- Department of Cardiovascular Surgery, The Second Hospital of Jilin University, Changchun, China
Primary cardiac synovial sarcoma (PCSS) is a rare and highly aggressive tumor with a significant mortality rate. Treatment guidelines have not been defined given the relative rarity of the condition, especially for pregnant women. Described herein is a 36-year-old pregnant woman at 29 weeks with gestation who was hospitalized due to chest tightness and nausea, and echocardiography found a mass involved in the right heart and the tricuspid valve. She had to undergo cardiac surgery because the mass almost blocked the opening of the tricuspid valve. She underwent a radical resection of the masses and tricuspid valve, followed by replacement of the tricuspid valve with a mechanical valve. She successfully delivered a healthy baby boy. The diagnosis of synovial sarcoma is confirmed by positive results indicating rearrangement of the SYT gene. The patient survived throughout the 30-month follow-up period. There are no reported cases of pregnant women diagnosed with cardiac synovial sarcoma and have undergone cardiac surgery and cesarean section. Our treatment plan not only maximizes patient survival but also ensures fetal survival. This situation is rare and needs documentation.
Introduction
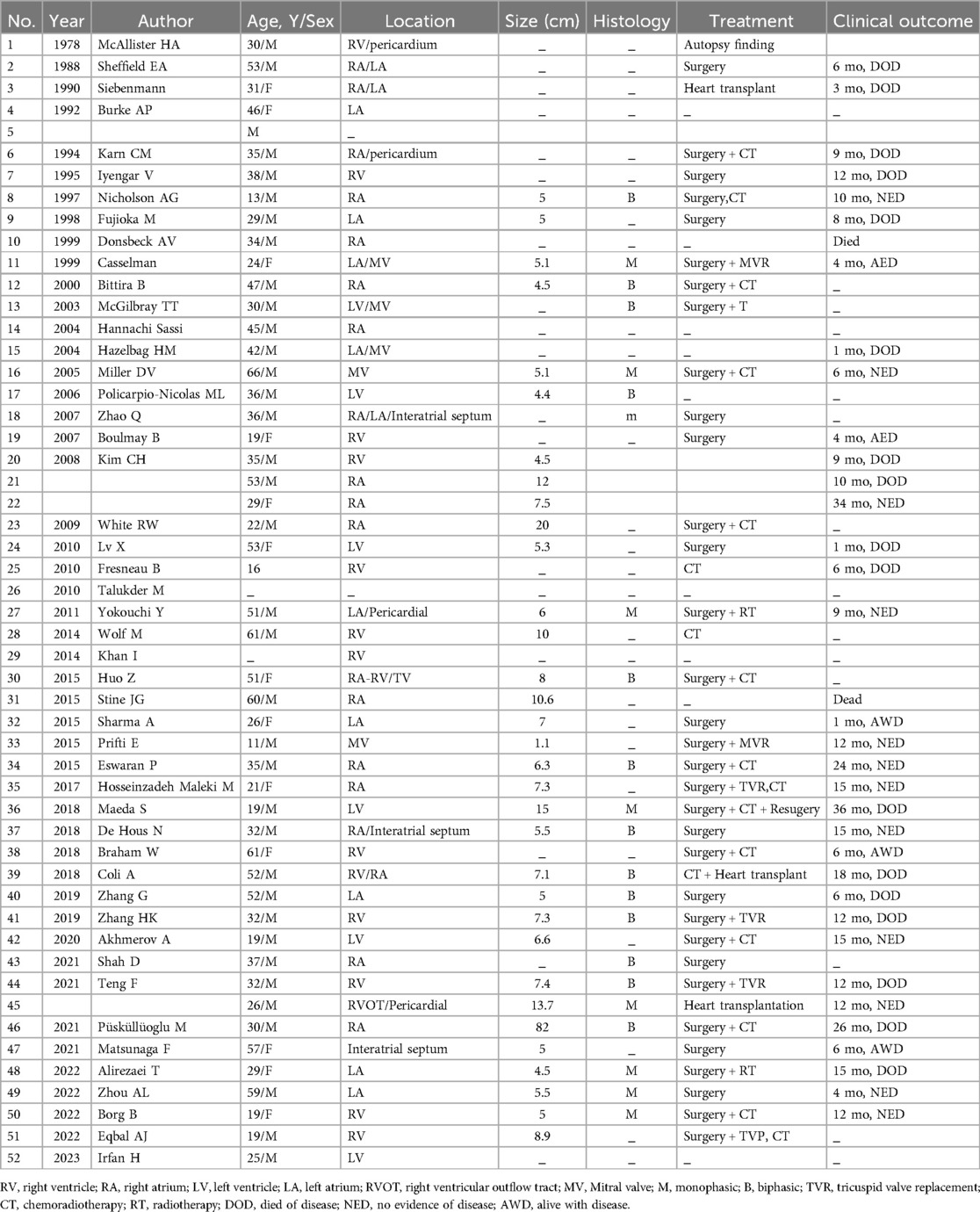
Synovial sarcoma is an aggressive malignant soft tissue tumor derived from primitive pluripotent mesenchyme capable of epithelial differentiation presenting in adolescents and young adults, with approximately 80% originating adjacent to the joints or tendon sheaths of the extremities (1). Primary cardiac synovial sarcomas (PCSS) represents an exceptionally rare subset. We reviewed the literature and found 52 cases of PCSS originated from the cardiac chambers or myocardium. Notably, There are no documented cases of PCSS in pregnant women. Surgical resection remains the first-line treatment, however, recurrence rates remain high due to incomplete macroscopic resection achieved in approximately one-third of patients (2). The need for cardiac surgery during pregnancy is rare, only 1% to 4% of pregnancies are complicated by maternal cardiac disease (3). Performing cardiac surgery on cardiopulmonary bypass (CPB) during pregnancy is high-risk for both the fetus and the mother.
Case presentation
A 36-year-old pregnant woman at 29 weeks gestation was admitted with complaint of chest tightness and nausea persisting for 3 days. Echocardiography confirmed the presence of an irregular mass measuring 85 mm × 40 mm arising from the tricuspid annulus, extending from the inlet to the outflow tract of the right ventricle (Figure 1A). Abdominal color ultrasound showed the congestion liver. Obstetric evaluation showed a uterine height of 30 cm and abdominal circumference of 90 cm; Biparietal diameter of the fetus (BPD) is 74 mm; Head circumferece (HC) is 277 mm; Abdominal circumferece (AC) is 248 mm; Femur length (FL) is 54 mm; The fetal heart rate was recorded as 152 beats/min. Because of her pregnancy, she did not undergo any other radiation tests.
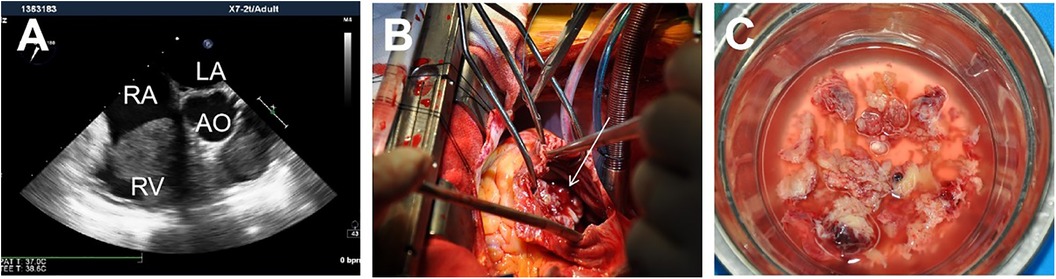
Figure 1. A mass adherent to the right heart (A); Intraoperative view showing the mass almost occupies the opening of the tricuspid valve (B). The characteristics of the gross specimen are soft, gray white, friable (4) (C).
After consultation with cardiologists, obstetricians, anesthesiologists, and perfusionists, we have made the decision to proceed with surgical intervention. The initial option entails tumor removal on CPB without inducing cardiac arrest while maintaining the pregnancy. Alternatively, the second option involves tumor excision on CPB under cardiac arrest and termination of the pregnancy.
After general anesthesia, median sternotomy was performed. After full heparinization, CPB was instituted through cannulations of the aorta, inferior vena cava, and superior vena cava. On atriotomy, the mass was almost occupying the opening of the tricuspid valve (Figure 1B). It was attached to the root of the anterior leaflet and septal leaflet, destroying the tricuspid valve entirely. We decided to remove the tumor and replace the tricuspid valve under cardiac arrest. Cold cardioplegic solution was instilled via the aortic root after cross clamping the aortic. Simultaneously, it was crucial to draw cardioplegia from the coronary sinus orifice. Remove the mass and irrigate the cardiac chambers thoroughly (Figure 1C). The valve has replaced with a 29-mm mechanical valve. The aortic clamp was opened and right atrium was sutured. After careful hemostasis and closing the wound in layers, The rest of surgery was completed routinely. Subsequently, the patient underwent a cesarean section successfully delivering a healthy baby boy. The CPB time was 74 min and cross clamp time was 36 min.
Subsequently, the postoperative pathology reveals the presence of monophasic synovial sarcoma, specifically confirmed by positive expression of Vimentin, TLE1, and BCL-2 in immunohistochemical analysis. The diagnosis of synovial sarcoma is further supported by positive results indicating SYT gene fracture rearrangement (Figure 2).
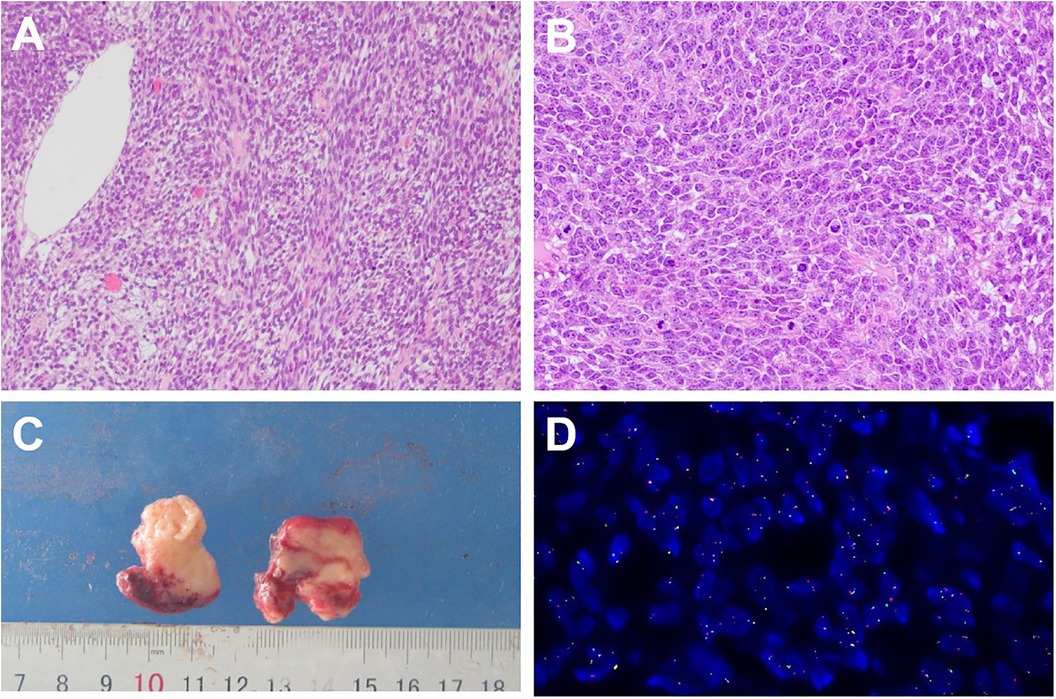
Figure 2. Histology appearance: uniform spindle cells arranged in fascicles. The spindle cells showing eosinophilic cytoplasm and round to oval nuclei.nuclear pleomorphism and with mitoses readily observed (hematoxylin and eosin stain × 100) (A); Poorly differentiated region((hematoxylin and eosin stain × 100) (B); Detection probe: LBP SYT gene fracture probe; 1G1R1F accounted for 48%; Chromosome site: 18q11. Probe labeling: green signal (G) is GSP SYT (Centromere), red signal (R) is GSP SYT (Telomere) (D).
She recovered without complication and was discharged on the 7th postoperative day. She received adjuvant chemotherapy consisting of a combination of anlotinib, toripalimab, and eribulin. The patient survived throughout the 30-month follow-up period. However, a echocardiography performed 26 months post-operatively revealed recurrence in the right ventricle and the right ventricular outflow tract. The patient refused to underwent re-operation to remove the mass.
Discussion
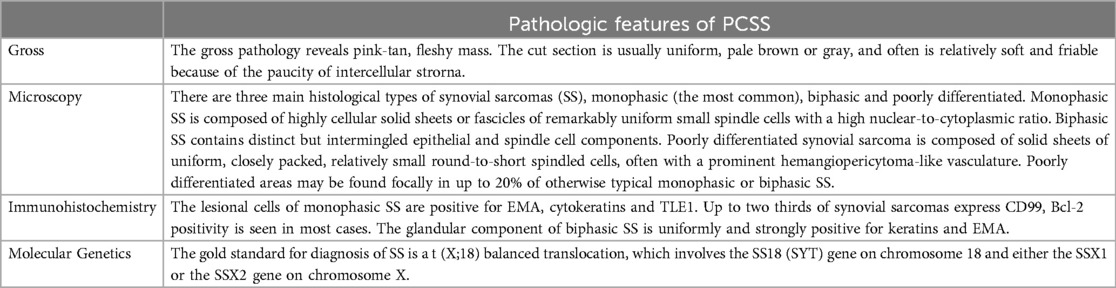
Primary cardiac synovial sarcomas (PCSS) is extremely rare, accounts for approximately 4.2% of all malignant primary cardiac tumors. They most commonly arise from the right atrium (24%) (5). PCSS has a 3:1 male predominance and a mean age of diagnosis in the fourth decade of life (1). These tumors are characterized by a t(X;18)(p11.2;q11.2) chromosomal translocation and the formation of a SS18-SSX fusion oncogene. There are three main histological types of PCSS, monophasic (the most common), biphasic and poorly differentiated (Table 1). In addition to histological characteristics, molecular demonstration of SYT gene rearrangement is essential in confirming the diagnosis (4). Complete resection is the treatment of choice, with additional chemotherapy and/or radiation therapy, when necessary. However, Cardiac surgery is inherently dangerous for both, the mother and fetus with mortality rates near 10% and 30%, respectively (3).
Given the rarity and heterogeneity of PCSS cases, there are no established treatment guidelines. Complete surgical resection is the best treatment choice for cardiac sarcomas. With the advantages of less trauma, less bleeding, and short hospital stay, minimally invasive is gaining popularity in the treatment of cardiac tumors. Moscarelli et al. (6) found that there was no difference in the occurrence of postoperative adverse cardiac events and neurological events between median sternotomy and minimally invasive. Considering that our patient is pregnant and the protection of the fetus, we needed to shorten the operation and CPB time as much as possible. Finally, we performed median sternotomy. Moreover, adjunctive radiotherapy and chemotherapy are associated with greater survival, and the most commonly used chemotherapy regimen is ifosfamide and doxorubicin (1, 2). Our patient received adjuvant chemotherapy consisting of a combination of anlotinib, toripalimab, and eribulin.
After an extensive review of the literature, no documented cases exist of PCSS occurring in pregnant women and being treated with surgery (Table 2). Cardiac surgery carried out on CPB in a pregnant woman is associated with poor neonatal outcomes although maternal outcomes are similar to cardiac surgery in non-pregnant women. Fetal mortality rates associated with maternal cardiac surgery during pregnancy range from 20% to 30% (3). Sustained forceful uterine contractions during CPB are considered as the most important contributors to fetal death (7). To mitigate this risk, we prioritize maintaining high perfusion flow rates and normothermia. Additionally, cardioplegia administration may elevate serum potassium levels, potentially leading to fetal hyperkalemia and subsequent cardiac arrest. To prevent this complication, we carefully avoid any entry of cardioplegia into the bloodstream by discarding it through the coronary sinus orifice. Our patient was at 29 weeks gestation. Considering that fetal development is acceptable, we performed a cesarean section. Primary cardiac sarcomas of all types have poor outcomes, PCSS is no different, with a mean survival of 9 to 16.5 months (8). As of now, our patients have survived for 30 months after surgery and the baby boy is also alive and well.
Conclusions
The current study reported a rare case of primary cardiac synovial sarcoma presenting in the right ventricle and the tricuspid valve in a pregnant woman. The tumor was complete excised through surgery, and the fetus has survived. The patient was still alive at 30-month follow-up. At present, there are no reported cases of pregnant women with cardiac synovial sarcoma who have underwent cardiac surgery and successfully delivered a surviving baby. Although limited, our contribution provides further data on the management of this rare malignant tumor and cardiac surgery during pregnancy.
Limitations
We removed the tumor in peacemeal rather than enbloc. Due to the mass almost completely occupies the opening of the tricuspid valve, we removed part of it first before we can proceed to the next section, followed by removal of involved muscle bands and the discrete fragments in the intermuscular space.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Second Hospital of Jilin University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ML: Conceptualization, Data curation, Formal Analysis, Methodology, Software, Writing – original draft, Writing – review & editing. MXH: Methodology, Writing – review & editing. HP: Methodology, Writing – review & editing, Conceptualization. YW: Conceptualization, Methodology, Writing – review & editing, Data curation. KL: Methodology, Writing – review & editing, Formal Analysis, Supervision.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang JG, Li NN. Primary cardiac synovial sarcoma. Ann Thorac Surg. (2013) 95(6):2202–9. doi: 10.1016/j.athoracsur.2013.01.030
2. Khan H, Chaubey S, Edlin J, Wendler O. Primary cardiac synovial sarcoma. A rare tumor with poor prognosis. Asian Cardiovasc Thorac Ann. (2014) 22(7):835–8. doi: 10.1177/0218492313483584
3. Shook LL, Barth WH. Cardiac surgery during pregnancy. Clin Obstet Gynecol. (2020) 63(2):1. doi: 10.1097/GRF.0000000000000533
4. Petts G, Keir H. Spindle cell and fibrohistiocytic soft tissue tumours of infancy and childhood. Diagnostic Histopathol. (2023) 29(12):533–43; ISSN 1756–2317. doi: 10.1016/j.mpdhp.2023.10.001
5. Habertheuer A, Laufer G, Wiedemann D, et al. Primary cardiac tumors on the verge of oblivion: a European experience over 15 years. J Cardiothorac Surg. (2015). doi: 10.1186/s13019-015-0255-4
6. Moscarelli M, Rahouma M, Nasso G, et al. Minimally invasive approaches to primary cardiac tumors: a systematic review and meta-analysis. J Card Surg. (2021) 36(2):483–92. doi: 10.1111/jocs.15224
7. Kapoor MC. Cardiopulmonary bypass in pregnancy. Ann Card Anaesth. (2014) 17(1):33–9. doi: 10.4103/0971-9784.124133
Keywords: synovial sarcoma, cardiac, pregnant woman, surgery, cardiopulmonary bypass, fetal outcomes
Citation: Li M, Huang M, Piao H, Wang Y and Liu K (2024) Case Report: Primary cardiac synovial sarcoma invading the tricuspid valve in a pregnant woman. Front. Cardiovasc. Med. 11:1437903. doi: 10.3389/fcvm.2024.1437903
Received: 24 May 2024; Accepted: 4 October 2024;
Published: 18 October 2024.
Edited by:
Reto Asmis, Wake Forest University, United StatesReviewed by:
Mohamed Rahouma, NewYork-Presbyterian, United StatesMahdi Saeedi-Moghadam, Shiraz University of Medical Sciences, Iran
Copyright: © 2024 Li, Huang, Piao, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kexiang Liu, a3hsaXU2NEBob3RtYWlsLmNvbQ==
 Mixia Li
Mixia Li Maoxun Huang
Maoxun Huang Hulin Piao
Hulin Piao Kexiang Liu
Kexiang Liu