- 1Cardiothoracic Surgery, Mayo Clinic, Jacksonville, FL, United States
- 2Mayo Clinic Libraries, Mayo Clinic, Jacksonville, FL, United States
- 3Heart and Lung Transplant National Recovery Program, United Network for Organ Sharing (UNOS), Jacksonville, FL, United States
- 4Division of Vascular Surgery, Oregon Health and Sciences University, Portland, OR, United States
Background: The number of patients living with left ventricular assist devices (LVADs) has gradually increased in the past decade. Non-cardiac surgery (NCS) in patients with LVAD poses a unique situation with its inherent challenges.
Aim: We conducted a comprehensive review to investigate the perioperative complications and mortality associated with emergent or elective NCS in patients with LVAD.
Method: A comprehensive literature search for any papers referring to continuous LVAD patients with NCS. All publications with at least five durable LVAD patients who had NCS were eligible for inclusion.
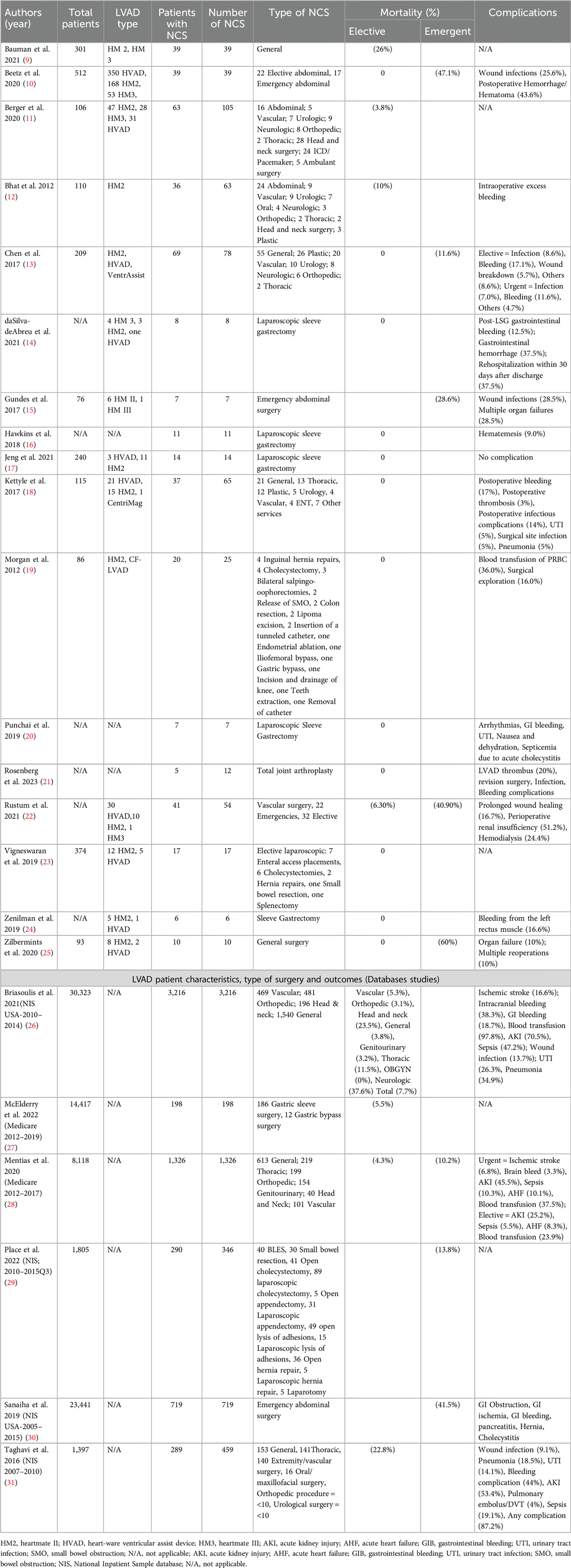
Result: Twenty articles matching our criteria were found and included in our study. This systematic review included 6,476 LVAD patients who underwent 6,824 NCS. There were 5–3,216 LVAD patients with NCS in each study. The median age was between 39 and 65 years, and most of the patients (78.8%) were male. Thirty-day postoperative mortality ranged from 0% to 60%. Eight studies reported no death within the 30 days of the operation. Common complications include gastrointestinal (GI) bleeding, intracranial bleeding, infection, acute kidney injury (AKI), urinary tract infection (UTI), stroke, sepsis, pneumonia, and VAD exchange. Emergent abdominal surgery had the highest (up to 60%) mortality rate, and vascular and neurological operations had the highest complication rates. Due to the diverse range of patients in each publication and the combination of outcomes presented in various publications, a meta-analysis was not conducted.
Conclusion: In LVAD patients, noncardiac surgery may be performed effectively and safely. LVAD patients who undergo non-cardiac surgery may require more transfusions due to their complex coagulopathies. However, perioperative management of LVAD patients undergoing emergent NCS should be optimized to reduce mortality.
Systematic Review Registration: https://osf.io/fetsb/.
Introduction
Heart failure due to various etiologies (ischemic and non-ischemic heart diseases) affects about 64.34 million people worldwide (8.52 per 1,000) (1) and 6.5 million Americans (2), with an incidence rate of 915,000 cases every year (3). End-stage heart failure is associated with a high mortality rate, and the treatment of choice for heart failure is heart transplantation. However, due to the severe shortage of organ donors, the criteria for selecting heart transplant recipients remain stringent. For patients with heart failure who are not suitable candidates for a heart transplant due to associated co-morbidities, LVAD can reduce mortality and improve 2-year survival significantly compared to maximal guideline-directed medical treatment (GDMT), whose progress has plateaued in recent years (4–6). Due to improvements in survival rates—over 70% for two years and 58.4% for five years with new generation LVADs—and the increasing number of patients undergoing LVAD implantation each year, the population of patients surviving with LVADs is growing rapidly (7). As a result, NCSs in LVAD patients become more common; therefore, clinicians caring for LVAD patients need to be aware of the perioperative management, potential complications, and outcomes of NCSs in these patients.
We limited our systematic review to continuous-flow LVADs since pulsatile LVADs are rarely implanted in contemporary clinical practice.
Methods
Studies were identified by a librarian developing and running searches in the MEDLINE (1946-Present), Embase (1974-Present), Cochrane Central Register of Controlled Trials (1991-Present), and Cochrane Database of Systematic Review (2005-Present) [all via the Ovid interface], Science Citation Index Expanded (1975-Present) and Emerging Sources Citation Index (2019-Present) [via the Web of Science interface], Scopus [via the Elsevier website interface] (1823-Present), Epistemonikos and the World Health Organizations (WHO) Global Index Medicus databases. Grey literature resources were included in the first search. There were no limits to language or publication date. Search filters to remove animal studies and case reports were used. The search strategies were created using a combination of keywords and standardized index terms. Search terms included MeSH, Embase/ Emtree terms, as well as keywords such as left ventricular assist device, LVAD, and non-cardiac surgical terms, with a particular focus on neurosurgery, abdominal surgery, and metabolic surgery terms. All databases, registers, and grey literature resources were searched on April 13th, 2022, and recently updated to May 31st, 2024. and all papers published until December 31, 2023, were reviewed. Forward citation searching was employed for both searches. A draft search strategy was peer reviewed by a second information professional, John Reynolds. The full search strategies are available here: https://osf.io/fetsb.
All studies with five or more LVAD patients undergoing NCS were eligible for inclusion. Exclusions included perioperative procedures and complications related to index implant hospitalization. Studies containing LVAD-related complications were excluded. Articles were included if more than 80% of the patients had continuous LVAD. The primary endpoint was 30-day postoperative or hospital mortality. Postoperative morbidity and complications, specifically bleeding and device malfunction, were secondary endpoints. All the abstracts without full text were excluded.
Results
A systematic review of the databases yielded 2,124 records, 599 of which were duplicates. Initial manual title screening excluded 1,488 off-topic citations. The remaining 37 records were assessed for eligibility, both screener groups identified 37 as meeting the inclusion criteria. Of those, 14 were excluded for the following reasons: minor surgical procedures (5), noncontinuous LVAD (3), series with less than 5 cases (3), systematic review (2), and treatment of mediastinitis (1). (Figure 1).
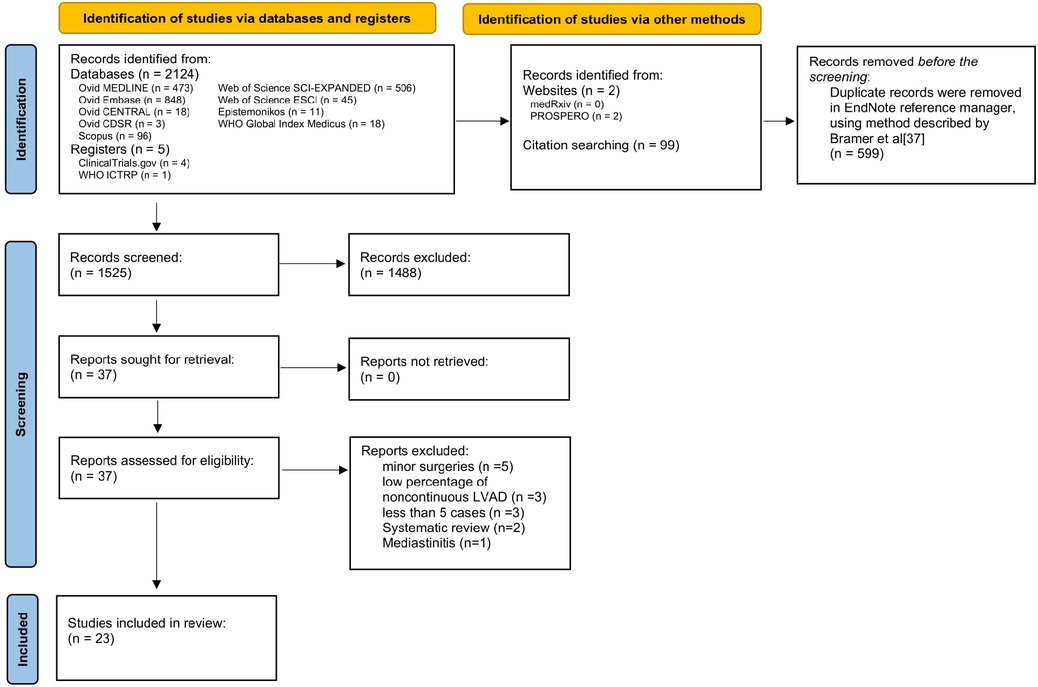
Figure 1. PRISMA flow diagram for literature searches (8).
In the end, 23 papers, with 6,476 LVAD patients who underwent 6,824 NCS, were included in this review. Table 1 summarize the results for restropective studies and large database analyses, repectively. The number of patients in each study ranged from 6 to 3,216 patients. The median ages of the patients in each article were between 39 and 65 years. Seven studies did not distinguish between the bridge to transplant or destination therapy in patients. Thirty-day postoperative mortality ranged from 0% to 60%. Eight studies reported no death within the 30 days of the operation.
Due to the wide heterogenicity in reports of the outcomes of the studies, we decided not to perform a meta-analysis or data synthesis. LVAD types, NCS, and perioperative anticoagulation management are among the variables reported with significant inconsistency, which could have reduced the validity of the formal analysis.
Because non-cardiac surgical procedures in LVAD comprise of a heterogenous group of patients, we categorized these procedures into general and abdominal surgeries, bariatric surgery, vascular surgery, and neurosurgery.
General surgical operations in LVAD patients range from superficial procedures on the skin and soft tissues to intra-abdominal procedures via laparoscopic or open operations (10, 14–17, 20, 23–25).
We found 21 out of 23 publications that reported the results in 4,151 general surgical procedures, with 244 gastric bypass operations that were discussed exclusively in 6 publications; therefore, we will discuss the latter operations separately in the bariatric surgery section. Some citations did not report data regarding different types of surgery, while some publications reported the mortality and complication in different kinds of operations. The mortality rates vary from 0% to 60% for bariatric operations (14, 16, 17, 20, 24) and emergency abdominal surgeries (25), respectively. More details about mortality rates and complications are available in Tables 1, 2. Next, we will discuss the outcomes of different non-cardiac operations in LVAD patients.
Discussions
General and abdominal surgeries
A study by Beets et al. focused on the outcome of abdominal surgery in LVAD patients. In their study, a total of 604 patients with LVAD from a single center were included, out of which 39 (6.5%) patients underwent abdominal surgery. A total of 22 patients (56.4%) underwent elective abdominal surgeries, including abdominal wall hernia repairs, partial colectomies, and cholecystectomies. There was no operative or postoperative death in patients undergoing elective abdominal surgery at a median follow-up of 23 months (1–78 months). A total of 17 patients (43.6%) underwent emergency surgery. The most common indications for emergency surgery were intestinal ischemia or perforation. In the first 30 days after abdominal surgery, eight patients (47.1%) died from sepsis and subsequent multiple organ failure, resulting in a dismal median survival rate of one month. Patients who underwent abdominal surgery tended to have significantly lower rates of a subsequent heart transplant and had a higher rate of VAD exchange due to thromboses or infections before or after the abdominal surgery (10). Another study, by Gundes et al., consists of 76 patients with LVAD implantation, seven patients underwent emergent laparotomy at 79.1 ± 79.4 days after LVAD implantation. Indication for emergent laparotomy in their series was abdominal compartment syndrome related to retroperitoneal hematoma (2), ileus (1), iatrogenic splenic injury (1), splenic abscess (1), acute abdomen (1), and pelvic abscess related to stump leakage in a patient with rectal cancer operation (1). Perioperative mortality was (28.6%) (15). The drivelines were removed from the lower right quadrant in 6 patients and the left lower quadrant in 1 patient.
For laparoscopic surgery, Vigneswaran et al. (10), reported the outcomes 17 patients who underwent a elective laparoscopic procedures: enteral access placement (7), cholecystectomy (6), hernia repair (2), small bowel resection (1) and splenectomy (1), there was no operative or postoperative mortality, LVAD related complication, or need for conversion to open or reoperation. No patient had thrombotic events, bleeding, or complications necessitating withholding anticoagulation. However, 5 of the 17 patients required intraoperative blood transfusion. These authors stated that they used the open periumbilical Hasson technique for port placement.
Place et al. conducted a study utilizing data from the National Inpatient Sample covering the period from 2010 to the third quarter of 2015. Their findings indicated a mortality rate of 13.8% for abdominal surgeries in LVAD patients (29).
Results from these studies reiterate that patients with LVAD can safely undergo elective abdominal procedures (23). However, every case needs to be well-planned by a multidisciplinary team, including general surgeons, heart failure cardiologists, cardiac surgeons, hematologists, anesthetists, and intensivists. Although the presence of LVAD does not preclude the patient from undergoing laparoscopic abdominal operations, a surgeon needs to be cognizant of bleeding and thrombotic risks, port placement sites, and perioperative management related to LVAD and anticoagulation.
Bariatric surgery
Many patients with heart failure suffer from morbid obesity, a contraindication for a heart transplant due to poor outcomes. It is advised that patients with a BMI above 35 kg/m2 receive counseling to decrease their BMI to below 35 kg/m2 before being listed for transplantation (32). However, limited physical activity due to heart failure makes it nearly impossible for the patient with heart failure to lose weight. Therefore, bariatric surgery has become a bridge to heart transplants in these patients. In addition, bariatric surgery has been shown to lower BMI, reduce heart failure symptoms, and enhance the overall quality of life (33).
Furthermore, with the widespread use of LVADs in the treatment of HF in patients with obesity, evidence of the efficacy of bariatric surgery in patients with severe HF and LVAD is growing (34, 35). In this systematic review, 46 patients (26 male and 20 female) underwent bariatric surgery in retrospective studies (14, 16, 17, 20, 24), and one study was conducted using the Medicare database. In retrospective studies, laparoscopic sleeve gastrectomy was the procedure that was performed the most. The procedure was associated with an excellent outcome with no perioperative or one-year mortality. There were only two (4.35%) deaths at a follow-up of two years. The most common complication in these patients was gastrointestinal bleeding. Other less common complications were urinary tract infections and arrhythmia due to hypokalemia. One patient required readmission within 30 days due to hematemesis.
McElderry et al. conducted a study using data from the Medicare database spanning from 2012 to 2019. The study focused on LVAD patients who underwent either gastric sleeve surgery or gastric bypass surgery. They reported a mortality rate of (5.5%) for these procedures (27).
The results of these studies show that bariatric surgery is a safe and viable option for morbidly obese patients with LVAD to lose weight. Hopefully, with new weight-loss medications becoming more widely available, the need for bariatric surgery in these patients becomes less (36).
Urgent vs. elective abdominal surgeries
There is a significant difference in outcomes in urgent vs. elective abdominal surgeries. As expected, elective surgeries have lower mortality and infection rates in LVAD patients (25). Chen et al., reported that the risk of infection and bleeding in elective operations is not higher than in urgent surgeries. However, emergent surgeries have a higher infection rate and higher mortality (13). There were no statistically significant differences among the groups in terms of postoperative ICU length of stay (LOS), postoperative length of time on the ventilator, hospital LOS, or postoperative thrombotic or bleeding complications (18). All groups, whether elective, urgent, or emergent, have higher early and late mortality rates comparing LVAD patients without NCS (28).
Sanaiha et al. showed that cachexia could increase the rates of emergency general surgeries in LVAD and heart transplant patients. LVAD patients with emergency surgery have higher mortality, hospitalization costs, and lengths of stay after adjusting for their comorbidities than non-emergency patients. In subgroup analysis, these authors reported that patients who required small bowel resection had the highest mortality rates, although wide confidence intervals limit the strength of the results (30).
Vascular surgery
There were 9 out of 20 articles that reported on outcome of 805 vascular procedures. Some reported specific results on vascular surgery, but others reported the outcomes along with other general surgeries. Two articles reported their experience in vascular surgery separately (22, 26).
Vascular surgery in LVAD patients carries a mortality rate of 5.3% (in hospital) (26) to 26.8% (30 days mortality) (22), and emergent procedures have higher mortality rates than elective ones (40.9% vs. 5.3%). Vascular surgeries in LVAD patients have a high ischemic stroke and gastrointestinal bleeding rates, 10.7% and 12.6%, respectively (22, 26).
Neurosurgery
Neurosurgical procedures in LVAD patients have the highest complication rate. LVAD patients required a craniotomy for an intracranial hemorrhage carry a 25%–50% mortality (11–13, 26). Most patients required blood product transfusions due to excessive blood loss during the procedure (12, 26). About 30%–40% of LVAD patients with neurosurgery have other complications, including acute kidney injury or sepsis, while 10% had a stroke after the neurosurgery. (26) Among non-cardiac surgeries, neurosurgery has the highest mortality rate in LAVD patients (12).
Conclusion
Patients with continuous-flow LVADs who had noncardiac surgeries were reviewed in this systematic study. Noncardiac surgeries can be performed effectively and safely on LVAD patients. Morbidity and mortality were caused by patient's conditions, complications such as bleeding, stroke, acute kidney injury, infections, and many others. The care for these complex patients requires multiple expertises and many resources. A multidisciplinary approach at tertiary centers with healthcare providers who have expertise in LVADs can significantly improve postoperative outcomes of these patients.
Our protocol for managing non-cardiac surgery (NCS) in patients with left ventricular assist devices (LVADs) is tailored based on the type of surgery, the specific LVAD model, and the patient's condition. Generally, the following steps are undertaken:
1. Preoperative:
Consultation with heart failure cardiologists, cardiac surgeons with expertise in LVAD, cardiac anesthesiologists, and potentially a hematologist.
Decisions regarding the reversal and bridging of anticoagulation therapy depend on whether the surgery is urgent or elective.
2. Intraoperative:
Implementation of hemodynamic monitoring
Meticulous surgical techniques to achieve hemostasis
Administration of blood products to help with hemostasis if needed.
3. Postoperative:
Continuation of hemodynamic monitoring as needed.
Transitioning the patient back to oral anticoagulation therapy.
Close monitoring for wound infection, stroke, and LVAD function.
By following this protocol, we aim to optimize patient outcomes and ensure the safety and efficacy of NCS in LVAD patients.
Limitations
Our study faced several limitations. All of the studies were retrospective in natures, and due to the data heterogeneity, we could not perform a meta-analysis. Additionally, the complications and mortality rates were not specified for the type of LVAD, preventing us from comparing the different LVAD types.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
EA-F: Writing – original draft. PG: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. JY: Writing – original draft. TB: Data curation, Methodology, Writing – review & editing. SJ: Writing – review & editing. IW: Resources, Validation, Writing – review & editing. SP: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to thank John Reynolds, MLIS, AHIP (https://orcid.org/0000-0001-6744-3517) from the Calder Memorial Library at the University of Miami Miller School of Medicine in Miami, FL, for peer review of the Medline database search strategy.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lippi G, Sanchis-Gomar F. Global epidemiology and future trends of heart failure. AME Med J. (2020) 5. doi: 10.21037/amj.2020.03.03
2. Kirklin JK, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, et al. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant. (2017) 36(10):1080–6. doi: 10.1016/j.healun.2017.07.005
3. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. (2017) 135(10):e146–603. doi: 10.1161/CIR.0000000000000485
4. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. (2020) 141(9):e139–596. doi: 10.1161/CIR.0000000000000757
5. Berardi C, Bravo CA, Li S, Khorsandi M, Keenan JE, Auld J, et al. The history of durable left ventricular assist devices and comparison of outcomes: heartware, heartmate II, heartmate 3, and the future of mechanical circulatory support. J Clin Med. (2022) 11(7):2022. doi: 10.3390/jcm11072022
6. Sidhu K, Lam PH, Mehra MR. Evolving trends in mechanical circulatory support: clinical development of a fully magnetically levitated durable ventricular assist device. Trends Cardiovasc Med. (2020) 30(4):223–9. doi: 10.1016/j.tcm.2019.05.013
7. Mehra MR, Goldstein DJ, Cleveland JC, Cowger JA, Hall S, Salerno CT, et al. Five-year outcomes in patients with fully magnetically levitated vs axial-flow left ventricular assist devices in the MOMENTUM 3 randomized trial. JAMA. (2022) 328(12):1233–42. doi: 10.1001/jama.2022.16197
8. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372. doi: 10.1136/bmj.n71
9. Bauman ZM, Cunningham R, Hodson A, Shostrom V, Evans CH, Schlitzkus LL. Emergent general surgery operations in patients with left ventricular assist devices. Am Surg. (2021) 87(1):8–14. doi: 10.1177/0003134820950683
10. Beetz O, Bajunaid A, Meißler L, Vondran FW, Kleine M, Cammann S, et al. Abdominal surgery in patients with ventricular assist devices: a single-center report. Asaio J. (2020) 66(8):890–8. doi: 10.1097/MAT.0000000000001085
11. Berger R, Nemeth A, Salewski C, Sandoval Boburg R, Acharya M, Weymann A, et al. Is it safe for patients with left ventricular assist devices to undergo non-cardiac surgery? Medicina (Kaunas). (2020) 56(9):424. doi: 10.3390/medicina56090424
12. Bhat G, Kumar S, Aggarwal A, Pauwaa S, Rossell G, Kurien S, et al. Experience with noncardiac surgery in destination therapy left ventricular assist devices patients. Asaio J. (2012) 58(4):396–401. doi: 10.1097/MAT.0b013e31825b8d36
13. Chen CW, Dumon KR, Shaked O, Acker MA, Atluri P, Dempsey DT. Non-cardiac surgery in patients with continuous-flow left ventricular assist devices: a single institutional experience. J Investig Med. (2017) 65(5):912–8. doi: 10.1136/jim-2016-000297
14. daSilva-deAbreu A, Garikapati K, Alhafez BA, Desai S, Eiswirth C, Krim S, et al. Laparoscopic sleeve gastrectomy in patients with obesity and ventricular assist devices: a comprehensive outcome analysis. Obes Surg. (2021) 31(2):884–90. doi: 10.1007/s11695-020-04948-9
15. Gündeş E, Uzun O, Çiyiltepe H, Aday U, Çetin DA, Gülmez S, et al. Emergency abdominal surgery in patients with left ventricular assist device: short- and long-term results. Postepy Kardiol Interwencyjnej. (2017) 13(4):313–9. doi: 10.5114/aic.2017.71613
16. Hawkins RB, Go K, Raymond SL, Ayzengart A, Friedman J. Laparoscopic sleeve gastrectomy in patients with heart failure and left ventricular assist devices as a bridge to transplant. Surg Obes Relat Dis. (2018) 14(9):1269–73. doi: 10.1016/j.soard.2018.04.005
17. Jeng EI, Miller AH, Friedman J, Tapia-Ruano SA, Reilly K, Parker A, et al. Ventricular assist device implantation and bariatric surgery: a route to transplantation in morbidly obese patients with End-stage heart failure. Asaio J. (2021) 67(2):163–8. doi: 10.1097/MAT.0000000000001212
18. Kettyle SM, Chervu NL, Rao AS, Sadi S, Majure D, Sava JA, et al. Outcomes following noncardiac surgery in patients with ventricular assist devices: a single-center experience. Am Surg. (2017) 83(8):842–6. doi: 10.1177/000313481708300833
19. Morgan JA, Paone G, Nemeh HW, Henry SE, Gerlach B, Williams CT, et al. Non-cardiac surgery in patients on long-term left ventricular assist device support. J Heart Lung Transplant. (2012) 31(7):757–63. doi: 10.1016/j.healun.2012.02.023
20. Punchai S, Nor Hanipah Z, Sharma G, Aminian A, Steckner K, Cywinski J, et al. Laparoscopic sleeve gastrectomy in heart failure patients with left ventricular assist device. Obes Surg. (2019) 29(4):1122–9. doi: 10.1007/s11695-018-3570-8
21. Rosenberg JH, Garvin KL, Hartman CW, Konigsberg BS. Total joint arthroplasty in patients with an implanted left ventricular assist device. Arthroplasty Today. (2023) 19:101005. doi: 10.1016/j.artd.2022.07.021
22. Rustum N, Schmitto J, Aper JD, Hanke T, Haverich JS, et al. A. Vascular procedures in patients with left ventricular assist devices: single-center experience. Indian J Thorac Cardiovasc Surg. (2021) 37(5):514–20. doi: 10.1007/s12055-021-01192-3
23. Vigneswaran Y, Wang V, Krezalek M, Prachand V, Wyers S, Juricek C, et al. Laparoscopic procedures in patients with cardiac ventricular assist devices. Surg Endosc. (2019) 33(7):2181–6. doi: 10.1007/s00464-018-6497-1
24. Zenilman A, Pechman D, Moran-Atkin E, Choi J, Camacho D. Bariatric surgery in patients with left ventricular assist devices: a safe and effective method of weight loss as a gateway to heart transplantation. Surg Obes Relat Dis. (2019) 15(10):1780–4. doi: 10.1016/j.soard.2019.08.003
25. Zilbermints V, Israeli O, Ben-Gal T, Rubchevsky V, Aravot D, Kashtan H, et al. Abdominal surgery in patients with a ventricular assist device: a single center experience in Israel. Isr Med Assoc J. (2020) 22(6):369–73. 32558443
26. Briasoulis A, Chehab O, Alvarez P. In-hospital outcomes of left ventricular assist devices (LVAD) patients undergoing noncardiac surgery. Asaio J. (2021) 67(2):144–8. doi: 10.1097/MAT.0000000000001205
27. McElderry B, Alvarez P, Hanna M, Chaudhury P, Bhat P, Starling RC. Outcomes of bariatric surgery in patients with left ventricular assist device. J Heart Lung Transplant. (2022) 41(7):914–8. doi: 10.1016/j.healun.2022.04.003
28. Mentias A, Briasoulis A, Sarrazin MSV, Alvarez PA. Trends, perioperative adverse events, and survival of patients with left ventricular assist devices undergoing noncardiac surgery. JAMA Netw Open. (2020) 3(11):e2025118. doi: 10.1001/jamanetworkopen.2020.25118
29. Place A, McCrum M, Bell T, Nirula R. EGS Plus: predicting futility in LVAD patients with emergency surgical disease. Am J Surg. (2022) 224(6):1421–5. doi: 10.1016/j.amjsurg.2022.10.031
30. Sanaiha Y, Xing H, Morales RR, Morchi R, Ragalie W, Benharash P. Abdominal operations after left ventricular assist device implantation and heart transplantation. J Surg Res. (2019) 243:481–7. doi: 10.1016/j.jss.2019.06.045
31. Taghavi S, Jayarajan SN, Ambur V, Mangi AA, Chan E, Dauer E, et al. Noncardiac surgical procedures after left ventricular assist device implantation. Asaio J. (2016) 62(4):370–4. doi: 10.1097/MAT.0000000000000366
32. Mehra MR, Canter CE, Hannan MM, Semigran MJ, Uber PA, Baran DA, et al. The 2016 international society for heart lung transplantation listing criteria for heart transplantation: a 10-year update. J Heart Lung Transplant. (2016) 35(1):1–23. doi: 10.1016/j.healun.2015.10.023
33. Miranda WR, Batsis JA, Sarr MG, Collazo-Clavell ML, Clark MM, Somers VK, et al. Impact of bariatric surgery on quality of life, functional capacity, and symptoms in patients with heart failure. Obes Surg. (2013) 23:1011–5. doi: 10.1007/s11695-013-0953-8
34. Ramani GV, McCloskey C, Ramanathan RC, Mathier MA. Safety and efficacy of bariatric surgery in morbidly obese patients with severe systolic heart failure. Clin Cardiol. (2008) 31(11):516–20. doi: 10.1002/clc.20315
35. Greene J, Tran T, Shope T. Sleeve gastrectomy and left ventricular assist device for heart transplant. JSLS. (2017) 21(3):e2017.00049. doi: 10.4293/JSLS.2017.00049
36. Mekhaimar M, Correa A, Hamo C, Doshi A, Young A, Roldan J, et al. Glp-1 receptor agonists are associated with weight loss among lvad patients with diabetes and obesity. J Card Fail. (2023) 29(4):617. doi: 10.1016/j.cardfail.2022.10.175
Keywords: noncardiac surgery, left ventricular assist device, mechanical circulatory support, outcome, complications
Citation: Alamouti-Fard E, Garg P, Yazji J, Brigham T, Jacob S, Wadiwala IJ and Pham SM (2024) Outcomes after noncardiac surgery in patients with left ventricular assist devices: a systematic review. Front. Cardiovasc. Med. 11:1414444. doi: 10.3389/fcvm.2024.1414444
Received: 8 April 2024; Accepted: 5 August 2024;
Published: 17 September 2024.
Edited by:
Antonio Loforte, University of Turin, ItalyReviewed by:
Carlotta Sorini Dini, University of Siena, ItalyAneesh Dhore-patil, Houston Methodist Hospital, United States
Copyright: © 2024 Alamouti-Fard, Garg, Yazji, Brigham, Jacob, Wadiwala and Pham. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Si M. Pham, c2ltYWlwaGFtQGdtYWlsLmNvbQ==
 Emad Alamouti-Fard
Emad Alamouti-Fard Pankaj Garg
Pankaj Garg John Yazji1
John Yazji1 Tara Brigham
Tara Brigham Si M. Pham
Si M. Pham