- Internal Medicine Research Unit, Pfizer Research and Development, Cambridge, MA, United States
The lymphatic vascular system spans nearly every organ in the body and serves as an important network that maintains fluid, metabolite, and immune cell homeostasis. Recently, there has been a growing interest in the role of lymphatic biology in chronic disorders outside the realm of lymphatic abnormalities, lymphedema, or oncology, such as cardiovascular-kidney-metabolic syndrome (CKM). We propose that enhancing lymphatic function pharmacologically may be a novel and effective way to improve quality of life in patients with CKM syndrome by engaging multiple pathologies at once throughout the body. Several promising therapeutic targets that enhance lymphatic function have already been reported and may have clinical benefit. However, much remains unclear of the discreet ways the lymphatic vasculature interacts with CKM pathogenesis, and translation of these therapeutic targets to clinical development is challenging. Thus, the field must improve characterization of lymphatic function in preclinical mouse models of CKM syndrome to better understand molecular mechanisms of disease and uncover effective therapies.
1 Lymphatic vascular biology
The vascular system plays a critical role in maintaining normal function of the human body, and it is made up of blood and lymphatic vasculature. As a complement to the blood vasculature that delivers oxygen and nutrients to tissues, the lymphatic vasculature consists of blind-ended vessels that form a unidirectional network to transport interstitial fluids, metabolites, and immune cells to secondary lymphoid organs and eventually back to the blood circulation (1). The blinded-ended vessels, or the lymphatic capillaries reside in the tissue, and they demonstrate discontinuous button-like junctions, which allow them to absorb tissue fluid and transport immune cells towards the collecting vessels (2). The lymphatic collecting vessels, on the other hand, have zipper-like junctions and contain valves, which allow a unidirectional transport of lymph against gravity towards the blood circulation (3). In addition to the distinct morphological features and function of lymphatic vessels, lymphatic endothelium is also distinguished from blood endothelium by the high expression of multiple lymphatic specific regulators, including prospero homeobox protein 1 (PROX1) and vascular endothelial growth factor receptor-3 (VEGFR3) (4). PROX1 is a key transcriptional regulator for lymphatic endothelial cell fate and identity, while VEGFR3 is the principal receptor for lymphangiogenesis (5). There are two well-known ligands for VEGFR3, which are vascular endothelial growth factor C (VEGFC) and vascular endothelial growth factor D (VEGFD), and the activation of VEGFR3 upon its binding to the ligands induces lymphangiogenesis (6). Moreover, lymphatic capillaries can also be distinguished from collecting vessels by the high expression of lymphatic vessel endothelial hyaluronan receptor type 1 (LYVE1) and chemokine (C-C motif) ligand 19 and 21 (CCL19/CCL21) (7). CCL19 and CCL21 are important in the interaction between lymphatic capillaries and C-C Chemokine Receptor 7 (CCR7)-expressing dendritic cells (DCs) (8). Although often overlooked compared to the blood vasculature, the lymphatic vasculature plays an important role in health and disease, and increasing evidence suggests that the lymphatics could be a potential new therapeutic target for cardiovascular-kidney-metabolic (CKM) syndrome.
2 CKM syndrome and lymphatics
CKM syndrome is the clinical concept that there is a connected pathology between cardiovascular disease, kidney disease and metabolic diseases such as obesity and diabetes, and that new approaches are necessary to treat the diseases as a whole entity (9). CKM syndrome is characterized by early dysfunctional adiposity that can progress to hyperglycemia and insulin resistance (10). This predisposes patients to systemic inflammatory and oxidative stresses, which, when combined with genetic and environmental factors, cascades into cardiorenal dysfunction (11, 12). At this point, the cardiovascular system, kidney, and systemic metabolic network cascade through a series of communications that ultimately lead to coronary artery disease, peripheral artery disease, stroke, heart failure, or renal failure (9). Improving the lymphatic vascular network, which spans throughout multiple organs and regulates several aspects of disease, may provide a novel therapeutic avenue for the treatment of CKM syndrome (Figure 1). The lymphatic vasculature has been shown in numerous studies to be dysfunctional in heart failure, atherosclerosis, kidney disease, and obesity that selective improvement of lymphatics can provide preclinical efficacy in individual models within CKM syndrome (13–15). However, the application of improving lymphatic vascular function has had limited attempts clinically. We outline here various reports of lymphatic vascular involvement in CKM health, techniques to directly interrogate lymphatic function for drug discovery, therapeutic pathways that may have clinical benefit in CKM, and the challenges of translating these approaches to clinical development.
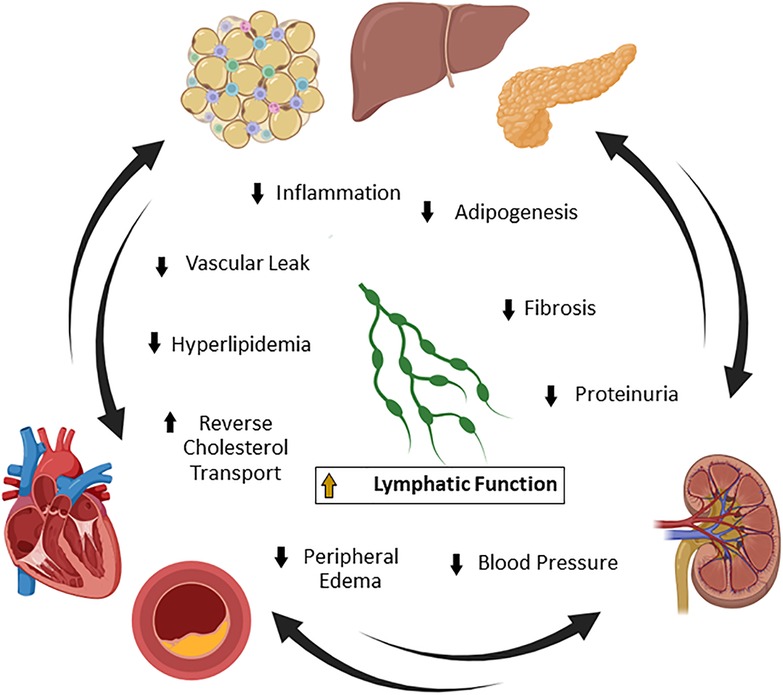
Figure 1. Lymphatic function and cardiovascular-kidney-metabolic (CKM) syndrome. CKM syndrome is a complex disease involving connected pathologies between metabolism, the cardiovascular network, and the renal system. Lymphatic vascular homeostasis is important for the maintenance of systemic lipid and metabolite levels, blood pressure, fluid transport, and immune trafficking. Many of these pathways contribute to the manifestation of CKM syndrome in mice and humans. Improvement of lymphatic function is a potentially novel strategy to simultaneously treat multiple drivers of pathology in CKM syndrome by reducing obesity, inflammation, fibrosis, proteinuria, blood pressure, and edema. Created with BioRender.com.
2.1 Heart failure
Heart failure is a complex syndrome with heterogenous clinical phenotypes, various underlying etiologies, and mechanisms of impairment (16, 17). Despite the heterogeneity, anti-hypertensive thiazide diuretics, along with angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers, remain one of the most commonly used medications for heart failure, especially in patients with elevated risk factors (18). This provides clear rationale for the improvement of lymphatic function in this large patient population as the lymphatic network controls the transport of interstitial fluid throughout the body (19). Second, pulmonary edema, which involves the buildup of fluid in the lungs, is a common symptom of congestive heart failure and results from increased venous and capillary pressure to the lungs secondary to the increase in left ventricular pressure (20). It has been suggested that about 80% of patients with congestive heart failure have pulmonary edema, which worsens patient symptoms, including exacerbated shortness of breath, chest pain, and fatigue (21). Increasing lymphatic function may directly enhance patient quality of life by clearing both peripheral and pulmonary interstitial fluid and absolving these symptoms. Among the heart failure sub-categories, heart failure with preserved ejection fraction (HFpEF) has become the most common form and is characterized by preserved ejection fraction and diastolic dysfunction (22). Patients with HFpEF demonstrated peripheral lymphatic rarefaction and reduced lymphatic drainage, suggesting a potential association between lymphatics and HFpEF etiology (23, 24).
Many recent studies have established a clear role for the lymphatic vasculature in rodent models of heart failure. In preclinical models of myocardial infarction, robust lymphangiogenesis was observed in injured heart and potentially represents an endogenous response to alleviate cardiac injury (25, 26). Consistently, in the setting of chronic heart failure induced by pressure overload, lymphangiogenesis was also observed in heart, although to a lesser extent (27, 28). Additionally, cardiac lymphatic dysfunction in mice induced diastolic dysfunction, a hallmark of HFpEF, and cardiac lymphangiogenesis stimulated by cell therapy restored diastolic dysfunction, supporting a potential therapeutic role of lymphatics in HFpEF (29). More interestingly, human heart failure cardiac tissues showed evidence of increased lymphatic density (27). However, these lymphatics displayed reduced lumen diameter, suggesting that although the heart can endogenously compensate in this disease, it may be insufficient to attenuate cardiac dysfunction (27). Moreover, administration of lymphangiogenic factors such as VEGFCC156S, a point mutant that is a specific VEGFR3 ligand, to further facilitate lymphangiogenesis and improve lymphatic function in rodent model of heart failure demonstrated beneficial effects, as we discuss in greater detail in another section of this review article, highlighting the potential of targeting lymphatics for the treatment of heart failure (30, 31).
2.2 Atherosclerosis
Emerging evidence indicates an important role of the lymphatic vasculature in the progression and regression of atherosclerotic plaques in large arteries. Robust lymphangiogenesis has been reported in aortas and coronary arteries of atherosclerotic mice and humans and correlates with atherosclerosis severity (32, 33). As atherosclerosis progresses, the lymphatic network expands along the aorta, forming a loose vascular plexus and breaching only as far as the adventitial layer of the vessel (33). Although mainly studied in rodent models of atherosclerosis, the lymphatic vasculature is thought to play two important roles in atherosclerosis: (1) control of systemic lipoprotein levels and (2) lymphatic clearance of plaque lipids and immune cells (33–36). The lymphatic system is highly important in the regulation of dietary lipid uptake as well as peripheral lipoprotein trafficking and can control levels of pro-atherogenic low-density lipoprotein (LDL) and athero-protective high-density lipoprotein (HDL) (37, 38). Soluble Vegfr3-expressing or Vegfr3-mutant (Chy) mice were crossed with low-density lipoprotein receptor, Ldlr, knockout mice, which led to significantly increased total serum cholesterol and triglycerides due to an increase in circulating very low-density lipoprotein (VLDL) and LDL (36). Furthermore, Vegfr3-specific agonism via VEGFCC156S significantly improved lymphatic function and lowered circulating LDL (35). Rodent atherosclerosis is highly dependent on circulating pro-atherogenic lipoprotein particles, and both studies showed that improved lymphatic function correlated with decreased LDL and decreased atherosclerotic burden (35, 36). Secondly, the lymphatic vasculature that forms on the outer edges of the growing atherosclerotic plaque may have a direct function of clearing lipids and immune cells as well as serving as an important conduit for reverse cholesterol transport. Martel et al. showed that the lymphatic vasculature directly transports cholesterol from the plaque to acceptor HDL in circulation, and inhibition of Vegfr3 signaling caused aortic cholesterol retention (34). Lastly, this aortic lymphatic vascular network is critical for the regression of atherosclerosis in mice given ezetimibe, which prevents cholesterol absorption (33). Enhancing lymphatic clearance and overall function may be a novel and effective strategy to reduce cardiovascular-related death beyond traditional lipid-lowering therapies.
2.3 Chronic kidney disease
Renal lymphatics, a largely neglected target in discussions regarding kidney diseases, has also drawn more attention in the past few years. There are abundant lymphatic vessels in kidney cortex of all species, which play a critical role in maintaining tissue homeostasis and regulating interstitial edema (13, 39). Similar to heart failure and atherosclerosis, robust lymphangiogenesis has been observed in preclinical models of acute kidney injury (AKI) and chronic kidney disease (CKD) as well as kidney biopsies from patients with AKI, nephropathy and diabetic kidney disease (DKD) (40–42). Increased lymphatic density was often observed at the site of tubulointerstitial lesions and areas of fibrosis and inflammation, suggesting that local lymphangiogenesis may represent an intrinsic response to resolve tissue damage, fibrosis and local inflammation (43, 44). In addition, reduced lymphatic vessel diameter and branching was observed in the kidney of an autosomal dominant polycystic kidney disease (ADPKD) mouse model, indicating that lymphatic function might be impaired in the pathogenesis of ADPKD (45). More interestingly, enhancing lymphangiogenesis in kidney through local Vegfd overexpression, using a genetic approach or kidney targeted nanoparticles, reduced blood pressure, increased sodium excretion and alleviated renal inflammation in hypertensive mouse models, implicating that renal lymphatics also contribute to hypertension and hypertensive kidney disease (46–48). Although there has been recent interrogation of the role of lymphatics in kidney biology, there remains much to be uncovered as to how the lymphatic vasculature changes in chronic kidney disease and how they can be manipulated to improve kidney function.
2.4 Obesity and metabolic syndrome
Many studies have identified an association between lymphatic dysfunction and metabolic disorders such as obesity and chronic liver disease. This relationship was first documented by the observation that mice lacking Prox1 display adult-onset obesity, increased adiposity, and elevated lipid levels and that restoration of Prox1 in lymphatic endothelium rescued these phenotypes (49, 50). In addition, humans with morbid obesity [body mass index (BMI) > 40] often display impaired lymphatic function and lymphedema, which may become irreversible, even after massive weight loss, in patients with BMI >50 (51, 52).
There is evidence that improving lymphatic function could also ameliorate obesity and its comorbidities by repairing lipid transport and attenuating low-grade inflammation. Disruption of intestinal lacteals, the lymphatic vessels that are critical for dietary lipid absorption and transport into blood stream as chylomicrons, increased the susceptibility to obesity and insulin resistance in mice (53). Conversely, promoting lacteal junction zippering to reduce chylomicron uptake ameliorated diet induced obesity in mice (54, 55). These studies provide evidence that modulation of lymphatic vascular function could be beneficial to control obesity or its comorbidities. Additional molecular mechanisms underlying metabolic disorders and lymphatic dysfunction have been thoroughly explored elsewhere (56–58).
3 Preclinical assays to monitor lymphatic function
Several assays have been created to characterize lymphatic vessel function in rodents (Table 1). However, many of these techniques have been used to interrogate mechanisms in lymphedema or lymphatic malformations, which is understandable since the lymphatic vasculature directly contributes to the pathology of these indications (59, 60). Only recently have these assays been applied to mouse models of CKM syndrome. This section describes methods to evaluate lymphatic function in rodents both terminally and non-terminally.
3.1 Imaging assays of peripheral lymphatic function
One of the main functions of the lymphatic vascular network is the transport of molecules and fluid from the interstitial space, through proximal lymph nodes, and back toward the venous circulation (1). Muscular tissue contraction and fluid pressure are the main forces responsible for this at the initial lymphatic level (61). However, collecting lymphatic vessels intrinsically produce lymph flow through the contractions of smooth muscle cells (SMCs) lining the vasculature, a process that is dependent on the health of both the SMCs as well as the endothelium (62). Autonomous lymphatic contraction and fluid transport have been used as a readout of overall lymphatic function in mouse models, and several assays have been developed to measure this process. Ex vivo methods to study lymphatic contractility include the single-vessel preparation. This procedure entails the isolation and dissection of a lymphatic vessel followed by cannulation in an ex vivo perfusion system (63). Vessel pressure and flow can be altered, and compounds can be applied to the bath to examine their functional effects (63). Measurements include vessel internal diameter (end diastolic and end systolic), amplitude, ejection fraction, contraction frequency, and fractional pump flow (63–65). Ex vivo preparations allow control of the environment and direct engagement of the lymphatics, which can be helpful in confirming immediate target engagement when therapeutics are directly applied to the vessel.
Although highly quantitative, ex vivo preparations of lymphatic vessels lack the surrounding biological components that may govern contraction in vivo. Several advances in imaging techniques and injectable tracers have allowed for the visualization and measurement of similar lymphatic parameters in live animals (66). Higher resolution techniques to measure lymphatic function that have potential for clinical translatability include magnetic functional imaging (MRI), photoacoustic (PA) imaging, optical coherence tomography (OCT), and positron emission tomography/computed tomography (PET/CT) (67). Many of these methods are lower throughput, utilize expensive or inflexible tracers, and have limited fields of view in the context of preclinical mouse experimentation (67–72). In recent years, near-infrared fluorescence (NIRF) imaging has gained popularity with the optimization of fluorescent dyes and ease of use. Fluorescein isothiocyanate (FITC)-dextran and indocyanine green (ICG) are two commonly used dyes in this method, with the latter routinely used clinically to visualize lymphatics in patients after surgery or to diagnose primary lymphedema (73). The dye is usually administered intradermally, which fills superficial lymphatics vessels and proximal lymph nodes. The resolution is sufficient to visualize lymphatics that lay closer to skin in mice, such as the collecting lymphatics distal to the popliteal lymph node or within the tail in mice (67). Vessel snapshots or live imaging can be acquired by fluorescence microscopy and used to quantify the rate of lymphatic transport and evaluate lymphatic function (74). High frequency of pigmentation in the C57Bl/6 strain and subcutaneous fat deposition in dysmetabolic mouse models are obstacles to vessel visualization and pose some limitations if this technique is to be used in mouse models of CKM syndrome.
Several nonterminal endpoints can be monitored once the lymphatic vasculature or lymph nodes are visualized utilizing the techniques listed above. Live imaging of the collecting lymphatic vessel allows the measurement of lymphatic function parameters similar to ex vivo techniques, such as vessel packet transport, amplitude, and contraction frequency (75). Although these measurements are popularly reported in mouse models and representative of overall lymphatic function, lymphatic contraction frequency is not a discreet clinical parameter and is rarely measured in patients (76, 77). Clinically, fluorescent lymphography is more commonly used as a qualitative measurement, where gross observations are made about morphology and permeability (73). This, too, can be measured in mice, but may lack reproducibility and explicit quantitation (66). Therefore, there is a need for the establishment of a standardized measurement of lymphatic function in patients, which will better guide mouse experimentation. Some examples of imaging endpoints exist that may translate between mouse and human. For instance, pulse-chase style experiments in which a dye or tracer bolus is injected intradermally and imaged live or at time intervals can measure pumping velocity and overall lymphatic clearance (66, 70). Secondly, application of a cuff around the limb (or tail) can allow researchers to calculate lymphatic pressure when combined with live lymphangiography (78, 79). However, a more uniform measurement of lymphatic function in humans would bring more confidence into applying the same measurement for investigating functional mechanisms in mice, which would enable therapeutic discovery.
3.2 Immune trafficking assays
A key function of the lymphatic vasculature is to serve as a conduit between immune cells in the periphery and the lymph node. Immune tolerance is tightly regulated by the cross-talk of surveilling DCs in the periphery that acquire and present antigen to T cells, which influence T cell differentiation and activation (80). To do this, the capillary lymphatic endothelium secretes chemokines, such as CCL19 and CCL21, which attract CCR7-expressing DCs and transports them through collecting lymphatic vessels to lymph nodes (8). Lymphatic dysfunction results in overall decreased lymphatic fluid transport, poor DC migration to secondary lymphoid organs, and exacerbated immune dysregulation (81, 82). Several mouse assays have been developed to monitor this crucial process of lymphatics.
A classic method to measure DC migration is through the tracing and quantification of labeled DCs to the lymph node, which can be achieved through several means. DCs can be purified from a donor animal such as Cd45.1+ mice, or mice expressing a fluorescent protein, such as GFP, and transferred to the skin of a recipient model (82, 83). Following transfer, the spleen or lymph node proximal to the area of transfer can be digested, and migrated DCs quantified as Cd45.1+ (or GFP+)Cd45+ Cd11chiMHCIIhi by flow cytometry (82, 83). To examine the migration of endogenous DCs, fluorescent macromolecules, such as FITC-conjugated dextran, ovalbumin, or albumin can be directly injected into the lung, tail, or skin, which will be taken up by DCs, and FITC+ Cd45+ Cd11chiMHCIIhi cells can be similarly identified by flow cytometry (84). These pulse-chase style experiments can potentially be performed at the end of a CKM syndrome study and serve as a terminal marker of lymphatic function in mice.
3.3 Mouse models of lymphedema
Lymphedema results from direct injury to the lymphatic vasculature and is characterized by the inability of the lymphatic network to clear lymph from distal portions of the body and swelling of the affected area (85). This disease can manifest from either genetic insufficiency (primary lymphedema) or after external insult, such as surgery, chemotherapy, or irradiation therapy (secondary lymphedema) (59, 86). Although lymphedema is a standalone indication, preclinical models of lymphedema allow researchers to interrogate the mechanism-of-action and horsepower of the ability of their target of interest to enhance lymphangiogenesis and overall lymphatic function and could be used to develop and refine preclinical assays to monitor lymphatic function. The Chy mouse model, which contains a loss-of-function mutation in the gene encoding Vegfr3, has been useful in studies of lymphatic dysfunction due its strong lymphatic dysfunction phenotype, including sparse lymphatic coverage, disrupted lymphatic flow, leaky lymphatic vessels, and swelling of the extremities (87). However, this model very closely models primary lymphedema patients and may not be suitable for therapeutic testing on a CKM mouse background. Furthermore, many targets converge on the VEGFR3 signaling axis and would require fully intact VEGFR3 for validation.
The mouse tail lymphedema model has become a commonly used in vivo model of secondary lymphedema (88). This surgical procedure requires the circumferential excision of tail skin –2 cm below the base of the tail and injection of Evans Blue dye to visualize the two main collecting lymphatic vessels that run parallel to the lateral veins (89). The lymphatic vessels are then ligated and excised, leading to accumulation of lymph distal to the surgery and swelling until the lymphatic network is reconnected. Edema in this model peaks around three weeks post-surgery, which slowly regresses as lymphatic networks are re-established and fibrous tissue is healed (90, 91). Both collecting lymphatic vessels that run along either side of the tail are commonly excised, but an alternate model has been developed, which excises only one vessel (dominant vessel), enabling functional analysis of the intact vessel (79). The benefit of this model is that the extent of edema is not only dependent on lymphangiogenesis, but also lymphatic output of the remaining vessel, allowing researchers to interrogate a broader spectrum of molecular mechanisms (79).
Aside from tail lymphedema, preclinical models of secondary lymphedema also include the removal of lymphatic vessels in the mouse hindlimb and popliteal lymph node (PLN) dissection (92, 93). However, removal of the PLN alone often does not result in chronic edema (88). Variations of this surgical model have been developed including the removal of several lymph nodes (superficial inguinal lymph node, popliteal lymph node, and deep inguinal lymph node) and excision of the hindlimb femoral lymphatic vessel (94). Both tail and hindlimb methods of inducing lymphedema recapitulate several of the clinical manifestations of prolonged edema, such as dermal thickening, lymphatic vessel expansion and dilation, immune infiltration, and fibrosis (95, 96). Thus, these models could be used to refine the currently available assays for evaluating lymphatic function, establish standardized measurements of lymphatic function between rodent models and patients with lymphedema, and assess the efficacy of therapeutic molecules that directly improve lymphatic function.
3.4 Molecular endpoints
Molecular biomarkers such as protein or mRNA that change in response to lymphatic improvement are highly valuable for the monitoring of lymphatic function in preclinical models and eventual clinical trials. Many studies have shown that upon stimulation of lymphangiogenesis or enhanced lymphatic contraction, markers such as Prox1, Pdpn (podoplanin), Vegfc and Flt4 (gene that encodes VEGFR3) become upregulated, likely due to overall enhanced lymphatic coverage in the tissue of interest, but also through increased transcriptional activity per cell from positive feedback loops (97–99). In addition, increased lymphatic vessel density and lymphatic lumen diameter, which could be visualized through immunofluorescent or whole mount stain, are often observed in rodent models upon stimulation of lymphangiogenesis (26). Furthermore, chemokines such as CCL19 and CCL21 are secreted from functional capillary lymphatics to attract antigen-presenting leukocytes in the parenchyma and could theoretically be used as biomarkers of lymphatic function (100, 101). However, it remains unclear when and to what degree these biomarkers change in adult rodent models of CKM syndrome and which tissue bed would produce the highest signal-to-noise. Many of the examples listed above are analyzed either in development, with chronic treatment of a lymphangiogenic factor or after long-term genetic overexpression or knockout, and how a singular therapy would change the expression of these biomarkers remain unknown (102–104). A similar conundrum occurs in the evaluation of lymphatics in mice through slide mounting and immunofluorescence/immunohistochemistry, where many studies focus on using developmental lymphangiogenesis as a surrogate for improved lymphatic function, while a parameter that can be quantified and clearly correlate with lymphatic function remain to be established (97, 105). Ideally, a clear and specific biomarker should be established so that upon lymphatic enhancement by a drug modality, a gene or protein could be measured within hours or days to confirm activity of the therapeutic and make quick, informed decisions on progressing forward with the study.
In summary, the lymphatic biology field has generated several rodent assays to monitor lymphatic function both terminally and non-terminally. Although some of these lymphatic assays have been used in mouse models of heart failure, atherosclerosis, chronic kidney disease, and obesity, there remains a large gap in knowledge of the level of lymphatic dysfunction in many of the individual disease models. Furthermore, many preclinical models of chronic disease do not develop consistent signs of lymphatic dysfunction that occur in humans, such as pulmonary edema or fluid retention (106, 107). Analyzing a large suite of lymphatic assays in CKM syndrome models may allow head-to-head comparisons of lymphatic contribution to disease.
4 Bridging preclinical studies to clinical outcomes in lymphatic anomalies
Many of the preclinical techniques that probe lymphatic function have shown translatability into the clinic and therapeutic advancement. Most of the successes have been made in the field of lymphatic anomalies, which is not unsurprising as this collection of diseases are primarily the direct result of lymphatic dysfunction (60, 108). Lymphatic anomaly is an umbrella term that includes diseases of lymphatic inactivation, such as primary lymphedema, and lymphatic overactivation, such as malformations (59). As mentioned previously, primary lymphedema is caused by a deficiency in the lymphatics, commonly congenital, which can manifest as decreased total body coverage, increased lymphatic leakiness, diminished ability to transport lymph, or enhanced inflammation and fibrosis (98). Primary lymphedema is the result of insufficient lymphatics and lymph absorption, which makes it an enticing indication to translate therapeutic targets into models of CKM syndrome. FLT4 is a one of the most well-known genetic drivers of primary lymphedema and will be discussed in-depth below in the context of CKM syndrome, but other targets that are causative for primary lymphedema include VEGFC, forkhead box protein C2 (FOXC2), angiopoietin-2 (ANGPT2), cadherin EGF LAG seven-pass G-type receptor 1 (CELSR1), and many others that lead to lymphatic valve dysfunction or defective lymphatic endothelial proliferation and survival (109–113). Lymphoscintigraphy utilizing either radiolabeled or fluorescent dyes is the proven method to diagnose primary lymphedema and can be used to quantify lymph velocity and qualitatively monitor the morphology of the lymphatic vasculature for abnormalities or lymph backflow (114, 115). This diagnostic assay has proven preclinical translatability, as discussed above. Unfortunately, primary lymphedema has seen little clinical advancement, with the current standard of care consisting of management of symptoms, manual compression, and surgery (59). This may soon be solved by recent advancements in genetic and diagnostic tools, which have greatly supported successes in lymphatic malformations.
Lymphatic malformations are a collection of lymphatic pathologies characterized by hyperactivation of the lymphatic vasculature. The subcategories include cystic lymphatic malformations (CLM), which manifest as focal lesions, and complex lymphatic anomalies (CLA), which affect multiple organs, including bone, have a wider spectrum of clinical features, and include dysfunction of the major conducting abdominal or thoracic lymphatic vessels (60). Lymphatic malformations are sporadic, making diagnosis and management difficult (60). However, several somatic drivers of the lymphatic malformations have been identified as causative genes, which has greatly advanced understanding of the disease and has led to clinical optimism (108). For example, next generation sequencing has identified somatic gain-of-function mutations in PIK3CA, which encodes phosphatidylinositol-4,5,-bisphosphate 3-kinase (PI3K), as causal for cystic lymphatic malformations and a variety of phenotypically distinct complex lymphatic anomalies (116, 117). Mice containing these Pi3kca mutations exhibit many of the same pathologies as humans with lymphatic malformations, which were attenuated with alpelisib, a Food and Drug Administration (FDA)-approved PI3K inhibitor for breast cancer patients (102). Alpelisib has shown been shown to reduce lymphatic malformation volume and alleviate symptoms in a small cohort of patients; it is currently ongoing phase II/III testing (NCT05948943) (102). T2-weighted magnetic resonance imaging (MRI) has proven to be the primary method to quantify lymphatic malformation volume in patients and was used to exhibit the efficacy of alpelisib in the Pik3ca mouse lymphatic malformation model prior injection into humans, serving as a critical translational tool to be used for target validation (102).
Other ongoing clinical efforts follow a similar paradigm and focus on using FDA-approved a cancer therapies, such as sirolimus and damentib/trametinib drugs to inhibit mTOR and MEK, respectively (108). As mentioned in previous sections, evidence suggests that lymphatic contribution to CKM syndrome may be due to lymphatic vascular insufficiency and rarefaction (23, 24). Interestingly, this is in direct contrast to molecular drivers of lymphatic malformations and suggests that lymphatic health most likely operates on a bell-curve spectrum, where under- or over-activation results in pathology. Therefore, fine-tuning is an important consideration for therapeutic exploration. However, ongoing clinical trials show a clear proof-of-concept that genetic testing and translatable imaging techniques can identify lymphatic drivers of disease and test the efficacy of targeted therapeutics (60, 108). Application of this strategy may allow quick progression of lymphatic-specific therapies in CKM syndrome.
5 Therapeutic targets and opportunities
There exist many reports in literature of promising targets that can be modulated to enhance lymphatic function. Several of these studies utilize genetic models to interrogate mechanisms and purely focus on diseases of direct lymphatic dysfunction, such as lymphedema. However, several targets have breached this paradigm and have also been examined in the context of chronic disease. These targets are also amenable to therapeutic development (receptors and enzymes) and have even seen advancement in human clinical trials. We describe the therapeutic opportunity of such targets in CKM syndrome.
5.1 VEGFR3
VEGFR3 is a key regulator of lymphangiogensis and function, and thus may be a promising target to enhance lymphatic function in CKM. VEGFR3 is a receptor tyrosine kinase that is enriched in lymphatic endothelial cells in adult animals, which provides specificity to the lymphatic vasculature, thereby sparing the blood endothelium (118). Extracellular binding of its natural ligands VEGFC or VEGFD bring together VEGFR3 homodimers, causing transphosphorylation of the kinase domains and propagating downstream signaling (119). Activation of the protein kinase B (AKT) and Mitogen-activated protein kinase (MAPK) signaling cascades are key nodes by which VEGFR3 activation promotes cellular responses, including lymphangiogenesis and proliferation (120). VEGFR3 may also form heterodimers with vascular endothelial growth factor receptor 2 (VEGFR2) to mediate signaling and angiogenesis, although the relative contribution of this complex in adulthood is not fully understood (121). VEGFR3 is critical to the early development of the lymphatic endothelium, and it also regulates lymphatic function in adulthood (122). Clinically, VEGFR3 loss-of-function mutations leads to primary lymphedema, most notably Milroy disease (123).
While VEGFC can engage both VEGFR2 and VEGFR3, an engineered point mutation in VEGFC (cysteine 156 to serine, VEGFCC156S), was found to abolish binding of VEGFC to VEGFR2 and selectively activate VEGFR3 (31). VEGFCC156S maintains lymphatic activation of VEGFR3, stimulates lymphangiogenesis in vivo and eliminates possible effects of VEGFC binding to the blood endothelium (26). There is clear evidence that VEGFCC156S improves cardiac function in the context of acute cardiac injury, such as myocardial infarction (MI). Numerous preclinical studies showed that VEGFCC156S treatment increased lymphangiogenesis, reduced cardiac congestion, decreased inflammation, attenuated fibrosis, and rescued cardiac dysfunction in mouse or rat coronary artery ligation and/or occlusion models of MI (25, 124–126). Notably, VEGFR3 inhibition via the kinase inhibitor MAZ51, soluble VEGFR3, or VEGFR3 blocking antibodies exacerbated cardiac dysfunction and disease pathogenesis (27). However, lymphangiogenesis blockade in heart using Flt4 knockout or Vegfc/Vegfd double knockout animals did not exacerbate cardiac dysfunction after myocardial infarction (MI), indicating that the beneficial effects observed with VEGFCC156S in MI could be mediated by alternative mechanisms (127).
In addition to acute cardiac injury, VEGFCC156S treatment is also effective in chronic heart failure preclinical models. In a six-week angiotensin II-induced model of hypertension and systolic dysfunction, VEGFCC156S improved ejection fraction, lowered blood pressure, and reduced inflammation (128). Consistent with this result, in a six-week chronic heart failure model generated by pressure overload, VEGFCC156S alleviated cardiac dysfunction, fibrosis, inflammation and edema (28).
Furthermore, VEGFR3 activation through VEGFCC156S administration alleviated fibrosis and inflammation in a CKD model induced by unilateral ureteral obstruction, and improved cystic disease and reduced inflammation in mouse models of ADPKD (40, 129). Moreover, VEGFR3 is expressed in human glomerular endothelial cells and attenuates VEGFR2 phosphorylation upon VEGFA stimulation, implicating a potential role for VEGFR3 in regulating glomerular filtration barrier function (130). In contrast, Vegfr3 inhibition using soluble VEGFR3, anti-VEGFR3 antibodies or transgenic Vegfr3 overexpression approach demonstrated beneficial effects in multiple clinical models of AKI (131). The context-dependent role of VEGFR3 in kidney diseases warrants further investigation.
Despite the extensive preclinical evidence that VEGFR3 activation may be beneficial in diseases with lymphatic dysfunction, only a few attempts have been made at translating this pathway into the clinic. One consideration for targeting VEGFR3 clinically may be safety concerns associated with chronic activation of a growth factor signaling pathway, such as enhanced vessel leakiness, or potentially carcinogenesis risk. This topic will be discussed further below. Secondly, questions remain regarding the horsepower of lymphatic vascular targeting to significantly improve CKM. Lastly, while VEGFR3 is enriched in the lymphatic endothelium, it can also be expressed in the bone as well as liver sinusoidal endothelial cells (104). VEGFR3 activation in these cell types may produce unintended consequences.
One method to overcome these potential risks includes targeted VEGFC gene delivery. Lymfactin is adenoviral-mediated VEGFC gene delivery, which is administered to breast cancer patients with high risk for upper extremity lymphedema via a lymph node flap that is collected from the groin area and treated ex vivo (132). The lymph node flap was then applied to area likely to undergo lymphedema. This therapy stimulated lymphatic growth and enhanced lymphatic flow in porcine preclinical studies (133). In humans, lymph node transfer and Lymfactin treatment was well tolerated and exhibited a 46% reduction in excess arm volume after a 24 month follow up (134). Targeted therapies such as this would be more challenging in CKM disorders, which are likely systemic diseases. Technological advancements will be necessary to increase the number of patients who would benefit from lymphatic-targeted therapies.
5.2 Adrenomedullin
Another growth factor that is important for lymphatic function and growth is adrenomedullin (AM). AM is a hormone peptide that enacts its signaling via binding to the G protein-coupled receptor calcitonin receptor-like receptor (CLR) in complex with either receptor activity modifying protein (RAMP) 2 or 3 (135). AM predominantly activates the Gs alpha signaling cascade, which directly increases intracellular cyclic AMP to transduce downstream signaling (136). CLR-RAMP2/3 complexes are found in many tissue types, and the biological effects of AM signaling depends on the cell type activated. Of note, AM can activate angiogenesis pathways in both blood and lymphatic endothelial cells (137). AM regulates vascular tone via downstream endothelial nitric oxide synthase (eNOS) activation, which increases vasodilation and endothelial barrier function in the cardiovascular system (138, 139).
AM is clearly associated with lymphatic health, as deletion of the gene encoding adrenomedullin (Adm), calcitonin receptor-like receptor (Calcrl), and Ramp2 all resulted in interstitial lymphedema, abnormal lymphatic vessels, and embryonic lethality (140). This finding was confirmed in adult mice, where Adm haploinsufficiency caused lymphedema in the hindlimbs after skin incision (141). Pharmacological delivery of AM via osmotic minipump attenuated tail swelling in a mouse model of surgically induced tail lymphedema (142). Furthermore, genetic AM overexpression improved cardiac function and decreased edema in a left anterior descending coronary artery ligation mouse model of myocardial infarction (143). Overall, AM signaling may be a potent mechanism to enhance lymphatic function and health in CKM.
Researchers have explored efficacy of enhancing AM signaling in the clinic due to its reported anti-inflammatory and anti-microbial effects (144). Adrecizumab is a humanized monoclonal antibody that binds and stabilizes AM without interfering with CLR engagement, which extends the normally short terminal half-life of AM to fourteen days (145). Adrecizumab has been used in clinical studies to test efficacy in sepsis, where it was reportedly safe and well-tolerated. In this study, adrecizumab improved organ function and significantly reduced patient mortality (146). Adrecizumab is also currently being tested clinically for acute heart failure as a proof of principle study that may lead to future studies in chronic heart failure (NCT04252937). Additional methods for overcoming the short half-life of AM involve continuous AM infusion or AM modification by PEGylation (147). Lastly, AM is displayed a protective role in kidney disease through its vasodilatory, natriuretic, and diuretic actions (148, 149). Combined with AM's reported ability to attenuate adipose inflammation and work synergistically with glucagon-like peptide 1 (GLP-1) in appetite regulation, AM has promise as a therapeutic target for CKM (148–151). Although the AM/CLR/RAMP signaling axis is complex, the specificity for receptor combinations that lead to certain biologic responses may allow researchers to develop more targeted therapeutics to interrogate this pathway.
5.3 Eicosanoids
Eicosanoids are lipid signaling molecules originally derived from arachidonic acid and polyunsaturated fatty acids that have a diverse set of functions, most notably in modulation of immune function and inflammatory processes (152). Leukotriene B4 (LTB4) is a bioactive lipid generated from the 5-lipoxygenase (5-LOX) branch of eicosanoid metabolism that exerts its biological effects via binding to its cognate LTB4 receptors, (LTB4Rs), and promoting g-protein signaling (153, 154). LTB4 core function includes leukocyte activation and pro-inflammatory signaling in the endothelium (155). LTB4 antagonism via ketoprofen significantly reduced tail swelling and pathogenesis in a mouse surgical tail lymphedema model (156). In pathogenic conditions, such as lymphedema, the authors found that LTB4 concentrations rose to a level that inhibits lymphatic coverage and worsens disease outcomes. Interestingly, this mechanism was dependent on Vegfr3 and Notch1, indicating that many lymphatic endothelial pathways converge on similar molecular targets (156).
LTB4 inhibition revealed promising preclinical efficacy in rodent models of lymphedema, which spurred a two-part clinical study to test effectiveness and safety in patients. In the open-label portion of the clinical study, patients with primary or secondary lymphedema were given oral ketoprofen three times a day for four months (157). After the four-month period, patient histopathology score and skin thickness were significantly reduced compared to baseline. In the second part of the study, a double-blind, placebo-controlled experiment, patients receiving ketoprofen had significantly reduced skin thickness, histopathology score, and circulating inflammatory marker expression compared to baseline than patients receiving placebo (157). Notably, ketoprofen also inhibits cyclooxygenases (COX), and pro-inflammatory signaling by these enzymes may contribute to the overall horsepower of these clinical results (158).
Prostaglandins generated by the COX arm of eicosanoid metabolism are also important for lymphatic function and regulation of CKM. One study found that COX2-generated lipid mediators and Vegfr3 signaling were responsible for high-fat diet-induced metabolic dysfunction in mice and erratic mesenteric lymphatic vessel growth (159). This led to unregulated leakage of fat-concentrated lymph into adipose, which worsened metabolic dysfunction. The authors of this study chemically modified the COX2 inhibitor celecoxib to increase targeting specifically to the mesenteric lymph and increase its bioavailability. This modified prodrug successfully blocked prostaglandins within the mesenteric lymph, decreased Vegfc levels, inhibited lymphatic leakage, and attenuated metabolic dysfunction (159). This study reveals the complex feedback mechanisms surrounding Vegfr3 in disease but serves as an important proof-of-concept that amelioration of lymphatic dysfunction may provide significant benefits in chronic diseases such as obesity or CKM.
5.4 Other pathways with early evidence for further investigation
In addition to VEGFR3, adrenomedullin, and eicosanoids, several other targets and pathways have also been reported to regulate lymphangiogenesis and lymphatic function. For example, angiopoietin 2 (Ang2) plays important role in regulating lymphatic vessel development and function. Ang2 is an antagonist ligand for TEK receptor tyrosine kinase (TIE2) receptor in blood endothelium, where the vascular endothelial protein tyrosine phosphatase (VE-PTP/PTPRβ) is expressed, but is an agonist ligand for TIE2 in lymphatic endothelium, where VE-PTP expression is lacking (160). Ang2-Tie2 signaling in the lymphatic endothelium is required for Vegfr3 expression and signaling as well as Vegfc-induced lymphangiogenesis in adult mice, and blocking Ang2 using antibodies reduced Vegfr3 expression and inhibited lymphangiogenesis, highlighting the important role of Angiopoietin-Tie2 signaling in the lymphatic endothelium (161–163). In addition, Epsins also regulate Vegfr3 degradation in the lymphatic endothelium. Lymphatic-specific Epsin knockout alleviated Vegfr3 degradation and subsequent inhibition of lymphangiogenesis in diabetic mice, suggesting that modulation of Vegfr3 localization could be a potential therapeutic approach to restore impaired lymphangiogenesis (91). Furthermore, several other lymphangiogenic factors, such as collagen and calcium-binding EGF domain-containing protein (CCBE1), Semaphorins and Neuropilins, fibroblast growth factor-2 (FGF2), sphingosine 1-phosphate (S1P), bone morphogenetic protein-9 (BMP9) and activin-like kinase receptor type I (ALK1), Notch1 and Ephrin B2 are studied and reviewed thoroughly elsewhere (164). In addition to lymphangiogenic factors, lymphoangiocrine molecules, such as Reelin, that are secreted from the lymphatics during injury are reported to mediate the beneficial effects of lymphangiogenesis in a disease context, and these factors and associated pathways could be worthwhile to investigate as novel therapeutic targets (165, 166).
6 Safety considerations
Although there is strong preclinical evidence that the enhancement of lymphatic function will lead to efficacy in disease outcomes, there exist many unknowns about safety and tolerability for several of the above-mentioned therapeutic opportunities. Firstly, most of the targets that enhance lymphatic function also classically activate lymphatic endothelial proliferation and lymphangiogenesis. Overactivation of this pathway may have potential oncogenic off-target effects. Tumors can express several lymphatic mitogenic factors, including VEGFC and AM, which correlate with disease severity (167, 168). These factors can play a role in tumor vascularization, survival, and metastasis (169, 170). Furthermore, there may be opposing biological functions of increasing lymphangiogenesis via classical mechanisms. One example of this is the regulation of vascular barrier function and angiogenesis by the VEGFA signaling pathway, which enhances vascular proliferation and angiogenesis and is simultaneously, a potent activator of vascular permeability (171, 172). VEGFC has pro-lymphangiogenic effects and may worsen the lymphatic barrier at certain concentrations or in specific disease settings (159). However, this pathway also reportedly enhances endothelial barrier function, which indicates that a balance may needed when targeting VEGFC therapeutically (173, 174). Thus, further investigation is needed to identify differences between VEGFA and VEGFC biological activity in the blood endothelium (173). Several targets, such as AM, LTB4, and COX2 activate pathways that are ubiquitously expressed in several other tissues, which could result in unwanted or unexpected outcomes, especially in a chronic dosing scenario. Furthermore, inhibition of classical inflammatory responses may increase risk for infection and could be detrimental in disease resolution. Methods to specifically target the lymphatic endothelium, such as antibody-drug conjugate (ADC), nanoparticles, or local lymph delivery may provide specificity to test mechanism of action while enhancing the therapeutic safety profile.
7 Conclusion
Enhancement of lymphatic function has the potential to bridge systemic and organ-specific pathologies within CKM syndrome. Restoration of lymphatic function can stabilize metabolite trafficking and attenuate inflammatory processes in metabolic disorders (98, 175). Furthermore, lymphatic restoration has the potential to simultaneously improve cardiac and kidney parameters directly. However, the role of lymphatics in human disease has only recently come into appreciation, and much remains unknown in different indications and patient populations. Animal models displaying key aspects of human CKM syndrome, such as edema, are also lacking, which makes identifying novel therapeutic mechanisms targeting lymphatic function challenging. Rodent models of atherosclerosis, heart failure, and kidney disease are continuing to be refined and optimized, and a complete picture of lymphatic function, from lymphatic contraction to DC trafficking, would help bring a better understanding of the role of lymphatics in these indications.
Although technological advances allow researchers to analyze lymphatic function with high resolution and fidelity, there are not yet any FDA-approved therapies to improve lymphatic function, even in lymphatic-specific diseases, such as lymphedema. One major challenge is specificity. The lymphatic vascular network differs greatly in function from the blood vasculature, yet many therapeutic targets may affect both (1). An ideal therapeutic target would be unique to lymphatic cells or lymph fluid and important in physiology or disease. To this end, the composition of lymph fluid, both in human disease and preclinical models of disease, has been widely understudied, in part due to difficulty in sampling in mice. How the lymph differs from the blood during pathogenesis may provide insights into specific pathways up- or down-regulated in the lymphatics. Furthermore, many preclinical mechanisms propose improvement of lymphatic coverage through enhanced lymphangiogenesis (176). Whether functional lymphangiogenesis can occur in adult humans, and if this can enhance fluid or immune clearance, is currently speculative. Nonetheless, significant progress has been made in both the understanding of lymphatics and CKM syndrome, and several targets have the promise for further testing in humans. Further study and refinement may one day identify a specific and safe lymphatic therapy with the potential to greatly benefit patient outcomes.
Author contributions
JF: Conceptualization, Writing – original draft, Writing – review & editing. LS: Conceptualization, Writing – original draft, Writing – review & editing. KT: Writing – original draft, Writing – review & editing. RR: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
Illustrations were generated with BioRender.
Conflict of interest
Authors JF, LS, KT, and RR were employed by company Pfizer Research & Development. JF, LS, and RR own Pfizer stock.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Oliver G, Kipnis J, Randolph GJ, Harvey NL. The lymphatic vasculature in the 21(st) century: novel functional roles in homeostasis and disease. Cell. (2020) 182(2):270–96. doi: 10.1016/j.cell.2020.06.039
2. Hu Z, Zhao X, Wu Z, Qu B, Yuan M, Xing Y, et al. Lymphatic vessel: origin, heterogeneity, biological functions, and therapeutic targets. Signal Transduct Target Ther. (2024) 9(1):9. doi: 10.1038/s41392-023-01723-x
3. Cueni LN, Detmar M. The lymphatic system in health and disease. Lymphat Res Biol. (2008) 6(3–4):109–22. doi: 10.1089/lrb.2008.1008
4. Ulvmar MH, Makinen T. Heterogeneity in the lymphatic vascular system and its origin. Cardiovasc Res. (2016) 111(4):310–21. doi: 10.1093/cvr/cvw175
5. Bui K, Hong YK. Ras pathways on Prox1 and lymphangiogenesis: insights for therapeutics. Front Cardiovasc Med. (2020) 7:597374. doi: 10.3389/fcvm.2020.597374
6. Holmes DI, Zachary I. The vascular endothelial growth factor (VEGF) family: angiogenic factors in health and disease. Genome Biol. (2005) 6(2):209. doi: 10.1186/gb-2005-6-2-209
7. Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. (1999) 144(4):789–801. doi: 10.1083/jcb.144.4.789
8. Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. (2005) 5(8):617–28. doi: 10.1038/nri1670
9. Ndumele CE, Neeland IJ, Tuttle KR, Chow SL, Mathew RO, Khan SS, et al. A synopsis of the evidence for the science and clinical management of cardiovascular-kidney-metabolic (CKM) syndrome: a scientific statement from the American heart association. Circulation. (2023) 148(20):1636–64. doi: 10.1161/CIR.0000000000001186
10. Ndumele CE, Rangaswami J, Chow SL, Neeland IJ, Tuttle KR, Khan SS, et al. Cardiovascular-kidney-metabolic health: a presidential advisory from the American heart association. Circulation. (2023) 148(20):1606–35. doi: 10.1161/CIR.0000000000001184
11. Khan MS, Shahid I, Anker SD, Fonarow GC, Fudim M, Hall ME, et al. Albuminuria and heart failure: JACC state-of-the-art review. J Am Coll Cardiol. (2023) 81(3):270–82. doi: 10.1016/j.jacc.2022.10.028
12. Zoccali C, Mallamaci F, Lightstone L, Jha V, Pollock C, Tuttle K, et al. A new era in the science and care of kidney diseases. Nat Rev Nephrol. (2024). doi: 10.1038/s41581-024-00828-y
13. Donnan MD, Kenig-Kozlovsky Y, Quaggin SE. The lymphatics in kidney health and disease. Nat Rev Nephrol. (2021) 17(10):655–75. doi: 10.1038/s41581-021-00438-y
14. Jiang X, Tian W, Nicolls MR, Rockson SG. The lymphatic system in obesity, insulin resistance, and cardiovascular diseases. Front Physiol. (2019) 10:1402. doi: 10.3389/fphys.2019.01402
15. Zhou Y, Huang C, Hu Y, Xu Q, Hu X. Lymphatics in cardiovascular disease. Arterioscler Thromb Vasc Biol. (2020) 40(11):e275–83. doi: 10.1161/ATVBAHA.120.314735
16. Murphy SP, Ibrahim NE, Januzzi JL Jr. Heart failure with reduced ejection fraction: a review. JAMA. (2020) 324(5):488–504. doi: 10.1001/jama.2020.10262
17. Redfield MM, Borlaug BA. Heart failure with preserved ejection fraction: a review. JAMA. (2023) 329(10):827–38. doi: 10.1001/jama.2023.2020
18. Shah SJ, Stafford RS. Current trends of hypertension treatment in the United States. Am J Hypertens. (2017) 30(10):1008–14. doi: 10.1093/ajh/hpx085
19. Wiig H, Swartz MA. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol Rev. (2012) 92(3):1005–60. doi: 10.1152/physrev.00037.2011
20. King KC, Goldstein S. Congestive heart failure and pulmonary edema. In: StatPearls. (2024). Available online at: https://www.ncbi.nlm.nih.gov/pubmed/32119444
21. Platz E, Jhund PS, Campbell RT, McMurray JJ. Assessment and prevalence of pulmonary oedema in contemporary acute heart failure trials: a systematic review. Eur J Heart Fail. (2015) 17(9):906–16. doi: 10.1002/ejhf.321
22. Borlaug BA. Evaluation and management of heart failure with preserved ejection fraction. Nat Rev Cardiol. (2020) 17(9):559–73. doi: 10.1038/s41569-020-0363-2
23. Cuijpers I, Simmonds SJ, van Bilsen M, Czarnowska E, Gonzalez Miqueo A, Heymans S, et al. Microvascular and lymphatic dysfunction in HFpEF and its associated comorbidities. Basic Res Cardiol. (2020) 115(4):39. doi: 10.1007/s00395-020-0798-y
24. Rossitto G, Mary S, McAllister C, Neves KB, Haddow L, Rocchiccioli JP, et al. Reduced lymphatic reserve in heart failure with preserved ejection fraction. J Am Coll Cardiol. (2020) 76(24):2817–29. doi: 10.1016/j.jacc.2020.10.022
25. Henri O, Pouehe C, Houssari M, Galas L, Nicol L, Edwards-Levy F, et al. Selective stimulation of cardiac lymphangiogenesis reduces myocardial edema and fibrosis leading to improved cardiac function following myocardial infarction. Circulation. (2016) 133(15):1484–97; discussion 1497. doi: 10.1161/CIRCULATIONAHA.115.020143
26. Klotz L, Norman S, Vieira JM, Masters M, Rohling M, Dube KN, et al. Cardiac lymphatics are heterogeneous in origin and respond to injury. Nature. (2015) 522(7554):62–7. doi: 10.1038/nature14483
27. Heron C, Dumesnil A, Houssari M, Renet S, Lemarcis T, Lebon A, et al. Regulation and impact of cardiac lymphangiogenesis in pressure-overload-induced heart failure. Cardiovasc Res. (2023) 119(2):492–505. doi: 10.1093/cvr/cvac086
28. Lin QY, Zhang YL, Bai J, Liu JQ, Li HH. VEGF-C/VEGFR-3 axis protects against pressure-overload induced cardiac dysfunction through regulation of lymphangiogenesis. Clin Transl Med. (2021) 11(3):e374. doi: 10.1002/ctm2.374
29. Pu Z, Shimizu Y, Hayashi T, Che Y, Suzuki J, Tsuzuki K, et al. Cardiac lymphatic insufficiency leads to diastolic dysfunction via myocardial morphologic change. JACC Basic Transl Sci. (2023) 8(8):958–72. doi: 10.1016/j.jacbts.2023.01.008
30. Harris NR, Balint L, Dy DM, Nielsen NR, Mendez HG, Aghajanian A, et al. The ebb and flow of cardiac lymphatics: a tidal wave of new discoveries. Physiol Rev. (2023) 103(1):391–432. doi: 10.1152/physrev.00052.2021
31. Joukov V, Kumar V, Sorsa T, Arighi E, Weich H, Saksela O, et al. A recombinant mutant vascular endothelial growth factor-C that has lost vascular endothelial growth factor receptor-2 binding, activation, and vascular permeability activities. J Biol Chem. (1998) 273(12):6599–602. doi: 10.1074/jbc.273.12.6599
32. Nakano T, Nakashima Y, Yonemitsu Y, Sumiyoshi S, Chen YX, Akishima Y, et al. Angiogenesis and lymphangiogenesis and expression of lymphangiogenic factors in the atherosclerotic intima of human coronary arteries. Hum Pathol. (2005) 36(4):330–40. doi: 10.1016/j.humpath.2005.01.001
33. Yeo KP, Lim HY, Thiam CH, Azhar SH, Tan C, Tang Y, et al. Efficient aortic lymphatic drainage is necessary for atherosclerosis regression induced by ezetimibe. Sci Adv. (2020) 6(50). doi: 10.1126/sciadv.abc2697
34. Martel C, Li W, Fulp B, Platt AM, Gautier EL, Westerterp M, et al. Lymphatic vasculature mediates macrophage reverse cholesterol transport in mice. J Clin Invest. (2013) 123(4):1571–9. doi: 10.1172/JCI63685
35. Milasan A, Smaani A, Martel C. Early rescue of lymphatic function limits atherosclerosis progression in ldlr(-/-) mice. Atherosclerosis. (2019) 283:106–19. doi: 10.1016/j.atherosclerosis.2019.01.031
36. Vuorio T, Nurmi H, Moulton K, Kurkipuro J, Robciuc MR, Ohman M, et al. Lymphatic vessel insufficiency in hypercholesterolemic mice alters lipoprotein levels and promotes atherogenesis. Arterioscler Thromb Vasc Biol. (2014) 34(6):1162–70. doi: 10.1161/ATVBAHA.114.302528
37. Huang LH, Elvington A, Randolph GJ. The role of the lymphatic system in cholesterol transport. Front Pharmacol. (2015) 6:182. doi: 10.3389/fphar.2015.00182
38. Randolph GJ, Miller NE. Lymphatic transport of high-density lipoproteins and chylomicrons. J Clin Invest. (2014) 124(3):929–35. doi: 10.1172/JCI71610
39. Russell PS, Hong J, Windsor JA, Itkin M, Phillips ARJ. Renal lymphatics: anatomy, physiology, and clinical implications. Front Physiol. (2019) 10:251. doi: 10.3389/fphys.2019.00251
40. Hasegawa S, Nakano T, Torisu K, Tsuchimoto A, Eriguchi M, Haruyama N, et al. Vascular endothelial growth factor-C ameliorates renal interstitial fibrosis through lymphangiogenesis in mouse unilateral ureteral obstruction. Lab Invest. (2017) 97(12):1439–52. doi: 10.1038/labinvest.2017.77
41. Uchiyama T, Takata S, Ishikawa H, Sawa Y. Altered dynamics in the renal lymphatic circulation of type 1 and type 2 diabetic mice. Acta Histochem Cytochem. (2013) 46(2):97–104. doi: 10.1267/ahc.13006
42. Zarjou A, Black LM, Bolisetty S, Traylor AM, Bowhay SA, Zhang MZ, et al. Dynamic signature of lymphangiogenesis during acute kidney injury and chronic kidney disease. Lab Invest. (2019) 99(9):1376–88. doi: 10.1038/s41374-019-0259-0
43. Rodas L, Barnadas E, Pereira A, Castrejon N, Saurina A, Calls J, et al. The density of renal lymphatics correlates with clinical outcomes in IgA nephropathy. Kidney Int Rep. (2022) 7(4):823–30. doi: 10.1016/j.ekir.2021.12.029
44. Zhang Y, Zhang C, Li L, Liang X, Cheng P, Li Q, et al. Lymphangiogenesis in renal fibrosis arises from macrophages via VEGF-C/VEGFR3-dependent autophagy and polarization. Cell Death Dis. (2021) 12(1):109. doi: 10.1038/s41419-020-03385-x
45. Jafree DJ, Moulding D, Kolatsi-Joannou M, Perretta Tejedor N, Price KL, Milmoe NJ, et al. Spatiotemporal dynamics and heterogeneity of renal lymphatics in mammalian development and cystic kidney disease. Elife. (2019) 8. doi: 10.7554/eLife.48183
46. Balasubbramanian D, Baranwal G, Clark MC, Goodlett BL, Mitchell BM, Rutkowski JM. Kidney-specific lymphangiogenesis increases sodium excretion and lowers blood pressure in mice. J Hypertens. (2020) 38(5):874–85. doi: 10.1097/HJH.0000000000002349
47. Goodlett BL, Kang CS, Yoo E, Navaneethabalakrishnan S, Balasubbramanian D, Love SE, et al. A kidney-targeted nanoparticle to augment renal lymphatic density decreases blood pressure in hypertensive mice. Pharmaceutics. (2021) 14(1). doi: 10.3390/pharmaceutics14010084
48. Lopez Gelston CA, Balasubbramanian D, Abouelkheir GR, Lopez AH, Hudson KR, Johnson ER, et al. Enhancing renal lymphatic expansion prevents hypertension in mice. Circ Res. (2018) 122(8):1094–101. doi: 10.1161/CIRCRESAHA.118.312765
49. Escobedo N, Proulx ST, Karaman S, Dillard ME, Johnson N, Detmar M, et al. Restoration of lymphatic function rescues obesity in Prox1-haploinsufficient mice. JCI Insight. (2016) 1(2). doi: 10.1172/jci.insight.85096
50. Harvey NL, Srinivasan RS, Dillard ME, Johnson NC, Witte MH, Boyd K, et al. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat Genet. (2005) 37(10):1072–81. doi: 10.1038/ng1642
51. Greene AK, Grant FD, Maclellan RA. Obesity-induced lymphedema nonreversible following massive weight loss. Plast Reconstr Surg Glob Open. (2015) 3(6):e426. doi: 10.1097/GOX.0000000000000398
52. Sudduth CL, Greene AK. Current overview of obesity-induced lymphedema. Adv Wound Care (New Rochelle). (2022) 11(7):392–8. doi: 10.1089/wound.2020.1337
53. Cifarelli V, Appak-Baskoy S, Peche VS, Kluzak A, Shew T, Narendran R, et al. Visceral obesity and insulin resistance associate with CD36 deletion in lymphatic endothelial cells. Nat Commun. (2021) 12(1):3350. doi: 10.1038/s41467-021-23808-3
54. Wang H, Chen S, Tang Y, Nie K, Gao Y, Wang Z, et al. Berberine promotes lacteal junction zippering and ameliorates diet-induced obesity through the RhoA/ROCK signaling pathway. Phytomedicine. (2024) 124:155268. doi: 10.1016/j.phymed.2023.155268
55. Zhang F, Zarkada G, Han J, Li J, Dubrac A, Ola R, et al. Lacteal junction zippering protects against diet-induced obesity. Science. (2018) 361(6402):599–603. doi: 10.1126/science.aap9331
56. Burchill MA, Goldberg AR, Tamburini BAJ. Emerging roles for lymphatics in chronic liver disease. Front Physiol. (2019) 10:1579. doi: 10.3389/fphys.2019.01579
57. Escobedo N, Oliver G. The lymphatic vasculature: its role in adipose metabolism and obesity. Cell Metab. (2017) 26(4):598–609. doi: 10.1016/j.cmet.2017.07.020
58. Norden PR, Kume T. The role of lymphatic vascular function in metabolic disorders. Front Physiol. (2020) 11:404. doi: 10.3389/fphys.2020.00404
59. Brouillard P, Witte MH, Erickson RP, Damstra RJ, Becker C, Quere I, et al. Primary lymphoedema. Nat Rev Dis Primers. (2021) 7(1):77. doi: 10.1038/s41572-021-00309-7
60. Makinen T, Boon LM, Vikkula M, Alitalo K. Lymphatic malformations: genetics, mechanisms and therapeutic strategies. Circ Res. (2021) 129(1):136–54. doi: 10.1161/CIRCRESAHA.121.318142
61. Muthuchamy M, Zawieja D. Molecular regulation of lymphatic contractility. Ann N Y Acad Sci. (2008) 1131:89–99. doi: 10.1196/annals.1413.008
62. von der Weid PY, Zawieja DC. Lymphatic smooth muscle: the motor unit of lymph drainage. Int J Biochem Cell Biol. (2004) 36(7):1147–53. doi: 10.1016/j.biocel.2003.12.008
63. Castorena-Gonzalez JA, Scallan JP, Davis MJ. Methods for assessing the contractile function of mouse lymphatic vessels ex vivo. Methods Mol Biol. (2018) 1846:229–48. doi: 10.1007/978-1-4939-8712-2_15
64. Scallan JP, Bouta EM, Rahimi H, Kenney HM, Ritchlin CT, Davis MJ, et al. Ex vivo demonstration of functional deficiencies in popliteal lymphatic vessels from TNF-transgenic mice with inflammatory arthritis. Front Physiol. (2021) 12:745096. doi: 10.3389/fphys.2021.745096
65. Scallan JP, Davis MJ. Genetic removal of basal nitric oxide enhances contractile activity in isolated murine collecting lymphatic vessels. J Physiol. (2013) 591(8):2139–56. doi: 10.1113/jphysiol.2012.250662
66. Sevick-Muraca EM, Kwon S, Rasmussen JC. Emerging lymphatic imaging technologies for mouse and man. J Clin Invest. (2014) 124(3):905–14. doi: 10.1172/JCI71612
67. Polomska AK, Proulx ST. Imaging technology of the lymphatic system. Adv Drug Deliv Rev. (2021) 170:294–311. doi: 10.1016/j.addr.2020.08.013
68. Ahn JH, Cho H, Kim JH, Kim SH, Ham JS, Park I, et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature. (2019) 572(7767):62–6. doi: 10.1038/s41586-019-1419-5
69. Blatter C, Meijer EFJ, Padera TP, Vakoc BJ. Simultaneous measurements of lymphatic vessel contraction, flow and valve dynamics in multiple lymphangions using optical coherence tomography. J Biophotonics. (2018) 11(8):e201700017. doi: 10.1002/jbio.201700017
70. Bruck J, Calaminus C, Hoffmann SHL, Schwenck J, Holstein J, Yazdi AS, et al. Non invasive in vivo monitoring of dimethyl fumarate treatment in EAE by assessing the glucose metabolism in secondary lymphoid organs. Eur J Immunol. (2021) 51(4):1006–9. doi: 10.1002/eji.202048879
71. Pamarthi V, Pabon-Ramos WM, Marnell V, Hurwitz LM. MRI Of the central lymphatic system: indications, imaging technique, and pre-procedural planning. Top Magn Reson Imaging. (2017) 26(4):175–80. doi: 10.1097/RMR.0000000000000130
72. Xia J, Yao J, Wang LV. Photoacoustic tomography: principles and advances. Electromagn Waves (Camb). (2014) 147:1–22. doi: 10.2528/pier14032303
73. Suami H, Chang DW, Yamada K, Kimata Y. Use of indocyanine green fluorescent lymphography for evaluating dynamic lymphatic status. Plast Reconstr Surg. (2011) 127(3):74e–6e. doi: 10.1097/PRS.0b013e3182063639
74. Proulx ST, Ma Q, Andina D, Leroux JC, Detmar M. Quantitative measurement of lymphatic function in mice by noninvasive near-infrared imaging of a peripheral vein. JCI Insight. (2017) 2(1):e90861. doi: 10.1172/jci.insight.90861
75. Robinson HA, Kwon S, Hall MA, Rasmussen JC, Aldrich MB, Sevick-Muraca EM. Non-invasive optical imaging of the lymphatic vasculature of a mouse. J Vis Exp. (2013) (73):e4326. doi: 10.3791/4326
76. Manrique OJ, Bustos SS, Ciudad P, Adabi K, Chen WF, Forte AJ, et al. Overview of lymphedema for physicians and other clinicians: a review of fundamental concepts. Mayo Clin Proc. (2022) 97(10):1920–35. doi: 10.1016/j.mayocp.2020.01.006
77. Snyder EJ, Sarma A, Borst AJ, Tekes A. Lymphatic anomalies in children: update on imaging diagnosis, genetics, and treatment. AJR Am J Roentgenol. (2022) 218(6):1089–101. doi: 10.2214/AJR.21.27200
78. Belgrado JP, Vandermeeren L, Vankerckhove S, Valsamis JB, Malloizel-Delaunay J, Moraine JJ, et al. Near-infrared fluorescence lymphatic imaging to reconsider occlusion pressure of superficial lymphatic collectors in upper extremities of healthy volunteers. Lymphat Res Biol. (2016) 14(2):70–7. doi: 10.1089/lrb.2015.0040
79. Weiler MJ, Cribb MT, Nepiyushchikh Z, Nelson TS, Dixon JB. A novel mouse tail lymphedema model for observing lymphatic pump failure during lymphedema development. Sci Rep. (2019) 9(1):10405. doi: 10.1038/s41598-019-46797-2
80. Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. (2001) 106(3):255–8. doi: 10.1016/s0092-8674(01)00449-4
81. Lim HY, Rutkowski JM, Helft J, Reddy ST, Swartz MA, Randolph GJ, et al. Hypercholesterolemic mice exhibit lymphatic vessel dysfunction and degeneration. Am J Pathol. (2009) 175(3):1328–37. doi: 10.2353/ajpath.2009.080963
82. Weitman ES, Aschen SZ, Farias-Eisner G, Albano N, Cuzzone DA, Ghanta S, et al. Obesity impairs lymphatic fluid transport and dendritic cell migration to lymph nodes. PLoS One. (2013) 8(8):e70703. doi: 10.1371/journal.pone.0070703
83. Torrisi JS, Hespe GE, Cuzzone DA, Savetsky IL, Nitti MD, Gardenier JC, et al. Inhibition of inflammation and iNOS improves lymphatic function in obesity. Sci Rep. (2016) 6:19817. doi: 10.1038/srep19817
84. Vermaelen KY, Carro-Muino I, Lambrecht BN, Pauwels RA. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J Exp Med. (2001) 193(1):51–60. doi: 10.1084/jem.193.1.51
85. Sleigh BC, Manna B. Lymphedema. In: StatPearls. (2024). Available online at: https://www.ncbi.nlm.nih.gov/pubmed/30725924
86. Rockson SG, Keeley V, Kilbreath S, Szuba A, Towers A. Cancer-associated secondary lymphoedema. Nat Rev Dis Primers. (2019) 5(1):22. doi: 10.1038/s41572-019-0072-5
87. Karkkainen MJ, Saaristo A, Jussila L, Karila KA, Lawrence EC, Pajusola K, et al. A model for gene therapy of human hereditary lymphedema. Proc Natl Acad Sci U S A. (2001) 98(22):12677–82. doi: 10.1073/pnas.221449198
88. Bucan A, Frendo M, Ngo MT, Sorensen JA, Holmich LR. Surgical lymphedema models in the mice hindlimb-A systematic review and quality assessment. Microsurgery. (2024) 44(1):e31088. doi: 10.1002/micr.31088
89. Hassanein AH, Sinha M, Neumann CR, Mohan G, Khan I, Sen CK. A murine tail lymphedema model. J Vis Exp. (2021) (168). doi: 10.3791/61848
90. Baik JE, Park HJ, Kataru RP, Savetsky IL, Ly CL, Shin J, et al. TGF-beta1 mediates pathologic changes of secondary lymphedema by promoting fibrosis and inflammation. Clin Transl Med. (2022) 12(6):e758. doi: 10.1002/ctm2.758
91. Wu H, Rahman HNA, Dong Y, Liu X, Lee Y, Wen A, et al. Epsin deficiency promotes lymphangiogenesis through regulation of VEGFR3 degradation in diabetes. J Clin Invest. (2018) 128(9):4025–43. doi: 10.1172/JCI96063
92. Campos JL, Pons G, Rodriguez E, Al-Sakkaf AM, Vela FJ, Pires L, et al. Popliteal vascular lymph node resection in the rabbit hindlimb for secondary lymphedema induction. J Vis Exp. (2022) (189). doi: 10.3791/64576
93. Kataru RP, Baik JE, Park HJ, Wiser I, Rehal S, Shin JY, et al. Regulation of immune function by the lymphatic system in lymphedema. Front Immunol. (2019) 10:470. doi: 10.3389/fimmu.2019.00470
94. Kuzminich Y, Dixon JB. Evaluation of longitudinal lymphatic function changes upon injury in the mouse tail with photodynamic therapy. Cardiovasc Eng Technol. (2023) 14(2):204–16. doi: 10.1007/s13239-022-00645-z
95. Li CY, Kataru RP, Mehrara BJ. Histopathologic features of lymphedema: a molecular review. Int J Mol Sci. (2020) 21(7). doi: 10.3390/ijms21072546
96. Zhou C, Su W, Han H, Li N, Ma G, Cui L. Mouse tail models of secondary lymphedema: fibrosis gradually worsens and is irreversible. Int J Clin Exp Pathol. (2020) 13(1):54–64. Available online at: https://www.ncbi.nlm.nih.gov/pubmed/3205527332055273
97. Cha B, Ho YC, Geng X, Mahamud MR, Chen L, Kim Y, et al. YAP and TAZ maintain PROX1 expression in the developing lymphatic and lymphovenous valves in response to VEGF-C signaling. Development. (2020) 147(23). doi: 10.1242/dev.195453
98. Montenegro-Navarro N, Garcia-Baez C, Garcia-Caballero M. Molecular and metabolic orchestration of the lymphatic vasculature in physiology and pathology. Nat Commun. (2023) 14(1):8389. doi: 10.1038/s41467-023-44133-x
99. Srinivasan RS, Escobedo N, Yang Y, Interiano A, Dillard ME, Finkelstein D, et al. The Prox1-Vegfr3 feedback loop maintains the identity and the number of lymphatic endothelial cell progenitors. Genes Dev. (2014) 28(19):2175–87. doi: 10.1101/gad.216226.113
100. Issa A, Le TX, Shoushtari AN, Shields JD, Swartz MA. Vascular endothelial growth factor-C and C-C chemokine receptor 7 in tumor cell-lymphatic cross-talk promote invasive phenotype. Cancer Res. (2009) 69(1):349–57. doi: 10.1158/0008-5472.CAN-08-1875
101. Iwami D, Brinkman CC, Bromberg JS. Vascular endothelial growth factor c/vascular endothelial growth factor receptor 3 signaling regulates chemokine gradients and lymphocyte migration from tissues to lymphatics. Transplantation. (2015) 99(4):668–77. doi: 10.1097/TP.0000000000000561
102. Delestre F, Venot Q, Bayard C, Fraissenon A, Ladraa S, Hoguin C, et al. Alpelisib administration reduced lymphatic malformations in a mouse model and in patients. Sci Transl Med. (2021) 13(614):eabg0809. doi: 10.1126/scitranslmed.abg0809
103. Martinez-Corral I, Zhang Y, Petkova M, Ortsater H, Sjoberg S, Castillo SD, et al. Blockade of VEGF-C signaling inhibits lymphatic malformations driven by oncogenic PIK3CA mutation. Nat Commun. (2020) 11(1):2869. doi: 10.1038/s41467-020-16496-y
104. Sung DC, Chen M, Dominguez MH, Mahadevan A, Chen X, Yang J, et al. Sinusoidal and lymphatic vessel growth is controlled by reciprocal VEGF-C-CDH5 inhibition. Nat Cardiovasc Res. (2022) 1(11):1006–21. doi: 10.1038/s44161-022-00147-0
105. Hominick D, Silva A, Khurana N, Liu Y, Dechow PC, Feng JQ, et al. VEGF-C promotes the development of lymphatics in bone and bone loss. Elife. (2018) 7. doi: 10.7554/eLife.34323
106. van den Borne SW, van de Schans VA, Strzelecka AE, Vervoort-Peters HT, Lijnen PM, Cleutjens JP, et al. Mouse strain determines the outcome of wound healing after myocardial infarction. Cardiovasc Res. (2009) 84(2):273–82. doi: 10.1093/cvr/cvp207
107. Villalba-Orero M, Lopez-Olaneta MM, Gonzalez-Lopez E, Padron-Barthe L, Gomez-Salinero JM, Garcia-Prieto J, et al. Lung ultrasound as a translational approach for non-invasive assessment of heart failure with reduced or preserved ejection fraction in mice. Cardiovasc Res. (2017) 113(10):1113–23. doi: 10.1093/cvr/cvx090
108. Petkova M, Ferby I, Makinen T. Lymphatic malformations: mechanistic insights and evolving therapeutic frontiers. J Clin Invest. (2024) 134:6. doi: 10.1172/JCI172844
109. Fang J, Dagenais SL, Erickson RP, Arlt MF, Glynn MW, Gorski JL, et al. Mutations in FOXC2 (MFH-1), a forkhead family transcription factor, are responsible for the hereditary lymphedema-distichiasis syndrome. Am J Hum Genet. (2000) 67(6):1382–8. doi: 10.1086/316915
110. Fastre E, Lanteigne LE, Helaers R, Giacalone G, Revencu N, Dionyssiou D, et al. Splice-site mutations in VEGFC cause loss of function and nonne-milroy-like primary lymphedema. Clin Genet. (2018) 94(1):179–81. doi: 10.1111/cge.13204
111. Gonzalez-Garay ML, Aldrich MB, Rasmussen JC, Guilliod R, Lapinski PE, King PD, et al. A novel mutation in CELSR1 is associated with hereditary lymphedema. Vasc Cell. (2016) 8:1. doi: 10.1186/s13221-016-0035-5
112. Irrthum A, Karkkainen MJ, Devriendt K, Alitalo K, Vikkula M. Congenital hereditary lymphedema caused by a mutation that inactivates VEGFR3 tyrosine kinase. Am J Hum Genet. (2000) 67(2):295–301. doi: 10.1086/303019
113. Leppanen VM, Brouillard P, Korhonen EA, Sipila T, Jha SK, Revencu N, et al. Characterization of ANGPT2 mutations associated with primary lymphedema. Sci Transl Med. (2020) 12:560. doi: 10.1126/scitranslmed.aax8013
114. Szuba A, Shin WS, Strauss HW, Rockson S. The third circulation: radionuclide lymphoscintigraphy in the evaluation of lymphedema. J Nucl Med. (2003) 44(1):43–57. Available online at: https://www.ncbi.nlm.nih.gov/pubmed/1251587612515876
115. Unno N, Nishiyama M, Suzuki M, Tanaka H, Yamamoto N, Sagara D, et al. A novel method of measuring human lymphatic pumping using indocyanine green fluorescence lymphography. J Vasc Surg. (2010) 52(4):946–52. doi: 10.1016/j.jvs.2010.04.067
116. Grenier JM, Borst AJ, Sheppard SE, Snyder KM, Li D, Surrey LF, et al. Pathogenic variants in PIK3CA are associated with clinical phenotypes of kaposiform lymphangiomatosis, generalized lymphatic anomaly, and central conducting lymphatic anomaly. Pediatr Blood Cancer. (2023):e30419. doi: 10.1002/pbc.30419
117. Luks VL, Kamitaki N, Vivero MP, Uller W, Rab R, Bovee JV, et al. Lymphatic and other vascular malformative/overgrowth disorders are caused by somatic mutations in PIK3CA. J Pediatr. (2015) 166(4):1048–54.e1–5. doi: 10.1016/j.jpeds.2014.12.069
118. Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, et al. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci U S A. (1995) 92(8):3566–70. doi: 10.1073/pnas.92.8.3566
119. Dixelius J, Makinen T, Wirzenius M, Karkkainen MJ, Wernstedt C, Alitalo K, et al. Ligand-induced vascular endothelial growth factor receptor-3 (VEGFR-3) heterodimerization with VEGFR-2 in primary lymphatic endothelial cells regulates tyrosine phosphorylation sites. J Biol Chem. (2003) 278(42):40973–9. doi: 10.1074/jbc.M304499200
120. Salameh A, Galvagni F, Bardelli M, Bussolino F, Oliviero S. Direct recruitment of CRK and GRB2 to VEGFR-3 induces proliferation, migration, and survival of endothelial cells through the activation of ERK, AKT, and JNK pathways. Blood. (2005) 106(10):3423–31. doi: 10.1182/blood-2005-04-1388
121. Nilsson I, Bahram F, Li X, Gualandi L, Koch S, Jarvius M, et al. VEGF Receptor 2/-3 heterodimers detected in situ by proximity ligation on angiogenic sprouts. EMBO J. (2010) 29(8):1377–88. doi: 10.1038/emboj.2010.30
122. Dumont DJ, Jussila L, Taipale J, Lymboussaki A, Mustonen T, Pajusola K, et al. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science. (1998) 282(5390):946–9. doi: 10.1126/science.282.5390.946
123. Gordon K, Spiden SL, Connell FC, Brice G, Cottrell S, Short J, et al. FLT4/VEGFR3 and Milroy disease: novel mutations, a review of published variants and database update. Hum Mutat. (2013) 34(1):23–31. doi: 10.1002/humu.22223
124. Houssari M, Dumesnil A, Tardif V, Kivela R, Pizzinat N, Boukhalfa I, et al. Lymphatic and immune cell cross-talk regulates cardiac recovery after experimental myocardial infarction. Arterioscler Thromb Vasc Biol. (2020) 40(7):1722–37. doi: 10.1161/ATVBAHA.120.314370
125. Shimizu Y, Polavarapu R, Eskla KL, Pantner Y, Nicholson CK, Ishii M, et al. Impact of lymphangiogenesis on cardiac remodeling after ischemia and reperfusion injury. J Am Heart Assoc. (2018) 7(19):e009565. doi: 10.1161/JAHA.118.009565
126. Vieira JM, Norman S, Del Campo V, Cahill C, Barnette TJ, Gunadasa-Rohling DN, et al. The cardiac lymphatic system stimulates resolution of inflammation following myocardial infarction. J Clin Invest. (2018) 128(8):3402–12. doi: 10.1172/JCI97192
127. Keller TCST, Lim L, Shewale SV, McDaid K, Marti-Pamies I, Tang AT, et al. Genetic blockade of lymphangiogenesis does not impair cardiac function after myocardial infarction. J Clin Invest. (2021) 131(20). doi: 10.1172/JCI147070
128. Song L, Chen X, Swanson TA, LaViolette B, Pang J, Cunio T, et al. Lymphangiogenic therapy prevents cardiac dysfunction by ameliorating inflammation and hypertension. Elife. (2020) 9. doi: 10.7554/eLife.58376
129. Huang JL, Woolf AS, Kolatsi-Joannou M, Baluk P, Sandford RN, Peters DJ, et al. Vascular endothelial growth factor C for polycystic kidney diseases. J Am Soc Nephrol. (2016) 27(1):69–77. doi: 10.1681/ASN.2014090856
130. Donnan MD, Deb DK, Onay T, Scott RP, Ni E, Zhou Y, et al. Formation of the glomerular microvasculature is regulated by VEGFR-3. Am J Physiol Renal Physiol. (2023) 324(1):F91–105. doi: 10.1152/ajprenal.00066.2022
131. Liu J, Yu C. Lymphangiogenesis and lymphatic barrier dysfunction in renal fibrosis. Int J Mol Sci. (2022) 23(13). doi: 10.3390/ijms23136970
132. Hartiala P, Suominen S, Suominen E, Kaartinen I, Kiiski J, Viitanen T, et al. Phase 1 lymfactin(®) study: short-term safety of combined adenoviral VEGF-C and lymph node transfer treatment for upper extremity lymphedema. J Plast Reconstr Aesthet Surg. (2020) 73(9):1612–21. doi: 10.1016/j.bjps.2020.05.009
133. Lahteenvuo M, Honkonen K, Tervala T, Tammela T, Suominen E, Lahteenvuo J, et al. Growth factor therapy and autologous lymph node transfer in lymphedema. Circulation. (2011) 123(6):613–20. doi: 10.1161/CIRCULATIONAHA.110.965384
134. Leppapuska IM, Hartiala P, Suominen S, Suominen E, Kaartinen I, Maki M, et al. Phase 1 lymfactin(R) study: 24-month efficacy and safety results of combined adenoviral VEGF-C and lymph node transfer treatment for upper extremity lymphedema. J Plast Reconstr Aesthet Surg. (2022) 75(11):3938–45. doi: 10.1016/j.bjps.2022.08.011
135. McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. (1998) 393(6683):333–9. doi: 10.1038/30666
136. Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, et al. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. (1993) 192(2):553–60. doi: 10.1006/bbrc.1993.1451
137. Balint L, Nelson-Maney NP, Tian Y, Serafin SD, Caron KM. Clinical potential of adrenomedullin signaling in the cardiovascular system. Circ Res. (2023) 132(9):1185–202. doi: 10.1161/CIRCRESAHA.123.321673
138. Nagaya N, Satoh T, Nishikimi T, Uematsu M, Furuichi S, Sakamaki F, et al. Hemodynamic, renal, and hormonal effects of adrenomedullin infusion in patients with congestive heart failure. Circulation. (2000) 101(5):498–503. doi: 10.1161/01.cir.101.5.498
139. Temmesfeld-Wollbruck B, Hocke AC, Suttorp N, Hippenstiel S. Adrenomedullin and endothelial barrier function. Thromb Haemost. (2007) 98(5):944–51. doi: 10.1160/th07-02-0128
140. Fritz-Six KL, Dunworth WP, Li M, Caron KM. Adrenomedullin signaling is necessary for murine lymphatic vascular development. J Clin Invest. (2008) 118(1):40–50. doi: 10.1172/JCI33302
141. Nikitenko LL, Shimosawa T, Henderson S, Makinen T, Shimosawa H, Qureshi U, et al. Adrenomedullin haploinsufficiency predisposes to secondary lymphedema. J Invest Dermatol. (2013) 133(7):1768–76. doi: 10.1038/jid.2013.47
142. Jin D, Harada K, Ohnishi S, Yamahara K, Kangawa K, Nagaya N. Adrenomedullin induces lymphangiogenesis and ameliorates secondary lymphoedema. Cardiovasc Res. (2008) 80(3):339–45. doi: 10.1093/cvr/cvn228
143. Trincot CE, Xu W, Zhang H, Kulikauskas MR, Caranasos TG, Jensen BC, et al. Adrenomedullin induces cardiac lymphangiogenesis after myocardial infarction and regulates cardiac edema via connexin 43. Circ Res. (2019) 124(1):101–13. doi: 10.1161/CIRCRESAHA.118.313835
144. Temmesfeld-Wollbruck B, Brell B, David I, Dorenberg M, Adolphs J, Schmeck B, et al. Adrenomedullin reduces vascular hyperpermeability and improves survival in rat septic shock. Intensive Care Med. (2007) 33(4):703–10. doi: 10.1007/s00134-007-0561-y
145. Geven C, van Lier D, Blet A, Peelen R, Ten Elzen B, Mebazaa A, et al. Safety, tolerability and pharmacokinetics/pharmacodynamics of the adrenomedullin antibody adrecizumab in a first-in-human study and during experimental human endotoxaemia in healthy subjects. Br J Clin Pharmacol. (2018) 84(9):2129–41. doi: 10.1111/bcp.13655
146. Laterre PF, Pickkers P, Marx G, Wittebole X, Meziani F, Dugernier T, et al. Safety and tolerability of non-neutralizing adrenomedullin antibody adrecizumab (HAM8101) in septic shock patients: the AdrenOSS-2 phase 2a biomarker-guided trial. Intensive Care Med. (2021) 47(11):1284–94. doi: 10.1007/s00134-021-06537-5
147. Kubo K, Tokashiki M, Kuwasako K, Tamura M, Tsuda S, Kubo S, et al. Biological properties of adrenomedullin conjugated with polyethylene glycol. Peptides. (2014) 57:118–21. doi: 10.1016/j.peptides.2014.05.005
148. Nishikimi T. Adrenomedullin in the kidney-renal physiological and pathophysiological roles. Curr Med Chem. (2007) 14(15):1689–99. doi: 10.2174/092986707780830943
149. Sogbe-Diaz ME, Diaz-Lopez EE. Adrenomedullin in the kidney: physiology and pathophysiology. Invest Clin. (2016) 57(1):66–76. Available online at: https://www.ncbi.nlm.nih.gov/pubmed/27382803 (Adrenomedulina en la fisiologia y fisiopatologia renales).27382803
150. Bech EM, Voldum-Clausen K, Pedersen SL, Fabricius K, Rudkjaer LCB, Hansen HH, et al. Adrenomedullin and glucagon-like peptide-1 have additive effects on food intake in mice. Biomed Pharmacother. (2019) 109:167–73. doi: 10.1016/j.biopha.2018.10.040
151. Dai HB, Wang FZ, Kang Y, Sun J, Zhou H, Gao Q, et al. Adrenomedullin attenuates inflammation in white adipose tissue of obese rats through receptor-mediated PKA pathway. Obesity (Silver Spring). (2021) 29(1):86–97. doi: 10.1002/oby.23012
152. Dennis EA, Norris PC. Eicosanoid storm in infection and inflammation. Nat Rev Immunol. (2015) 15(8):511–23. doi: 10.1038/nri3859
153. Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. (1997) 387(6633):620–4. doi: 10.1038/42506
154. Yokomizo T, Kato K, Terawaki K, Izumi T, Shimizu T. A second leukotriene B(4) receptor, BLT2. A new therapeutic target in inflammation and immunological disorders. J Exp Med. (2000) 192(3):421–32. doi: 10.1084/jem.192.3.421
155. Shimizu T. Lipid mediators in health and disease: enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu Rev Pharmacol Toxicol. (2009) 49:123–50. doi: 10.1146/annurev.pharmtox.011008.145616
156. Tian W, Rockson SG, Jiang X, Kim J, Begaye A, Shuffle EM, et al. Leukotriene B(4) antagonism ameliorates experimental lymphedema. Sci Transl Med. (2017) 9(389). doi: 10.1126/scitranslmed.aal3920
157. Rockson SG, Tian W, Jiang X, Kuznetsova T, Haddad F, Zampell J, et al. Pilot studies demonstrate the potential benefits of antiinflammatory therapy in human lymphedema. JCI Insight. (2018) 3(20). doi: 10.1172/jci.insight.123775
158. Kantor TG. Ketoprofen: a review of its pharmacologic and clinical properties. Pharmacotherapy. (1986) 6(3):93–103. doi: 10.1002/j.1875-9114.1986.tb03459.x
159. Cao E, Watt MJ, Nowell CJ, Quach T, Simpson JS, De Melo Ferreira V, et al. Mesenteric lymphatic dysfunction promotes insulin resistance and represents a potential treatment target in obesity. Nat Metab. (2021) 3(9):1175–88. doi: 10.1038/s42255-021-00457-w
160. Souma T, Thomson BR, Heinen S, Carota IA, Yamaguchi S, Onay T, et al. Context-dependent functions of angiopoietin 2 are determined by the endothelial phosphatase VEPTP. Proc Natl Acad Sci U S A. (2018) 115(6):1298–303. doi: 10.1073/pnas.1714446115
161. Holopainen T, Saharinen P, D'Amico G, Lampinen A, Eklund L, Sormunen R, et al. Effects of angiopoietin-2-blocking antibody on endothelial cell-cell junctions and lung metastasis. J Natl Cancer Inst. (2012) 104(6):461–75. doi: 10.1093/jnci/djs009
162. Korhonen EA, Murtomaki A, Jha SK, Anisimov A, Pink A, Zhang Y, et al. Lymphangiogenesis requires Ang2/tie/PI3K signaling for VEGFR3 cell-surface expression. J Clin Invest. (2022) 132:15. doi: 10.1172/JCI155478
163. Zheng W, Nurmi H, Appak S, Sabine A, Bovay E, Korhonen EA, et al. Angiopoietin 2 regulates the transformation and integrity of lymphatic endothelial cell junctions. Genes Dev. (2014) 28(14):1592–603. doi: 10.1101/gad.237677.114
164. Zheng W, Aspelund A, Alitalo K. Lymphangiogenic factors, mechanisms, and applications. J Clin Invest. (2014) 124(3):878–87. doi: 10.1172/JCI71603
165. Liu C, Li J. The physiological functions of lymphangiocrine signals. Trends Endocrinol Metab. (2023) 34(6):319–20. doi: 10.1016/j.tem.2023.03.004
166. Liu X, De la Cruz E, Gu X, Balint L, Oxendine-Burns M, Terrones T, et al. Lymphoangiocrine signals promote cardiac growth and repair. Nature. (2020) 588(7839):705–11. doi: 10.1038/s41586-020-2998-x
167. Akagi K, Ikeda Y, Miyazaki M, Abe T, Kinoshita J, Maehara Y, et al. Vascular endothelial growth factor-C (VEGF-C) expression in human colorectal cancer tissues. Br J Cancer. (2000) 83(7):887–91. doi: 10.1054/bjoc.2000.1396
168. Nouguerede E, Berenguer C, Garcia S, Bennani B, Delfino C, Nanni I, et al. Expression of adrenomedullin in human colorectal tumors and its role in cell growth and invasion in vitro and in xenograft growth in vivo. Cancer Med. (2013) 2(2):196–207. doi: 10.1002/cam4.51
169. Su JL, Yen CJ, Chen PS, Chuang SE, Hong CC, Kuo IH, et al. The role of the VEGF-C/VEGFR-3 axis in cancer progression. Br J Cancer. (2007) 96(4):541–5. doi: 10.1038/sj.bjc.6603487
170. Vazquez R, Riveiro ME, Berenguer-Daize C, O'Kane A, Gormley J, Touzelet O, et al. Targeting adrenomedullin in oncology: a feasible strategy with potential as much more than an alternative anti-angiogenic therapy. Front Oncol. (2020) 10:589218. doi: 10.3389/fonc.2020.589218
171. Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. (1983) 219(4587):983–5. doi: 10.1126/science.6823562
172. Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. (2006) 312(5):549–60. doi: 10.1016/j.yexcr.2005.11.012
173. Heinolainen K, Karaman S, D'Amico G, Tammela T, Sormunen R, Eklund L, et al. VEGFR3 modulates vascular permeability by controlling VEGF/VEGFR2 signaling. Circ Res. (2017) 120(9):1414–25. doi: 10.1161/CIRCRESAHA.116.310477
174. Onions KL, Gamez M, Buckner NR, Baker SL, Betteridge KB, Desideri S, et al. VEGFC Reduces glomerular albumin permeability and protects against alterations in VEGF receptor expression in diabetic nephropathy. Diabetes. (2019) 68(1):172–87. doi: 10.2337/db18-0045
175. Randolph GJ, Ivanov S, Zinselmeyer BH, Scallan JP. The lymphatic system: integral roles in immunity. Annu Rev Immunol. (2017) 35:31–52. doi: 10.1146/annurev-immunol-041015-055354
Keywords: lymphatics, drug discovery, pharmacology, preclinical model of cardiovascular disease, cardiovascular-kidney-metabolic syndrome (CKM)
Citation: Fowler JWM, Song L, Tam K and Roth Flach RJ (2024) Targeting lymphatic function in cardiovascular-kidney-metabolic syndrome: preclinical methods to analyze lymphatic function and therapeutic opportunities. Front. Cardiovasc. Med. 11:1412857. doi: 10.3389/fcvm.2024.1412857
Received: 5 April 2024; Accepted: 24 May 2024;
Published: 10 June 2024.
Edited by:
Hong Chen, Boston Children's Hospital and Harvard Medical School, United StatesReviewed by:
Pengchun Yu, Oklahoma Medical Research Foundation, United StatesYao Gao, Boston Children's Hospital and Harvard Medical School, United States
© 2024 Fowler, Song, Tam and Roth Flach. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rachel J. Roth Flach, cmFjaGVsLnJvdGhmbGFjaEBwZml6ZXIuY29t
 Joseph Wayne M. Fowler
Joseph Wayne M. Fowler LouJin Song
LouJin Song Kelly Tam
Kelly Tam Rachel J. Roth Flach
Rachel J. Roth Flach