
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 18 November 2024
Sec. General Cardiovascular Medicine
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1439239
 Jie An1,†
Jie An1,† Zikan Zhong2,†
Zikan Zhong2,† Bingquan Xiong3
Bingquan Xiong3 Dandan Yang4
Dandan Yang4 Youquan Li5
Youquan Li5 Ya Luo1
Ya Luo1 Hao Li1
Hao Li1 Yang Jiao1
Yang Jiao1 Genqing Zhou2
Genqing Zhou2 Min Xu1
Min Xu1 Shaowen Liu2*
Shaowen Liu2* Jie Li1*
Jie Li1*
Background: The prognostic significance of utilizing both the systemic inflammatory response index (SIRI) and pulse pressure (PP) collectively in assessing cardiovascular mortality (CVM) across populations remains to be elucidated.
Methods: Multivariate Cox proportional hazards analysis investigated the SIRI, PP, and CVM association. Receiver operating characteristic (ROC) curves evaluated the predictive performance of the combined SIRI and PP for CVM in the broader demographic. Subsequently, the area under the ROC curve (AUC) was compared using the Z-test, and a novel nomogram was developed to assess its accuracy in predicting CVM. Restricted cubic spline (RCS) was used to evaluate the association between SIRI and PP.
Results: The study involved 19,086 NHANES database individuals, with 9,531 males (49.94%). During the follow-up period, 456 CVM instances (2.39%) occurred. Multivariate Cox proportional hazards analysis revealed both the SIRI [adjusted hazard ratio (HR) 1.16, P < 0.001] and PP (HR = 1.01, P = 0.004) as independent CVM predictors. A 0.1-unit SIRI increase and 10 mmHg PP escalation correlated with 2% (adjusted HR = 1.02, P < 0.001) and 7% (adjusted HR = 1.07, P = 0.004) CVM enhancements, respectively. The combined SIRI and PP area under the curve was 0.77, ranging from 0.77 to 0.79 in female cohorts, non-smokers, and non-pathological contexts. High SIRI and PP, either high SIRI or PP, were associated with 3 and 2 times the CVM risk compared to low SIRI and PP. Adding the SIRI and PP to general risk factors improved CVM predictive efficacy (Z = 4.17, P < 0.001). The novel nomogram's concordance index was 0.90, indicating excellent discrimination. The predicted probabilities’ calibration plot aligned with actual CVM rates at 1, 5, and 10 years. RCS showed an S-shaped relationship between SIRI and PP.
Conclusions: Integrating the SIRI with PP demonstrates substantial predictive efficacy for CVM within the broader United States community, notably in female cohorts, non-smokers, and non-pathological contexts.
Cardiovascular disease (CVD) poses a significant global health challenge, accounting for approximately 22.9% of all deaths (1). The rising incidence of CVD necessitates effective diagnostic and prognostic tools. Chronic inflammation is central to CVD pathogenesis, contributing to endothelial dysfunction, atherosclerosis, and increased cardiovascular mortality (CVM) (2–5). Among inflammatory markers, the systemic inflammatory response index (SIRI) has emerged as a notable predictor of adverse cardiovascular outcomes. Elevated SIRI levels correlate with worse cardiovascular outcomes, indicating its potential as a diagnostic marker of inflammatory status and its implications for cardiovascular health (2, 6–9).
Simultaneously, pulse pressure (PP) often occupies a subordinate position in clinical assessments compared to its systolic and diastolic counterparts. Nonetheless, it is a pivotal indicator of arterial stiffness and cardiovascular risk. Beyond its portrayal of arterial blood flow culpability, PP provides intricate insights into the dynamic interplay among cardiac output, vascular compliance, and peripheral resistance. Extensive literature has elucidated the close association of PP with the risk of stroke, myocardial infarction, heart failure, diabetes, and CVM (10–14). However, investigations into the relationship between PP and CVM in elderly patient cohorts remain limited, yielding disparate findings (15). Thus, PP serves not only as a measure of blood pressure but also as an indicator of underlying vascular pathology.
The interplay between SIRI and PP is compelling, as both metrics reflect distinct yet interrelated aspects of cardiovascular health. SIRI encapsulates systemic inflammation, while PP provides insights into hemodynamic changes and vascular compliance. Chronic inflammation may induce vascular remodeling and stiffening (16), potentially linking elevated SIRI with increased PP in at-risk populations. Additionally, inflammation disrupts blood pressure regulation, contributing to elevations in both systolic and diastolic pressures, thereby influencing PP.
Despite the established prognostic value of SIRI and PP, research exploring their combined predictive power for CVM is limited. Utilizing both SIRI and PP as complementary indicators could enhance risk stratification, aiding clinicians in identifying individuals at heightened risk for cardiovascular events. Ultimately, in this context, we propose a novel clinical prediction model that incorporates both PP and SIRI. Prior nomograms have demonstrated an AUC ranging from 0.70 to 0.84 in predicting all-cause mortality and CVM. We aim to investigate a novel nomogram that integrates SIRI and PP to ascertain whether it offers enhanced diagnostic value in forecasting CVM, potentially providing clinicians with a more robust tool for identifying individuals at increased risk (17–19).
NHANES, an acronym for the National Health and Nutrition Examination Survey in the US, integrates an extensive range of data collection methodologies. These encompass questionnaire surveys, physical examinations, analysis of biological specimens, and nutritional assessments. This survey serves as a pivotal tool for gaining insights into the health status of the American populace, assessing the efficacy of public health policies, and informing strategic initiatives in clinical practice. Our study utilized data spanning from 2007 to 2016, which can be accessed through the official website. All data are integrated using unique respondent sequence number (SEQN) identifiers, with follow-up outcomes recorded up to December 31, 2019. Following the exclusion of missing values and outliers, a total of 19,086 individuals were included in the study cohort. A detailed flowchart depicting participant selection is provided in Supplementary Figure 1.
Data were collected by matching unique SEQN identifiers and including information on age, gender, race, educational attainment, marital status, smoking status, drinking habits, income-to-poverty ratio (PIR ≥ 2.5, <2.5), diabetes mellitus (DM), congestive heart failure (CHF), cancer, coronary heart disease (CHD), hypertension, stroke, chronic kidney disease (CKD), body mass index (BMI), daily energy intake, sleep time, systemic inflammatory response index (SIRI), systemic immune-inflammation index (SII), systemic immune-inflammation response index (SIIRI), systolic blood pressure (SBP), diastolic blood pressure (DBP), mean artery pressure (MAP), pulse pressure (PP), and cardiovascular mortality. Data containing anomalies or missing values is excluded. Drinking habits are operationally defined as the consumption of a minimum of 12 alcoholic beverages within the preceding year. Smoking status is operationally defined as having a cumulative history of tobacco consumption amounting to a minimum of 100 cigarettes for one's lifetime. Body mass index (BMI) is computed by dividing an individual's weight (measured in kilograms) by the square of their height (measured in meters) (20). SBP and DBP were assessed on at least three separate occasions, and the mean of these measurements was determined by at least two researchers. The calculation formulas for SIRI, SII, and SIIRI are as follows (21):
The study's endpoint is CVM, which signifies death from disorders and conditions affecting the heart and blood vessels. This includes fatal occurrences like heart attacks, strokes, and other cardiovascular diseases.
Continuous variables that adhere to a normal distribution are presented as mean ± standard deviation. Analysis entails either an independent two-sample t-test or an analysis of variance. Conversely, median and interquartile ranges are utilized for non-normally distributed data, and analysis is conducted using non-parametric tests. Categorical data are represented as percentages and analyzed employing the chi-square test. Univariate and multivariate Cox proportional hazards regression analyses were employed to examine the risk factors linked with CVM. The area under the receiver operating characteristic (ROC) curve (AUC) served to evaluate the predictive capacity for mortality. Cut-off values (i.e., the optimal threshold corresponding to the maximum Youden index) were then calculated for SIRI and PP. Participants were categorized into high SIRI and low SIRI, high PP and low PP, low SIRI and PP, either high SIRI or PP, and both high SIRI and PP based on these cut-off values. A sensitivity analysis was conducted to explore the robust predictive value of SIRI combined with PP for cardiovascular mortality. This was done in various risk factor models, excluding individuals with a follow-up period of less than 3 years, and in populations where missing SIRI and PP values were imputed. Kaplan-Meier curves were plotted accordingly and the differences between groups were compared using the log-rank test. Calculate the AUC for the SIRI and PP combined to assess their predictive value for CVM, as well as in subgroup analyses for different populations. The ROC curve AUC was compared using the Z-test. RCS was utilized to investigate the nonlinear associations between continuous SIRI and PP. The determination of the number and placement of knots in the RCS was based on the Akaike information criterion (AIC), which helps to find a balance between achieving a good fit and avoiding overfitting (22). By jointly constructing a Nomogram risk prediction model based on multiple independent risk factors, we utilize the C-index to assess discrimination. The C-index is evaluated by conducting 1,000 bootstrap samples to calculate the index, with values ranging between 0.5 and 1. A value of 0.5 indicates complete inconsistency, suggesting that the model lacks predictive power, while a value of 1 signifies perfect consistency, indicating that the model's predictions align entirely with the actual outcomes (23). Additionally, we utilized calibration curves to assess the concordance between the Nomogram's predictions of CVM at 1, 5, and 10 years and the observed mortality rates. The NHANES population was divided into a training set and a validation set to enhance the robustness of model validation (24). All statistical analyses were conducted utilizing SPSS 26, GraphPad Prism 8.0, and R 4.3.1. Two-sided P-values < 0.05 indicate significance.
Out of 50,588 data points, 28,675 had missing or anomalous values, and 2,827 were missing SIRI and PP. Finally, 19,086 participants were included in the analysis, with 456 deaths (2.39%). The distribution included individuals age (48.9 ± 17.5 years), males (49.94%), Non-Hispanic White ethnicity (44.86%), individuals with a college degree or above (53.83%), widowed/divorced/separated individuals (21.43%), smokers (44.96%), individuals reporting drinking (72.82%), and so on. Detailed information can be found in Table 1. The SIRI and PP did not follow a normal distribution, with median and interquartile range values for SIRI being 1.02 (0.69–1.50) and for PP 50 (41–61), respectively. Based on the cutoff values of SIRI (1.55) and PP (60 mmHg), the population was divided into three groups (low SIRI and PP, either high SIRI or PP, and both high SIRI and PP). In the high SIRI and high PP groups, there were higher proportions of individuals age, smokers, individuals with PIR <2.5, and higher rates of diseases (DM, CHF, CHD, stroke, cancer, CKD, hypertension). Additionally, these individuals exhibited higher BMI and higher SBP. High SIRI and high PP, either high SIRI or high PP, and low SIRI and low PP groups had CVM rates of 9.38%, 3.61%, and 0.66%, respectively. The intergroup differences were statistically significant (P < 0.001), as shown in the Table 2. In the Supplementary Table 1, it was observed that the rates of smoking, drinking, DM, CHF, CKD, and CVM were lower in females when grouped by gender.
Incorporating indicators such as SIRI, SII, and SIIRI for assessing CVM, the AUC values were 0.67, 0.60, and 0.62 (Supplementary Figure 2). Incorporating indicators such as PP, SBP, DBP, and MAP for assessing CVM, the AUC values were 0.73, 0.67, 0.61, and 0.52, respectively (Supplementary Figure 3). There were significant differences observed between the alive group and the CVM group in both SIRI [1.00 (0.69–1.48) vs. 1.54 (0.97–2.26), P < 0.001] and PP [50 (41–61) vs. 66 (53–81), P < 0.001]. RCS showed an S-shaped relationship between SIRI and PP (P < 0.0001). When SIRI exceeded 0.51, PP sharply increased as SIRI increased, followed by a gradual rise (Figure 1). The cutoff value for SIRI was 1.55, with a sensitivity of 50.0% and a specificity of 77.4%. For PP, the cutoff value was 60 mmHg, with a sensitivity of 65.4% and a specificity of 73.0%. When combining SIRI and PP, the AUC was 0.77 (95% CI: 0.75–0.80), with a sensitivity of 70.2% and specificity of 74.4% (Figure 2). Using the Z-test to compare the area under the ROC curve (AUC), statistical differences were found between the combined SIRI and PP and SIRI (Z = 6.78, P < 0.001), as well as between the combined SIRI and PP and PP (Z = 6.86, P < 0.001) (Supplementary Table 2a).
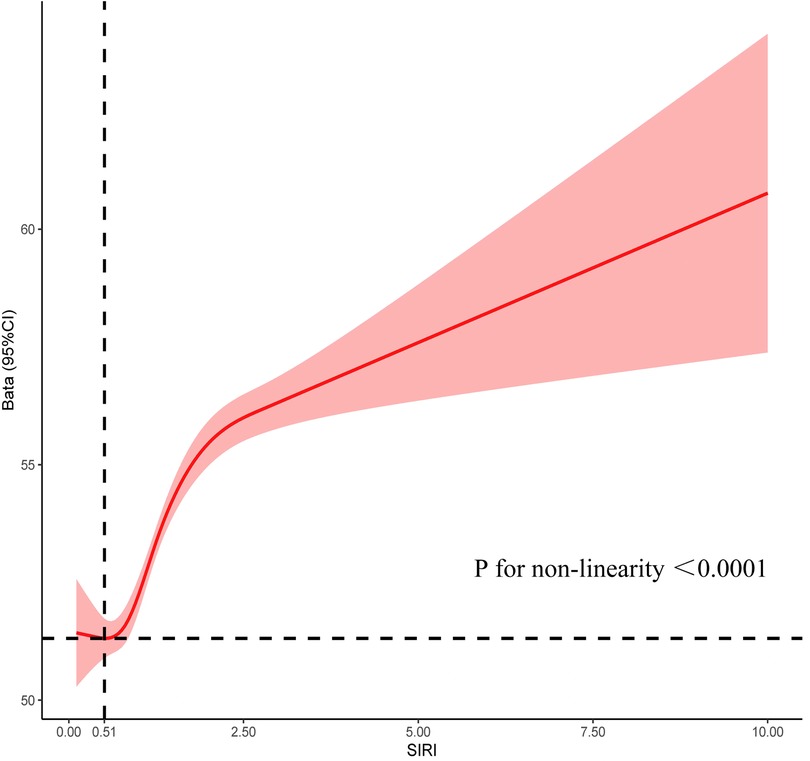
Figure 1. Restrictive cubic spline explores the relationship between SIRI and PP, with SIRI on the X-axis and PP on the Y-axis.
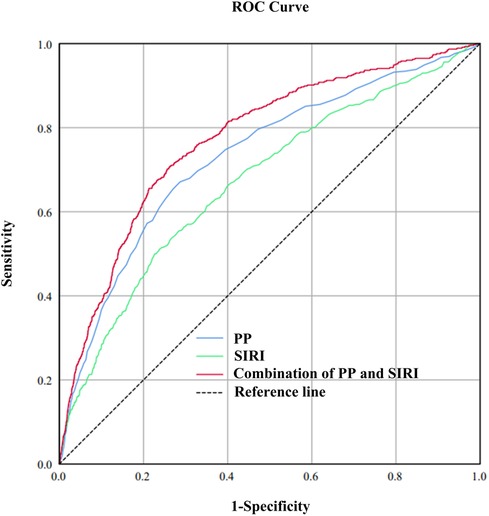
Figure 2. To compare the predictive value of SIRI, PP, and the combined SIRI and PP for cardiovascular mortality, the area under the receiver operating characteristic (ROC) curve (AUC) was calculated. The calculated AUC values were 0.67, 0.73, and 0.77 for SIRI, PP, and the combined SIRI and PP, respectively.
Multivariate Cox regression analysis revealed that gender, age, race, marital status, DM, CHF, CHD, cancer, hypertension, smoke status, PIR, SIRI, and PP were independent risk factors for CVM (Table 3). The general risk factors are defined as age, gender, race, marital status, DM, CHF, CHD, cancer, hypertension, and PIR. Here are the definitions for the groups: Group 1: High SIRI and high PP; Group 2: Either high SIRI or high PP; Group 3: Low SIRI and low PP. In the unadjusted model, a 0.1-unit increase in SIRI had an HR of 1.03, and a 10 mmHg increase in PP had an HR of 1.47. When comparing Group 1 to Group 3, the HR was 18.18. For comparisons of Group 2 against Group 1, the HR was 5.97. In model 1, adjusting for age and gender, for each 0.1-unit increase in SIRI, the HR was 1.02. For every 10 mmHg increase in PP, the HR was 1.10. When comparing Group 3 with Group 1, the HR was 2.28. The comparison of Group 2 with Group 1 resulted in an HR of 3.80. In model 2, adjusting for general risk factors and PP, for each 0.1-unit increase in SIRI, the HR was 1.02. Additionally, adjusting for general risk factors and SIRI, for every 10 mmHg increase in PP, the HR was 1.07. When adjusting for general risk factors and comparing Group 3 with Group 1, the HR was 3.17. The comparison of Group 2 with Group 1 resulted in an HR of 1.99. The comparison among these three groups yielded a P for trend <0.001 (Table 4).
Imputation of missing values for SIRI and PP: The HR for Group 3 vs. Group 1 in the unadjusted model, Model 1, and Model 2 were 13.60, 2.83, and 2.45, respectively. For Group 2 vs. Group 1, the HRs were 4.97, 1.91, and 1.71, respectively. After excluding data with follow-up times of less than 3 years, the HR for Group 3 vs. Group 1 in the unadjusted model, Model 1, and Model 2 were 14.86, 3.24, and 2.74, respectively. For Group 2 vs. Group 1, the HRs were 4.71, 2.42, and 2.09, respectively.
As depicted in forest plot Figure 3, SIRI combined with PP demonstrated uniform predictive value for CVM across all subgroups. The overall area under the curve (AUC) was 0.77, with sensitivity of 70.2% and specificity of 74.4%. The highest predictive value was observed in non-smokers (AUC: 0.79), while the predictive value was high and stable in female and non-pathological contexts (AUC: 0.77–0.78).
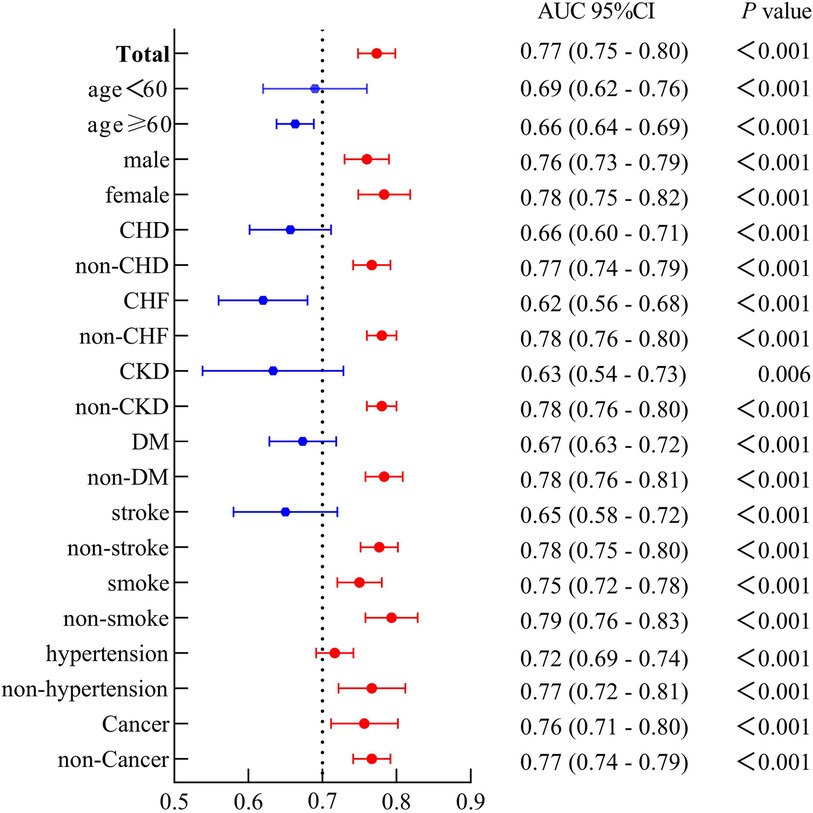
Figure 3. The forest plot demonstrated the combined predictive value of SIRI and PP among different subgroups.
Kaplan-Meier survival curves were generated based on cutoff values of SIRI and PP, along with data on CVM. The high SIRI had a higher mortality compared to the low SIRI, with a Log-rank P < 0.001 and an HR of 3.65 (95% CI: 2.92–4.56). Similarly, the high PP had a higher mortality compared to the low PP, with a Log-rank P < 0.001 and an HR of 5.43 (95% CI: 4.41–6.70). The groups comprising high SIRI and high PP, either high SIRI or high PP, exhibited higher mortality than those of the low SIRI and low PP groups (Log-rank P < 0.001), as shown in Figure 4.
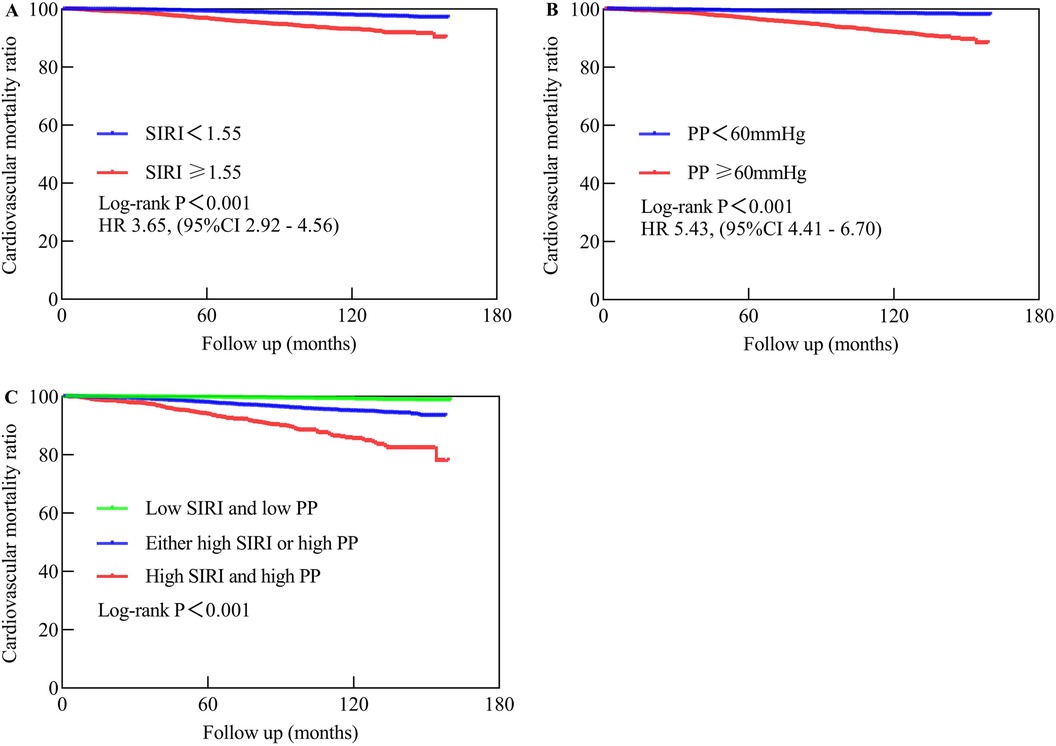
Figure 4. The Kaplan-Meier analysis showed the cardiovascular mortality in different populations. (A) Represents low SIRI compared to high SIRI. (B) Compares low PP to high PP, while (C) compares high SIRI and high PP to either high SIRI or high PP vs. low SIRI and low PP. Low SIRI is defined as an SIRI of less than 1.55, while high SIRI is defined as an SIRI of 1.55 or higher. Low PP is defined as a PP of less than 60 mmHg, while high PP is defined as a PP of 60 mmHg or higher.
Our nomogram comprises 11 independent risk factors, including general risk factors, SIRI, and PP (Figure 5). The AUC for the combined general risk factors was 0.88 (95% CI: 0.87–0.90), while the AUC for general risk factors + SIRI + PP was 0.89 (95% CI: 0.87–0.90). Using the Z-test to compare general risk factors + SIRI + PP and general risk factors, a statistically notable disparity was detected (Z = 4.17, P < 0.001) (Supplementary Figure 4 and Table 2B). We also employed 1,000 bootstrap samples to calculate the C-index for assessing the predictive accuracy of the nomogram, yielding a C-index of 0.90, suggesting excellent predictive value. The C-index of the internal validation cohort was 0.90, and the external validation cohort was 0.91. As depicted in Figure 6, the calibration plot of predicted probabilities demonstrated excellent alignment with the actual CVM rates at 1, 5, and 10 years.
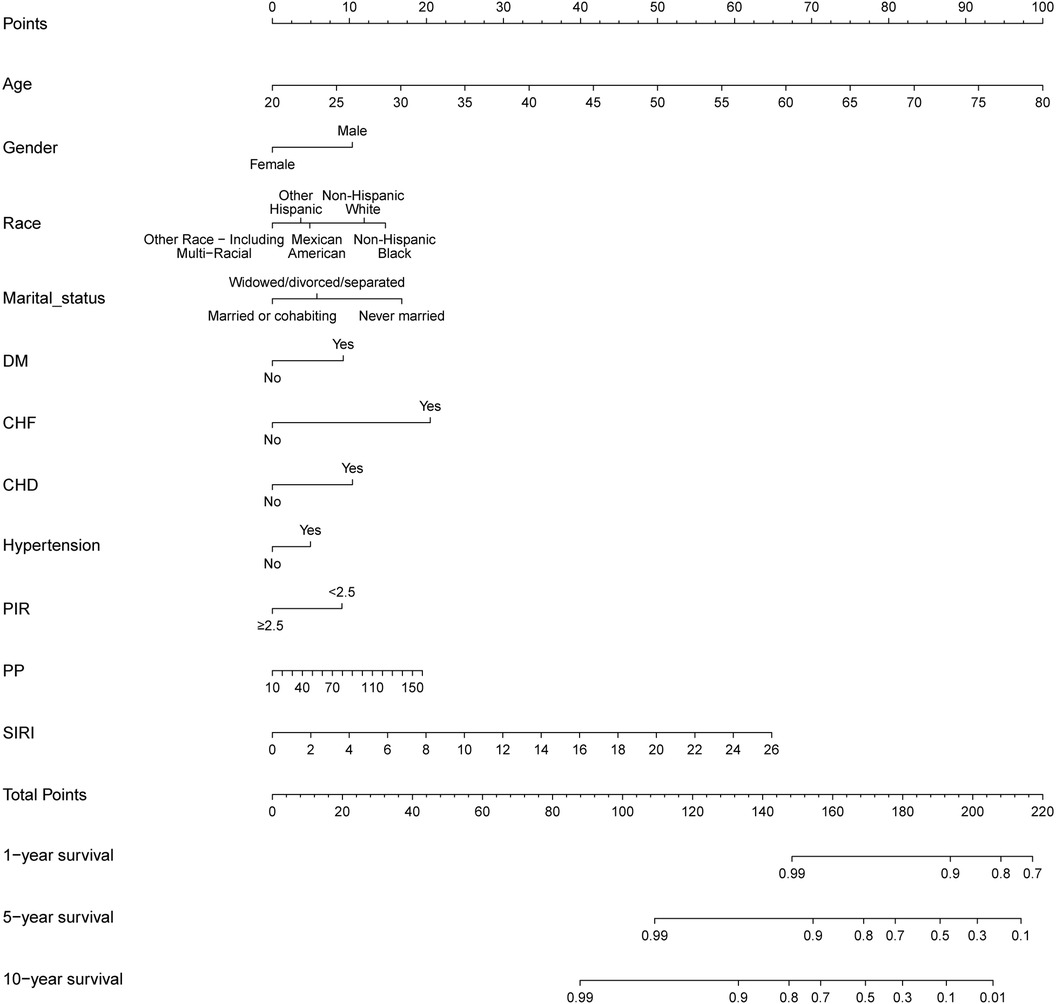
Figure 5. A novel prognostic nomogram for cardiovascular mortality across 1, 5, and 10-year intervals, wherein the cumulative score aligned with the probability of cardiovascular mortality depicted at the base.
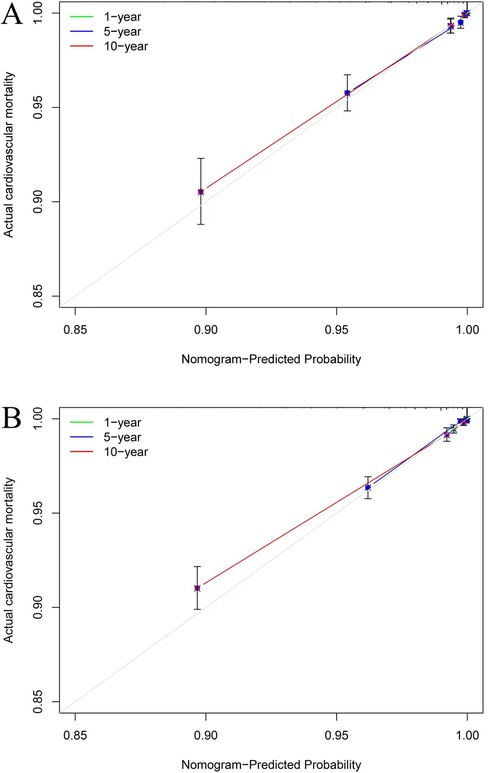
Figure 6. The calibration plots for cardiovascular mortality (CVM). The x-axis represents the predicted risk of CVM. The y-axis represents the actual rate of CVM. The grey line indicates a perfect prediction by an ideal model. The green, blue, and red solid lines represent the performance of the predicting model, with a closer fit to the grey line suggesting better prediction for 1-year, 5-year, and 10-year CVM. The C-index of the internal validation cohort was 0.90 (A), and the external validation cohort was 0.91 (B).
Our research findings suggest that the accuracy of predicting cardiovascular mortality (CVM) may be enhanced through the simultaneous integration of systemic inflammatory response index (SIRI) and pulse pressure (PP). This improvement appears to show a considerable degree of robustness and consistency, particularly in female cohorts, non-smokers, and non-pathological contexts. We observe that higher levels of SIRI and PP are associated with increased mortality. Our study offers preliminary evidence that SIRI may perform better than systemic immune-inflammation index and systemic immune-inflammation response index in predicting CVM within the general population and indicates an S-shaped relationship with PP. Furthermore, we have developed an innovative nomogram to provide precise predictive capabilities for CVM at 1, 5, and 10 years.
Chronic inflammation plays a pivotal role in the pathogenesis of cardiovascular diseases (CVD) and associated mortality, with the SIRI emerging as a significant biomarker reflecting the overall inflammatory state. Elevated SIRI levels have been demonstrated to correlate closely with CVM (2), indicating that persistent inflammation contributes not only to endothelial dysfunction but also to the progression of atherosclerosis (25, 26). Under conditions of chronic inflammation, endothelial cell injury disrupts vascular function and hemodynamics, thereby increasing the risk of cardiovascular events (27).
Concurrently, elevated PP is also significantly linked to the risk of CVM (14). PP serves as an indicator of arterial elasticity and compliance; its elevation is typically associated with arterial stiffness and a decline in vascular compliance (28). Research has established that high PP is not merely a risk factor for cardiovascular diseases and CVM but can exacerbate cardiac workload, leading to alterations in cardiac structure and function (14, 29). Furthermore, PP elevation is significantly associated with various inflammatory markers, such as C-reactive protein and interleukin-6, suggesting that inflammation may exacerbate cardiovascular risk by affecting vascular compliance (30–32).
Importantly, the interrelationship between SIRI and PP highlights their collective capacity to reflect the state of chronic inflammation and vascular health. In our study, when SIRI is greater than 0.51, PP gradually increases with the increase in SIRI, indicating that under inflammatory conditions, vascular functionality may be severely compromised. Specifically, chronic inflammation leads to endothelial activation and the infiltration of inflammatory cells, thereby heightening mechanical stress on the vascular wall and contributing to increased PP (30, 32). Conversely, elevated PP can impose additional tensile stress on the vessel wall, potentially triggering further endothelial damage and inflammatory responses, thus perpetuating a vicious cycle.
In our study, the synergistic application of SIRI and PP demonstrates a stronger predictive efficacy compared to their individual use. Our findings confirm our hypothesis that the simultaneous presence of high SIRI and low PP can further increase the risk of CVM, and this conclusion is consistent across various sensitivity analyses. Therefore, integrating SIRI and PP as predictive markers for CVM holds significant clinical relevance. By assessing these two indices, clinicians can obtain a comprehensive understanding of a patient's cardiovascular risk, facilitating the development of personalized intervention strategies. This combined assessment enhances the predictive accuracy of cardiovascular events but also aids in identifying high-risk individuals, enabling early intervention and improved prognostic outcomes.
Subgroup analysis shows that compared to individuals with lower SIRI and PP levels, individuals in the high SIRI and high PP subgroup have a higher proportion of widowed, divorced, or separated, higher smoking rates, increased comorbidity rates, higher BMI, and lower proportions of individuals with a college education or above. Following adjustments for numerous confounding variables, high SIRI and PP, as well as either high SIRI or PP, were associated with increased CVM compared to low SIRI and PP, with robust results also obtained in sensitivity analyses. Notably, as the levels of SIRI and PP increased, there was a discernible upward trend in the risk of CVM within the wider community, which achieved statistical significance. Furthermore, subgroup analysis shows that the combined use of the SIRI and PP has predictive value (AUC 0.77–0.79) in different populations, particularly evident among female cohorts, non-smokers, and non-pathological contexts. Potential explanations may involve the relatively diminished significance attributed to SIRI and PP concerning mortality among patients burdened with comorbidities. Additionally, given that individuals with comorbidities undergo frequent assessments and hospitalizations, aberrations in SIRI and PP are more readily identifiable, thereby facilitating the management of these parameters within a more favorable range through lifestyle adjustments adherence to standardized medication regimens, and even timely surgical interventions. Lastly, in contrast to males, females demonstrate lower rates of smoking, alcohol consumption and BMI, along with decreased incidences of diabetes mellitus (DM), CHF, CHD, and CVM. Similarly, comorbidities are more prevalent among smokers. Consequently, the relative importance of SIRI and PP in mortality is accentuated among females and non-smokers. This implies that timely identification, diagnosis, and management of abnormal SIRI and PP are imperative in non-pathological conditions and among females. Moreover, the joint utilization of SIRI and PP demonstrates applicability in predicting CVM within the general citizenry.
We know that ischemic heart disease, stroke, self-inflicted injury, chronic obstructive pulmonary disease, and cancer are the main causes of mortality (33). Major risk factors contributing to years of life lost due to disability encompass elevated BMI, smoking, hypertension, high sodium diet, elevated fasting blood glucose, medication usage, and environmental particulate pollution. Considering these risk factors, our nomogram amalgamates general risk factors while integrating two novel risk factors identified in our study, namely SIRI and PP, totaling 11 independent risk factors. Our investigation has unveiled that the incorporation of SIRI and PP into the novel clinical prediction model enhances its prognostic capability, with statistically significant disparities noted. Furthermore, the concordance index of 0.90 indicates commendable discriminative ability, consistent with the cumulative area under the curve calculations and surpassing previous study reports (17–19). Calibration curves evince optimal alignment between predicted and actual CVM rates at 1, 5, and 10 years.
First, SIRI and PP are easily obtainable indicators in clinical practice, and their combination has a higher predictive value for CVM. Second, multiple sensitivity analyses confirmed that the group with higher SIRI and PP had a higher CVM rate. Finally, the new nomogram model demonstrated a higher c-index, providing valuable guidance in clinical work.
However, notable limitations exist. Primarily, the study encountered challenges related to missing data, potentially introducing bias into the results. Additionally, parameters such as SIRI and PP mainly represent momentary values susceptible to influence by other confounding factors, thus potentially failing to reflect the body's long-term metabolic status. The integration of 24-h dynamic blood pressure monitoring may bolster the robustness of the study's findings. Furthermore, despite the favorable predictive value of the combined SIRI and PP, their sensitivity and specificity remain relatively modest. Lastly, future research could further explore the shared pathways between inflammatory and hypertension pathways. This will help guide subsequent studies in this field.
The concurrent utilization of SIRI and PP emerges as a robust predictor of CVM within the general populace of the US. Particular attention to assessment and management may be warranted in female cohorts, non-smokers, and non-pathological contexts. Both SIRI and PP are autonomous risk factors for CVM in the broader demographic, with elevated levels of both indicators correlating with heightened risks of CVM. The novel nomogram exhibits relatively high precision in forecasting CVM.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The studies involving humans were approved by The National Center for Health Statistics and Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
JA: Data curation, Formal Analysis, Resources, Writing – original draft. ZZ: Data curation, Formal Analysis, Resources, Writing – original draft. BX: Investigation, Writing – review & editing. DY: Investigation, Methodology, Visualization, Writing – review & editing. YLi: Software, Writing – review & editing. YLu: Investigation, Software, Supervision, Validation, Writing – review & editing. HL: Supervision, Validation, Writing – review & editing. YJ: Methodology, Visualization, Writing – review & editing. GZ: Writing – review & editing. MX: Methodology, Visualization, Writing – review & editing. SL: Conceptualization, Funding acquisition, Project administration, Writing – review & editing. JL: Conceptualization, Funding acquisition, Project administration, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by National Natural Science Foundation of China, grant number 82370324; the Clinical Research Plan of SHDC (SHDC2023CRD008 and SHDC2024CRI015) and the Clinical Research Plan of Shanghai General Hospital (No. CCTR-2022B09). Fund Project of the Health Commission of Guizhou Province: gzwkj2023-303.
All data for this study were obtained from the NHANES database. Special thanks to the staff and participants who worked hard on this database.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1439239/full#supplementary-material
Supplementary Figure 1 | The flowchart of the study.
Supplementary Figure 2 | Incorporating indicators like SIRI, SII, and SIIRI to assess cardiovascular mortality, resulted in AUC values of 0.67, 0.60, and 0.62, respectively.
Supplementary Figure 3 | Incorporating indicators like PP, SBP, DBP, and MAP to assess cardiovascular mortality, resulted in AUC values of 0.73, 0.67, 0.61, and 0.52, respectively.
Supplementary Figure 4 | The cumulative AUCs for cardiovascular mortality for general risk factors and general risk factors + PP + SIRI were 0.85 and 0.86, respectively.
1. Acharya A, Chowdhury HR, Ihyauddin Z, Mahesh PKB, Adair T. Cardiovascular disease mortality based on verbal autopsy in low- and middle-income countries: a systematic review. Bull W H O. (2023) 101(9):571–86. doi: 10.2471/BLT.23.289802
2. Nascimento MAL, Ferreira LGR, Alves TVG, Rios DRA. Inflammatory hematological indices, cardiovascular disease and mortality: a narrative review. Arq Bras Cardiol. (2024) 121(7):e20230752. doi: 10.36660/abc.20230752
3. Gonzalez AL, Dungan MM, Smart CD, Madhur MS, Doran AC. Inflammation resolution in the cardiovascular system: arterial hypertension, atherosclerosis, and ischemic heart disease. Antioxid Redox Signaling. (2024) 40(4–6):292–316. doi: 10.1089/ars.2023.0284
4. Speer T, Dimmeler S, Schunk SJ, Fliser D, Ridker PM. Targeting innate immunity-driven inflammation in CKD and cardiovascular disease. Nat Rev Nephrol. (2022) 18(12):762–78. doi: 10.1038/s41581-022-00621-9
5. Soehnlein O, Libby P. Targeting inflammation in atherosclerosis—from experimental insights to the clinic. Nat Rev Drug Discovery. (2021) 20(8):589–610. doi: 10.1038/s41573-021-00198-1
6. Ma M, Wu K, Sun T, Huang X, Zhang B, Chen Z, et al. Impacts of systemic inflammation response index on the prognosis of patients with ischemic heart failure after percutaneous coronary intervention. Front Immunol. (2024) 15:1324890. doi: 10.3389/fimmu.2024.1324890
7. Dong W, Gong Y, Zhao J, Wang Y, Li B, Yang Y. A combined analysis of TyG index, SII index, and SIRI index: positive association with CHD risk and coronary atherosclerosis severity in patients with NAFLD. Front Endocrinol (Lausanne). (2024) 14:1281839. doi: 10.3389/fendo.2023.1281839
8. Zhou H, Li X, Wang W, Zha Y, Gao G, Li S, et al. Immune-inflammatory biomarkers for the occurrence of MACE in patients with myocardial infarction with non-obstructive coronary arteries. Front Cardiovasc Med. (2024) 11:1367919. doi: 10.3389/fcvm.2024.1367919
9. Zhao S, Dong S, Qin Y, Wang Y, Zhang B, Liu A. Inflammation index SIRI is associated with increased all-cause and cardiovascular mortality among patients with hypertension. Front Cardiovasc Med. (2023) 9:1066219. doi: 10.3389/fcvm.2022.1066219
10. Zhang L, Wang B, Wang C, Li L, Ren Y, Zhang H, et al. High pulse pressure is related to risk of type 2 diabetes mellitus in Chinese middle-aged females. Int J Cardiol. (2016) 220:467–71. doi: 10.1016/j.ijcard.2016.06.233
11. Liu FD, Shen XL, Zhao R, Tao XX, Wang S, Zhou JJ, et al. Pulse pressure as an independent predictor of stroke: a systematic review and a meta-analysis. Clin Res Cardiol. (2016) 105(8):677–86. doi: 10.1007/s00392-016-0972-2
12. Vaccarino V, Holford TR, Krumholz HM. Pulse pressure and risk for myocardial infarction and heart failure in the elderly. J Am Coll Cardiol. (2000) 36(1):130–8. doi: 10.1016/S0735-1097(00)00687-2
13. Haider AW, Larson MG, Franklin SS, Levy D. Systolic blood pressure, diastolic blood pressure, and pulse pressure as predictors of risk for congestive heart failure in the Framingham heart study. Ann Intern Med. (2003) 138(1):10–6. doi: 10.7326/0003-4819-138-1-200301070-00006
14. Zhao L, Song Y, Dong P, Li Z, Yang X, Wang S. Brachial pulse pressure and cardiovascular or all-cause mortality in the general population: a meta-analysis of prospective observational studies. J Clin Hypertens (Greenwich). (2014) 16(9):678–85. doi: 10.1111/jch.12375
15. Domanski M, Mitchell G, Pfeffer M, Neaton JD, Norman J, Svendsen K, et al. Pulse pressure and cardiovascular disease-related mortality: follow-up study of the multiple risk factor intervention trial (MRFIT). JAMA. (2002) 287(20):2677–83. doi: 10.1001/jama.287.20.2677
16. Lacolley P, Regnault V, Laurent S. Mechanisms of arterial stiffening: from mechanotransduction to epigenetics. Arterioscler Thromb Vasc Biol. (2020) 40(5):1055–62. doi: 10.1161/ATVBAHA.119.313129
17. Liu L, Lo K, Huang C, Feng YQ, Zhou YL, Huang YQ. Derivation and validation of a simple nomogram prediction model for all-cause mortality among middle-aged and elderly general population. Ann Palliat Med. (2021) 10(2):1167–79. doi: 10.21037/apm-20-580
18. Shen X, Zhang XH, Yang L, Wang PF, Zhang JF, Song SZ, et al. Development and validation of a nomogram of all-cause mortality in adult Americans with diabetes. Sci Rep. (2024) 14(1):19148. doi: 10.1038/s41598-024-69581-3
19. Yang J, Wang X, Jiang S. Development and validation of a nomogram model for individualized prediction of hypertension risk in patients with type 2 diabetes mellitus. Sci Rep. (2023) 13(1):1298. doi: 10.1038/s41598-023-28059-4
20. Kroenke CH, Neugebauer R, Meyerhardt J, Prado CM, Weltzien E, Kwan ML, et al. Analysis of body mass index and mortality in patients with colorectal cancer using causal diagrams. JAMA Oncol. (2016) 2(9):1137–45. doi: 10.1001/jamaoncol.2016.0732
21. Mangalesh S, Dudani S, Mahesh NK. Development of a novel inflammatory index to predict coronary artery disease severity in patients with acute coronary syndrome. Angiology. (2024) 75(3):231–9. doi: 10.1177/00033197231151564
22. Johannesen CDL, Langsted A, Mortensen MB, Nordestgaard BG. Association between low density lipoprotein and all cause and cause specific mortality in Denmark: prospective cohort study. BMJ. (2020) 371:m4266. doi: 10.1136/bmj.m4266
23. Xiong S, Chen Q, Chen X, Hou J, Chen Y, Long Y, et al. Adjustment of the GRACE score by the triglyceride glucose index improves the prediction of clinical outcomes in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Cardiovasc Diabetol. (2022) 21(1):145. doi: 10.1186/s12933-022-01582-w
24. Zhang W, Ji L, Wang X, Zhu S, Luo J, Zhang Y, et al. Nomogram predicts risk and prognostic factors for bone metastasis of pancreatic cancer: a population-based analysis. Front Endocrinol (Lausanne). (2022) 12:752176. doi: 10.3389/fendo.2021.752176
25. Gimbrone MA Jr, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. (2016) 118(4):620–36. doi: 10.1161/CIRCRESAHA.115.306301
26. Xu S, Ilyas I, Little PJ, Li H, Kamato D, Zheng X, et al. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: from mechanism to pharmacotherapies. Pharmacol Rev. (2021) 73(3):924–67. doi: 10.1124/pharmrev.120.000096
27. Peng Z, Shu B, Zhang Y, Wang M. Endothelial response to pathophysiological stress. Arterioscler Thromb Vasc Biol. (2019) 39(11):e233–43. doi: 10.1161/ATVBAHA.119.312580
28. Niiranen TJ, Kalesan B, Mitchell GF, Vasan RS. Relative contributions of pulse pressure and arterial stiffness to cardiovascular disease. Hypertension. (2019) 73(3):712–7. doi: 10.1161/HYPERTENSIONAHA.118.12289
29. Said MA, Eppinga RN, Lipsic E, Verweij N, van der Harst P. Relationship of arterial stiffness Index and pulse pressure with cardiovascular disease and mortality. J Am Heart Assoc. (2018) 7(2). doi: 10.1161/JAHA.117.007621
30. Li X, Zhang H, Huang J, Xie S, Zhu J, Jiang S, et al. Gender-specific association between pulse pressure and C-reactive protein in a Chinese population. J Hum Hypertens. (2005) 19(4):293–9. doi: 10.1038/sj.jhh.1001818
31. Amar J, Ruidavets JB, Peyrieux JC, Mallion JM, Ferrières J, Safar ME, et al. C-reactive protein elevation predicts pulse pressure reduction in hypertensive subjects. Hypertension. (2005) 46(1):151–5. doi: 10.1161/01.HYP.0000171165.80268.be
32. Chang CS, Kuo CL, Huang CS, Cheng YS, Lin SS, Liu CS. The relationship between pulse pressure with plasma PCSK9 and interleukin-6 among patients with acute ischemic stroke and dyslipidemia. Brain Res. (2022) 1795:148080. doi: 10.1016/j.brainres.2022.148080
Keywords: systemic inflammatory response index, pulse pressure, cardiovascular mortality, prognostication, nomogram
Citation: An J, Zhong Z, Xiong B, Yang D, Li Y, Luo Y, Li H, Jiao Y, Zhou G, Xu M, Liu S and Li J (2024) Integration of the systemic inflammatory response index with pulse pressure enhances prognostication of cardiovascular mortality in the general population of the United States: insights from the NHANES database. Front. Cardiovasc. Med. 11:1439239. doi: 10.3389/fcvm.2024.1439239
Received: 5 June 2024; Accepted: 28 October 2024;
Published: 18 November 2024.
Edited by:
Dragos Cretoiu, Carol Davila University of Medicine and Pharmacy, RomaniaReviewed by:
Botang Guo, Harbin Medical University, ChinaCopyright: © 2024 An, Zhong, Xiong, Yang, Li, Luo, Li, Jiao, Zhou, Xu, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaowen Liu, c2hhb3dlbi5saXVAaG90bWFpbC5jb20=; Jie Li, enVueWlsakAxMjYuY29t
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.