Abstract
Objective:
Preemptive thoracic endovascular aortic repair (TEVAR) has the potential to improve the prognosis of Stanford type B aortic dissection (TBAD), however it is important to determine whether it could be safely performed as a prophylactic treatment. This study aimed to determine the short- and long-term outcomes of preemptive TEVAR for uncomplicated TBAD with a small aortic aneurysm.
Design:
Retrospective multicenter analysis.
Methods:
We analyzed 212 patients with medically treated uncomplicated subacute TBAD between July 2004 and October 2019 in two Japanese academic centers. The short- and long-term prognosis of patients who underwent preemptive TEVAR and the changes in aortic diameter over time after TEVAR were analyzed. Aorta-related complications, aortic-related death and postoperative complications were recorded and analyzed. Analysis was performed on an intension-to-treat basis.
Results:
During follow-up, patients were divided into two groups: optimal medical treatment [OMT; n = 185 (87%)] and preemptive TEVAR [n = 27 (13%)]. In all cases, aortic enlargement was the reason for therapeutic intervention in the preemptive TEVAR group. Propensity score matching yielded a cohort of 27 control patients with OMT (group A) and 27 patients who underwent preemptive TEVAR (group B). Preoperative characteristics were similar between groups. In group B, only one patient developed type A dissection at a late stage and died from aortic rupture. Freedom from aortic-related death at 1/5/10 years was 100%/92%/92% in group B. Overall growth (mm/year) of max aorta was significantly smaller in the TEVAR group than in the control group (−3.7 ± 2.9 vs. 0.4 ± 5.6, p < 0.01), and the diameter of the false lumen was reduced (−8 ± 4.8 vs. −1.3 ± 8.0, p < 0.001).
Conclusions:
Short- and long-term outcomes of TEVAR for uncomplicated TBAD with a small aortic aneurysm were excellent, with few postoperative complications. After TEVAR, aortic remodeling was observed in the short term, suggesting that it may contribute to the prevention of aortic-related death due to rupture.
Introduction
Acute aortic dissection, the most common catastrophic aortic event affects per 100,000 people is around 2.9%–4.4% (1, 2), the number of cases reported from Japan ranges from 3 to 10% (3, 4). Although the traditional treatment methods for complicated acute Stanford type B aortic dissection such as impending rupture or a malperfusion syndrome have been surgical treatment, open graft replacement of the proximal descending thoracic aorta. In-hospital mortality is reported to be 29% when open surgery is performed in these patients with complicated acute TBAD (5, 6), and the outcome of treatment for acute complications still needs to be improved. On the other hand, the treatment of acute uncomplicated TBAD has historically been well established (7–9), and recent reports indicate that optimal medical treatment (OMT) is a treatment with good survival rates, including in the later phase (10). However, even with good survival rates in the remote stage, aortic complications such as developing aneurysms and rupture are not infrequent (11), and the pros and cons of early intervention are still being controversial (12). Subacute or chronic aneurysm enlargement in patients with uncomplicated TBAD has been reported to be treated with TEVAR with better outcomes and survival rates (13), however which patients are particularly well treated is not clear. We aggressively perform preemptive TEVAR in patients with uncomplicated TBAD who do not have large aortic diameters but have a tendency toward aneurysmal enlargement. The occurrence of acute complications and remote prognosis of these patients are unknown. In this study, we utilized the resources of two institutions to analyze the short-term and long-term outcomes of patients with uncomplicated TBAD treated with preemptive TEVAR compared with those undergoing OMT.
Methods
Approval from the Yamagata University Hospital Ethical Committee and patient written consent were obtained (Institutional Review Board #2018-245). All patients were informed about the use of their data for clinical research.
Two centers in Yamagata prefecture participated in this study. Data were collected between July 2004 and October 2019 in Yamagata University Hospital and between February 2016 and May 2019 in Nihonkai General Hospital (Figure 1). Acute aortic dissection was defined as a case in which the patient was examined and diagnosed with pain and other symptoms as the main complaints. Acute uncomplicated TBAD was defined as the absence of malperfusion (both dynamic obstruction, which is improved by false lumen decompression, and static obstruction, which is not improved) or signs of early disease progression (14) such as type A dissection presenting within 14 days of symptom onset. We divided the time course of aortic dissection into acute (<14 days), sub-acute (15–90 days), and chronic (>90 days) phases (14). For blood pressure control, all patients received an intravenous calcium-channel blocker, nitroglycerine, β-blockers or a combination of these after admission. The systolic blood pressure was controlled to less than 120 mmHg and the heart rate to less than 60, with careful monitoring of urine output starting 2 weeks after onset. Two weeks later, blood pressure was controlled to less than 130 mmHg and heart rate was in the range of 60 to 80. On admission, patients were treated in the intensive care unit (ICU) or a high care unit (HCU). All patients underwent contrast computed tomography (CT) scanning at emergency admission, and on the 1st and 7th days after admission. All patients were administered oral medications starting on the 1st day after CT screening, and were encouraged to take a short walk starting on the 7th day after onset. Patients were eligible for discharge 4 weeks after onset. That protocol was developed using Japanese guidelines as a reference (3). During follow-up, patients who had an aortic adverse event, despite medical management, underwent an aortic intervention. The term “aortic adverse event” includes enlargement of the aortic diameter (≥ 55 mm enlargement and/or ≥ 5 mm enlargement in 6 months), malperfusion and aortic rupture (3, 14). CT angiography (CTA) images at presentation and all follow-up CT scans were reviewed in all patients. The standard scan regimen was as follows: at symptom onset, at discharge, 3 or 6 months after discharge, 12 months after discharge and yearly thereafter. The scan regimen differed for individual patients, depending on findings. All imaging studies were reviewed for radiologic signs of adverse events, such as organ malperfusion or rupture, as well as imaging evidence of pathology resolution. A total of 212 CT images and their analysis were available for routine surveillance. Follow-up CT was absent in 9 patients. Four of them had plain CT, and 13 were excluded from the measurement of cross-sectional area because surgical intervention was performed before the first follow-up (one month after onset).
Figure 1
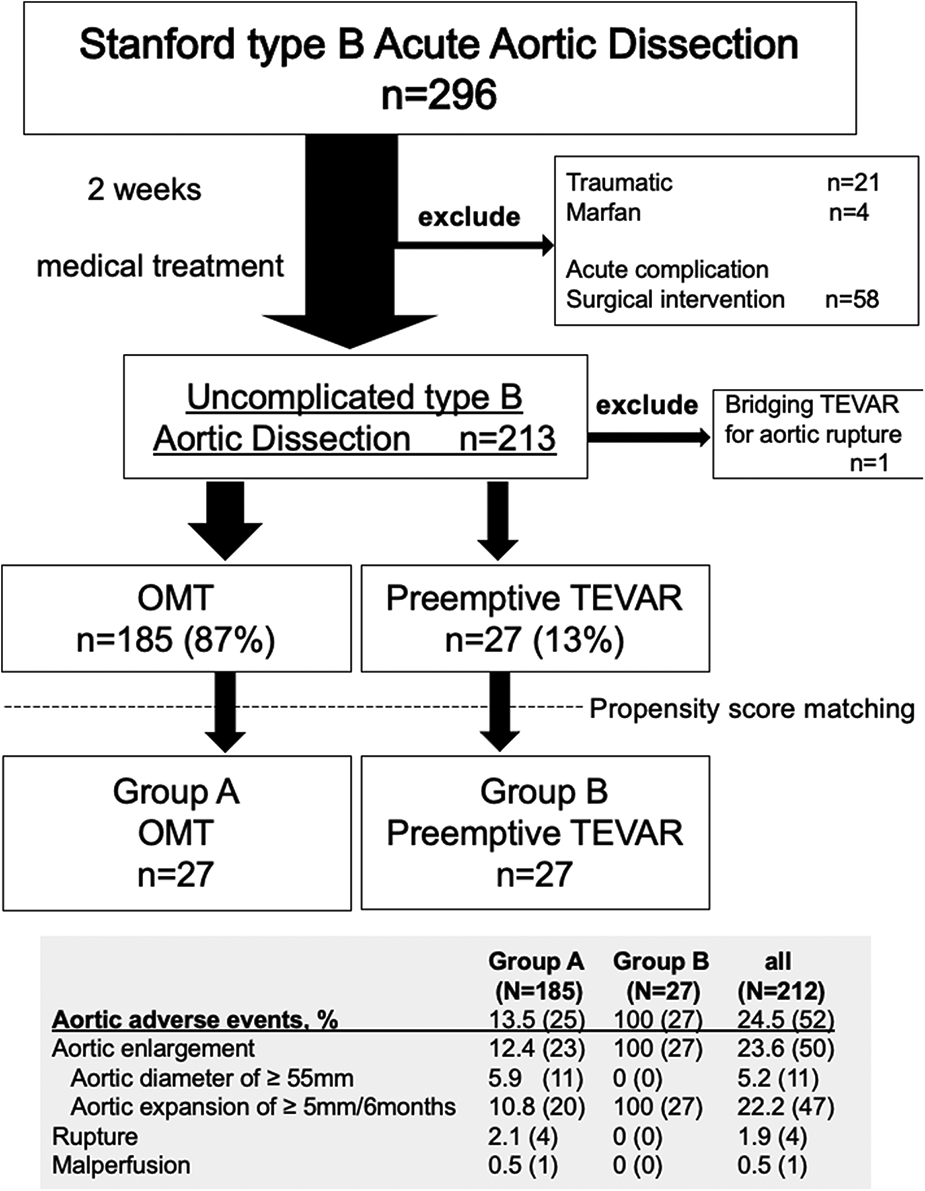
Flow diagram of the entire series of patients with Stanford type B aortic dissection. The study comprised 212 consecutive patients with uncomplicated type B aortic dissection. Propensity score matching was performed and adjusted for the background characteristics of each patient group. Patients were divided into 2 groups according to the treatment option, Group A consisted of 27 patients who received optimal medical treatment (OMT), and Group B consisted of 27 patients who underwent preemptive TEVAR.
Image analysis was performed on a SYNAPSE VINCENT system (Tokyo Japan, FUJIFILM Holding Corporation) with a dedicated 3D image analyzer. All CTA measurements were obtained using multiplanar reconstruction (MPR) images in an axial plane perpendicular to the aortic median centerline. The aortic median centerline was generated by software on the VINCENT system that uses a 3D algorithm to perform MPR centered on the contrast-enhanced aortic lumen. In no instance did the maximum aortic diameter occur in a non-dissected segment.
The areas of the true and false lumen were measured. Measurements were performed on the orthogonal cross-section in which the aortic diameter was maximal. Aortic growth rates were calculated by dividing the change in aortic diameter one year after TEVAR by the aortic diameter immediately after TEVAR.
The status of the false lumen on imaging was classified as patent if flow was present in the absence of thrombus. “Partial thrombosis” was evaluated at a later phase when an enhanced CT scan was performed and also classified as “patent”. “Complete thrombosis” was classified as “thrombosed” (15).
Intramural hematomas and penetrating aortic ulcers were not included in this study.
The incidence and percentage for variables of each classification were recorded using descriptive statistics. For continuous variables, we recorded the sample size, mean, standard deviation, and minimum and maximum values. Survival rates were compared using Kaplan– Meier curves and the log-rank test. This study was performed under intention-to-treat analysis. A matched group analysis was performed by propensity matching preemptive TEVAR cases and OMT cases. Propensity scores were generated using logistic regression analyses in 2 steps. Potential predictors were selected from a published data review, known confounding covariates for the outcomes of interest, differences between the 2 patients groups (Table 1), and clinical judgement. The groups were matched with age, sex, height, weight, body mass index, history of chronic obstructive pulmonary disease, hypertension, diabetes mellitus and stroke. Univariate analysis was performed using ANOVA or Student's t-test and the Chi-square test. The analysis of aortic diameter with follow-up outcomes was calculated with Repeated Measures Analysis of Variance (RMANOVA). All statistical analyses were performed with JMP software, ver. 17 (SAS Institute, Tokyo, Japan).
Table 1
| Patient characteristics | All patients (n = 212) | OMT (N = 27) | Preemptive TEVAR (N = 27) | p value |
|---|---|---|---|---|
| Age, y, mean ± SD | 70.0 ± 11.9 | 68.6 ± 15.2 | 68.0 ± 9.8 | 0.865 |
| Male, % | 70 (148 of 212) | 85 (23 of 27) | 70 (19 of 27) | 0.327 |
| Height, cm, mean ± SD | 161.0 ± 9.7 | 164.2 ± 8.6 | 161.8 ± 9.4 | 0.326 |
| Weight, kg, mean ± SD | 61.2 ± 14.5 | 68.2 ± 15.0 | 66.6 ± 14.5 | 0.7 |
| BMI (kg/m2), mean ± SD | 23.4 ± 4.3 | 25.2 ± 4.6 | 25.3 ± 4.4 | 0.914 |
| COPD, % | 39 (82 of 210) | 37 (10 of 27) | 33 (9 of 27) | 1 |
| HTN, % | 51 (109 of 212) | 70 (19 of 27) | 70 (19 of 27) | 1 |
| Diabetes mellitus, % | 6.1 (13 of 212) | 11.1 (3 of 27) | 14.8 (4 of 27) | 1 |
| History of stroke, % | 3.8 (8 of 212) | 0 (0 of 27) | 0 (0 of 27) | – |
| Renal insufficiency, % | 6.6 (14 of 212) | 0 (0 of 27) | 3.7 (1 of 27) | 1 |
| Coronary artery disease, % | 3.8 (8 of 212) | 3.7 (1 of 27) | 7.4 (2 of 27) | 1 |
| Hyperlipidemia, % | 33 (70 of 212) | 55.6 (15 of 27) | 44 (12 of 27) | 0.587 |
| Follow up period, months, mean ± SD | 55 ± 45 | 47.3 ± 40 | 51.3 ± 31 | 0.681 |
| Acute course of disease | ||||
| Delirium, % | 44 (89 of 204) | 26 (7 of 27) | 44 (11 of 25) | 0.245 |
| NPPV required, % | 27 (55 of 204) | 30 (8 of 27) | 40 (10 of 25) | 0.562 |
| Tracheal Intubation, % | 10 (20 of 204) | 0 (0 of 27) | 8 (2 of 25) | 0.226 |
| Positive pressure ventilation required (NPPV or mechanical ventilation), % | 32 (65 of 204) | 22 (6 of 27) | 32 (8 of 25) | 0.536 |
| ICU + HCU stay, day Mean ± SD | 6.2 ± 4.9 | 5.4 ± 4.6 | 5.9 ± 3.8 | 0.687 |
| Medication at discharge from the hospital | ||||
| Beta blockers n, % | 78 (161 of 207) | 92 (24 of 26) | 82 (22 of 27) | 0.25 |
| Angiotensin converting enzyme inhibitors, % | 19 (39 of 207) | 7.7 (2 of 26) | 22 (6 of 27) | 0.604 |
| Angiotensin II receptor blockers, % | 65 (135 of 207) | 69 (18 of 26) | 59 (16 of 27) | 0.569 |
| Calcium channel blockers, % | 77 (160 of 207) | 73 (19 of 26) | 67 (18 of 27) | 0.766 |
| Statins, % | 31 (64 of 207) | 58 (15 of 26) | 40 (11 of 27) | 0.276 |
| Steroids, % | 4 (8 of 211) | 0 (0 of 26) | 8 (2 of 26) | 0.236 |
| Anticoagulants, % | 5 (11 of 212) | 0 (0 of 27) | 4 (1 of 27) | 1 |
Baseline patient characteristics of all patients with or without an aortic adverse event.
TEVAR, thoracic endovascular aortic repair; OMT, optimal medical treatment; SD, standard deviation; BMI, body mass index; COPD, chronic obstructive pulmonary disease; HTN, hypertension; NPPV, noninvasive positive pressure ventilation; ICU; intensive care unit; HCU, high care unit.
Results
A total of 296 patients were admitted with a diagnosis of TBAD. A total of 212 medically treated patients were enrolled. All were Japanese, and 148 (70%) were males. The mean (range) age of all patients was 70 (62.0–79.0) years. The most common comorbidity was hypertension (n = 109, 51%); diabetes mellitus and renal insufficiency were present in 6.1% (13 of 212) and 6.6% (14 of 212) of the study population, respectively. The average observation period was 55 ± 45 months (Table 1). One of the patients who underwent TEVAR in the subacute phase had TEVAR as a bridging procedure until graft replacement for aortoesophageal fistula and was excluded from the present study.
During follow-up, a total of 27 patients (12.7%; group B) underwent preemptive TEVAR. All of the indications for TEVAR were aortic enlargement, none of which met the definition of maximum aortic diameter of >55 mm due to short-term enlargement. In the OMT group (group A), the incidence of aortic rupture was 1.9% (n = 4), and 1 patient (0.5%) required surgical management within 1 month of onset of dissection due to malperfusion (Figure 1). Four patients died from aortic adverse events (1.9%). An 84-year-old man died of aortobronchial fistulation 2 months after onset. He had dementia and could not stand by himself, and his family did not request surgical intervention. Three other patients died of aortic rupture, two of whom died after graft replacement. Aortic interventions were needed in 39 patients (18%), and these included 13 with graft replacement, 27 with endovascular repair, 2 with hybrid (open and endovascular) repair and 1 with an extra-anatomical bypass. Two patients who developed Stanford type A aortic dissection during the follow-up period underwent graft replacement, and both survived. One death occurred from type A dissection 14 months after preemptive TEVAR, but the relationship to TEVAR was unclear. The survival rate after surgical intervention was 92% (35 of 38). The 1-, 5-, and 10-year aortic-related death-free rates for groups A and B were 99%/94%/92% and 100%/92%/92%, respectively (Figure 2A).
Figure 2
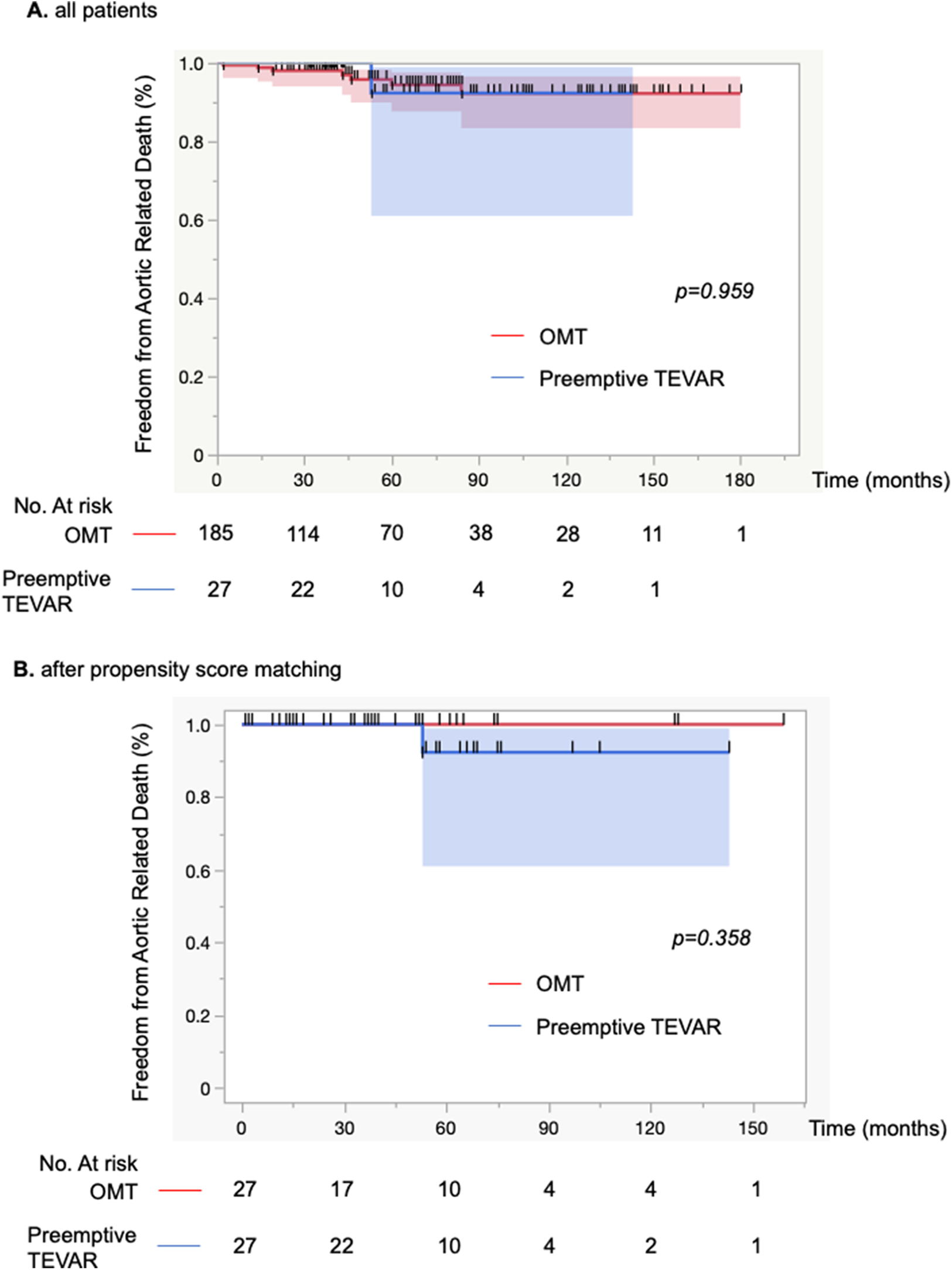
(A) Kaplan-Meier curve of freedom from aortic-related death for 212 patients. Optimal medical treatment (OMT) was performed on the basis of conservative treatment, and if aortic adverse events occurred during the course of the disease, graft replacement was performed. (B) Kaplan-Meier curve of freedom from aortic-related deaths for 54 patients. Propensity score matching was performed and adjusted for the background characteristics of each patient group. Comparisons between groups were made with the log-rank test.
Demographics, comorbid conditions, and dissection characteristics based on the initial CT findings were compared between groups with propensity matching (Tables 1, 2). There were no differences in patient's background. The Kaplan-Meier curve of freedom from aortic-related death after propensity matching also showed no significant difference between groups (Figure 2B). Marfan syndrome was present in 4 patients overall, and those patients were excluded from the analysis. Acute observations showed no difference between the groups in occurrence of delirium, tracheal intubation, and length of stay in the HCU and ICU (Table 1). At discharge, no differences between groups were detected in medication (Table 1). From the initial CT findings, no significant difference between groups was found in the number of intimal tears (group A: 1.8 ± 1.1 vs. group B: 2.3 ± 1.4, p = 0.21), but patent false lumen was more common in group A vs. group B [70% (19 of 27)] vs. 33% [9 of 27], respectively; p = 0.014; Table 2). There was no significant difference between groups in initial aortic diameter on admission (group A: 38 ± 6 vs. group B: 38 ± 6, p = 0.775), maximum aortic area (group A: 1,251 ± 557 mm2 vs. group B: 1,220 ± 363 mm2, p = 0.814) and the location of entry tears (Table 2) in the late-phase CT findings. The maximum aortic diameter at the initial CT scan of group B was small, with a maximum value of 47 mm and a minimum value of 20.5 mm. Aortic diameter was significantly enlarged in group B (group A: 1.8 ± 5.6 vs. group B: 10.8 ± 8, p < 0.0001). Primary entry was most common in the distal arch (78%, 21 of 27), followed by the celiac artery (15%, 4 of 27) and diaphragm levels (7%, 2 of 27). All TEVARs were performed with the objective of closing the primary entry, and 100% were successful. All false lumens at the location of the entry tear were thrombosed after TEVAR, and the average time required for complete thrombosis to form was 1.8 ± 2.1 months. Results of aortic diameter shows overall growth (mm/year) of max aorta was significantly smaller in the TEVAR group than the control group (−3.7 ± 2.9 vs. 0.4 ± 5.6, p < 0.01). The monthly post-TEVAR aortic diameter (within and between the group interaction) was significantly reduced after TEVAR (p < 0.001) (Figure 3C).
Table 2
| Initial findings | All patients (n = 212) | OMT (N = 26) | Preemptive TEVAR (N = 27) | p value |
|---|---|---|---|---|
| Aortic diameter on admission, mm, Mean ± SD | 38 ± 6 | 38 ± 6 | 38 ± 6 | 0.755 |
| Initial true and false lumen area of maximum aortic diameter (mm2) | 1,232 ± 495 | 1,223 ± 603 | 1,287 ± 350 | 0.634 |
| True lumen area of maximum aortic diameter (mm2) | 644 ± 284 | 640 ± 300 | 584 ± 253 | 0.469 |
| False lumen area of maximum aortic diameter (mm2) | 588 ± 404 | 629 ± 450 | 702 ± 275 | 0.478 |
| True lumen area ratio (true/false + true) | 0.58 ± 0.05 | 0.43 ± 0.19 | 0.018 | |
| Frue lumen area ratio (false/false + true) | 0.43 ± 0.23 | 0.56 ± 0.21 | 0.034 | |
| Entry located distal arch, % | 79 (157 of 200) | 73 (19 of 26) | 78 (21 of 27) | 0.757 |
| Entry located tracheal bifurcation level, % | 3 (6 of 200) | 0 (0 of 26) | 0 (0 of 27) | - |
| Entry located diaphragm level, % | 10 (20 of 200) | 23 (6 of 26) | 7 (2 of 27) | 0.142 |
| Entry located celiac artery level, % | 6 (12 of 200) | 0 (0 of 26) | 15 (4 of 27) | 0.111 |
| Entry located abdominal aorta, % | 3 (6 of 200) | 4 (1 of 26) | 0 (0 of 27) | 0.491 |
| Number of intimal tears | 1.7 ± 1.1 | 1.8 ± 1.1 | 2.3 ± 1.4 | 0.21 |
| Diameter of primary entry, mm, Mean ± SD | 12.7 ± 7.3 | 10.8 ± 3.3 | 12.7 ± 7.0 | 0.506 |
| Patent false lumen, % | 40 (85 of 212) | 33 (9 of 27) | 70 (19 of 27) | 0.014 |
| Follow-up findings | ||||
| Aortic growth rate (mm/month) | 0.48 ± 1.12 | 0.3 ± 0.93 | 1.8 ± 1.33 | <0.0001 |
Initial computed tomography findings for all patients with or without an aortic adverse event.
OMT, optimal medical treatment; TEVAR, thoracic endovascular aortic repair; SD, standard deviation.
Figure 3
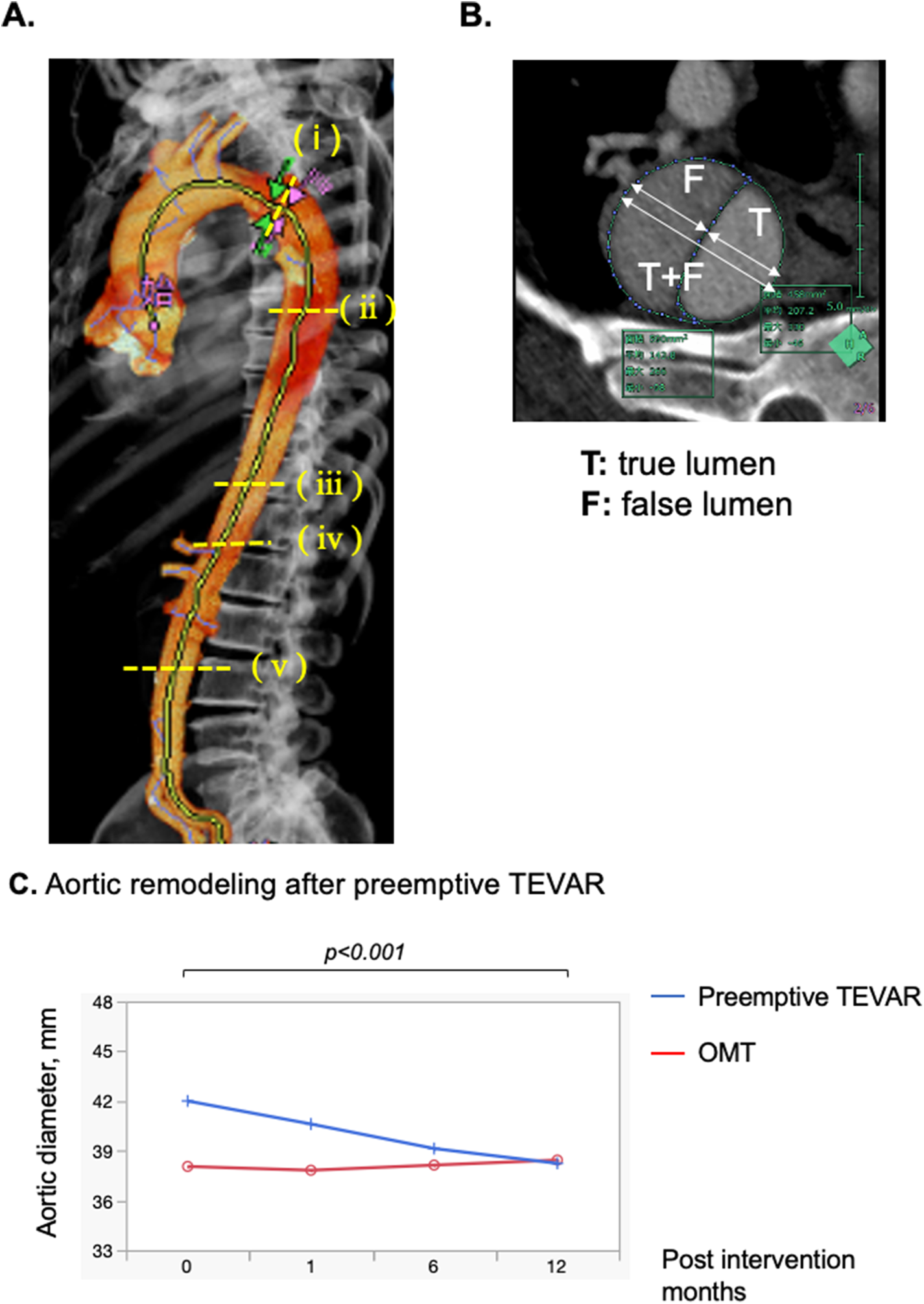
(A) Computed tomography (CT) measurement techniques. Aortic dissection was diagnosed via contrast-enhanced CT in all patients. On CT images obtained at admission, the aortic diameter and aortic area were measured, and false lumen patency was assessed. 3D image of the aorta after generation of an aortic centerline from the proximal aortic arch to the left iliac artery by software on the VINCENT system. The aortic diameters and area were measured at 5 locations based on the anatomical site, comprising the distal aortic arch, tracheal bifurcation, diaphragm, celiac artery and abdominal aorta (level of inferior mesenteric artery branch off). (B) Measurement of diameter of the true and false lumens and the diameter of the true and false lumens (C) maximum aortic diameter (mm) before TEVAR and 1, 3, 6, and 12 months after TEVAR were measured, and measurements were plotted. Compared with Preemptive TEVAR group and optimal medical treatment group. The 95% confidence interval for the mean is shown with the graph.
Although the diameter of the true lumen was not changed (3.5 ± 0.7 vs. 2.8 ± 7.1, p = 0.261), the diameter of the false lumen was reduced (−8 ± 4.8 vs. −1.3 ± 8.0, p < 0.001) (Table 3). Remodeling of the stenting site was significantly more pronounced in the first month (true lumen: 2.8 ± 2.7 mm, p < 0.0001, false lumen: −4.0 ± 3.9 mm, p < 0.0001, true + false lumen: −1.4 ± 2.2 mm, p < 0.0001) than in the subsequent six months for true lumen diameter, false lumen diameter and true + false lumen aortic diameter (true lumen: 0.7 ± 2.1 mm, p = 0.706, false lumen: −2.0 ± 2.9 mm, p = 0.912, true + false: −1.5 ± 2.0 mm, p = 0.0295; Supplementary Figure S1). Overall growth (mm/year) and growth rates (%) of distal aortic arch (DAA), level of tracheal bifurcation, diaphragm, celiac artery, and inferior mesenteric artery (IMA) after TEVAR were −4.8 ± 7.7 and (−11.5 ± 19.9), −4.2 ± 7.7 and (−10.7 ± 20.1), −2.6 ± 2.8 and (−6.9 ± 7.7), −1.1 ± 2.7 and (−3.2 ± 8.7), and 0 ± 2.5 and (0.6 ± 9.7), respectively in group A sub analysis (Table 4, Supplementary Figures S2–S6). Growth of the abdominal aorta was minimal. The true lumen tended to enlarge after TEVAR, with the DAA (2.7 ± 7.6 mm/year, 10.6 ± 27.3%) more enlarged and peripherally less enlarged (inferior mesenteric artery level: 1.1 ± 1.9 mm/year, 6.0 ± 11.1%). The diameter of the true lumen of the DAA (p = 0.0003) and the level of tracheal bifurcation (p = 0.0054) increased significantly during the first year after TEVAR, but no significant difference was observed in the periphery from the diaphragm. In contrast, false lumens tended to shrink after TEVAR, with a greater change in DAA (−7.1 ± 5.3 mm/year, −61.6 ± 35.7%) and less change in peripheral IMA levels (−0.9 ± 3.6 mm/year, 35.1 ± 251.2%) (Supplementary Figures S2–S5). In addition, the diameter of the false lumen of the DAA (p < 0.0001), level of tracheal bifurcation (p < 0.0001) and diaphragm (p < 0.0001) decreased significantly during the first year after TEVAR, but no significant difference was observed in the periphery from the celiac artery.
Table 3
| Image findings in 12 months | OMT (N = 26) | Preemptive TEVAR (N = 27) | p value |
|---|---|---|---|
| Aortic diameter before intervention | 38 ± 5 | 43 ± 7 | 0.0141 |
| Aortic growth (mm/year) | 0.4 ± 5.6 | −3.7 ± 2.9 | 0.0019 |
| Aortic growth rates (%) | 2.0 ± 12.5 | −8.7 ± 6.5 | 0.0003 |
| True lumen | |||
| Aortic growth (mm/year) | 2.8 ± 7.1 | 3.5 ± 0.7 | 0.261 |
| Aortic growth rate (%) | 15.8 ± 35.2 | 18.1 ± 15.0 | 0.763 |
| False lumen | |||
| Aortic growth (mm/year) | −1.3 ± 8.0 | −8 ± 4.8 | 0.0009 |
| Aortic growth rates (%) | −2.9 ± 63.1 | −65.7 ± 30.4 | <0.0001 |
Changes in aortic growth and growth rates.
OMT, optimal medical treatment; TEVAR, thoracic endovascular aortic repair.
Optimal medical treatment group vs. preemptive thoracic endovascular aortic repair group.
Table 4
| Aortic levels | True + False lumen | True lumen | False lumen | |||
|---|---|---|---|---|---|---|
| Aortic growth (mm/year) | Aortic growth rates (%) | Aortic growth (mm/year) | Aortic growth rates (%) | Aortic growth (mm/year) | Aortic growth rates (%) | |
| maximum aorta | −3.6 ± 3.0 | −8.7 ± 6.5 | 4.7 ± 3.5 | 18.1 ± 15.0 | −8.0 ± 4.8 | −65.8 ± 30.4 |
| distal aortic arch | −4.8 ± 7.7 | −11.5 ± 19.9 | 2.7 ± 7.6 | 10.6 ± 27.3 | −7.1 ± 5.3 | −61.6 ± 35.7 |
| tracheal bifurcation | −4.2 ± 7.7 | −10.7 ± 20.1 | 2.6 ± 8.4 | 12.6 ± 28.2 | −6.4 ± 5.6 | −50.2 ± 57.8 |
| diaphragm | −2.6 ± 2.8 | −6.9 ± 7.7 | 1.5 ± 3.7 | 7.6 ± 15.1 | −4.0 ± 3.7 | −44.7 ± 35.7 |
| celiac artery | −1.1 ± 2.7 | −3.2 ± 8.7 | 1.0 ± 3.0 | 6.7 ± 18.6 | −1.7 ± 4.9 | −22.1 ± 149.7 |
| inferior mesenteric artery | 0 ± 2.5 | 0.6 ± 9.7 | 1.1 ± 1.9 | 6.0 ± 11.1 | −0.9 ± 3.6 | 35.1 ± 251.2 |
Changes in aortic growth and growth rates.
TEVAR, thoracic endovascular aortic repair.
Sub analysis of post preemptive thoracic endovascular aortic repair.
Discussion
In this study, we showed that preemptive TEVAR for uncomplicated TBAD is associated with few postoperative complications, aneurysmal progression, and increased aortic remodeling.
Treatment of uncomplicated TBAD is divided into acute, subacute, and chronic phases depending on the time of disease onset. The 30-day mortality rate for acute type B aortic dissection (AD) is reported to be 10% (16), and malperfusion is responsible for half of this mortality (6). TEVAR in the acute treatment of TBAD is indicated when aortic complications occur, but it is reported that 50% of patients require intervention after 1 year; thus, further improvement is needed. The Investigation of Stent Grafts in Aortic Dissection (INSTEAD) trial comparing TEVAR + best medical treatment (BMT) with BMT alone is well-known for TEVAR outcomes in subacute uncomplicated TBAD, showing the superiority of endovascular aortic repair over BMT alone in terms of survival and remodeling in long-term outcomes, but the number of participants was small (136 patients with chronic dissection), and the use of TEVAR for subacute uncomplicated TBAD has not yet been established (12). A recent meta-analysis confirmed favorable results for uncomplicated and complicated TBAD, showing a significant early mortality advantage of TEVAR over open surgical reconstruction (7.3% vs. 19.0%, respectively; p = 0.024) (17). This was due to the lower incidence of paraplegia and stroke with TEVAR (18). Early intervention with TEVAR is a growing indication, but intervention in asymptomatic, uncomplicated patients should be done with caution, and it has not yet been established which patients should be treated. Recently reported predictive factors for aortic enlargement include the number of vessels originating from the false lumen (19) and the number of intercostal arteries (20). Factors that predict aortic events and mortality in patients with chronic TBAD include male sex (21), partial thrombosis of the false lumen (22), aortic diameter >40 mm (7, 23, 24), early aortic expansion after onset (25), younger age (13, 21, 26), a primary entry tear located on the concavity of the distal aortic arch (27) and dissection entry tears >10 mm (25). These risk factors are indications for surgical intervention (28, 29). There are many reports on the aortic diameter at the onset of acute TBAD, and it is an especially important independent risk factor (30); it is highly likely that the diameter of the aneurysm will increase over the long term in patients with an aortic diameter >40 mm at the site of dissection at the initial examination (10). In the case of a small aortic diameter, early complications from preemptive TEVAR are undesirable, but in the present study, patients had good outcomes after TEVAR, and no major complications, such as cerebral infarction or spinal cord paralysis, occurred. The reasons for this may include the fact that we adhered to the TEVAR instructions for use, cases considered technically difficult were not targeted for treatment, and many cases were treated in the subacute phase of the intervention (Table 5). Nienaber et al. reported acceptable early outcomes with preemptive TEVAR, with a mortality of 91.3% and incidence of neurologic adverse events of 4.2% (31). In our study, mortality from preemptive TEVAR within 1 year was 0%, and no adverse events occurred. This is thought to be due to an advantage in patient selection, and demonstrates that preemptive TEVAR can be performed safely when the indications are appropriate. Nienaber et al. reported an average maximum aortic diameter of 44.1 ± 9.6 mm for treated patients, whereas we treated patients with a smaller average diameter of 38 ± 6 mm. In our study, there were no treatment-related complications, indicating that preemptive TEVAR is safe and feasible in patients with early aortic dissection. Aortic remodeling was also mentioned by Nienaber et al., who found that 92.6% had remodeling within 1 year after stenting (31). Our results also showed true lumen expansion and false lumen shrinkage, with remodeling being greater on the proximal side and smaller on the distal side. In our study, early postoperative and long-term outcomes of preemptive TEVAR were favorable, with early aortic remodeling obtained. A particularly important finding of this study was that thrombosis of the largest false lumen of the aorta was obtained in all cases within 1 year after closure of the primary entry with TEVAR. Specifically, 80% (21 of 26) of these cases had thrombosis of the false lumen within 1 month after the procedure. The results of the current study also suggest that performing TEVAR before the dissected aorta undergoes anatomic changes that would result in a massive aneurysm may reduce the risk of mortality and aortic adverse events, such as rupture.
Table 5
| Preemptive TEVAR (N = 28) | |
|---|---|
| Subacute,% | 89 (24 of 27) |
| Chronic,% | 11 (3 of 27) |
Timing of therapeutic intervention.
TEVAR, thoracic endovascular aortic repair.
The term “preemptive TEVAR” is used when the strategy is an alternative to prevention and aims to prevent progression of the disease once it has occurred (12, 32). The TEVAR in our study is considered to be included in the preemptive TEVAR, as it is mainly a prophylactic aspect of aneurysm. The definition of “preemptive TEVAR” may require further discussion.
Our study is limited by its retrospective design, relatively small number of patients, incomplete follow-up (9%), and varying number of CT scans among patients. Three cases of Ulcer-like projection type were observed in the present study, all of which became patent false lumen type over time. Therefore, these three cases are classified as patent false lumen in this study. In some cases, surgical intervention was performed when aneurysmal enlargement was observed at a rate equivalent to 5 mm/6 months before 6 months after onset, so we have included 0.83 mm/month as a criterion for aneurysmal enlargement.
These findings highlight the potential of preemptive TEVAR to achieve early favorable outcomes and to prevent future aortic-related events. This work, however, is hypothesis generating and requires prospective validation in larger cohorts. Although our treatment was performed in accordance with the instructions for the use of stent grafts, further studies should be conducted to determine which patients have a better prognosis when preemptive TEVAR is performed.
Conclusions
Early postoperative complication and survival rates after preemptive TEVAR for subacute or early chronic TBAD were favorable. Rates of future aortic intervention were low, and preemptive TEVAR was performed safely, according to anatomic indications. TEVAR for TBADs with small aortic diameters results in early reduction of the false lumen and is particularly prone to remodeling in the aortic arch and proximal descending aorta. Preemptive TEVAR in appropriate cases may be an progressive option for subacute or chronic TBAD.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The studies involving humans were approved by Yamagata University Hospital Ethical Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
KN: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal Analysis, Data curation, Conceptualization. KK: Writing – original draft, Data curation. SN: Writing – original draft, Data curation. RS: Writing – original draft, Supervision, Formal Analysis. SA: Writing – original draft, Data curation. AI: Writing – original draft, Data curation. DW: Writing – original draft, Data curation. SH: Writing – original draft, Data curation. EO: Writing – original draft, Data curation. MM: Writing – original draft, Data curation. YK: Writing – original draft, Data curation. CK: Writing – original draft, Formal Analysis, Data curation. HU: Writing – original draft, Formal Analysis. TS: Writing – review & editing, Validation, Supervision. TU: Writing – review & editing, Validation, Supervision, Investigation, Formal Analysis.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank JAM Post (www.jamp.com) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1442800/full#supplementary-material
Supplemental Figures
Combined diameter of the true and false lumens and the diameter of the true and false lumens before TEVAR and 1, 3, 6, and 12 months after TEVAR were measured, respectively.
Supplementary Figure S1Maximum aortic diameter.
Supplementary Figure S2Distal arch of the aorta.
Supplementary Figure S3Tracheal bifurcation level of the aorta.
Supplementary Figure S4Diaphragm level of the aorta.
Supplementary Figure S5Celiac artery level of the aorta.
Supplementary Figure S6Inferior mesenteric artery level of the aorta.
Abbreviations
TBAD, Stanford type B aortic dissection; TEVAR, thoracic endovascular aortic repair; OMT, optimal medical treatment; CT, computed tomography; ICU, intensive care unit; HCU, high care unit; CTA, CT angiography; IMA, inferior mesenteric artery; BMT, best medical treatment; MPR, multiplanar reconstruction; BMI, body mass index; COPD, chronic obstructive pulmonary disease; HTN, hypertension; NPPV, noninvasive positive pressure ventilation.
References
1.
MészárosIMóroczJSzláviJSchmidtJTornóciLNagyLet alEpidemiology and clinicopathology of aortic dissection. Chest. (2000) 117:1271–8. 10.1378/chest.117.5.1271
2.
BrooksM. Review of studies reporting the incidence of acute type B aortic dissection. Hearts. (2020) 1:152–65. 10.3390/hearts1030016
3.
OginoHIidaOAkutsuKChibaYHayashiHIshibashi-UedaHet alJCS/JSCVS/JATS/JSVS 2020 guideline on diagnosis and treatment of aortic aneurysm and aortic dissection. Circ J. (2023) 87(10):1410–621. 10.1253/circj.CJ-22-0794
4.
Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery, ShimizuHOkadaMTangokuADokiYEndoSFukudaHet alThoracic and cardiovascular surgeries in Japan during 2017: annual report by the Japanese association for thoracic surgery. Gen Thorac Cardiovasc Surg. (2020) 68:414–49. 10.1007/s11748-020-01298-2
5.
TsaiTTFattoriRTrimarchiSIsselbacherEMyrmelTEvangelistaAet alLong-term survival in patients presenting with type B acute aortic dissection: insights from the international registry of acute aortic dissection. Circulation. (2006) 114:2226–31. 10.1161/CIRCULATIONAHA.106.622340
6.
TrimarchiSNienaberCARampoldiVMyrmelTSuzukiTBossoneEet alRole and results of surgery in acute type B aortic dissection: insights from the international registry of acute aortic dissection (IRAD). Circulation. (2006):1357–64. 10.1161/CIRCULATIONAHA.105.000620
7.
DailyPOTruebloodHWStinsonEBWuerfleinRDShumwayNE. Management of acute aortic dissections. Ann Thorac Surg. (1970) 10:237–47. 10.1016/s0003-4975(10)65594-4
8.
CoselliJS. Thoracoabdominal aortic aneurysms: experience with 372 patients. J Card Surg. (1994) 9:638–47. 10.1111/j.1540-8191.1994.tb00898.x
9.
UmañaJPLaiDTMitchellRSMooreKARodriguezFRobbinsRCet alIs medical therapy still the optimal treatment strategy for patients with acute type B aortic dissections?J Thorac Cardiovasc Surg. (2002) 124:896–910. 10.1067/mtc.2002.123131
10.
NakamuraKUchidaTShoRHamasakiAHayashiJSadahiroM. Analysis of risk factors for aortic enlargement in patients with chronic type B aortic dissection. Ann Vasc Dis. (2018) 11:490–5. 10.3400/avd.oa.18-00115
11.
DurhamCACambriaRPWangLJErgulEAAransonNJPatelVIet alThe natural history of medically managed acute type B aortic dissection. J Vasc Surg. (2015) 61:1192–8. 10.1016/j.jvs.2014.12.038
12.
NienaberCAKischeSRousseauHEggebrechtHRehdersTCKundtGet alEndovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv. (2013) 6:407–16. 10.1161/CIRCINTERVENTIONS.113.000463
13.
LuebkeTBrunkwallJ. Type B aortic dissection: a review of prognostic factors and meta-analysis of treatment options. Aorta Stamford Conn. (2014) 2:265–78. 10.12945/j.aorta.2014.14-040
14.
ErbelRAboyansVBoileauCBossoneEDi BartolomeoREggebrechtHet al2014 ESC guidelines on the diagnosis and treatment of aortic diseases. Kardiol Pol. (2014) 72:1169–252. 10.5603/KP.2014.0225
15.
HughesGCAndersenNDMcCannRL. Management of acute type B aortic dissection. J Thorac Cardiovasc Surg. (2013) 145:S202–7. 10.1016/j.jtcvs.2012.11.078
16.
TrimarchiSTolenaarJLTsaiTTFroehlichJPegorerMUpchurchGRet alInfluence of clinical presentation on the outcome of acute B aortic dissection: evidences from IRAD. J Cardiovasc Surg (Torino). (2012) 53:161–8.
17.
MoulakakisKGMylonasSNDalainasIKakisisJKotsisTLiapisCD. Management of complicated and uncomplicated acute type B dissection. A systematic review and meta-analysis. Ann Cardiothorac Surg. (2014) 3:234–46. 10.3978/j.issn.2225-319X.2014.05.08
18.
ThrumurthySGKarthikesalingamAPattersonBOHoltPJEHinchliffeRJLoftusIMet alA systematic review of mid-term outcomes of thoracic endovascular repair (TEVAR) of chronic type B aortic dissection. Eur J Vasc Endovasc Surg Off J Eur Soc Vasc Surg. (2011) 42:632–47. 10.1016/j.ejvs.2011.08.009
19.
KammanAVBrunkwallJVerhoevenELHeijmenRHTrimarchiStrialistsADSORB. Predictors of aortic growth in uncomplicated type B aortic dissection from the acute dissection stent grafting or best medical treatment (ADSORB) database. J Vasc Surg. (2017) 65:964–971.e3. 10.1016/j.jvs.2016.09.033
20.
SailerAMvan KuijkSMJNelemansPJChinASKinoAHuiningaMet alComputed tomography imaging features in acute uncomplicated Stanford type-B aortic dissection predict late adverse events. Circ Cardiovasc Imaging. (2017) 10:e005709. 10.1161/CIRCIMAGING.116.005709
21.
TolenaarJLvan KeulenJWJonkerFHWvan HerwaardenJAVerhagenHJMollFLet alMorphologic predictors of aortic dilatation in type B aortic dissection. J Vasc Surg. (2013) 58:1220–5. 10.1016/j.jvs.2013.05.031
22.
TsaiTTEvangelistaANienaberCAMyrmelTMeinhardtGCooperJVet alPartial thrombosis of the false lumen in patients with acute type B aortic dissection. N Engl J Med. (2007) 357:349–59. 10.1056/NEJMoa063232
23.
OnitsukaSAkashiHTayamaKOkazakiTIshiharaKHiromatsuSet alLong-term outcome and prognostic predictors of medically treated acute type B aortic dissections. Ann Thorac Surg. (2004) 78:1268–73. 10.1016/j.athoracsur.2004.02.031
24.
van BogerijenGHWTolenaarJLRampoldiVMollFLvan HerwaardenJAJonkerFHWet alPredictors of aortic growth in uncomplicated type B aortic dissection. J Vasc Surg. (2014) 59:1134–43. 10.1016/j.jvs.2014.01.042
25.
SailerAMNelemansPJHastieTJChinASHuiningaMChiuPet alPrognostic significance of early aortic remodeling in acute uncomplicated type B aortic dissection and intramural hematoma. J Thorac Cardiovasc Surg. (2017) 154:1192–200. 10.1016/j.jtcvs.2017.04.064
26.
NakamuraKUchidaTHamasakiASadahiroM. How should we treat uncomplicated subacute type B aortic dissection in octogenarians?J Cardiothorac Surg. (2019) 14:44. 10.1186/s13019-019-0869-z
27.
CambriaRPConradMF. Thoracic endovascular aneurysm repair for uncomplicated type B dissection. J Vasc Surg. (2016) 64:1558–9. 10.1016/j.jvs.2016.08.002
28.
SchwartzSIDurhamCClouseWDPatelVILancasterRTCambriaRPet alPredictors of late aortic intervention in patients with medically treated type B aortic dissection. J Vasc Surg. (2018) 67:78–84. 10.1016/j.jvs.2017.05.128
29.
LeeJHJungJCSohnBChangHWKimDJKimJSet alChanges in aortic growth rate and factors influencing aneurysmal dilatation after uncomplicated acute type B aortic dissection. Interact Cardiovasc Thorac Surg. (2022) 35:ivac126. 10.1093/icvts/ivac126
30.
GeragotellisAAl-TawilMJubouriMTanSZCPWilliamsIBashirM. Risk profile analysis of uncomplicated type B aortic dissection patients undergoing thoracic endovascular aortic repair: laboratory and radiographic predictors. J Card Surg. (2022) 37:2811–20. 10.1111/jocs.16655
31.
NienaberCAKischeSAkinIRousseauHEggebrechtHFattoriRet alStrategies for subacute/chronic type B aortic dissection: the investigation of stent grafts in patients with type B aortic dissection (INSTEAD) trial 1-year outcome. J Thorac Cardiovasc Surg. (2010) 140:S101–8. 10.1016/j.jtcvs.2010.07.026
32.
SachsCVecchiniFCorniquetMBartoliMBarralP-AMasiMet alPreemptive treatment in the acute and early subacute phase of uncomplicated type B aortic dissections with poor prognosis factors. Front Cardiovasc Med. (2024) 11:1362576. 10.3389/fcvm.2024.1362576
Summary
Keywords
uncomplicated Stanford type B aortic dissection, conservative treatment, preemptive thoracic endovascular aortic repair, aortic aneurysm, aortic remodeling
Citation
Nakamura K, Kobayashi K, Nakai S, Sho R, Arai S, Ishizawa A, Watanabe D, Hirooka S, Ohba E, Mizumoto M, Kuroda Y, Kim C, Uchino H, Shimanuki T and Uchida T (2024) Safe and favorable prognosis of thoracic endovascular aortic repair for the low-risk patients with non-acute type B aortic dissection. Front. Cardiovasc. Med. 11:1442800. doi: 10.3389/fcvm.2024.1442800
Received
02 June 2024
Accepted
14 September 2024
Published
28 October 2024
Volume
11 - 2024
Edited by
Lin Chang, University of Michigan, United States
Reviewed by
Yasunori Iida, Saiseikai Yokohamashi Tobu Hospital, Japan
Jia Hu, Sichuan University, China
Updates
Copyright
© 2024 Nakamura, Kobayashi, Nakai, Sho, Arai, Ishizawa, Watanabe, Hirooka, Ohba, Mizumoto, Kuroda, Kim, Uchino, Shimanuki and Uchida.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ken Nakamura ken.nakamura622@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.