
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cardiovasc. Med. , 08 August 2024
Sec. Structural Interventional Cardiology
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1437626
This article is part of the Research Topic Reviews in Transcatheter Aortic Valve Implantation View all 6 articles
Aortic Stenosis (AS) is a common condition with an estimated pooled prevalence of all AS in the elderly population at around 12.4%, with that of severe AS estimated to be around 3.4%. In the past, surgical aortic valve replacement was the primary treatment option for severe AS for decades. However, with the compelling evidence on the safety and efficacy of transcatheter aortic valve replacement (TAVR), it has become the gold standard treatment option for many patients with symptomatic severe AS. Transfemoral access has been the preferred method for transcatheter heart valve delivery. However, the prevalent use of TAVR on a diverse patient profile with different risk factors, such as peripheral artery disease, precluded the possibility of a transfemoral approach despite the improvement of valves and delivery systems technology. Therefore, alternative TAVR approaches have gained increasing utility in cases where transfemoral access is unfavorable. We review the journey, evolution, and techniques for different approaches of percutaneous TAVR, including transfemoral, transcarotid, transsubclavian/transaxillary, and transcaval approaches, in addition to the traditional “surgical” transaortic and transapical accesses. Consolidating these data highlights each approach's practicality and limitations, providing additional grounding for case-by-case utilization and future clinical research.
Aortic stenosis (AS) is an insidious disease characterized by a long latency period, followed by rapid progression after the appearance of symptoms, resulting in a high mortality rate among untreated patients (1). Although the etiology of AS can vary, cases due to calcific degeneration predominantly affect those above the age of 65. The pooled prevalence of all AS in the elderly (75 years or older) is 12.4%, with that of severe AS estimated to be around 3.4%. Moreover, the number of patients with calcific AS is projected to more than double by 2050 in developed nations based on simulation models (2). Without definitive management via valvular replacement, the four-year mortality rate is around 45% (3). Over the past decade, transcatheter aortic valvular replacements (TAVR) have exceeded the number of isolated surgical aortic valvular replacements (SAVR) (4). The PARTNER 3 and EVOLUT Low-Risk trials have demonstrated compelling evidence regarding the safety and effectiveness of TAVR compared to SAVR in low-risk patients at 5 and 4 years, respectively (5–8). With the increase in TAVR procedures worldwide across a diverse patient profile with different risk factors, physiological and anatomic constraints challenges have come to the forefront. Traditionally, transfemoral access has shown superior results and is the only TAVR method that showed equal or superior outcomes as compared to SAVR. However, alternative approaches have garnered increasing utility in cases where transfemoral is high risk, or the anatomy is unfavorable. We review the journey, evolution, and the techniques for different approaches of percutaneous TAVR, including transfemoral, transcarotid, transsubclavian/transaxillary, and transcaval approaches, in addition to the traditional “surgical” transaortic and transapical accesses. Consolidating these data highlights each approach's practicality and limitations, thereby providing additional grounding for case-by-case utilization and future clinical research, which will help address significant knowledge gaps.
Historically, patients with severe AS who were considered high risk for surgery were treated either conservatively with medical therapy or by adding an invasive method with balloon aortic valvuloplasty, which was first developed by Dr. Alain Cribier in 1985 (9, 10). Balloon aortic valvuloplasty provided a short-term improvement of valvular function and symptoms and was associated with a high degree of valvular restenosis (11–13). Given the limited therapeutic effect, with substantial risks related to this treatment option, more interest in developing better and more durable solutions with a percutaneous delivery system for a bioprosthetic aortic heart valve was seen. The concept of transcatheter balloon-expandable valves was introduced in the 1980s by Danish researcher Dr. Henning Rud Andersen, who tested the concept with handmade metal stents in pigs (14, 15). Since then, many investigators tested the concept of transcatheter aortic heart valve delivery with prosthetic valves of different designs in animal models (16–20). With that, Dr. Alain Cribier and colleagues performed the first-in-human and proof of concept of the TAVR procedure on April 16, 2002, in Rouen, France (21). That patient had multiple comorbidities and was hemodynamically unstable, which precluded him from obtaining a surgical valve replacement. Due to the large size of the delivery system, a 24F sheath (outer diameter 26F), a venous trans-septal antegrade approach was performed. It was successfully performed with excellent immediate results. Transesophageal echocardiography at nine weeks demonstrated a securely implanted prosthetic valve with continued sustainable function. With that, the TAVR Journey started with Cribier et al. demonstrating the feasibility of safely and successfully implanting a prosthetic heart valve in a native diseased valve using standard interventional techniques (21).
This successful catheter-based approach ushered in a new era of treating aortic stenosis with minimally invasive approaches. The company Criber co-founded, Percutaneous Valve Technologies, was then acquired in 2004 by Edwards Lifesciences Corporation (Irvine, CA, USA), which has continued the development of TAVR valves that are in use today (22).
In 2003, Dr. David Paniagua and his team performed the first human TAVR using the retrograde approach in Texas, USA (23, 24). The Paniagua Heart Valve (PHV) was developed by Endoluminal Technology Research in Miami, Florida. The valve was compatible with 11–16F sheaths. PHV's durability was initially tested in vitro on a systemic circulation simulator and multiple animal models, showing proper valve function (23). PHV was mounted on a 16F sheath, allowing a retrograde approach through the femoral artery. The valve immediately functioned normally, improving the patient's clinical status. However, three days post-procedure, the patient died of respiratory failure of unclear etiology (24).
Other pioneer physicians also contributed to the evolution of TAVR technology. In Vancouver, Canada, Dr. John Webb introduced a modified delivery system - a deflectable RetroFlex catheter - which permits easy crossing across the aortic arch and the stenotic valve. It was done with the balloon-expandable Cribier-Edwards Sapien valve retrograde through the femoral artery in 2005 (22, 25, 26). Consequently, Eberhard Grube performed his first human TAVR using another valve design, the self-expanding CoreValve™ device, later acquired by Medtronic, Minneapolis, in 2009 (25, 27).
Subsequently, Friedrich Mohr and Michael Mack evaluated a new alternative approach, the minimally invasive transapical approach, in 2006 in Leipzig, Germany, using the Edwards Ascendra delivery system on 30 patients deemed high risk for surgery (22, 23, 28).
Figure 1 highlights the timeline of the first reported cases of each TAVR access site with their corresponding valve type.
With improvements in TAVR technology seen throughout the years, such as in device size and delivery systems, there have been improvements in clinical outcomes and a reduction in procedural complications (34–36). An improvement in valve size, design, and delivery systems, which are compatible with 14–16 French sheaths, have led to reduced paravalvular leaks and conduction disturbances from valve design and implantation techniques perspective, in addition to lower rates of vascular complications with regards to lower profile deliver systems (35). Different valve designs, such as a self-expanding platform, have improved the flexibility of device delivery and decreased vascular complications due to their compatibility with an Inline sheath. The Inline sheath enables the operator to insert the device without the need for a separate larger access sheath, reducing the overall profile of the system. These diverse valves and systems have distinct features that enable their use in different clinical and anatomical situations (34).
Transfemoral access has been the most utilized approach for TAVR, accounting for about 95% of cases (4). TAVR access sites can be divided into two categories based on how the arteriotomy is achieved: percutaneous or surgical cut-down. Percutaneous access can be achieved via transfemoral (TF), transsubclavian, or transaxillary (TAx) approaches. Surgical cut-down is typically used for transcarotid (TC), TAx, direct aortic, apical, and, in some instances, transfemoral approaches. A novel percutaneous approach involves transvenous access, with a cross-over to the transaortic, known as transcaval (TCv) access (4).
Surgical cut-down to the femoral artery was a method predominantly used in the first-generation heart valve system. However, with the improvement of vascular closure devices and a decrease in the size of the delivery sheaths, TF TAVR has evolved to percutaneous access. Despite improvement in TAVR technology, challenging access site anatomies such as severely calcified femoral arteries or small femoral artery diameter may sometimes require surgical cut-down with a 4–5 cm skin incision (37). However, this is rarely done anymore. Percutaneous TF access has a higher rate of major vascular complications than TF with surgical cut-down. A study showed that TF with percutaneous access group had a 10% risk of major vascular complications while TF with surgical cut-down had none (37). It may be due to increased vessel wall trauma during sheath exchange. Consequently, surgical access allows the insertion of a sheath under direct field vision, ultimately decreasing injury to the vessel wall. On the contrary, surgical cut-down TF was associated with higher access site infection risk than percutaneous TF, requiring prolonged antibiotics use or wound debridement (38). In addition, surgical cut-down TF access may still warrant the use of general anesthesia.
Preprocedural diagnostic testing often includes cross-sectional imaging and reconstruction of the aortoiliac vessels with multidetector computed tomography (MDCT). An MDCT became the gold standard pre-TAVR test and is required to accurately measure the inner vessel diameters of each femoral, subclavian, and axillary artery. It also enables proper assessment of the aortic root and valve annulus sizing and critical assessment of related structures (39). A multidisciplinary heart team's role is crucial to assessing valvular replacement's suitability, including evaluation of comorbidities, surgical risk, and anatomical characteristics to choose the optimal therapy and access to be used (40).
Studies have frequently proved the superior clinical outcomes associated with a transfemoral approach for TAVR with respect to other alternative approaches (41, 42). However, in some scenarios, complex vasculature anatomy and conditions have limited the transfemoral approach. Factors such as severe iliofemoral tortuosity, severe vessel wall calcification, inadequate lumen diameter, and significant focal stenosis have been considered challenges, but in some instances, relative contraindications to transfemoral TAVR (39).
Severe iliac tortuosity can be overcome using a bilateral stiff wire technique (39). The success behind this technique is that the bilateral stiff wires promote vessel straightening due to their passive resistance to curvature, although sometimes unsuccessful due to the iliac's high rigidity, making it difficult to straighten them with the bilateral stiff wire technique.
On the other hand, another challenge is severe calcific peripheral artery disease, which can interfere with the ability to deliver prosthetic valves through the femoral vessels (43). Therefore, facilitated TF TAVR can be used, which is TF, following percutaneous interventions using multiple dilators, transluminal angioplasty, iliofemoral stenting, or intravascular lithotripsy (IVL) (44). The use of IVL before attempting transfemoral TAVR has been a topic of interest as a potential method to overcome severe ilio-femoral calcification (45).
Lithotripsy has been used for over 30 years to overcome calcific pathologies such as renal calculi. It delivers pulsatile sonic pressure waves to disrupt calcium deposited in the wall of the vessel (46). Till today, there are no guidelines to specify when it is suitable to perform IVL before TF TAVR. However, Experts have highlighted some CT angiography criteria that enable IVL pre-TF TAVR (46). Kempton et al. stated that if the calcification is localized (less than 20 mm in length) and circumferential (360° calcification circumference), a minimum luminal diameter of 4 mm is required. If the calcification is localized and has a circumference between 270 and 360°, a minimum luminal diameter of 3 mm is needed. If the calcification is diffused (greater than 20 mm in length) and circumferential, a minimum luminal diameter of 4.5 mm is advised. Consequently, if the lesion is diffuse and between 270° and 360° in calcification circumference, a luminal diameter of 3.5 mm is needed (46). A multi-center study was done to assess the safety and efficiency of IVL before transfemoral delivery of a TAVR system in patients with severe aortoiliac calcification. All patients had a successful sheath passage and valve implantation. Access site complications were as low as 4%, with no iliofemoral arterial perforation or dissection seen. Facilitated TF was associated with a lower 30-day rate of major adverse events as compared to trans-thoracic options. Consequently, facilitated TF had a lower 1-year stroke/TIA rate compared to the other extra-thoracic alternative access sites (44). However, with the compelling evidence that highlights the effectiveness and safety of IVL-assisted TF TAVR, complications such as perforation and dissection of the artery can still be seen, which can be life-threatening (47). Multidisciplinary discussion is recommended to assess the best candidates for IVL-assisted TF TAVR through a case-by-case approach.
The rate of IVL use prior to transfemoral TAVR has recently significantly increased. Data from the European TAVR Registry highlighted that IVL before TAVR increased from 2.4% in 2018 to 6.5% in 2020 (48).
The 2019 STS-ACC TVT (Society of Thoracic Surgeons-American College of Cardiology Transcatheter Valve Therapy Registry) Registry report included 276,316 patients undergoing TAVR at sites across the United States. Temporal trends in vascular access sites from the early TAVR period in 2011 till 2019 were analyzed. Femoral access TAVR increased from 57% to 95% in 2019. Consequently, the types of alternative access sites used shifted substantially. Transapical and direct aortic approaches decreased significantly to 0.3% and 0.5%, respectively.
On the other hand, subclavian access was the most used alternative access site at around 2.5%. Carotid access increased to 0.9%. In addition, transcaval access was added, with 121 cases reported in 2019 (4). The observed temporal trend in TAVR access sites highlights a transformational change in TAVR approaches. Figure 2 highlights the available options for TAVR access sites. The following sections will review the available alternative access sites for TAVR.
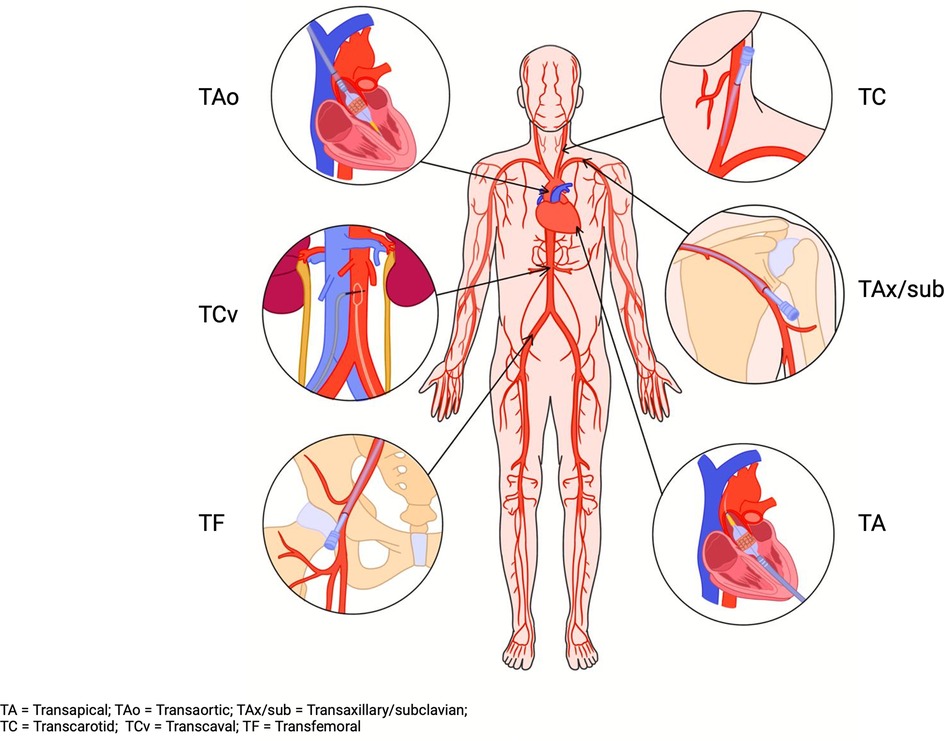
Figure 2. TAVR access sites. TA, transapical; TAo, transaortic; TAx/sub, transaxillary/subclavian; TC, transcarotid; TCv, transcaval; TF, transfemoral.
TA was first performed in 2005 in Vancouver, Canada (25, 29). The initial impression of this procedure was that it is an off-pump procedure, and large delivery systems can be used regardless of the state of the iliofemoral system or aorta. The operative technique consists of an anterolateral mini-thoracotomy of 5 mm in the fifth intercostal space to access the apex of the heart. The pericardium is incised longitudinally, and the left ventricular apex is punctured. A sheath is then introduced and positioned across the aortic valve, and then the valve is deployed. Then, the transapical sheath is removed with safe suturing of the apex of the ventricle with purse-string sutures to ensure secure hemostasis (49). Contraindications of the TA approach are the presence of left ventricle apical thrombus and severely reduced left ventricular function.
This approach gained recognition at first since it used an antegrade approach of introducing the valve, which was preferred when compared to the first-generation devices of the retrograde transfemoral approach, which were associated with significant peripheral vascular complications, and the other available transseptal femoral venous approach which was associated with procedural complexity and potential injury to the mitral valve. However, TA TAVR lost traction with time due to its high rate of mortality and morbidity, similar to that of surgical aortic valve replacement, which eliminates the benefit of TAVR. The most extensive study to date on TA TAVR showed that the 30-day mortality of TA TAVR was around 8.8%, and the 1-year mortality was 25.6% (50). With the surge of other alternative access sites for TAVR and improvement in the technology of the TAVR valve and their delivery systems, which led to better outcomes, TA TAVR has almost vanished. This is reflected by the 0.3% of TAVR cases reported by the 2019 STS-ACC TVT Registry.
With time, at the TAVR's expense, TAo TAVR rates increased. TAo TAVR was first performed in 2009 in Munich, Germany. It was done in an 80-year-old female patient with critical AS with severe calcification of the iliofemoral system and subclavian arteries (30). TAo can be done through mini sternotomy or mini-thoracotomy. A 6-cm skin incision is done with an extension into the third intercostal space at the level of the innominate artery. Surgeons dissect through the pectoralis muscle, exposing and accessing the ascending aorta through the Seldinger technique. A stiff guidewire is introduced and positioned in the left ventricle to help advance the sheath. Then, the prosthetic valve is deployed in place as per the routine retrograde TAVR technique (51). In order to perform TAo, it is vital to identify a suitable calcium-free target site at the anterolateral ascending aorta with a minimum distance of 5 cm from the aortic annular plane and a convenient trajectory to the landing zone, with the angle of puncture in line with the left ventricular outflow tract (52). TAo has several advantages over TA TAVR, such as the absence of direct myocardial injury and less respiratory compromise while preserving the benefit of having a short distance for the landing zone and better valve manipulation (53). However, the TAo approach has some relative contraindications, such as the presence of a porcelain aorta, short ascending aorta, previous cardiac surgery, and thoracic deformities (52). Supplementary Figure S1 shows access points of the transapical and transaortic TAVR.
TAo TAVR's clinical evidence made it an unattractive TAVR approach. A study showed that the all-cause mortality rate at 30 days of TAo compared to the TF group was 10.9% vs. 4.1% respectively. Major or life-threatening bleeding was significantly higher in the TAo group at 30 days (66.7% vs. 35.4%, p < 0.001) (51). In addition, major stroke rate at 30 days was significant for TAo at 5.7% vs. 2.6% for TF. The relatively poor clinical outcomes associated with TAo and TA AVR and the rise in other alternative sites for TAVR with their respective benefits have somehow led to the disappearance of TAo and TA TAVRs.
TAo and TA TAVR are very rarely used and are now mostly mentioned for their historical significance related to the evolution of TAVR therapy.
The following sections will explore contemporary extra-thoracic alternative approaches for TAVR, including transsubclavian/axillary (which can also be accessed through surgical cut-down), transcarotid, and transcaval approaches.
Transaxillary access was first reported in 2008 in Munich, Germany (31). Axillary and subclavian access for TAVR has often been combined as one alternative TAVR access site. Most cases in reported registries have described the axillary access (infraclavicular) approach rather than the subclavian approach (supraclavicular) (54). Therefore, throughout the rest of the manuscript, the term transaxillary (TAx) will be used to refer to either transsubclavian or transaxillary. As mentioned, the 2019 STS-ACC TVT Registry reported that TAx was the most utilized site in the alternative access approach for TAVR. An increase in its use as an alternative access site of TAVR was observed, increasing from 20% in 2015 to 49% in 2017 for non-TF-TAVR cases (4).
Atherosclerosis of the iliofemoral system is often more pronounced than that of the axillary or subclavian arteries. In addition, the axillary artery has a vessel diameter similar to that of the iliofemoral artery, around 6 mm. These anatomical characteristics make them reasonable access target sites for TAVR (55).
Pre-procedural screening for TAx TAVR should be done using MDCT, similar to other access types. In patients with chronic kidney disease or other conditions that disallow them to undergo MDCT, arterial duplex ultrasound of the axillary vessels and a non-contrast CT can be done carefully to assess vessel caliber. CT vascular reconstruction of the axillary and subclavian vessels should be done, as typically done for the iliofemoral system. Anatomical characteristics are warranted to perform TAx TAVR, such as the presence of a minimal luminal diameter >5 mm (axillary diameter >5 mm for self-expanding and >5.5 mm for balloon-expandable valves), absence of severely calcified axillary artery, absence of excessive arterial tortuosity, absence of any preexisting vascular injury (i.e., dissection), and presence of an appropriate takeoff angle from the aortic arch (subclavian to arch angulation should be <80°). Consequently, Patients with a known history of coronary artery bypass graft and a patent left internal mammary artery can be considered potential candidates for TAx TAVR if needed (56, 57).
It is essential to identify the segments of the axillary artery during TAx TAVR. Anatomically, the first segment of the axillary artery is the most suitable site for access because, in this area, there are fewer vascular branches than in other areas of the axillary artery (58). Supplementary Figure S2 shows a zoomed-in section of the recommended target zone for the axillary artery access point. Consequently, in this area, the brachial plexus is present as a discrete bundle lying cranial to the axillary artery before dividing into significant branches. Regardless of which axillary segment is accessed, care should be taken to avoid injury to the brachial plexus. On the other hand, if supraclavicular access of the subclavian artery is done, extra caution should be made for the phrenic nerve, which runs superior to the subclavian vessel. Furthermore, the left axillary artery is preferred over the right side. One reason is a better angle for valve delivery in the aortic annulus. In addition, the left-side access site is usually suggested as it closely mimics the transfemoral approach (58).
Axillary artery can be accessed either through surgical cut-down of the artery or percutaneously. For open access, usually, under general anesthesia, operators undergo a sub clavicular skin incision in the deltopectoral groove, followed by subcutaneous tissue dissection through the pectoralis muscle to reveal and isolate the axillary artery. Subsequently, the artery is accessed through the Seldinger technique, a catheter is inserted, and an extra- or super-stiff wire is positioned within the left ventricular apex. A sheath is inserted into the axillary artery, and TAVR follows any regular procedure. At the end of the procedure, the sheath is removed, and the axillary artery is clamped and repaired using interrupted 5-0 Prolene sutures.
On the other hand, for complete percutaneous access, only conscious sedation is needed. The technique used for percutaneous axillary artery access is similar to percutaneous TF TAVR. The artery is punctured with a micropuncture needle under fluoroscopic and ultrasound guidance (57). Proper positioning of the wire in the axillary artery is confirmed under fluoroscopy. The inner cannula of the micropuncture dilator is advanced in the artery. A micropuncture wire is introduced again, and the dilator is upsized. Two Perclose ProGlide devices are placed in the axillary artery for suture-mediated artery closure (57). A sheath is then introduced and placed in the ascendant aorta. After the TAVR procedure, a peripheral balloon is advanced into the left axillary artery for percutaneous access closure and inflated to 1–4 atmospheres at the percutaneous insertion site to allow dry closure. It is done to facilitate proper hemostasis and minimize vascular access complications, but it can also be used in case of residual bleeding to tamponade the vessel. Perclose device is then used for closure (57).
A recent meta-analysis highlighted several observations comparing TAx TAVR to other access sites. The risk of major or life-threatening bleeding was higher via TAx TAVR compared with TF TAVR (OR = 1.51, p = 0.034). It might be due to the anatomical location of the subclavian artery, which requires a deep dissection for it to be exposed. In addition, TAx TAVR had a higher 30-day mortality rate (OR = 1.66%, p = 0.0002) and all-cause mortality (6–18 month follow-up) (OR = 1.2, p = 0.002) than the TF TAVR. The risk of stroke was significantly higher for TAx TAVR compared with TF TAVR and caval approaches (59). Consequently, a cohort study using the Society of Thoracic Surgeons/American College of Cardiology TVT Registry which included 1249 patients who underwent TAVR using TAx access showed a significantly higher stroke rate at 6.3% (54). This elevated stroke rate is consistent with the CoreValve Extreme Risk US Pivotal Trial data. However, the stroke rate was much higher than recorded in the PARTNER 2 trial. This discrepancy in stroke rates among several studies can be due to factors that need further investigation. Several questions should be asked, such as whether there is a benefit to using cerebral embolic protection devices during TAx access or whether there is a relationship between higher stroke rates and the side of the axillary access site used.
The transcarotid approach was first described in 2010 in France in a patient with severe tortuosity of the iliofemoral and subclavian arteries (32). It was then considered as a last resort due to the theoretical association of increased stroke risk from embolic events arising from carotid atheroma and valvular calcification.
Routine preoperative evaluations are needed, including carotid artery ultrasonography and computed tomography angiography of the chest, abdomen, and pelvis. Carotid site selection is determined according to several criteria, including minimal lumen diameter, degree of tortuosity, calcification, and percentage of contralateral carotid artery stenosis. A lumen diameter of 6.5 mm is considered the minimum accepted diameter for TC TAVR (60). Absence of vertebral, subclavian, and contralateral common carotid stenosis and congenital variants of the aortic arch is also recommended. If there is stenosis of more than 50% at the selected common carotid artery, this warrants using this side as the preferred access one to maximize cerebral flow from the contralateral patent artery. Some experts recommend the evaluation of patients who might be prone to cerebral hypoperfusion using cerebral magnetic resonance angiography with transcranial Doppler ultrasound to evaluate the circle of Willis and collateral cerebral blood flow. Intraoperatively, monitoring cerebral perfusion through cerebral oximetry or electroencephalography might be used to evaluate the need for carotid shunting (61). However, these perioperative neurovascular workups are not routinely done. Until today, no general consensus exists on using the previously mentioned diagnostic tools to accurately stratify candidates' risks of the TC TAVR procedure and its neurological burden.
This so-called “minimally-invasive nature of the procedure” is done through a cut-down approach with a 4 cm incision medial to the sternocleidomastoid muscle just above the clavicle to expose the common carotid artery. Care should be taken when dissecting the carotid sheath due to the potential risk of injuring nearby neurovascular structures, notably the vagus nerve. The carotid artery is accessed, and the sheath is placed using the Seldinger technique (60). Supplementary Figure S3 highlights steps in obtaining access to common carotid artery for TAVR. The TAVR sheath is positioned in place, and the valve is deployed. Upon the removal of the sheath, the common carotid artery is clamped proximally and distally, and the arteriotomy is repaired.
Allen et al. highlighted an important observation regarding the stroke risk and TC TAVR technique (62). The initial stroke risk with TC access in the French Transcarotid TAVR registry, which included 96 patients, was 6.3% (63). The French technique used by Mylotte and his colleagues included sequentially dilating the carotid artery before introducing the delivery sheath without clamping the distal portion of the common carotid artery during the procedure. However, the contemporary technique used for TC access evolved and includes distally clamping the common carotid artery during the procedure and using a transverse arteriotomy without serial dilation of the artery. After valve delivery, the artery is flushed for potential embolic remnants and repaired before the carotid clamp is released. This technique was described as a single-center experience report in two different institutions and was associated with stroke rates as low as 0%–2.4% (60, 64). Furthermore, an updated report from the French Transcarotid TAVR registry showed a significantly lower stroke rate of 1.6% compared to their initial report of 6.3%. This can be attributed to the TC access technique, which included transverse carotid arteriotomy with carotid clamping (65). Therefore, it is essential to highlight the observed association between the TC access technique and stroke rate.
Several studies have tried to evaluate the safety and outcomes of the transcarotid approach as an alternative access site for TAVR. Supplementary Figure S4 summarizes the results of a meta-analysis by Usman et al., which displays the incidence of different outcomes in TC TAVR (66). The study also highlighted an important observation where the incidence of stroke and TIA decreased throughout the years of performing TC TAVR which might be attributed to improved skill acquisition and operator experience. Furthermore, a lower major vascular complication was seen (pooled estimate 2.4%) when compared with the PARTNER 2 trial (7.9%) (67) and SURTAVI trial (6%) (33). A recent meta-analysis compared TC TAVR data against each alternative access site. The key findings were as follows: TC had higher short-term mortality rates and lower rates of vascular complications than transfemoral. TC was associated with a lower mortality rate than that of transaortic and transapical access. Compared with transaxillary access, TC had lower contrast volume, faster procedure time, and lower rate of major vascular complications. However, TC was associated with higher major bleeding than transaxillary access. More importantly, when it comes to TC access, no significant increase in CVA events was seen compared to other accesses (68).
With the increase in operator experience with TC TAVR and the compelling evidence of its safety and effectiveness, TC TAVR has increased compared to other alternative accesses. Some centers have utilized TC access as the preferred alternative access site for TAVR (60).
Transcaval (TCv) access initially originated as a concept in 2010 as an alternate method to deliver larger caliber delivery devices to the abdominal aorta from extra-thoracic access. With additional testing in animal models, the first-in-human TCv TAVR procedure was performed in 2013 (69, 70). Since then, in addition to TAVR, TCv access use has expanded to help deliver transcatheter endovascular aortic repair (TEVAR), transcatheter temporary mechanical circulatory support (tMCS) when limb ischemia is of concern, and in pediatric interventional cardiology. TCv TAVR involves the placement of an introducer sheath into the femoral vein with the advancement of an electrosurgical guidewire into the inferior vena cava (IVC). Pre-procedural MDCT-based evaluation of a calcium-free target window in the infrarenal abdominal aorta is localized with fluoroscopy. The electrosurgical wire is then used to traverse the wall of the IVC, through the retroperitoneal space, and into the aorta at the planned aortotomy site, thereby creating an arteriovenous fistula. The electrosurgical wire is then snared and exchanged for a rigid wire, and the procedure proceeds via a standard approach for retrograde TAVR. Post-delivery, the TCv tract is closed with a nitinol occluder device, and a closure aortogram is completed to identify any complications. Often, if existent, minimal residual aorto-caval fistula is acceptable. However, severe extravasation may require covered stenting or rarely surgical repair (69). Figure 3 highlights a schematic depiction of caval-aortic access as described above.
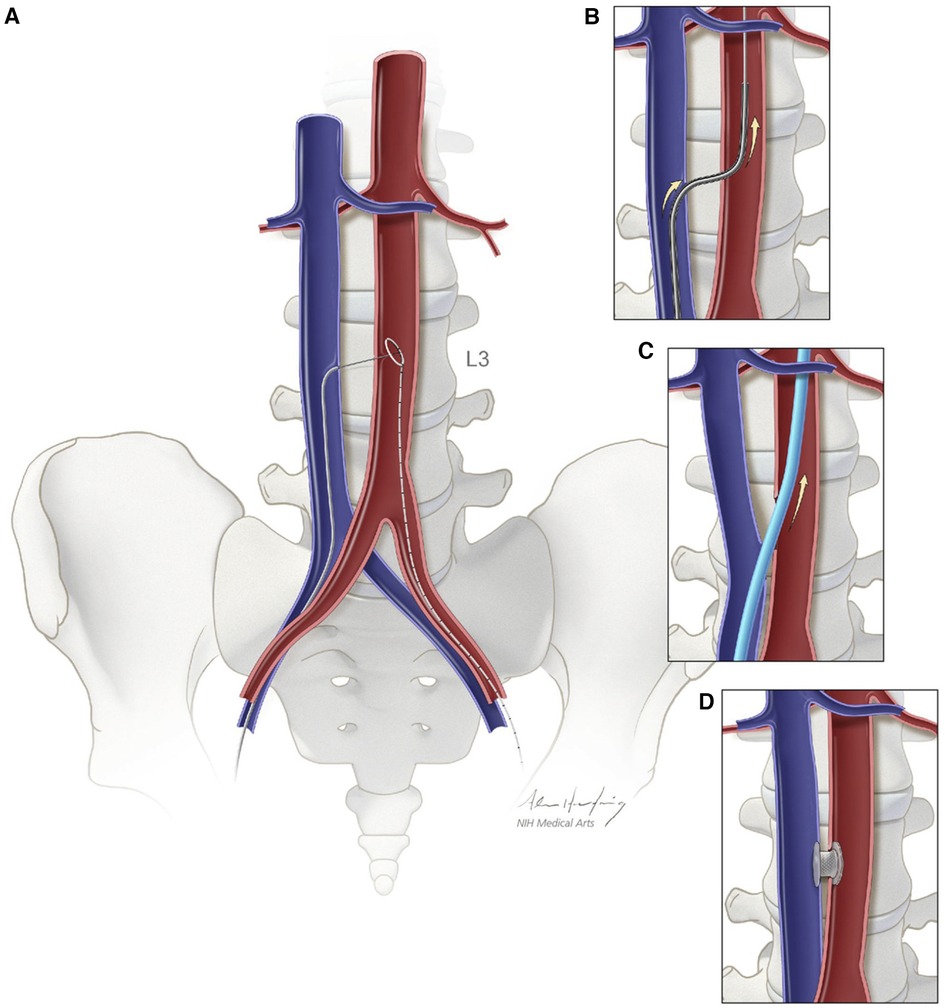
Figure 3. Schematic depiction of caval-aortic access. (A) A catherter directs a transfemoral vein guidewire from the inferior vena cava towards a snare target positioned in the adjoining abdominal aorta. (B) A catheter is advanced over the guidewire into the aorta and used to introduce a more rigid guidewire. (C) The valve introducer sheath is advanced from the vena cava into the aorta. (D) After completion of TAVR, the aorto-caval access tract is closed with a nitinol occluder. Reprinted from Greenbaum AB, O'Neill WW, Paone G, Guerrero ME, Wyman JF, Cooper RL, Lederman RJ. Caval-aortic access allow transcatheter aortic valve replacement in otherwise ineligible patients: initial human experience. J AM Coll Cardiol. 2014 Jul 1:63., with permission from Elsevier.
The TCv approach utilizes the physiology of the retroperitoneal space to its advantage. In prior observation surgical experience, it was often thought that aortic perforation in the retroperitoneum would cause life-threatening hemorrhage. However, this is only true for an open retroperitoneum, as the hydrostatic pressure of the space is compromised. In situ, the retroperitoneal hydrostatic pressure is around 20 mmHg, higher than that of the IVC, around ten mmHg. Thus, any aortic blood extravasation will favor the IVC instead of the retroperitoneum, creating a well-tolerated shunt without significant hemodynamic consequences (71, 72).
Transcaval target selection is primarily based on sufficiently large non-circumferential calcium-free aortic window (typically >2 mm larger than delivery sheath), absence of interposed tissue such as bowel or mesenteric arteries, and adequate distance from aortic branches in case there is a need for covered stent bailout. Non-ideal targets include those with synthetic grafted endovascular repair or aortic aneurysm; however, these are still suitable. Relatively contraindicated targets include aortic dissection, pedunculated atheromata, or aortic stents (72). IVC filters are considered soft contraindications because TCv can be done above, below, or alongside the filter. It is recommended to identify bony landmarks like the lumbar spines and the iliac crest on CT scan and angiographic landmarks (aortoiliac bifurcation and renal artery) by fluoroscopy to ensure safe TCv access and closure. These images can be displayed during the procedure to help guide the operator.
In current practice, the aorto-caval tract is closed using the off-label nitinol cardiac occluder device - Amplatzer Duct Occluder. This device demonstrated favorable and acceptable results (72). To be noted, this occluder is incompletely hemostatic directly after deployment, prone to pull-through, and may result in the need for blood transfusion or covered stent usage (73). In addition, some patients with significantly impaired right ventricular function may poorly tolerate persistent left-right shunting and should be closely monitored. A first-in-human test of a dedicated Transcaval Closure Device was developed and studied for 12 patients undergoing TCv. The study showed promising outcomes, where all patients had complete closure of the transcaval tract 30 days post-discharge and the absence of vascular or bleeding complications (73). However, further trials with a larger sample size are needed if widespread adoption of this approach will take place.
In clinical outcomes studies, transcaval access is a safe approach compared to other alternative access techniques. Barbash et al. investigated 185 patients, of whom 12% underwent transcaval TAVR, and 82% underwent alternative access TAVR. Their results noted a lower incidence of AKI in the transcaval cohort and shorter hospital stays but no difference in early or 30-day mortality (74). Lederman et al. conducted a meta-analysis of transcaval access with transaxillary access and concluded lower stroke and similar bleeding rates across eight centers in the United States (75). A recent systematic review comparing TCv TAVR against supra-aortic (TC and TAx) TAVR found no statistically significant difference between in-hospital or 30-day all-cause mortality, major bleeding, need for blood transfusions, major vascular complications, and kidney injury. Notably, TCv TAVR was associated with a lower rate of neurovascular complications but failed to reach statistical significance (76).
With increasing technological advancements in TAVR devices and delivery systems, in addition to growing operator experience, the number of TF TAVR procedures across a diverse patient profile with different risk factors continues to increase, becoming the vast majority of daily cases performed. Alternative access site approaches have been an essential and needed solution for the minority of patients who cannot undergo TAVR through the femoral approach. Facilitated TF TAVR, with the use of multiple dilators and/or IVL-assisted TF access, has somehow limited the need for alternative access sites for patients previously considered unable to undergo TF TAVR. It has demonstrated promising outcomes (44, 45, 47, 77). However, randomized studies on its use are still lacking. When alternative access is needed, current evidence strongly recommends an extra-thoracic approach rather than a thoracic one, which limits the role of both TAo and TA TAVR (Graphical Abstract) (50, 51, 53). TAx and TC approaches have been the most utilized alternative approaches (4). Many centers have depended on the TC approach as their first alternative access site (60). The TC approach's strong evidence on stroke risks has made it a compelling alternative for patients with unfavorable anatomy for TF TAVR (60, 64–66). On the other hand, TCv access has demonstrated favorable outcomes (74–76). However, some limitations remain that should be addressed, such as its significant learning curve, available data regarding its bleeding risk, effect of the potential remaining large vessel shunt, availability of specialized materials needed, and its relatively higher cost. TCv is currently considered to be an off-label treatment option for TAVR. Tables 1, 2 highlight the major studies on outcomes for alternative TAVR access methods and their procedural characteristic, mainly operator position and anesthesia mode, respectively (52, 57, 74, 83, 84).
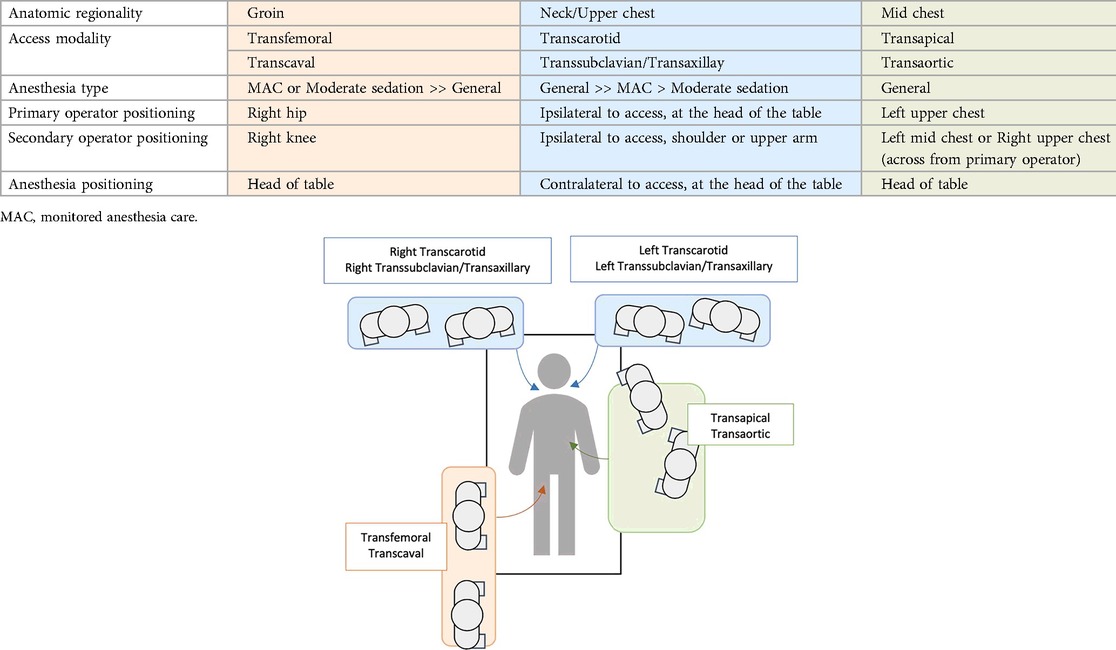
Table 2. Summary of procedural characteristics of different TAVR access methods- operator position and anesthesia mode (52, 57, 74, 83, 84).
Only observational studies have compared the different alternative approaches. Therefore, randomized controlled trials are still warranted to safely ensure an optimal approach for choosing the appropriate access site for a given patient profile. However, conducting such studies would be complex since this alternative approach comes as a second choice when the transfemoral approach is not an option, which would limit inclusion (61). In addition, anatomical conditions that enable an alternative approach over the other would promote selection bias in a comparative study. Subsequently, institutional expertise in one approach over another would yield inconsistent results.
Notably, a multidisciplinary heart team consisting of imaging experts, interventionalists, and cardiac surgeons should be the cornerstone of any TAVR center of excellence. Proper planning of TAVR procedures yields superior outcomes. Expert consensus regarding operator and institutional recommendations for TAVR has emphasized the importance of a TAVR center to expand to at least one alternative access method other than TF TAVR (85). With the available data, no one extra-thoracic alternative approach for TF TAVR is preferred over the other. Each approach has its advantages and risks, which should be assessed on a case-by-case basis. Every TAVR center should have an algorithm for the TAVR access site strategy. This depends on the TAVR volume, expertise, and personal preference of the operators with each access site. Therefore, TAVR centers can rely on their team's expertise to safely choose the best alternative access site in each case, based on available data, personal preference, and skills set.
Figure 4 represents an algorithm solely based on the authors' personal opinions on what they use in their practice, highlighting the approach used to choose TAVR access sites.
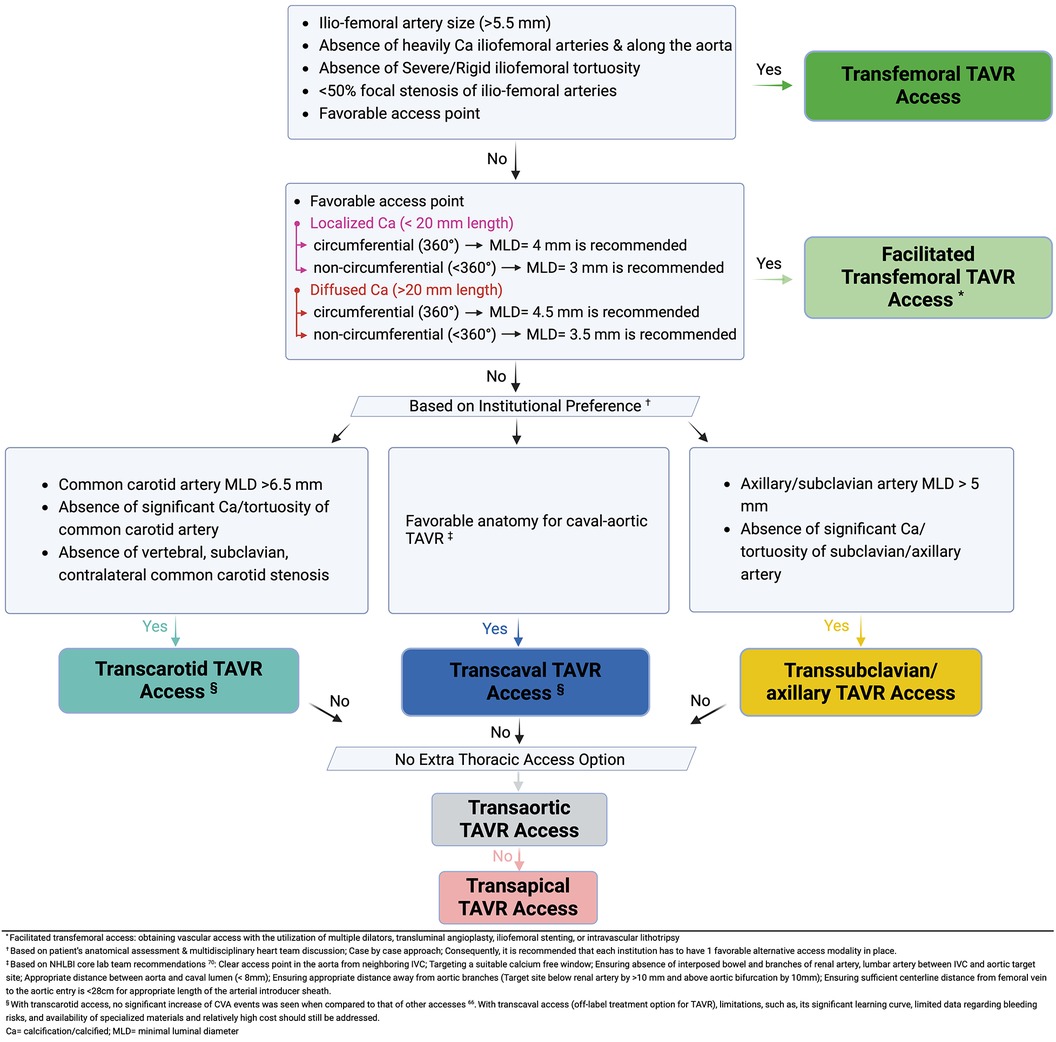
Figure 4. Multidisciplinary structural heart team algorithm for TAVR access site suitability (46, 70).
Interventionalists continue to explore TAVR through other alternative access sites. Recently published reports describing attempted TAVR through the brachial artery had promising early results (86–88). Although access through the brachial artery may present as an attractive option, multiple limitations and risks are associated with this approach (86, 87). On the other hand, biotechnology research and development innovators are constantly working on novel TAVR delivery systems, devices, and leaflet technologies to safely expand TF TAVR to almost all patients, regardless of their risk profile, which may ultimately limit alternative access approaches even more (34).
Alternative approaches for transfemoral TAVR currently remain an essential option in a subset population with unfavorable iliofemoral vasculature anatomy. The ongoing development of future pipeline technologies in TAVR valves and delivery systems may help minimize the current limitations and refine procedural techniques. Future studies on upcoming novel TAVR devices and delivery systems would be interesting to see how they will affect our current alternative approaches for TAVR.
MA: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. VP: Conceptualization, Writing – original draft, Methodology. PP: Project administration, Resources, Supervision, Validation, Writing – review & editing. AK: Supervision, Validation, Writing – review & editing. AF: Supervision, Validation, Writing – review & editing, Conceptualization, Writing – original draft.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1437626/full#supplementary-material
1. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. (2010) 363(17):1597–607. doi: 10.1056/NEJMoa1008232
2. Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, et al. Heart disease and stroke statistics-2023 update: a report from the American Heart Association. Circulation. (2023) 147(8):e93–621. doi: 10.1161/CIR.0000000000001123
3. Généreux P, Sharma RP, Cubeddu RJ, Aaron L, Abdelfattah OM, Koulogiannis KP, et al. The mortality burden of untreated aortic stenosis. J Am Coll Cardiol. (2023) 82(22):2101–9. doi: 10.1016/j.jacc.2023.09.796
4. Carroll JD, Mack MJ, Vemulapalli S, Herrmann HC, Gleason TG, Hanzel G, et al. STS-ACC TVT registry of transcatheter aortic valve replacement. J Am Coll Cardiol. (2020) 76(21):2492–516. doi: 10.1016/j.jacc.2020.09.595
5. Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O'Hair D, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. (2019) 380(18):1706–15. doi: 10.1056/NEJMoa1816885
6. Forrest JK, Deeb GM, Yakubov SJ, Gada H, Mumtaz MA, Ramlawi B, et al. 3-year outcomes after transcatheter or surgical aortic valve replacement in low-risk patients with aortic stenosis. J Am Coll Cardiol. (2023) 81(17):1663–74. doi: 10.1016/j.jacc.2023.02.017
7. Mack MJ, Leon MB. Transcatheter aortic-valve replacement in low-risk patients at five years. Reply. N Engl J Med. (2024) 390(9):866. doi: 10.1056/NEJMc2314291
8. Forrest JK, Deeb GM, Yakubov SJ, Gada H, Mumtaz MA, Ramlawi B, et al. 4-Year Outcomes of patients with aortic stenosis in the evolut low risk trial. J Am Coll Cardiol. (2023) 82(22):2163–5. doi: 10.1016/j.jacc.2023.09.813
9. Cribier A, Eltchaninoff H, Tron C, Bauer F, Agatiello C, Sebagh L, et al. Early experience with percutaneous transcatheter implantation of heart valve prosthesis for the treatment of end-stage inoperable patients with calcific aortic stenosis. J Am Coll Cardiol. (2004) 43(4):698–703. doi: 10.1016/j.jacc.2003.11.026
10. Cribier A, Savin T, Saoudi N, Rocha P, Berland J, Letac B. Percutaneous transluminal valvuloplasty of acquired aortic stenosis in elderly patients: an alternative to valve replacement? Lancet. (1986) 1(8472):63–7. doi: 10.1016/S0140-6736(86)90716-6
11. McKay RG, Investigators MSAVR. The mansfield scientific aortic valvuloplasty registry: overview of acute hemodynamic results and procedural complications. J Am Coll Cardiol. (1991) 17(2):485–91. doi: 10.1016/S0735-1097(10)80120-2
12. Cribier A, Savin T, Berland J, Rocha P, Mechmeche R, Saoudi N, et al. Percutaneous transluminal balloon valvuloplasty of adult aortic stenosis: report of 92 cases. J Am Coll Cardiol. (1987) 9(2):381–6. doi: 10.1016/S0735-1097(87)80392-3
13. Block PC, Palacios IF. Clinical and hemodynamic follow-up after percutaneous aortic valvuloplasty in the elderly. Am J Cardiol. (1988) 62(10):760–3. doi: 10.1016/0002-9149(88)91218-0
14. Svensson LG, Tuzcu M, Kapadia S, Blackstone EH, Roselli EE, Gillinov AM, et al. A comprehensive review of the PARTNER trial. J Thorac Cardiovasc Surg. (2013) 145(3 Suppl):S11–6. doi: 10.1016/j.jtcvs.2012.11.051
15. Andersen HR, Knudsen LL, Hasenkam JM. Transluminal implantation of artificial heart valves. Description of a new expandable aortic valve and initial results with implantation by catheter technique in closed chest pigs. Eur Heart J. (1992) 13(5):704–8. doi: 10.1093/oxfordjournals.eurheartj.a060238
16. Bonhoeffer P, Boudjemline Y, Saliba Z, Hausse AO, Aggoun Y, Bonnet D, et al. Transcatheter implantation of a bovine valve in pulmonary position: a lamb study. Circulation. (2000) 102(7):813–6. doi: 10.1161/01.CIR.102.7.813
17. Boudjemline Y, Bonhoeffer P. Steps toward percutaneous aortic valve replacement. Circulation. (2002) 105(6):775–8. doi: 10.1161/hc0602.103361
18. Šochman J, Peregrin JH, Pavčnik D, Timmermans H, Rösch J. Percutaneous transcatheter aortic disc valve prosthesis implantation: a feasibility study. Cardiovasc Intervent Radiol. (2000) 23:384–8. doi: 10.1007/s002700010060
19. Boudjemline Y, Bonhoeffer P. Percutaneous implantation of a valve in the descending aorta in lambs. Eur Heart J. (2002) 23(13):1045–9. doi: 10.1053/euhj.2001.3076
20. Cribier A, Eltchaninoff H, Bareinstein N, Daniel P, Laborde F, Leon M. Trans-catheter implantation of balloon-expandable prosthetic heart valves: early results in an animal model. Circulation. (2001) 104(17):552.
21. Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer F, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. (2002) 106(24):3006–8. doi: 10.1161/01.cir.0000047200.36165.b8
22. Cribier A. The development of transcatheter aortic valve replacement (TAVR). Glob Cardiol Sci Pract. (2016) 2016(4):e201632. doi: 10.21542/gcsp.2016.32
23. Bourantas CV, Serruys PW. Evolution of transcatheter aortic valve replacement. Circ Res. (2014) 114(6):1037–51. doi: 10.1161/CIRCRESAHA.114.302292
24. Paniagua D, Condado JA, Besso J, Vélez M, Burger B, Bibbo S, et al. First human case of retrograde transcatheter implantation of an aortic valve prosthesis. Tex Heart Inst J. (2005) 32(3):393.16392228
25. Figulla HR, Franz M, Lauten A. The history of transcatheter aortic valve implantation (TAVI)-A personal view over 25 years of development. Cardiovasc Revasc Med. (2020) 21(3):398–403. doi: 10.1016/j.carrev.2019.05.024
26. Webb JG, Pasupati S, Humphries K, Thompson C, Altwegg L, Moss R, et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation. (2007) 116(7):755–63. doi: 10.1161/CIRCULATIONAHA.107.698258
27. Grube E, Laborde JC, Zickmann B, Gerckens U, Felderhoff T, Sauren B, et al. First report on a human percutaneous transluminal implantation of a self-expanding valve prosthesis for interventional treatment of aortic valve stenosis. Catheter Cardiovasc Interv. (2005) 66(4):465–9. doi: 10.1002/ccd.20544
28. Walther T, Falk V, Borger MA, Dewey T, Wimmer-Greinecker G, Schuler G, et al. Minimally invasive transapical beating heart aortic valve implantation–proof of concept. Eur J Cardiothorac Surg. (2007) 31(1):9–15. doi: 10.1016/j.ejcts.2006.10.034
29. Ye J, Cheung A, Lichtenstein SV, Carere RG, Thompson CR, Pasupati S, et al. Transapical aortic valve implantation in humans. J Thorac Cardiovasc Surg. (2006) 131(5):1194–6. doi: 10.1016/j.jtcvs.2006.01.026
30. Bauernschmitt R, Schreiber C, Bleiziffer S, Ruge H, Mazzitelli D, Hutter A, et al. Transcatheter aortic valve implantation through the ascending aorta: an alternative option for no-access patients. Heart Surg Forum. (2009) 12(1):E63–4. doi: 10.1532/HSF98.20081112
31. Ruge H, Lange R, Bleiziffer S, Hutter A, Mazzitelli D, Will A, et al. First successful aortic valve implantation with the CoreValve ReValving system via right subclavian artery access: a case report. Heart Surg Forum. (2008) 11(5):E323–4. doi: 10.1532/HSF98.20081021
32. Modine T, Lemesle G, Azzaoui R, Sudre A. Aortic valve implantation with the CoreValve ReValving system via left carotid artery access: first case report. J Thorac Cardiovasc Surg. (2010) 140(4):928–9. doi: 10.1016/j.jtcvs.2010.03.001
33. Reardon MJ, Mieghem NMV, Popma JJ, Kleiman NS, Søndergaard L, Mumtaz M, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. (2017) 376(14):1321–31. doi: 10.1056/NEJMoa1700456
34. Zaid S, Atkins MD, Kleiman NS, Reardon MJ, Tang GHL. What’s new with TAVR? An update on device technology. Methodist Debakey Cardiovasc J. (2023) 19(3):4–14. doi: 10.14797/mdcvj.1230
35. Chamandi C, Puri R, Rodriguez-Gabella T, Rodés-Cabau J. Latest-generation transcatheter aortic valve replacement devices and procedures. Can J Cardiol. (2017) 33(9):1082–90. doi: 10.1016/j.cjca.2017.03.012
36. Cahill TJ, Chen M, Hayashida K, Latib A, Modine T, Piazza N, et al. Transcatheter aortic valve implantation: current status and future perspectives. Eur Heart J. (2018) 39(28):2625–34. doi: 10.1093/eurheartj/ehy244
37. Burri M, Ruge H, Erlebach M, Lange R. Surgical cutdown avoids vascular complications in transcatheter aortic valve replacement in calcified and small femoral arteries. Thorac Cardiovasc Surg. (2022) 70(3):199–204. doi: 10.1055/s-0041-1725202
38. Nakamura M, Chakravarty T, Jilaihawi H, Doctor N, Dohad S, Fontana G, et al. Complete percutaneous approach for arterial access in transfemoral transcatheter aortic valve replacement: a comparison with surgical cut-down and closure. Catheter Cardiovasc Interv. (2014) 84(2):293–300. doi: 10.1002/ccd.25130
39. Michel J, Frangieh AH, Ott I, Kasel AM. Bending the rules in transfemoral TAVI with the SAPIEN 3: overcoming severe iliac tortuosity. Heart Lung Circ. (2017) 26(8):e50–3. doi: 10.1016/j.hlc.2017.02.007
40. Kindzelski B, Mick SL, Krishnaswamy A, Kapadia SR, Attia T, Hodges K, et al. Evolution of alternative-access transcatheter aortic valve replacement. Ann Thorac Surg. (2021) 112(6):1877–85. doi: 10.1016/j.athoracsur.2021.02.018
41. Blackstone EH, Suri RM, Rajeswaran J, Babaliaros V, Douglas PS, Fearon WF, et al. Propensity-matched comparisons of clinical outcomes after transapical or transfemoral transcatheter aortic valve replacement: a placement of aortic transcatheter valves (PARTNER)-I trial substudy. Circulation. (2015) 131(22):1989–2000. doi: 10.1161/CIRCULATIONAHA.114.012525
42. Conrotto F, D'Ascenzo F, Francesca G, Colaci C, Sacciatella P, Biondi-Zoccai G, et al. Impact of access on TAVI procedural and midterm follow-up: a meta-analysis of 13 studies and 10,468 patients. J Interv Cardiol. (2014) 27(5):500–8. doi: 10.1111/joic.12141
43. Beurtheret S, Karam N, Resseguier N, Houel R, Modine T, Folliguet T, et al. Femoral versus nonfemoral peripheral access for transcatheter aortic valve replacement. J Am Coll Cardiol. (2019) 74(22):2728–39. doi: 10.1016/j.jacc.2019.09.054
44. Palmerini T, Saia F, Kim WK, Renker M, Iadanza A, Fineschi M, et al. Vascular access in patients with peripheral arterial disease undergoing TAVR: the hostile registry. JACC Cardiovasc Interv. (2023) 16(4):396–411. doi: 10.1016/j.jcin.2022.12.009
45. Di Mario C, Goodwin M, Ristalli F, Ravani M, Meucci F, Stolcova M, et al. A prospective registry of intravascular lithotripsy-enabled vascular access for transfemoral transcatheter aortic valve replacement. JACC Cardiovasc Interv. (2019) 12(5):502–4. doi: 10.1016/j.jcin.2019.01.211
46. Kempton H, Roy A, Watson A, Evans D, Muller D, Roy D. Using intravascular lithotripsy to facilitate transfemoral arterial access for transcatheter aortic valve implantation. Heart Lung Circ. (2022) 31(11):e135–9. doi: 10.1016/j.hlc.2022.07.016
47. Nardi G, De Backer O, Saia F, Søndergaard L, Ristalli F, Meucci F, et al. Peripheral intravascular lithotripsy for transcatheter aortic valve implantation: a multicentre observational study. EuroIntervention. (2022) 17(17):e1397–406. doi: 10.4244/EIJ-D-21-00581
48. Wong CK, Chiu ACH, Chan KE, Sze SY, Tam FC, Un KC, et al. Advances in technology and techniques for transcatheter aortic valve replacement with concomitant peripheral arterial disease. Front Med Technol. (2022) 4:959249. doi: 10.3389/fmedt.2022.959249
49. Nakatsuka D, Tabata M. Transapical approach for transcatheter aortic valve implantation. Ann Cardiothorac Surg. (2017) 6(5):553–4. doi: 10.21037/acs.2017.09.01
50. Thourani VH, Jensen HA, Babaliaros V, Suri R, Vemulapalli S, Dai D, et al. Transapical and transaortic transcatheter aortic valve replacement in the United States. Ann Thorac Surg. (2015) 100(5):1718–26; discussion 26–7. doi: 10.1016/j.athoracsur.2015.05.010
51. O'Hair DP, Bajwa TK, Popma JJ, Watson DR, Yakubov SJ, Adams DH, et al. Direct aortic access for transcatheter aortic valve replacement using a self-expanding device. Ann Thorac Surg. (2018) 105(2):484–90. doi: 10.1016/j.athoracsur.2017.07.051
52. Stastny L, Krapf C, Dumfarth J, Gasser S, Bauer A, Friedrich G, et al. Minireview: transaortic transcatheter aortic valve implantation: is there still an indication? Front Cardiovasc Med. (2022) 9:798154. doi: 10.3389/fcvm.2022.798154
53. Lanz J, Greenbaum A, Pilgrim T, Tarantini G, Windecker S. Current state of alternative access for transcatheter aortic valve implantation. EuroIntervention. (2018) 14(AB):AB40–52. doi: 10.4244/EIJ-D-18-00552
54. Dahle TG, Kaneko T, McCabe JM. Outcomes following subclavian and axillary artery access for transcatheter aortic valve replacement: society of the thoracic surgeons/American College of Cardiology TVT registry report. JACC Cardiovasc Interv. (2019) 12(7):662–9. doi: 10.1016/j.jcin.2019.01.219
55. Edelman JJ, Meduri C, Yadav P, Thourani VH. Current evidence for alternative access transcatheter aortic valve replacement. Struct Heart. (2020) 4(6):453–7. doi: 10.1080/24748706.2020.1821936
56. Bapat V, Tang GHL. Axillary/subclavian transcatheter aortic valve replacement: the default alternative access? JACC Cardiovasc Interv. (2019) 12(7):670–2. doi: 10.1016/j.jcin.2019.02.017
57. Harloff MT, Percy ED, Hirji SA, Yazdchi F, Shim H, Chowdhury M, et al. A step-by-step guide to trans-axillary transcatheter aortic valve replacement. Ann Cardiothorac Surg. (2020) 9(6):510–21. doi: 10.21037/acs-2020-av-79
58. Mathur M, Krishnan SK, Levin D, Aldea G, Reisman M, McCabe JM. A step-by-step guide to fully percutaneous transaxillary transcatheter aortic valve replacement. Struct Heart. (2017) 1(5):209–15. doi: 10.1080/24748706.2017.1370156
59. Yokoyama Y, Sakata T, Mikami T, Misumida N, Scotti A, Takagi H, et al. Vascular access for transcatheter aortic valve replacement: a network meta-analysis. J Cardiol. (2023) 82(4):227–33. doi: 10.1016/j.jjcc.2023.04.015
60. Kirker EB, Hodson RW, Spinelli KJ, Korngold EC. The carotid artery as a preferred alternative access route for transcatheter aortic valve replacement. Ann Thorac Surg. (2017) 104(2):621–9. doi: 10.1016/j.athoracsur.2016.12.030
61. Overtchouk P, Modine T. A comparison of alternative access routes for transcatheter aortic valve implantation. Expert Rev Cardiovasc Ther. (2018) 16(10):749–56. doi: 10.1080/14779072.2018.1524295
62. Allen KB, Watson D, Vora AN, Mahoney P, Chhatriwalla AK, Schwartz JG, et al. Transcarotid versus transaxillary access for transcatheter aortic valve replacement with a self-expanding valve: a propensity-matched analysis. JTCVS Tech. (2023) 21:45–55. doi: 10.1016/j.xjtc.2023.07.019
63. Mylotte D, Sudre A, Teiger E, Obadia JF, Lee M, Spence M, et al. Transcarotid transcatheter aortic valve replacement: feasibility and safety. JACC Cardiovasc Interv. (2016) 9(5):472–80. doi: 10.1016/j.jcin.2015.11.045
64. Allen KB, Chhatriwalla AK, Cohen D, Saxon J, Hawa Z, Kennedy KF, et al. Transcarotid versus transapical and transaortic access for transcatheter aortic valve replacement. Ann Thorac Surg. (2019) 108(3):715–22. doi: 10.1016/j.athoracsur.2019.02.007
65. Overtchouk P, Folliguet T, Pinaud F, Fouquet O, Pernot M, Bonnet G, et al. Transcarotid approach for transcatheter aortic valve replacement with the sapien 3 prosthesis: a multicenter French registry. JACC Cardiovasc Interv. (2019) 12(5):413–9. doi: 10.1016/j.jcin.2018.11.014
66. Usman MS, Rawasia WF, Siddiqi TJ, Mujeeb FA, Nadeem S, Alkhouli M. Meta-analysis evaluating the safety and efficacy of transcarotid transcatheter aortic valve implantation. Am J Cardiol. (2019) 124(12):1940–6. doi: 10.1016/j.amjcard.2019.09.015
67. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. (2016) 374(17):1609–20. doi: 10.1056/NEJMoa1514616
68. Abraham B, Sous M, Sedhom R, Megaly M, Roman S, Sweeney J, et al. Meta-analysis on transcarotid versus transfemoral and other alternate accesses for transcatheter aortic valve implantation. Am J Cardiol. (2023) 192:196–205. doi: 10.1016/j.amjcard.2023.01.023
69. Greenbaum AB, O'Neill WW, Paone G, Guerrero ME, Wyman JF, Cooper RL, et al. Caval-aortic access to allow transcatheter aortic valve replacement in otherwise ineligible patients: initial human experience. J Am Coll Cardiol. (2014) 63(25 Pt A):2795–804. doi: 10.1016/j.jacc.2014.04.015
70. Halabi M, Ratnayaka K, Faranesh AZ, Chen MY, Schenke WH, Lederman RJ. Aortic access from the vena cava for large caliber transcatheter cardiovascular interventions: pre-clinical validation. J Am Coll Cardiol. (2013) 61(16):1745–6. doi: 10.1016/j.jacc.2013.01.057
71. Muhammad KI, Tokarchik GC. Transcaval transcatheter aortic valve replacement: a visual case review. J Vis Surg. (2018) 4:102. doi: 10.21037/jovs.2018.04.02
72. Lederman RJ, Greenbaum AB, Khan JM, Bruce CG, Babaliaros VC, Rogers T. Transcaval access and closure best practices. JACC Cardiovasc Interv. (2023) 16(4):371–95. doi: 10.1016/j.jcin.2022.12.005
73. Rogers T, Greenbaum AB, Babaliaros VC, Stine AM, Khan JM, Schenke WH, et al. Dedicated closure device for transcaval access closure: from concept to first-in-human testing. JACC Cardiovasc Interv. (2019) 12(21):2198–206. doi: 10.1016/j.jcin.2019.05.053
74. Barbash IM, Segev A, Berkovitch A, Fefer P, Maor E, Elian D, et al. Clinical outcome and safety of transcaval access for transcatheter aortic valve replacement as compared to other alternative approaches. Front Cardiovasc Med. (2021) 8:731639. doi: 10.3389/fcvm.2021.731639
75. Lederman RJ, Babaliaros VC, Lisko JC, Rogers T, Mahoney P, Foerst JR, et al. Transcaval versus transaxillary TAVR in contemporary practice: a propensity-weighted analysis. JACC Cardiovasc Interv. (2022) 15(9):965–75. doi: 10.1016/j.jcin.2022.03.014
76. Antiochos P, Kirsch M, Monney P, Tzimas G, Meier D, Fournier S, et al. Transcaval versus supra-aortic vascular accesses for transcatheter aortic valve replacement: a systematic review with meta-analysis. J Clin Med. (2024) 13(2):455. doi: 10.3390/jcm13020455
77. Armstrong EJ, Soukas PA, Shammas N, Chamberlain J, Pop A, Adams G, et al. Intravascular lithotripsy for treatment of calcified, stenotic iliac arteries: a cohort analysis from the disrupt PAD III study. Cardiovasc Revasc Med. (2020) 21(10):1262–8. doi: 10.1016/j.carrev.2020.02.026
78. Fröhlich GM, Baxter PD, Malkin CJ, Scott DJ, Moat NE, Hildick-Smith D, et al. Comparative survival after transapical, direct aortic, and subclavian transcatheter aortic valve implantation (data from the UK TAVI registry). Am J Cardiol. (2015) 116(10):1555–9. doi: 10.1016/j.amjcard.2015.08.035
79. Kirker E, Korngold E, Hodson RW, Jones BM, McKay R, Cheema M, et al. Transcarotid versus subclavian/axillary access for transcatheter aortic valve replacement with SAPIEN 3. Ann Thorac Surg. (2020) 110(6):1892–7. doi: 10.1016/j.athoracsur.2020.05.141
80. Folliguet TA, Teiger E, Beurtheret S, Modine T, Lefevre T, Van Belle E, et al. Carotid versus femoral access for transcatheter aortic valve implantation: a propensity score inverse probability weighting study. Eur J Cardiothorac Surg. (2019) 56(6):1140–6. doi: 10.1093/ejcts/ezz216
81. Debry N, Trimech TR, Gandet T, Vincent F, Hysi I, Delhaye C, et al. Transaxillary compared with transcarotid access for TAVR: a propensity-matched comparison from a French multicentre registry. EuroIntervention. (2020) 16(10):842–9. doi: 10.4244/EIJ-D-20-00117
82. Paone G, Eng M, Kabbani LS, Borgi J, Peterson E, Novitsky B, et al. Transcatheter aortic valve replacement: comparing transfemoral, transcarotid, and transcaval access. Ann Thorac Surg. (2018) 106(4):1105–12. doi: 10.1016/j.athoracsur.2018.04.029
83. McKiernan M, Grubb KJ, Guyton RA, Paone G. Transcarotid transcatheter aortic valve replacement. Oper Tech Thorac Cardiovasc Surg. (2021) 26(2):224–43. doi: 10.1053/j.optechstcvs.2020.12.001
84. Mieghem NMV, Tijssen J. Alternative access for TAVR. JACC Cardiovasc Interv. (2022) 15(9):976–8. doi: 10.1016/j.jcin.2022.04.001
85. Deeb GM. 2018 AATS/ACC/SCAI/STS expert consensus systems of care document: operator and institutional recommendations and requirements for transcatheter aortic valve replacement. (2019).
86. Topalo R, Hájek P, Vik K, Adlová R, Horn M, Veselka J. Brachial artery access for transcatheter aortic valve implantation. Postepy Kardiol Interwencyjnej. (2021) 17:124–5. doi: 10.5114/aic.2021.104782
87. Saad M, Elhakim A, Rusch R, Berndt R, Panholzer B, Lutter G, et al. Trans-brachial TAVI in a patient with aortic isthmus stenosis: a case report. J Clin Med. (2024) 13(2):308. doi: 10.3390/jcm13020308
Keywords: TAVR, TAVR - current and future, TAVI, TAVR alternative access, aortic stenosis, TAVR history, transcaval access
Citation: Abdelnour MW, Patel V, Patel PM, Kasel AM and Frangieh AH (2024) Alternative access in transcatheter aortic valve replacement—an updated focused review. Front. Cardiovasc. Med. 11:1437626. doi: 10.3389/fcvm.2024.1437626
Received: 24 May 2024; Accepted: 22 July 2024;
Published: 8 August 2024.
Edited by:
Alfonso Ielasi, IRCCS Ospedale Galeazzi Sant'Ambrogio, ItalyReviewed by:
Droc Ionel, Central Military Hospital Bucharest Romania, Romania© 2024 Abdelnour, Patel, Patel, Kasel and Frangieh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonio H. Frangieh, YWZyYW5naWVAaHMudWNpLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.