- 1The Second Clinical Medical School, Lanzhou University, Lanzhou, China
- 2Department of Cardiology, The Second Hospital of Lanzhou University, Lanzhou, China
Background: The weight adjusted waist index (WWI) represents a novel indicator for assessing central obesity. The objective of this study is to investigate the association between WWI and coronary heart disease (CHD).
Method: The data of 44,528 participants in total were gathered from NHANES database from 1999 to 2020. WWI is calculated as the waist circumference (WC, cm) divided by the square root of the body weight (kg), and CHD was determined based on participants’ self-reports. The association between WWI and CHD was examined using multiple logistic regression analysis, restrictive cubic spline (RCS), receiver operating characteristic (ROC) curve, mediation analysis, subgroup and interaction analyses.
Result: This was a cross-sectional investigation. A total of 44,528 participants were included [50.23% male; mean WWI 10.89 (0.01) cm/√kg]. The multivariate logistic regression analysis revealed that in three models, one-standard-deviation increment in WWI was associated with an increased probability of CHD occurrence by 2.39 (2.22,2.57),1.47 (1.32,1.65), and 1.15 (1.00,1.32) times, respectively. Additionally, RCS analysis indicated a linear relationship between WWI and CHD. and the ROC analysis results showed that the discriminatory power of WWI for CHD was superior to that of body mass index (BMI) and WC. Glycated hemoglobin (HbA1c) partially mediated the relationship between WWI and CHD. Subgroup and interaction analyses confirmed that age, systolic blood pressure, and diabetes status had a significant impact on the association between WWI and CHD (P for interaction <0.05).
Conclusion: The level of WWI has been demonstrated to be associated with an increased risk of CHD. Specifically, as WWI increases, the risk of CHD becomes higher. On this basis, it is hypothesized that WWI may potentially serve as an independent risk factor for CAD, thereby highlighting the substantial value of WWI in the identification and management of CHD.
1 Introduction
Coronary heart disease (CHD) is defined as a cardiovascular disorder arising from ischemia and hypoxia of myocardial tissue due to stenosis or occlusion of the coronary arteries. CHD, along with its associated complications such as myocardial infarction and heart failure, remains a major contributor to global morbidity and mortality rates (1, 2).The investigation revealed that the incidence rate of CHD had increased by 33% over the period from 1990 to 2019 (3),Additionally, in 2019, approximately 360,900 deaths in the American population were attributed to coronary heart disease (4).These data emphasize the detrimental effects of CHD and underscore the importance and urgency of proactive implementation of public health interventions for its prevention. Currently, a multitude of risk factors have been identified as being associated with CHD, encompassing smoking, hyperlipidemia, hypertension, diabetes, snoring, and obesity (1, 5, 6).
Obesity, which is recognized as one of the key risk factors for chronic diseases, poses a significant public health concern and demonstrates a close association with cardiovascular diseases. The primary etiologies underlying obesity relate to the increase in the amount and/or size of adipose tissue, as well as inappropriate adipose tissue distribution (7).According to the investigation, since 1980, the number of obese individuals has demonstrated a doubling trend. It is anticipated that by 2025, the prevalence of obesity among men will reach 18%, whereas among women, the prevalence is projected to exceed 21%. Furthermore, forecasts indicate that more than 6% of men and 9% of women will develop severe obesity (8, 9). The traditional metrics for assessing obesity, BMI and WC, are widely used. BMI, in particular, is the most commonly employed index due to its cost-effectiveness and ease of use. However, certain studies have identified a paradoxical relationship between BMI and the mortality rate associated with cardiovascular diseases (CVDs). Specifically, within certain populations, a higher BMI is not always directly associated with an increased risk of CVDs. In some instances, overweight or mildly obese individuals may even exhibit a lower risk or mortality rate of CVDs, a phenomenon referred to as the “obesity paradox” (10).Given the limitation of BMI in accurately distinguishing between fat mass and muscle mass, it is prudent to exercise caution when elucidating the relationship between obesity and CVDs, particularly focusing on CHD (10–12).WC is regarded as a preferred surrogate marker for quantifying visceral fat accumulation. However, it fails to account for the influence of height, thereby limiting its accuracy in assessing abdominal obesity across populations with varying stature (13).
In 2018, Park et al. introduced the WWI as an innovative metric to quantify the degree of obesity (14).This index integrates the advantages of WC while reducing the correlation with BMI, thereby enabling a more precise characterization of fat and muscle composition. Despite prior studies showing an association between WWI and CVDs mortality (15), the relationship between WWI and the incidence of CHD, as well as its comparative discriminatory ability for CHD vs. traditional indicators, remains unclear. Therefore, the aim of this study is to evaluate whether WWI can improve the detection rate of CHD in the general population.
2 Materials and methods
2.1 Description of the study
In this study, we utilized data from the NHANES database from 1999 to 2020. NHANES, administered by the National Center for Health Statistics (NCHS), aims to assess the health and nutritional status of adults and children in the United States. To ensure the representativeness of the sample, NHANES employed a multi-stage, stratified, clustered probability sampling design. Each participant underwent a series of assessments, and the collected data were subsequently categorized into five main sections: demographic information, dietary intake, physical examination results, laboratory test outcomes, and questionnaire responses. The NHANES protocol was approved by the Institutional Review Board of NCHS, and all participants provided written informed consent prior to their participation. Consequently, no further ethical review was required. Additionally, the data relevant to this study are publicly available on the NHANES official website (NHANES—National Health and Nutrition Examination Survey Homepage).
2.2 Study population
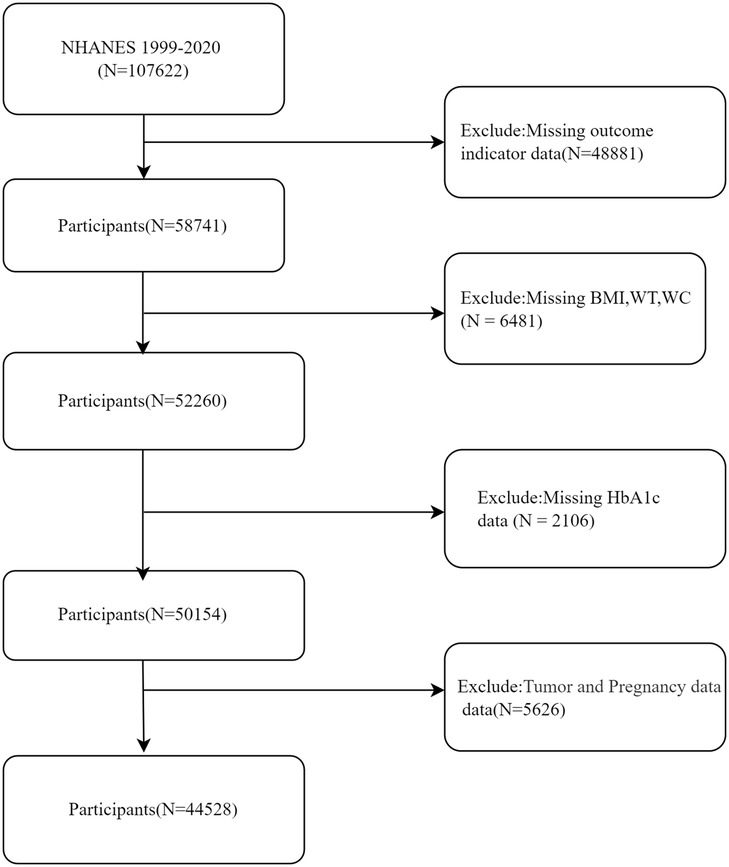
The present study included a comprehensive sample of 107,622 individuals sourced from the NHANES database, covering the period from 1999 to 2020. After rigorous exclusion criteria were applied, which involved removing individuals with missing or incomplete data on outcome measures (n = 48,881), physical morphological parameters (BMI, WC, weight) (n = 6,481), HbA1c (n = 2,106), as well as those with a history of tumors or current pregnancy (n = 5,626), a final cohort of 44,528 participants was included in the definitive analysis (Figure 1).
2.3 Assessment of WWI
In this study, the WWI (cm/√kg) was utilized as the exposure variable. It was calculated by dividing the WC (cm) by the square root of the body weight (kg). The WWI represents a novel metric for assessing obesity, with a particular emphasis on central obesity. A higher WWI ratio signifies a more severe degree of central obesity (14). The anthropometric parameters were collected by skilled health technicians within the Mobile Examination Center (MEC). During each physical measurement session, the technicians were assisted by recorders. Body weight was measured using a digital scale with a precision of 0.1 kg. Participants were positioned at the center of the scale, wearing standardized examination attire, with their hands placed alongside their bodies and gazing straight ahead. WC was assessed using a standard tape measure. A horizontal marking was made above the outermost edge of the right iliac crest, and the tape measure was positioned at the intersection of this line with the right mid-axillary line. The measurement was taken at the end of a normal exhalation and was recorded with a precision of 0.1 cm (16).
2.4 Assessment of CHD
The definition of CHD in our study was based on the response to the question in the questionnaire: “Has a doctor ever informed you that you have coronary heart disease?” Participants who responded “yes” were classified as belonging to the group with CHD. In our research, CHD was designated as the outcome variable.
2.5 Assessment of covariates
The other covariates included in this study were: age (≥60 years or <60 years), gender (male or female), race (non-Hispanic white, non-Hispanic black, other/multiracial, Mexican American, and other Hispanic American), alcohol consumption (no drinking, 1–5 times per month, 5–10 times per month, 10 + times per month), BMI (underweight: <18.5 kg/m2, normal weight: 18.5–24.9 kg/m2, overweight: 25.0–29.9 kg/m2, obese: ≥30.0 kg/m2), education level, smoking status, vigorous physical activity (yes or no), systolic blood pressure (SBP), diastolic blood pressure (DBP), cholesterol, creatinine, glucose, triglyceride, poverty index ratio (PIR), high-density lipoprotein cholesterol (HDL-C), uric acid, albumin level, HbA1c, heart failure status, and stroke status.
2.6 Statistical analysis
The statistical methodologies employed in this study adhered to the standards outlined on the NHANES official website. Given the multi-stage, stratified, and clustered probability sampling design, we applied appropriate weights to the analysis. In the baseline characteristics table, continuous variables were presented as mean (standard errors), while categorical variables were presented as weighted frequencies (weighted percentages). Descriptive statistical analysis of the WWI quartiles was conducted using the t-test or chi-square test. To examine the relationship between WWI and CHD, multiple logistic regression models were constructed. Prior to model building, variables with significant collinearity were excluded based on the correlation matrix and variance inflation factor (VIF). Three models were developed, guided by clinical expertise, AIC values, and likelihood ratio tests: Model 1, unadjusted for covariates; Model 2, adjusted for age, gender, race, education, and PIR; and Model 3, further adjusted for smoking, alcohol consumption, SBP, DBP, HDL-C, uric acid, triglycerides, albumin, physical activity, HbA1c, heart failure, stroke, BMI, and cholesterol. To assess the potential nonlinear relationship between WWI and CHD, RCS analysis was performed. Additionally, the ROC curve was utilized to compare the discriminatory abilities of BMI, WC, and WWI in determining CHD. Subsequently, mediation analysis was conducted to explore the role of HbA1c, as a mediator between WWI and CHD. Finally, subgroup analysis and interaction testing were employed to evaluate differences in the relationship between WWI and CHD across various subgroups, including age, gender, race, education, smoking status, alcohol consumption, SBP, DBP, and diabetes mellitus (DM) status. All statistical analyses were conducted using R version 4.3.3, with statistical significance set at a two-sided P < 0.05.
3 Result
3.1 Baseline data
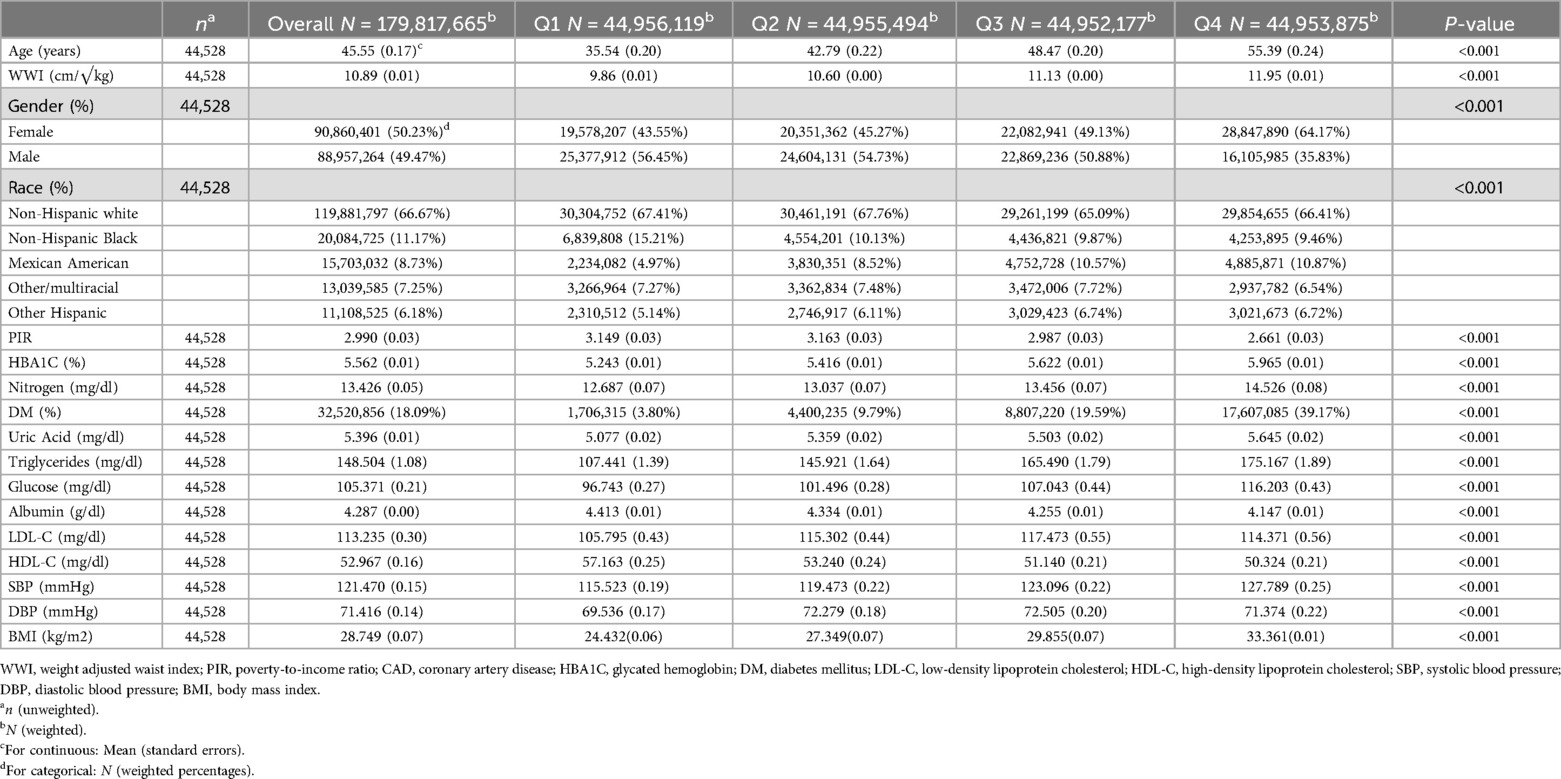
The characteristics of the study population are presented in Table 1. A total of 44,528 participants were enrolled in this study [50.23% male; mean age 45.55(0.17)]. All covariates examined in this study demonstrated statistical significance in relation to the baseline characteristics across the WWI quartiles. The mean WWI value for all participants was 10.89(0.01) (cm/√kg). The specific quartile values of WWI were as follows: Q1:9.86 (0.01); Q2:10.60 (0.00); Q3:11.13 (0.00); and Q4:11.95 (0.01). Compared to individuals in the lowest WWI quartile, those in the highest quartile were more likely to be older, female, non-Hispanic white, and had a higher propensity for CHD and DM. Additionally, they exhibited higher levels of BMI, HbA1c, blood urea nitrogen, uric acid, triglycerides, blood glucose, SBP, and DBP. Concurrently, as the WWI increased, the PIR and HDL-C levels decreased.
3.2 Logistic analysis results
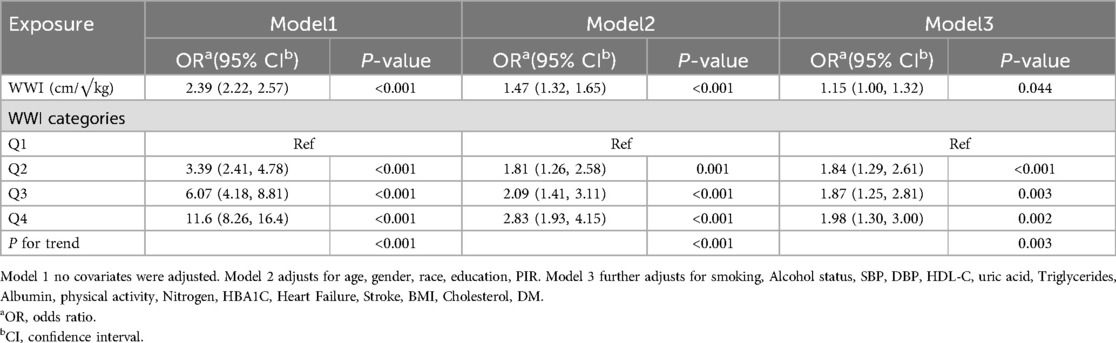
This study employed logistic regression analysis to assess the correlation between WWI and CHD, with the detailed results presented in Table 2. When WWI was treated as a continuous variable in the analysis, Model 1, which did not include any covariate adjustments, revealed that for each standard deviation increment in WWI, the odds of CHD occurrence significantly increased [OR (95%CI):2.39 (2.22, 2.57), P < 0.001]. After adjusting for factors such as age, gender, race, education, and PIR in Model 2, the increase in odds was attenuated to an OR of 1.47 (95%CI: 1.32, 1.65, P < 0.001). Upon further covariate adjustment based on Model 2, the odds increased to an OR of 1.15 (95% CI: 1.00, 1.32, P < 0.044) for each standard deviation increment in WWI. When WWI was analyzed using quartile grouping, a consistent trend was observed across all three models. Taking Model 1 as an example, with Q1 serving as the reference group, the odds of CHD occurrence in the Q2 group substantially increased [OR (95%CI): 3.39(2.41, 4.78), P < 0.001]; in the Q3 group, the odds further increased [OR (95%CI):6.07(4.18, 8.81), P < 0.001]; and in the Q4 group, the highest odds of CHD occurrence were observed [OR (95%CI):11.6(8.26, 16.4), P < 0.001]. After adjusting for corresponding covariates in Model 2 and Model 3, a similar trend of significantly increasing odds of CHD occurrence with increasing WWI quartiles was demonstrated, with all trend test P-values less than 0.05.
3.3 RCS analysis result
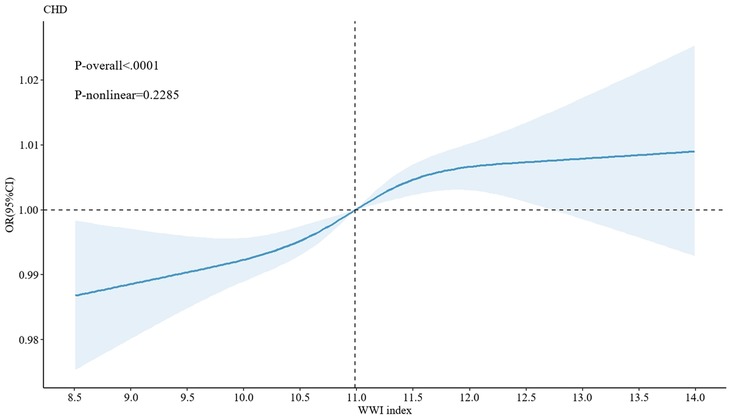
Additionally, following the adjustment of all covariates in Model 3, an in-depth exploration was conducted using the generalized additive model (GAM) with smooth curve fitting capabilities. The results demonstrated a significant linear correlation between WWI and CHD (P for nonlinear = 0.2285, P < 0.05) (Figure 2). This finding further complemented and reinforced the results obtained from the previous logistic regression analysis, shedding light on the association between WWI and CHD from diverse perspectives.
3.4 ROC curve analysis and mediation analysis
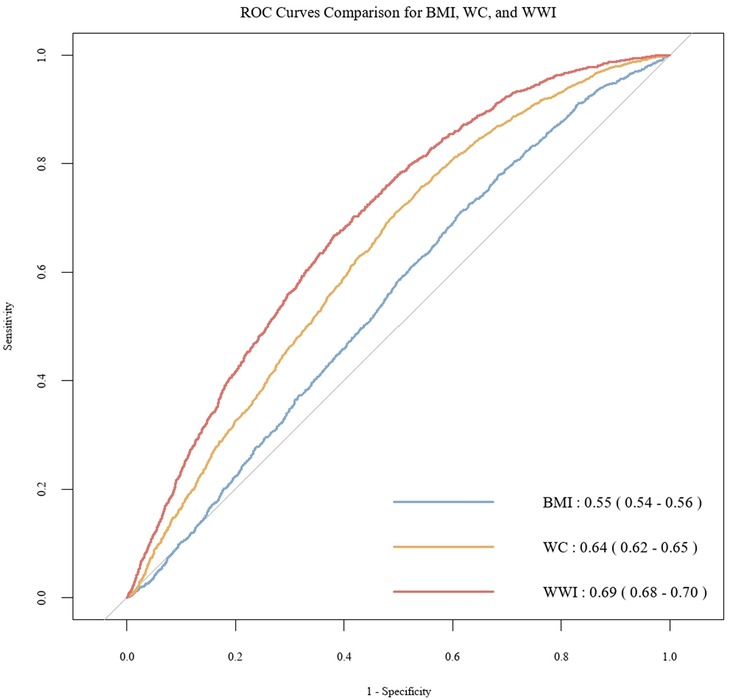
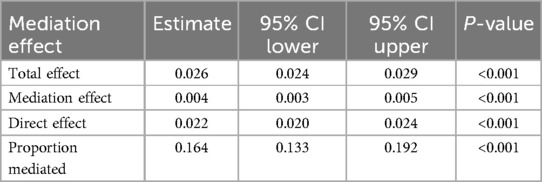
To further investigate the discriminatory abilities of WWI, BMI, and WC in relation to the risk of CHD, and to verify the superiority of WWI, we constructed ROC curves. The results demonstrated that WWI exhibited significantly stronger discriminatory power for CHD compared to BMI and WC [WWI: AUC(95%CI): 0.69(0.68, 0.70); BMI: AUC(95%CI): 0.55(0.54, 0.56); WC: AUC(95%CI): 0.64(0.62, 0.65)] (Figure 3). Additionally, the Table 3 illustrates the mediation analysis, The findings indicated that HbA1c partially mediated the association between WWI and CHD, with a mediation effect size of 16.4% (P < 0.001).
3.5 Subgroup analyses and interactions
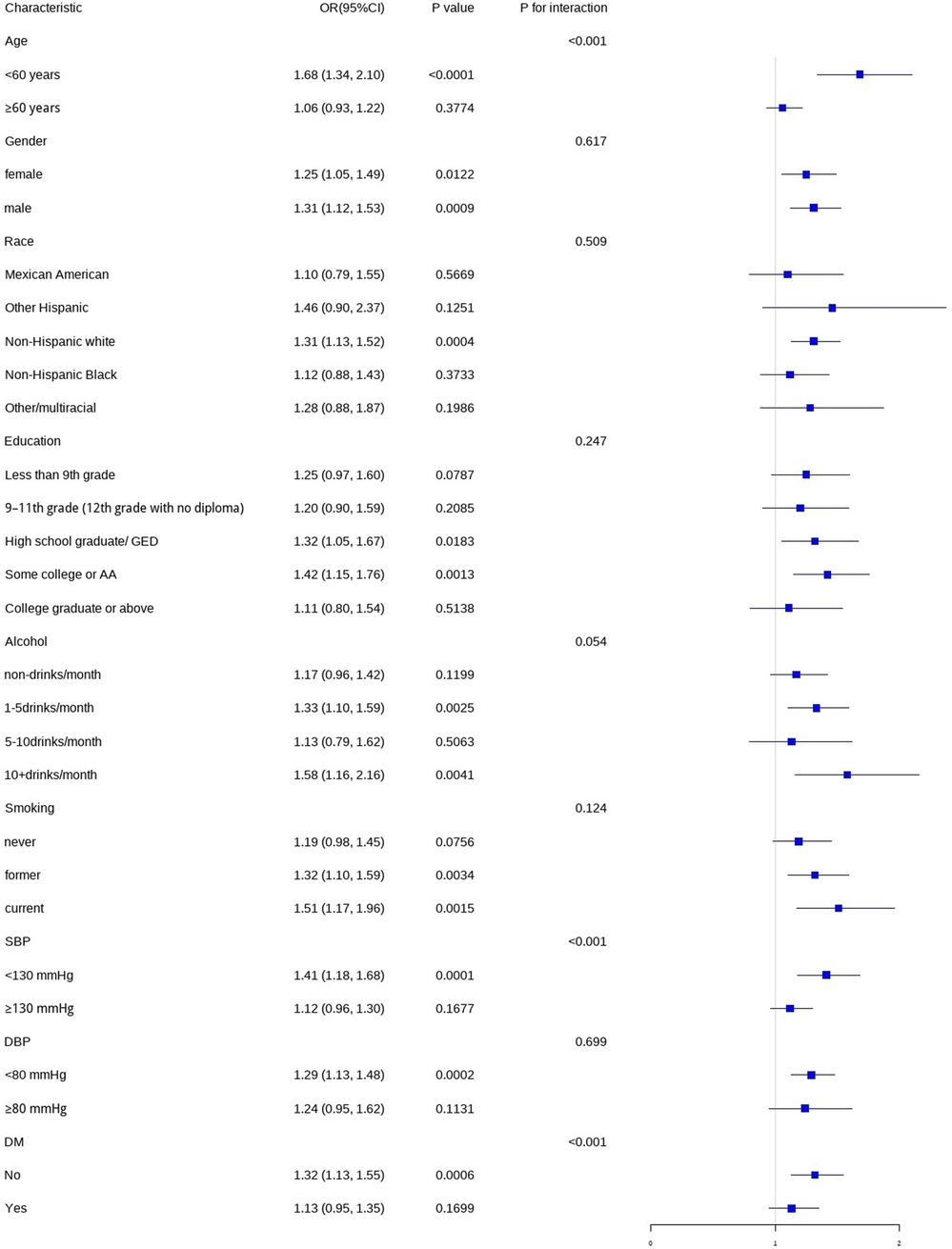
In this study, we conducted subgroup analyses and interaction tests, stratified by age, gender, race, education level, smoking status, alcohol consumption, SBP, DBP, and DM status, to assess the robustness of the association between WWI and CHD and to detect any potential disparities (Figure 4). The results indicated that gender, race, education level, smoking status, alcohol consumption, and DBP did not significantly modify the relationship between WWI and CHD (P > 0.05). However, age, SBP, and DM status had a significant impact on the association between WWI and CHD (P < 0.05).
4 Discussion
In this cross-sectional study, which included 44,528 participants, we observed a linear and positive correlation between the WWI and the prevalence of CHD. This suggests that across the entire range of WWI values, individuals with higher WWI scores are more susceptible to developing CHD. Additionally, the results from the subgroup analysis and interaction tests indicated that age, SBP, and DM status had a specific impact on the stability of the relationship between WWI and CHD. The mediation analysis revealed that HbA1c partially mediated the association between WWI and CHD. When compared to BMI and WC, WWI demonstrated superior diagnostic utility for identifying an increased risk of CHD. Consequently, based on these findings, we hypothesize that an elevated WWI may serve as an independent risk factor for CHD, and its diagnostic accuracy for CHD surpasses that of BMI and WC, thereby highlighting the pivotal role of WWI in the prevention and management of CHD.
In addition to the present study, numerous prior investigations have explored the correlation between the WWI and various diseases. For instance, a study involving 12,447 participants demonstrated that a high WWI level (≥ 11.2 cm/√kg) was associated with an increase in all-cause mortality and cardiovascular mortality in southern China (17).In the field of cardiovascular diseases, Hu et al. also identified a significant positive correlation between the WWI and the risk of stroke. An increase in WWI indicates a higher risk of incident stroke (18).Concurrently, the study conducted by Zhang et al. also established a linear positive correlation between the WWI and the risk of heart failure. Specifically, for each standard deviation increment in WWI, the corresponding risk of heart failure increased by 19.5% (15).Furthermore, WWI may potentially serve as an intervention factor for the prevention and treatment of arteriosclerosis (19). in addition to its role in blood pressure management. Moreover, in a cohort study among patients with hypertensive obstructive sleep apnea (OSA), a J-shaped association was observed between WWI and CVDs. Notably, when WWI exceeded 11.5 cm/√kg, the risk of CVDs increased significantly (13).The correlations between WWI and a range of diseases, including bone mineral density (20), depression (21), chronic kidney disease (22), kidney stones (23), and fractures (24), have also been successively confirmed. Our study further confirmed the relationship between WWI and CHD, which aligns with previous research findings regarding the adverse effects of elevated WWI on CVDs.
Numerous prior studies have unequivocally demonstrated that obesity is a significant risk factor for CHD. Among these, BMI, serving as a conventional indicator for assessing obesity, has broad applications. Meyer et al. focused on children and adolescents aged 2–19 years and determined that BMI within this age range was positively correlated with the incidence of CHD in adulthood (25). Lamon-Fava et al. investigated the association between BMI and CHD separately in male and female groups, and their findings likewise demonstrated that an increase in BMI was adversely associated with the primary risk factors for CHD (26). In another study involving 15,828 patients with stable CHD, when BMI exceeded 25 kg/m2, cardiometabolic and inflammatory risk factors increased in a gradual manner concomitant with the increment in BMI (27). However, a study on the Korean population revealed a U-shaped association between BMI and all-cause mortality, CVDs mortality, and cancer mortality. The phenomenon whereby the mortality risk of moderate obesity is lower than that of normal weight, underweight, or overweight individuals is termed the “obesity paradox” (28). This phenomenon is partly attributed to methodological biases or the confounding effects of other factors, and it also highlights the limitations of BMI in differentiating between fat mass and lean body mass (10, 12). On the basis of traditional indicators, researchers have been persistently exploring new obesity assessment indicators, such as WC, Although WC is also a commonly used measure of obesity, due to its high correlation with BMI, it has not emerged as an outstanding obesity assessment indicator independent of BMI (29).
A pivotal finding of this study is that HbA1c partially mediates the association between WWI and CHD. HbA1c serves as an indicator of blood glucose levels over the preceding 2–3 months and is a crucial diagnostic criterion for DM. Studies have shown a positive correlation between HbA1c levels and the incidence and mortality rates of cardiovascular diseases (30). Therefore, maintaining optimal HbA1c levels is beneficial for the prevention of CHD and the improvement of prognosis in CHD patients (31).
As a novel indicator for assessing central obesity, the WWI demonstrates significant advantages over the conventional BMI and WC in determining the risk of CHD. By combining WC with the square root of body weight, the WWI not only retains the strengths of WC in evaluating fat distribution but also reduces its correlation with BMI, thereby providing a more comprehensive reflection of an individual's muscle mass and fat mass. This advantage persists even among individuals with varying BMI levels (32). Furthermore, WWI mitigates the influence of the obesity paradox, Consequently, it emerges as a more comprehensive and precise tool for CHD risk assessment, possessing substantial clinical application value (18). A vast array of studies has established a notable correlation between central obesity and the risk and mortality of CHD. For example, Coutinho et al. conducted a comprehensive analysis of 2,188 studies and found that central obesity was directly linked to CHD mortality (33).Similarly, a study following 130,473 participants aged 60–69 in the UK Biobank further confirmed this finding (34). As research progresses, an ever-growing body of evidence suggests that WWI is associated with a range of diseases, including hypertension, arteriosclerosis, abnormal uric acid levels, heart failure, and depression (15, 19, 35–37).Our study also confirms that WWI may serve as an independent risk factor for CHD. Compared to the traditional BMI anthropometric parameter, WWI more accurately reflects the distribution of body fat and proposes a novel strategy for the prevention and treatment of coronary heart disease (16).
The WWI exhibits a positive correlation with abdominal fat mass and a negative correlation with abdominal muscle mass, enabling it to reflect the ratio of fat to muscle mass rather than an absolute fat mass alone (38).Consequently, compared to BMI and WC, WWI is more adept at accurately capturing “true obesity,” specifically the form of obesity intimately associated with metabolic disturbances. The mechanisms underlying the increased risk of CHD associated with elevated WWI values can be elucidated from two perspectives: Firstly, abdominal fat cells, particularly visceral fat cells, have a greater propensity to directly release free fatty acids (FFAs) into the portal vein compared to other anatomical regions. This results in prolonged exposure of the liver to a high FFA milieu, stimulating the synthesis of very low-density lipoprotein (VLDL), which subsequently elevates plasma triglyceride and cholesterol levels, leading to dyslipidemia. Additionally, the high FFA environment may induce insulin resistance, further augmenting blood pressure and insulin levels (hypertension and hyperinsulinemia), and elevating blood glucose, thereby increasing HbA1c levels. The chronic hyperglycemic state indicated by high HbA1c levels intensifies the inflammatory response, collectively promoting the development of atherosclerosis (33, 39, 40). Secondly, the augmentation in central obesity denoted by WWI may reflect perturbations in fat metabolism. This, in turn, prompts the body to release more reactive oxygen species (ROS). These ROS interact with nitric oxide (NO) to produce cytotoxic peroxynitrite, damaging vascular endothelial cells. Furthermore, the increase in ROS accelerates the oxidation of low-density lipoprotein (LDL), making it more susceptible to phagocytosis by macrophages and the formation of foam cells, thereby exacerbating vascular endothelial damage and facilitating atherosclerosis (41–43).In summary, WWI, as an index that concurrently reflects the ratio of fat to muscle mass, provides a novel perspective for understanding and assessing obesity and its association with metabolic disorders, such as CHD. With further research and validation, WWI is anticipated to become a crucial tool in the management of obesity and the prevention of CHD.
One of the prominent strengths of this study is its large-sample size, which is based on the NHANES database. The stratified, multi-stage, and clustered probability sampling paradigm employed by this database ensures the representativeness and reliability of the data. During the data analysis process, we utilized a correlation matrix and variance inflation factor to preempt multicollinearity and further compared the discriminative abilities of WWI, BMI, and WC for CHD using the ROC curve. Additionally, our study determined that HbA1c partially mediates the correlation between WWI and CHD. Furthermore, we conducted subgroup analyses and interaction assessments to thoroughly investigate the disparities in the correlation between WWI and CHD across diverse populations. Despite these strengths, our study also has certain limitations. Firstly, since the data is primarily sourced from the American population, caution should be exercised when generalizing the results to populations in other countries and regions. Secondly, due to the cross-sectional nature of the NHANES database, our study is unable to directly infer a causal relationship between WWI and coronary heart disease. Additionally, the outcome variable used in this study was derived from a questionnaire survey, which may introduce recall bias or misclassification. Finally, although we made efforts to adjust for potential covariates during data analysis, there may still be other unaccounted factors that could impact the results. Therefore, in future studies, we will further explore these issues and work to resolve these limitations.
5 Conclusion
In conclusion, it clearly demonstrates a positive correlation between the level of WWI and the risk of CHD. Specifically, an elevation in WWI is accompanied by a corresponding increase in the risk of CHD. Notably, compared to traditional indices, WWI exhibits superior diagnostic potential for CHD. Furthermore, the study also established that HbA1c partially mediates the relationship between WWI and CHD. These findings strongly suggest that WWI may be an independent risk factor for CHD, thereby opening up novel potential strategic pathways for the prevention and treatment of coronary heart disease. Given that WWI possesses notable advantages such as ease of calculation, convenient acquisition, and high cost-effectiveness, its widespread application within various medical and health institutions is highly feasible. Especially in regions with relatively scarce medical and economic resources, the implementation of WWI may yield substantial social and economic benefits. Consequently, further in-depth exploration of the relationship between WWI and CHD is of great significance. Looking ahead, research could be considered to advance in the following areas: Firstly, conduct large-scale longitudinal studies to elucidate the causal link between WWI and CHD, thereby providing more compelling evidence for disease prevention and control. Secondly, perform studies among different ethnic and regional populations to validate the universality of WWI in predicting CHD and make the research findings more widely applicable. Thirdly, deeply investigate the underlying mechanism through which WWI influences CHD, dissect the changes at the cellular and molecular levels, and concurrently explore the impact of lifestyle interventions on the relationship between WWI and CHD. Through these efforts, it is anticipated that the value of WWI as a CHD predictor will be more comprehensively and profoundly affirmed, thereby establishing a solid theoretical and practical foundation for the precise prevention and treatment of CHD.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The protocol for the “National Health and Nutrition Examination Survey” has obtained approval from the Institutional Review Board of the National Center for Health Statistics (NCHS). Additionally, all participants provided written informed consent prior to their participation in the study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Supervision, Writing – original draft, Writing – review & editing. SS: Data curation, Funding acquisition, Supervision, Validation, Writing – review & editing. QZ: Conceptualization, Data curation, Formal Analysis, Writing – review & editing. TT: Project administration, Supervision, Writing – review & editing. DL: Conceptualization, Data curation, Writing – review & editing. GH: Data curation, Writing – review & editing. ZW: Data curation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by Gansu Provincial Science and Technology Program (22YF7FA088).
Acknowledgments
The authors appreciate the NHANES participants, staff, and investigators. This article has utilized the Wenxin Da Model within ERNIE Bot 3.5 for language adjustment.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1445802/full#supplementary-material
References
1. Malakar AK, Choudhury D, Halder B, Paul P, Uddin A, Chakraborty S. A review on coronary artery disease, its risk factors, and therapeutics. J Cell Physiol. (2019) 234(10):16812–23. doi: 10.1002/jcp.28350
2. Riaz H, Khan MS, Siddiqi TJ, Usman MS, Shah N, Goyal A, et al. Association between obesity and cardiovascular outcomes: a systematic review and meta-analysis of mendelian randomization studies. JAMA Netw Open. (2018) 1(7):e183788. doi: 10.1001/jamanetworkopen.2018.3788
3. Manla Y, Almahmeed W. The pandemic of coronary heart disease in the Middle East and north Africa: what clinicians need to know. Curr Atheroscler Rep. (2023) 25(9):543–57. doi: 10.1007/s11883-023-01126-x
4. Manohar H, Potter AS, Koutroumpakis E, Deswal A, Palaskas NL. Can we mitigate coronary heart disease risk in patients with cancer? Curr Atheroscler Rep. (2022) 24(8):599–606. doi: 10.1007/s11883-022-01035-5
5. Wang M, Wang M, Zhu Q, Yao X, Heizhati M, Cai X, et al. Development and validation of a coronary heart disease risk prediction model in snorers with hypertension: a retrospective observed study. Risk Manag Healthc Policy. (2022) 15:1999–2009. doi: 10.2147/RMHP.S374339
6. Zhao J, Wang M, Li N, Luo Q, Yao L, Cai X, et al. Development and validation of a novel model for predicting coronary heart disease in snoring hypertensive patients with hyperhomocysteinemia. Int Heart J. (2023) 64(6):970–8. doi: 10.1536/ihj.23-384
7. Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol. (2013) 62(10):921–5. doi: 10.1016/j.jacc.2013.06.027
8. Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metab Clin Exp. (2019) 92:6–10. doi: 10.1016/j.metabol.2018.09.005
9. NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. (2016) 387(10026):1377–96. doi: 10.1016/S0140-6736(16)30054-X
10. Antonopoulos AS, Oikonomou EK, Antoniades C, Tousoulis D. From the BMI paradox to the obesity paradox: the obesity-mortality association in coronary heart disease. Obes Rev. (2016) 17(10):989–1000. doi: 10.1111/obr.12440
11. Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. (2006) 355(8):763–78. doi: 10.1056/NEJMoa055643
12. Hainer V, Aldhoon-Hainerová I. Obesity paradox does exist. Diabetes Care. (2013) 36(Suppl 2):S276–281. doi: 10.2337/dcS13-2023
13. Zhao J, Cai X, Hu J, Song S, Zhu Q, Shen D, et al. J-shaped relationship between weight-adjusted-waist index and cardiovascular disease risk in hypertensive patients with obstructive sleep apnea: a cohort study. Diabetes Metab Syndr Obes. (2024) 17:2671–81. doi: 10.2147/DMSO.S469376
14. Park Y, Kim NH, Kwon TY, Kim SG. A novel adiposity index as an integrated predictor of cardiometabolic disease morbidity and mortality. Sci Rep. (2018) 8(1):16753. doi: 10.1038/s41598-018-35073-4
15. Zhang D, Shi W, Ding Z, Park J, Wu S, Zhang J. Association between weight-adjusted-waist index and heart failure: results from national health and nutrition examination survey 1999–2018. Front Cardiovasc Med. (2022) 9:1069146. doi: 10.3389/fcvm.2022.1069146
16. Xie F, Xiao Y, Li X, Wu Y. Association between the weight-adjusted-waist index and abdominal aortic calcification in United States adults: results from the national health and nutrition examination survey 2013–2014. Front Cardiovasc Med. (2022) 9:948194. doi: 10.3389/fcvm.2022.948194
17. Ding C, Shi Y, Li J, Li M, Hu L, Rao J, et al. Association of weight-adjusted-waist index with all-cause and cardiovascular mortality in China: a prospective cohort study. Nutr Metab Cardiovasc Dis. (2022) 32(5):1210–7. doi: 10.1016/j.numecd.2022.01.033
18. Hu J, Cai X, Song S, Zhu Q, Shen D, Yang W, et al. Association between weight-adjusted waist index with incident stroke in the elderly with hypertension: a cohort study. Sci Rep. (2024) 14(1):25614. doi: 10.1038/s41598-024-76709-y
19. Xiong Y, Shi W, Huang X, Yu C, Zhou W, Bao H, et al. Association between weight-adjusted waist index and arterial stiffness in hypertensive patients: the China H-type hypertension registry study. Front Endocrinol (Lausanne). (2023) 14:1134065. doi: 10.3389/fendo.2023.1134065
20. Zhang Y, Wu H, Li C, Liu C, Liu M, Liu X, et al. Associations between weight-adjusted waist index and bone mineral density: results of a nationwide survey. BMC Endocr Disord. (2023) 23(1):162. doi: 10.1186/s12902-023-01418-y
21. Fei S, Liu M, Shanshan H, Xie R, Danni W, Ningying Z. Association between weight-adjusted-waist index and depression: a cross-sectional study. Endocr Connect. (2024) 13(6):e230450. doi: 10.1530/EC-23-0450
22. Li X, Wang L, Zhou H, Xu H. Association between weight-adjusted-waist index and chronic kidney disease: a cross-sectional study. BMC Nephrol. (2023) 24(1):266. doi: 10.1186/s12882-023-03316-w
23. Lin W, Ye Q, Lin M-E. Relationship between the weight-adjusted-waist index and kidney stone: a population-based study. World J Urol. (2023) 41(11):3141–7. doi: 10.1007/s00345-023-04620-8
24. Tao J, Zhang Y, Tan C, Tan W. Associations between weight-adjusted waist index and fractures: a population-based study. J Orthop Surg Res. (2023) 18(1):290. doi: 10.1186/s13018-023-03776-8
25. Meyer JF, Larsen SB, Blond K, Damsgaard CT, Bjerregaard LG, Baker JL. Associations between body mass index and height during childhood and adolescence and the risk of coronary heart disease in adulthood: a systematic review and meta-analysis. Obes Rev. (2021) 22(9):e13276. doi: 10.1111/obr.13276
26. Lamon-Fava S, Wilson PW, Schaefer EJ. Impact of body mass index on coronary heart disease risk factors in men and women. The framingham offspring study. Arterioscler Thromb Vasc Biol. (1996) 16(12):1509–15. doi: 10.1161/01.ATV.16.12.1509
27. Held C, Hadziosmanovic N, Aylward PE, Hagström E, Hochman JS, Stewart RAH, et al. Body mass index and association with cardiovascular outcomes in patients with stable coronary heart disease - A STABILITY substudy. J Am Heart Assoc. (2022) 11(3):e023667. doi: 10.1161/JAHA.121.023667
28. Kim NH, Lee J, Kim TJ, Kim NH, Choi KM, Baik SH, et al. Body mass index and mortality in the general population and in subjects with chronic disease in Korea: a nationwide cohort study (2002–2010). PLoS One. (2015) 10(10):e0139924. doi: 10.1371/journal.pone.0139924
29. Clark AL, Fonarow GC, Horwich TB. Waist circumference, body mass index, and survival in systolic heart failure: the obesity paradox revisited. J Card Fail. (2011) 17(5):374–80. doi: 10.1016/j.cardfail.2011.01.009
30. Sinning C, Makarova N, Völzke H, Schnabel RB, Ojeda F, Dörr M, et al. Association of glycated hemoglobin A1c levels with cardiovascular outcomes in the general population: results from the BiomarCaRE (biomarker for cardiovascular risk assessment in Europe) consortium. Cardiovasc Diabetol. (2021) 20(1):223. doi: 10.1186/s12933-021-01413-4
31. Meng Q, Yang J, Wang F, Li C, Sang G, Liu H, et al. Development and external validation of nomogram to identify risk factors for CHD in T2DM in the population of northwestern China. Diabetes Metab Syndr Obes. (2023) 16:1271–82. doi: 10.2147/DMSO.S404683
32. Kim NH, Park Y, Kim NH, Kim SG. Weight-adjusted waist index reflects fat and muscle mass in the opposite direction in older adults. Age Ageing. (2021) 50(3):780–6. doi: 10.1093/ageing/afaa208
33. Coutinho T, Goel K, de Sá D C, Kragelund C, Kanaya AM, Zeller M, et al. Central obesity and survival in subjects with coronary artery disease: a systematic review of the literature and collaborative analysis with individual subject data. J Am Coll Cardiol. (2011) 57(19):1877–86. doi: 10.1016/j.jacc.2010.11.058
34. Bowman K, Atkins JL, Delgado J, Kos K, Kuchel GA, Ble A, et al. Central adiposity and the overweight risk paradox in aging: follow-up of 130,473 UK biobank participants. Am J Clin Nutr. (2017) 106(1):130–5. doi: 10.3945/ajcn.116.147157
35. Li M, Yu X, Zhang W, Yin J, Zhang L, Luo G, et al. The association between weight-adjusted-waist index and depression: results from NHANES 2005–2018. J Affect Disord. (2024) 347:299–305. doi: 10.1016/j.jad.2023.11.073
36. Li H, Fang G, Huang C, An W, Bai X, Huang Y. Association between the weight-adjusted waist index and serum uric acid: a cross-sectional study. Int J Clin Pract. (2023) 2023:8215866. doi: 10.1155/2023/8215866
37. Zhao P, Shi W, Shi Y, Xiong Y, Ding C, Song X, et al. Positive association between weight-adjusted-waist index and hyperuricemia in patients with hypertension: the China H-type hypertension registry study. Front Endocrinol (Lausanne). (2022) 13:1007557. doi: 10.3389/fendo.2022.1007557
38. Kim JY, Choi J, Vella CA, Criqui MH, Allison MA, Kim NH. Associations between weight-adjusted waist index and abdominal fat and muscle mass: multi-ethnic study of atherosclerosis. Diabetes Metab J. (2022) 46(5):747–55. doi: 10.4093/dmj.2021.0294
39. Cameron AJ, Zimmet PZ. Expanding evidence for the multiple dangers of epidemic abdominal obesity. Circulation. (2008) 117(13):1624–6. doi: 10.1161/CIRCULATIONAHA.108.775080
40. Kosmas CE, Bousvarou MD, Kostara CE, Papakonstantinou EJ, Salamou E, Guzman E. Insulin resistance and cardiovascular disease. J Int Med Res. (2023) 51(3):3000605231164548. doi: 10.1177/03000605231164548
41. Azumi H, Inoue N, Ohashi Y, Terashima M, Mori T, Fujita H, et al. Superoxide generation in directional coronary atherectomy specimens of patients with angina pectoris: important role of NAD(P)H oxidase. Arterioscler Thromb Vasc Biol. (2002) 22(11):1838–44. doi: 10.1161/01.ATV.0000037101.40667.62
42. Stocker R, Keaney JF. Role of oxidative modifications in atherosclerosis. Physiol Rev. (2004) 84(4):1381–478. doi: 10.1152/physrev.00047.2003
Keywords: weight adjusted waist index, coronary heart disease, NHANES, obesity, epidemiology
Citation: Liu Y, Sun S, Zou Q, Tao T, Li D, Han G and Wei Z (2025) Correlation between weight-adjusted waist index and coronary heart disease: NHANES 1999–2020. Front. Cardiovasc. Med. 11:1445802. doi: 10.3389/fcvm.2024.1445802
Received: 8 June 2024; Accepted: 19 December 2024;
Published: 10 January 2025.
Edited by:
Xintian Cai, People’s Hospital of Xinjiang Uygur Autonomous Region, ChinaReviewed by:
Jiabin Tu, Fujian Provincial Hospital, ChinaAo Wu, Shandong University of Traditional Chinese Medicine, China
Di Shen, People’s Hospital of Xinjiang Uygur Autonomous Region, China
Copyright: © 2025 Liu, Sun, Zou, Tao, Li, Han and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shougang Sun, c3Vuc2hnQGx6dS5lZHUuY24=
 Yan Liu
Yan Liu Shougang Sun2*
Shougang Sun2* Qi Zou
Qi Zou Guodong Han
Guodong Han