
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 18 June 2024
Sec. Cardiovascular Surgery
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1427930
This article is part of the Research Topic Minimally Invasive Cardiac Surgery: State of the art and current challenges View all 10 articles
 Ali Fatehi Hassanabad1*
Ali Fatehi Hassanabad1* Justyna Fercho2
Justyna Fercho2 Mortaza Fatehi Hassanabad1
Mortaza Fatehi Hassanabad1 Melissa King1
Melissa King1 Morgan Sosniuk3
Morgan Sosniuk3 Dominique de Waard4
Dominique de Waard4 Corey Adams1
Corey Adams1 William D. T. Kent1
William D. T. Kent1 Wojtek Karolak2
Wojtek Karolak2
Background: Right anterior mini thoracotomy (RAMT) for aortic valve replacement (AVR) is a minimally invasive procedure that avoids sternotomy. Herein, we report the outcomes of patients who underwent redo-cardiac via a RAMT approach for AVR.
Methods: This case series reports the clinical outcomes of 14 consecutive redo operations, done in Calgary (Canada) and Gdansk (Poland) between 2020 and 2023. Primary outcomes were 30-day mortality and disabling stroke. Secondary outcomes included surgical times, hemodynamics, permanent pacemaker implantation (PPM), length of ICU and hospital stay, new post-operative atrial fibrillation (POAF), post-operative blood transfusion, incidence of acute respiratory distress syndrome (ARDS), rate of continuous renal replacement therapy (CRRT) and/or dialysis, and chest tube output in the first 12-hours after surgery.
Results: Nine patients were male, and the mean age was 64.36 years. There were no deaths, while one patient had a disabling stroke postoperatively. Mean cardiopulmonary bypass and cross clamp-times were 136 min and 90 min, respectively. Three patients needed a PPM, 3 patients needed blood transfusions, and 2 developed new onset POAF. Median lengths of ICU and hospital stays were 2 and 12 days, respectively. There was no incidence of paravalvular leak greater than trace and the average transvalvular mean gradient was 12.23 mmHg.
Conclusion: The number of patients requiring redo-AVR is increasing. Redo-sternotomy may not be feasible for many patients. This study suggests that the RAMT approach is a safe alternative to redo-sternotomy for patients that require an AVR.
Aortic valve replacement (AVR) is the gold standard treatment for severe, symptomatic aortic valve stenosis (AS). Despite an aging population, surgical and transcatheter advances have facilitated repeat interventions on dysfunctional native and prosthetic aortic valves. When considering re-intervening on a diseased prosthetic aortic valve, options include redo-surgical aortic valve replacement (SAVR) or valve-in-valve (ViV) transcatheter aortic valve replacement (TAVR). Several studies over the past 10 years have demonstrated favourable outcomes with each of these strategies (1–5). Generally, it is believed TAVR offers a minimally-invasive low risk procedure, but with limited durability, whereas redo-SAVR is associated with higher risk, but greater durability. Redo-SAVR via RAMT may represent a compromise, offering a less invasive option with greater durability.
Conventional SAVR is performed via full median sternotomy, while minimally-invasive SAVR can be done through either a hemi-sternotomy or a right anterior mini thoracotomy (RAMT). Although the current literature on redo-SAVR is mainly focused on redo-full median sternotomy or hemi-sternotomy approaches, there is a paucity of data reporting the clinical outcomes of redo-AVR, performed through a RAMT incision. When compared to conventional SAVR, RAMT has been shown to have similar clinical outcomes, less pain, and less blood transfusions (6–10). There is also evidence showing that patients undergoing RAMT can have an expedited return to their functional baseline secondary to quicker mobilization, better pain control, and no sternal precautions (11). RAMT access is well-liked by patients, as many associate full median sternotomy with increased morbidity and prolonged rehabilitation time. For these reasons, in the appropriate patient, RAMT is our preferred approach for redo-AVR.
Herein, we present the clinical outcomes of redo-AVR, performed via RAMT (redo-RAMT AVR) at two centers in North America and Europe. We show that a redo-AVR can be safely performed in appropriately selected patients through a RAMT approach. Our study provides original, real-world data on redo-RAMT AVR from two vastly different regions.
This case series involved retrospective collection of data to review the clinical outcomes of patients undergoing redo-RAMT AVR at a Canadian and a Polish center. All redo-operations were performed by 3 surgeons, who routinely perform minimally invasive valve surgery, between June 2020 and August 2023. This study was approved by the Conjoint Health Research Ethics Board at the University of Calgary and the Medical University of Gdansk underlying the Declaration of Helsinki (Ethics IDs: REB18-0042 and 062/2022, respectively).
Primary outcomes were death secondary to cardiac cause within 30-days of surgery and disabling post-operative stroke. Secondary outcomes included surgical times, permanent pacemaker implantation (PPM), length of intensive care unit (ICU) stay, length of hospital stay, new post-operative atrial fibrillation (POAF), post-operative blood transfusion, incidence of acute respiratory distress syndrome (ARDS), rate of continuous renal replacement therapy (CRRT) and/or dialysis, and chest tube output in the first 12-hours after surgery. Echocardiographic parameters indicating correct valve implantation was assessed, including incidence of paravalvular leak and residual mean transvalvular gradient.
Perioperative considerations for RAMT AVR have been described in detail previously (12). The same considerations are generally applicable for redo-operations through a RAMT incision (Figure 1) and are indicated for an AVR. Briefly, the ideal candidate will not have an elevated body mass index, their aorta will not be shifted left-ward, the distance from the aortic valve to the incision is less than 9 cm, and the peripheral vessels are suitable for instituting CPB. While the authors of this study believe that a RAMT incision will provide similar exposure to the aortic valve irrespective of first-time vs. redo-surgery, since this is a complex operation, surgeons should be selective early in their experience. While no particular steps are taken in redo- vs. first-time RAMT, an important factor in selecting patients for a potential redo-RAMT AVR is the index cardiac operation. It is essential to be prepared when encountering a hostile intra-thoracic cavity with a RAMT approach as exposure, dissection, and access to the aortic valve may all be affected by dense pericardial adhesions. It may also be unsafe or unfeasible to remove a prosthetic aortic valve through a RAMT incision. In such situations, conversion to a sternotomy would be recommended.
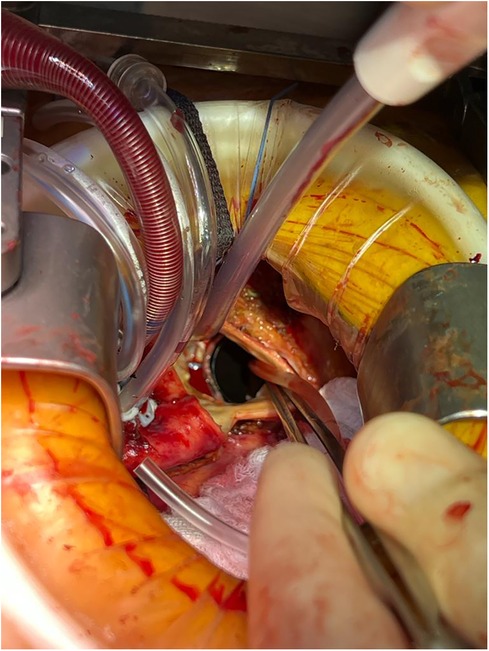
Figure 1 Redo-RAMT for a patient with a mechanical prosthetic aortic valve in-situ who presented with valve thrombosis. The mechanical valve was replaced with a bioprosthetic valve through a RAMT incision.
In this case series, patients were considered as possible candidates for redo-surgery via a RAMT approach if they met the anatomical requirements noted before (12). If the risk of redo-sternotomy was deemed to be too high on preoperative imaging, a stronger consideration was given for a RAMT. Furthermore, this cohort of patients were determined to have a quicker return to their functional baseline, and voiced a preference to avoid a sternotomy if it did not place them at a higher surgical risk. Patients with active infective endocarditis, previous bypass grafts, and those requiring concomitant procedures were not considered for a RAMT incision. A CT chest, abdomen, and pelvis with contrast run-off was obtained for this cohort of patients. There were no patients with missing data, so all 14 consecutive patients were included in the cohort.
Fourteen consecutive patients underwent redo-cardiac surgery for an AVR through a RAMT incision. Index operations were done through sternotomy (n = 12), mini-sternotomy (n = 1), and left thoracotomy (n = 1, for repair of coarctation of the aorta). Nine were male and the average age of the patient cohort was 64.36 ± 11.08 years. In the cohort, 9 patients had had a previous AVR; 1 had previously undergone mitral valve replacement (MVR), tricuspid valve replacement (TVR), and aortic valve repair; 1 had undergone MVR, tricuspid valve repair, and aortic valve repair; 1 had a mechanical MVR; 1 had undergone a left thoracotomy as a child to repair coarctation of the aorta; and 1 had undergone aortic valvulotomy. Finally, the mean European System for Cardiac Operative Risk Evaluation (EuroSCORE) II was 3.77% ± 3.54% for this case series. Patient demographics are listed in Table 1.
Different types of valves were used in this case series. The type and size of the valves that were used is summarized in Table 2. A femoral cutdown was performed to establish peripheral CPB in all patients. The third rib was detached in 10 cases. There was no conversion to sternotomy and there were no concomitant procedures. The mean CPB and cross-clamp times were 137.69 ± 54.41 min and 90.47 ± 34.97 min, respectively. There was no incidence of paravalvular leak (PVL) greater than trace and the mean and peak transvalvular pressure gradients were 12.57 ± 5.94 mmHg and 25.69 ± 9.89 mmHg, respectively. Intraoperative details are summarized in Table 3.
There were no deaths at 30-days postoperatively, but 4 patients did have a neurological event postoperatively, with only 1 being disabling. The causes for the neurological events were hypoxic brain injury secondary to hypotension for 1 patient while they were undergoing continuous renal replacement therapy (CRRT); cortical laminar necrosis causing hypoxic brain injury in 1 patient; and self-limiting postoperative seizures in 2 patients. Three patients received blood products in the ICU: one patient was transfused 2 units of packed red blood cells (pRBCs), 1 patient received 4 units of pRBCs, and 1 patient received 1 unit of pRBCs. On the ward, 2 patients were transfused 2 units of pRBCs each. Three patients experienced new onset postoperative atrial fibrillation (POAF) after their operation and 3 required a permanent pacemaker (PPM). The average chest tube output in the first 12-hours after surgery was 271.43 ± 329.22 ml; of note, only one patient was taken back to the operating room emergently perioperatively for excessive bleeding. None of the patients had acute respiratory distress syndrome (ARDS). One of the patients required CRRT. Median length of ICU and hospital stays were 2 (IQR: 5) and 11 (IQR: 9) days, respectively. Postsurgical findings have been summarized in Table 4.
With an aging population, repeat interventions for cardiac diseases are becoming more frequent. In most cases, the index operation is performed through a full median sternotomy. Although preoperative planning (13) and identifying patients at risk of injury during re-entry can mitigate the risk of redo sternotomy (14), it is still associated with a higher rate of complications (15, 16). Results from the multicenter European RECORD (REdo Cardiac Operation Research Database) initiative showed that conventional redo sternotomy for AVR was associated with a hospital mortality of 5.1%, major re-entry cardiovascular complications at 4.9%, and stroke at 6.6% (17). The same study found that the risk of ARDS was 10.6%; acute kidney injury (AKI) was 19.3% (where the need for CRRT was 7.2%), the need for transfusions was 66.9%, and the PPM implantation rate was 12.7% (17).
With the growth of TAVR, repeat interventions on the aortic valve are more commonly done with a ViV transcatheter approach. While there are accumulating studies that compare first time and repeat transcatheter strategies to redo-SAVR (3, 18–21), a RAMT approach should offer an important alternative for these patients for several reasons. First, the long-term outcomes of transcatheter valves is not known; second, some patients may not be suitable candidates for transcatheter approaches and transcatheter valves; third, RAMT can facilitate excellent hemodynamic results with respect to PVL and trans-valvular pressure gradients; fourth, small prosthetic aortic valves may be excised and removed through a RAMT incision when the ViV TAVR option is not feasible; and fifth, RAMT can mitigate the risks associated with proper valve deployment during TAVR, especially in patients with a prior mechanical mitral valve replacement (22).
The RAMT approach has been demonstrated to be safe for first time AVR in diverse patient populations, including octogenarians (23–26). A small number of studies have assessed the outcomes of minimally-invasive redo-AVR through hemi-sternotomy and RAMT (27–29). In a sub-population analysis of the Sutureless and Rapid-Deployment Aortic Valve Replacement International Registry (SURD-IR), Santarpino and colleagues focused on the sutureless and rapid deployment valves and reported the outcomes of 20 patients who underwent redo-RAMT AVR (27). In this registry study, among the redo-RAMT cohort, there were no deaths, while postoperative stroke rate was 4.8%, 3.6% of the patients required PPM, and bleeding requiring reoperation occurred in 8.9% of the patients (27). In a single-center study, Pindeda et al. compared the outcomes of redo-AVR via RAMT vs. median sternotomy (29). They found that in-hospital mortality was zero for the RAMT cohort vs. four (10%) in the median sternotomy group (p = 0.08), whereas postoperative complications occurred in six (17%) vs. 19 (46%) (p = 0.005) of these two groups, respectively. The median ICU and total hospital length of stay were 48-hours vs. 69-hours (p = 0.03), and 7-days vs. 9-days (p = 0.03) for the minimally-invasive and median sternotomy group, respectively (29). Although these are registry and single-center studies, respectively, they do support the safety of redo-AVR via RAMT.
The present study combines outcomes of redo-operations via a RAMT incision from a North American and a European center. We show that none of the patients died perioperatively and only one patient had a disabling stroke. Importantly, in our cohort the transfusion rate was lower that quoted in the European RECORD initiative (50% vs. 70%) (17). The same trend was noted for rate of ARDS, while similar rates were noted for CRRT in our study and the RECORD initiative. It is important to note that in this cohort, 6 of 14 patients received a mechanical prosthetic valve, highlighting the possibility of sewing in such a prosthetic through a RAMT incision in a patient with previous surgery. As expected, the transvalvular pressure gradients for these 6 patients was high, thus increasing the cohort's intraoperative valve hemodynamics. With respect to the neurological events observed in our cohort, while high (4/14 patients), their underlying cause cannot be fully attributed to intraoperative complications. Nevertheless, future studies should closely monitor and report the incidence, cause, and severity of any neurological events in patients undergoing this type of high-risk operation.
To further highlight the safety of employing a RAMT approach after prior cardiac surgery, the operations were performed by 3 different surgeons, suggesting that this strategy can be considered in carefully selected patients. These 3 surgeons routinely perform minimally invasive valve surgery, so were comfortable with a RAMT incision for redo-operations. With respect to RAMT as a first-time operation, both centers perform approximately 60 cases on an annual basis. While our cohort included patients with previous valvular operations and one patient with a previous coarct repair, none had a prior CABG surgery. Although there is a case report of a patient who underwent RAMT for redo-AVR after CABG with bilateral internal thoracic arteries (30), patent grafts can significantly increase the operative risk and these patients may be best served with a TAVR if indicated. The authors of this study believe that patent grafts and especially patent bilateral internal mammary artery grafts stand as a contraindication for redo-RAMT AVR. Nevertheless, it will be important to make note of any larger studies that report the outcomes of patients with prior CABG surgery who undergo a redo-operation through a RAMT incision. Finally, the authors would like to acknowledge that there may be concerns of encountering extensive right-sided pleural adhesions via RAMT. Surprisingly, however, very little adhesions are usually encountered through a RAMT incision even in patients whose right pleural space was opened or manipulated during their primary sternotomy.
Our study includes several limitations. First, the study size is small, which is reflective of RAMT being a relatively new approach for treating aortic valve disease. Second, the study does not report the long-term outcomes of the patient cohort. Third, the study lacks a comparator group, namely redo-sternotomy and/or redo hemi-sternotomy AVR. While comparing between surgical approaches is important, it is essential to have large sample sizes that can be propensity-matched to ensure appropriate analyses can be done when interpreting the results.
With an ageing population, patients requiring redo-cardiac surgery will continue to increase. In select patients where a redo-sternotomy is not safe or feasible, a RAMT incision may be considered. Although larger studies with longer follow-up period are needed, our study suggests that RAMT can yield similar clinical outcomes to a conventional redo-sternotomy in carefully selected patients.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by This study was approved by the Conjoint Health Research Ethics Board at the University of Calgary and the Medical University of Gdansk underlying the Declaration of Helsinki (Ethics IDs: REB18-0042 and 062/2022, respectively). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
AH: Conceptualization, Data curation, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. JF: Data curation, Writing – review & editing. MH: Data curation, Writing – review & editing. MK: Data curation, Writing – review & editing. MS: Writing – review & editing. DD: Writing – review & editing. CA: Writing – review & editing. WDK: Supervision, Writing – review & editing. WOK: Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Fukuhara S, Kim KM, Yang B, Romano M, Ailawadi G, Patel HJ, et al. Reoperation following transcatheter aortic valve replacement: insights from 10 years’ experience. J Thorac Cardiovasc Surg. (2023). doi: 10.1016/j.jtcvs.2023.04.029
2. Tang GHL, Zaid S, Kleiman NS, Goel SS, Fukuhara S, Marin-Cuartas M, et al. Explant vs redo-TAVR after transcatheter valve failure: mid-term outcomes from the EXPLANTORREDO-TAVR international registry. JACC Cardiovasc Interv. (2023) 16(8):927–41. doi: 10.1016/j.jcin.2023.01.376
3. Gatta F, Haqzad Y, Gradinariu G, Malvindi PG, Khalid Z, Suelo-Calanao RL, et al. Redo aortic valve replacement vs valve-in-valve trans-catheter aortic valve implantation: a UK propensity-matched analysis. Monaldi Arch Chest Dis. (2023) 94(1). doi: 10.4081/monaldi.2023.2546
4. Fukunaga N, Al-Sarraf A, Jawad K, Lafreniere-Roula M, Rao V. Early and mid-term outcomes after aortic valve intervention in patients with previous stentless or stented bioprostheses. J Cardiothorac Surg. (2023) 18(1):34. doi: 10.1186/s13019-023-02118-3
5. Glauber M, Kent WDT, Asimakopoulos G, Troise G, Padrò JM, Royse A, et al. Sutureless valve in repeated aortic valve replacement: results from an international prospective registry. Innovations (Philadelphia, Pa). (2021) 16(3):273–9. doi: 10.1177/1556984521999323
6. Mikus E, Calvi S, Campo G, Pavasini R, Paris M, Raviola E, et al. Full sternotomy, hemisternotomy, and minithoracotomy for aortic valve surgery: is there a difference? Ann Thorac Surg. (2018) 106(6):1782–8. doi: 10.1016/j.athoracsur.2018.07.019
7. Stoliński J, Plicner D, Grudzień G, Wąsowicz M, Musiał R, Andres J, et al. A comparison of minimally invasive and standard aortic valve replacement. J Thorac Cardiovasc Surg. (2016) 152(4):1030–9. doi: 10.1016/j.jtcvs.2016.06.012
8. Gilmanov D, Farneti PA, Ferrarini M, Santarelli F, Murzi M, Miceli A, et al. Full sternotomy versus right anterior minithoracotomy for isolated aortic valve replacement in octogenarians: a propensity-matched study †. Interact Cardiovasc Thorac Surg. (2015) 20(6):732–41. doi: 10.1093/icvts/ivv030
9. Bowdish ME, Hui DS, Cleveland JD, Mack WJ, Sinha R, Ranjan R, et al. A comparison of aortic valve replacement via an anterior right minithoracotomy with standard sternotomy: a propensity score analysis of 492 patients. Eur J Cardiothorac Surg. (2016) 49(2):456–63. doi: 10.1093/ejcts/ezv038
10. Ghoreishi M, Thourani VH, Badhwar V, Massad M, Svensson L, Taylor BS, et al. Less-invasive aortic valve replacement: trends and outcomes from the society of thoracic surgeons database. Ann Thorac Surg. (2021) 111(4):1216–23. doi: 10.1016/j.athoracsur.2020.06.039
11. Olds A, Saadat S, Azzolini A, Dombrovskiy V, Odroniec K, Lemaire A, et al. Improved operative and recovery times with mini-thoracotomy aortic valve replacement. J Cardiothorac Surg. (2019) 14(1):91. doi: 10.1186/s13019-019-0912-0
12. Hassanabad A F, Vasanthan V, Kent WDT. Minimally invasive surgical aortic valve replacement: an overview of recent advances. Can J Cardiol. (2019) 35(2):225–8. doi: 10.1016/j.cjca.2018.11.027
13. Hamid U I, Digney R, Soo L, Leung S, Graham AN. Incidence and outcome of re-entry injury in redo cardiac surgery: benefits of preoperative planning. Eur J Cardiothorac Surg. (2015) 47(5):819–23. doi: 10.1093/ejcts/ezu261
14. Park CB, Suri RM, Burkhart HM, Greason KL, Dearani JA, Schaff HV, et al. Identifying patients at particular risk of injury during repeat sternotomy: analysis of 2555 cardiac reoperations. J Thorac Cardiovasc Surg. (2010) 140(5):1028–35. doi: 10.1016/j.jtcvs.2010.07.086
15. Elahi M, Dhannapuneni R, Firmin R, Hickey M. Direct complications of repeat median sternotomy in adults. Asian Cardiovasc Thorac Ann. (2005) 13(2):135–8. doi: 10.1177/021849230501300208
16. Christiansen S, Schmid M, Autschbach R. Perioperative risk of redo aortic valve replacement. Ann Thorac Cardiovasc Surg. (2009) 15(2):105–10.19471224
17. Onorati F, Biancari F, De Feo M, Mariscalco G, Messina A, Santarpino G, et al. Mid-term results of aortic valve surgery in redo scenarios in the current practice: results from the multicentre European RECORD (REdo cardiac operation research database) initiative†. Eur J Cardiothorac Surg. (2015) 47(2):269–80. doi: 10.1093/ejcts/ezu116
18. Demal TJ, Gordon C, Bhadra OD, Linder M, Ludwig S, Grundmann D, et al. Contemporary outcome trends in transcatheter aortic valve-in-valve implantation versus redo aortic valve replacement. Am J Cardiol. (2022) 171:115–21. doi: 10.1016/j.amjcard.2022.01.049
19. Choi CH, Cao K, Malaver D, Kincaid EH, Lata A, Kon N, et al. Redo-aortic valve replacement in prior stentless prosthetic aortic valves: transcatheter versus surgical approach. Catheter Cardiovasc Interv. (2022) 99(1):181–92. doi: 10.1002/ccd.29921
20. Malvindi P G, Luthra S, Santarpino G, Ramadan T, Hunduma G, Olevano C, et al. Early- and mid-term outcomes of reinterventions for aortic bioprosthesis failure. Asian Cardiovasc Thorac Ann. (2022) 30(7):788–96. doi: 10.1177/02184923221094974
21. Dokollari A, Cameli M, Mandoli GE, Kalra DS, Poston R, Coku L, et al. Early and midterm clinical outcomes of transcatheter valve-in-valve implantation versus redo surgical aortic valve replacement for aortic bioprosthetic valve degeneration: two faces of the same medal. J Cardiothorac Vasc Anesth. (2021) 35(11):3223–31. doi: 10.1053/j.jvca.2021.05.029
22. Scholtz S, Piper C, Horstkotte D, Furukawa N, Börgermann J, Gummert J, et al. Transcatheter aortic valve implantation in patients with pre-existing mechanical mitral valve prostheses. J Invasive Cardiol. (2019) 31(9):260–4.31478891
23. Bakhtiary F, El-Sayed Ahmad A, Amer M, Salamate S, Sirat S, Borger MA. Video-assisted minimally invasive aortic valve replacement through right anterior minithoracotomy for all comers with aortic valve disease. Innovations (Philadelphia, Pa). (2021) 16(2):169–74. doi: 10.1177/1556984520977212
24. Amirjamshidi H, Vidovich C, Goodman A, Knight PA. Early outcomes of isolated aortic valve replacement through right anterior minithoracotomy using the latest-generation biological prosthesis. Innovations (Philadelphia, Pa). (2021) 16(1):52–7. doi: 10.1177/1556984520975889
25. Hassanabad A F, Aboelnazar N, Maitland A, Holloway DD, Adams C, Kent WDT. Right anterior mini thoracotomy approach for isolated aortic valve replacement: early outcomes at a Canadian center. J Card Surg. (2021) 36(7):2365–72. doi: 10.1111/jocs.15571
26. Krishna RK, Santana O, Mihos CG, Pineda AM, Weiss UK, Lamelas J. Minimally invasive aortic valve replacement in octogenarians performed via a right anterior thoracotomy approach. J Heart Valve Dis. (2014) 23(6):671–4.25790612
27. Santarpino G, Berretta P, Kappert U, Teoh K, Mignosa C, Meuris B, et al. Minimally invasive redo aortic valve replacement: results from a multicentric registry (SURD-IR). Ann Thorac Surg. (2020) 110(2):553–7. doi: 10.1016/j.athoracsur.2019.11.033
28. Oezpeker C, Barbieri F, Zujs V, Grimm M, Lio A, Glauber M, et al. Minimally invasive redo-aortic valve replacement: reduced operative times as compared to full sternotomy. Thorac Cardiovasc Surg. (2020) 68(2):141–7. doi: 10.1055/s-0038-1668497
29. Pineda AM, Santana O, Reyna J, Sarria A, Lamas GA, Lamelas J. Outcomes of reoperative aortic valve replacement via right mini-thoracotomy versus median sternotomy. J Heart Valve Dis. (2013) 22(1):50–5.23610989
Keywords: right anterior mini thoracotomy, aortic valve replacement, redo-surgery, minimally-invasive valve surgery, minimally-invasive surgery
Citation: Fatehi Hassanabad A, Fercho J, Fatehi Hassanabad M, King M, Sosniuk M, de Waard D, Adams C, Kent WDT and Karolak W (2024) Right anterior mini thoracotomy for redo cardiac surgery: case series from North America and Europe. Front. Cardiovasc. Med. 11:1427930. doi: 10.3389/fcvm.2024.1427930
Received: 5 May 2024; Accepted: 3 June 2024;
Published: 18 June 2024.
Edited by:
Payam Akhyari, University Hospital Essen, GermanyReviewed by:
Farhad Bakhtiary, University of Bonn, Germany© 2024 Fatehi Hassanabad, Fercho, Fatehi Hassanabad, King, Sosniuk, de Waard, Adams, Kent and Karolak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ali Fatehi Hassanabad, YWxpLmZhdGVoaWhhc3NhbmFiYWRAYWhzLmNh
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.