- 1Division of Cardiology, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada
- 2Department of Health Science, Queen University, Kingston, ON, Canada
- 3Translational Medicine Program, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada
- 4Department of Biophysics, Faculty of Medicine, University of Toronto, Toronto, ON, Canada
- 5Department of Physiology, Faculty of Medicine, University of Toronto, Toronto, ON, Canada
- 6Research Institute, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada
- 7Department of Diagnostic Imaging, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada
- 8Early Origins of Adult Health Research Group, Health and Biomedical Innovation, Clinical and Health Sciences, University of South Australia, Adelaide, SA, Australia
Placental function plays a crucial role in fetal development, as it serves as the primary interface for delivery of nutrients and oxygen from the mother to fetus. Magnetic resonance imaging (MRI) has significantly improved our ability to visualize and understand the placenta's complex structure and function. This review provides an up-to-date examination of the most common and novel placental MRI techniques. It will also discuss the clinical applications of MRI in diagnosing and monitoring placental insufficiency, as well as its implications for fetal growth restriction (FGR) and congenital heart disease (CHD). Ongoing research using multi-parametric MRI techniques aims to develop novel biomarkers and uncover the relationships between placental parameters and pre-onset diseased states, ultimately contributing to better maternal and fetal health outcomes, which is essential to better guide clinical judgement.
Introduction
The placenta is a transient, yet complex organ, involved in the proper growth and development of the fetus. The attachment of the uterine vessels to the basal plate of the placenta, controls the maternal-placental exchange of oxygen and nutrients (1). Similarly, the attachment of the umbilical vessels to the chorionic plate are responsible for placental-fetal transmission (1). Collectively, these vascular beds function through the chorionic villus, the functional unit of the placenta, which mediates the interaction between the uterine and umbilical vessels. Within the placenta, the uterine artery supplies maternal blood to the intervillous space, which surrounds the chorionic villus, and facilitates the transfer of oxygen and nutrients. The physiological relationship between structure and function is evident within placental anatomy, which fundamentally supports the maternal-fetal interchange.
Placental interactions between the maternal and fetal vasculature are essential in the efficient exchange of nutrients and gases to support fetal growth and development. The initial formation of the chorionic villi, representing the functional unit of the placenta, begins 13 days post-conception and is complete around 24–26 weeks, where mature intermediate villi (MIV) differentiate into terminal villi specialized in nutrient and gas exchange (2, 3). The placental vasculature closely parallels villi development, with the initial presence of fetoplacental capillaries at three weeks post-conception, followed by gradual capillary growth throughout the transition phase from immature intermediate villi (IIV) to MIV to terminal villi (3, 4). In particular, non-branching angiogenesis is a crucial process during the late-second to early-third trimester, occurring within the terminal villi, that minimizes the diffusion distance between maternal and fetal blood and further facilitates the growing needs of the fetus for oxygen and nutrients (3).
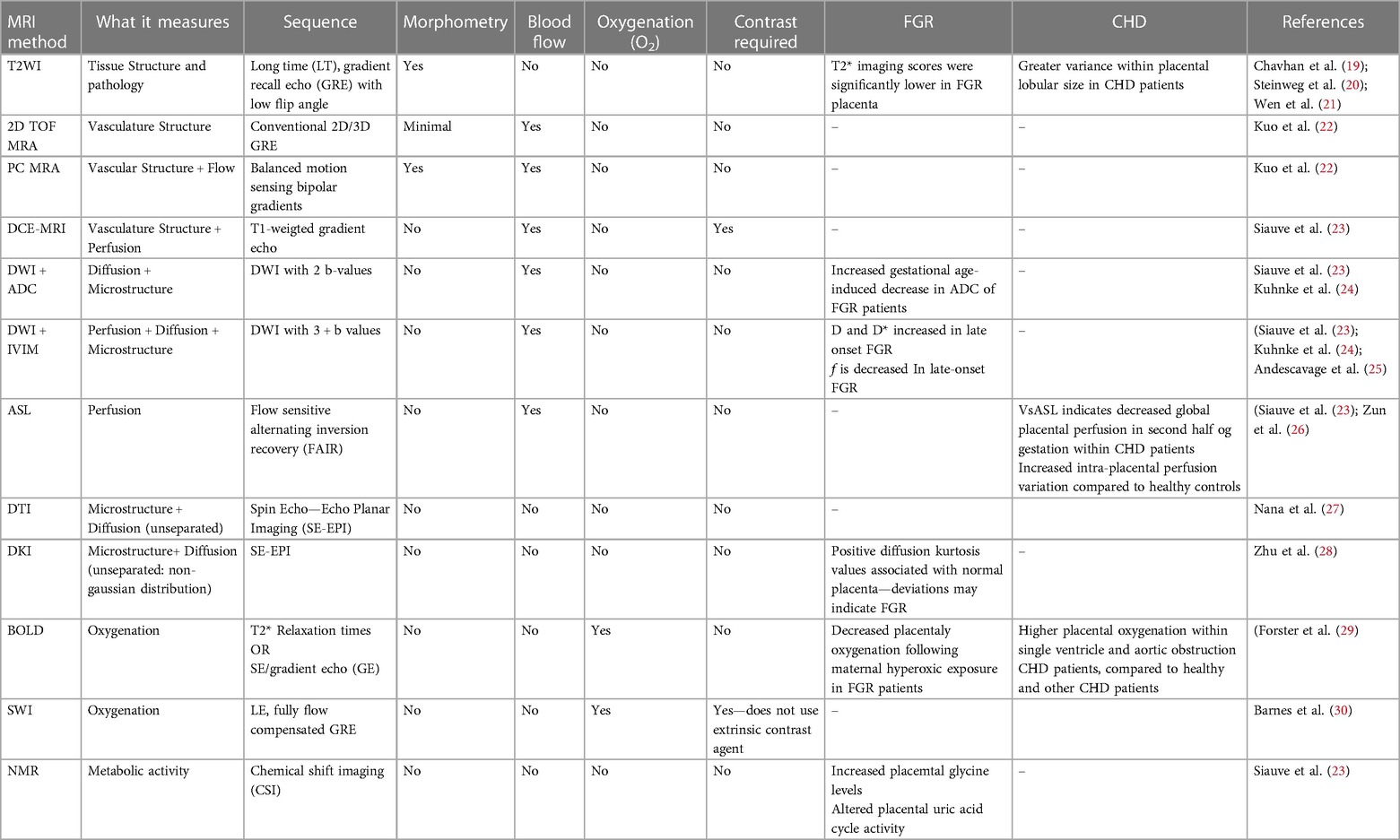
Placental insufficiency occurs when there is a deviation from the normal processes of villous and vasculature development, leading to impaired transfer of nutrients and oxygen to the fetus. These physiological changes associated with placental insufficiency can lead to fetal chronic hypoxemia and/or hypoglycemic as well as FGR and CHD (5–8). Alongside placental insufficiency, issues involving dysfunctional implantation, such as placenta accreta spectrum and previa, or variant morpholgies, such as circumvallate placenta, may pose a risk to proper fetal development (9–11). The dependence of the fetus on maternal supply of nutrients and oxygen places a high demand for optimal placental function and effectively necessitates a lack of compromised blood flow. The placenta lacks neural innervation, which places a high reliance on hemodynamic forces and downstream vaso-regulatory mediator products, such as nitric oxide (NO), in facilitating proper development (8, 12). As such, variable hemodynamic forces caused by faulty fetoplacental vasculature formation may hinder necessary regulatory mechanisms to prevent FGR and CHD, among other developmental abnormalities (5, 8, 13). Figure 1 illustrates theoretical changes in placental hemodynamics that could occur in response to different maternal, placental, and fetal conditions, potentially leading to chronic fetal hypoxemia. These alterations can be detected using advanced magnetic resonance imaging (MRI) techniques, providing insights into the physiological adaptations and pathological processes affecting placental function. Saini et al., demonstrated the ability of MRI to measure blood flow and oxygen levels in the uterus and umbilical vessels, enabling the calculation of oxygen delivery to the pregnant uterus, placenta, and fetus (14) (Figure 2). Further study explored the feasibility of using MRI to assess maternal-fetal oxygen transport and consumption in conditions with altered uterine artery blood flow due to maternal position (15) or pharmacologic treatment (16). Relation to maternal positioning during healthy late-stage pregnancies has been applied to understanding the impact of maternal position or pharmacologic treatment to increase uterine artery blood flow on placental oxygen consumption (15–18). MRI offers a non-invasive way to gather detailed information about placental function and fetal well-being, which could aid in monitoring pregnancies and identifying potential complications (15).
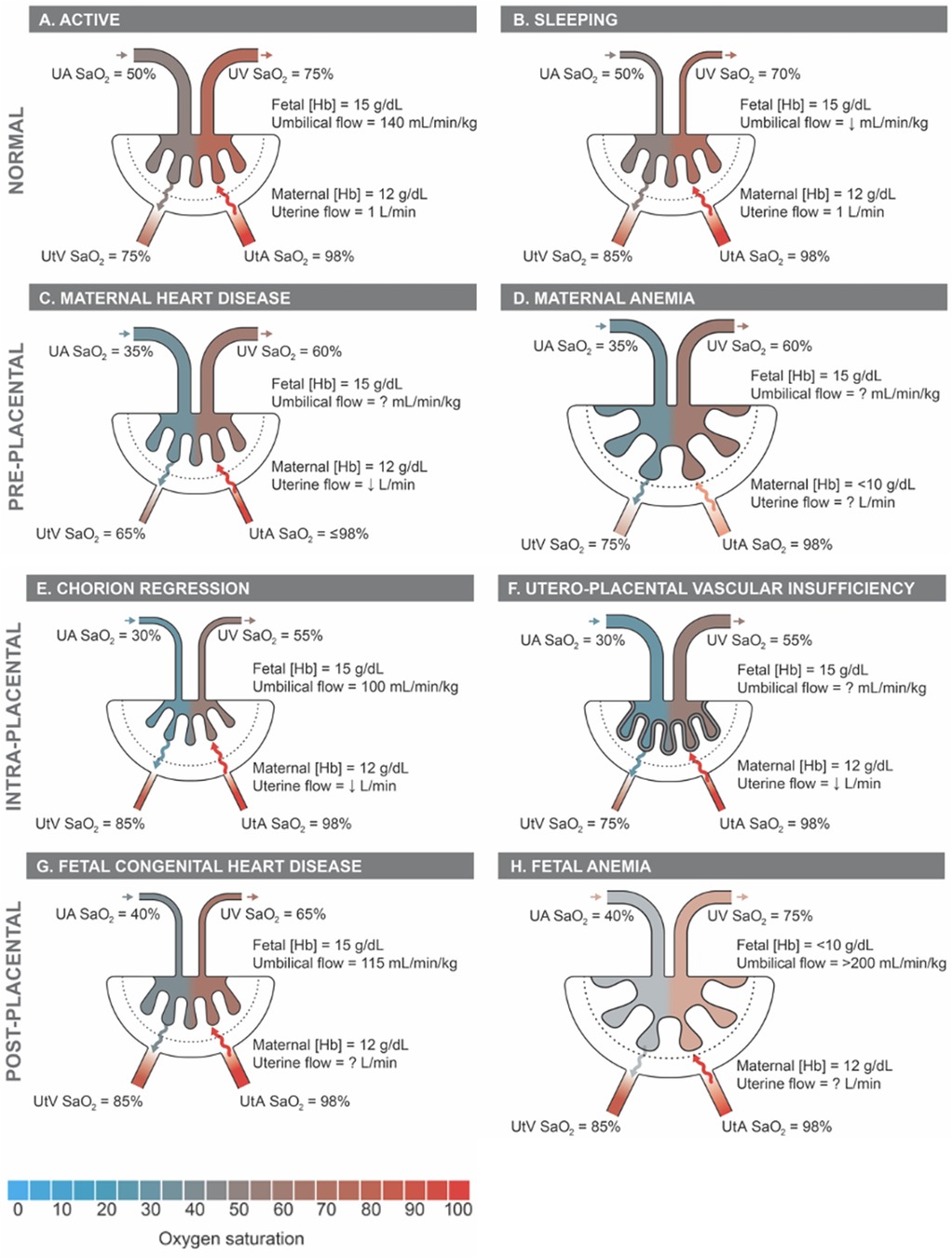
Figure 1 Proposed placental hemodynamics in normals and examples of pre-, intra-, and post-placental causes of impaired oxygen transport. Reference values from animal and human literature included where available with unknown values indicated as “?”. Q, flow; SaO2, oxygen saturation; Hb, hemoglobin concentration; UtA, uterine arteries; UtV, uterine veins; UA, umbilical arteries; UV, umbilical vein.
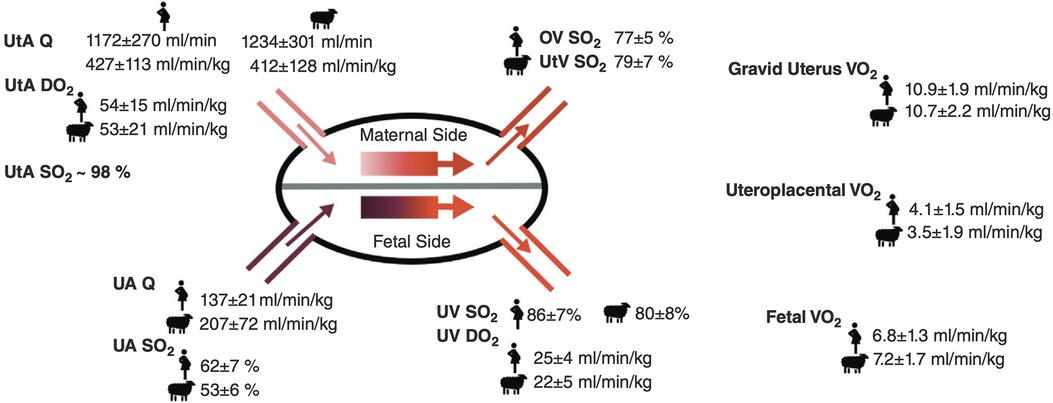
Figure 2 Placental hemodynamics, oxygen transport, and consumption in healthy normal human and sheep pregnant subjects in late gestation, Re-produced from Saini et al. (14) with permission from John Wiley and Sons. Q, blood flow; DO2, oxygen delivery; SO2, oxygen saturation; VO2, oxygen consumption; UtA, uterine artery; UtV, uterine vein; OV, ovarian vein; UA, umbilical artery; UV, umbilical vein; Hgb, [hemoglobin]; m, maternal; f, fetal.
Given the significance of fetoplacental vasculature development in the proper functioning and maintenance of the fetal environment, an update on the most current advances within the field of placental imaging is necessary. In this review, we will summarize the progress of various MRI technologies in assessing fetoplacental vascular composition and function, including levels of trans-placental perfusion and diffusion, as well as their abilities to measure oxygenation within the fetal blood (Table 1). This will be coupled with an analysis of the clinical implications of applicable MRI technologies on pregnancy complications associated with placental dysfunction such as FGR and CHD, involving the benefits and limitations of their use, as well as future directions.
Progression of placenta MRI
MRI technology has come a long way since its inception in 1984, allowing a clear understanding of placental physiology. Placental imaging has presented unique challenges since its inception, often associated with the variability of placenta location, size, shape and vascular composition. Further, the need to maintain fetal safety limits the progression of the field. However, over the past four decades, researchers have developed increasingly complex MRI techniques (Figure 3), such as diffusion kurtosis imaging (DKI), which can measure non-normal water diffusivity within placental vasculature (31, 32). This parallels our growing understanding of the role that MRI may play in a better understanding of placental physiology and the impact of disease states on its structure and function.
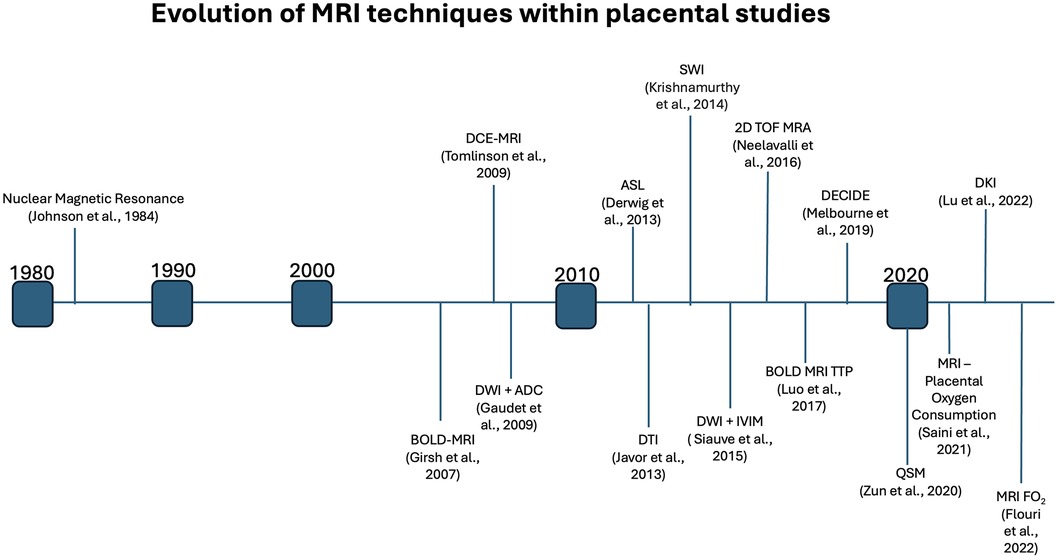
Figure 3 Timeline indicating the first noted usage of various MRI methods within placental studies, highlighting the extensive growth of placental MRI over the past decades.
Current utility of MRI in the human placenta
Placenta structural and morphometric assessments
Although ultrasound is commonly used in pregnancy to measure placental and fetal growth and blood flow velocity in major vessels, it may offer a decreased ability for functional assessment, and limited viewing capabilities. The use of MRI offers an advantage in the antenatal study of the macrostructural and morphometric properties of the placenta (33). In particular, a broader viewing frame, along with greater contrast of soft tissue, and decreased operator-dependency provides benefits compared to ultrasound (33–35). Despite a slightly blurred boundary between the placenta and myometrium, the ability to clearly distinguish between the placenta and the amniotic fluid is significant and allows for precise measurements of placental shape and size at any gestational age (36, 37). The clinical relevance of placental morphometric properties is underscored—numerous studies have found correlations between placental weight and volume and postnatal properties such as birthweight, as well as health in later life (38–43). Furthermore, studies have indicated significant associations between properties such as placental weight, volume and surface area with placental insufficiency, fetal growth restriction (FGR) and Congenital Heart Disease (CHD) (44–47).
The use of T2-weighted imaging (T2WI) in MRI offers advantages for placental imaging compared to other techniques such as T1-weighted imaging (T1WI) (36). With T2WI, the normal placenta appears homogenous, particularly during the second trimester, providing a more effective baseline when compared to the heterogenous appearance with T1WI (36, 37, 48, 49). The transition to a more heterogenous appearance on T2WI, especially as the third trimester approaches, may indicate placental maturation—specifically, better-defined cotyledons with a more lobular appearance (48, 49). However, these observations should be integrated with additional structural and functional data to ensure a comprehensive understanding. Methods have been devised that combine 2D MRI images from axial, sagittal and coronal planes to construct a super-resolution 3D view of the placenta, enhancing segmentation and visualization capabilities (37, 50, 51). Super-resolution reconstruction methods aimed at improving the imaging of structural and morphometric properties hold significant potential to improve the diagnostic and analytic capabilities of placental MRI (37). Further, particular 3D-MRI models are capable of providing 3D views of the placenta, without the need for fusing 2D stacks (52). Usage of 3D-MRI models may decrease processing times, and improve image quality, due to decreased dependence on operator segmentation expertise.
Vascular structure and function
The success of many quantitative MRI techniques in placental imaging relies on the use of efficient vascular localizers to properly visualize the fetal, placental, and maternal vasculature (53). Time-of-flight (TOF) magnetic resonance angiography (MRA) represents a conventional, non-contrasting MRA approach that facilitates the ability to visualize vasculature in 3D (53). Ultrasound is a commonly used modality in clinical practice for imaging the fetal and placental vasculature due to its non-invasive nature, safety, and relatively low cost. However, ultrasound has limitations, particularly when visualizing deeper structures or those obscured by overlying tissues, bones, or gas. TOF-MRA offers an alternative approach that can overcome these limitations by acting as a supplemental measure to ultrasound results (54, 55). In the past, a study by Neelavalli et al. indicated the success of TOF-MRA in visualizing the fetal, placental and maternal vasculature when using 3.0 T MRI during the third trimester (53). While this study showed success in observing the vasculature of the fetus and the placenta, it was limited due to its testing parameters. For instance, the clinical reality of fetal imaging at that time relied on access to 1.5 T MRIs during earlier gestational periods. This was addressed by Qu et al., who conducted a study involving 2D TOF-MRA at 1.5 T and 3.0 T while also including an experimental group to determine the impact of gestational age. Regardless of 1.5 T or 3.0 T usage, the umbilical arteries were observed in all cases, and chorionic arteries were observed in most cases. Further, the repeated use of 3.0 T on three patients within the second and third trimesters revealed consistent images of the radial and spiral arteries, indicating feasible usage within the second trimester (55). This study led the way to the view that 1.5 and 3.0 T MRI can be used in fetal and placental imaging.
The limitations of TOF-MRA involve a bias towards higher-speed blood flow, which limits the resolution of less rapid blood vessels, as well as a clinical need for faster imaging times, which assists in combatting excessive fetal motion (53, 55). Future research should focus on improved resolution of lower-speed blood vessels using TOF-MRA techniques to address vascular localization concerns with modern usage.
Phase contrast (PC) MRA is a non-contrasting sequence that offers capabilities for the quantification and localization of blood velocity (37, 56). PC-MRA offers similar limitations as TOF-MRA, including long acquisition times; however, its 3D usage provides advantages over TOF-MRA, particularly related to enhanced detection of collateral flow or slower flow (57). PC-MRA is typically only used in cases where vascular structure and flow are essential, such as within the detection of stenosis (22). A study by Nii et al., which used 2D PC-MRA, emphasized its clinical utility by indicating a change in uterine blood in pregnant women, following administration of tadalafil, a phosphodiesterase-5 inhibitor, for treatment of FGR (58).
Placental perfusion
Dynamic contrast-enhanced (DCE) MRI is a valuable technique for imaging the placental vasculature, offering both visualization and quantitative analysis of microcirculatory parameters, through fast-imaging sequences that coincide with repeated administration of a contrasting agent (59). However, the use of gadolinium as a contrast agent in DCE-MRI during pregnancy has been associated with risks to the fetus due to its secretion into the amniotic fluid (60, 61). Some nonhuman studies have indicated low gadolinium concentrations within amniotic fluid following administration; however, human trials are necessary to confirm safe usage (62, 63). To address this issue, recent research has focused on developing alternate contrasting agents that are safer for use during pregnancy. One such alternative is a manganese-based contrast agent, Mn-PyC3A, which has been tested in non-human primates and found to be comparable in efficacy to gadolinium-based contrast agents (GBCAs) (64). Mn-PyC3A offers the advantage of dual excretion through both renal and hepatobiliary systems, reducing the risk of accumulation in the body (64). Further studies are needed to validate the long-term safety and sensitivity of Mn-PyC3A within various tissues and MRI techniques. An alternative, ferumoxytol, has been used safely in pregnant women for iron-deficient anemia and shows promise in recent studies using DCE-MRI with rhesus macaques (65–67). Prior studies have indicated that ferumoxytol has a safe administration profile through its lack of effect on placental and fetal growth and histopathology (68–70). DCE-MRI contributes significantly to assessing placental perfusion with studies demonstrating its ability to quantify maternal-placental blood flow in non-human primates and detect perfusion abnormalities in growth-restricted fetuses (71–73). A linear relationship between DCE-MRI perfusion metrics and gestational age suggests that this technique could be used to monitor proper fetal growth and identify developmental issues early (71). Recent research also highlights the potential of DCE-MRI for detailed spatiotemporal depiction of the placental lobes, offering new clinical perspectives for the early detection of abnormalities (74). However, the use of gadolinium as a contrast agent during pregnancy poses risks, promoting the exploration of safer alternatives like ferumoxytol for future applications in prenatal diagnostics.
Arterial Spin Labelling (ASL) represents a non-invasive MRI alternative to assess placental perfusion by comparing signal intensity within the placenta to blood travelling to the placenta (23). Common techniques within advanced ASL include spatial-selective labelling, which tags the blood based on location, and velocity-selective labelling, which tags the blood based on its velocity. Traditional ASLs, like pulse ASL (pASL) and continuous ASL (cASL), face limitations in accurate placental perfusion measurement due to inefficient labelling and signal loss (75). Pseudo-continuous (pcASL) and velocity-sensitive (vsASL) ASLs are advanced techniques that address some of these limitations, with pcASL offering improved signal-to-noise ratio (SNR) while relying on spatial-selective labelling (75–77). A study by Liu et al. used pcASL to demonstrate a relative decrease in placental lobule perfusion in high-risk pregnancies, indicating its clinical utility to identify placental dysfunction (78–80). Moreover, vsASL differs from the other ASLs through its replacement of spatial-selective labelling with velocity-selective labelling, which consequently prevents the measurement of arterial transit times (ATT) (80–83). This modification, alongside a decreased sensitivity to ATT, has allowed for a higher SNR in vsASL compared to pcASL (75, 84). Despite this, pcASL remains the clinical standard due to the novel nature of vsASL (75). The VESPA ASL technique combines pcASL and vsASL to optimize perfusion analysis while still measuring ATTs, offering a comprehensive approach to assessing placental health (83).
Placental microstructure
Diffusion-weighted imaging (DWI) is a non-invasive MRI technique that offers insight into placental microstructure and function by detecting the molecular motion of water protons (85). This method is particularly useful for identifying tissues with compromised membrane integrity in necrosis with placental failure (86). Consequently, DWI has clinical relevance in the early detection of changes related to various placental disorders, including placental insufficiency, FGR and CHD (25, 87–89).
Studies have utilized DWI to analyze placental health, with the apparent diffusion constant (ADC) and the intravoxel incoherent motion (IVIM) being key quantitative models for interpreting DWI data. The ADC reflects the overall mobility of water molecules within a tissue, while IVIM provides more detailed information about the microcirculation within the placenta. The ADC is a generalized measure of placental diffusion that cannot differentiate between true diffusion (D) and pseudo-diffusion (D*). Higher ADC values indicate less restriction for water movement and may suggest the presence of fluid-filled spaces, while lower ADC values indicate higher restrictions of water movement, typically in areas of high cellular density (90). ADC values have an inverse relationship with gestational age due to the normal maturation of the placenta during fetal growth (32, 50, 85). In growth-restricted fetuses, the gestational age decrease in ADC is larger and occurs earlier in gestation, offering an approach to distinguish between restricted and normal placental development (17, 50, 91–94). The Dmean and D*minimum values derived from IVIM are also negatively correlated with gestational age (GA), which parallels the development of a fibrotic or calcified placental vasculature, and constitutes decreased diffusion and perfusion (32). The measurement of perfusion fraction (f) has revealed novel insight into placental dysfunction, with an inverse relationship between f and placenta dysfunction, which emphasizes the importance of perfusion within a healthy placenta (95, 96). Proper perfusion within the healthy placenta facilitates the transfer of oxygen and nutrients that are responsible for supporting normal fetal growth. This relationship allows for more effective identification of at risk of pregnancies, potentially enabling earlier intervention and improving outcomes. Anisotropic IVIM models account for the orientation of microvascular tissue and assume differences in results based on this (97). These models are indicated as a better fit for placental DWI and explain the diffusive properties more effectively than ADC modelling (97, 98). However, a limitation of the IVIM approach is increased total acquisition time, which can negatively impact patient compliance and may lead to motion artifacts (99).
DKI and the hybrid IVIM-DKI provide an enhanced view of placental health during pregnancy (32). These methods extend beyond traditional Diffusion Tensor Imaging (DTI) by accounting for the non-Gaussian distribution of water molecules within the placenta, offering a more detailed analysis of microstructural changes and tissue heterogeneity (100–102). However, recent validations have shown that DTI-based motion correction can effectively address misalignments in DWI caused by the movement of the fetus and mother, leading to more accurate quantification of placenta microstructures (103). Despite this clinical utility, DTI has limited biological specificity of various placental microstructural features (101). Within DKI, DK values are positively associated with gestational age, which indicates the potential of DKI in distinguishing cases of restricted fetal growth through low DK values (32).
Placenta oxygenation
Assessing placental oxygenation is a crucial aspect of monitoring placental and fetal development during pregnancy. The dynamic changes in oxygen within fetal and maternal placental vasculature across the three trimesters support the growth and development of the placenta and the fetus. In the first trimester, lower partial pressure of oxygen (PO2) around 20mmHg are physiologically normal and contribute to trophoblast proliferation (104, 105). As pregnancy progresses to the second trimester, there is an increase in PO2 to approximately 60 mmHg, which aids in trophoblast invasion and ensures proper placental development (104, 105). In the third trimester, PO2 begins to decrease and fluctuate, reaching an average of ∼40 mmHg. This decline correlates with the increasing oxygen demands of the growing fetus (104, 105). The importance of maintaining appropriate oxygenation is underscored by its strong association with conditions such as placental insufficiency, FGR, and CHD (106–109).
T1 and T2 mapping are common techniques associated with analysis of placental oxygenation and are utilized in a variety of signal sequences. T1 and T2 mapping is sensitive to oxygen saturation (SO2) levels, as well as hematocrit, which has led to its role in the evaluation of these measures (110). T1 and T2 relaxation times offer insight to placental function, and consequently may supplement current practices for determining placental function and corresponding fetal outcomes (95, 111). For instance, a study by Schabel et al., found a strong association between placental T2* and pregnancy outcomes within cases of placental insufficiency (111). Additional studies have indicated a predictive ability of T1 within cases of small for gestational age (SGA), which indicates its clinical potential (95). Further, the use of T1 and T2 in combination, allowed for significant distinctions between healthy- and FGR-pregnancies, compared to the isolated use of either (112).
Blood Oxygen Level Dependant (BOLD)-MRI is a sophisticated diagnostic tool that measures placenta oxygenation by detecting T2* relaxation time changes. This technique exploits the fact that deoxyhemoglobin, a component of red blood cells, has paramagnetic properties that affect the MRI signal intensity (113–115). Luo et al. used BOLD MRI to indirectly assess regional placental oxygen delivery, modelling the oxygenation response and generating time-to-plateau (TTP) maps (114). They found distinct TTP patterns in placentas with pathology vs. healthy ones, suggesting BOLD MRI could non-invasively detect placental dysfunction, complementing histopathological diagnosis (Figure 4). The R2* (1/T2*) parameter derived from BOLD-MRI scans is particularly important because it reflects the amount of deoxyhemoglobin present and indirectly measures placenta oxygenation: higher R2* values indicate lower oxygen levels.
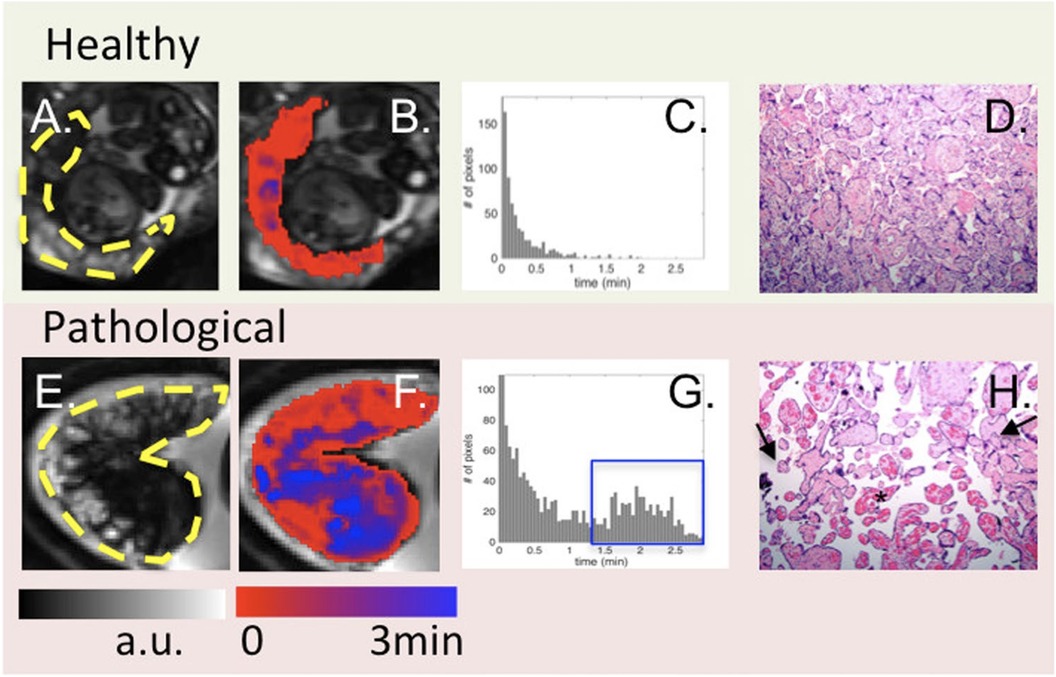
Figure 4 From left to right: BOLD images (A), time to peak (TTP) maps (B), histogram of TTP distribution (C) and histology (10X) (D). One control (top) is compared to one case with abnormal placental pathology (bottom). Yellow dashes in (A, E) outline the placenta. For healthy subjects, TTP values were short and placental histology was normal. For pathological cases, TTP values were longer and less uniform [blue regions in (F) and blue box in (G)]. Arrows in (H) point to the vascular villi, and the star identifies chorangiosis, re-produced from Luo et al. (114) used under CC BY 4.0.
Several studies have demonstrated that BOLD-MRI can quickly and directly observe changes in placental oxygenation in response to increased maternal oxygenation, whether achieved through supplemental oxygen or maternal breathing manipulation (107, 116). The rapid peak in ΔR2* values following oxygen administration underscores the sensitivity of BOLD-MRI in capturing real-time alterations in placenta function (107, 116). Furthermore, Saini et al. show that the maternal position during the BOLD-MRI scan influences placental oxygenation. These findings indicate that the supine position may impair oxygenation compared to the left lateral position (15), which supports standard clinical recommendations for the left lateral tilt position, aimed at optimizing blood flow between the mother and fetus (117). Additionally, Turk et al. point out the necessity of accounting for Braxton Hicks contractions within the interpretation of signal intensity due to their association with decreased global R2* levels within the placenta (118).
Another significant development in this field is the assessment of Placental Vascular Reactivity (PlVR), a novel and non-invasive index measured by BOLD-MRI that gauges the adaptability of the placental vasculature to changes in maternal oxygen levels. PlVR is primarily influenced by fluctuations in maternal CO2 levels, revealing a unidirectional relationship between mother and fetus regarding vascular responses (119). As an indicator of placental vascular integrity, PlVR quantification holds promise for clinical application due to its straightforward validation and use in a clinical setting (119). Collectively, this evidence portrays the significant clinical potential of BOLD-MRI in revolutionizing the real-time analyses of placental function and development.
Susceptibility Weighted Imaging (SWI) offers contrast capabilities by qualitatively interpreting the distortions in magnetic fields (86, 120). SWI is particularly sensitive to deoxygenated blood, which aids in distinguishing arteries from veins, making it valuable for oxygen saturation studies (121). Building on this, Quantitative Susceptibility Mapping (QSM) extends the capabilities of SWI by mapping the distribution of magnetic materials within tissues (120, 122), using both magnitude and phase data to achieve higher sensitivity to blood oxygenation compared to BOLD-MRI (123, 124). QSM has been used clinically to study the brain but has also shown potential in placenta research, where it has revealed positive correlations between spatial variation in oxygenation and gestational age. Effective use of QSM includes measuring baseline susceptibility, with amniotic fluid serving this role in placenta studies (122, 125).
The overlap of maternal and fetal blood within placental scans presents difficulties in functional analysis (93). Diffusion-Relaxation Combined Imaging for Detailed Placental Evaluation (DECIDE) is a novel multiparametric MRI technology that combines T2 relaxometry and DWI to compartmentalize the placenta into regions of maternal blood flow, fetal blood flow and placental tissue to address this issue (126). The clinical value of DECIDE has been validated in sheep studies and used to test efficacy of potential FGR therapies (16, 18, 50, 93). A study by Darby et al. used DECIDE to analyze the effects of tadalafil, on placental perfusion and found no change in SO2 of fetal blood in the placenta but an increase in maternal blood volume in the placenta, further supporting the clinical feasibility of DECIDE. DECIDE provides functional biomarkers capable of analyzing the SO2 of fetal blood in the placenta (FO2), which is directly approximated by T2 relaxation times (126, 127). Moreover, DECIDE detected decreased FO2 in the placentas of FGR patients and has indicated an inverse relationship with FGR severity (18, 91). Overall, DECIDE FO2 offers significant potential in differentiating FGR from small but normoxemic fetuses, which offers precise predictions of fetal growth up to three weeks in advance and offers significant potential for early diagnosis within FGR patients (93).
Placenta metabolism
The study of placental metabolomics represents an emerging field of research that has the potential to enhance our understanding of biomarkers and pathogenesis related to placenta disease (128). This is a burgeoning area with potential for deepening our comprehension of conditions such as FGR and CHD (129–131). Nuclear Magnetic Resonance (NMR) is a key analytical tool in this domain, enabling the real-time and in vivo examination of placental metabolites (128). Within the scope of imaging technologies, NMR may also be referred to as MR spectroscopy (MRS) (94). Proton MRS (H-MRS), a common metabolomic technique, has been instrumental in detecting disturbances in metabolite concentrations and biochemical pathway activities within the placenta (129, 132). Deuterium MRS (D-MRS), a non-invasive alternative to H-NMR, holds promise for clinical applications due to deuterium's inertness in metabolism and non-radioactive nature (133, 134). Studies using D-MRS have identified increased lactate in preeclamptic placentas, suggesting heightened glycolysis and hypoxic stress (134). High-resolution magic angle spinning (HR-MAS) MRS has emerged as an ex vivo method capable of measuring tricarboxylic acid (TCA) cycle placental metabolic analysis. Using this method, the HR-MAS MRS could identify and quantify various TCA cycle intermediates in placentas from mothers with hypercortisolemia (135, 136). Building on HR-MAS MRS, Comprehensive Multiphase (CMP) MRS enhances the differentiation of various phases within complex tissues like the placenta, offering detailed characterization that can improve the detection of abnormalities (137, 138). Clinical applicability of CMP MRS was demonstrated through the identification of altered amino acid concentrations in preterm birth placentas, underscoring its value in elucidating metabolic changes associated with placental pathologies (138, 139).
Clinical implications in FGR and CHD
FGR occurs in ∼10% of pregnancies and is associated with poor outcomes including an increased risk of stillbirth, premature birth and admission to the neonatal intensive care unit. Furthermore, there is an association between poor growth in utero and the risk of non-communicable chronic disease in adulthood (140–142). Although FGR has maternal, placental and fetal causes, each generally results in reduced substrate (oxygen and/or nutrients) delivery to the fetus such that the fetus does not reach its genetic growth potential and has a birth weight less than the 10th centile, although Delphi consensus reports suggest the 3rd centile is most clinically relevant (143). The fetus mounts a hemodynamic and endocrine response to reduced substrates that results in changes in the structural and functional development of most organ systems (6, 144). These responses are the basis of fetal programming of increased risk of hypertension, coronary artery disease, diabetes, and obesity in adulthood.
Strikingly, nearly 50% of all FGR fetuses go undetected until after birth (106), despite improvements in obstetric imaging and management (145). Distinguishing SGA from FGR can be difficult (146). Thus, clinical decisions about when to deliver the FGR baby may not be optimal (145). To avoid stillbirth, for example, many FGR/SGA babies are delivered preterm and may face poor outcomes associated with immature organs (147).
Thus, early detection of placental dysfunction to allow proper clinical care is essential (148–150). Despite the lack of a gold-standard definition for FGR diagnosis, improved early detection and fetal monitoring are within the scope of modern MRI techniques (151). Even when FGR is identified, suboptimal monitoring protocols hinder the precise determination of the most advantageous timing for intervention (106, 148). Innovations within MRI techniques have facilitated their use for assessing placental macro- and microstructure, as well as placental oxygenation and metabolism (14, 23, 25, 32, 53, 59, 71, 85, 94, 115, 120, 129, 132, 152). Studies on placental morphometric characteristics and vascular structure have indicated significant relationships involving decreased placental volume (44). This is coupled with studies showing increased D and D* levels, with decreased f signals in late-onset FGR patients (25). These novel biomarkers of FGR may assist in guiding clinical judgements regarding proper care practices. Further, studies using BOLD-MRI have identified decreased placental oxygenation in the placentas of fetuses with FGR following periods of maternal hyperoxygenation (106, 107). This underscores the clinical relevance of oxygenation within more effective FGR care. Moreover, studies involving H-NMR have indicated elevated glycine levels and altered urea cycle activity within FGR patients, highlighting the prevalence of metabolic shifts (129). The future of MRI within FGR patients will likely involve multi-parametric and multi-compartment MRI techniques to allow for more optimal diagnostic and monitoring abilities. For instance, a study by Aughwane et al. used DECIDE, to not only indicate decreased placental oxygenation within FGR patients but also account for differences across varying gestational ages to produce more accurate parameters (91). Collectively, the use of various MRI technologies has provided more specific parameters and physiological biomarkers that can significantly advance the detection and monitoring of FGR, which may decrease the prevalence of stillbirths and faulty postnatal development within these patients.
CHD has been increasing in incidence over the last two decades (153, 154), and its diagnosis and management has been improved through MRI analysis (155–159), and the assessment of placental function. This variation in case incidence could reflect changes in diagnostic protocols for CHD or advancements in medical diagnostic capabilities; however, it highlights the need for improving clinical diagnostic and monitoring technologies. The development of the fetal heart is closely related to the development of the placenta, which emphasizes the importance of placental imaging (5, 160, 161). Consequently, CHD diagnosis and management may be more optimally approached through MRI analysis of placental structure and oxygenation (45, 47, 89, 108, 109). A study by Steinweg et al. used a T2*-based MRI to find that fetuses with CHD displayed greater variance within placental lobular size compared to healthy controls (20). Similarly, placental volume exhibits a positive relation with fetal birth weight; however, within CHD, placental volume displays a steeper positive relation with birth weight, which may indicate a compensation effect in the placenta (162). These structural observations may offer enhanced phenotypical indications of CHD, which may support diagnostic and monitoring efforts. Further, a study by You et al. used BOLD-MRI to demonstrate a relatively higher increase in placental oxygenation within single ventricle (SV) and aortic obstruction CHD compared to healthy controls and other CHD patients (116). In particular, this finding offers potential therapy for improving placental oxygenation within SV and aortic obstruction CHD and highlights the need to differentiate between various types of CHD. Various types of CHD exist, each with unique compensatory mechanisms that add to the clinical complexity of managing these diseases (163). MRI usage within CHD appears limited due to the lack of studies examining placental metabolism as a physiological biomarker, but its clinical implementation offers benefits and would function effectively as a supplement to current clinical protocols based on its ability to provide an understanding of placental function.
Challenges and future directions
The challenges associated with placental MRI largely stem from patient-based limitations, particularly those related to excessive fetal and maternal motion (48, 164). This motion can lead to signal acquisition issues and result in image degradation (165, 166). Typical examples of this non-rigid motion involve placental deformation, maternal respiration patterns, uncontrolled uterine contractions and fetal body and breathing movements (34, 118). Techniques like Deformable slice-to-volume registration (DSVR) have been developed to correct misaligned or degraded images. However, it presents significant limitations involving improper handling of severe motion-induced degradations and automatic rejection of outliers, which may exclude useful information (167). To address these concerns, researchers are exploring the use of artificial intelligence (AI) for automatic segmentation of the placenta (168, 169), which could reduce the time-consuming and complex nature of manual segmentation practices (169).
Another issue is the presence of tissue interfaces within the placental environment, which can further degrade signals and images. The transition to 3 T MRI has made this a growing concern, as it increases image distortion compared to 1.5 T MRIs. Concerns including extreme acoustic effects and prolonged exposure to 3 T signals have been cited as potential safety hazards; however, some of these have been addressed (170, 171). Previously, 3 T was believed to be harmful to fetal development, but recent studies have found it safe for use within the fetal environment without demonstrating an excessive specific absorption rate (170).
To promote standardization in placental MRIs, implementing a validated scoring system should be a goal, as this could improve inter-observer reproducibility. Studies have shown that using MRI scoring systems can increase predictive values in determining future outcomes, offering strong clinical potential for their use in placental MRIs (172, 173).
Conclusion
Evolving placental MRI technology now enables earlier detection and management of fetal conditions, featuring improved imaging clarity, minimized motion interference, and an array of diagnostic biomarkers. These advances aim to predict pre-dysfunction disease onset, enhancing therapeutic efficacy across gestational stages. Future directions focus on refining protocols and discovering new biomarkers to integrate placental MRI as a standard in clinical practice for optimal perinatal health.
Author contributions
ES: Writing – original draft, Writing – review & editing. LS: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. CM: Conceptualization, Writing – original draft, Writing – review & editing. MS: Conceptualization, Writing – original draft, Writing – review & editing. JM: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Huppertz B. The anatomy of the normal placenta. J Clin Pathol. (2008) 61:1296–302. doi: 10.1136/jcp.2008.055277
2. Gude NM, Roberts CT, Kalionis B, King RG. Growth and function of the normal human placenta. Thromb Res. (2004) 114:397–407. doi: 10.1016/j.thromres.2004.06.038
3. Kingdom J, Huppertz B, Seaward G, Kaufmann P. Development of the placental villous tree and its consequences for fetal growth. Eur J Obstet Gynecol Reprod Biol. (2000) 92:35–43. doi: 10.1016/S0301-2115(00)00423-1
4. Demir R, Kaufmann P, Castellucci M, Erbengi T, Kotowski A. Fetal vasculogenesis and angiogenesis in human placental villi. Acta Anat (Basel). (2008) 136:190–203. doi: 10.1159/000146886
5. Courtney JA, Cnota JF, Jones HN. The role of abnormal placentation in congenital heart disease; cause, correlate, or consequence? Front Physiol. (2018) 9:1045. doi: 10.3389/fphys.2018.01045
6. Darby JRT, Varcoe TJ, Orgeig S, Morrison JL. Cardiorespiratory consequences of intrauterine growth restriction: influence of timing, severity and duration of hypoxaemia. Theriogenology. (2020) 150:84–95. doi: 10.1016/j.theriogenology.2020.01.080
7. Malhotra A, Allison BJ, Castillo-Melendez M, Jenkin G, Polglase GR, Miller SL. Neonatal morbidities of fetal growth restriction: pathophysiology and impact. Front Endocrinol. (2019) 10:55. doi: 10.3389/fendo.2019.00055
8. Morley LC, Debant M, Walker JJ, Beech DJ, Simpson NAB. Placental blood flow sensing and regulation in fetal growth restriction. Placenta. (2021) 113:23–8. doi: 10.1016/j.placenta.2021.01.007
9. Taniguchi H, Aoki S, Sakamaki K, Kurasawa K, Okuda M, Takahashi T, et al. Circumvallate placenta: associated clinical manifestations and complications—a retrospective study. Obstet Gynecol Int. (2014) 2014:986230. doi: 10.1155/2014/986230
10. Silver RM. Abnormal placentation: placenta previa, vasa previa, and placenta accreta. Obstet Gynecol. (2015) 126:654. doi: 10.1097/AOG.0000000000001005
11. Morlando M, Collins S. Placenta accreta spectrum disorders: challenges, risks, and management strategies. Int J Womens Health. (2020) 12:1033–45. doi: 10.2147/IJWH.S224191
12. Tropea T, Wareing M, Greenwood SL, Feelisch M, Sibley CP, Cottrell EC. Nitrite mediated vasorelaxation in human chorionic plate vessels is enhanced by hypoxia and dependent on the NO-sGC-cGMP pathway. Nitric Oxide. (2018) 80:82–8. doi: 10.1016/j.niox.2018.08.009
13. Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “great obstetrical syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. (2011) 204:193–201. doi: 10.1016/j.ajog.2010.08.009
14. Saini BS, Darby JRT, Marini D, Portnoy S, Lock MC, Yin Soo J, et al. An MRI approach to assess placental function in healthy humans and sheep. J Physiol. (2021) 599:2573–602. doi: 10.1113/JP281002
15. Saini BS, Ducas R, Darby JRT, Marini D, Sun L, Macgowan CK, et al. Feasibility of MRI assessment of maternal-fetal oxygen transport and consumption relative to maternal position in healthy late gestational pregnancies. J Physiol. (2023) 601:5413–36. doi: 10.1113/JP285097
16. Darby JRT, Flouri D, Cho SKS, Williams GK, Holman SL, Meakin AS, et al. Maternal tadalafil treatment does not increase uterine artery blood flow or oxygen delivery in the pregnant ewe. Exp Physiol. (2024) 109:980–91. doi: 10.1113/EP091593
17. Couper S, Clark A, Thompson JMD, Flouri D, Aughwane R, David AL, et al. The effects of maternal position, in late gestation pregnancy, on placental blood flow and oxygenation: an MRI study. J Physiol. (2021) 599:1901–15. doi: 10.1113/JP280569
18. Jani D, Clark A, Couper S, Thompson JMD, David AL, Melbourne A, et al. The effect of maternal position on placental blood flow and fetoplacental oxygenation in late gestation fetal growth restriction: a magnetic resonance imaging study. J Physiol. (2023) 601:5391–411. doi: 10.1113/JP284269
19. Chavhan GB, Babyn PS, Thomas B, Shroff MM, Haacke EM. Principles, techniques, and applications of T2*-based MR imaging and its special applications. Radiogr Rev Publ Radiol Soc N Am Inc. (2009) 29:1433–49. doi: 10.1148/rg.295095034
20. Steinweg JK, Hui GTY, Pietsch M, Ho A, Van Poppel MP, Lloyd D, et al. T2* placental MRI in pregnancies complicated with fetal congenital heart disease. Placenta. (2021) 108:23–31. doi: 10.1016/j.placenta.2021.02.015
21. Wen T, Chang Q, He J, Chen Z, Xu L, Guan Y, et al. Differentiating between normal and fetal growth restriction-complicated placentas: is T2∗ imaging imaging more accurate than conventional diffusion-weighted imaging? Clin Radiol. (2023) 78:362–8. doi: 10.1016/j.crad.2023.01.013
22. Kuo AH, Nagpal P, Ghoshhajra BB, Hedgire SS. Vascular magnetic resonance angiography techniques. Cardiovasc Diagn Ther. (2019) 9:S28–36. doi: 10.21037/cdt.2019.06.07
23. Siauve N, Chalouhi GE, Deloison B, Alison M, Clement O, Ville Y, et al. Functional imaging of the human placenta with magnetic resonance. Am J Obstet Gynecol. (2015) 213:S103–14. doi: 10.1016/j.ajog.2015.06.045
24. Kuhnke M, Langner S, Khaw AV, Angermaier A, Hosten N, Kirsch M. Diffusion-weighted MRI - how many B-values are necessary? ROFO Fortschr Geb Rontgenstr Nuklearmed. (2012) 184:303–10. doi: 10.1055/s-0031-1299103
25. Andescavage N, You W, Jacobs M, Kapse K, Quistorff J, Bulas D, et al. Exploring in vivo placental microstructure in healthy and growth-restricted pregnancies through diffusion-weighted magnetic resonance imaging. Placenta. (2020) 93:113–8. doi: 10.1016/j.placenta.2020.03.004
26. Zun Z, Zaharchuk G, Andescavage NN, Donofrio MT, Limperopoulos C. Non-invasive placental perfusion imaging in pregnancies complicated by fetal heart disease using velocity-selective arterial spin labeled MRI. Sci Rep. (2017) 7:16126. doi: 10.1038/s41598-017-16461-8
27. Nana R, Zhao T, Hu X. Single-shot multi-echo parallel EPI for DTI with improved SNR and reduced distortion. Magn Reson Med. (2008) 60:1512–7. doi: 10.1002/mrm.21770
28. Zhu L-H, Zhang Z-P, Wang F-N, Cheng Q-H, Guo G. Diffusion kurtosis imaging of microstructural changes in brain tissue affected by acute ischemic stroke in different locations. Neural Regen Res. (2019) 14:272–9. doi: 10.4103/1673-5374.244791
29. Forster BB, MacKay AL, Whittall KP, Kiehl KA, Smith AM, Hare RD, et al. Functional magnetic resonance imaging: the basics of blood-oxygen-level dependent (BOLD) imaging. Can Assoc Radiol J J Assoc Can Radiol. (1998) 49:320–9.
30. Barnes SRS, Haacke EM. Susceptibility-weighted imaging: clinical angiographic applications. Magn Reson Imaging Clin N Am. (2009) 17:47–61. doi: 10.1016/j.mric.2008.12.002
31. Johnson IR, Symonds EM, Kean DM, Worthington BS, Broughton Pipkin F, Hawkes RC, et al. Imaging the pregnant human uterus with nuclear magnetic resonance. Am J Obstet Gynecol. (1984) 148:1136–9. doi: 10.1016/0002-9378(84)90642-2
32. Lu T, Wang Y, Guo A, Wei C, Chen Y, Wang S, et al. Standard diffusion-weighted, diffusion kurtosis and intravoxel incoherent motion MR imaging of the whole placenta: a pilot study of volumetric analysis. Ann Transl Med. (2022) 10:269. doi: 10.21037/atm-22-1037
33. Salavati N, Smies M, Ganzevoort W, Charles AK, Erwich JJ, Plösch T, et al. The possible role of placental morphometry in the detection of fetal growth restriction. Front Physiol. (2019) 9:1884. doi: 10.3389/fphys.2018.01884
34. Andescavage N, Limperopoulos C. Emerging placental biomarkers of health and disease through advanced magnetic resonance imaging (MRI). Exp Neurol. (2022) 347:113868. doi: 10.1016/j.expneurol.2021.113868
35. Gatta G, Di Grezia G, Cuccurullo V, Sardu C, Iovino F, Comune R, et al. MRI in pregnancy and precision medicine: a review from literature. J Pers Med. (2021) 12:9. doi: 10.3390/jpm12010009
36. Arthuis C, Millischer A-E, Bussières L, Mahallati H, Henry C, Ville Y, et al. MRI based morphological examination of the placenta. Placenta. (2021) 115:20–6. doi: 10.1016/j.placenta.2021.08.056
37. Aughwane R, Ingram E, Johnstone ED, Salomon LJ, David AL, Melbourne A. Placental MRI and its application to fetal intervention. Prenat Diagn. (2020) 40:38–48. doi: 10.1002/pd.5526
38. Barker DJP, Osmond C, Thornburg KL, Kajantie E, Eriksson JG. The lifespan of men and the shape of their placental surface at birth. Placenta. (2011) 32:783–7. doi: 10.1016/j.placenta.2011.07.031
39. Eriksson JG, Gelow J, Thornburg KL, Osmond C, Laakso M, Uusitupa M, et al. Long-term effects of placental growth on overweight and body composition. Int J Pediatr. (2012) 2012:324185. doi: 10.1155/2012/324185
40. Thornburg KL, Marshall N. The placenta is the center of the chronic disease universe. Am J Obstet Gynecol. (2015) 213:S14–20. doi: 10.1016/j.ajog.2015.08.030
41. Myatt L, Thornburg KL. Effects of prenatal nutrition and the role of the placenta in health and disease. Methods Mol Biol Clifton NJ. (2018) 1735:19–46. doi: 10.1007/978-1-4939-7614-0_2
42. Sathasivam R, Selliah P, Sivalingarajah R, Mayorathan U, Munasinghe BM. Placental weight and its relationship with the birth weight of term infants and body mass index of the mothers. J Int Med Res. (2023) 51:03000605231172895. doi: 10.1177/03000605231172895
43. Tankala M, Rao MK, Senapati S, Behera SS. Morphometric evaluation of human placental and umbilical cord for neonatal indices: a cross-sectional study. Cureus. (2023) 15:e48959. doi: 10.7759/cureus.48959
44. Andescavage N, duPlessis A, Metzler M, Bulas D, Vezina G, Jacobs M, et al. In vivo assessment of placental and brain volumes in growth-restricted fetuses with and without fetal Doppler changes using quantitative 3D MRI. J Perinatol. (2017) 37:1278–84. doi: 10.1038/jp.2017.129
45. Matthiesen NB, Henriksen TB, Agergaard P, Gaynor JW, Bach CC, Hjortdal VE, et al. Congenital heart defects and indices of placental and fetal growth in a nationwide study of 924 422 liveborn infants. Circulation. (2016) 134:1546–56. doi: 10.1161/CIRCULATIONAHA.116.021793
46. Ohgiya Y, Nobusawa H, Seino N, Miyagami O, Yagi N, Hiroto S, et al. MR imaging of fetuses to evaluate placental insufficiency. Magn Reson Med Sci. (2016) 15:212–9. doi: 10.2463/mrms.mp.2015-0051
47. Rychik J, Goff D, McKay E, Mott A, Tian Z, Licht DJ, et al. Characterization of the placenta in the newborn with congenital heart disease: distinctions based on type of cardiac malformation. Pediatr Cardiol. (2018) 39:1165–71. doi: 10.1007/s00246-018-1876-x
48. Novis MI, Moura APC, Watanabe ADPF, Pereira LCLE, Warmbrand G, D’Ippolito G. Placental magnetic resonance imaging: normal appearance, anatomical variations, and pathological findings. Radiol Bras. (2021) 54:123–9. doi: 10.1590/0100-3984.2020.0010
49. Otake Y, Kanazawa H, Takahashi H, Matsubara S, Sugimoto H. Magnetic resonance imaging of the human placental cotyledon: proposal of a novel cotyledon appearance score. Eur J Obstet Gynecol Reprod Biol. (2019) 232:82–6. doi: 10.1016/j.ejogrb.2018.11.011
50. Flouri D, Darby JRT, Holman SL, Perumal SR, David AL, Morrison JL, et al. Magnetic resonance imaging of placentome development in the pregnant ewe. Placenta. (2021) 105:61–9. doi: 10.1016/j.placenta.2021.01.017
51. Torrents-Barrena J, Piella G, Masoller N, Gratacós E, Eixarch E, Ceresa M, et al. Segmentation and classification in MRI and US fetal imaging: recent trends and future prospects. Med Image Anal. (2019) 51:61–88. doi: 10.1016/j.media.2018.10.003
52. Cao Y, Wei Y, Yu Y, Wang Z. Safety and efficacy of a novel three-dimensional magnetic resonance imaging model for uterine incision in placenta previa. Int J Gynecol Obstet. (2017) 139:336–41. doi: 10.1002/ijgo.12311
53. Neelavalli J, Krishnamurthy U, Jella PK, Mody SS, Yadav BK, Hendershot K, et al. Magnetic resonance angiography of fetal vasculature at 3.0 T. Eur Radiol. (2016) 26:4570–6. doi: 10.1007/s00330-016-4243-4
54. Hendler I, Blackwell SC, Bujold E, Treadwell MC, Mittal P, Sokol RJ, et al. Suboptimal second-trimester ultrasonographic visualization of the fetal heart in obese women: should we repeat the examination? J Ultrasound Med. (2005) 24:1205–9. doi: 10.7863/jum.2005.24.9.1205
55. Qu F, Sun T, Marin-Concha J, Jaiman S, Jiang L, Mody S, et al. Fetal-placental MR angiography at 1.5 T and 3 T. Magn Reson Imaging. (2023) 102:133–40. doi: 10.1016/j.mri.2023.05.003
56. Wymer DT, Patel KP, Burke WF, Bhatia VK. Phase-contrast MRI: physics, techniques, and clinical applications. Radiographics. (2020) 40:122–40. doi: 10.1148/rg.2020190039
57. Özsarlak Ö, Van Goethem JW, Maes M, Parizel PM. MR angiography of the intracranial vessels: technical aspects and clinical applications. Neuroradiology. (2004) 46:955–72. doi: 10.1007/s00234-004-1297-9
58. Nii M, Enomoto N, Ishida M, Magawa S, Takakura S, Maki S, et al. Two-dimensional phase-contrast MRI reveals changes in uterine arterial blood flow in pregnant women administered tadalafil for fetal growth restriction. Placenta. (2024) 146:1–8. doi: 10.1016/j.placenta.2023.12.013
59. Clark A, Flouri D, Mufti N, James J, Clements E, Aughwane R, et al. Developments in functional imaging of the placenta. Br J Radiol. (2023) 96:20211010. doi: 10.1259/bjr.20211010
60. Little JT, Bookwalter CA. Magnetic resonance safety: pregnancy and lactation. Magn Reson Imaging Clin. (2020) 28:509–16. doi: 10.1016/j.mric.2020.06.002
61. Ray JG, Vermeulen MJ, Bharatha A, Montanera WJ, Park AL. Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA. (2016) 316:952–61. doi: 10.1001/jama.2016.12126
62. Prola-Netto J, Woods M, Roberts VHJ, Sullivan EL, Miller CA, Frias AE, et al. Gadolinium chelate safety in pregnancy: barely detectable gadolinium levels in the juvenile nonhuman primate after in utero exposure. Radiology. (2018) 286:122–8. doi: 10.1148/radiol.2017162534
63. Oh KY, Roberts VHJ, Schabel MC, Grove KL, Woods M, Frias AE. Gadolinium chelate contrast material in pregnancy: fetal biodistribution in the nonhuman primate. Radiology. (2015) 276:110–8. doi: 10.1148/radiol.15141488
64. Gale EM, Wey H-Y, Ramsay I, Yen Y-F, Sosnovik DE, Caravan P. A manganese-based alternative to gadolinium: contrast-enhanced MR angiography, excretion, pharmacokinetics, and metabolism. Radiology. (2018) 286:865–72. doi: 10.1148/radiol.2017170977
65. Gerb J, Strauss W, Derman R, Short V, Mendelson B, Bahrain H, et al. Ferumoxytol for the treatment of iron deficiency and iron-deficiency anemia of pregnancy. Ther Adv Hematol. (2021) 12:20406207211018042. doi: 10.1177/20406207211018042
66. Seiter DP, Nguyen SM, Morgan TK, Mao L, Dudley DM, O’connor DH, et al. Ferumoxytol dynamic contrast enhanced magnetic resonance imaging identifies altered placental cotyledon perfusion in rhesus macaques†. Biol Reprod. (2022) 107:1517–27. doi: 10.1093/biolre/ioac168
67. Toth GB, Varallyay CG, Horvath A, Bashir MR, Choyke PL, Daldrup-Link HE, et al. Current and potential imaging applications of ferumoxytol for magnetic resonance imaging. Kidney Int. (2017) 92:47–66. doi: 10.1016/j.kint.2016.12.037
68. Ahmad F, Treanor L, McGrath TA, Walker D, McInnes MDF, Schieda N. Safety of off-label use of ferumoxtyol as a contrast agent for MRI: a systematic review and meta-analysis of adverse events. J Magn Reson Imaging. (2021) 53:840–58. doi: 10.1002/jmri.27405
69. Nguyen SM, Wiepz GJ, Schotzko M, Simmons HA, Mejia A, Ludwig KD, et al. Impact of ferumoxytol magnetic resonance imaging on the rhesus macaque maternal-fetal interface†. Biol Reprod. (2020) 102:434–44. doi: 10.1093/biolre/ioz181
70. Zhu A, Reeder SB, Johnson KM, Nguyen SM, Golos TG, Shimakawa A, et al. Evaluation of a motion-robust 2D chemical shift-encoded technique for R2* and field map quantification in ferumoxytol-enhanced MRI of the placenta in pregnant rhesus macaques. J Magn Reson Imaging JMRI. (2020) 51:580–92. doi: 10.1002/jmri.26849
71. Deloison B, Arthuis C, Benchimol G, Balvay D, Bussieres L, Millischer A-E, et al. Human placental perfusion measured using dynamic contrast enhancement MRI. PLoS One. (2021) 16:e0256769. doi: 10.1371/journal.pone.0256769
72. Frias AE, Schabel MC, Roberts VHJ, Tudorica A, Grigsby PL, Oh KY, et al. Using dynamic contrast-enhanced MRI to quantitatively characterize maternal vascular organization in the primate placenta. Magn Reson Med. (2015) 73:1570–8. doi: 10.1002/mrm.25264
73. Lo JO, Roberts VHJ, Schabel MC, Wang X, Morgan TK, Liu Z, et al. Novel detection of placental insufficiency by magnetic resonance imaging in the nonhuman primate. Reprod Sci. (2018) 25:64–73. doi: 10.1177/1933719117699704
74. Melbourne A, Schabel MC, David AL, Roberts VHJ. Magnetic resonance imaging of placental intralobule structure and function in a preclinical nonhuman primate model†. Biol Reprod. (2024) 110:1065–76. doi: 10.1093/biolre/ioae035
75. Zun Z, Limperopoulos C. Placental perfusion imaging using velocity-selective arterial spin labeling. Magn Reson Med. (2018) 80:1036–47. doi: 10.1002/mrm.27100
76. Shao X, Liu D, Martin T, Chanlaw T, Devaskar SU, Janzen C, et al. Measuring human placental blood flow with multi-delay 3D GRASE pseudo-continuous arterial spin labeling at 3 tesla. J Magn Reson Imaging JMRI. (2018) 47:1667–76. doi: 10.1002/jmri.25893
77. van Osch MJ, Teeuwisse WM, Chen Z, Suzuki Y, Helle M, Schmid S. Advances in arterial spin labelling MRI methods for measuring perfusion and collateral flow. J Cereb Blood Flow Metab. (2018) 38:1461–80. doi: 10.1177/0271678X17713434
78. Grevent D, Poujol J, Millischer A, Sonigo P, Boddaert N, Salomon L. OP19.08: pseudo-continuous labelling (pCASL) of arterial spins for the non-invasive assessment of placental perfusion: a feasibility study. Ultrasound Obstet Gynecol. (2018) 52:124. doi: 10.1002/uog.19572
79. Liu D, Shao X, Danyalov A, Chanlaw T, Masamed R, Wang DJJ, et al. Human placenta blood flow during early gestation with pseudocontinuous arterial spin labeling MRI. J Magn Reson Imaging. (2020) 51:1247–57. doi: 10.1002/jmri.26944
80. Taso M, Aramendía-Vidaurreta V, Englund EK, Francis S, Franklin S, Madhuranthakam AJ, et al. Update on state-of-the-art for arterial spin labeling (ASL) human perfusion imaging outside of the brain. Magn Reson Med. (2023) 89:1754–76. doi: 10.1002/mrm.29609
81. Harteveld AA, Hutter J, Franklin SL, Jackson LH, Rutherford M, Hajnal JV, et al. Systematic evaluation of velocity-selective arterial spin labeling settings for placental perfusion measurement. Magn Reson Med. (2020) 84:1828–43. doi: 10.1002/mrm.28240
82. Hutter J, Harteveld AA, Jackson LH, Franklin S, Bos C, van Osch MJP, et al. Perfusion and apparent oxygenation in the human placenta (PERFOX). Magn Reson Med. (2020) 83:549–60. doi: 10.1002/mrm.27950
83. Woods JG, Wong EC, Boyd EC, Bolar DS. VESPA ASL: VElocity and SPAtially selective arterial spin labeling. Magn Reson Med. (2022) 87:2667–84. doi: 10.1002/mrm.29159
84. Qin Q, Alsop DC, Bolar DS, Hernandez-Garcia L, Meakin J, Liu D, et al. Velocity-selective arterial spin labeling perfusion MRI: a review of the state of the art and recommendations for clinical implementation. Magn Reson Med. (2022) 88:1528–47. doi: 10.1002/mrm.29371
85. Capuani S, Guerreri M, Antonelli A, Bernardo S, Porpora MG, Giancotti A, et al. Diffusion and perfusion quantified by magnetic resonance imaging are markers of human placenta development in normal pregnancy. Placenta. (2017) 58:33–9. doi: 10.1016/j.placenta.2017.08.003
86. Andescavage NN, Du Plessis A, Limperopoulos C. Advanced MR imaging of the placenta: exploring the in utero placenta–brain connection. Semin Perinatol. (2015) 39:113–23. doi: 10.1053/j.semperi.2015.01.004
87. Jouannic JM, Blondiaux E, Senat MV, Friszer S, Adamsbaum C, Rousseau J, et al. Prognostic value of diffusion-weighted magnetic resonance imaging of brain in fetal growth restriction: results of prospective multicenter study. Ultrasound Obstet Gynecol. (2020) 56:893–900. doi: 10.1002/uog.21926
88. Mansour S, Hamed S, Sayed S, Hosny S. Role of diffusion MR imaging (DWI) and three-dimensional ultrasound (3DUS) in the assessment of placental insufficiency in the gestational hypertension. Egypt J Radiol Nucl Med. (2019) 50:1–10. doi: 10.1186/s43055-019-0062-3
89. Ren J-Y, Ji H, Zhu M, Dong S-Z. DWI in brains of fetuses with congenital heart disease: a case-control MR imaging study. AJNR Am J Neuroradiol. (2021) 42:2040–5. doi: 10.3174/ajnr.A7267
90. Jacobs MA, Ouwerkerk R, Petrowski K, Macura KJ. Diffusion weighted imaging with ADC mapping and spectroscopy in prostate cancer. Top Magn Reson Imaging TMRI. (2008) 19:261–72. doi: 10.1097/RMR.0b013e3181aa6b50
91. Aughwane R, Mufti N, Flouri D, Maksym K, Spencer R, Sokolska M, et al. Magnetic resonance imaging measurement of placental perfusion and oxygen saturation in early-onset fetal growth restriction. BJOG Int J Obstet Gynaecol. (2021) 128:337–45. doi: 10.1111/1471-0528.16387
92. Bonel HM, Stolz B, Diedrichsen L, Frei K, Saar B, Tutschek B, et al. Diffusion-weighted MR imaging of the placenta in fetuses with placental insufficiency. Radiology. (2010) 257:810–9. doi: 10.1148/radiol.10092283
93. Flouri D, Darby JRT, Holman SL, Cho SKS, Dimasi CG, Perumal SR, et al. Placental MRI predicts fetal oxygenation and growth rates in sheep and human pregnancy. Adv Sci. (2022) 9:2203738. doi: 10.1002/advs.202203738
94. Song F, Wu W, Qian Z, Zhang G, Cheng Y. Assessment of the placenta in intrauterine growth restriction by diffusion-weighted imaging and proton magnetic resonance spectroscopy: a pilot study. Reprod Sci. (2017) 24:575–81. doi: 10.1177/1933719116667219
95. Andersen AS, Anderson KB, Hansen DN, Sinding M, Petersen AC, Peters DA, et al. Placental MRI: longitudinal relaxation time (T1) in appropriate and small for gestational age pregnancies. Placenta. (2021) 114:76–82. doi: 10.1016/j.placenta.2021.08.057
96. Malmberg M, Kragsterman E, Sinding M, Hansen DN, Peters DA, Frøkjær JB, et al. Perfusion fraction derived from IVIM analysis of diffusion-weighted MRI in the assessment of placental vascular malperfusion antenatally. Placenta. (2022) 119:1–7. doi: 10.1016/j.placenta.2022.01.005
97. Slator PJ, Hutter J, McCabe L, Gomes ADS, Price AN, Panagiotaki E, et al. Placenta microstructure and microcirculation imaging with diffusion MRI. Magn Reson Med. (2018) 80:756–66. doi: 10.1002/mrm.27036
98. Giza SA, Sethi S, Smith LM, Empey M-EET, Morris LE, McKenzie CA. The application of in utero magnetic resonance imaging in the study of the metabolic and cardiovascular consequences of the developmental origins of health and disease. J Dev Orig Health Dis. (2021) 12:193–202. doi: 10.1017/S2040174420001154
99. Maiuro A, Ercolani G, Di Stadio F, Antonelli A, Catalano C, Manganaro L, et al. Two-compartment perfusion MR IVIM model to investigate normal and pathological placental tissue. J Magn Reson Imaging. (2024) 59:879–91. doi: 10.1002/jmri.28858
100. Jensen JH, Helpern JA. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed. (2010) 23:698–710. doi: 10.1002/nbm.1518
101. Martinez-Heras E, Grussu F, Prados F, Solana E, Llufriu S. Diffusion-weighted imaging: recent advances and applications. Semin Ultrasound CT MRI. (2021) 42:490–506. doi: 10.1053/j.sult.2021.07.006
103. Sun Z, Wu W, Zhao P, Odibo A, Wang Q, Wang Y. Diffusion tensor based motion correction enables multi-parametric imaging of human placenta microstructures. Placenta Reprod Med. (2022) 1:2–9. doi: 10.54844/prm.2022.0125
104. Burton GJ, Cindrova-Davies T, Yung HW, Jauniaux E. Hypoxia and reproductive health: oxygen and development of the human placenta. Reprod Camb Engl. (2021) 161:F53–65. doi: 10.1530/REP-20-0153
105. Huppertz B. Placental physioxia is based on spatial and temporal variations of placental oxygenation throughout pregnancy. J Reprod Immunol. (2023) 158:103985. doi: 10.1016/j.jri.2023.103985
106. Al Darwish FM, Coolen BF, van Kammen CM, Alles LK, de Vos J, Schiffelers RM, et al. Assessment of feto-placental oxygenation and perfusion in a rat model of placental insufficiency using T2* mapping and 3D dynamic contrast-enhanced MRI. Placenta. (2024) 151:19–25. doi: 10.1016/j.placenta.2024.04.008
107. Magawa S, Nii M, Enomoto N, Takakura S, Maki S, Tanaka H, et al. Evaluation of placental oxygenation in fetal growth restriction using blood oxygen level-dependent magnetic resonance imaging. Placenta. (2022) 126:40–5. doi: 10.1016/j.placenta.2022.06.005
108. Peyvandi S, Xu D, Wang Y, Hogan W, Moon-Grady A, Barkovich AJ, et al. Fetal cerebral oxygenation is impaired in congenital heart disease and shows Variable response to maternal hyperoxia. J Am Heart Assoc Cardiovasc Cerebrovasc Dis. (2021) 10:e018777. doi: 10.1161/JAHA.120.018777
109. Sun L, Lee F-T, van Amerom JFP, Freud L, Jaeggi E, Macgowan CK, et al. Update on fetal cardiovascular magnetic resonance and utility in congenital heart disease. J Congenit Cardiol. (2021) 5:4. doi: 10.1186/s40949-021-00059-x
110. Portnoy S, Seed M, Sled JG, Macgowan CK. Non-invasive evaluation of blood oxygen saturation and hematocrit from T1 and T2 relaxation times: in vitro validation in fetal blood. Magn Reson Med. (2017) 78:2352–9. doi: 10.1002/mrm.26599
111. Schabel MC, Roberts VHJ, Gibbins KJ, Rincon M, Gaffney JE, Streblow AD, et al. Quantitative longitudinal T2* mapping for assessing placental function and association with adverse pregnancy outcomes across gestation. PLoS One. (2022) 17:e0270360. doi: 10.1371/journal.pone.0270360
112. Ingram E, Morris D, Naish J, Myers J, Johnstone E. MR imaging measurements of altered placental oxygenation in pregnancies complicated by fetal growth restriction. Radiology. (2017) 285:953–60. doi: 10.1148/radiol.2017162385
113. Li L-P, Halter S, Prasad PV. BOLD MRI of the kidneys. Magn Reson Imaging Clin N Am. (2008) 16:613–viii. doi: 10.1016/j.mric.2008.07.008
114. Luo J, Abaci Turk E, Bibbo C, Gagoski B, Roberts DJ, Vangel M, et al. In vivo quantification of placental insufficiency by BOLD MRI: a human study. Sci Rep. (2017) 7:3713. doi: 10.1038/s41598-017-03450-0
115. Ward PGD, Orchard ER, Oldham S, Arnatkevičiūtė A, Sforazzini F, Fornito A, et al. Individual differences in haemoglobin concentration influence bold fMRI functional connectivity and its correlation with cognition. NeuroImage. (2020) 221:117196. doi: 10.1016/j.neuroimage.2020.117196
116. You W, Andescavage NN, Kapse K, Donofrio MT, Jacobs M, Limperopoulos C. Hemodynamic responses of the placenta and brain to maternal hyperoxia in fetuses with congenital heart disease by using blood oxygen–level dependent MRI. Radiology. (2020) 294:141–8. doi: 10.1148/radiol.2019190751
117. Fujita N, Higuchi H, Sakuma S, Takagi S, Latif MAHM, Ozaki M. Effect of right-lateral versus left-lateral tilt position on compression of the inferior vena Cava in pregnant women determined by magnetic resonance imaging. Anesth Analg. (2019) 128:1217–22. doi: 10.1213/ANE.0000000000004166
118. Turk EA, Abulnaga SM, Luo J, Stout JN, Feldman HA, Turk A, et al. Placental MRI: effect of maternal position and uterine contractions on placental BOLD MRI measurements. Placenta. (2020) 95:69–77. doi: 10.1016/j.placenta.2020.04.008
119. Rajagopalan V, Truong V, Wang S, Lopez J, Rosas V, Borzage M, et al. Non-invasive in-utero quantification of vascular reactivity in human placenta. Ultrasound Obstet Gynecol. (2024) 63:481–8. doi: 10.1002/uog.27512
120. Ruetten PPR, Gillard JH, Graves MJ. Introduction to quantitative susceptibility mapping and susceptibility weighted imaging. Br J Radiol. (2019) 92:20181016. doi: 10.1259/bjr.20181016
121. Uzianbaeva L, Yan Y, Joshi T, Yin N, Hsu C-D, Hernandez-Andrade E, et al. Methods for monitoring risk of hypoxic damage in fetal and neonatal brains: a review. Fetal Diagn Ther. (2021) 49:1–24. doi: 10.1159/000520987
122. Dimov AV, Li J, Nguyen TD, Roberts AG, Spincemaille P, Straub S, et al. QSM throughout the body. J Magn Reson Imaging. (2023) 57:1621–40. doi: 10.1002/jmri.28624
123. Sørensen A, Hutter J, Seed M, Grant PE, Gowland P. T2*-weighted placental MRI: basic research tool or emerging clinical test for placental dysfunction? Ultrasound Obstet Gynecol. (2020) 55:293–302. doi: 10.1002/uog.20855
124. Turk EA, Stout JN, Ha C, Luo J, Gagoski B, Yetisir F, et al. Placental MRI: developing accurate quantitative measures of oxygenation. Top Magn Reson Imaging TMRI. (2019) 28:285–97. doi: 10.1097/RMR.0000000000000221
125. Zun Z, Kapse K, Quistorff J, Andescavage N, Gimovsky AC, Ahmadzia H, et al. Feasibility of QSM in the human placenta. Magn Reson Med. (2021) 85:1272–81. doi: 10.1002/mrm.28502
126. Melbourne A, Aughwane R, Sokolska M, Owen D, Kendall G, Flouri D, et al. Separating fetal and maternal placenta circulations using multiparametric MRI. Magn Reson Med. (2019) 81:350–61. doi: 10.1002/mrm.27406
127. Portnoy S, Osmond M, Zhu MY, Seed M, Sled JG, Macgowan CK. Relaxation properties of human umbilical cord blood at 1.5 tesla. Magn Reson Med. (2017) 77:1678–90. doi: 10.1002/mrm.26231
128. Mohammad S, Bhattacharjee J, Vasanthan T, Harris CS, Bainbridge SA, Adamo KB. Metabolomics to understand placental biology: where are we now? Tissue Cell. (2021) 73:101663. doi: 10.1016/j.tice.2021.101663
129. Bahado-Singh RO, Turkoglu O, Yilmaz A, Kumar P, Zeb A, Konda S, et al. Metabolomic identification of placental alterations in fetal growth restriction. J Matern Fetal Neonatal Med. (2022) 35:447–56. doi: 10.1080/14767058.2020.1722632
130. Mires S, Reddy S, Skerritt C, Caputo M, Eastwood K. Maternal metabolomic profiling and congenital heart disease risk in offspring: a systematic review of observational studies. Prenat Diagn. (2023) 43:647–60. doi: 10.1002/pd.6301
131. Troisi J, Symes SJK, Lombardi M, Cavallo P, Colucci A, Scala G, et al. Placental metabolomics of fetal growth restriction. Metabolites. (2023) 13:235. doi: 10.3390/metabo13020235
132. Walejko JM, Chelliah A, Keller-Wood M, Gregg A, Edison AS. Global metabolomics of the placenta reveals distinct metabolic profiles between maternal and fetal placental tissues following delivery in non-labored women. Metabolites. (2018) 8:10. doi: 10.3390/metabo8010010
133. De Feyter HM, Behar KL, Corbin ZA, Fulbright RK, Brown PB, McIntyre S, et al. Deuterium metabolic imaging (DMI) for MRI-based 3D mapping of metabolism in vivo. Sci Adv. (2018) 4:eaat7314. doi: 10.1126/sciadv.aat7314
134. Markovic S, Roussel T, Neeman M, Frydman L. Deuterium magnetic resonance imaging and the discrimination of fetoplacental metabolism in normal and L-NAME-induced preeclamptic mice. Metabolites. (2021) 11:376. doi: 10.3390/metabo11060376
135. Austdal M, Thomsen LCV, Tangerås LH, Skei B, Mathew S, Bjørge L, et al. Metabolic profiles of placenta in preeclampsia using HR-MAS MRS metabolomics. Placenta. (2015) 36:1455–62. doi: 10.1016/j.placenta.2015.10.019
136. Joseph S, Walejko JM, Zhang S, Edison AS, Keller-Wood M. Maternal hypercortisolemia alters placental metabolism: a multiomics view. Am J Physiol-Endocrinol Metab. (2020) 319:E950–60. doi: 10.1152/ajpendo.00190.2020
137. Courtier-Murias D, Farooq H, Masoom H, Botana A, Soong R, Longstaffe JG, et al. Comprehensive multiphase NMR spectroscopy: basic experimental approaches to differentiate phases in heterogeneous samples. J Magn Reson. (2012) 217:61–76. doi: 10.1016/j.jmr.2012.02.009
138. Mercer GV, Stapleton D, Barrett C, Ringer LCM, Lambe S, Critch A, et al. Identifying placental metabolic biomarkers of preterm birth using nuclear magnetic resonance of intact tissue samples. Placenta. (2023) 143:80–6. doi: 10.1016/j.placenta.2023.10.006
139. Regnault TRH, de Vrijer B, Battaglia FC. Transport and metabolism of amino acids in placenta. Endocrine. (2002) 19:23–41. doi: 10.1385/ENDO:19:1:23
140. Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. Br Med J. (1989) 298:564–7. doi: 10.1136/bmj.298.6673.564
141. Barker DJ. The fetal and infant origins of adult disease. Br Med J. (1990) 301:1111. doi: 10.1136/bmj.301.6761.1111
142. McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. (2005) 85:571–633. doi: 10.1152/physrev.00053.2003
143. Gordijn SJ, Beune IM, Thilaganathan B, Papageorghiou A, Baschat AA, Baker PN, et al. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol. (2016) 48:333–9. doi: 10.1002/uog.15884
144. Morrison JL. Sheep models of intrauterine growth restriction: fetal adaptations and consequences. Clin Exp Pharmacol Physiol. (2008) 35:730–43. doi: 10.1111/j.1440-1681.2008.04975.x
145. Kajdy A, Modzelewski J, Jakubiak M, Pokropek A, Rabijewski M. Effect of antenatal detection of small-for-gestational-age newborns in a risk stratified retrospective cohort. PLoS One. (2019) 14:e0224553. doi: 10.1371/journal.pone.0224553
146. Society for Maternal-Fetal Medicine (SMFM), Martins JG, Biggio JR, Abuhamad A. Society for maternal-fetal medicine consult series #52: diagnosis and management of fetal growth restriction: (replaces clinical guideline number 3, April 2012). Am J Obstet Gynecol. (2020) 223:B2–B17. doi: 10.1016/j.ajog.2020.05.010
147. Gardosi J, Madurasinghe V, Williams M, Malik A, Francis A. Maternal and fetal risk factors for stillbirth: population based study. Br Med J. (2013) 346:f108. doi: 10.1136/bmj.f108
148. King VJ, Bennet L, Stone PR, Clark A, Gunn AJ, Dhillon SK. Fetal growth restriction and stillbirth: biomarkers for identifying at risk fetuses. Front Physiol. (2022) 13:959750. doi: 10.3389/fphys.2022.959750
149. Morrison JL, Ayonrinde OT, Care AS, Clarke GD, Darby JRT, David AL, et al. Seeing the fetus from a DOHaD perspective: discussion paper from the advanced imaging techniques of DOHaD applications workshop held at the 2019 DOHaD world congress. J Dev Orig Health Dis. (2020) 12:153–67. doi: 10.1017/S2040174420000884
150. Öztürk HNO, Türker PF. Fetal programming: could intrauterin life affect health status in adulthood? Obstet Gynecol Sci. (2021) 64:473–83. doi: 10.5468/ogs.21154
151. Wilcox AJ, Snowden JM, Ferguson K, Hutcheon J, Basso O. On the study of fetal growth restriction: time to abandon SGA. Eur J Epidemiol. (2024) 39:233–9. doi: 10.1007/s10654-024-01098-5
152. Zhu MY, Milligan N, Keating S, Windrim R, Keunen J, Thakur V, et al. The hemodynamics of late-onset intrauterine growth restriction by MRI. Am J Obstet Gynecol. (2016) 214:367.e1–e17. doi: 10.1016/j.ajog.2015.10.004
153. Hoffman JIE, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. (2002) 39:1890–900. doi: 10.1016/s0735-1097(02)01886-7
154. Wu W, He J, Shao X. Incidence and mortality trend of congenital heart disease at the global, regional, and national level, 1990–2017. Medicine (Baltimore). (2020) 99:e20593. doi: 10.1097/MD.0000000000020593
155. Seed M, Bradley T, Bourgeois J, Jaeggi E, Yoo S-J. Antenatal MR imaging of pulmonary lymphangiectasia secondary to hypoplastic left heart syndrome. Pediatr Radiol. (2009) 39:747–9. doi: 10.1007/s00247-009-1223-8
156. Al Nafisi B, van Amerom JF, Forsey J, Jaeggi E, Grosse-Wortmann L, Yoo S-J, et al. Fetal circulation in left-sided congenital heart disease measured by cardiovascular magnetic resonance: a case–control study. J Cardiovasc Magn Reson. (2013) 15:65. doi: 10.1186/1532-429X-15-65
157. Sun L, Macgowan CK, Sled JG, Yoo S-J, Manlhiot C, Porayette P, et al. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation. (2015) 131:1313–23. doi: 10.1161/CIRCULATIONAHA.114.013051
158. Lee F-T, Seed M, Sun L, Marini D. Fetal brain issues in congenital heart disease. Transl Pediatr. (2021) 10:2182–96. doi: 10.21037/tp-20-224
159. Panigrahy A, Votava-Smith JK, Licht DJ. Need for “one-stop-shop” heart-brain-placental imaging in fetal congenital heart disease: fetal hemodynamics portend neurodevelopmental outcome. J Am Coll Cardiol. (2024) 83:1240–2. doi: 10.1016/j.jacc.2024.02.022
160. Burton GJ, Jauniaux E. Pathophysiology of placental-derived fetal growth restriction. Am J Obstet Gynecol. (2018) 218:S745–61. doi: 10.1016/j.ajog.2017.11.577
161. Camm EJ, Botting KJ, Sferruzzi-Perri AN. Near to one’s heart: the intimate relationship between the placenta and fetal heart. Front Physiol. (2018) 9:629. doi: 10.3389/fphys.2018.00629
162. Andescavage N, Yarish A, Donofrio M, Bulas D, Evangelou I, Vezina G, et al. 3-D volumetric MRI evaluation of the placenta in fetuses with complex congenital heart disease. Placenta. (2015) 36:1024–30. doi: 10.1016/j.placenta.2015.06.013
163. Schoen FJ. Introduction to congenital heart disease articles in cardiovascular pathology. Cardiovasc Pathol. (2010) 19:257–8. doi: 10.1016/j.carpath.2010.04.008
164. Kühle H, Cho SKS, Barber N, Goolaub DS, Darby JRT, Morrison JL, et al. Advanced imaging of fetal cardiac function. Front Cardiovasc Med. (2023) 10:1206138. doi: 10.3389/fcvm.2023.1206138
165. Christiaens D, Slator PJ, Cordero-Grande L, Price AN, Deprez M, Alexander DC, et al. In utero diffusion MRI: challenges, advances, and applications. Top Magn Reson Imaging. (2019) 28:255–64. doi: 10.1097/RMR.0000000000000211
166. You W, Evangelou IE, Zun Z, Andescavage N, Limperopoulos C. Robust preprocessing for stimulus-based functional MRI of the moving fetus. J Med Imaging. (2016) 3:026001. doi: 10.1117/1.JMI.3.2.026001
167. Uus A, Zhang T, Jackson LH, Roberts TA, Rutherford MA, Hajnal JV, et al. Deformable slice-to-volume registration for motion correction of fetal body and placenta MRI. IEEE Trans Med Imaging. (2020) 39:2750–9. doi: 10.1109/TMI.2020.2974844
168. Abulnaga SM, Abaci Turk E, Bessmeltsev M, Grant PE, Solomon J, Golland P. Placental flattening via volumetric parameterization. In: Shen D, Liu T, Peters TM, Staib LH, Essert C, Zhou S, Yap P-T, Khan A, editors. Medical Image Computing and Computer Assisted Intervention – MICCAI 2019. Cham: Springer International Publishing (2019). p. 39–47. doi: 10.1007/978-3-030-32251-9_5
169. Kulseng CPS, Hillestad V, Eskild A, Gjesdal K-I. Automatic placental and fetal volume estimation by a convolutional neural network. Placenta. (2023) 134:23–9. doi: 10.1016/j.placenta.2023.02.009
170. Chartier AL, Bouvier MJ, McPherson DR, Stepenosky JE, Taysom DA, Marks RM. The safety of maternal and fetal MRI at 3 T. Am J Roentgenol. (2019) 213:1170–3. doi: 10.2214/AJR.19.21400
171. McJury MJ. Acoustic noise and magnetic resonance imaging: a narrative/descriptive review. J Magn Reson Imaging. (2022) 55:337–46. doi: 10.1002/jmri.27525
172. Andorka C, Barta H, Sesztak T, Nyilas N, Kovacs K, Dunai L, et al. The predictive value of MRI scores for neurodevelopmental outcome in infants with neonatal encephalopathy. Pediatr Res. (2024):1–8. doi: 10.1038/s41390-024-03189-1
Keywords: fetal growth restriction, congenital heart disease, MRI, human placenta, BOLD, DWI, SWI, placenta oxygenation
Citation: Sadiku E, Sun L, Macgowan CK, Seed M and Morrison JL (2024) Advanced magnetic resonance imaging in human placenta: insights into fetal growth restriction and congenital heart disease. Front. Cardiovasc. Med. 11: 1426593. doi: 10.3389/fcvm.2024.1426593
Received: 11 May 2024; Accepted: 26 June 2024;
Published: 23 July 2024.
Edited by:
Paolo Montaldo, Imperial College London, United KingdomReviewed by:
Silvia Visentin, University of Padua, ItalyVictoria Roberts, Oregon Health & Science University, United States
Anderson H. Kuo, Midland Memorial Hospital, United States
© 2024 Sadiku, Sun, Macgowan, Seed and Morrison. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liqun Sun, bGlxdW4uc3VuQHNpY2traWRzLmNh
†These authors have contributed equally to this work and share senior authorship
 Eric Sadiku
Eric Sadiku Liqun Sun
Liqun Sun Christopher K. Macgowan
Christopher K. Macgowan Mike Seed
Mike Seed Janna L. Morrison
Janna L. Morrison