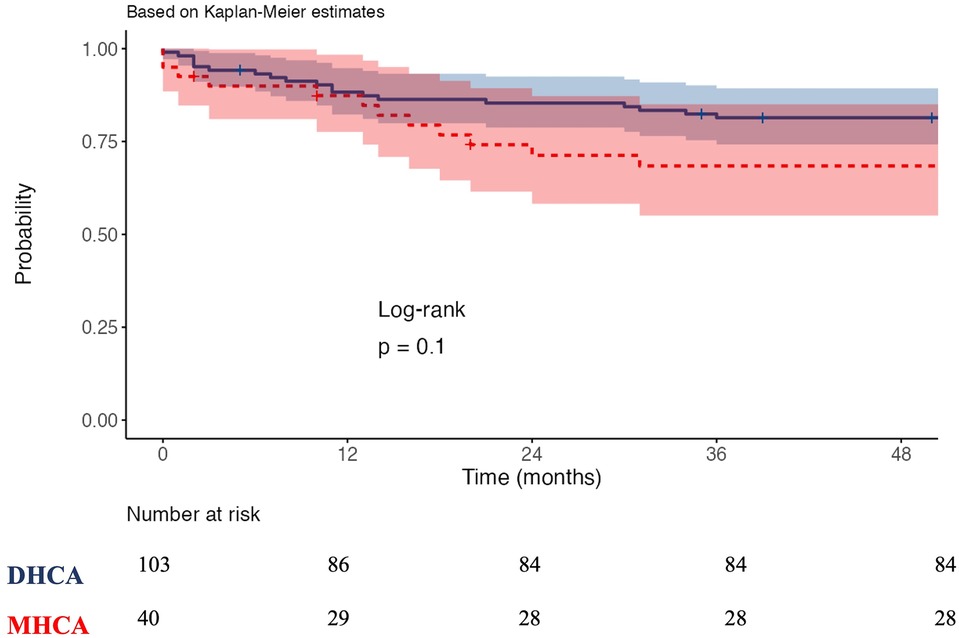
Figure 1. Kaplan-Meier survival curves. DHCA: Deep hypothermia circulatory arrest, MHCA: moderate hypothermia circulatory arrest.
Regression analysis
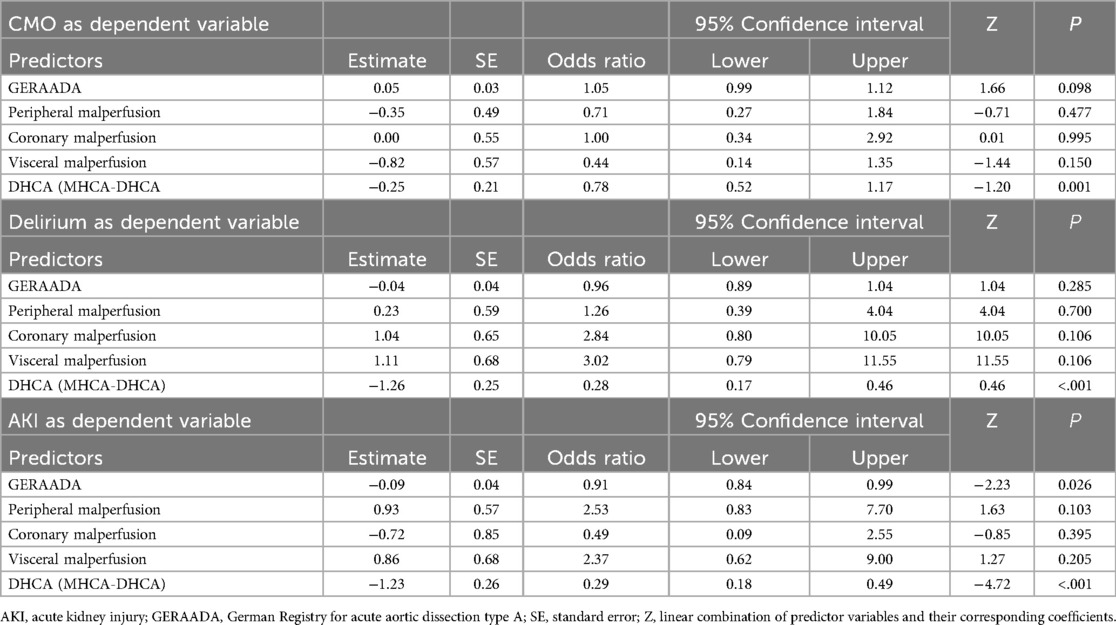
To identify independent predictors of CME, we conducted univariable logistic analyses, which were subsequently followed by stepwise backward multivariable logistic analyses using the GERAADA score and malperfusion status as covariates to calculate the adjusted odds ratio. Table 3 presents the adjusted multivariable logistic analyses. The adjusted odds ratio for CME in the MHCA group was 0.78 (95% CI: 0.52–1.17, p = 0.001). The use of MHCA demonstrated a protective effect on reducing postoperative delirium (OR: 0.28, 95% CI: 0.14–0.46, p < 0.01) and postoperative AKI (OR: 0.29, 95% CI: 0.14–0.49; p < 0.01).
Subanalysis comparing patients with uSCP and those without uSCP
Supplementary Table S1 summarizes the comparison of preoperative characteristics and postoperative outcomes between patients who underwent ATAAD repair with or without uSCP. The incidence of ATAAD only in the ascending aorta was significantly greater in patients who underwent surgery without uSCP (8 (57.1%) vs. 1 (0.8), p < 0.001). The most common operation performed without uSCP was supracoronary replacement of the ascending aorta (13 (92.8%) vs. 35 (27.1), p = 0.001). The CPB time and cross-clamp time were significantly shorter in the group without uSCP than in the uSCP group (CPB: 208.8 ± 29.3 min. vs. 245.8 ± 53.5 min, p = 0.012; cross-clamp time: 117.9 ± 36.2 min vs. 145.2 ± 38.2 min, p = 0.012, respectively). There was no significant difference in postoperative complications between the two groups (Supplementary Table S1). In-hospital mortality did not differ between the two groups, but two-year survival was better in the uSCP group (log-rank, p = 0.029) (Supplementary Figure S1).
Propensity matching and the treatment effect analysis
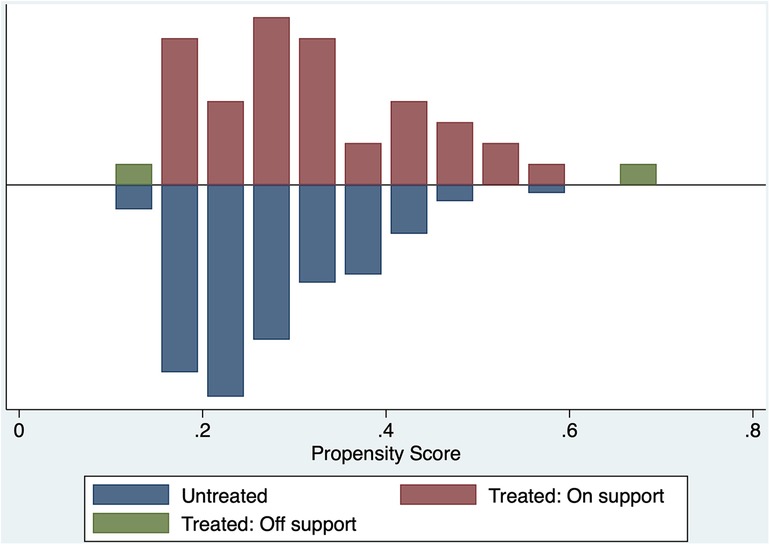
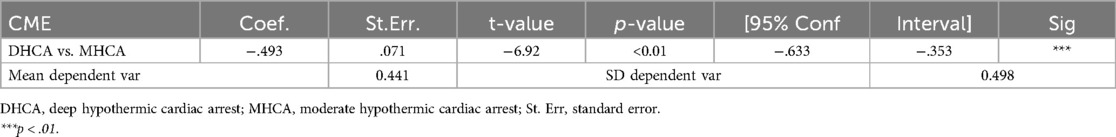
After we performed the propensity matching and matched the 40 patients from the MHCA group to 40 patients from the DHCA group. Figure 2 demonstrate the balance in the matching. After matching we compared both groups in terms of CME using the treatment effect analysis (Table 4). The treatment effect estimation showed a significant difference between DHCA vs. MHCA in the incidence of CME (coefficient:−.493, 95%-CI: −.633 to −.353, p < 0.01).
Discussion
Considering the brain's susceptibility to hypoxemia and the long-standing surgical experience in neuroprotection after aortic surgical treatment based on HCA, numerous surgeons worldwide aim to minimize the oxygen demand with more extensive hypothermia (7).
Compared with the DHCA group, the MHCA group exhibited better preservation of renal function and fewer cases of AKI. DHCA patients not only had worse kidney function parameters but also required external renal replacement therapy substantially more often (15). In contrast to our findings regarding the higher incidence of AKI in the DHCA group, Tsai JY et al. (16) analyzed data from 221 consecutive patients who underwent aortic arch replacement and found no difference in the incidence of AKI between patients who received DHCA and those who received MHCA. Tsai JY et al. (16) also reported that temperature did not notably affect the need for blood transfusions or rethoracotomy, which contrasts with our findings. In accordance with our results, Zhou et al. (17) investigated the outcomes of 533 patients who underwent total arch replacement combined with a frozen Elefant trunk, either with MHCA or DHCA. Zhou and colleagues reported that the incidence of AKI was higher in the DHCA group and was associated with higher mortality (17).
On the other hand, Wu et al. (18) analyzed data from 438 patients with ATAAD who were treated either with DHCA or MHCA. Similar to our findings, they reported that MHCA has a protective effect on reducing postoperative AKI and in-hospital mortality. In accordance with our findings, they also reported that patients treated with DHCA had longer ICU stays and hospital stays (18).
Cao et al. (19) performed a meta-analysis to analyze the effect of DHCA vs. MHCA in aortic arch surgery on postoperative renal function. They (19) included a total of 14 observational studies with 4,142 patients, and similar to our findings, they reported that compared with DHCA, MHCA reduces the incidence of renal failure and the need for renal replacement.
Li et al. (20) proposed that hypothermia during circulatory arrest techniques may serve as an independent risk factor for acute kidney injury (AKI). In accordance with our findings, independent investigations by other research groups have also shown a correlation between AKI and DHCA (21, 22). Li et al. (20) found that in the event of post-surgical AKI. Mechanistically, increased bradykinin, resulting from a temperature decrease during HCA, inhibited the Nrf2-xCT pathway and heightened oxidative stress, culminating in postoperative AKI.
The larger temperature span allows more re-warming, consequently increasing the total CPB time, leading to detrimental multifactorial coagulopathy (23, 24). There was no discernible decrease in the incidence of cardiac tamponade or pericardial effusion in the MHCA group, whereas the causes of surgical bleeding were evenly prevalent between the two groups. However, the DHCA group had a greater re-exploration rate due to major bleeding during the ICU stay. Re-exploration for excessive bleeding after cardiothoracic surgery is associated with worse outcomes, including significantly higher postoperative mortality and morbidity, and should be avoided (25).
Additionally, the study revealed that individuals in the DHCA group required prolonged mechanical ventilation. One significant factor may be the superior vascular constriction in the peripheral body areas at lower temperatures, leading to continuous cold blood return, resulting in prolonged hypothermia during the initial hours of the patient's stay in the ICU. In accordance with our findings, Leshnower et al. (26) analyzed 288 patients who underwent ATAAD and compared outcomes between patients who received DHCA and those who received MHCA. They reported a reduction in postoperative mechanical ventilation time and ICU stay in the MHCA group. Leschnower et al. also reported an association between prolonged CPB time in the DHCA group and postoperative pulmonary dysfunction (26).
A recent study by Liu et al. (27) suggested that a prolonged postoperative ICU stay was associated with emergency surgery, preoperative urea nitrogen levels, and CPB time. Compared with the regular ICU stay group, the prolonged ICU stay group experienced a significantly higher incidence of adverse events, including tracheotomy, reintubation, 72 h of tracheal extubation after surgery, 12 h of consciousness recovery after surgery, ICU re-entry, and irregular discharge (27).
In contrast to our results, Hameed et al. (28) conducted a large meta-analysis that included 26,968 patients from 68 studies. They found that antegrade cerebral perfusion and retrograde cerebral perfusion were associated with substantially lower postoperative stroke and operative mortality rates compared with DHCA. They also reported that the duration of circulatory arrest was linked to the neuroprotective benefits of antegrade and retrograde cerebral perfusion when compared with deep hypothermic circulatory arrest. In our study, we did not identify any correlation between the HCA time and postoperative outcomes.
Another crucial aspect is the higher occurrence of delirium among the DHCA group. Postoperative delirium is a common complication that may occur after cardiac surgery. It extends the duration of the patient's ICU stay, poses a risk to the safety of both the patient and medical staff, and causes considerable emotional distress to patients and their relatives. While profound hypothermia might reduce metabolic demand in situations where energy substrates are scarce, it can also have several negative repercussions. Both human and animal investigations have provided evidence indicating that neuronal damage is associated with profound hypothermia followed by rewarming alone (29). Our findings regarding the lower incidence of postoperative delirium and ICU stay in the MHCA group are similar to the findings of Khaladj et al. and Tsai et al. (16, 30).
Prolonged CPB duration is a consistent independent factor associated with a higher occurrence of delirium (31–33). Additionally, the prolonged mechanical ventilation observed in the DHCA group is another factor that contributes to delirium (34).
Hughes et al. (35) studied 309 patients in a randomized single-blind trial [GOT ICE (Cognitive Effects of Body Temperature During Hypothermic Circulatory Arrest)] of patients undergoing arch surgery with HCA plus antegrade cerebral perfusion at 4 US referral aortic centers. Hughes et al. (35) found a notable regional postoperative alteration in cortical thickness, which wasdetected in 18 locations of focus, often within the 0.05–0.10 mm range. Most of these variations were bilaterally manifested in the inferior frontal and dorsolateral prefrontal cortices (35). These findings may give more insights information about the possible postoperative cognitive and neurological function, which can occur after DHCA or MHCA.
On the other hand, the lower temperature used in HCA did not provide any additional advantages in terms of neuroprotection. There was no increase in the occurrence of either ischemic or hemorrhagic stroke among individuals with less hypothermia. The overall incidence of other adverse effects was similar in both groups. These findings are consistent with those of Leshnower et al. and Tsai et al. (16, 26), who reported no significant differences between DHCA and MHCA with respect to adverse permanent neurological outcomes.
The findings of the present study strongly support the idea that the combination of SCP and MHCA provides adequate protection to the brain and internal organs of patients undergoing ATAAD repair, eliminating the need for severe hypothermia. A prospective, randomized study comparing DHCA and MHCA with uSCP could help determine if DHCA offers any benefits over MHCA in terms of patient outcomes. While this study and others have reported favorable results with MHCA for treating ATAAD, it is challenging to persuade surgeons to subject patients to the additional CPB times and potential risks of DHCA, including multiorgan endothelial dysfunction (36). Thus, comprehensive retrospective assessments of alternative approaches will likely continue to shape circulatory therapy in acute type A aortic dissection surgery.
Study limitations
This study was limited as it was a retrospective cohort study conducted at a single institution. Most patients who received surgery earlier in the research period were subjected to deep hypothermia, whereas those who underwent surgery more recently experienced moderate hypothermia. Therefore, it is plausible that perioperative care approaches have changed slightly over time, thereby biasing our results. However, the degree of uncertainty regarding the extent of aortic replacement and other concomitant procedures necessary to ensure patient survival is one of the most difficult aspects of repairing ATAAD. These decisions are frequently made intraoperatively after evaluating the aortic intima from the valve level to the left subclavian artery. Consequently, the creation of two homogeneous groups for comparing perfusion strategies is challenging due to the high degree of variability in the operations performed to repair ATAAD. Finally, we did not find any difference in the two years survival but due to the lack of follow-up information such as quality of life, dialysis and neurological findings, necessitates additional research to assess the advantages of moderate hypothermia during follow-up.
Conclusion
The principal findings of our study indicate that DHCA elevates the risk of postoperative AKI and delirium. As a result, the duration of hospitalization and intensive care unit stay was markedly extended. Consequently, MHCA should be favored over DHCA when the clinical circumstances permit, since DHCA remains a secure alternative in intricate dissection instances.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was authorized by the Research Ethics Committee at RWTH University Aachen, Germany (EK 20-003). The ethics board waived the requirement for informed consent, considering the retrospective nature of the study.
Author contributions
HA: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Methodology, Investigation, Formal Analysis, Data curation, Conceptualization. AK: Writing – review & editing, Visualization, Validation, Software, Resources, Project administration, Methodology, Investigation, Formal Analysis, Data curation. AH: Writing – review & editing, Supervision, Methodology, Conceptualization. HK: Methodology, Writing – review & editing, Supervision, Resources, Project administration. AM: Writing – review & editing, Supervision, Resources, Project administration, Methodology, Conceptualization. MA: Writing – review & editing, Resources, Methodology, Conceptualization. MS: Writing – review & editing, Validation, Software, Resources, Project administration. RZ: Conceptualization, Writing – review & editing, Validation, Supervision, Software, Resources, Project administration, Methodology. MK: Writing – review & editing, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Formal Analysis, Data curation, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1447007/full#supplementary-material
References
1. Harris KM, Nienaber CA, Peterson MD, Woznicki EM, Braverman AC, Trimarchi S, et al. Early mortality in type a acute aortic dissection: insights from the international registry of acute aortic dissection. JAMA Cardiol. (2022) 7(10):1009–15. doi: 10.1001/jamacardio.2022.2718
2. Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, et al. 2014 Esc guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The task force for the diagnosis and treatment of aortic diseases of the European Society of Cardiology (esc). Eur Heart J. (2014) 35(41):2873–926. doi: 10.1093/eurheartj/ehu281
3. McClure RS, Ouzounian M, Boodhwani M, El-Hamamsy I, Chu MWA, Pozeg Z, et al. Cause of death following surgery for acute type a dissection: evidence from the Canadian thoracic aortic collaborative. Aorta (Stamford. (2017) 5(2):33–41. doi: 10.12945/j.aorta.2017.16.034
4. Conzelmann LO, Weigang E, Mehlhorn U, Abugameh A, Hoffmann I, Blettner M, et al. Mortality in patients with acute aortic dissection type A: analysis of Pre- and intraoperative risk factors from the German registry for acute aortic dissection type a (GERAADA). Eur J Cardiothorac Surg. (2016) 49(2):e44–52. doi: 10.1093/ejcts/ezv356
5. Chan PG, Seese L, Aranda-Michel E, Sultan I, Gleason TG, Wang Y, et al. Operative mortality in adult cardiac surgery: is the currently utilized definition justified? J Thorac Dis. (2021) 13(10):5582–91. doi: 10.21037/jtd-20-2213
6. Yan TD, Bannon PG, Bavaria J, Coselli JS, Elefteriades JA, Griepp RB, et al. Consensus on hypothermia in aortic arch surgery. Ann Cardiothorac Surg. (2013) 2(2):163–8. doi: 10.3978/j.issn.2225-319X.2013.03.03
7. Stecker MM, Cheung AT, Pochettino A, Kent GP, Patterson T, Weiss SJ, et al. Deep hypothermic circulatory arrest: I. Effects of cooling on electroencephalogram and evoked potentials. Ann Thorac Surg. (2001) 71(1):14–21. doi: 10.1016/s0003-4975(00)01592-7
8. McCullough JN, Zhang N, Reich DL, Juvonen TS, Klein JJ, Spielvogel D, et al. Cerebral metabolic suppression during hypothermic circulatory arrest in humans. Ann Thorac Surg. (1999) 67(6):1895–9. discussion 919-21. doi: 10.1016/s0003-4975(99)00441-5
9. Madhavan S, Chan SP, Tan WC, Eng J, Li B, Luo HD, et al. Cardiopulmonary bypass time: every minute counts. J Cardiovasc Surg (Torino). (2018) 59(2):274–81. doi: 10.23736/s0021-9509.17.09864-0
10. Roberts A, Duncan EC, Hargrave P, Kingery DR, Barnes J, Horstemeyer DL, et al. Complications of cardiopulmonary bypass from an anesthesia perspective: a clinical review. HCA Healthc J Med. (2023) 4(1):13–21. doi: 10.36518/2689-0216.1525
11. Hu J, Liu Y, Huang L, Song M, Zhu G. Association between cardiopulmonary bypass time and mortality among patients with acute respiratory distress syndrome after cardiac surgery. BMC Cardiovasc Disord. (2023) 23(1):622. doi: 10.1186/s12872-023-03664-3
12. Czerny M, Grabenwöger M, Berger T, Aboyans V, Della Corte A, Chen EP, et al. EACTS/STS guidelines for diagnosing and treating acute and chronic syndromes of the aortic organ. Eur J Cardiothorac Surg. (2024) 65(2):2–99. doi: 10.1093/ejcts/ezad426
13. Luehr M, Merkle-Storms J, Gerfer S, Li Y, Krasivskyi I, Vehrenberg J, et al. Evaluation of the GERAADA score for prediction of 30-day mortality in patients with acute type a aortic dissection. Eur J Cardiothorac Surg. (2021) 59(5):1109–14. doi: 10.1093/ejcts/ezaa455
14. StataCorp. Stata Causal Inference and Treatment-Effects Estimation Reference Manual Release 18. Texas: A Stata Press Publication StataCorp LLC College Station (2023). Available online at: https://www.stata.com/manuals/causal.pdf (cited 11.09.2024).
15. Luckraz H, Gravenor MB, George R, Taylor S, Williams A, Ashraf S, et al. Long and short-term outcomes in patients requiring continuous renal replacement therapy post cardiopulmonary bypass. Eur J Cardiothorac Surg. (2005) 27(5):906–9. doi: 10.1016/j.ejcts.2005.01.057
16. Tsai JY, Pan W, Lemaire SA, Pisklak P, Lee VV, Bracey AW, et al. Moderate hypothermia during aortic arch surgery is associated with reduced risk of early mortality. J Thorac Cardiovasc Surg. (2013) 146(3):662–7. doi: 10.1016/j.jtcvs.2013.03.004
17. Zhou H, Wang G, Yang L, Shi S, Li J, Wang M, et al. Acute kidney injury after total arch replacement combined with frozen elephant trunk implantation: incidence, risk factors, and outcome. J Cardiothorac Vasc Anesth. (2018) 32(5):2210–7. doi: 10.1053/j.jvca.2018.02.026
18. Wu J, Qiu J, Fang Z, Luo Q, Huang Y, Yu C, et al. Optimal degree of hypothermia in total arch replacement for type a aortic dissection. Front Cardiovasc Med. (2021) 8:668333. doi: 10.3389/fcvm.2021.668333
19. Cao L, Guo X, Jia Y, Yang L, Wang H, Yuan S. Effect of deep hypothermic circulatory arrest versus moderate hypothermic circulatory arrest in aortic arch surgery on postoperative renal function: a systematic review and meta-analysis. J Am Heart Assoc. (2020) 9(19):e017939. doi: 10.1161/jaha.120.017939
20. Li J, Wang M, Wang M, Sang H, Wang W, Gong M, et al. Bradykinin induces acute kidney injury after hypothermic circulatory arrest through the repression of the Nrf2-Xct pathway. iScience. (2024) 27(6):110075. doi: 10.1016/j.isci.2024.110075
21. Brown JA, Serna-Gallegos D, Navid F, Thoma FW, Zhu J, Kumar R, et al. The long-term impact of acute renal failure after aortic arch replacement for acute type a aortic dissection. J Card Surg. (2022) 37(8):2378–85. doi: 10.1111/jocs.16614
22. Augoustides JG, Floyd TF, McGarvey ML, Ochroch EA, Pochettino A, Fulford S, et al. Major clinical outcomes in adults undergoing thoracic aortic surgery requiring deep hypothermic circulatory arrest: quantification of organ-based perioperative outcome and detection of opportunities for perioperative intervention. J Cardiothorac Vasc Anesth. (2005) 19(4):446–52. doi: 10.1053/j.jvca.2005.05.004
23. Bick RL. Alterations of hemostasis associated with cardiopulmonary bypass: pathophysiology, prevention, diagnosis, and management. Semin Thromb Hemost. (1976) 3(2):59–82. doi: 10.1055/s-0028-1086129
24. Bartoszko J, Karkouti K. Managing the coagulopathy associated with cardiopulmonary bypass. J Thromb Haemost. (2021) 19(3):617–32. doi: 10.1111/jth.15195
25. Heimisdottir AA, Nielsen SJ, Karlsson M, Jeppsson A, Gudbjartsson T. Long-term outcome of patients undergoing re-exploration for bleeding following cardiac surgery: a swedeheart study. Eur J Cardiothorac Surg. (2022) 62(5):2–10. doi: 10.1093/ejcts/ezac208
26. Leshnower BG, Thourani VH, Halkos ME, Sarin EL, Keeling WB, Lamias MJ, et al. Moderate versus deep hypothermia with unilateral selective antegrade cerebral perfusion for acute type a dissection. Ann Thorac Surg. (2015) 100(5):1563–8. discussion 8-9. doi: 10.1016/j.athoracsur.2015.05.032
27. Liu H, Zhang S, Zhang C, Gao Q, Liu Y, Liao F, et al. Risk factors for prolonged postoperative ICU stay in the patients with Stanford type a aortic dissection. J Cardiothorac Surg. (2024) 19(1):46. doi: 10.1186/s13019-024-02548-7
28. Hameed I, Rahouma M, Khan FM, Wingo M, Demetres M, Tam DY, et al. Cerebral protection strategies in aortic arch surgery: a network meta-analysis. J Thorac Cardiovasc Surg. (2020) 159(1):18–31. doi: 10.1016/j.jtcvs.2019.02.045
29. Warren DE, Bickler PE, Clark JP, Gregersen M, Brosnan H, McKleroy W, et al. Hypothermia and rewarming injury in hippocampal neurons involve intracellular Ca2+and glutamate excitotoxicity. Neuroscience. (2012) 207:316–25. doi: 10.1016/j.neuroscience.2011.12.034
30. Khaladj N, Peterss S, Oetjen P, von Wasielewski R, Hauschild G, Karck M, et al. Hypothermic circulatory arrest with moderate, deep or profound hypothermic selective antegrade cerebral perfusion: which temperature provides best brain protection? Eur J Cardiothorac Surg. (2006) 30(3):492–8. doi: 10.1016/j.ejcts.2006.05.031
31. O'Neal JB, Billings FT 4th, Liu X, Shotwell MS, Liang Y, Shah AS, et al. Risk factors for delirium after cardiac surgery: a historical cohort study outlining the influence of cardiopulmonary bypass. Can J Anaesth. (2017) 64(11):1129–37. doi: 10.1007/s12630-017-0938-5
32. Segernäs A, Skoog J, Ahlgren Andersson E, Almerud Österberg S, Thulesius H, Zachrisson H. Prediction of postoperative delirium after cardiac surgery with a quick test of cognitive speed, mini-mental state examination and hospital anxiety and depression scale. Clin Interv Aging. (2022) 17:359–68. doi: 10.2147/cia.S350195
33. Dzemali O, Graves K, Loeblein H, Zientara A, Kostorz A, Häussler A, et al. Risk factors and incidence for postop delirium in patients undergoing cardiac surgery with cardiopulmonary bypass support vs. off pump surgery. Thorac Cardiovasc Surg. (2013) 61(S 01):OP159. doi: 10.1055/s-0032-1332398
34. Lin S-M, Huang C-D, Liu C-Y, Lin H-C, Wang C-H, Huang P-Y, et al. Risk factors for the development of early-onset delirium and the subsequent clinical outcome in mechanically ventilated patients. J Crit Care. (2008) 23(3):372–9. doi: 10.1016/j.jcrc.2006.09.001
35. Hughes GC, Chen EP, Browndyke JN, Szeto WY, DiMaio JM, Brinkman WT, et al. Cognitive effects of body temperature during hypothermic circulatory arrest trial (got ice): a randomized clinical trial comparing outcomes after aortic arch surgery. Circulation. (2024) 149(9):658–68. doi: 10.1161/circulationaha.123.067022
Keywords: hypothermic circulatory arrest, acute type a aortic dissection, deep hypothermia, aortic surgery, moderate hypothermia
Citation: Abdulwahab HAM, Kolashov A, Haneya A, Klump H, Moza A, Arab MF, Shoaib M, Zayat R and Khattab MA (2024) Temperature management in acute type A aortic dissection treatment: deep vs. moderate hypothermic circulatory arrest. Is colder better?. Front. Cardiovasc. Med. 11:1447007. doi: 10.3389/fcvm.2024.1447007
Received: 10 June 2024; Accepted: 16 September 2024;
Published: 27 September 2024.
Edited by:
Gabor Erdoes, University Hospital of Bern, SwitzerlandReviewed by:
Alessandro Belletti, IRCCS San Raffaele Scientific Institute, ItalySelim Mosbahi, Inselspital University Hospital Bern, Switzerland
Dragana Unic Stojanovic, Institute for Cardiovascular Diseases Dedinje, Serbia
Copyright: © 2024 Abdulwahab, Kolashov, Haneya, Klump, Moza, Arab, Shoaib, Zayat and Khattab. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alish Kolashov, YS5rb2xhc2hvdkBiYnRncnVwcGUuZGU=
†These authors have contributed equally to this work and share first authorship
 Hend Abdulwahab Muftah Abdulwahab
Hend Abdulwahab Muftah Abdulwahab Alish Kolashov
Alish Kolashov Assad Haneya
Assad Haneya Hannes Klump
Hannes Klump Ajay Moza3
Ajay Moza3 Mohamad Fateh Arab
Mohamad Fateh Arab Rashad Zayat
Rashad Zayat