- 1Department of Ultrasound Medicine, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Clinical Research Center for Medical Imaging in Hubei Province, Wuhan, China
- 3Hubei Province Key Laboratory of Molecular Imaging, Wuhan, China
Background and aims: Transcatheter tricuspid valve replacement (TTVR) has recently emerged as a novel therapeutic approach for managing severe tricuspid regurgitation (TR). However, surgical tricuspid valve replacement (STVR) continues to be the predominant treatment modality. There are limited comparative data on both procedures. This study aimed to compare clinical and echocardiographic outcomes between patients who underwent mini-thoracotomy transatrial LuX-Valve TTVR and those who underwent STVR.
Methods: This study prospectively collected patients with severe TR who underwent TTVR (n = 29) or isolated STVR (n = 59) at Wuhan Union Hospital from 2019 to 2022. All TTVR patients received the LuX-Valve via a mini-thoracotomy and transatrial approach. The clinical and echocardiographic outcomes were compared at 30-day and one-year follow-ups.
Results: At baseline, patients with LuX-Valve TTVR had higher surgical risk scores and a greater proportion of right ventricular dysfunction compared with STVR. In the early postoperative period, the STVR group had a greater decrease in right ventricular function. Hospital length of stay (LOS), intensive care unit LOS, total procedure time, and tracheal intubation time were shorter in the TTVR than in the STVR group. The incidence of postoperative paravalvular leaks was higher among patients who underwent TTVR. Compared to the STVR group, the pacemaker implantation rate was lower in the TTVR group. During follow-up, the peak tricuspid valve velocity and mean gradient in the TTVR group were consistently lower than those in the STVR group. There was similar mortality between TTVR and STVR at 30-day and one-year follow-ups.
Conclusions: The mini-thoracotomy transatria LuX-Valve TTVR has a higher incidence of paravalvular leaks and a lower rate of pacemaker implantation than STVR, with similar 30-day and one-year mortality rates. In some respects, mini-thoracotomy transatrial LuX-Valve TTVR may be a feasible and safe treatment option for specific populations, or it could potentially serve as an alternative therapy to supplement conventional STVR. Further follow-up is required to assess differences in long-term clinical outcomes and valve durability.
Introduction
Clinically significant tricuspid regurgitation (TR) is common in the general population, with a prevalence of 0.55%, and its incidence increases with age, reaching a prevalence of 4% in people over 75 years of age (1). Moderate or greater TR is associated with increased mortality regardless of pulmonary artery systolic pressure, left ventricular ejection fraction, and right heart function (2, 3). Previous evidences demonstrated that untreated severe TR can decrease overall survival, with a 5-year survival rate of less than 50% (4–6). However, the treatment and management of this issue continue to be insufficient. Tricuspid valve surgery is recommended for treating severe TR based on the current guidelines (7, 8), but the timing for surgical intervention is still debated. About 86% of tricuspid valve surgeries are usually combined with left heart valve surgery, and isolated tricuspid valve surgeries are relatively rare (9). The mortality rate of isolated tricuspid valve surgery is high, due to the delayed referral, numerous complications, and the remodeling of the right ventricle (10–12).
Following the great success of transcatheter techniques in the aortic and mitral valve procedures, transcatheter tricuspid valve intervention (TTVI) has also recently developed rapidly as an option for symptomatic, inoperable, anatomically eligible patients with severe TR. A previous study showed no significant disparity in long-term survival rates between patients with isolated severe TR who underwent surgical intervention and those who solely received medical management (13). A clinical study utilizing propensity score matching showed that patients who received TTVI had lower rates of all-cause mortality and one-year rehospitalization than those who received medication (14). The 2021 ESC/EACTS Guidelines indicate that patients with severe TR who are not eligible for surgical intervention should consider transcatheter treatment at Heart Valve Centers with expertise in treating tricuspid valve disease (7). Previous studies have shown favorable results regarding the safety and efficacy of transcatheter tricuspid valve replacement (TTVR) (15–19). The ongoing advancement of TTVR technology offers an additional minimally invasive therapeutic option for individuals with severe TR. However, there are limited data comparing patients undergoing TTVR with those undergoing surgical tricuspid valve replacement (STVR) for severe TR.
Therefore, the present study aimed to assess the clinical and echocardiographic outcomes at 30 days and one year in a mini-thoracotomy transatria LuX-Valve TTVR cohort and compare these outcomes with those of conventional isolated STVR.
Patients and methods
Study population
Between January 2019 and December 2022, 88 patients underwent TTVR or isolated STVR at our institution. All the study subjects were divided into the TTVR group (n = 29) and the STVR group (n = 59).
All enrolled patients had severe, massive, or torrential TR. A multidisciplinary heart team selected each operation approach based on the comprehensive assessments of patient risk and anatomical findings. The inclusion criteria for the TTVR group were as follows: after a thorough discussion with the cardiac team, patients were deemed at high or extremely high risk for surgical procedures. Based on the comprehensive evaluations from echocardiographic and computed tomography analyses, the cardiac team determined that the patient's tricuspid valve anatomy was unsuitable for transcatheter edge-to-edge repair (TEER). This decision was informed by the presence of a coaptation gap exceeding 10 mm, significant leaflet tethering, or TR induced by a pacemaker. Conversely, the patient's tricuspid valve anatomy was deemed appropriate for LuX-Valve implantation. The exclusion criteria were as follows: patients with poor left or right ventricular function (left ventricular ejection fraction (LVEF) < 50%, tricuspid annular plane systolic excursion (TAPSE) < 10 mm or right ventricular fractional area change (RVFAC) < 20%), severe pulmonary hypertension (pulmonary artery systolic pressure [PASP] > 60 mm Hg [by right heart catheterization]), and untreated severe coronary artery disease.
The inclusion criteria for the STVR group were as follows: to minimize selection bias and obtain a clinically homogeneous cohort, the STVR group selected patients with isolated STVR. Patients with active infective endocarditis, those requiring concurrent surgery for coronary artery disease, those needing additional valve repair or replacement procedures, and those with combined congenital heart disease were excluded.
All the patients were transferred to the intensive care unit postoperatively and managed according to the approved postoperative care pathway. All the patients received anticoagulants or antiplatelet agents for a certain period after the procedure. The study was approved by the institutional ethics committee, and written informed consent was obtained from each participant.
Echocardiographic evaluation
Echocardiographic evaluations were conducted at baseline, 30 days and one year after TTVR or STVR. Transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE) were utilized for the screening phase, intraoperative guidance, and outcomes assessment. Echocardiographic parameters were obtained according to the definitional guidelines of the American Society of Echocardiography (20). Based on Simpson's method measurements of the apical 2-chamber and 4-chamber views, left ventricular (LV) volumes were determined, and the left ventricular ejection fraction (LVEF) was calculated. The biplane-modified Simpson's method was used to calculate left atrial (LA) volume. Single-plane disks were used to obtain right atrial (RA) volume. RV dimensions, RV end-systolic and RV end-diastolic areas, and RVFAC were measured in the RV-focused apical 4-chamber view. TAPSE was determined on M-mode recordings of the lateral tricuspid annulus in the RV-focused apical 4-chamber view. The PASP was calculated from the peak velocity of the TR jet by applying the Bernoulli equation and adding RA pressure according to the diameter and collapsibility of the inferior vena cava (21). RV-free wall longitudinal strain (RVFWLS) was assessed in the RV-focused apical 4-chamber view using Tom Tec Image-Arena (TOMTEC Corp., Chicago, IL, USA). The abnormal cutoff values for RV function parameters were set as TAPSE < 17 mm, RVFAC < 35%, and RVFWLS > −20% (20). RV dysfunction was defined as meeting any one of the above criteria.
The severity of TR was assessed using qualitative, semi-quantitative, and quantitative methods, as outlined in the American Society of Echocardiography guidelines (22). Furthermore, a 5-class grading scheme was employed for the grading of TR: mild, moderate, severe, massive, and torrential (23). Our study reported paravalvular leaks and total TR (including paravalvular leaks and transvalvular TR) incidences.
Data collection and follow-up
The data were collected prospectively as a part of our institutional database. They included detailed information on patients' demographics, baseline clinical characteristics, laboratory and echocardiographic findings, intraoperative variables, and postoperative outcomes. The TRI-SCORE value was calculated based on the patient's preoperative characteristics, laboratory values, and echocardiographic parameters (24). The survival data for patients were obtained through telephonic communication with either the patients themselves or their respective family members.
Operative techniques
All the transcatheter procedures were performed with the LuX-Valve TTVR system (Ningbo Jens Care Biotechnology Co., Ningbo, China), which were implanted through a minimally invasive thoracotomy and transatrial approach as previously described (15, 17) without the use of cardiopulmonary bypass (Supplementary Tables S1 and S2). Preprocedural multidetector CT and echocardiographic findings were utilized to ascertain valve size. All the procedures were conducted under general anesthesia and were facilitated by TEE and fluoroscopic guidance. TEE guided the catheter delivery, valve release, and valve position adjustment throughout the procedure.
The LuX-Valve System comprised a delivery system and a stent bioprosthesis. Specifically, the stent bioprosthesis was composed of four components: (1) a triple leaflet prosthetic valve with treated bovine pericardium; (2) a self-expanding nitinol valve stent coated with polytetrafluoroethylene fabric; (3) an interventricular septal anchoring component shaped like a bird's tongue; (4) two polytetrafluoroethylene-covered graspers. The stent bioprosthesis comed in 4 sizes (30–55 mm) and 8 skirt models for tricuspid annulus diameters between 25 and 50 mm. The diameter of the delivery system was 32 Fr, comprising three main components: the inner sheath, the outer sheath, and the core (15).
STVR procedures were conducted under extracorporeal circulation, utilizing median sternotomy and thoracotomy. The valve type was determined preoperatively, and the valve size was determined intraoperatively by calibration with a proprietary valve measuring device (Supplementary Table S1).
Study endpoints
The primary endpoints of this analysis included all-cause mortality within 30 days and at one year, as well as readmissions due to heart failure (HF) within one year. Thirty-day mortality was defined as all-cause mortality within 30 days post-procedure, regardless of discharge. Secondary endpoints encompassed adverse events at 30 days and one year, including stroke, acute kidney injury with dialysis, bleeding requiring transfusion, reoperation for bleeding, permanent pacemaker implantation, myocardial infarction, deep sternal wound infection, device migration, TV reoperation, and valve thrombosis.
Statistical analysis
Continuous variables are displayed as means ± SDs or medians (interquartile ranges) and were compared using a two-sample Student's t-test (for normally distributed data) or the Wilcoxon rank-sum test (for non-normally distributed data). Categorical variables are displayed as frequencies (percentages) and were compared using the chi-squared or Fisher exact tests. Paired t-tests were utilized to compare continuous variables before and after the operation within groups, while one-way analysis of variance was employed to compare continuous variables among the three groups. In the event of statistically significant overall results, post hoc multiple comparison tests were conducted using the Bonferroni correction. Kaplan-Meier analysis was used to analyze survival curves, and log-rank testing was used to compare them. Two-tailed tests were performed to test the hypotheses, and the significance level was set at 0.05. All statistical analyses were conducted using SPSS statistics version 26.0 (IBM, Armonk, New York).
Results
Baseline characteristics
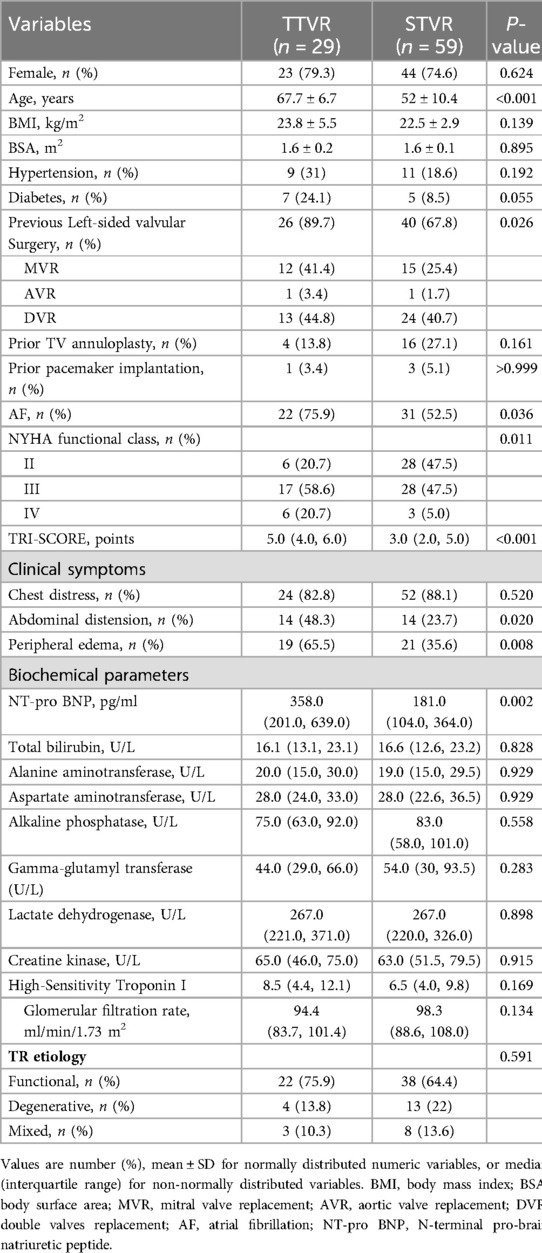
Tricuspid valve replacement (TVR) was conducted in a total of 88 patients, with 29 patients receiving TTVR and 59 patients undergoing STVR. The baseline clinical characteristics are presented in Table 1. The TTVR patients were older and had exhibited a higher prevalence of prior left-sided valvular surgery, and higher TRI-SCORE and NT-pro BNP level than the STVR patients. Likewise, the TTVR group had a higher incidence of chronic atrial fibrillation, abdominal distension, and peripheral edema compared to the STVR group. Patients in the TTVR group exhibited more severe symptoms before the procedure, with a higher proportion in New York Heart Association (NYHA) functional class III/IV (79.3% vs. 52.5%, P = 0.011). The etiology of TR was primarily functional (75.9% in the TTVR group, 64.4% in the STVR group, P = 0.591). However, there were no significant differences in sex ratio, body mass index, incidence of hypertension or diabetes, prior pacemaker implantation, and hepatic and renal function parameters between the TTVR and STVR groups.
Procedural data and in-hospital outcomes
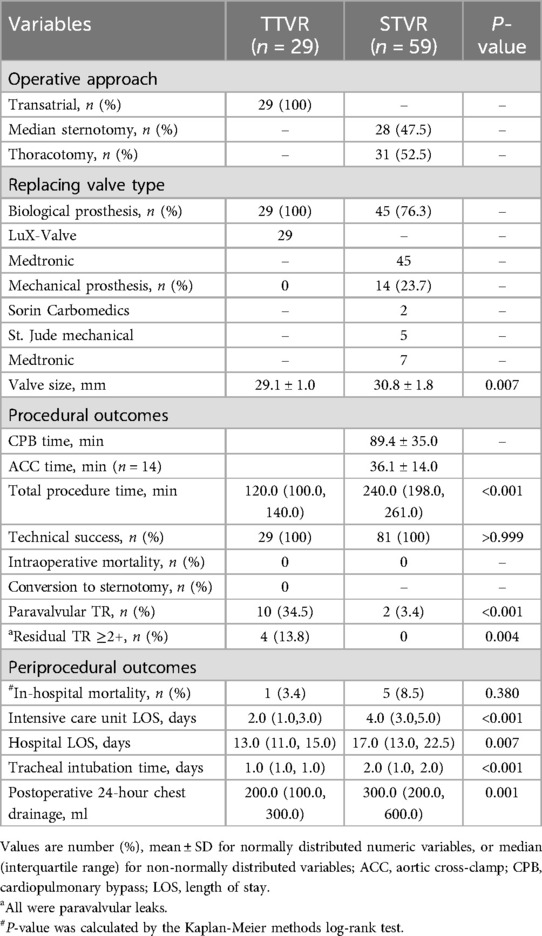
Table 2 summarises procedural characteristics and in-hospital outcomes. All the transcatheter procedures were performed with the LuX-Valve TTVR system, implanted via a minimally invasive right thoracotomy and transatrial approach. In the STVR group, 31 patients underwent thoracotomy, and 28 patients underwent median sternotomy. In the STVR group, 45 patients had a biological prosthesis implanted, while 14 patients received a mechanical prosthesis. Total procedure time was significantly shorter in the TTVR group compared to the STVR group [120.0 (100.0, 140.0) min vs. 240.0 (198.0, 261.0) min, P < 0.001].
There were no intraoperative deaths in either group. Intensive care unit LOS [2.0 (1.0, 3.0) days vs. 4.0 (3.0, 5.0) days, P < 0.001] and hospital LOS [13.0 (11.0, 15.0) days vs. 17.0 (13.0, 22.5) days, P = 0.007] were significantly shorter in the TTVR group. In addition, patients in the TTVR group had shorter tracheal intubation time and less postoperative 24-hour chest drainage than those in the STVR group. Immediate postoperative echocardiography showed residual TR ≥ 2 + in 4 patients, all of which were paravalvular leaks, and all occurred in the TTVR group. In-hospital mortality was similar between the TTVR and STVR groups (3.4% vs. 8.5%, P = 0.380).
30-day and one-year follow-up
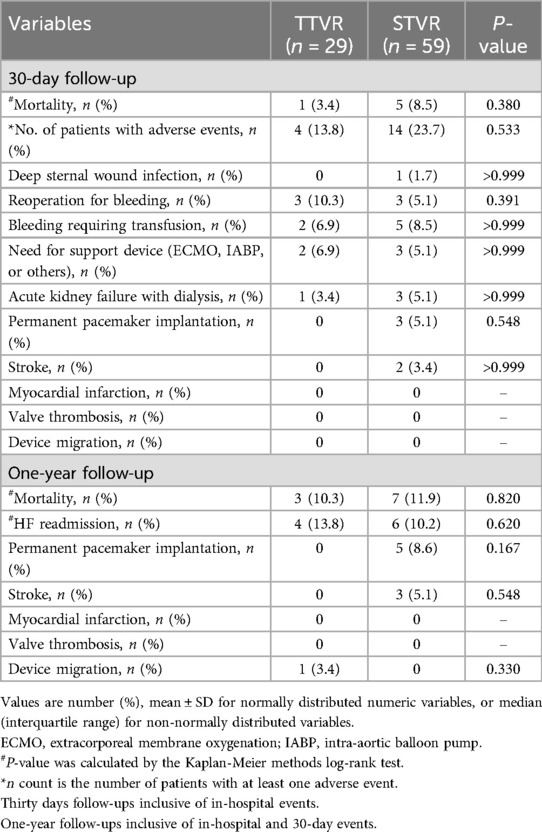
Table 3 describes the 30-day and one-year mortality and adverse events for the TTVR and STVR groups. The STVR group had a higher frequency of 30-day adverse events than the TTVR group, but this difference did not attain statistical significance (23.7% vs. 13.8%, P = 0.533). During the 30-day follow-up period, 6 patients needed reoperation for bleeding, with 3 patients (10.1%) in the transcatheter group and 3 patients (5.1%) in the surgical group (P = 0.391). Although not statistically significant, it was noteworthy that 3 patients (5.1%) in the STVR group developed complete AV block and underwent permanent pacemaker implantation at the 30-day postoperative follow-up, which did not occur in the TTVR group. Furthermore, two patients experienced strokes, and one patient developed deep sternal wound infection in the STVR group, with no such events in the TTVR group.
One patient in the TTVR group was found to have device migration at 330 days postoperatively and subsequently underwent surgical tricuspid valve replacement. The incidence of readmission due to HF was comparable between the TTVR group and the STVR group at one-year follow-up, with 4 (13.8%) and 6 (10.2%), respectively. In addition, in the STVR group, 5 patients had permanent pacemakers implanted, and 3 patients had strokes, which did not occur in the TTVR group (TTVR 0% vs. STVR 8.6%, P = 0.167; TTVR 0% vs. STVR 5.1%, P = 0.548). Among the stroke patients, two underwent bioprosthetic valve replacement, and one underwent mechanical valve replacement. Mortality at 30 days (TTVR 3.4% vs. STVR 8.5%, P = 0.380) and one year (TTVR 10.3% vs. STVR 11.9%, P = 0.820) were similar between the TTVR group and the STVR group (Table 3, Figure 1).
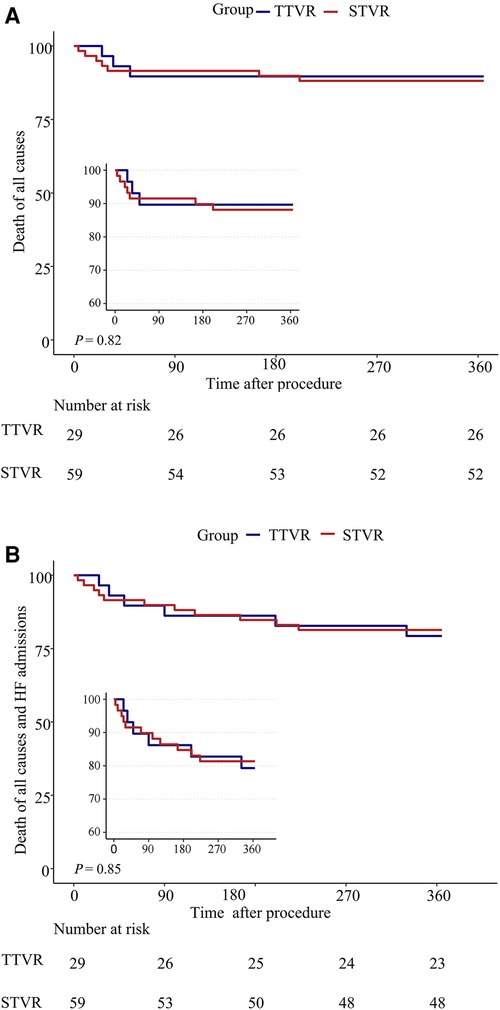
Figure 1. One-year outcomes of TTVR vs. STVR. (A) Death of all causes; (B) Death of all causes and HF readmissions.
Echocardiographic characteristics
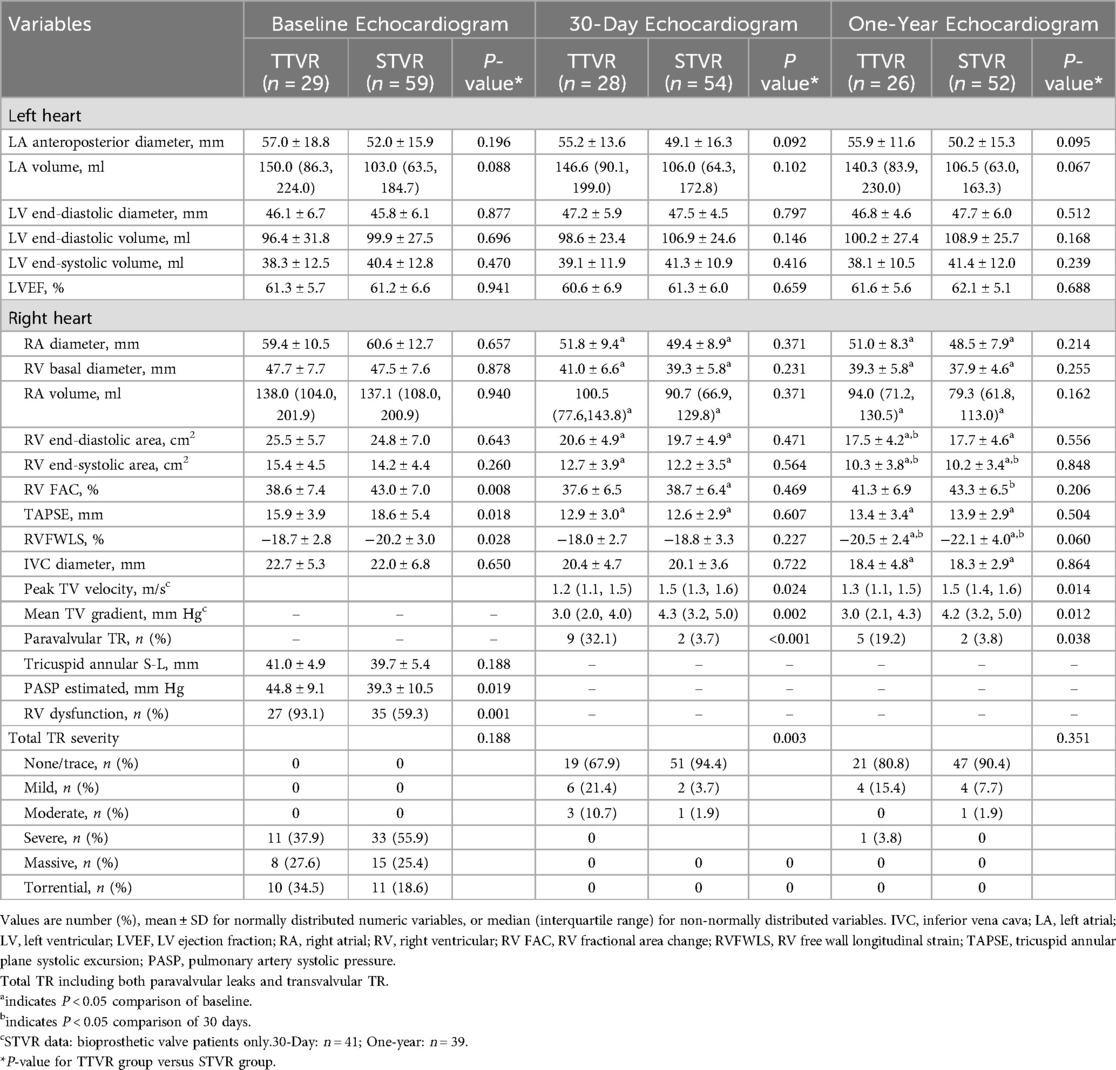
Echocardiographic data at baseline, 30 days, and one year following TTVR and STVR are shown in Table 4 and Figure 2. At baseline, the TTVR group had lower RVFAC, TAPSE and RVFWLS, a higher proportion of RV dysfunction (93.1% vs. 59.3%, P = 0.001), and higher PASP (44.8 ± 9.1 vs. 39.3 ± 10.5 mmHg, P = 0.019) as compared with the STVR group. At 30 days postoperatively, TAPSE decreased from baseline in both groups and did not significantly improve at one-year follow-up. Despite a decrease in RVFAC from baseline at 30 days, there was an improvement at the one-year follow-up. RVFWLS remained relatively stable in both groups at 30 days, with a significant improvement observed at one-year follow-up compared to the preoperative period. RV function was similar at 30 days and one-year follow-ups between the TTVR and STVR patients. Both groups demonstrated good right heart remodelling postoperatively. Additionally, patients who underwent TTVR and STVR had similar left and right heart sizes and LVEF at baseline, 30-day, and one-year follow-ups.

Figure 2. Changes in echocardiographic measures from baseline to 30 days and one year in the TTVR and STVR. (A) LVEF, left ventricular ejection fraction; (B) RAD, right atrial diameter; (C) RAV, right atrial volume; (D) RVD, right ventricular basal diameter; (E) RVEDA, right ventricular end-diastolic area; (F) RVESA, right ventricular end-systolic area; (G) TAPSE, tricuspid annular plane systolic excursion; (H) RVFAC, right ventricular fractional area change; (I) RVFWLS, RV free wall longitudinal strain. a indicates P < 0.05 comparison of baseline; b indicates P < 0.05 comparison of 30 days.
With hemodynamics, the TTVR group showed lower peak tricuspid valve velocity and mean gradient than the STVR group at 30 days and one year. The peak tricuspid valve velocity and mean gradient remained stable in both groups over the 30-day and one-year follow-up periods. At baseline, the TR severity was similar in both TTVR and STVR groups, with all the patients having severe or greater TR. The distribution of total TR severity (paravalvular leaks and transvalvular TR) in the two groups at all time points is shown in Figure 3. Immediate postoperative TEE showed that residual TR ≥ 2 + and paravalvular leaks were more prevalent in the TTVR group than in the STVR group (13.8% vs. 0% and 34.5% vs. 3.4%, P < 0.05 for both). The percentage of ≥moderate paravalvular TR was higher after TTVR than STVR. However, both groups showed a noteworthy amelioration in the severity of TR immediately postoperatively compared to baseline. In the TTVR group, four patients had moderate paravalvular leaks, and three of them died during follow-up. One patient died within 30 days post-procedure from lung infection, and the other two died from right heart failure during the follow-up of 30 days to one year. The fourth patient with moderate paravalvular leaks also underwent STVR due to device migration within one year post-procedure. The TTVR group had a higher proportion of paravalvular leaks than the STVR group at both 30-day ansd one-year follow-ups (P < 0.05) (Table 4). At the one-year follow-up, the severity of total TR (paravalvular leaks and transvalvular TR) in the TTVR group did not appear to be significantly different from that in the STVR group (Table 4, Figure 3).
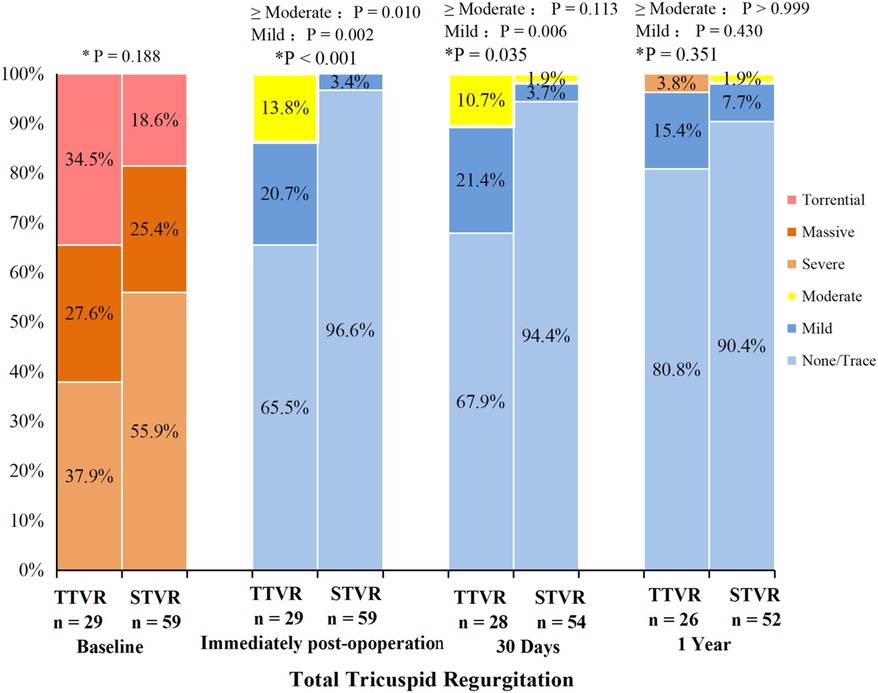
Figure 3. Total tricuspid regurgitation from baseline to 30 days and one year in the TTVR and STVR. * P value for TTVR group versus STVR group. Total TR including both paravalvular leaks and transvalvular TR.
Discussions
This study compared clinical and echocardiographic data between mini-thoracotomy transatrial LuX-Valve transcatheter and surgical tricuspid valve replacement and found the following major findings: (1) TTVR offers significantly shorter total procedure time, Intensive care unit LOS, tracheal intubation time, and hospital LOS compared to STVR; (2) compared with STVR, TTVR had a lower rate of pacemaker implantation, but a higher incidence of paravalvular TR; (3) despite higher preprocedural risk scores in the TTVR group, there was no significant difference in mortality between the TTVR and STVR groups at 30 days and one year.
Our results indicate that TTVR patients exhibited lower peak tricuspid valve velocity and mean tricuspid valve gradient when compared to STVR patients. It is probable that the differences in valve sizing and the slight ability for expansion of transcatheter valves, which is not feasible with a fixed-sized surgical sewing ring, are contributing factors. The long-term impacts of lower peak trans prosthetic velocity and mean trans prosthetic gradients on valve durability remain uncertain and will necessitate continued long-term follow-up.
The incidence and severity of paravalvular TR were higher after TTVR than after surgical intervention. The procedure outcomes showed four cases with residual TR ≥ 2+, all occurring in the TTVR group, and these were all paravalvular leaks. The TRILUMINATE trial indicated that patients with residual TR ≥ 2 + at 30 days had a threefold increased risk of experiencing death or hospitalization for HF (25). In our study, all three patients who died in the TTVR group had residual TR ≥ 2+, further demonstrating the association of this with mortality. At the one-year follow-up, the distribution of total TR (paravalvular leaks and transvalvular TR) was similar between the two groups. Moreover, the degree of TR in the TTVR group appeared to have improved compared to the 30-day follow-up. The observed improvement in the data could be attributed to deaths among patients with moderate paravalvular leaks in the TTVR group during the follow-up. Continued advancements in device technology and refined procedural strategies could potentially decrease the occurrence of paravalvular leaks.
The size of the left heart and LV function remained stable in both groups throughout the follow-up period. Both groups exhibited favorable right heart remodelling throughout the follow-up period, as evidenced by a noteworthy decrease in right heart dimensions and areas from the baseline. These findings align with prior research on transcatheter or surgical interventions (16, 26). Although the TTVR group had lower TAPSE and RV FAC at baseline compared to the STVR group, both groups manifested a reduction in these parameters after 30 days, with a more conspicuous decline in the STVR group. The baseline RV function of TTVR patients was worse due to chronic TR leading to RV myocardial injury and decreased RV function. This dysfunction became evident postoperatively following TR correction, as the preload decreased without initial compensation, resulting in a temporary decline (27). The decline observed in the surgical group may also be related to the use of cardiopulmonary bypass and pericardiotomy (28, 29). However, after one year, it seems to have improved with compensation. In patients with pre-existing severe RV dysfunction, the impact of TTVR on postoperative RV function may be less than that of STVR. The TTVR may be a favorable treatment option for patients with pre-existing severe RV dysfunction.
The present study reports a pacemaker implantation rate of approximately 8.4% (5/59) in the surgical group at the one-year follow-up, consistent with prior research (30). The EVOQUE System's one-year FIM Study revealed the rate of pacemaker implantation was 11% (3/27) (16) In a one-year FIM multicenter study of the LuX-Valve TTVR system, the rate of pacemaker implantation was 3.23% (1/31), and the implantation was considered device-independent (31). Notably, none of the patients in the transcatheter group in our study required postoperative pacemaker implantation, which could potentially be attributed to the unique design of the LuX-Valve. Previously reported implantation modalities for TTVR devices relied on radial forces between the device and the tricuspid annulus (32, 33). This type of fixation can lead to complications such as conduction block and right coronary artery impingement (34, 35). LuX-Valve utilizes a non-radial force design to reduce the incidence of these complications (36).
Despite the absence of significant differences between the two groups in bleeding requiring transfusion or reoperation for bleeding, bleeding remains a noteworthy adverse event that warrants attention. Our study indicated that two of three patients in the TTVR group who died required reoperation due to bleeding, which could be attributed to the transcatheter access approach. The transatrial approach may increase the likelihood of bleeding. Utilizing a large delivery sheath may require a transatrial approach, potentially leading to bleeding in the surgical or chest wall areas in individuals with coagulopathy. The presence of bleeding was a significant and independent prognostic indicator following the transcatheter intervention (37). As the approaches and planning of the TTVR procedure develop, it will become less invasive and more secure.
In our study, patients in the transcatheter group were significantly older and had a higher TRI-SCORE than those in the surgical group. However, it is essential to note that mortality from all causes at 30 days and one year was similar between the groups. Prior studies on TTVR using the GATE, EVOQUE, and LuX-Valve systems showed mortality ranging from 7% to 17.5% during the 6-month to one-year follow-up period (12–14, 29). The mortality for isolated STVR was between 10.9% and 12% and did not improve over time or with increasing surgical volume, which was consistent with the findings of our study (10, 11, 30). This finding provides additional evidence regarding the safety and effectiveness of TTVR. However, the potential applicability of TTVR to populations beyond high-risk groups and the possibility of expanding its indications necessitate further observation and clinical trials.
Currently, there have been only reports on the short-term efficacy of TTVR (16–18). However, there is still a lack of long-term data on the durability of prostheses in TTVR. Two-year observations of the EVOQUE system showed a 71% survival rate, accompanied by consistent symptom improvement and sustained efficacy in reducing TR (38). Furthermore, the LuX-Valve TTVR system demonstrated promising prospects for sustained efficacy and safety over a mid-to-long-term duration (39). However, these findings were based on a four-year follow-up involving a single patient. The data from our study suggest that TTVR may offer short-term efficacy equivalent to STVR, and may even be superior in some respects. Nevertheless, further longer-term follow-up studies are needed to assess the advantages and disadvantages of both approaches.
Study limitations
Our study was a non-randomized, single-center study, which may introduce selection bias and limit generalizability. The small sample size also limits the power of our analyses. More reliable estimates in larger patient populations are needed. Several TTVR valves are available on the market, each utilizing different materials, designs, and implantation methods, and therefore, each carries various possible complications that may affect the treatment outcomes. Further technological advances (e.g., device iterations, smaller sheaths, transfemoral or transjugular approaches) may favorably influence the results of TTVR. The LuX-Valve transcatheter implantation via right atrial approach through minimally invasive thoracotomy carries a higher risk compared to the less invasive transfemoral/transjugular catheterization approach. It is noted that the LuX-valve Plus (transjugular approach), the next generation of LuX-Valve used in this trial, has entered clinical trials. Furthermore, prospective studies with larger cohorts are necessary to validate the long-term safety and efficacy of TTVR.
Conclusions
Although the mini-thoracotomy transatrial LuX-Valve TTVR group has a higher surgical risk score than the STVR group, there is no significant difference in mortality between the two groups during the 30-day and one-year follow-ups. Compared to STVR, the LuX-Valve TTVR group has a higher incidence of paravalvular leaks and a lower pacemaker implantation rate. In some respects, the LuX-Valve TTVR may be a feasible and safe treatment option for specific population or it could potentially serve as an alternative therapy to supplement conventional STVR. However, long-term clinical outcomes and valve durability still require further follow-up studies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Wuhan Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (Ethics No. 0086). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LH: Writing – review & editing, Data curation, Resources, Software, Writing – original draft. ZS: Data curation, Funding acquisition, Resources, Writing – review & editing, Formal Analysis. YC: Resources, Writing – review & editing. YX: Data curation, Writing – review & editing. ZZ: Resources, Writing – review & editing. WS: Resources, Writing – review & editing. HL: Resources, Writing – review & editing. LF: Resources, Writing – review & editing. LH: Resources, Writing – review & editing. LZ: Funding acquisition, Writing – review & editing. YY: Resources, Writing – review & editing. JW: Resources, Writing – review & editing. QL: Resources, Writing – review & editing. YL: Resources, Writing – review & editing, Methodology. MX: Conceptualization, Funding acquisition, Methodology, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 81701716; 82230066; 81727805; 81922033) and the National Natural Science Foundation of Hubei (Grant No. 2018CFB568).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1417757/full#supplementary-material
Abbreviations
TTVR, transcatheter tricuspid valve replacement; STVR, surgical tricuspid valve replacement; TR, tricuspid regurgitation; LOS, length of stay; LVEF, left ventricular ejection fraction; RAD, right atrial diameter; RAV, right atrial volume; RVD, right ventricular basal diameter; RVEDA, right ventricular end-diastolic area; RVESA, right ventricular end-systolic area; TAPSE, tricuspid annular plane systolic excursion; RVFAC, right ventricular fractional area change; RVFWLS, right ventricular free wall longitudinal strain.
References
1. Topilsky Y, Maltais S, Medina Inojosa J, Oguz D, Michelena H, Maalouf J, et al. Burden of tricuspid regurgitation in patients diagnosed in the community setting. JACC Cardiovasc Imaging. (2019) 12(3):433–42. doi: 10.1016/j.jcmg.2018.06.014
2. Wang N, Fulcher J, Abeysuriya N, McGrady M, Wilcox I, Celermajer D, et al. Tricuspid regurgitation is associated with increased mortality independent of pulmonary pressures and right heart failure: a systematic review and meta-analysis. Eur Heart J. (2019) 40(5):476–84. doi: 10.1093/eurheartj/ehy641
3. Bar N, Schwartz LA, Biner S, Aviram G, Ingbir M, Nachmany I, et al. Clinical outcome of isolated tricuspid regurgitation in patients with preserved left ventricular ejection fraction and pulmonary hypertension. J Am Soc Echocardiogr. (2018) 31(1):34–41. doi: 10.1016/j.echo.2017.09.010
4. Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol. (2004) 43(3):405–9. doi: 10.1016/j.jacc.2003.09.036
5. Chorin E, Rozenbaum Z, Topilsky Y, Konigstein M, Ziv-Baran T, Richert E, et al. Tricuspid regurgitation and long-term clinical outcomes. Eur Heart J Cardiovasc Imaging. (2020) 21(2):157–65. doi: 10.1093/ehjci/jez216
6. Benfari G, Antoine C, Miller WL, Thapa P, Topilsky Y, Rossi A, et al. Excess mortality associated with functional tricuspid regurgitation complicating heart failure with reduced ejection fraction. Circulation. (2019) 140(3):196–206. doi: 10.1161/CIRCULATIONAHA.118.038946
7. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease: developed by the task force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Rev Esp Cardiol (Engl Ed). (2022) 75(6):524. doi: 10.1016/j.rec.2022.05.006
8. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. (2021) 143(5):e72–e227. doi: 10.1161/CIR.0000000000000923
9. Kilic A, Saha-Chaudhuri P, Rankin JS, Conte JV. Trends and outcomes of tricuspid valve surgery in North America: an analysis of more than 50,000 patients from the Society of Thoracic Surgeons database. Ann Thorac Surg. (2013) 96(5):1546–52. doi: 10.1016/j.athoracsur.2013.06.031
10. Alqahtani F, Berzingi CO, Aljohani S, Hijazi M, Al-Hallak A, Alkhouli M. Contemporary trends in the use and outcomes of surgical treatment of tricuspid regurgitation. J Am Heart Assoc. (2017) 6(12):e007597. doi: 10.1161/JAHA.117.007597
11. Zack CJ, Fender EA, Chandrashekar P, Reddy YNV, Bennett CE, Stulak JM, et al. National trends and outcomes in isolated tricuspid valve surgery. J Am Coll Cardiol. (2017) 70(24):2953–60. doi: 10.1016/j.jacc.2017.10.039
12. Dreyfus J, Flagiello M, Bazire B, Eggenspieler F, Viau F, Riant E, et al. Isolated tricuspid valve surgery: impact of aetiology and clinical presentation on outcomes. Eur Heart J. (2020) 41(45):4304–17. doi: 10.1093/eurheartj/ehaa643
13. Axtell AL, Bhambhani V, Moonsamy P, Healy EW, Picard MH, Sundt TM 3rd, et al. Surgery does not improve survival in patients with isolated severe tricuspid regurgitation. J Am Coll Cardiol. (2019) 74(6):715–25. doi: 10.1016/j.jacc.2019.04.028
14. Taramasso M, Benfari G, van der Bijl P, Alessandrini H, Attinger-Toller A, Biasco L, et al. Transcatheter versus medical treatment of patients with symptomatic severe tricuspid regurgitation. J Am Coll Cardiol. (2019) 74(24):2998–3008. doi: 10.1016/j.jacc.2019.09.028
15. Sun Z, Li H, Zhang Z, Li Y, Zhang L, Xie Y, et al. Twelve-month outcomes of the LuX-valve for transcatheter treatment of severe tricuspid regurgitation. Eurointervention. (2021) 17(10):818–26. doi: 10.4244/EIJ-D-21-00095
16. Webb JG, Chuang AM, Meier D, von Bardeleben RS, Kodali SK, Smith RL, et al. Transcatheter tricuspid valve replacement with the evoque system: 1-year outcomes of a multicenter, first-in-human experience. JACC Cardiovasc Interv. (2022) 15(5):481–91. doi: 10.1016/j.jcin.2022.01.280
17. Lu FL, An Z, Ma Y, Song ZG, Cai CL, Li BL, et al. Transcatheter tricuspid valve replacement in patients with severe tricuspid regurgitation. Heart. (2021) 107(20):1664–70. doi: 10.1136/heartjnl-2020-318199
18. Hahn RT, Kodali S, Fam N, Bapat V, Bartus K, Rodes-Cabau J, et al. Early multinational experience of transcatheter tricuspid valve replacement for treating severe tricuspid regurgitation. JACC Cardiovasc Interv. (2020) 13(21):2482–93. doi: 10.1016/j.jcin.2020.07.008
19. Sondergaard L, Moller JE, De Backer O, Moller-Sorensen PH, Cheng Y, Rossing K, et al. First-in-human implantation of a new transcatheter tricuspid valve replacement system. JACC Case Rep. (2023) 14:101841. doi: 10.1016/j.jaccas.2023.101841
20. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. (2015) 16(3):233–70. doi: 10.1093/ehjci/jev014
21. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. (2010) 23(7):685–713. doi: 10.1016/j.echo.2010.05.010
22. Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. (2017) 30(4):303–71. doi: 10.1016/j.echo.2017.01.007
23. Hahn RT, Zamorano JL. The need for a new tricuspid regurgitation grading scheme. Eur Heart J Cardiovasc Imaging. (2017) 18(12):1342–3. doi: 10.1093/ehjci/jex139
24. Dreyfus J, Audureau E, Bohbot Y, Coisne A, Lavie-Badie Y, Bouchery M, et al. TRI-SCORE: a new risk score for in-hospital mortality prediction after isolated tricuspid valve surgery. Eur Heart J. (2022) 43(7):654–62. doi: 10.1093/eurheartj/ehab679
25. Lurz P, Stephan von Bardeleben R, Weber M, Sitges M, Sorajja P, Hausleiter J, et al. Transcatheter edge-to-edge repair for treatment of tricuspid regurgitation. J Am Coll Cardiol. (2021) 77(3):229–39. doi: 10.1016/j.jacc.2020.11.038
26. Mukherjee D, Nader S, Olano A, Garcia MJ, Griffin BP. Improvement in right ventricular systolic function after surgical correction of isolated tricuspid regurgitation. J Am Soc Echocardiogr. (2000) 13(7):650–4. doi: 10.1067/mje.2000.103958
27. Orban M, Wolff S, Braun D, Stolz L, Higuchi S, Stark K, et al. Right ventricular function in transcatheter edge-to-edge tricuspid valve repair. JACC Cardiovasc Imaging. (2021) 14(12):2477–9. doi: 10.1016/j.jcmg.2021.06.026
28. Zanobini M, Saccocci M, Tamborini G, Veglia F, Di Minno A, Poggio P, et al. Postoperative echocardiographic reduction of right ventricular function: is pericardial opening modality the main culprit? Biomed Res Int. (2017) (2017):4808757. doi: 10.1155/2017/4808757
29. Singh A, Huang X, Dai L, Wyler D, Alfirevic A, Blackstone EH, et al. Right ventricular function is reduced during cardiac surgery independent of procedural characteristics, reoperative status, or pericardiotomy. J Thorac Cardiovasc Surg. (2020) 159(4):1430–8. doi: 10.1016/j.jtcvs.2019.04.035
30. Scotti A, Sturla M, Granada JF, Kodali SK, Coisne A, Mangieri A, et al. Outcomes of isolated tricuspid valve replacement: a systematic review and meta-analysis of 5,316 patients from 35 studies. Eurointervention. (2022) 18(10):840–51. doi: 10.4244/EIJ-D-22-00442
31. Taramasso M. Transcatheter tricuspid valve replacement with the LuX-Valve system 1-year results of a multicenter, TTVR experience. Pcr London valves (2022).
32. Chen J, Abudupataer M, Hu K, Maimaiti A, Lu S, Wei L, et al. Risk factors associated with perioperative morbidity and mortality following isolated tricuspid valve replacement. J Surg Res. (2018) 221:224–31. doi: 10.1016/j.jss.2017.08.014
33. Navia JL, Kapadia S, Elgharably H, Maluenda G, Bartus K, Baeza C, et al. Transcatheter tricuspid valve implantation of navigate bioprosthesis in a preclinical model. JACC Basic Transl Sci. (2018) 3(1):67–79. doi: 10.1016/j.jacbts.2017.08.003
34. Fam NP, von Bardeleben RS, Hensey M, Kodali SK, Smith RL, Hausleiter J, et al. Transfemoral transcatheter tricuspid valve replacement with the EVOQUE system: a multicenter, observational, first-in-human experience. JACC Cardiovasc Interv. (2021) 14(5):501–11. doi: 10.1016/j.jcin.2020.11.045
35. Taramasso M, Alessandrini H, Latib A, Asami M, Attinger-Toller A, Biasco L, et al. Outcomes after current transcatheter tricuspid valve intervention: mid-term results from the international trivalve registry. JACC Cardiovasc Interv. (2019) 12(2):155–65. doi: 10.1016/j.jcin.2018.10.022
36. Asmarats L, Puri R, Latib A, Navia JL, Rodes-Cabau J. Transcatheter tricuspid valve interventions:landscape, challenges, and future directions. J Am Coll Cardiol. (2018) 71(25):2935–56. doi: 10.1016/j.jacc.2018.04.031
37. Piccolo R, Pilgrim T, Franzone A, Valgimigli M, Haynes A, Asami M, et al. Frequency, timing, and impact of access-site and non-access-site bleeding on mortality among patients undergoing transcatheter aortic valve replacement. JACC Cardiovasc Interv. (2017) 10(14):1436–46. doi: 10.1016/j.jcin.2017.04.034
38. Stolz L, Weckbach LT, Hahn RT, Chatfield AG, Fam NP, von Bardeleben RS, et al. 2-year outcomes following transcatheter tricuspid valve replacement using the EVOQUE system. J Am Coll Cardiol. (2023) 81(24):2374–6. doi: 10.1016/j.jacc.2023.04.014
Keywords: transcatheter tricuspid valve replacement, surgical tricuspid valve replacement, tricuspid regurgitation, paravalvular leaks, tricuspid valve
Citation: Huang L, Sun Z, Cai Y, Xie Y, Zhang Z, Sun W, Li H, Fang L, He L, Zhang L, Yang Y, Wang J, Lv Q, Li Y and Xie M (2024) Comparison of clinical and echocardiographic outcomes between mini-thoracotomy transatrial LuX-Valve transcatheter and surgical tricuspid valve replacement. Front. Cardiovasc. Med. 11:1417757. doi: 10.3389/fcvm.2024.1417757
Received: 15 April 2024; Accepted: 24 July 2024;
Published: 5 August 2024.
Edited by:
Francesco Cannata, Monzino Cardiology Center (IRCCS), ItalyReviewed by:
Sun Kyun Ro, Hanyang University, Republic of KoreaJeffrey Khoo, University Hospitals of Leicester NHS Trust, United Kingdom
© 2024 Huang, Sun, Cai, Xie, Zhang, Sun, Li, Fang, He, Zhang, Yang, Wang, Lv, Li and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenxing Sun, c3VuemhlbnhpbmdAaHVzdC5lZHUuY24=; Yuman Li, bGl5bUBodXN0LmVkdS5jbg==; Mingxing Xie, eGllbXhAaHVzdC5lZHUuY24=
†These authors share first authorship
 Lei Huang1,2,3,†
Lei Huang1,2,3,† Zhenxing Sun
Zhenxing Sun Li Zhang
Li Zhang Yali Yang
Yali Yang Jing Wang
Jing Wang Qing Lv
Qing Lv Yuman Li
Yuman Li Mingxing Xie
Mingxing Xie