- 1Institute of Innovation and Applied Research in Chinese Medicine, Hunan University of Chinese Medicine, Changsha, China
- 2Scientific Research Department, The First Hospital of Hunan University of Chinese Medicine, Changsha, China
In China and other Asian nations, traditional medicine has long been utilized in the treatment of cardiovascular diseases (CVD). While Chinese authorities have incorporated traditional Chinese medicine (TCM) treatment experiences as a supplementary guide for CVD, its international recognition remains limited due to a scarcity of high-quality and reliable randomized controlled trials (RCTs) evidence. The purpose of this study was to examine the clinical outcomes with TCM for CVD after the recent publication of large trials adding >20,000 individuals to the published data. Here, we systematically reviewed 55 published RCTs (modified Jadad scores > 4) in the past 20 years, involving a total of 36,261 patients. In most studies, TCM has been associated with significant improvements in alternative endpoints such as hypertension, coronary heart disease, stroke and heart failure. A total of 19 trials reported on primary outcomes such as cardiovascular events and death events. During the follow-up period, some Chinese patent medicines can effectively reduce the “hard” endpoints of coronary heart disease, stroke, and heart failure, the overall trend of cardiovascular outcomes is lower. The risk of adverse effects was not significantly increased compared to the control group, suggesting its potential as an alternative approach for primary and secondary prevention of CVD based on the available evidence.
1 Introduction
Cardiovascular disease (CVD) is a leading cause of death and disability, accounting for approximately one-third of all deaths globally with 19.05 million fatalities in 2020 (1). The burden of CVD disproportionately impacts low- and middle-income countries. For instance, the incidence of CVD in middle-income European Society of Cardiology member countries is estimated to be 30% higher than in high-income countries, yet the resources available to tackle this issue are frequently limited (2, 3). According to the latest clinical guidelines for CVD, current control mainly focuses on lifestyle management, medication and revascularization. Although some medicines are effective in reducing all-cause death, a significant proportion of patients remain at high risk of cardiovascular events, and there is still much room to improve efficacy and reduce adverse effects (4, 5). Given that the current demand for CVD control has not been met, clinicians are considering the potential role of traditional and natural medicines in CVD prevention and treatment (6).
Traditional Chinese Medicine (TCM) has been developed and used continuously for more than 2,000 years and has become increasingly popular in Asia and Western countries over the past few decades, especially acupuncture (7). At present, the integration of traditional Chinese and Western medicine has become China's unique medical system and the most commonly accepted treatment approaches by the Chinese public (8, 9). Past reviews of TCM for CVD have shown that TCM was associated with significant improvements in surrogate end points for hypertension, coronary heart disease (CHD), cardiac arrhythmias, and heart failure. Some Chinese patent medicines could help prevent cardiovascular risk factors and might be used as complementary and alternative approaches for primary and secondary prevention of CVD. However, only a few previous studies have included reports of adverse cardiovascular events and cardiovascular death (6, 10–12). Due to the late development of RCT designs, previous studies are generally of low quality, and their conclusions are not sufficiently reliable or convincing (13).
With the publication of TCM Consort and acupuncture standardized design, reports of high-quality RCTs have gradually increased in recent years (14, 15). TCM follows an ancient physiological system, and believes that health is the result of harmony between body functions and between the body and nature (16). A recent study published in Nature clarified the neuroanatomical basis of the relative specificity of acupuncture points by showing that stimulating different acupoints can activate their respective neural pathways, thus providing preliminary evidence for the concepts of acupoints and acupuncture (17). A review published in the British Medical Journal in 2022 showed that acupuncture has significant effects on 8 diseases, but it is largely absent from clinical practice and policy considerations (18). In recent years, a series of high-quality acupuncture studies have demonstrated the efficacy of acupuncture in digestive, urinary system, and cancer pain (19–22). Similarly, some studies for CVD such as stable angina pectoris, hypertension, myocardial ischemia, and stroke have been gradually published (23–26). In this study, we examine the available evidence from RCTs on the effects of TCM in patients with CVD over the past two decades. We further appraised past RCTs by means of modified Jadad scores, retained high-quality studies, incorporated the most recent high-quality trials, and explored primary outcome events.
2 Search strategy and selection criteria
Relevant studies were identified by searching for papers published from January 2004 to January 2024 in MEDLINE (Ovid); EMBASE; the Cochrane Library; Global Health, International Pharmaceutical Abstracts; the China National Knowledge Internet; and the China Biology Medicine, Wanfang, and VIP databases. We also considered the Chinese Clinical Trials Registry (www.chictr.org.cn) and the US Clinical Trials Registry (clinicaltrials.gov).
The search algorithm for MEDLINE was as follows: (“traditional Chinese medicine” OR “traditional Chinese medication” OR “TCM”) AND (“cardiovascular disease” OR “hypertension” OR “blood pressure” OR “angina” OR “coronary heart disease” OR “coronary artery disease” OR “myocardial infarction” OR “stroke” OR “heart failure”) AND (“randomized” OR “randomized controlled trial”) AND “blind,” with no restriction on subheadings. The reference lists of all retrieved papers were checked for other potentially relevant citations, and studies not included in the electronic sources mentioned previously were searched manually. Authors of identified papers will be contacted for further information if necessary.
We included reports of clinical studies with the following criteria: (1) study patients with a definite diagnosis of essential hypertension, myocardial infarction, angina pectoris, coronary artery disease (CAD), stroke or heart failure who were randomized to receive TCM, contemporary medication, or placebo; (2) total sample size ≥ 60 cases; (3) follow-up in each study group ≥ 4 weeks; and (4) quantitative measurements of surrogate endpoints and/or adverse cardiovascular events and/or adverse drug effects available to facilitate outcome analysis. We excluded reports of studies with the following features: (1) studies were nonrandomized and/or non-blinded; (2) patients enrolled had no definite diagnosis; (3) studies compared different TCM medications; and (4) studies reported only symptomatic changes of patients, without objective laboratory measurements or physical examination. (5) methodological quality was evaluated for each study with a Modified Jadad score between 0 (weakest) to 7 (strongest), as described previously; any study with a score < 4 was considered to be of poor quality and was excluded. In addition, when 2 papers reported the results of the same study, the paper with less data was excluded.
Finally, we included 55 eligible reports of RCTs: 39 in English and 16 in Chinese. The time of publication of these 55 studies ranged from 2004 to 2024. Overall, there were 13 reports of hypertension, 21 of CHD, 10 of stroke, and 11 of heart failure.
3 Hypertension
High blood pressure currently affects more than 1 billion people worldwide. Hypertension is associated with increased risk of CVD events (coronary heart disease, heart failure, and stroke) and death. The consensus for first-line treatment of hypertension is lifestyle changes, including weight loss, dietary sodium reduction and potassium supplementation, healthy eating patterns, physical activity, and limited alcohol consumption (27). At present, many patients still have uncontrolled blood pressure (BP) due to poor compliance, which increases the risk of cardiovascular disease (28, 29). Risk factors for poor adherence are multifactorial and include side effects of antihypertensive medications and hypertension-related symptoms (30–32). Chinese medicine treats hypertension by identifying the patient's symptoms and physique to develop a personalized treatment plan.
We included 13 randomized controlled trial studies on TCM and essential hypertension, including 10 studies on Chinese patent medicines and 3 studies on acupuncture. The sample sizes ranged from 120 to 628 participants, and the mean follow-up ranged from 4 weeks to 52 weeks. The methodological quality of the included studies was generally high: 9 of 13 reports had a modified Jadad score of 7, 2 of 13 reports had a score of 6, 1 report had a score of 5, and 1 trial on the Xuemaikang capsule had a score of 4 (Supplementary Table S1).
The intervention group was treated with Zhongfu jiangya capsule, Jiangya capsule, Qiqilian capsule, Jiangyabao tablet, Tiankuijiangya tablet, Gastrodia-uncaria granules, xuemaikang capsule, Angong jiangya pill, and acupuncture. Data summaries for all high-quality RCTs of TCM interventions for hypertension are listed in Figure 1.
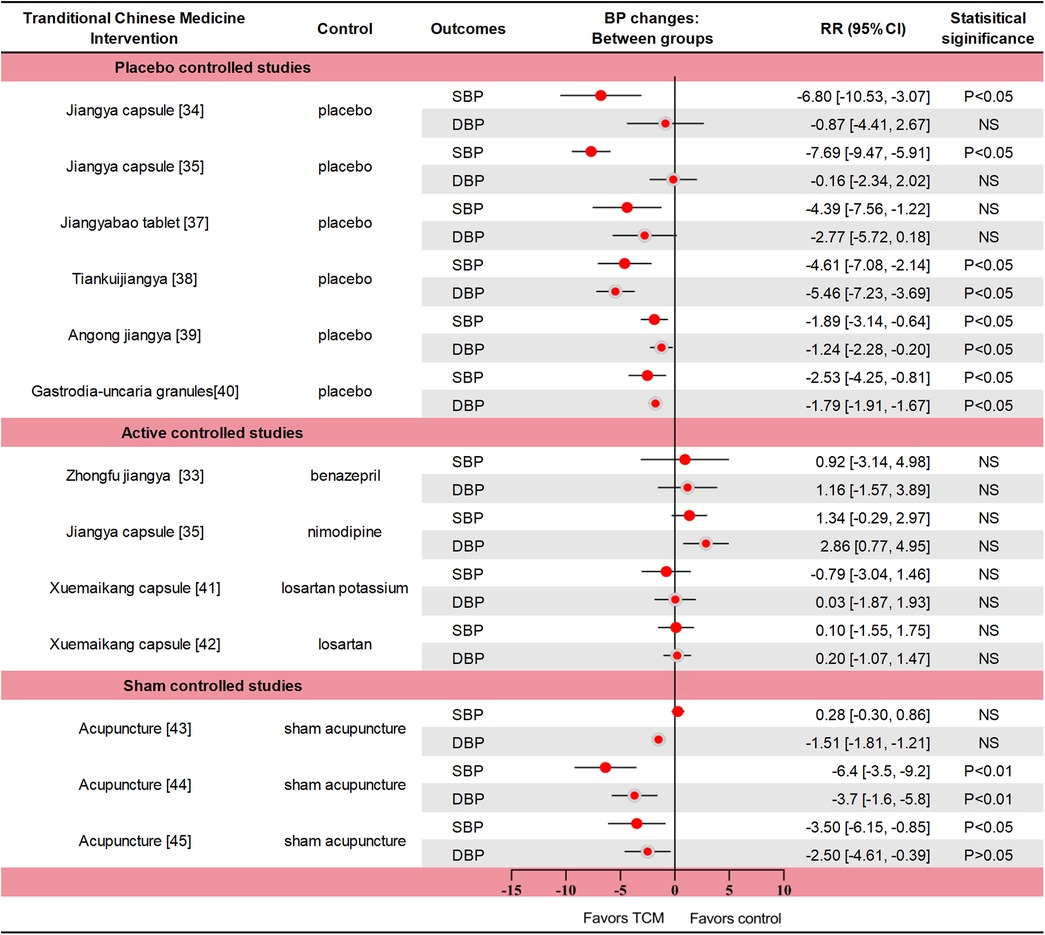
Figure 1. Data summaries From the high-quality RCTs of TCM interventions for hypertension. Numbers in parentheses=reference numbers. BP, blood pressure; CI, confidence interval; DBP, diastolic blood pressure; NS, not significant; RCT, randomized controlled trial; RR, risk ratio; SBP, systolic blood pressure; TCM, traditional Chinese medicine.
A multicenter RCT (n = 418) compared Zhongfu jiangya capsule with Benazepril. The change of blood pressure in the two groups was similar (p = 0.661 for SBP and p = 0.409 for DBP). In the Zhongfu jiangya group, the 24-hour mean SBP and DBP decreased by 10.67 mm Hg and 7.49 mm Hg from baseline (p < 0.01) (33). In a RCT, Jiangya capsule significantly reduced SBP (p < 0.05) but did not reduce DBP (p > 0.05) compared with placebo (34). In another comparison with placebo, nimodipine, Jiangya capsules reduced 24-h average SBP and day-average DBP compared with placebo (both p < 0.05), but not DBP or night-average SBP (both p > 0.05). The therapeutic effect of Jiangya capsules and nimodipine were similar (both p > 0.05), and the adverse effects were also similar (35). The rate of effective response (defined as a reduction ≥ 10 mmHg in DBP or to the normal range and/or a reduction ≥ 30 mmHg in SBP) to treatment with Qiqilian capsule was higher than with placebo (p < 0.05) (36). Although the effective response to Jiangyabao tablet treatment during the daytime did not differ significantly from that of placebo under standard antihypertensive treatment (p > 0.05), both SBP and DBP were significantly lower with Jiangyabao than with placebo at night (both p < 0.05) (37). After 8 weeks of Tiankuijiangya tablet treatment, morning SBP and DBP were reduced by 17.64 mm Hg and 11.85 mm Hg from baseline, respectively, showing statistical significance compared with placebo (p < 0.05 for both comparisons). The incidence of adverse reactions was 3.13% (5/160) in the Tiankuijiangya group compared to 2.50% (2/80) in the placebo group (38). Liver-fire hyperactivity syndrome is one of the TCM syndroms of hypertension, mainly accompanied by headache (especially both temples), dizziness, irritability, and chest tightness symptoms. In one study (n = 338), the antihypertensive effective rate of Angongjiangya group was 65.68% (the range of BP was 80%∼120%) after four weeks, and the SBP and DBP were decreased by 9.33 mmHg and 5.57 mmHg (p < 0.01), the incidence of adverse events was 1.48% and 3.25%, respectively (39). In a trial of 251 patients with masked hypertension, daytime SBP/DBP was reduced by 5.44/3.39 and 2.91/1.60 mmHg in the Gastrodia-uncaria granules and placebo groups (p < 0.05). The between-group difference in BP reductions was significant for the daytime (2.52 vs. 1.79 mmHg; p ≤ 0.025) and 24-h BP (2.33 vs. 1.49 mmHg; p ≤ 0.012), but not for the clinic and nighttime BPs (p ≥ 0.162). Only 1 adverse event (sleepiness during the day) was reported (40). One trial compared the efficacy of Xuemaikang (SXC) and losartan potassium in the treatment of primary hypertension (n = 276). After 8 weeks, the SBP decreased by 10.8 mmHg and 10.01 mmHg, and the DBP decreased by 8.36 mmHg and 8.39 mmHg, respectively (p < 0.05) (41). In another RCT of SXC vs. losartan in the treatment of mild hypertension (n = 628), DBP changes were similar after 8 weeks of treatment with two groups, decreasing by 7.9 mmHg and 8.1 mmHg respectively. In total cholesterol, SXC group had a significant decrease compared with losartan group, which was −0.1 mmol/L and 0.1 mmol/L (p = 0.025). Adverse events during follow-up were similar in both groups (42). Thus, SXC might be an alternative for mild hypertension, particularly for patients with a preference for natural medicine.
The SHARP study (n = 192) used the principles of TCM acupuncture to perform active or invasive sham acupuncture for 6 weeks. Active acupuncture was divided into personalized acupuncture and standardized acupuncture, and the treatment effects were similar. The average blood pressure reduction between active and sham acupuncture was not significantly different after 10 weeks (all p > 0.05). Acupuncture also showed no advantage over sham acupuncture after 12 months (43). Another acupuncture study (n = 140) showed a significant difference in blood pressure after 6 weeks of treatment between the active and sham acupuncture groups, with 24-h SBP and DBP differences of 6.4 mm Hg and 3.7 mm Hg, respectively (p < 0.01). In the active acupuncture group, mean 24 h after treatment dynamic SBP and DBP decreased significantly by 5.4 mm Hg and 3.0 mm Hg, respectively. No study-related adverse events occurred (44). In an acupuncture for mild hypertension trial (n = 415), personalized acupuncture treatment was similar to standard acupuncture treatment. Compared with the sham acupuncture and the waiting-list group, patients in the active acupuncture group had more reduction in SBP at week 6 (7.2 vs. 4.1 vs. 4.1 mm Hg, p > 0.05); However, acupuncture was superior to sham acupuncture (P = 0.035) and waiting-list control (p < 0.001) at week 9. A total of 9 mild adverse events were reported, most of which were local hematoma and one case of nausea (24).
The theory and practice of acupuncture has been practiced in China for more than 2,000 years, treating diseases by needling specific points on the human body. In fact, the mechanism by which acupuncture affects blood pressure is not well understood. The specific mechanism may be related to the regulation of renin-angiotensin-aldosterone system (RAAS), metabolic disorders, central nervous system, oxidative stress, inflammatory response and vascular endothelial function. In previously published guidance on alternative approaches of reducing blood pressure, acupuncture works similarly to some relaxation techniques (Class III, Level of Evidence B) (45). In recent years, the quality of trials on acupuncture intervention for hypertension has been low, and the conclusions are not sufficiently convincing and can only be regarded as a possible potential benefit (46–48). In the three studies we included, there was insufficient evidence that acupuncture significantly reduced blood pressure.
Current evidence shows that some Chinese patent medicines have antihypertensive effects and a good safety profile, and may be used as an alternative approach. As far as the available clinical evidence is concerned, acupuncture for the treatment of hypertension is still in the early stage of exploration. Differences in the efficacy between personalized (meridian) and standard (non-meridian) acupuncture in treating hypertension are not well understood. Additionally, differences in acupoint specificity and clinical efficacy remain unclear. A total of six trials reported adverse effects or events (all p > 0.05); however, long-term effects on blood pressure and cardiovascular (CV) events were less reported.
4 CHD
We included 21 RCTs on TCM and coronary heart disease, including angina pectoris, myocardial infarction (MI), CAD, acute coronary syndrome (ACS), and stable myocardial ischemia. 16 of 21 reports had a Modified Jadad score of 7, 2 of 21 reports had a score of 6, 3 of 21 reports had a score of 5. Sample sizes ranged from 66 to 4,870, and follow-up periods ranged from 4 weeks to 4.5 years (Supplementary Table S2).
The Chinese patent medicines in the study included Danlou tablet, Wufu xinnaoqing capsule, Shenzhuguanxin capsule, Guanxin shutong capsule, Xuefuzhuyu capsule, Shenmai capsule, Xuezhikang capsule, Tongxinluo capsule, Qishenyiqi dropping pills, Xiongshao capsule, Qingxin jieyu tablets, Yugengtongyu capsule, Guanxin danshen, Suxiao jiuxin pills and Shexiangbaoxin pills. In addition, several acupuncture trials are covered, including stable angina and ischemic heart disease.
4.1 Angina pectoris
The most common clinical manifestation of CHD is angina pectoris and its alleviation is a vital component in the management of patients with CHD. Stable angina is defined here as symptoms that may be attributed to myocardial ischemia, for example, chest discomfort, but lack the duration and severity that one may associate with acute myocardial infarction. The current aim of pharmacologic management of stable angina is to prevent myocardial ischemia episodes, control symptoms, improve quality of life, and prevent cardiovascular events (49). Because of limited medical resources and lack of significant improvement in angina relief from percutaneous coronary intervention, Chinese clinicians choose traditional Chinese medicine and acupuncture in addition to antianginal treatment for chronic stable angina (CSA). Acupuncture in China has been used as non-pharmacological treatment for several decades, especially to relieve symptoms of myocardial ischemia, improve cardiac function, and prevent recurrence (50–57).
In an RCT (n = 66), Danlou tablet compared with placebo reduced angina pectoris (Phlegm and Stasis Mutual Obstruction Syndrome) attack frequency, pain duration, consumption of nitroglycerin and the level of hs-CRP was decreased (all p < 0.05) (58). On the basis of conventional treatment, 240 patients with chronic stable angina pectoris were treated with Wufuxinnaoqing capsule and placebo. After 12 weeks, the total effective rate of the treatment group, the rate of nitroglycerin withdrawal and reduction was superior to that of the control group (all p < 0.01) (59). In a RCT (n = 187) comparing Shenzhuguanxin capsule with placebo after 12 months, APS was reduced by 76.7% in treatment and 53.8% in placebo group, and CM symptom scores were reduced by 18.3% and 16.1%, respectively (60).
One RCT (n = 232) that Xinlin pills significantly increased the total duration of treadmill exercise after 4 weeks (p < 0.05) by FAS analysis. In addition, the reduction of nitroglycerin dose was 2.45 tablets and 0.5 tablets per week (p < 0.05), the frequency and duration of angina pectoris attacks were also significantly reduced (p < 0.05) (61). A total of 4 weeks of treatment with Guanxinshutong (GXST) resulted in a statistically significant lowering of the time of angina attacks and the consumption of nitroglycerin dose (p < 0.01). The incidence of adverse events was similar between the two groups (p = 0.17). Five adverse events (AEs) were reported in the GXST group, all of which were gastrointestinal disturbances (62). In a small sample (n = 90) RCT of unstable angina pectoris after PCI, compared with placebo, Xuefuzhuyu granule had a lower BSS score after 4 weeks (p < 0.01), and were beneficial in terms of physical pain, angina stability, and angina pectoris (p < 0.05) (63). The three trials had low rates of adverse events and reactions, and good safety profile. Another trial of 324 patients with stable angina from 13 hospitals in China evaluated the efficacy of Suxiao jiuxin pills. Compared with the control group, the curative efficacy rate of stable angina, the curative efficacy rate of TCMSS significantly increased, and the total score of angina pectoris symptoms and TCMSS significantly reduced in the Suxiao jiuxin pills group, accompanied by the statistically significant improvement in the curative efficacy rate based on CCS grade reduction (all P < 0.05). The medication compliance, concomitant medication, and rates of adverse events were similar between the two groups (P > 0.05) (64).
In the 2019 high-quality acupuncture trial for stable angina pectoris, 398 participants (mean age = 62.6 years) were divided into four groups (DAM: Disease & Meridians; NAM: non-disease affecting meridian points; SA: false acupuncture; WL: not receiving acupuncture), after 16 weeks of intention-to-treat, compared with other group, acupuncture on the DAM as adjunctive treatment to antianginal therapy showed superior benefits in alleviating angina (p < 0.01). 16 patients reported mild to moderate adverse events related to acupuncture, which did not require medical intervention (23). Due to the complexity and difficulty of the acupuncture trials, the large sample of nearly 400 people did not show significant side effects, thus, the superior benefits of acupuncture in alleviating angina are considered highly convincing.
In these RCTs of angina pectoris, TCM interventions including Danlou tablet, Wufuxinnaoqing capsule, Shenzhuguanxin capsule, Xinlin Pill, GXST capsule, Xuefuzhuyu granule and acupuncture treatment had positive effects on angina pectoris attack frequency and nitroglycerin consumption during a follow-up period ranging from 4 to 52 weeks. Six studies reported adverse effects, two of which reported cardiovascular events. Compared with placebo, Wufuxinnaoqing and Shenzhuguanxin could effectively decrease cardiovascular events. Furthermore, based on Eastern acupuncture theory, the incidence of angina pectoris in the DAM group (Disease & Meridians) was lower than that in the NAM group (non-disease affected meridian acupoint group), and there were no adverse CV events during the treatment.
4.2 Myocardial infarction
Myocardial infarction is also a major life-threatening condition worldwide. Despite reperfusion therapy and optimal medical management, patients with STEMI still face high risks of inhospital mortality and recurrent cardiovascular events (65–67). Tongxinluo was initially evaluated and approved in China for angina pectoris and ischemic stroke in 1996 (68). After that, some Chinese patent medicines were gradually used for acute coronary syndrome (ACS).
The 2008 CCSPS trial evaluated Xuezhikang for MI with major adverse CV events as the endpoint. They were randomly assigned to receive daily treatment with Xuezhikang (n = 2,429) or placebo (n = 2,441) and were followed for an average of 4.5 years. The primary endpoint included CV death and non-fatal MI. The incidence of the primary CV events was significantly lower with Xuezhikang treatment than with placebo (p < 0.05). Serum total cholesterol, low-density lipoprotein cholesterol and triglyceride levels in the Xuezhikang group were lower than those in the placebo group (p < 0.001). Xuezhikang was deemed safe and well tolerated, with no treatment-related serious adverse events reported (69).
One study (n = 219) showed that Tongxinluo capsule reduced myocardial non-reflow and infarct size after acute MI direct PCI, improved myocardial perfusion. Tongxinluo significantly improved ST-segment recovery 6 h after reperfusion (p < 0.05) and lasted until 24 h after reperfusion (p < 0.01) (70). In another recent study on Tongxinluo, published in JAMA in 2023, researchers conducted a 12-month randomized, double-blind, placebo-controlled trial in China in patients (n = 3,797, mean age = 61.1 years) who developed STEMI within 24 h. The primary endpoint was Major Adverse Cardiovascular and Cerebrovascular Events (MACCEs), including cardiac death, myocardial reinfarction, emergency coronary revascularization, and stroke. MACCEs occurrence was lower in the Tongxinluo group than in the control group during the 30-day and 1 year period (both p < 0.01), Cardiac death was also lower than in the placebo group (both p < 0.05). Adverse drug reactions were more frequent in the Tongxinluo group compared to the placebo group (2.1% vs. 1.1%, p = 0.02), mainly driven by gastrointestinal symptoms. The trial demonstrated that Tongxinluo can be used as a supplement to STEMI guidelines, significantly alleviating clinical outcomes at 30 days and 1 year with mild adverse effects (71).
One large RCT (n = 3,505) compared the efficacy and safety of Qishenyiqi (QSYQ) dropping pills with aspirin in secondary prevention of myocardial infarction. The results showed that QSYQ had similar effects to aspirin and less adverse reactions. The incidence rates of primary outcomes at 12 and 18 months was 2.98% and 3.67% for the QSYQ group and 2.96% and 3.81% for the aspirin group, respectively. Cardiovascular death and mild adverse effects were similar between the two groups (p > 0.05) (72). Eighty-three MI patients were randomly assigned to receive either Danlou tablets or placebo for 90 days of treatment. Danlou tablet could significantly reduce left ventricular end-diastolic volume index and left ventricular end-systolic volume index (both p < 0.01), and increase left ventricular ejection fraction (p < 0.01). In terms of safety, the incidence of major adverse cardiovascular events in the Danlou group was lower compared with placebo (p < 0.05), and the composite incidence of two groups was 11.9% and 34.15%, respectively (73).
MI studies have shown that TCM could effectively improve hard endpoints and/or surrogate endpoints, while its adverse effects are acceptable. Four studies reported adverse cardiovascular events and composite endpoints, especially in the three large-sample RCTs, Xuesaitong, Tongxinluo and QSYQ were proven to reduce cardiovascular events and all-cause mortality.
4.3 CAD
Either incidence or mortality of coronary artery disease (CAD) accounts for a large part of cardiovascular diseases (74–76). According to the latest ESC clinical practice guidelines, and the treatment is mainly based on lifestyle management, medical treatment, and revascularization (77). However, despite the use of medical treatment and revascularization, there is still much room for improving efficacy and reducing adverse reactions (5). In China, TCM is a potential add-on treatment and has a long history being used for the treatment of CAD (6, 78).
In one RCT, patients with restenosis after PCI were randomized to receive Xiongshao or placebo, 6 months of treatment with Xiongshao ameliorated restenosis (p < 0.05) and decreased cardiovascular events with borderline statistical significance (p = 0.051) after PCI, compared with placebo without any drug-related adverse events (79). In a multicentre, double-blind, randomized trial, patients with stable CHD (n = 1,500, mean age = 60.3 years) were randomly assigned to receive Qingxin jieyu capsules or placebo for 6 months. The primary outcomes were similar between the two groups (p > 0.05). However, the absolute risk of composite “hard” endpoints was reduced by 0.99% in Qinxin jieyu groups (80). In one RCT involving 114 patients with CHD, the incidence of the comprehensive outcome of Yugengtongyu granules was significantly lower (p < 0.05). The primary outcomes and independent events such as non-fatal MI showed a decreasing trend. A total of 11 adverse events occurred at one year of follow-up, the most common event being constipation (81).
Two hundred CHD patients with depression and anxiety after PCI were included and randomly assigned to receive Guanxin Danshen or placebo. After 12 weeks of treatment, the PHQ-9 and GAD-7 scores in the Guanxin danshen were significantly lower than those in the control group (p < 0.05), and the incidence of MACE in the two groups was similar (p > 0.05) (82). A multicenter RCT investigated the safety and efficacy of Suxiaojiuxin pills, the most frequently used drug in the cardiovascular acute phase in China, in patients with ACS with early PCI (n = 200, mean age = 61.2 years). The occurrence of MACE in Suxiaojiuxin Pills group was lower than that in placebo group (p < 0.05). In addition, Suxiaojiuxin pills might improve patients' LVEF levels and SAQ scores during long-term follow-up (83). 151 patients (mean age = 63 years) with stable ischemic heart disease were randomized to TA (tranditional acupuncture), sham acupuncture and waiting control. For markers of parasympathetic tone, withdrawal stress HRV was higher in the TA group than in the SA group (p < 0.05), with vagus activity 17% higher (p = 0.008) (54).
MUSKARDIA trail included 2,674 patients with stable CHD randomly received Shexiangbaoxin pills and placebo, the incidence of MACE at was 1.9% and 2.6% after 24 months. Shexiangbaoxin pills had a 26.9% reduction in the incidence of MACE compared to the placebo group. Angina frequency was significantly reduced in the Shexiangbaoxin group at 18 months (p = 0.0362). Other secondary endpoints and adverse events were similar between the two groups(both p > 0.05) (84). Another study on the MUSKARDIA trial evaluated the efficacy and safety of patients with stable CHD and diabetes mellitus (DM or FBG ≥ 7.0 mmol/L). Compared with placebo groups, Shexiangbaoxin pills were significantly lower in the incidence of MACE (p < 0.05) and secondary composite outcomes (p < 0.05). In patients with uncontrolled DM (≥ 4 measurements of FBG ≥ 7 mmol/L in five times of follow up), the risk of secondary outcomes was also significantly lower than in the placebo group (p < 0.05) (85).
In these CAD trials, 7 studies reported similar adverse effects within the groups. Xiongshao capsule, Qingxin jieyu capsule, Xinkeshu, Yuyuetongyu, Guanxin danshen, and acupuncture have certain effects on improving CAD-related surrogate endpoints. Qingxin jieyu capsule and Suxiaojiuxin pills demonstrated significant advantages in reducing cardiovascular (CV) events (p < 0.05).
5 Stroke
Stroke, characterized by high morbidity, disability, and mortality, is the leading cause of death in China, contributing substantially to the global burden of diseases (86). The disease burden of stroke is still severe in China, although the age-standardised incidence and mortality rates have decreased since 1990 (87).
Currently, recanalization therapy via intravenous thrombolysis and mechanical thrombectomy has allowed a great breakthrough in improving the neurologic prognosis of patients with ischemic stroke (88). However, recanalization therapy benefits a limited number of patients because of the strict time window and high operational requirements (8). Therefore, the discovery and development of some effective and safe alternative therapies are needed to further improve the prognosis of patients with ischemic stroke.
A total of 10 RCTs on TCM and ischemic stroke were included, including 1 study on acupuncture. Sample sizes ranged from 100 to 2,966, and follow-up periods ranged from 4 to 102 weeks. The modified Jadad scores of the included studies were all >5, 7 of 10 reports had a score 7, 2 of 10 reports had a score 6, 1 of 10 report had a score 5. (Supplementary Table S3). The intervention group was treated with Naoxinduotai capsule, Sanqitongshu capsule, acupuncture, Ginkgo tablet, Neuroaid, Dihuangyinzi, YangyinYiqi Huoxue granule, Xuesaitong capsule. The control group received placebo and sham acupuncture.
Compared with placebo, Naoxinduotai capsule could significantly improve nerve function and Barthel index in ischemic stroke (p < 0.05), and no drug-related adverse reactions were found in the Naoxinduotai group (89). An RCT included 605 ischemic stroke patients who were given Danqi Piantang (DJ) capsules or placebo for 1 month. Functional outcome was measured by the Stroke Diagnosis and Treatment Effect Scale's comprehensive function score, which showed that the DJ group was better than the control group (p < 0.05), its adverse effects and tolerability were acceptable (90). 140 patients with anterior circulation ischemic stroke within 30 days of onset were randomly treated with Sanqitongshu capsules or placebo in addition to aspirin treatment. Sanqitongshu capsule could significantly improve the neurological deficit score and BI score (both p < 0.05). The adverse reactions manifested by gastrointestinal discomfort were similar (91). 290 patients with initial acute ischemic stroke (≥24 h but within 14 days) were randomly divided into acupuncture group and control group (sham acupoints) on the basis of standard treatment. There were significant differences in mean BI and quality of life scores between the two groups at 6 months (both p < 0.01). The NIHSS scores of the two groups showed no significant differences at 2 weeks (p > 0.05), but there was a significant difference at 4 weeks (p < 0.01). In terms of recurrent stroke, 6 cases in the acupuncture group and 34 cases in the control group (p < 0.01) (25). Patients with acute ischemic stroke (n = 102) were randomized to receive Ginkgo tablet and placebo for 4 months. At 4-month follow-up, the NIHSS score in the Ginkgo tablet group decreased by 50% from baseline, significantly more than the placebo group (p < 0.05) (92).
In an RCT of the efficacy of Chinese medicine Neuroaid in stroke recovery, MLC601 was statistically no better than placebo in improving outcomes at 3 months when used among patients with acute ischemic stroke of intermediate severity (93). The CHIMES-E trial (n = 880, mean age = 61.8 years) was designed to evaluate the effect of Neuroaid treatment on long-term outcomes in subjects with cerebral infarction (moderate severity within 72 h) after 3 months of treatment. The likelihood of achieving functional independence was significantly increased after 6 months of treatment with Neuroaid compared with placebo and the benefits continued until 18 months after stroke. However, the outcomes at 24 months were similar. In addition, overall mortality and incidence of vascular events were also similar between two groups (both p > 0.05) (94). One hundred patients with ischemic stroke (less than 30 days) were randomly assigned to receive Dihuangyinzi or placebo for 12 weeks. The Fugl-Meyer score and BI in Dihuangyinzi group were significantly improved compared with those in the placebo group (p < 0.05) (95). An RCT (n = 288) evaluated the efficacy of Yangyin Yiqi Huoxue granule (YYHG) in treating Ischemic Stroke with Qi-Yin Deficiency and Blood Stasis Syndrome. The comprehensive cure rates in the high-dose YYHG treatment group were significantly higher than in the other three groups (P < 0.01). The improvement of NIHSS, ADL, QLI, and CMS scores in both the high-dose and low-dose YYHG groups was significantly superior to that of the positive control group and the placebo control group (P < 0.05). Regarding safety, adverse reactions after YYHG treatment were generally mild (3.78%), and no serious adverse reactions were reported (96). A large-scale RCT included 2,966 Chinese adults with ischemic stroke, treated with Xuesaitong capsule (Panax notoginseng Saponins) and placebo; At 3 months, Xuesaitong capsule significantly increased the likelihood of functional independence in ischemic stroke patients (p < 0.01). Serious adverse events were similar between the two groups (p > 0.05). This study suggests that Xuesaitong is a safe alternative therapy for improving the prognosis of ischemic stroke (97).
In these RCTs, two studies reported on the primary outcome; the outcome events and adverse events of TCM intervention were similar to those of the control group (P > 0.05); One of the large-sample, high-quality trials showed that Xuesaitong was slightly lower than the placebo group in terms of cardiovascular events and mortality, but there was no significant difference (p > 0.05).
6 Heart failure
Ischaemic heart disease has become the main cause of heart failure (HF) in China in recent decades (98, 99). Patients with ischaemic HF (IHF) have a poorer quality of life and worse prognosis than those without IHF, even after receiving the standard international guideline-directed medical therapy. Traditional Chinese medicine may have a complementary effect in improving exercise tolerance and cardiac function among patients with HF (100–104). It has been reported that TCM may have complementary effects in improving exercise tolerance and cardiac function in patients with heart failure.
We included and evaluated 11 RCTs of TCM and heart failure (HF). Sample sizes ranged from 80 to 3,110 patients, and follow-up periods ranged from 4 weeks to 36 months. 9 of 11 reports had a Modified Jadad score of 7, 2 of 11 reports had a score of 6 (Supplementary Table S4). The intervention group was treated with Nuanxin capsule, Shencaotongmai granule, Qiangxintongmai, Qiliqiangxin, Zhuanshenling, Qishenyiqi, Shensongyangxin granule, Qishen granules, Buyanghuanwu. Standard treatment was based on clinical guidelines for HF, and all control groups received placebo.
In one RCT of 150 patients with chronic HF, based on standard treatment, the total effective rate was 85.9% in Nuanxin capsule group and 63.0% in control group. The decrease in NYHA score was more significant in the Nuanxin group (p < 0.01), and it improved heart function in chronic HF with fewer adverse reactions (105). A total of 280 patients with chronic HF (NYHA classes II and III) were randomized to receive Shencaotongmai granules or placebo for 12 weeks. The added value of LVEF in Shencaotongmai group was significantly higher than placebo (p < 0.05). The incidence of adverse reactions in both groups was 0.71% (106). 280 patients with chronic HF were randomized to Qiangxintongmai and placebo. After 12 weeks of treatment, the Qiangxintongmai group had more significant improvement in 6-minute walking test and LHFQ score (both p < 0.01), no related adverse reactions were reported (107). A RCT was conducted in 140 patients with chronic HF (heart-kidney Yang deficiency). After 12 weeks of external application of Zhuangshenling formula, the improvement of BNP level was significantly better than control group (p < 0.05). 6-min walking distance between the two groups after treatment was similar (p > 0.05), and no adverse events were reported (108). In a RCT (n = 411), Shensongyangxin granule caused a significantly greater decline in the total number of ventricular premature complexes (VPCs) than the placebo did (p < 0.05), The secondary endpoints of the LVEF, NYHA classification, NT-proBNP, 6MWD, and MLHFQ scores showed a greater improvements in the Shensongyangxin group than in the placebo group (p < 0.05). In this 12-week study, Shensongyangxin was demonstrated to have the benefits of VPCs suppression and cardiac function improvement with good compliance on a background of standard treatment for CHF. The analysis of drug-induced adverse events revealed no differences between the two groups (109).
One RCT randomly divided 228 patients with HF (NYHA class of II to III) into two groups, to be treated with Yangxinkang tablet or placebo for 4 weeks. Compared with placebo, scores on the Minnesota Heart Failure Living Questionnaire (MLHFQ) showed a significant improvement (p < 0.05), with no treatment-related adverse events (110). A total of 640 HF patients were randomly assigned to receive QSYQ or placebo for six months. Compared with placebo, the QSYQ group had a significant increase in 6-minute walking distance and Minnesota heart failure questionnaire scores at 6 months (both p < 0.01). The incidence of the composite endpoint was similar between the two groups at 6 and 12 months (111). In a twelve-week RCT (n = 191), the Qishen granules group demonstrated a considerably greater reduction in NT-proBNP than the placebo group (p = 0.011). Patients who received Qishen granules performed better in the NYHA functional rank, 6MWD, TCM syndrome integral scale, and quality of life (p < 0.05). The Qishen granules performed better in HFrEF patients regarding the efficiency of NT-proBNP (112). A trial has proven that Buyanghuanwu treatment can further improve cardiac dysfunction and clinical symptoms of IHF on the basis of standard treatment without obvious adverse reactions. After 3 months of treatment, the NYHA classification, TCM syndrome scores, and the percentage of subjects with at least 30% reduction in NT-ProBNP were significantly improved in the BYHW group, compared with the control group (p < 0.05) (113).
A multicenter RCT study evaluated the efficacy of Qiliqiangxin capsule in patients with chronic HF (n = 512). During the 12-week follow-up, the reduction of NT-proBNP in Qiliqiangxin capsule group was significantly greater than that in placebo group (p = 0.002); in terms of reducing NT-proBNP by at least 30%, the proportion in the Qiliqiangxin capsule group was greater than that in the placebo group (p < 0.001). The serious adverse events and drug-related adverse events were similar (p > 0.01) (102). The recent QUEST trial (n = 3,110) evaluated the clinical efficacy and safety of Qiliqiangxin capsule for major heart failure outcomes in patients with HFrEF (LVEF ≤ 40%; NT-pro BNP ≥ 450 pg/ml). During a median follow-up of 18.3 months, the incidence of MACE in Qiliqiangxin group was significantly lower than that in placebo group (p < 0.001). In terms of secondary endpoints, serum NT-proBNP in Qiliqiangxin group decreased significantly more than that in control group during 3 months of follow-up (p = 0.047). Qiliqiangxin capsule was well tolerated, safety endpoint analysis in two groups showed that was similar (p = 0.058) (114).
In summary, these studies suggest that Chinese patent medicines, such as Qiliqiangxin, Nuanxin, Shencao tongmai, Yangxinkang, might be effective in improving cardiac remodeling and function in patients with chronic heart failure. Six studies reported similar adverse effects or events, and with safe profile.
7 Adverse cardiovascular events and death events
A total of 19 of the included studies reported adverse CV events, all-cause mortality, or CV death, including heart failure, stroke, and CAD (CV events for Figure 2, death events for Figure 3).
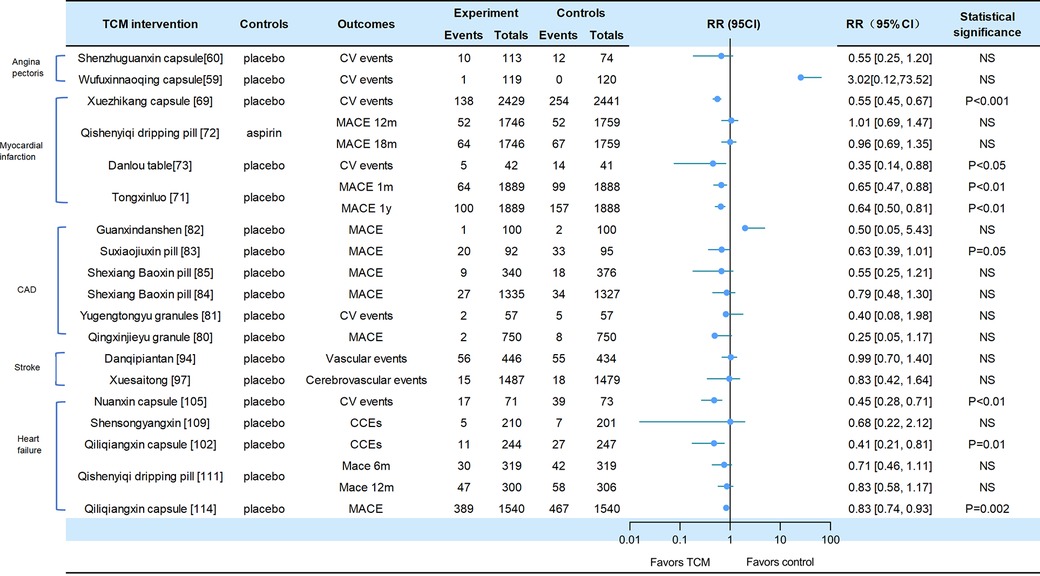
Figure 2. Cv events in traditional Chinese medicine interventions for CVD. Numbers in parentheses=reference numbers. CV, cardiovascular; CVD, cardiovascular disease; TCM, traditional Chinese medicine; NS, not significant; RR, risk ratio.
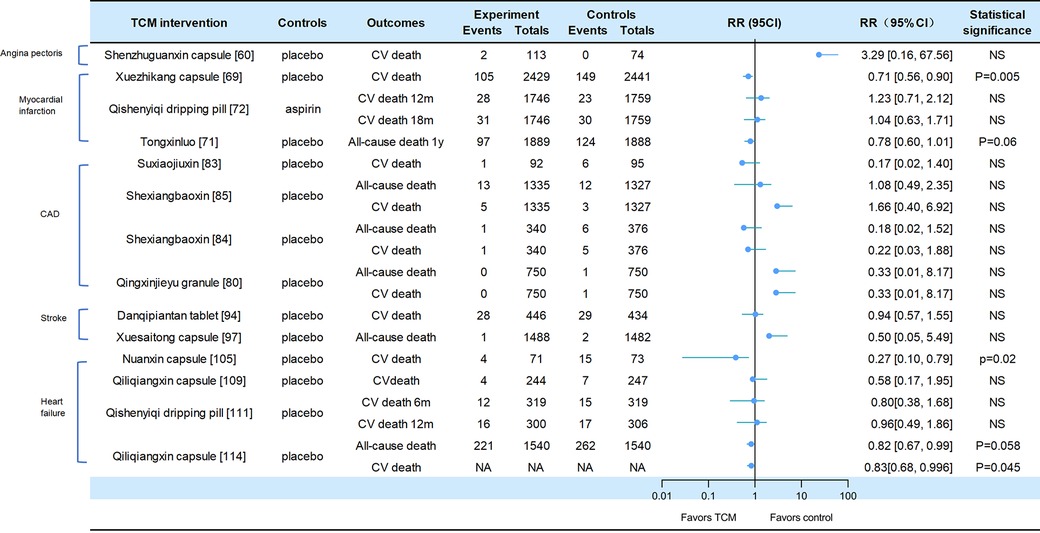
Figure 3. Death events in traditional Chinese medicine interventions for CVD. Numbers in parentheses=reference numbers. CV, cardiovascular; CVD, cardiovascular disease; TCM, traditional Chinese medicine; NS, not significant; RR, risk ratio.
Adverse cardiovascular events were reported in two trials of angina pectoris. Compared with placebo, SZGX group had a lower risk of CV event (8.85% [10/113] vs. 16.22% [12/74], p > 0.05), and CV death (1.8% [2/113] vs. 0 [0/74], p > 0.05) (60). Additionally, Wufuxinnaqing showed similar rates of CV events compared to the placebo (0.84% [1/119] vs. 0 [0/120], p > 0.05) (59).
Cardiovascular events and mortality were reported in four trials of myocardial infarction. The Xuezhikang group had a significantly lower incidence of primary cardiovascular events compared to the placebo group, including major coronary events [5.7% (138/2,429)] vs. 10.4% [254/2,441], p < 0.001), and CV death [4.3% (105/2,429)] vs. 6.1% [149/2,441], p = 0.005). During the 4.5-year follow-up period, Xuezhikang capsule not only reduced cardiovascular mortality and total mortality by 30% and 33%, respectively, but also lowered the risk of non-fatal MI compared to the placebo (p < 0.001) (69). In the Shang 2013 trial, the incidence of MACE was similar between Qishen yiqi dripping pills and aspirin group at 12 months (p = 0.872) and at 18 months (p = 0.957). The CV mortality rates were also similar in two groups at 12 months (p = 0.411) and at 18 months (p = 0.782). The incidence rates of non-fatal MI and non-fatal stroke were also significantly different (p > 0.05) (72). In the Mao 2016 trial, Danlou had significantly lower CV events (11.90% [5/42] vs. 34.15% [14/41], p = 0.02); as well as significantly lower rates of non-fatal MI (11.9% [5/42] vs. 21.95% [9/41]) and severe heart failure (2.38% [1/42] vs. 12.2% [5/41]) (73). In the 2023 trial of Tongxinluo for MI, the occurrence of MACCEs was lower in the Tongxinluo group than in the control group during the 30-day period (64 [3.4%] vs. 99 [5.2%], p < 0.01). Cardiac death was also lower in the Tongxinluo group (56 [3.0%] vs. 80 [4.2%], p < 0.05). After 1 year, the incidence of MACCE (100 [5.3%] vs. 157 [8.3%], p < 0.01) and cardiac death (85 [4.5%] vs. 116 [6.1%], p < 0.05) remained lower (71).
Six trials reported cardiovascular events and mortality in CAD. The Wang 2023 trial reported that the incidence of MACE was similar between the GXDS and placebo groups (1% [1/100] vs. 2% [2/100], p > 0.05), and all patients experienced cardiogenic rehospitalization (82). The Shen 2020 trial reported a lower incidence of major cardiovascular events in SJP groups compared to placebo group (21.7% [20/92] vs. 34.7% [33/95], p < 0.05). The cases of MACE (death/myocardial infarction/stroke/heart failure rehospitalization) in the SJP and placebo groups were 1/2/12/5 and 6/4/9/14, respectively. The analysis showed the SJP group had a lower incidence of MACE than the placebo group (MACE/not MACE, 20/70 vs. 33/62, p < 0.05) (83). The Zhou 2023 trial reported that the incidence of MACE (2.6% [9/340] vs. 4.8% [18/376], p = 0.192) and all-cause mortality (0.3% [1/340] vs. 1.6% [6/376], p = 0.127) was similar between the MUSKARDIA and placebo groups. In addition, there was no significant difference in CV death (0.3% [1/340] vs. 1.3% [5/376], p = 0.665) and composite outcomes (15.3% [52/340] vs. 22.6% [85/376]), p = 0.192) (85). In the MUSKARDIA subgroup analysis, the incidence of MACE at 24 months was similar in both groups (p = 0.2869). Furthermore, there were similar rates in all-cause mortality (p = 0.8526) and CV death (p = 0.7119). After two years, the incidence of MACE events decreased by 26.9%. The incidence of non-fatal MI (p = 0.3656) and no-fatal stroke (p = 0.9898) was similar in both groups (84). In Wang 2021 trial, the major outcomes of Yugengtongyu granules and placebo were similar (p = 0.435). There were no cardiovascular deaths or all-cause deaths in both groups during the follow-up period; compared with placebo, all composite outcomes were lower in Yugengtongyu group (p = 0.013), but was similar in non-fatal MI [0 vs. 8.77% (5/57), p = 0.067] (81). In Li 2019 trial, Qingxin Jieyu granule showed a significantly lower incidence of MACE compared to the placebo (p = 0.012); the hard endpoint was reduced by 0.99% during the follow-up. Additionally, there were no differences in all-cause mortality [0 vs. 0.16% (1/750), p = 0.95] and CV death [0 vs. 0.16% (1/750), p = 0.95] (80).
Two trials focused on stroke reported cardiovascular events. In the CHIMES-E trial, vascular events (12.6% [56/446] vs. 12.7% [55/434], p > 0.05) and all-cause mortality (6.3% [28/446] vs. 6.7% [29/434], p > 0.05) in NeuroAid were similar to those in the placebo group (94). In a 2023 study of Xuesaitong for stroke, the incidence of cerebrovascular events (1% [15/1,487] vs. 1.2% [18/1,479], p = 0.59) and all-cause death (0.1% vs. 0.1%, p = 0.56) were similar between two groups (97).
Cardiovascular and mortality events were reported in the five trials of heart failure. The Nuanxin group had lower rates of cardiovascular events (23.9% [17/71] vs. 53.4% [39/73], p < 0.05) compared to the placebo group; however, cardiovascular death rates were similar in both groups (5.63% [4/71] vs. 8.95% [15/73], p > 0.05) (105). The incidence of CCEs in Shensongyangxin and placebo groups were 2.2% and 3.5%, respectively (p > 0.05) (109). Qiliqiangxin showed a lower incidence of CCEs compared to the placebo (4.51% [11/244] vs. 10.93% [27/247], p = 0.008), while the mortality rate was similar in both groups (1.64% [4/244] vs. 2.83% [7/247]) (102). In the trial of QSYQ for heart failure, the incidence of MACE at 6 months was not significantly lower in QSYQ group compared to the placebo (9.40% [30/319] vs. 13.17% [42/319], p = 0.255); the incidence of MACE at one year was similar (15.67% [47/300] vs. 19.12% [58/306]) (111). In Li 2023 trial, the incidence of CV death events was similar at six months (3.76% [12/319] vs. 4.70% [15/319], p = 0.689) and at one year (5.33% [16/300] vs. 5.64% [17/306], p > 0.05); the incidence of composite endpoint events was similar at 6 months (13.17% vs. 16.61%) and 12 months (20.38% vs. 22.88%). Additionally, Qiliqiangxin reduced cardiovascular death (p = 0.045) (114).
8 Conclusions
We initially conducted a search for studies on other cardiovascular risk factors and diseases in recent years, such as hyperlipidemia, hyperglycemia, and arrhythmia. There were only a limited number of valuable studies. For example, the SS-AFRF study, as reported in ESC 2023, also recently published in the European Heart Journal, showed that Shensong Yangxin capsule significantly reduced atrial fibrillation (AF) recurrence after perAF ablation (115). Therefore, we focused on including high-quality studies that have been updated in recent years for our evaluation.
The current evidence from RCTs indicates that Chinese patent medicines could effectively reduce the blood pressure of hypertensive patients, decrease the occurrence of coronary artery events in patients with a history of myocardial infarction (especially with the use of Xuesaitong, Qishenyiqi pills and Tongxinluo), alleviate the severity of angina pectoris and myocardial infarction, and reduce the frequency of angina attacks and consumption of nitroglycerin (especially Shensong yangxin capsule and acupuncture). TCM interventions have also shown promise in improving the functional independence of stroke patients (particularly with the use of Xuesaitong granules) and enhancing heart function in patients with heart failure (particularly with the use of Qiliqiangxin capsule). Furthermore, acupuncture may contribute to the restoration of limb function following a stroke and mitigate the onset of angina pectoris.
Regarding primary outcomes, TCM interventions, compared to placebo or Western medicines, did not increase the risks of CV events or CV death in included trials, except for hypertension trials (which were no mentioned). Some of these TCM interventions, including xuezhikang, Qishenyiqi pills, tongxinluo, suxiaojiuxin pills, qiliqiangxin, have been found effective in reducing cardiovascular events.
Furthermore, the occurrence of adverse reactions or events did not increase in most trials. It is worth mentioning that our review also has some limitations, some studies with short follow-up periods and small sample sizes, which may result in the long-term prognosis of TCM treatment remaining unclear. Additionally, some of the trials included in our review were published in Chinese, and due to language barriers, this may lead to the findings being easily overlooked by researchers whose native language is English. Of course, in the future, more high-quality randomized controlled trials will be needed to validate our conclusions.
Author contributions
YL: Writing – review & editing, Methodology. YH: Data curation, Writing – review & editing. YW: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the following projects: National Natural Science Foundation of China (No. 82174357); Research Project of Traditional Chinese Medicine Administration of Hunan Province (No. B2023141); Hunan Province Science and Medicine Joint Fund (No. 2023JJ60476); Discipline Construction "Open bidding for selecting the best candidates" project (No. 22JBZ06).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1419169/full#supplementary-material
References
1. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation. (2022) 145:e153–639. doi: 10.1161/CIR.000000000000105235078371
2. Walli-Attaei M, Rosengren A, Rangarajan S, Breet Y, Abdul-Razak S, Sharief WA, et al. Metabolic, behavioural, and psychosocial risk factors and cardiovascular disease in women compared with men in 21 high-income, middle-income, and low-income countries: an analysis of the PURE study. Lancet. (2022) 400:811–21. doi: 10.1016/S0140-6736(22)01441-636088949
3. Timmis A, Vardas P, Townsend N, Torbica A, Katus H, De Smedt D, et al. European Society of cardiology: cardiovascular disease statistics 2021. Eur Heart J. (2022) 43:716–99. doi: 10.1093/eurheartj/ehab89235016208
4. Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. (2021) 42:3227–337. doi: 10.1093/eurheartj/ehab48434458905
5. Davies A, Fox K, Galassi AR, Banai S, Ylä-Herttuala S, Lüscher TF. Management of refractory angina: an update. Eur Heart J. (2021) 42:269–83. doi: 10.1093/eurheartj/ehaa82033367764
6. Hao P, Jiang F, Cheng J, Ma L, Zhang Y, Zhao Y. Traditional Chinese medicine for cardiovascular disease. J Am Coll Cardiol. (2017) 69:2952–66. doi: 10.1016/j.jacc.2017.04.04128619197
7. World Health Organization. WHO traditional Medicine Strategy: 2014-2023. Geneva: World Health Organization (2013). Available online at: https://iris.who.int/handle/10665/92455 (Accessed January 19, 2024).
8. Xu Y, Lin S, Liu T, Cui Y, Li X, Mo M. Promoting the development of integrated Chinese and western medicine through crossing innovation. J Beijing Univ Tranditional Chin Med. (2023) 46:1653–7. doi: 10.3969/j.issn.1006-2157.2023.12.005
9. Chen K, Lv A. Situation of integrative medicine in China: results from a national survey in 2004. Chin J Integr Med. (2004) 12:161–5. doi: 10.1007/BF02836514
10. Hao P-P, Jiang F, Chen Y-G, Yang J, Zhang K, Zhang M-X, et al. Traditional Chinese medication for cardiovascular disease. Nat Rev Cardiol. (2015) 12:115–22. doi: 10.1038/nrcardio.2014.17725384847
11. Liang B, Gu N. Traditional Chinese medicine for coronary artery disease treatment: clinical evidence from randomized controlled trials. Front Cardiovasc Med. (2021) 8:702110. doi: 10.3389/fcvm.2021.70211034422929
12. Guo X, Chen X, Chen J, Tan Z, Yang Y, Zhang H. Current status and evaluation of randomized clinical trials of traditional Chinese medicine in the treatment of cardiovascular diseases. Evid Based Complement Alternat Med. (2022) 2022:1–13. doi: 10.1155/2022/6181862
13. Liu L, Leung EL-H, Tian X. Perspective: the clinical trial barriers. Nature. (2011) 480:S100–S100. doi: 10.1038/480S100a22190082
14. Cheng C, Wu T, Shang H, Li Y, Altman DG, Moher D, et al. CONSORT Extension for Chinese herbal medicine formulas 2017: recommendations, explanation, and elaboration. Ann Intern Med. (2017) 167:112. doi: 10.7326/M16-297728654980
15. Zhang Y-Q, Jiao R-M, Witt CM, Lao L, Liu J-P, Thabane L, et al. How to design high quality acupuncture trials—a consensus informed by evidence. Br Med J. (2022):376:e067476. doi: 10.1136/bmj-2021-067476
16. Berman BM, Langevin HM, Witt CM. Acupuncture for chronic low back pain. N Engl J Med. (2010) 363:454–61. doi: 10.1056/NEJMct080611420818865
17. Liu S, Wang Z, Su Y, Qi L, Yang W, Fu M, et al. A neuroanatomical basis for electroacupuncture to drive the vagal–adrenal axis. Nature. (2021) 598:641–5. doi: 10.1038/s41586-021-04001-434646018
18. Lu L, Zhang Y, Tang X, Ge S, Wen H, Zeng J, et al. Evidence on acupuncture therapies is underused in clinical practice and health policy. Br Med J. (2022) 376:1–5. doi: 10.1136/bmj-2021-067475
19. Liu Z, Yan S, Wu J, He L, Li N, Dong G, et al. Acupuncture for chronic severe functional constipation: a randomized, controlled trial. Ann Intern Med. (2016) 165:761–9. doi: 10.7326/M15-311827618593
20. Yang Z, Wang H, Edwards D, Ding C, Yan L, Brayne C, et al. Association of blood lipids, atherosclerosis and statin use with dementia and cognitive impairment after stroke: a systematic review and meta-analysis. Ageing Res Rev. (2020) 57:100962. doi: 10.1016/j.arr.2019.10096231505259
21. He Y, Guo X, May BH, Zhang AL, Liu Y, Lu C, et al. Clinical evidence for association of acupuncture and acupressure with improved cancer pain: a systematic review and meta-analysis. JAMA Oncol. (2020) 6:271–8. doi: 10.1001/jamaoncol.2019.523331855257
22. Zhao L, Chen J, Li Y, Sun X, Chang X, Zheng H, et al. The long-term effect of acupuncture for migraine prophylaxis: a randomized clinical trial. JAMA Intern Med. (2017) 177:508. doi: 10.1001/jamainternmed.2016.937828241154
23. Zhao L, Li D, Zheng H, Chang X, Cui J, Wang R, et al. Acupuncture as adjunctive therapy for chronic stable angina: a randomized clinical trial. JAMA Intern Med. (2019) 179:1388–97. doi: 10.1001/jamainternmed.2019.240731355870
24. Zheng H, Li J, Li Y, Zhao L, Wu X, Chen J, et al. Acupuncture for patients with mild hypertension: a randomized controlled trial. J Clin Hypertens. (2019) 21:412–20. doi: 10.1111/jch.13490
25. Shen P-F, Kong L, Ni L-W, Guo H-L, Yang S, Zhang L-L, et al. Acupuncture intervention in ischemic stroke: a randomized controlled prospective study. Am J Chin Med. (2012) 40:685–93. doi: 10.1142/S0192415X1250051622809024
26. C LIU. Evidence-based medicine and future design ideas for acupuncture treatment of cardiovascular diseases. Chin J Integr Tradit West Med. (2019) 39:1306–8. doi: 10.7661/j.cjim.20191015.324
27. Carey RM, Moran AE, Whelton PK. Treatment of hypertension: a review. JAMA. (2022) 328:1849–61. doi: 10.1001/jama.2022.1959036346411
28. Yang Q, Chang A, Ritchey MD, Loustalot F. Antihypertensive medication adherence and risk of cardiovascular disease among older adults: a population-based cohort study. J Am Heart Assoc. (2017) 6:e006056. doi: 10.1161/JAHA.117.006056
29. Tiffe T, Wagner M, Rücker V, Morbach C, Gelbrich G, Störk S, et al. Control of cardiovascular risk factors and its determinants in the general population- findings from the STAAB cohort study. BMC Cardiovasc Disord. (2017) 17:276. doi: 10.1186/s12872-017-0708-x29096615
30. Tedla YG, Bautista LE. Drug Side effect symptoms and adherence to antihypertensive medication. Am J Hypertens. (2016) 29:772–9. doi: 10.1093/ajh/hpv18526643686
31. Huang Z, Xu A, Cheung BMY. The potential role of fibroblast growth factor 21 in lipid metabolism and hypertension. Curr Hypertens Rep. (2017) 19:28. doi: 10.1007/s11906-017-0730-528337713
32. Chen S-L, Lee W-L, Liang T, Liao I-C. Factors associated with gender differences in medication adherence: a longitudinal study. J Adv Nurs. (2014) 70:2031–40. doi: 10.1111/jan.1236124506542
33. Kou Q-A. Clinical observation on treatment of hypertension (yin-deficiency with sthenic-yang syndrome type) with zhongfu hypotension capsule [Chinese]. Chin J Integr Tradit West Med. (2007) 27:745–8. doi: 10.3321/j.issn:1003-5370.2007.08.022
34. H L. Effects of jiangya capsule combined with nimodipine on depressurization and quality of life in elderly patients with isolated systolic hypertension [Chinese]. Chin J Inf TCM. (2008) 15:8–11. doi: 10.3969/j.issn.1005-5304.2008.09.004
35. Li H, Liu L, Zhao W, Liu J. Tranditional Chinese versus integrative treatment in elderly patients with isolated systolic hypertension: a multicenter, randomized, double-blind controlled trial. J Chin Integr Med. (2010) 8:410–6. doi: 10.3736/jcim20100503
36. Ma J, Yue G, Xu M, Ma X. A double-blind controlled study of the additional use of qiqilian capsules in the treatment of hypertension [Chinese]. J Guangxi Univ Chin Med. (2014) 17:1–2.
37. Chen X, Cheng G, Fan J. Treatment of level 2 hypertension by diagnosis and treatment program of integrative medicine: a multicentre. Randomized Controlled Trial [Chinese]. Chin J Integr Tradit West Med. (2015) 35:801–5. doi: 10.7661/CJIM.2015.07.0801
38. Wang S, Li P, Ran D, Wang X, Xue J. Randomized, double-blind, placebo control and multicenter clinical trials for tiankui antihypertension tablets combined with chemical drugs in the treatment of hypertension [Chinese]. China Pharm. (2015) 18:964–7. doi: 10.3969/j.issn.1008-049X.2015.06.025
39. Zhang D MX, Gao R LF. The effect of angong jiangya pill in the treatment of grade-1 hypertension with liver-fire hyperactivity syndrome: a randomized controlled clincial trial [Chinese]. Chin J Integr Med Cardio-Cerebrovasc Dis. (2017) 15:6–10. doi: 10.3969/j.issn.1672-1349.2017.01.002
40. Zhang D-Y, Cheng Y-B, Guo Q-H, Shan X-L, Wei F-F, Lu F, et al. Treatment of masked hypertension with a Chinese herbal formula: a randomized, placebo-controlled trial. Circulation. (2020) 142:1821–30. doi: 10.1161/CIRCULATIONAHA.120.04668533019798
41. Ma J WD. Clinical study on songlingxuemaikang capsule for the treatment of hypertension with losartan potassium tablets controlled [Chinese]. Drug Eval Res. (2018) 41:836–40.
42. Lai X, Dong Z, Wu S, Zhou X, Zhang G, Xiong S, et al. Efficacy and safety of Chinese herbal medicine compared with losartan for mild essential hypertension: a randomized, multicenter, double-blind, noninferiority trial. Circ Cardiovasc Qual Outcomes. (2022) 15:e007923. doi: 10.1161/CIRCOUTCOMES.121.00792335105177
43. Macklin EA, Wayne PM, Kalish LA, Valaskatgis P, Thompson J, Pian-Smith MCM, et al. Stop hypertension with the acupuncture research program (SHARP): results of a randomized, controlled clinical trial. Hypertension. (2006) 48:838–45. doi: 10.1161/01.HYP.0000241090.28070.4c17015784
44. Flachskampf FA, Gallasch J, Gefeller O, Gan J, Mao J, Pfahlberg AB, et al. Randomized trial of acupuncture to lower blood pressure. Circulation. (2007) 115:3121–9. doi: 10.1161/CIRCULATIONAHA.106.66114017548730
45. Brook RD, Appel LJ, Rubenfire M, Ogedegbe G, Bisognano JD, Elliott WJ, et al. Beyond medications and diet: alternative approaches to lowering blood pressure: a scientific statement from the American Heart Association. Hypertension. (2013) 61:1360–83. doi: 10.1161/HYP.0b013e318293645f23608661
46. Wang J, Xiong X, Liu W. Acupuncture for essential hypertension. Int J Cardiol. (2013) 169:317–26. doi: 10.1016/j.ijcard.2013.09.00124060112
47. Yang J, Chen J, Yang M, Yu S, Ying L, Liu GJ, et al. Acupuncture for hypertension. Cochrane Database Syst Rev. (2018) 2018:1–69. doi: 10.1002/14651858.CD008821.pub2
48. Fan M, Dai G, Li R, Wu X. Efficacy of acupuncture in the treatment of essential hypertension: an overview of systematic reviews and meta-analyses. Cardiovasc Ther. (2023) 2023:1–19. doi: 10.1155/2023/2722727
49. Ferraro R, Latina JM, Alfaddagh A, Michos ED, Blaha MJ, Jones SR, et al. Evaluation and management of patients with stable angina: beyond the ischemia paradigm: jACC state-of-the-art review. J Am Coll Cardiol. (2020) 76:2252–66. doi: 10.1016/j.jacc.2020.08.07833153586
50. Al-Lamee R, Thompson D, Dehbi H-M, Sen S, Tang K, Davies J, et al. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet Lond Engl. (2018) 391:31–40. doi: 10.1016/S0140-6736(17)32714-9
51. Wang Q, Liang D, Wang F, Li W, Han Y, Zhang W, et al. Efficacy of electroacupuncture pretreatment for myocardial injury in patients undergoing percutaneous coronary intervention: a randomized clinical trial with a 2-year follow-up. Int J Cardiol. (2015) 194:28–35. doi: 10.1016/j.ijcard.2015.05.04326011261
52. Bueno EA, Mamtani R, Frishman WH. Alternative approaches to the medical management of angina pectoris: acupuncture, electrical nerve stimulation, and spinal cord stimulation. Heart Dis Hagerstown Md. (2001) 3:236–41. doi: 10.1097/00132580-200107000-00006
53. Ho FM, Huang PJ, Lo HM, Lee FK, Chern TH, Chiu TW, et al. Effect of acupuncture at nei-kuan on left ventricular function in patients with coronary artery disease. Am J Chin Med. (1999) 27:149–56. doi: 10.1142/S0192415X9900019710467449
54. Mehta PK, Polk DM, Zhang X, Li N, Painovich J, Kothawade K, et al. A randomized controlled trial of acupuncture in stable ischemic heart disease patients. Int J Cardiol. (2014) 176:367–74. doi: 10.1016/j.ijcard.2014.07.01125103909
55. Mannheimer C, Carlsson CA, Emanuelsson H, Vedin A, Waagstein F, Wilhelmsson C. The effects of transcutaneous electrical nerve stimulation in patients with severe angina pectoris. Circulation. (1985) 71:308–16. doi: 10.1161/01.CIR.71.2.3083871177
56. Richter A, Herlitz J, Hjalmarson A. Effect of acupuncture in patients with angina pectoris. Eur Heart J. (1991) 12:175–8. doi: 10.1093/oxfordjournals.eurheartj.a0598652044550
57. Ballegaard S, Pedersen F, Pietersen A, Nissen VH, Olsen NV. Effects of acupuncture in moderate, stable angina pectoris: a controlled study. J Intern Med. (1990) 227:25–30. doi: 10.1111/j.1365-2796.1990.tb00114.x2105371
58. Wang S, Wang J, Li J, Xiong X. Efficacy assessment of treating patients with coronary heart disease angina of phlegm and stasis mutual obstruction syndrome by danlou tablet [Chinese]. Chin J Integr Tradit West Med. (2012) 32:1051–5.
59. Zhang Z, Xu F, Liu H, Wang F, Zhao M, Sun L, et al. A multicenter, randomized, double-blind clinical study on Wufuxinnaoqing soft capsule in treatment of chronic stable angina patients with blood stasis syndrome. Chin J Integr Med. (2015) 21:571–8. doi: 10.1007/s11655-014-1953-925555593
60. Xu D, Wu H, Lan T, Wang X, Sheng X, Lin Y, et al. Effect of Shenzhu Guanxin recipe on patients with angina pectoris after percutaneous coronary intervention: a prospective, randomized controlled trial. Chin J Integr Med. (2015) 21:408–16. doi: 10.1007/s11655-015-2040-626063318
61. Gao J, Gao X, Zou T, Zhao T, Wang D, Wu Z, et al. Effect of Xinling Wan in treatment of stable angina pectoris: a randomized, double-blinded, placebo parallel-controlled, multicenter trial [Chinese]. China J Chin Mater Medica. (2018) 43:1268–75. doi: 10.19540/j.cnki.cjcmm.20171226.002
62. Li Y, Zhang L, Lv S, Wang X, Zhang J, Tian X, et al. Efficacy and safety of oral Guanxinshutong capsules in patients with stable angina pectoris in China: a prospective, multicenter, double-blind, placebo-controlled, randomized clinical trial. BMC Complement Altern Med. (2019) 19:363. doi: 10.1186/s12906-019-2778-z31829173
63. Chu F, Wang J, Yao K, Li Z. Effect of Xuefu Zhuyu capsule on the symptoms and signs and health-related quality of life in the unstable angina patients with blood-stasis syndrome after percutaneous coronary intervention: a randomized controlled trial. Chin J Integr Med. (2010) 16:399–405. doi: 10.1007/s11655-010-9999-920535581
64. Sun Y, Yao Y, Jia M, Sun Y, Li H, Ruan X, et al. Evaluation of the efficacy and safety of Suxiao Jiuxin pill in the treatment of stable angina: a randomized, double-blind, placebo-controlled, multi-center clinical trial. J Ethnopharmacol. (2024) 318:116959. doi: 10.1016/j.jep.2023.11695937487965
65. Heusch G. Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat Rev Cardiol. (2020) 17:773–89. doi: 10.1038/s41569-020-0403-y32620851
66. Figtree GA, Vernon ST, Hadziosmanovic N, Sundström J, Alfredsson J, Arnott C, et al. Mortality in STEMI patients without standard modifiable risk factors: a sex-disaggregated analysis of SWEDEHEART registry data. Lancet Lond Engl. (2021) 397:1085–94. doi: 10.1016/S0140-6736(21)00272-5
67. Hillerson D, Li S, Misumida N, Wegermann ZK, Abdel-Latif A, Ogunbayo GO, et al. Characteristics, process metrics, and outcomes among patients with ST-elevation myocardial infarction in rural vs urban areas in the US: a report from the US national cardiovascular data registry. JAMA Cardiol. (2022) 7:1016–24. doi: 10.1001/jamacardio.2022.277436044196
68. Karalliedde LD, Kappagoda CT. The challenge of traditional Chinese medicines for allopathic practitioners. Am J Physiol Heart Circ Physiol. (2009) 297:H1967–1969. doi: 10.1152/ajpheart.00944.200919855052
69. Lu Z, Kou W, Du B, Wu Y, Zhao S, Brusco OA, et al. Effect of Xuezhikang, an extract from red yeast Chinese rice, on coronary events in a Chinese population with previous myocardial infarction. Am J Cardiol. (2008) 101:1689–93. doi: 10.1016/j.amjcard.2008.02.05618549841
70. Zhang H, Jia Z, Zhang J, Ye Z, Yang W, Tian Y. No-reflow protection and long-term efficacy for acute myocardial infarction with tongxinluo: a randomized double-blind placebo-controlled multicenter clinical trial (ENLEAT trial). Chin Med J (Engl). (2010) 123:2858–64. doi: 10.3760/cma.j.issn.0366-6999.2010.20.02121034597
71. Yang Y, Li X, Chen G, Xian Y, Zhang H, Wu Y, et al. Traditional Chinese medicine compound (tongxinluo) and clinical outcomes of patients with acute myocardial infarction: the CTS-AMI randomized clinical trial. JAMA. (2023) 330:1534–45. doi: 10.1001/jama.2023.1952437874574
72. Shang H, Zhang J, Yao C, Liu B, Gao X, Ren M, et al. Qi-Shen-Yi-Qi dripping pills for the secondary prevention of myocardial infarction: a randomised clinical trial. Evid Based Complement Alternat Med. (2013) 2013:1–9. doi: 10.1155/2013/738391
73. Mao S, Wang L, Ouyang W, Zhou Y, Qi J, Guo L, et al. Traditional Chinese medicine, danlou tablets alleviate adverse left ventricular remodeling after myocardial infarction: results of a double-blind, randomized, placebo-controlled, pilot study. BMC Complement Altern Med. (2016) 16:447. doi: 10.1186/s12906-016-1406-427825334
74. Liu S, Li Y, Zeng X, Wang H, Yin P, Wang L, et al. Burden of cardiovascular diseases in China, 1990–2016: findings from the 2016 global burden of disease study. JAMA Cardiol. (2019) 4:342–52. doi: 10.1001/jamacardio.2019.029530865215
75. Shen C, Ge J. Epidemic of cardiovascular disease in China: current perspective and prospects for the future. Circulation. (2018) 138:342–4. doi: 10.1161/CIRCULATIONAHA.118.03348430571361
76. Chen K-B, Chen K-C, Chang Y-L, Chang K-L, Chang P-C, Chang T-T, et al. In silico investigation of traditional Chinese medicine for potential lead compounds as SPG7 inhibitors against coronary artery disease. Mol Basel Switz. (2016) 21:588. doi: 10.3390/molecules21050588
77. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. (2020) 41:407–77. doi: 10.1093/eurheartj/ehz42531504439
78. Wang J, Lin F, Guo L-L, Xiong X-J, Fan X. Cardiovascular disease, mitochondria, and traditional Chinese medicine. Evid-Based Complement Altern Med ECAM. (2015) 2015:143145. doi: 10.1155/2015/143145
79. Lu X, Shi D, Xu H, Chen K, Lv S. Clinical study on effect of Xiongshao capsule on restenosis after percutaneous coronary intervention. Chin J Integr Tradit West Med. (2006) 26:13–7. doi: 10.3321/j.issn:1003-5370.2006.01.006
80. Li J, Gao Z, Zhang L, Li S, Yang Q, Shang Q, et al. Qing-Xin-Jie-Yu granule for patients with stable coronary artery disease (QUEST trial): a multicenter, double-blinded, randomized trial. Complement Ther Med. (2019) 47:102209. doi: 10.1016/j.ctim.2019.10220931780034
81. Wang D, Li C, Xu X, Xu H, Guo C, Wang J, et al. Effect of yugengtongyu granules in patients with stable coronary artery disease on reducing adverse cardiovascular events: a double-blind controlled trial. J Altern Complement Med. (2021) 27:142–9. doi: 10.1089/acm.2020.036133259734
82. Wang C, Huan N, Wang P, Geng Q, Ma W, Ma L, et al. Guanxin danshen dripping pills improve quality of life and cardiovascular prognoses of CHD patients after PCI with anxiety or depression (GLAD study): a randomized double-blind placebo-controlled study. Chin J Integr Med. (2023) 29:195–204. doi: 10.1007/s11655-022-3688-336301456
83. Shen Z, Chen T, Deng B, Fan M, Hua J, Zhang M, et al. Effects on suxiao jiuxin pills in the treatment of patients with acute coronary syndrome undergoing early percutaneous coronary intervention: a multicenter randomized double-blind placebo-controlled trial. J Altern Complement Med. (2020) 26:1055–63. doi: 10.1089/acm.2020.001432716206
84. Ge J-B, Fan W-H, Zhou J-M, Shi H-M, Ji F-S, Wu Y, et al. Efficacy and safety of shexiang baoxin pill (MUSKARDIA) in patients with stable coronary artery disease: a multicenter, double-blind, placebo-controlled phase IV randomized clinical trial. Chin Med J (Engl). (2021) 134:185–92. doi: 10.1097/CM9.000000000000125733273369
85. Zhou J, Shi H, Ji F, Wu Y, Zhao Y, Qian J, et al. Effectiveness and safety of shexiang baoxin pill (MUSKARDIA) in patients with stable coronary artery disease and concomitant diabetes mellitus: a subgroup analysis of a randomized clinical trial. Chin Med J (Engl). (2023) 136:82–7. doi: 10.1097/CM9.000000000000252736752805
86. Zhou M, Wang H, Zhu J, Chen W, Wang L, Liu S, et al. Cause-specific mortality for 240 causes in China during 1990–2013: a systematic subnational analysis for the global burden of disease study 2013. Lancet Lond Engl. (2016) 387:251–72. doi: 10.1016/S0140-6736(15)00551-6
87. Ma Q, Li R, Wang L, Yin P, Wang Y, Yan C, et al. Temporal trend and attributable risk factors of stroke burden in China, 1990–2019: an analysis for the global burden of disease study 2019. Lancet Public Health. (2021) 6:e897–906. doi: 10.1016/S2468-2667(21)00228-034838196
88. Goyal M, Menon BK, van Zwam WH, Dippel DWJ, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet Lond Engl. (2016) 387:1723–31. doi: 10.1016/S0140-6736(16)00163-X
89. Han L. Clinical study on Naoxin Duotai capsule in the treatment of hyperactivity of liver yang and blood stasis and obstruction of collaterals in the recovery period of ischemic stroke [Chinese]. Tianjin J Tradit Chin Med. (2007) 24:101–3. doi: 10.3969/j.issn.1672-1519.2007.02.00
90. Chen C, Venketasubramanian N, Gan RN, Lambert C, Picard D, Chan BPL, et al. Danqi piantang jiaonang (DJ), a traditional Chinese medicine, in poststroke recovery. Stroke. (2009) 40:859–63. doi: 10.1161/STROKEAHA.108.53161619164787
91. He L, Chen X, Zhou M, Zhang D, Yang J, Yang M, et al. Radix/rhizoma Notoginseng extract (sanchitongtshu) for ischemic stroke: a randomized controlled study. Phytomedicine. (2011) 18:437–42. doi: 10.1016/j.phymed.2010.10.00421094030
92. Oskouei DS, Rikhtegar R, Hashemilar M, Sadeghi-Bazargani H, Sharifi-Bonab M, Sadeghi-Hokmabadi E, et al. The effect of Ginkgo biloba on functional outcome of patients with acute ischemic stroke: a double-blind, placebo-controlled, randomized clinical trial. J Stroke Cerebrovasc Dis. (2013) 22:e557–63. doi: 10.1016/j.jstrokecerebrovasdis.2013.06.01023871729
93. Chen CLH, Young SHY, Gan HH, Singh R, Lao AY, Baroque AC, et al. Chinese medicine neuroaid efficacy on stroke recovery: a double-blind, placebo-controlled, randomized study. Stroke. (2013) 44:2093–100. doi: 10.1161/STROKEAHA.113.00205523780952
94. Venketasubramanian N, Young SH, Tay SS, Umapathi T, Lao AY, Gan HH, et al. Chinese medicine NeuroAiD efficacy on stroke recovery—extension study (CHIMES-E): a multicenter study of long-term efficacy. Cerebrovasc Dis. (2015) 39:309–18. doi: 10.1159/00038208225925713
95. Yu M, Sun Z-J, Li L-T, Ge H-Y, Song C-Q, Wang A-J. The beneficial effects of the herbal medicine Di-huang-yin-zi (DHYZ) on patients with ischemic stroke: a randomized, placebo controlled clinical study. Complement Ther Med. (2015) 23:591–7. doi: 10.1016/j.ctim.2015.06.00326275652
96. Wang Y, Yang J, Wan H, He Y, Xu B, Ai C, et al. Efficacy of yangyin yiqi huoxue granule in treatment of ischemic stroke patients with qi-yin deficiency and blood stasis syndrome: a randomized, double-blind, multicenter, phase-2 clinical trial. Chin J Integr Med. (2021) 27:811–8. doi: 10.1007/s11655-021-2857-033881715
97. Wu L, Song H, Zhang C, Wang A, Zhang B, Xiong C, et al. Efficacy and safety of Panax notoginseng saponins in the treatment of adults with ischemic stroke in China: a randomized clinical trial. JAMA Netw Open. (2023) 6:e2317574. doi: 10.1001/jamanetworkopen.2023.1757437338907
98. Jian Z, Zhang Y. China heart failure registry study——A multicenter, prospective investigation for preliminary analysis on etiology, clinical features and treatment in heart failure patients. Chin Circ J. (2015) 30:413–6. doi: 10.3969/j.issn.1000-3614.2015.05.002
99. Ma W, Ma H, Wang Y, Wang J, Wang H, Wang S, et al. 2021 Medical quality report of cardiovascular diseases in China: an executive summary. Chin Circ J. (2021) 36:1041–64. doi: 10.3969/j.issn.1000-3614.2021.11.001
100. Wang X, Hou Y, Mao J, Zhang Y, Li Z, Zhao Y, et al. Western medication plus traditional Chinese medicine preparations in patients with chronic heart failure: a prospective, single-blind, randomized, controlled, and multicenter clinical trial. J Tradit Chin Med Chung Tsa Chih Ying Wen Pan. (2017) 37:756–66. doi: 10.1016/s0254-6272(18)30038-4
101. Mao J, Hou Y, Shang H, Wang H, Wang X, Zhao Y, et al. Study on the evaluation of the clinical effects of traditional Chinese medicine in heart failure by complex intervention: protocol of SECETCM-HF. Trials. (2009) 10:122–7. doi: 10.1186/1745-6215-10-12220030859
102. Li X, Zhang J, Huang J, Ma A, Yang J, Li W, et al. A multicenter, Randomized122, double-blind, parallel-group, placebo-controlled study of the effects of qili qiangxin capsules in patients with chronic heart failure. J Am Coll Cardiol. (2013) 62:1065–72. doi: 10.1016/j.jacc.2013.05.03523747768
103. Wang Y, Xiong J, Yan X, Li X, Luo Z. Meta-analysis of supplemented zhenwu decoction for treating congestive heart-failure. China J Chin Mater Medica. (2016) 41:3679–85. doi: 10.4268/cjcmm20161930
104. Gao K, Zhao H, Gao J, Wen B, Jia C, Wang Z, et al. Mechanism of Chinese medicine herbs effects on chronic heart failure based on metabolic profiling. Front Pharmacol. (2017) 8:864. doi: 10.3389/fphar.2017.0086429213243
105. Zou X, Pang G, Sheng X, Lin X, Wu H, Li S. Double blinded randomized and controlled study on treatment of chronic heart failure by nuanxin capsule [Chinese]. Chin J Integr Tradit West Med. (2011) 31:19–22.
106. Wang C, Zhang Y, Gong L. Treatment of chronic heart failure by shencao tongmai granule: a multi-centered, double-blinded, randomized, parallel controlled trial [Chinese]. Chin J Integr Tradit West Med. (2012) 32:612–5.
107. Zhang Y, Gong L, Fan L, Wang C, Liao J. A randomized clinical trail: the improvement of qi-reinforcing-blood-activating drugs on life quality in patients with chronic heart failure [Chinese]. Chin Arch Tradit Chin Med. (2012) 30:1193–5. doi: 10.13193/j.archtcm.2012.06.11.zhangy.058
108. Fu X, Lu J, Yang F, Xiao W. Treatment of chronic heart failure of Xin-shen yang deficiency. Interior retention of water-fuild syndrome by external application of zhuangshenling recipe combined with Western medicine: a clinical study [Chinese]. Chin J Integr Tradit West Med. (2014) 34:808–11. doi: 10.7661/CJIM.2014.07.0808
109. Wang X, Hu D, Dang S, Huang H, Huang C-X, Yuan M-J, et al. Effects of traditional Chinese medicine shensong yangxin capsules on heart rhythm and function in congestive heart failure patients with frequent ventricular premature complexes: a randomized, double-blind, multicenter clinical trial. Chin Med J (Engl). (2017) 130:1639–47. doi: 10.4103/0366-6999.20990628685712
110. Xian S, Yang Z, Ren P, Ye X, Ye S, Wang Q, et al. Effect of yangxinkang tablets on chronic heart failure: a multi-center randomized double-blind placebocontrolled trial. Chin J Integr Med. (2015) 21:733–42. doi: 10.1007/s11655-015-2170-x25948124
111. Mao J, Zhang J, Lam CSP, Zhu M, Yao C, Chen S, et al. Qishen yiqi dripping pills for chronic ischaemic heart failure: results of the CACT-IHF randomized clinical trial. ESC Heart Fail. (2020) 7:3881–90. doi: 10.1002/ehf2.1298032954647
112. Du K, Liu J, Tan N, Huang X, Wang J, Zhao H, et al. The effects of qishen granules for patients with chronic heart failure: a multicenter randomized double-blind placebo-controlled trial. Front Pharmacol. (2022) 13:1017734. doi: 10.3389/fphar.2022.101773436618925
113. Zhu M, Wei J, Li Y, Wang Y, Ren J, Li B, et al. Efficacy and mechanism of buyang huanwu decoction in patients with ischemic heart failure: a randomized, double-blind, placebo-controlled trial combined with proteomic analysis. Front Pharmacol. (2022) 13:831208. doi: 10.3389/fphar.2022.83120835370712
114. Li X. First Randomised Trial of Traditional Chinese Medicine for Heart Failure Shows Benefit. Amsterdam, Netherlands: ESC Congress (2023). Available online at: https://www.escardio.org/The-ESC/Press-Office/Press-releases/First-randomised-trial-of-traditional-Chinese-medicine-for-heart-failure-shows-benefit
Keywords: cardiovascular diseases, Chinese patent medicine, hypertension, cardiovascular events, death events
Citation: Lin Y, Han Y and Wang Y (2024) Traditional Chinese medicine for cardiovascular disease: efficacy and safety. Front. Cardiovasc. Med. 11:1419169. doi: 10.3389/fcvm.2024.1419169
Received: 17 April 2024; Accepted: 25 October 2024;
Published: 3 December 2024.
Edited by:
Xiaofeng Yang Temple University, United StatesReviewed by:
James David Adams, Independent Researcher, Benicia, United StatesYouhua Wang, Shanghai University of Traditional Chinese Medicine, China
Copyright: © 2024 Lin, Han and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuhong Wang, d2FuZ3loMTA3QGZveG1haWwuY29t
 Youwei Lin
Youwei Lin Yuanshan Han2
Yuanshan Han2