- 1Department of Critical Care Medicine, Shanghai East Hospital, Tongji University School of Medicine, Shanghai, China
- 2Health Department, Beijing Armed PAP Corps, Beijing, China
Background: Cardiopulmonary bypass (CPB) triggers a strong inflammatory response in cardiovascular surgery patients during the perioperative period. This article mainly focuses on the perioperative application of novel inflammatory biomarkers in cardiovascular surgeries involving CPB.
Methods: Patients were divided into a CPB group and a non-CPB group according to whether they underwent CPB during cardiovascular surgery. Novel inflammatory biomarkers and clinical results were recorded. The neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), platelet × neutrophil/lymphocyte ratio (SII), and monocyte × platelet × neutrophil/lymphocyte ratio (PIV) were calculated. The primary outcomes were perioperative prognosis between the CPB and non-CPB groups. The secondary outcomes included perioperative alterations of novel inflammatory biomarkers in the CPB group and predictive values of novel inflammatory biomarkers for postoperative infection and acute kidney injury.
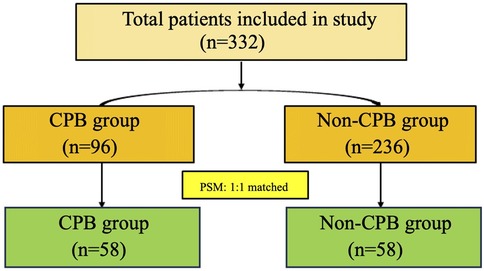
Results: A total of 332 patients were initially included in the study. Before propensity score matching (PSM), there were 96 patients in the CPB group and 236 patients in the non-CPB group. After PSM, both groups included 58 patients each. Compared with the non-CPB group, the CPB group had a higher proportion of intraoperative transfusion of blood products (63.79% vs. 6.90%, P < 0.001), specifically for red blood cells (58.62% vs. 3.45%, P < 0.001) and plasma (41.38% vs. 1.72, P < 0.001), exhibited a higher drainage fluid volume within 24 h [380 (200–550) ml vs. 200 (24–330) ml, P = 0.002], and required longer durations of mechanical ventilation [14.3 (6.6–21.3) h vs. 5.75 (4.08–10.1) h, P < 0.001] and ICU stay [48.78 (44.92–89.38) h vs. 27.16 (21.67–46.25) h, P < 0.001]. After surgery, NLR [14.00 (9.93–23.08) vs. 11.55 (7.38–17.38), P = 0.043] was higher in the CPB group, while the PIV, PLR, and SII in the CPB group were lower than those in the non-CPB group on the first day after surgery.
Conclusions: Cardiovascular surgeries involving CPB exhibit a poorer prognosis compared to non-CPB procedures. Novel inflammatory biomarkers, including PLR, PIV, and SII, may offer valuable insights into the degree of postoperative inflammation, with NLR emerging as a potentially reliable prognostic indicator.
Introduction
Most cardiovascular surgeries need to be assisted by cardiopulmonary bypass (CPB). Currently, relevant studies have confirmed that CPB triggers a strong inflammatory response in patients during the perioperative period, thus affecting the prognosis of patients (1). With the development of surgical counting, an increasing number of surgical operations are being performed without CPB, but the postoperative inflammatory response remains strong. Inflammation is closely related to the prognosis of patients after cardiovascular surgery. Previous studies have attempted to combine multiple biomarkers to develop more accurate indicators to improve the clinical application of biomarkers, including the neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), and systemic inflammation index (SII) (2). Furthermore, in recent years, the pan-immune inflammatory value (PIV) has also emerged as a popular indicator of inflammation. First proposed in 2022 as a novel prognostic biomarker for metastatic colorectal cancer (3), its application in cardiovascular surgery is rare. This article mainly focuses on the perioperative application of novel inflammatory markers in cardiovascular surgeries involving CPB and explores the perioperative changes in these biomarkers to guide their clinical application.
Methods
Study design
Patients undergoing open cardiovascular surgery in Shanghai East Hospital from August 2022 to June 2023 were included in this study. The clinical data of these patients were retrospectively analyzed, and the patients were divided into a CPB group and a non-CPB group according to whether CPB was used during the operation. The perioperative clinical results of the two groups were compared. This study was approved by the Ethics Committee of Biomedical Research at Shanghai East Hospital, Tongji University School of Medicine (Approval No: 2024YS-043). Given the observational nature of the study, the ethics committee waived the requirement for individual patient consent.
Data collection
Preoperative data, including age, body mass index (BMI), height, underlying diseases, and laboratory test results (e.g., leukocytes, lymphocytes, monocytes, platelets, bilirubin, creatinine, etc.), were collected. We also gathered relevant intraoperative data of the patients, including intraoperative blood transfusion details. In addition, we recorded postoperative inflammatory biomarkers and clinical outcomes, such as acute kidney injury (AKI), postoperative infections, mechanical ventilation (MV) duration, ICU stay duration, and in-hospital mortality.
Definitions
In our study, the following novel inflammatory biomarkers were utilized to assess the perioperative inflammatory response:
NLR: neutrophil/lymphocyte ratio, PLR: platelet/lymphocyte ratio, SII: platelet × neutrophil/lymphocyte ratio, and PIV: monocyte × platelet × neutrophil/lymphocyte ratio.
Furthermore, AKI is defined as any of the following: an increase in creatinine (SCr) by ≥0.3 mg/dl (≥26.5 μmol/L) within 48 h; an increase in SCr to ≥1.5 times the baseline level, which is known or presumed to have occurred within the prior 7 days; or urine volume <0.5 ml/kg/h for 6 h (4).
Postoperative infections include pulmonary infection, bloodstream infection, and urinary system infection.
Statistical analysis
Propensity score matching (PSM) analysis is a method used to reduce selection bias between two groups of patients. We used a logistic regression model to calculate the propensity score for each patient and performed 1:1 matching between the two groups, with a caliper width of 0.2 standard deviations (SD). Baseline demographic data, preoperative data, intraoperative data, and postoperative data were compared between the two groups both before and after PSM. The results are presented as the mean ± SD or median [interquartile range (IQR)] for continuous variables, as appropriate, and as the total number (%) for categorical variables. Comparisons between groups were made using the χ2 test or Fisher’s exact test for categorical variables and the Student’s t-test or Mann–Whitney U test for continuous variables, as appropriate. The ability of NLR, PLR, SII, and PIV to predict postoperative clinical outcomes (mainly in postoperative AKI and infection) was analyzed by receiver operating characteristics (ROC) curves and the resulting area under the curve (AUC). All statistical analyses were performed with R 4.3.2 (R Foundation). A two-tailed P < 0.05 was considered statistically significant.
Results
Baseline demographic and clinical data of patients
The flowchart of patient screening is shown in Figure 1. Initially, 332 patients were included in the study. Before PSM, there were 96 patients in the CPB group and 236 patients in the non-CPB group. After PSM, both groups included 58 patients each.
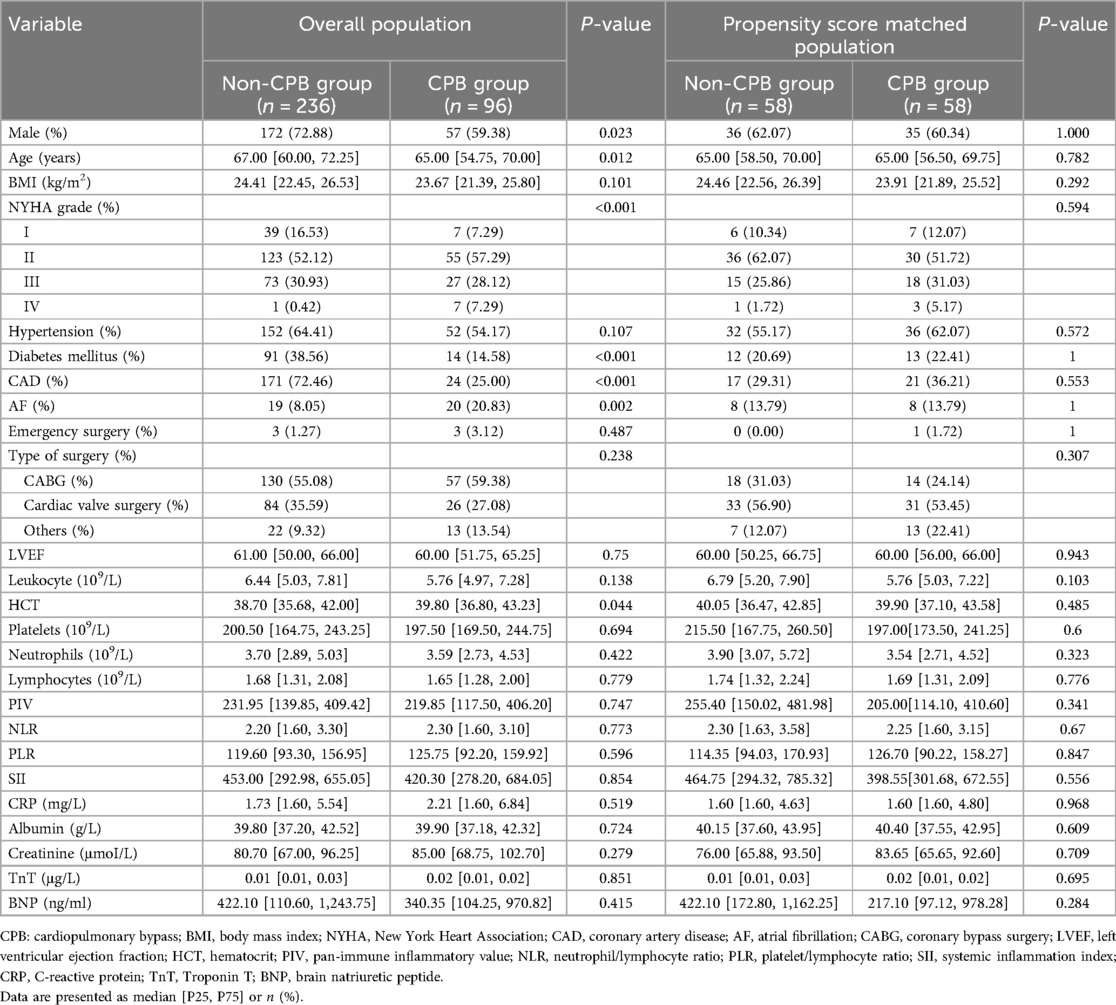
The baseline data analysis is presented in Table 1. Before PSM, there were statistically significant differences in gender, age, New York Heart Association (NYHA) grade, and diabetes mellitus rate between the CPB and non-CPB groups. After PSM, there was no statistical difference between the two groups in terms of preoperative characteristics.
Comparison of intraoperative data and postoperative clinical outcomes between the two groups
Intraoperative data and postoperative clinical outcomes for the two groups included in the final analysis were compared before and after PSM. Compared with the non-CPB group, the CPB group had a higher proportion of intraoperative transfusion of blood products (63.79% vs. 6.90%, P < 0.001), specifically for red blood cells (58.62% vs. 3.45%, P < 0.001) and plasma (41.38% vs. 1.72%, P < 0.001). In addition, the CPB group had a higher volume of drainage fluid within 24 h [380 (200–550) ml vs. 200 (24–330) ml, P = 0.002] and required longer durations of mechanical ventilation [14.3 (6.6–21.3) h vs. 5.75 (4.08–10.1) h, P < 0.001] and ICU stay [48.78 (44.92–89.38) h vs. 27.16 (21.67–46.25) h, P < 0.001]. However, there was no significant difference in mortality between the two groups (Table 2).
Comparison of postoperative clinical laboratory findings between the two groups
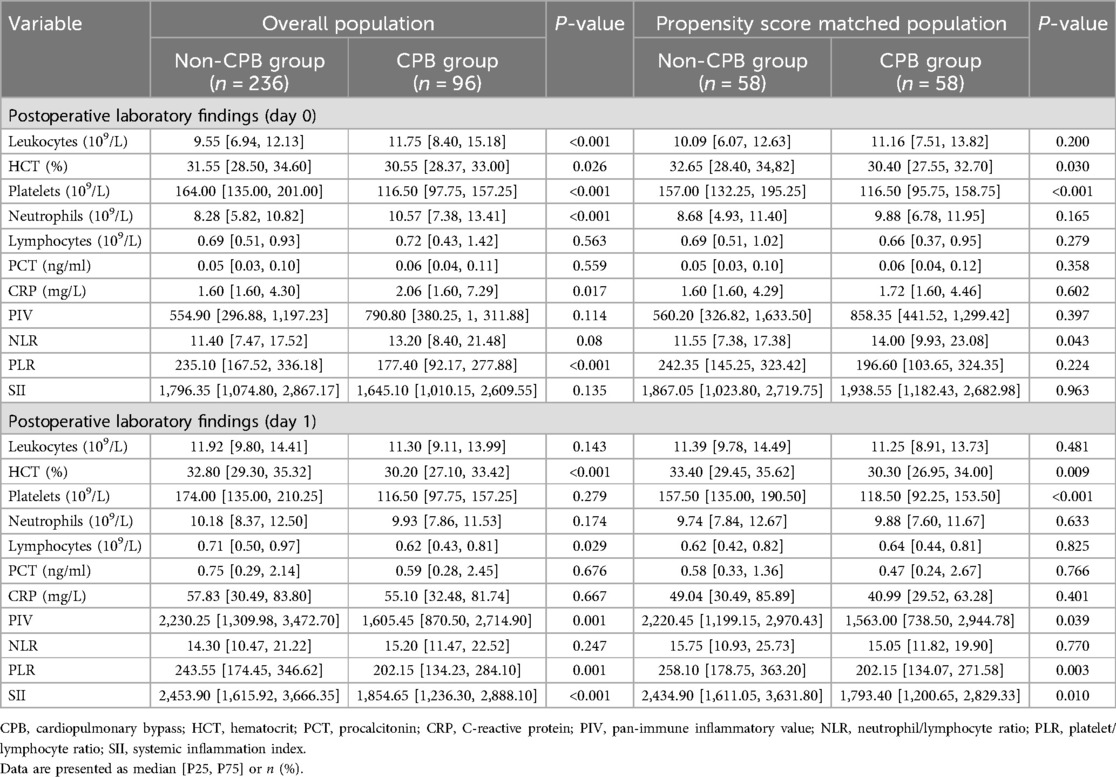
In terms of inflammation biomarkers, on the day after surgery, NLR was significantly higher in the CPB group [14.00 (9.93–23.08) vs. 11.55 (7.38–17.38), P = 0.043], whereas there were no significant differences in other indicators. On the first postoperative day, the non-CPB group exhibited higher values for several indicators: including PIV [2,220.45 (1,199.15–2,970.43) vs. 1,563.00 (738.50–2,944.78), P = 0.039], PLR [258.10 (178.75–363.20) vs. 202.15 (134.07–271.58), P = 0.003], and SII [2,434.90 (1,611.05–3,631.80) vs. 1,793.40 (1,200.65–2,829.33), P = 0.01]. Nevertheless, no statistical difference in NLR was found between the two groups on that day (Table 3).
Comparison of perioperative inflammatory biomarkers in the CPB group
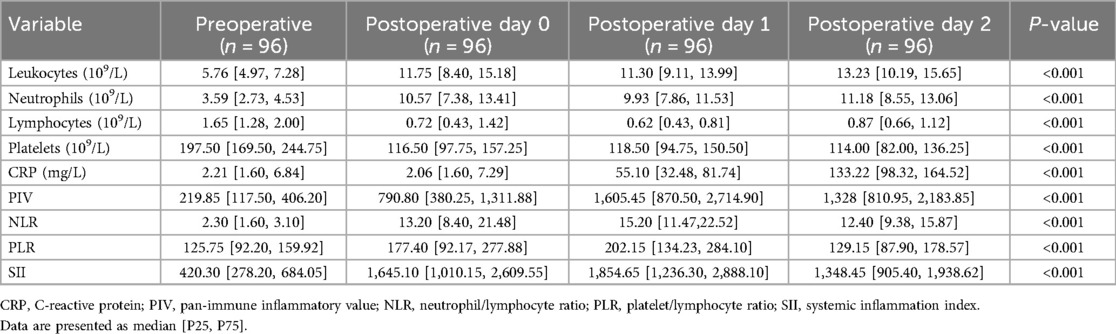
Perioperative inflammation biomarkers in the CPB group were compared. All novel inflammatory biomarkers were significantly elevated compared to presurgery levels, peaking on the first day after surgery and then entering a downward trend (Table 4).
Predictive value of inflammatory markers for postoperative infection and AKI
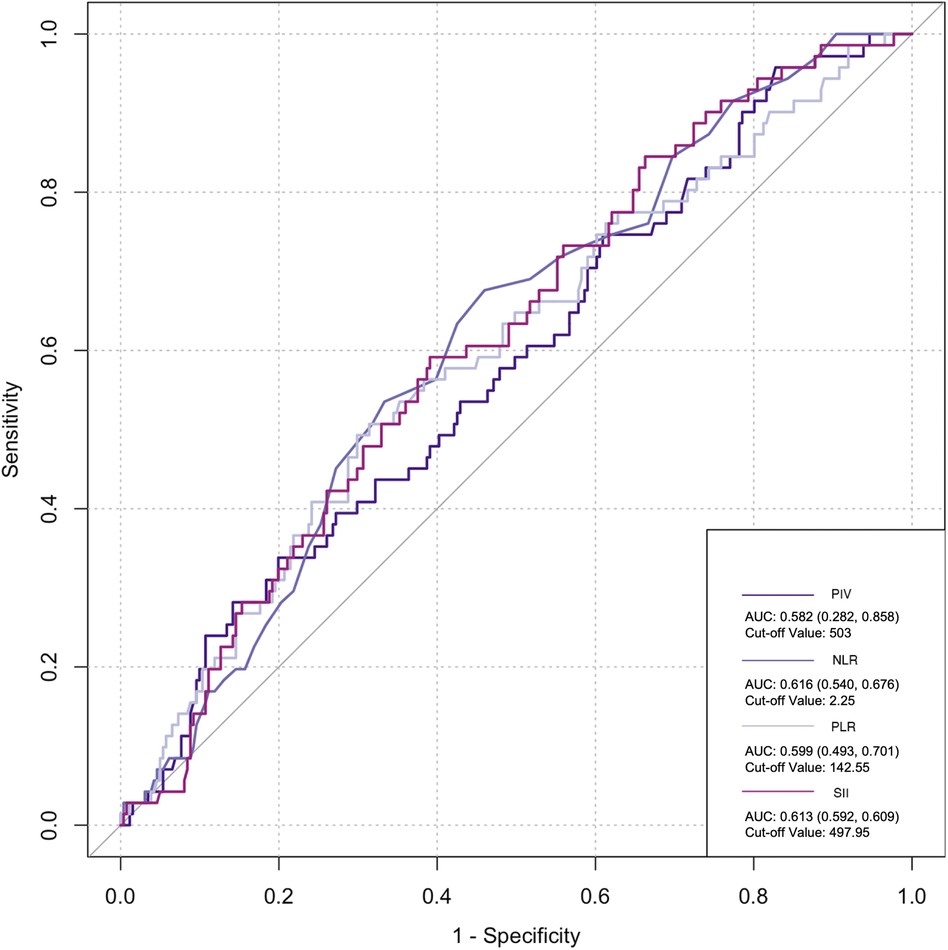
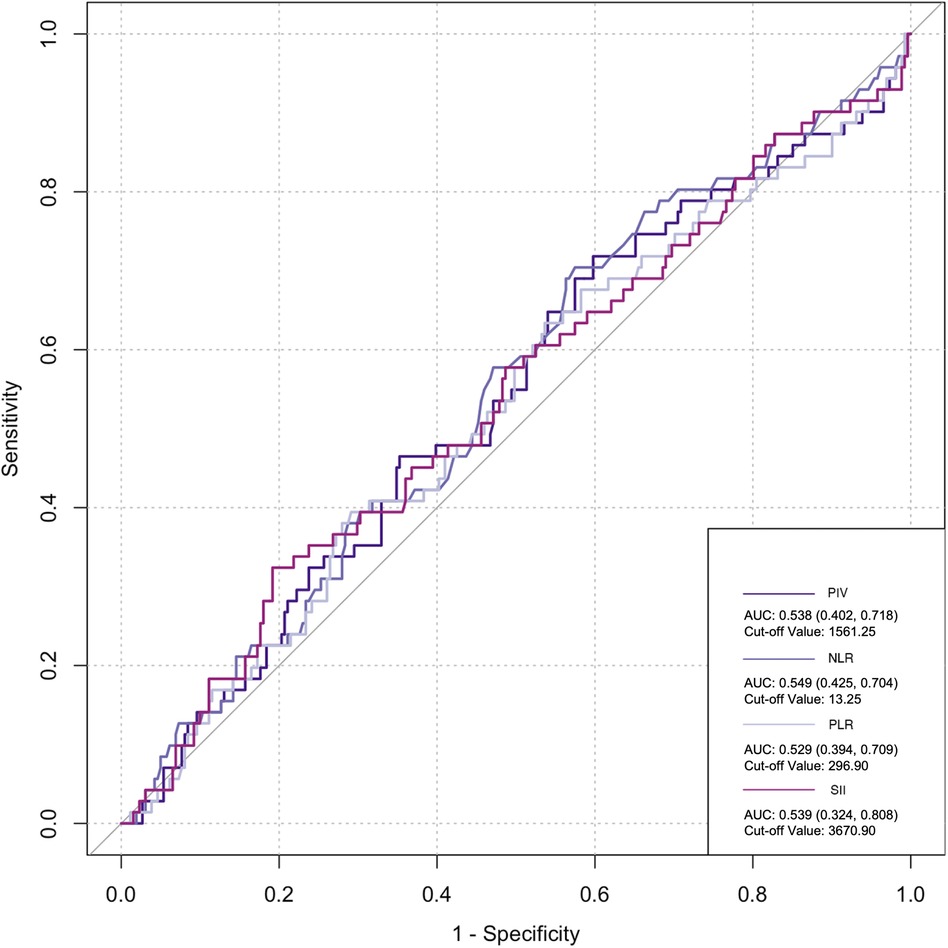
The ROC curve was employed to assess the predictive ability of postoperative infection and AKI. Among the inflammatory biomarkers analyzed, namely, PIV, PLR, and SII, their predictive values were not notably high. Specifically, only preoperative NLR demonstrated moderate predictive power, with an AUC of 0.616 [95% confidence interval (CI): 0.459–0.742] for postoperative infection, using a cutoff value of 3.05. Similarly, for postoperative AKI, preoperative NLR also yielded an AUC of 0.616 (95% CI: 0.54–0.676), with a cutoff value of 2.25 (Figures 2–5).
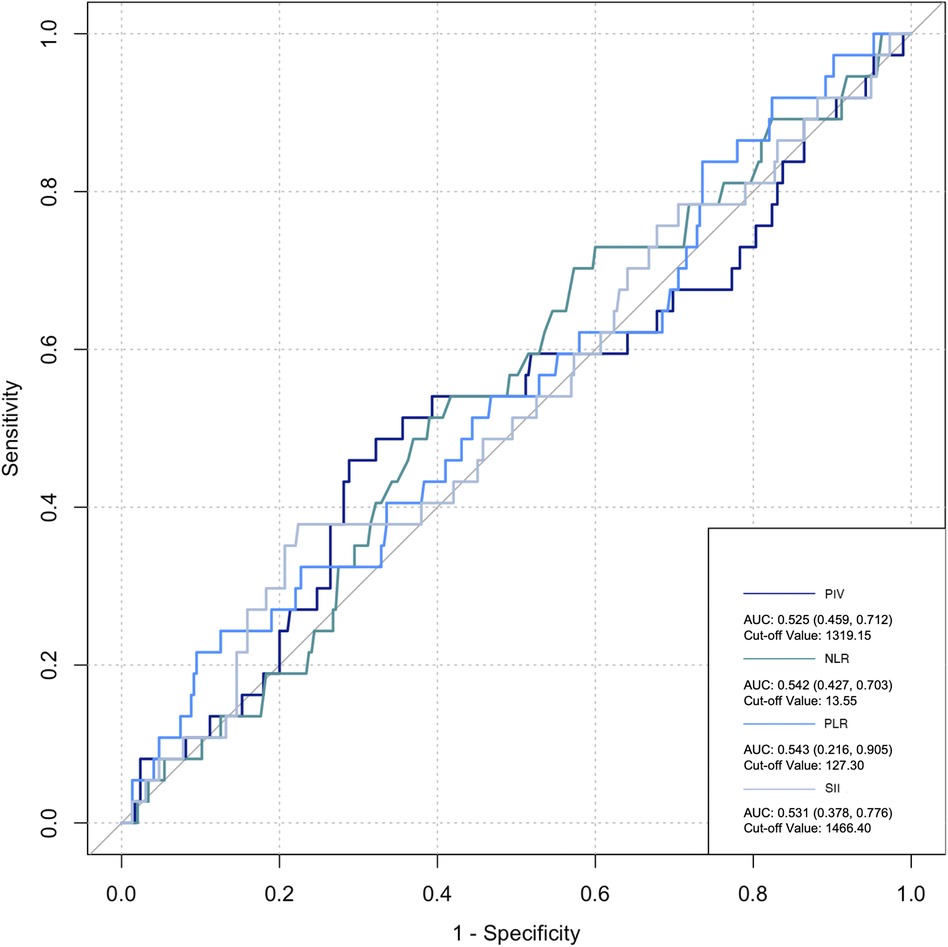
Figure 5. Postoperative biomarkers’ predictive value for infection. PIV, pan-immune inflammatory value; NLR, neutrophil/lymphocyte ratio; PLR, platelet/lymphocyte ratio; SII, systemic inflammation index.
Discussion
Currently, the majority of open cardiovascular surgeries and extensive vascular procedures still involve CPB. Most patients exhibit a systemic inflammatory response after CPB, characterized by elevated levels of circulating inflammatory cytokines and the activation of inflammatory cells (5). Clinical investigations have revealed a profound correlation between the intensity of this inflammatory response and unfavorable patient outcomes (6). Our investigation further indicates that individuals undergoing CPB surgery are at a higher risk of requiring perioperative blood transfusions and experiencing increased postoperative drainage within the first 24 h. In addition, these patients often require prolonged mechanical ventilation support and extended ICU stays due to the physiological impact of CPB. However, our study did not detect any significant increase in the likelihood of postoperative complications, such as AKI or infection, among patients who underwent CPB surgery. Moreover, there was no significant difference in the in-hospital mortality rate between the CPB and non-CPB groups.
Formerly, within the cardiovascular field, cellular inflammatory markers, including interleukin-6 (IL-6), tumor necrosis factor (TNF), C-reactive protein (CRP), and procalcitonin (PCT), have been extensively researched. Notably, an elevated IL-6 level above 421 pg/ml has been associated with a substantial increase in the risk of postoperative mortality among patients (OR = 12.6, 95% CI: 2.96–53.55) (7). In addition, IL-6 is a reliable predictor of postoperative delirium (8). IL-6 levels surge immediately following the commencement of CPB (9, 10), peaking at the end of surgery and gradually returning to baseline levels by the third postoperative day (11). On the other hand, CRP levels peak 48 h postoperatively, followed by a decline after 72 h, with a maximum value of 58.82 ± 42.23 mg/L, representing a 3–10-fold increase from baseline (12). Conventionally, CRP is considered more sensitive for the early diagnosis of inflammation, and a higher concentration often indicates a poorer prognosis for patients (13). However, some studies have contradicted this notion, revealing no significant correlation between elevated CRP levels and the occurrence of postoperative inflammation or clinical outcomes (14).
PCT, primarily utilized for the early diagnosis of infection, attains its peak value within 24 h postoperatively (15), averaging at 0.77 ± 0.49 ng/ml, approximately two to four times higher than the baseline level (12); however, its concentration levels are also positively associated with organ dysfunction. Studies have shown that patients with PCT >2.5 ng/L have a 4.5-fold increase in mortality at 28 days after surgery (16). When PCT concentrations exceed 0.7 ng/ml, postoperative organ failure can be predicted (with a sensitivity of 85% and specificity of 58%), while when PCT concentrations exceed 7.7 ng/ml, both sensitivity and specificity reach 100% (17). Serum PCT concentrations in patients with multiple organ dysfunction can reach 20 ng/ml (18). These inflammatory markers, although informative, require careful interpretation in the context of individual patient characteristics and surgical procedures to ensure accurate prognostication and tailored treatment strategies, especially in cardiovascular surgery.
In recent years, numerous research studies have focused on developing novel inflammatory markers by integrating multiple biomarkers, including the PLR, NLR, SII, and PIV. These biomarkers have demonstrated unique roles in various studies. Many factors can affect the occurrence of AKI after cardiac surgery, such as preoperative neopterin levels, EuroSCORE II, and clamp time, all of which have been identified as independent predictors of postoperative AKI (19). Previous studies have highlighted the NLR on the first postoperative day as a robust and independent predictor of early AKI in patients undergoing isolated off-pump coronary artery bypass (OPCAB) (20). Meanwhile, PIV has garnered significant attention in the context of ST-elevation myocardial infarction (STEMI). Recently, relevant studies have expanded the application of PIV to patients with acute heart failure and STEMI, establishing its close association with patient prognosis in cardiology (21, 22). However, there is relatively little research on these indicators in the cardiovascular surgery field. Our study showed that novel inflammatory biomarkers consistently increase after cardiovascular surgeries involving CPB, peaking on the first day after surgery. However, compared to non-CPB cardiovascular surgery, our analysis of PLR, SII, and PIV revealed a paradoxical finding: a seemingly attenuated postoperative inflammatory response in the CPB group. This observation is clearly in contrast with reality and is inconsistent with our impression. A careful analysis suggests that this discrepancy may be closely linked to the destruction of platelets during CPB. Current understanding suggests that platelet destruction, adhesion, and aggregation during CPB are primary factors contributing to reduced platelet counts after surgery (23). Prolonged CPB time further increases the risk of postoperative thrombocytopenia (24). Given these considerations, NLR, without the confounding influence of platelets, has emerged as a more informative metric for assessing perioperative inflammatory responses.
Previous studies have revealed that inflammatory cells contribute significantly to organ damage following CPB, primarily through two distinct mechanisms. Initially, monocytes adhere to vascular endothelial cells by upregulating CD11b expression and subsequently migrate from the blood vessels into tissues. Upon reaching the tissues, these monocytes upregulate the production of various inflammatory cytokines, including IL-6, IL-8, and TNF-α, creating a localized high-concentration zone (25). Notably, the concentration of these cytokines differs significantly from that observed intravascularly. A portion of these locally produced soluble factors enters the circulation, activating other immune effector cells, such as neutrophils. These activated neutrophils, guided by chemotactic signals from high-concentration cytokines in the tissue, migrate to the inflamed tissues through upregulated surface adhesion molecules like Mac-1 (CD11b/CD18). Upon arrival, they release oxygen free radicals, myeloperoxidase, elastase, and other agents, causing damage to surrounding tissues (26). Collectively, these observations suggest that monocytes and neutrophils are the primary immune effector cells mediating systemic inflammatory response (SIR) induced by CPB. Notably, the inclusion of more indices in research introduces more interfering factors, particularly considering the damage to platelets and blood cells caused by CPB, thus leading to potential data deviations. However, NLR solely comprises the ratio of neutrophils to lymphocytes. Neutrophils play an important role in inflammation, while lymphocyte count reflects physiological stress and is negatively correlated with inflammation (27). Dynamic changes in NLR are attributed to systemic inflammation. High NLR significantly increases the risk of mortality, postoperative re-intubation, and atrial fibrillation after cardiovascular surgery (28, 29). A meta-analysis estimated an AUC of 0.65 for NLR in predicting AKI following cardiovascular surgery (30). Lafçi et al. emphasized the utility of NLR as a simple and effective marker for predicting outcomes in a high-risk cardiovascular cohort (31), which is consistent with our research. In the present study, ROC curve analysis yielded a similar prediction for postoperative AKI and infection with NLR (AUC: 0.616), supporting NLR as a potentially reliable prognostic indicator.
Limitations
Our study, despite its valuable contributions, has several limitations that need to be addressed. First, the clinical data collected for the study may not be comprehensive enough to provide a complete picture of the subject matter. This could be due to various reasons such as limited access to patient records, incomplete reporting of symptoms, or the absence of certain critical information. Second, the results obtained from this study may not be generalizable to other populations, given the specific characteristics of the sample population and the conditions under which the study was conducted. To overcome these limitations and further validate the findings of this study, larger, randomized controlled trials are urgently needed. Such trials would involve a more diverse and representative sample population, allowing for more robust and generalizable results. In addition, the use of randomized allocation of participants to different treatment groups would help minimize potential biases and confounding factors, ensuring that the observed effects are truly attributable to the intervention being studied. By conducting such rigorous trials, we can gain a deeper understanding of the subject matter and make more informed decisions about the effectiveness of different treatment options.
Conclusions
In conclusion, patients undergoing cardiovascular surgery with CPB exhibit a poorer prognosis compared to those without CPB. Novel inflammatory biomarkers, including PLR, PIV, and SII, may offer valuable insights into the degree of postoperative inflammation, with NLR emerging as a potentially reliable prognostic indicator. Certainly, these findings necessitate further rigorous research and validation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Shanghai East Hospital Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
WZ: Conceptualization, Writing – original draft. HW: Conceptualization, Data curation, Writing – original draft. CL: Data curation, Methodology, Writing – original draft. Q-mM: Data curation, Investigation, Writing – original draft. Y-hG: Formal Analysis, Writing – original draft. S-yS: Conceptualization, Formal Analysis, Writing – review & editing. S-lM: Project administration, Resources, Supervision, Writing – review & editing. FZ: Project administration, Writing – review & editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the peak supporting clinical discipline of Shanghai health bureau (2023ZDFC0104 to L.T) and the National Key R&D Program “Stem Cell and Transformation Research” Key Special Project (2019YFA0110601).
Conflict of interest
Y-hG was employed by the Security Department of the Beijing Armed Police Corps.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wan S, LeClerc JL, Vincent JL. Inflammatory response to cardiopulmonary bypass: mechanisms involved and possible therapeutic strategies. Chest. (1997) 112(3):676–92. doi: 10.1378/chest.112.3.676
2. Eltohami YI, Kao HK, Lao WW, Huang Y, Abdelrahman M, Liao CT, et al. The prediction value of the systemic inflammation score for oral cavity squamous cell carcinoma. Otolaryngol Head Neck Surg. (2018) 158:1042–50. doi: 10.1177/0194599817751678
3. Fucà G, Guarini V, Antoniotti C, Morano F, Moretto R, Corallo S, et al. The pan-immune-inflammation value is a new prognostic biomarker in metastatic colorectal cancer: results from a pooled-analysis of the Valentino and TRIBE first-line trials. Br J Cancer. (2020) 123(3):403–9. doi: 10.1038/s41416-020-0894-7
4. Kellum JA, Lameire N, KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (part 1). Crit Care. (2013) 17(1):204. doi: 10.1186/cc11454
5. Hatami S, Hefler J, Freed DH. Inflammation and oxidative stress in the context of extracorporeal cardiac and pulmonary support. Front Immunol. (2022) 13:831930. doi: 10.3389/fimmu.2022.831930
6. Lindman BR, Goldstein JS, Nassif ME, Zajarias A, Novak E, Tibrewala A, et al. Systemic inflammatory response syndrome after transcatheter or surgical aortic valve replacement. Heart. (2015) 101(7):537–45. doi: 10.1136/heartjnl-2014-307057
7. Bauer A, Korten I, Juchem G, Kiesewetter I, Kilger E, Heyn J. EuroScore and IL-6 predict the course in ICU after cardiac surgery. Eur J Med Res. (2021) 26(1):29. doi: 10.1186/s40001-021-00501-1
8. Chen Y, Lu S, Wu Y, Shen Y, Zhao H, Ding S, et al. Change in serum level of interleukin 6 and delirium after coronary artery bypass graft. Am J Crit Care. (2019) 28(6):462–70. doi: 10.4037/ajcc2019976
9. Presta P, Bolignano D, Coppolino G, Serraino F, Mastroroberto P, Andreucci M, et al. Antecedent ACE inhibition, inflammatory response, and cardiac surgery associated acute kidney injury. Rev Cardiovasc Med. (2021) 22(1):207–13. doi: 10.31083/j.rcm.2021.01.288
10. Puchinger J, Ryz S, Nixdorf L, Edlinger-Stanger M, Lassnigg A, Wiedemann D, et al. Characteristics of interleukin-6 signaling in elective cardiac surgery: a prospective cohort study. J Clin Med. (2022) 11(3):590. doi: 10.3390/jcm11030590
11. Franke A, Lante W, Fackeldey V, Becker HP, Kurig E, Zöller LG, et al. Pro-inflammatory cytokines after different kinds of cardio-thoracic surgical procedures: is what we see what we know? Eur J Cardiothorac Surg. (2005) 28(4):569–75. doi: 10.1016/j.ejcts.2005.07.007
12. Arkader R, Troster EJ, Abellan DM, Lopes MR, Júnior RR, Carcillo JA, et al. Procalcitonin and C reactive protein kinetics in postoperative pediatric cardiac surgical patients. J Cardiothorac Vasc Anesth. (2004) 18(2):160–5. doi: 10.1053/j.jvca.2004.01.021
13. Gutiérrez-Gutiérrez B, Morales I, Pérez-Galera S, Fernández-Riejos P, Retamar P, de Cueto M, et al. Predictive value of the kinetics of procalcitonin and C-reactive protein for early clinical stability in patients with bloodstream infections due to gram-negative bacteria. Diagn Microbiol Infect Dis. (2019) 93(1):63–8. doi: 10.1016/j.diagmicrobio.2018.07.019
14. Allan CK, Newburger JW, McGrath E, Elder J, Psoinos C, Laussen PC, et al. The relationship between inflammatory activation and clinical outcome after infant cardiopulmonary bypass. Anesth Analg. (2010) 111(5):1244–51. doi: 10.1213/ANE.0b013e3181f333aa
15. Amouzeshi A, Abedi F, Zardast M, Rezaeian Bilondi Y, Amouzeshi Z. Prognostic value of 16 procalcitonin for morbidity and mortality in patients after cardiac surgery. Cardiol Res Pract. (2021) 2021:1542551. doi: 10.1155/2021/1542551
16. Fritz HG, Brandes H, Bredle DL, Bitterlich A, Vollandt R, Specht M, et al. Postoperative hypoalbuminaemia and procalcitonin elevation for prediction of outcome in cardiopulmonary bypass surgery. Acta Anaesthesiol Scand. (2003) 47(10):1276–83. doi: 10.1046/j.1399-6576.2003.00239.x
17. Celebi S, Koner O, Menda F, Balci H, Hatemi A, Korkut K, et al. Procalcitonin kinetics in pediatric patients with systemic inflammatory response after open heart surgery. Intensive Care Med. (2006) 32(6):881–7. doi: 10.1007/s00134-006-0180-z
18. Sablotzki A, Dehne MG, Friedrich I, Grond S, Zickmann B, Mühling J, et al. Different expression of cytokines in survivors and non-survivors from MODS following cardiovascular surgery. Eur J Med Res. (2003) 8(2):71–6.12626284
19. Çiçek ÖF, Akyürek F, Akbayrak H, Orhan A, Kaya EC, Büyükateş M. Can preoperative neopterin levels predict acute kidney injury in patients undergoing on-pump cardiac surgery? Turk J Biochem. (2023) 48(5):531–40. doi: 10.1515/tjb-2023-0074
20. Ishikawa M, Iwasaki M, Namizato D, Yamamoto M, Morita T, Ishii Y, et al. The neutrophil to lymphocyte ratio and serum albumin as predictors of acute kidney injury after coronary artery bypass grafting. Sci Rep. (2022) 12(1):15438. doi: 10.1038/s41598-022-19772-7
21. Murat B, Murat S, Ozgeyik M, Bilgin M. Comparison of pan-immune-inflammation value with other inflammation markers of long-term survival after ST-segment elevation myocardial infarction. Eur J Clin Invest. (2023) 53(1):e13872. doi: 10.1111/eci.13872
22. Inan D, Erdogan A, Pay L, Genc D, Demırtola AI, Yıldız U, et al. The prognostic impact of inflammation in patients with decompensated acute heart failure, as assessed using the pan-immune inflammation value (PIV). Scand J Clin Lab Invest. (2023) 83(6):371–8. doi: 10.1080/00365513.2023.2233890
23. Solis RT, Kennedy PS, Beall AC Jr, Noon GP, DeBakey ME. Cardiopulmonary bypass. Microembolization and platelet aggregation. Circulation. (1975) 52(1):103–8. doi: 10.1161/01.CIR.52.1.103
24. Hamid M, Akhtar MI, Naqvi HI, Ahsan K. Incidence and pattern of thrombocytopenia in cardiac surgery patients. J Pak Med Assoc. (2017) 67(7):1019–23.28770879
25. Brix-Christensen V. The systemic inflammatory response after cardiac surgery with cardiopulmonary bypass in children. Acta Anaesthesiol Scand. (2001) 45(6):671–9. doi: 10.1034/j.1399-6576.2001.045006671.x
26. Du L, Zhou J, Zhang J, Yan M, Gong L, Liu X, et al. Actin filament reorganization is a key step in lung inflammation induced by systemic inflammatory response syndrome. Am J Respir Cell Mol Biol. (2012) 47(5):597–603. doi: 10.1165/rcmb.2012-0094OC
27. Kurtul A, Ornek E. Platelet to lymphocyte ratio in cardiovascular diseases: a systematic review. Angiology. (2019) 70(9):802–18. doi: 10.1177/0003319719845186
28. Gibson PH, Cuthbertson BH, Croal BL, Rae D, El-Shafei H, Gibson G, et al. Usefulness of neutrophil/lymphocyte ratio as predictor of new-onset atrial fibrillation after coronary artery bypass grafting. Am J Cardiol. (2010) 105(2):186–91. doi: 10.1016/j.amjcard.2009.09.007
29. Gibson PH, Croal BL, Cuthbertson BH, Small GR, Ifezulike AI, Gibson G, et al. Preoperative neutrophil-lymphocyte ratio and outcome from coronary artery bypass grafting. Am Heart J. (2007) 154(5):995–1002. doi: 10.1016/j.ahj.2007.06.043
30. Chen D, Xiao D, Guo J, Chahan B, Wang Z. Neutrophil-lymphocyte count ratio as a diagnostic marker for acute kidney injury: a systematic review and meta-analysis. Clin Exp Nephrol. (2020) 24(2):126–35. doi: 10.1007/s10157-019-01800-y
Keywords: cardiovascular surgery, inflammation, perioperative, pan-immune inflammatory value (PIV), neutrophil/lymphocyte ratio (NLR)
Citation: Zhou W, Wang H, Li C, Ma Q-m, Gu Y-h, Sheng S-y, Ma S-l and Zhu F (2024) Alterations in novel inflammatory biomarkers during perioperative cardiovascular surgeries involving cardiopulmonary bypass: a retrospective propensity score matching study. Front. Cardiovasc. Med. 11:1433011. doi: 10.3389/fcvm.2024.1433011
Received: 15 May 2024; Accepted: 11 September 2024;
Published: 27 September 2024.
Edited by:
Zhonghua Sun, Curtin University, AustraliaReviewed by:
Ömer Faruk Çiçek, Selcuk University, TürkiyeVladimir M. Pisarev, Federal Research and Clinical Center of Intensive Care Medicine and Rehabilitation, Russia
Copyright: © 2024 Zhou, Wang, Li, Ma, Gu, Sheng, Ma and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shu-yue Sheng, c2hlbmdzaHV5dWVAMTI2LmNvbQ==; Shaolin Ma, bXNsaW5Ac29odS5jb20=; Feng Zhu, YWxleHpodWp1bmNoaUBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Wei Zhou
Wei Zhou He Wang1,†
He Wang1,† Qi-min Ma
Qi-min Ma Shao-lin Ma
Shao-lin Ma Feng Zhu
Feng Zhu