- 1Cardiovascular R&D Centre—UnIC@RISE, Department of Surgery and Physiology, Faculty of Medicine of the University of Porto, Porto, Portugal
- 2Department of Cardiology, Local Health Unit of Gaia and Espinho, Vila Nova de Gaia, Portugal
- 3Department of Cardiology, Local Health Unit of Sao Joao, Porto, Portugal
- 4MEDCIDS—Department of Community Medicine, Information and Health Decision Sciences, Faculty of Medicine, University of Porto, Porto, Portugal
- 5CINTESIS@RISE—Health Research Network, MEDCIDS, Faculty of Medicine, University of Porto, Porto, Portugal
Reverse left ventricular (LV) remodeling after aortic valve replacement (AVR), in patients with aortic stenosis, is well-documented as an important prognostic factor. With this systematic review and meta-analysis, we aimed to characterize the response of the unloaded LV after AVR. We searched on MEDLINE/PubMed and Web of Science for studies reporting echocardiographic findings before and at least 1 month after AVR for the treatment of aortic stenosis. In total, 1,836 studies were identified and 1,098 were screened for inclusion. The main factors of interest were structural and dynamic measures of the LV and aortic valve. We performed a random-effects meta-analysis to compute standardized mean differences (SMD) between follow-up and baseline values for each outcome. Twenty-seven studies met the eligibility criteria, yielding 11,751 patients. AVR resulted in reduced mean aortic gradient (SMD: −38.23 mmHg, 95% CI: −39.88 to −36.58, I2=92%), LV mass (SMD: −37.24 g, 95% CI: −49.31 to −25.18, I2=96%), end-diastolic LV diameter (SMD: −1.78 mm, 95% CI: −2.80 to −0.76, I2=96%), end-diastolic LV volume (SMD: −1.6 ml, 95% CI: −6.68 to 3.51, I2=91%), increased effective aortic valve area (SMD: 1.10 cm2, 95% CI: 1.01 to 1.20, I2=98%), and LV ejection fraction (SMD: 2.35%, 95% CI: 1.31 to 3.40%, I2=94.1%). Our results characterize the extent to which reverse remodeling is expected to occur after AVR. Notably, in our study, reverse remodeling was documented as soon as 1 month after AVR.
1 Introduction
Aortic stenosis (AS) is the most common acquired valvopathy in the Western world (1). Its incidence increases with age, and its prevalence is expected to rise in the future (2).
AS is not an isolated valve disease but a more complex and broad pathology involving the myocardium. AS progression is associated with left ventricular (LV) remodeling, which is the myocardial response to increased afterload (2). Initially, LV remodeling is a compensatory response to a persistent obstacle to systolic ejection. The sustained increased pressure and hemodynamic load lead to the classical development of LV hypertrophy. This initial adaptation allows for a reduction in wall stress and maintenance of cardiac output. After this stage, persistent obstruction leads to maladaptive LV remodeling, causing gradual deterioration of diastolic and systolic functions (1). Clinically, this process can translate into various symptoms, including death due to heart failure or arrhythmic events (2). In other words, maladaptive LV response negatively impacts the prognosis of AS patients regarding survival and cardiovascular events (3).
The only effective treatment for severe AS is aortic valve replacement (AVR), which can be performed either surgically (SAVR) or percutaneously via transcatheter AV implantation (TAVI). AVR aims to eliminate the LV obstruction and ultimately revert this inadequate LV response (2). After AVR, the extension of the achieved reverse LV remodeling is a major determinant of symptoms and outcomes (2). Its prognostic importance has been reported in several randomized trials (2, 4, 5). Transthoracic echocardiography (TTE) is the gold standard method to characterize AS severity, LV remodeling, and LV reverse remodeling after AVR. These LV adaptations comprise several changes in echocardiographic parameters, such as LV mass, cavity dimensions and volumes, wall thicknesses, and left ventricular ejection fraction (LVEF) (1). Unfortunately, data to predict LV response after AVR are lacking.
In this systematic review and meta-analysis, we aim to assess the extent of left ventricular remodeling at pre-determined time points post-procedure in patients with aortic stenosis who underwent AVR. The measured variables of interest included effective aortic valve area (AVA), mean aortic gradient (MAG), left ventricular mass (LVM), LVEF, and end-diastolic left ventricular diameter (EDLVD) and volume (EDLVV).
2 Methods
2.1 Eligibility and search strategy
This systematic review and meta-analysis was conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement (6).
The literature search was conducted on 15 March 2022 in two electronic databases: MEDLINE (through PubMed) and Web of Science. The search was conducted with no restrictions on language or year of publication. Full details of the search are presented in Table 1.

Table 1. Keywords used to perform the query in the two databases used in this study (date of search: 15 March 2022).
Studies were included if they reported echocardiographic findings before and at least 1 month after SAVR or TAVI for the treatment of AS. This time interval was chosen to allow acute changes after the procedure to resolve and for reverse remodeling to occur (7). Furthermore, patient evaluation had to be performed at pre-determined time points post-procedure, i.e., at either 1, 3, 6, or 12 months.
Studies also needed to report at least one outcome variable of interest for the measurement of the left ventricle reverse remodeling to be included, namely, left ventricular dimensions or ejection fraction.
We excluded all non-human studies, case–control studies, case reports, and reviews. Studies without a predefined follow-up period and with fewer than 100 patients were also excluded.
2.2 Study selection, data collection process, and study outcomes
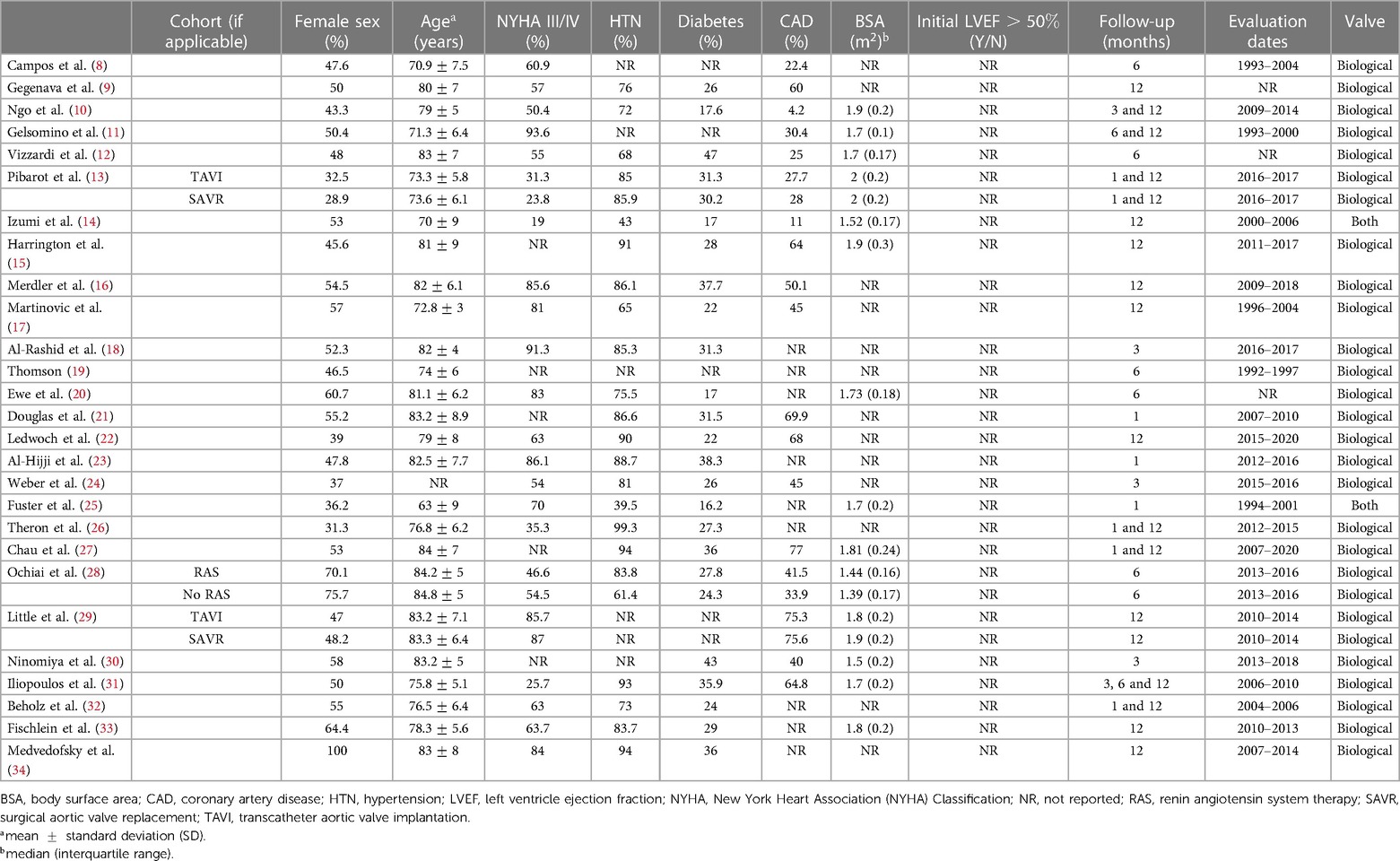
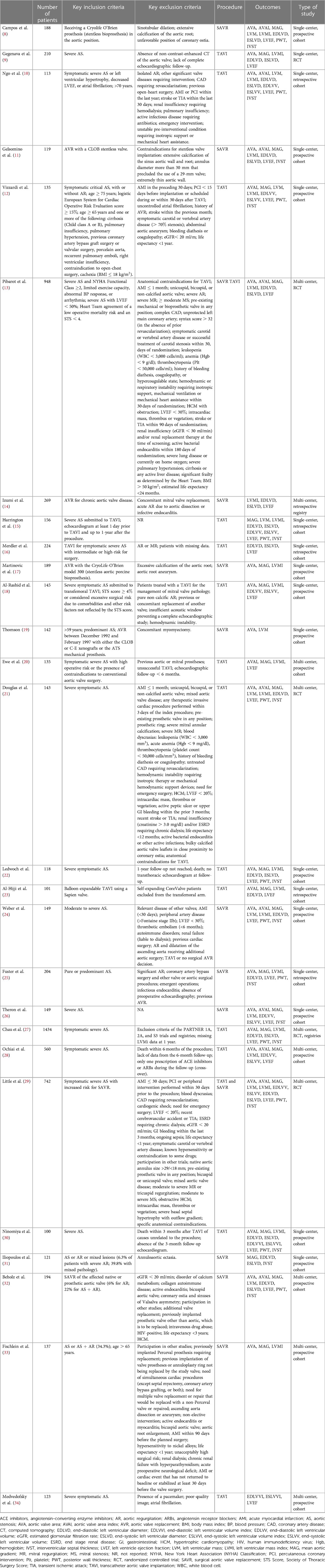
Two investigators (FSN and CAM) independently reviewed each study by title and abstract and then by full-text reading. Discordant decisions were managed by consensus. Authors of primary studies were contacted for clarification if relevant data were missing. For each primary study, two investigators (FSN and CAM) independently performed data extraction. We extracted the following information: study design (clinical setting, duration of follow-up, and number of patients included), Baseline characteristics of the population (Table 2) [eligibility criteria; age; gender; New York Heart Association (NYHA) class; body surface area (BSA); and frequency of hypertension, diabetes mellitus (DM), coronary heart disease, and other comorbidities], intervention (details on SAVR or TAVI procedures), and outcome data of interest. The latter included effective AVA, MAG, EDLVD, EDLVV, LVM, and LVEF.
2.3 Risk of bias assessment
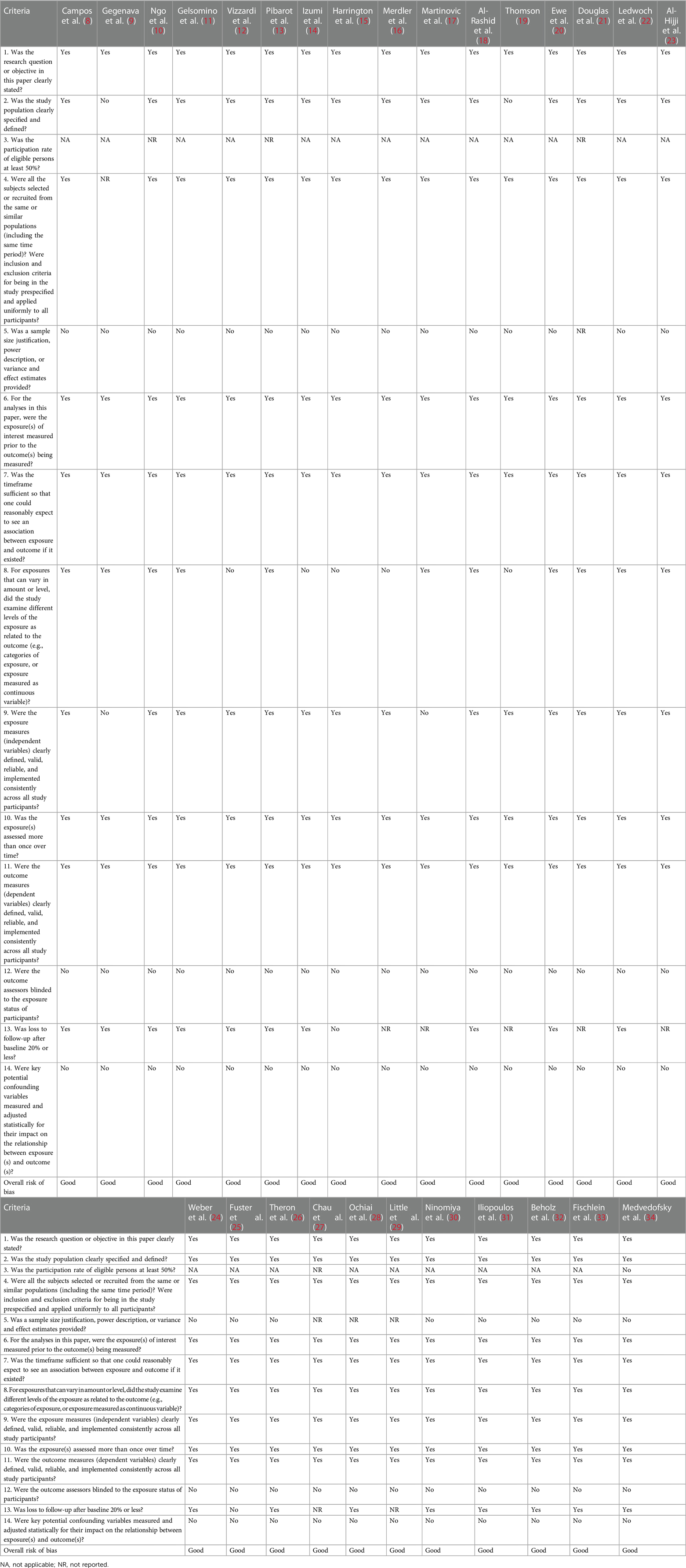
We used the Study Quality Assessment Tool for Observational Cohort Studies from the National Institutes of Health to categorize several domains for all the eligible studies. The overall risk of bias was independently assigned to each study by two investigators (FSN, CAM) and classified into “good,” “fair,” and “poor”, as detailed in Table 3.
2.4 Statistical analysis
We performed a random-effects meta-analysis using the restricted maximum likelihood approach to compute pooled mean differences (MD) or standardized mean differences (SMD) between post-follow-up and baseline values for each outcome. Heterogeneity was assessed by the Cochran Q statistic p-value and the I2 statistic: a p-value <0.10 and an I2 >50% were considered to represent substantial heterogeneity. Sources of heterogeneity were explored using univariable meta-regression models, with tested covariates including the publication year, mean age of the participants, percentage of females, average BSA, percentage of patients in NYHA classes III/IV, and percentage of patients with other comorbidities such as hypertension, diabetes, and coronary heart disease. In addition, we performed subgroup analyses for the follow-up period and the initial LVEF (classes were categorized into two groups: lower than 50% and higher than 50%). All statistical analyses were performed using the meta package of R software (35, 36).
3 Results
In total, 1,836 publications were identified through our search of MEDLINE/PubMed (944 records) and Web of Science (892 records) databases. After removing the duplicates, 1,098 records remained. Following the title and abstract screening, we selected 67 articles for full-text review. After excluding articles that did not meet the inclusion criteria, we ended up with 27 primary studies (see Figure 1 for the PRISMA 2020 flow diagram and Table 4 for a summary table of the included studies) (8–34).
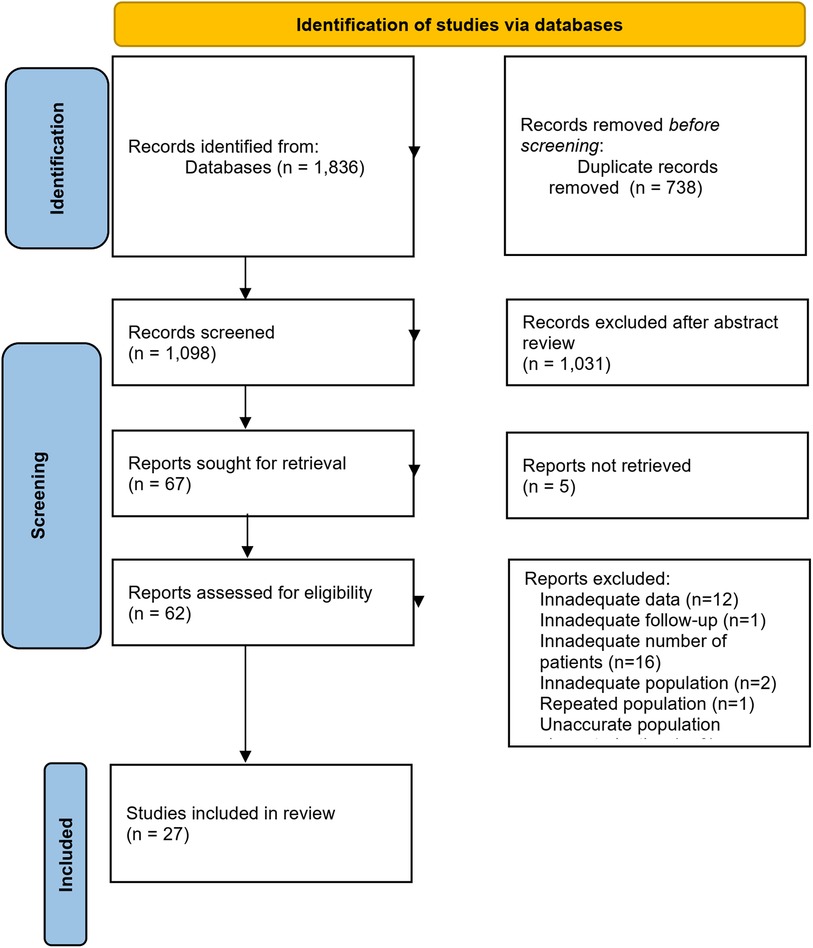
Figure 1. Flowchart for the study selection process. From: (37).
Since some studies contained more than one distinct population, the search yielded 39 independent patient cohorts. The studies were published between 1998 and 2020, assessing 11,751 patients who completed echocardiographic assessment before and at least 1 month post-AVR.
3.1 Effective aortic valve area and mean aortic gradient
While this work is related to left ventricular remodeling after AVR, we chose to start by reporting measures related to AVR, such as aortic valve area and gradient. This ensures that the studies assessed comparable conditions and demonstrated similar improvements after valve obstruction is resolved. By doing so, we aimed to establish a consistent baseline for analyzing left ventricular remodeling parameters.
Our meta-analytical results indicate that, after AVR, there was an increase in the effective aortic valve area and a decrease in the mean aortic gradient. Based on 26 cohorts (n=6,726 at baseline, Figure 2), the pooled SMD for effective aortic valve area was 1.10 cm2 (95% CI: 1.01–1.20, p<0.0001, I2=98%, Cochran’s Q p-value <0.0001), corresponding to a significant increase after AVR, albeit with substantial heterogeneity.Univariate meta-regression identified publication year, age, hypertension, NYHA class III or IV, DM, type of AVR, and EF >50% as potential moderators of heterogeneity (see Supplementary Table S1 for subgroup and heterogeneity analysis and Supplementary Table S2 for meta-regression).
In studies assessing SAVR (15 cohorts), AVA increased by 1.19 cm2 (95% CI: 1.05– 1.33), while in TAVI patients (11 cohorts), AVA increased by 0.99 cm2 (95% CI: 0.91– 1.06). The results were significantly different between SAVR and TAVI patients (p=0.01). No significant differences were observed when our results were stratified according to the follow-up period (Figure 2).
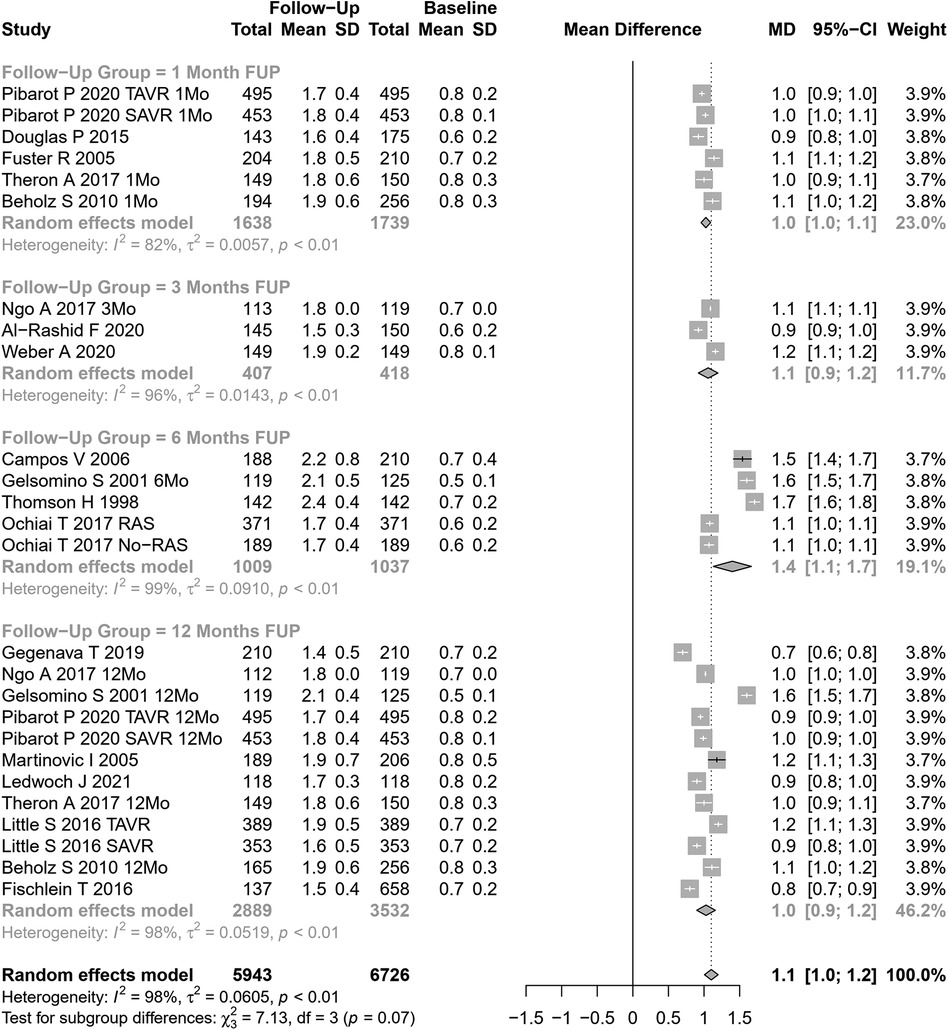
The mean aortic gradient was assessed in 33 cohorts (n=10,480 patients at baseline, Figure 3). The pooled SMD for mean aortic gradient was −38.23 mmHg (95% CI: −39.88 to −36.58 mmHg, p<0.0001, I2=92%, Cochran’s Q p-value <0.0001), indicating a significant decrease after AVR, but with substantial heterogeneity. Univariate meta-regression identified publication year and coronary artery disease as potential moderators of heterogeneity (see Supplementary Table S1 for subgroup and heterogeneity analysis and Supplementary Table S2 for meta-regression). Subgroup analyses showed a trend for differences according to follow-up periods (p=0.06; Figure 3) but not according to the type of AVR (p=0.16).
3.2 Parameters on left ventricular reverse remodeling
3.2.1 Left ventricular mass
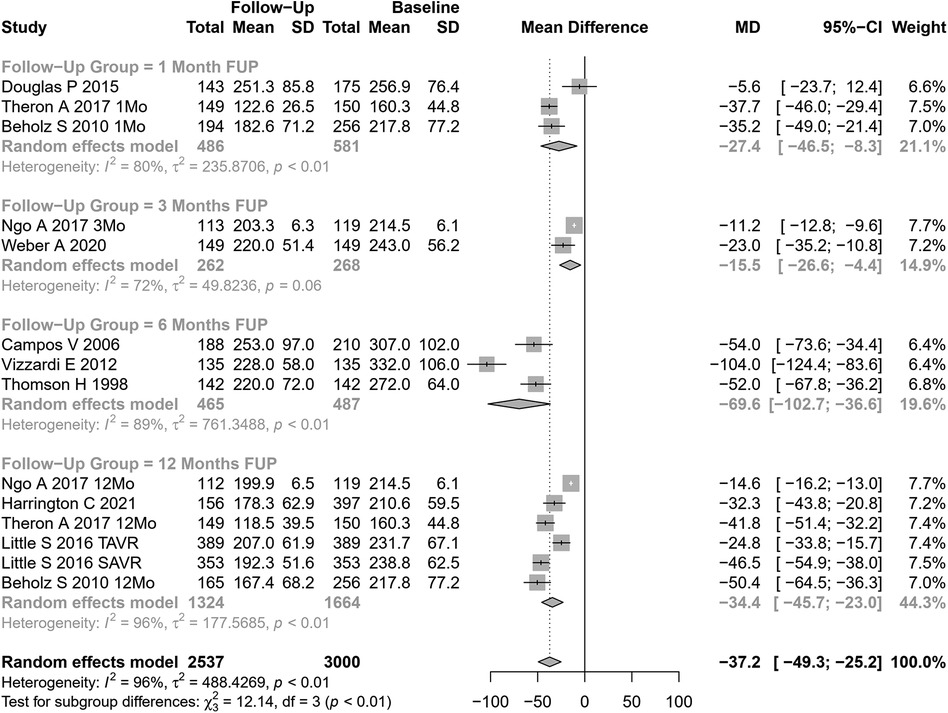
LVM change after AVR was analyzed in 14 cohorts (Figure 4). The pooled SMD for LVM was −37.24 g (95% CI: −49.31 to −25.18, p<0.0001; I2=96%, Cochran’s Q p-value <0.0001), indicating a significant decrease after AVR, albeit with substantial heterogeneity.
Performing subgroup analysis according to follow-up periods, significant differences were observed (p=0.007). However, the values involved were relatively small (and may represent different samples evaluated at various time points and not a cohort evaluated prospectively through time): LVM reduction of 27 g at 1 month, 16 g at 3 months, 70 g at 6 months, and 34 g at 12 months. Performing subgroup analysis according to the type of AVR, no significant differences were observed (p=0.49).
Univariate meta-regression identified publication year and DM as potential moderators of heterogeneity (see Supplementary Table S1 for subgroup and heterogeneity analysis and Supplementary Table S2 for meta-regression).
3.2.2 Left ventricular ejection fraction
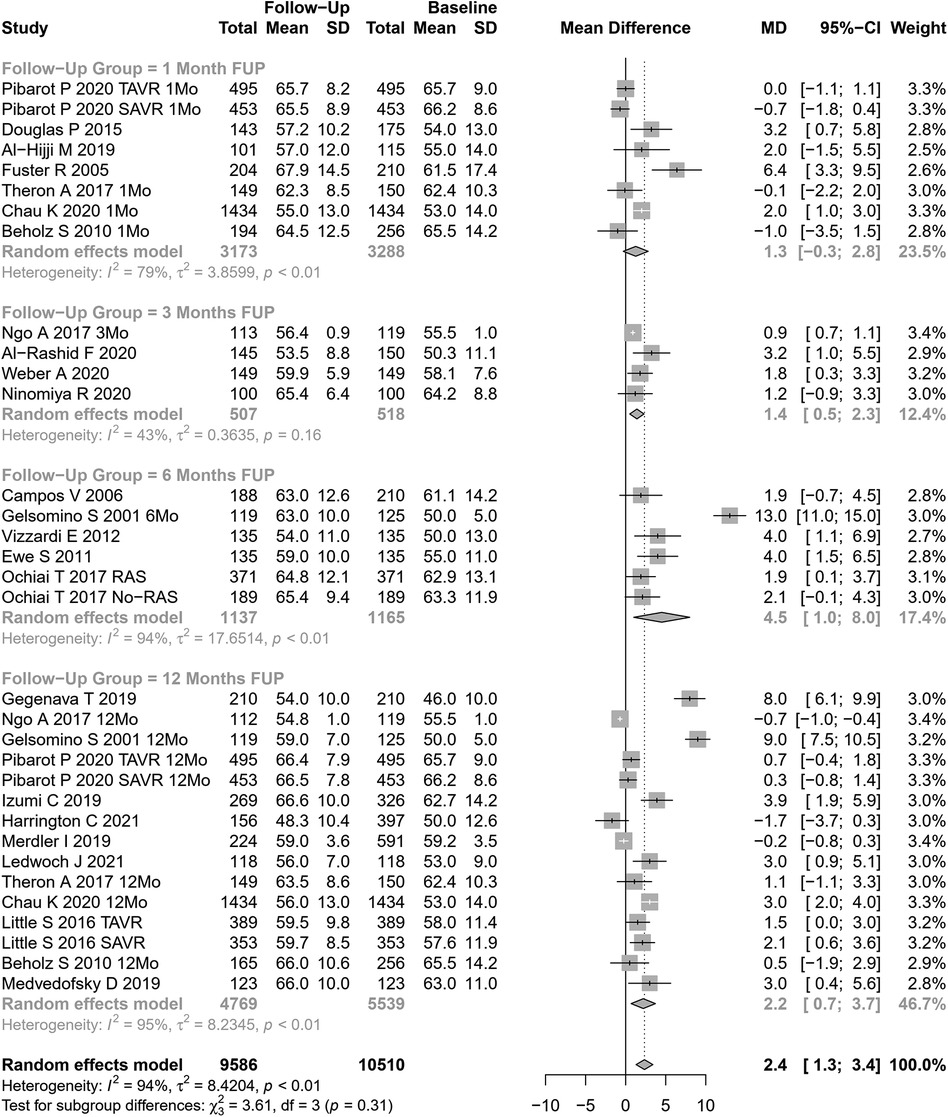
LVEF change after AVR was assessed in 33 cohorts (n=10,510 participants at baseline, Figure 5). The pooled SMD for LVEF was 2.35% (95% CI: 1.31%–3.40%, p<0.0001; I2=94.1%, Cochran’s Q p-value <0.0001), indicating a significant increase after AVR, although with substantial heterogeneity. Performing subgroup analysis according to follow-up periods or the type of AVR, no significant differences were observed (p=0.31 and p=0.42, respectively).
Univariate meta-regression identified publication year and NYHA classification III or IV as potential moderators of heterogeneity (Supplementary Table S1 for subgroup and heterogeneity analysis and Supplementary Table S2 for meta-regression).
3.2.3 End-diastolic left ventricular diameter and volume
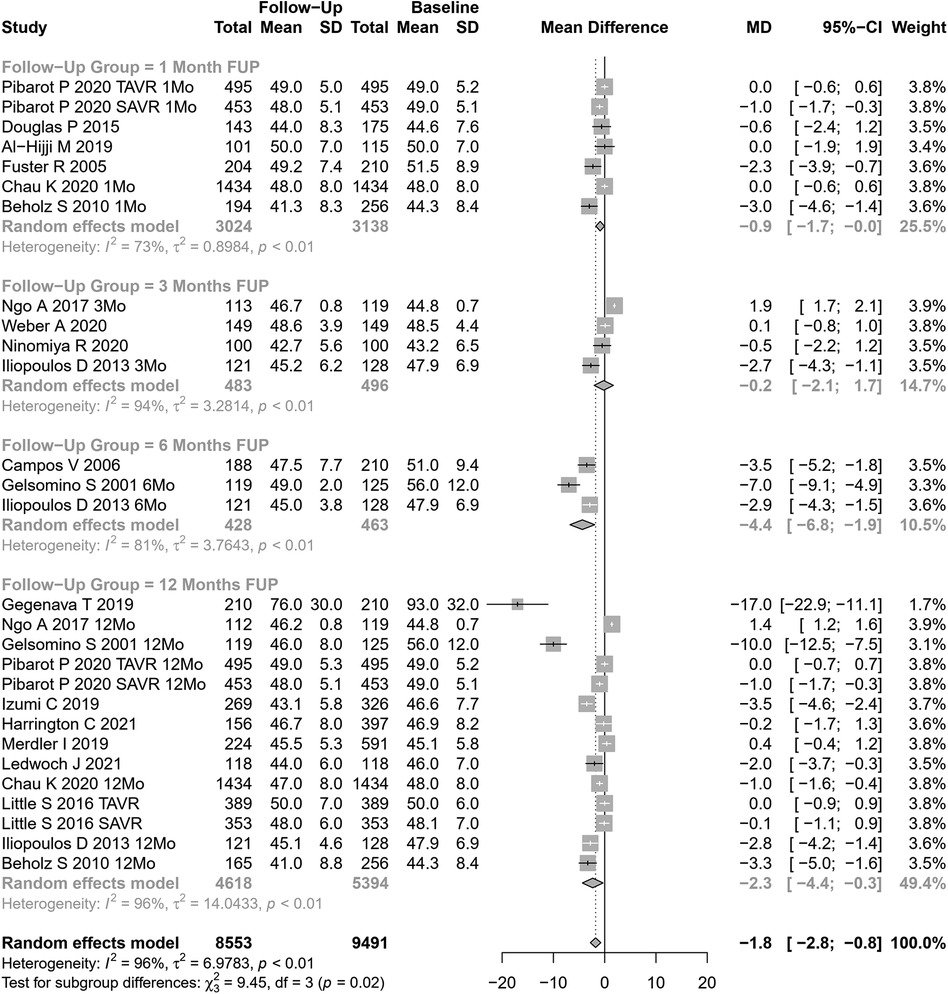
EDLVD change after AVR was assessed in 28 cohorts (n=9,491 participants at baseline, Figure 6). The pooled SMD for EDLVD was −1.78 mm (95% CI: −2.80 to −0.76, p=0.0006; I2=96%, Cochran’s Q p-value <0.0001), indicating a significant decrease after AVR, although with substantial heterogeneity.
Stratifying our results according to follow-up periods, significant differences were observed (p=0.02). However, the values involved were relatively small (and may represent different samples evaluated at various time points, rather than a cohort evaluated prospectively through time): EDLVD decreased by 0.88 mm at 1 month, 0.18 mm at 3 months, 6.77 mm at 6 months, and 2.33 mm at 12 months.
Significant differences were also observed in performing subgroup analysis according to the type of AVR (p=0.0002). In studies assessing SAVR (14 cohorts), EDLVD decreased by 2.92 mm (95% CI: −4.21 to −1.63) vs 0.16 mm in TAVI patients (14 cohorts; 95% CI: −0.87 to −0.55). Univariable meta-regression identified publication year, age, and coronary artery disease as potential moderators of heterogeneity (see Supplementary Table S1 for subgroup and heterogeneity analysis and Supplementary Table S2 for meta-regression).
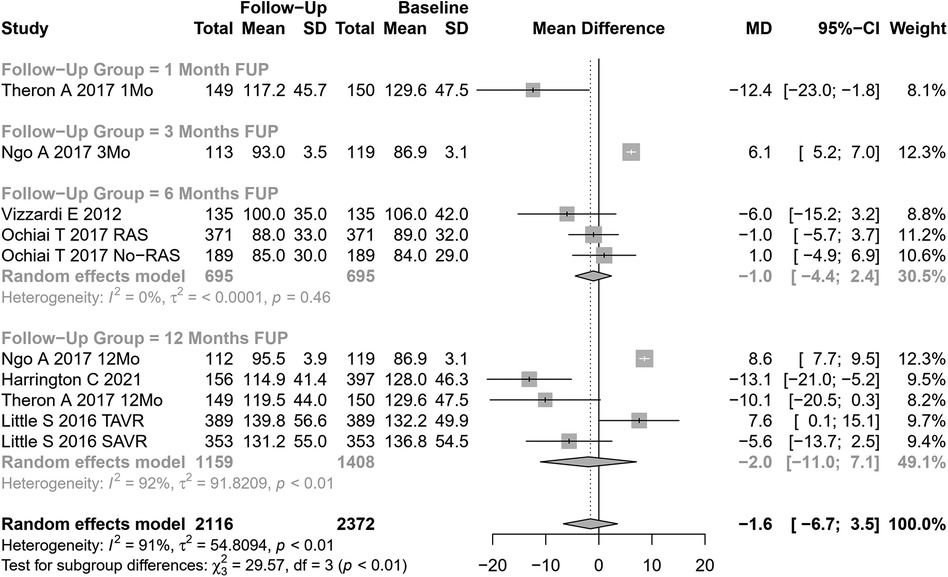
EDLVV change after AVR was assessed in 10 cohorts (n=2,116 participants at baseline, Figure 7). The pooled SMD for EDLVV was −1.6 ml (95% CI: −6.68 to 3.51, p=0.54; I2=91%, Cochran’s Q p-value <0.001), indicating a non-significant decrease after AVR.
Univariate meta-regression identified the type of AVR, coronary artery disease, and hypertension as potential moderators of heterogeneity (see Supplementary Table S1 for subgroup and heterogeneity analysis and Supplementary Table S2 for meta-regression).
4 Discussion
In this study, we assessed the echocardiographic parameters of the unloaded LV after AVR. Notably, LV reverse remodeling was evident at the earliest time point evaluated (1 month after AVR). Several of the evaluated parameters were consistent with reverse remodeling, namely, the significant reduction observed in LVM and EDLVD, and LVEF improvement. A trend for EDLVV reduction was also observed. Our results are consistent with those from Mehdipoor et al. (38), who reported indexed LVM reduction and increased LVEF within 6–15 months after TAVI on 10 primary studies involving 305 patients.
Patient follow-up after AVR typically focusses on monitoring valve hemodynamics over time, specifically the evolution of the effective aortic valve area, gradient, and left ventricular function. Reverse left ventricular remodeling is not commonly assessed in routine clinical practice post-AVR. This is partly due to the lack of established norms for what constitutes “normal” left ventricular remodeling after AVR. This study aimed to establish a framework for the expected changes in certain parameters following AVR.
Finally, it is important to note that, despite its infrequent use, the extent of left ventricular remodeling has significant prognostic implications post-AVR. Patients who do not exhibit improvements in LVEF and reductions in left ventricular mass and dimensions after AVR are at a higher risk for increased cardiovascular events (14, 39). In our opinion, further attention should be paid to the predictors of inadequate left ventricular remodeling after AVR, as this may aid in defining other criteria for AVR other than the severity of obstruction and left ventricular function.
4.1 Strengths and limitations
To our knowledge, this is the most extensive systematic review and meta-analysis conducted to assess the reverse LV remodeling profile in patients who underwent AVR. We excluded studies without a predefined follow-up period to obtain the most robust results possible. We performed meta-regression and subgroup analyses to explore sources of heterogeneity, identifying several variables in this context. To minimize publication and information bias, we searched different electronic bibliographic databases without applying exclusion criteria based on the date or language of publication and contacted authors whenever relevant information was missing.
Limitations of this meta-analysis are related to three main factors: the inherent source of variability regarding to measurements performed by echocardiography, the incomplete characterization of patients in some of the included studies, and the significant heterogeneity observed in our results.
First, a significant source of variability may be related to the fact that primary studies used TTE as the imaging LV assessment method, which is affected by inter-observer and intra-observer variability that can be a source of heterogeneity. For example, the non-significant reduction in LV volume compared to a significant reduction in LV diameter likely reflects the higher variability in echocardiographic measurements of three-dimensional parameters like LV volume, which tend to have a higher standard deviation compared to two-dimensional measurements like LV diameter. This variability could obscure significant findings. An analysis based on studies using CMR to evaluate LV could possibly reduce the heterogeneity across studies. However, it would be an undoubtedly less clinically useful analysis (40–42). Finally, another possible source of heterogeneity is the presence of prosthesis–patient mismatch (PPM), which could influence the results by leading to worse hemodynamic function and LV reverse remodeling. Our study did not analyze PPM because it was not reported in most studies.
Second, other non-evaluated factors may influence the extent of left ventricular remodeling after AVR. In this work, we showed that LV reverse remodeling may differ according to several patient characteristics, namely, age, hypertension, diabetes, coronary heart disease, and NYHA classification. However, the data available for analysis were sparse on information regarding the severity and duration of aortic stenosis, pre-existing LV remodeling, the presence of atrial fibrillation, associated valvular heart diseases, diastolic function, and patient–prosthesis mismatch that may also contribute to the extent of reverse remodeling. Furthermore, by using a summary or aggregate data from study publications, our meta-analysis may fail to identify patient characteristics that might be significant predictors of adequate LV remodeling. For example, previous works have shown that women have a more favorable LV remodeling after AVR than men (43). However, the available aggregate data were insufficient to characterize the impact of gender on LV reverse remodeling after AVR.
Finally, significant heterogeneity among studies was observed. Even though meta-regression and subgroup analysis were performed to identify possible variables that differed between studies and could explain the differences between primary studies, it must be noted that the included studies were mainly observational studies and included patients based on convenient criteria (i.e., patients who underwent AVR at a given institution), which added significant heterogeneity that cannot be controlled using regression techniques.
5 Conclusion
This is the most extensive systematic review and meta-analysis assessing reverse LV remodeling after AVR. Echocardiography demonstrates reverse LV remodeling as soon as 1 month after AVR, with reductions in MAG, LVM, and EDLVD, and improvement in AVA and LVEF.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
FSN: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. CAM: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. AIP: Writing – original draft, Writing – review & editing. BS-P: Writing – original draft, Writing – review & editing. AB: Writing – original draft, Writing – review & editing. JRS: Writing – original draft, Writing – review & editing. FS: Writing – original draft, Writing – review & editing. FM: Writing – original draft, Writing – review & editing. AL-M: Writing – original draft, Writing – review & editing. CS: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
CS is supported by a grant from Bolsas de Doutoramento em Medicina from José de Mello Saúde, Portugal.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1407566/full#supplementary-material
References
1. Abecasis J, Pinto DG, Ramos S, Masci PG, Cardim N, Gil V, et al. Left ventricular remodeling in degenerative aortic valve stenosis. Curr Probl Cardiol. (2021) 46:100801. doi: 10.1016/j.cpcardiol.2021.100801
2. Treibel TA, Badiani S, Lloyd G, Moon JC. Multimodality imaging markers of adverse myocardial remodeling in aortic stenosis. JACC Cardiovasc Imaging. (2019) 12:1532–48. doi: 10.1016/j.jcmg.2019.02.034
3. Jin XY, Petrou M, Hu JT, Nicol ED, Pepper JR. Challenges and opportunities in improving left ventricular remodelling and clinical outcome following surgical and trans-catheter aortic valve replacement. Front Med. (2021) 15(3):416–37. doi: 10.1007/s11684-021-0852-7
4. Gavina C, Falcao-Pires I, Pinho P, Manso MC, Goncalves A, Rocha-Goncalves F, et al. Relevance of residual left ventricular hypertrophy after surgery for isolated aortic stenosis. Eur J Cardiothorac Surg. (2016) 49:952–9. doi: 10.1093/ejcts/ezv240
5. Ikonomidis I, Tsoukas A, Parthenakis F, Gournizakis A, Kassimatis A, Rallidis L, et al. Four year follow up of aortic valve replacement for isolated aortic stenosis: a link between reduction in pressure overload, regression of left ventricular hypertrophy, and diastolic function. Heart. (2001) 86:309–16. doi: 10.1136/heart.86.3.309
6. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. (2009) 6:e1000100. doi: 10.1371/journal.pmed.1000100
7. Dobson LE, Musa TA, Uddin A, Fairbairn TA, Swoboda PP, Erhayiem B, et al. Acute reverse remodelling after transcatheter aortic valve implantation: a link between myocardial fibrosis and left ventricular mass regression. Can J Cardiol. (2016) 32:1411–8. doi: 10.1016/j.cjca.2016.04.009
8. Campos V, Adrio B, Estévez F, Mosquera VX, Pérez J, Cuenca JJ, et al. Aortic valve replacement with a Cryolife O’Brien stentless bioprosthesis. Rev Esp Cardiol. (2007) 60:45–50. doi: 10.1157/13097925
9. Gegenava T, Vollema EM, van Rosendael A, Abou R, Goedemans L, van der Kley F, et al. Changes in left ventricular global longitudinal strain after transcatheter aortic valve implantation according to calcification burden of the thoracic aorta. J Am Soc Echocardiogr. (2019) 32:1058–66. doi: 10.1016/j.echo.2019.05.011
10. Ngo A, Hassager C, Thyregod HGH, Søndergaard L, Olsen PS, Steinbrüchel D, et al. Differences in left ventricular remodelling in patients with aortic stenosis treated with transcatheter aortic valve replacement with CoreValve prostheses compared to surgery with porcine or bovine biological prostheses. Eur Heart J Cardiovasc Imaging. (2018) 19:39–46. doi: 10.1093/ehjci/jew321
11. Gelsomino S, Frassani R, Porreca L, Morocutti G, Morelli A, Livi U. Early and midterm results of model 300 Cryolife O’Brien stentless porcine aortic bioprosthesis. Ann Thorac Surg. (2001) 71:S297–301. doi: 10.1016/s0003-4975(01)02526-7
12. Vizzardi E, D’Aloia A, Fiorina C, Bugatti S, Parrinello G, Carlo MD, et al. Early regression of left ventricular mass associated with diastolic improvement after transcatheter aortic valve implantation. J Am Soc Echocardiogr. (2012) 25:1091–8. doi: 10.1016/j.echo.2012.06.010
13. Pibarot P, Salaun E, Dahou A, Avenatti E, Guzzetti E, Annabi MS, et al. Echocardiographic results of transcatheter versus surgical aortic valve replacement in low-risk patients. Circulation. (2020) 141:1527–37. doi: 10.1161/CIRCULATIONAHA.119.044574
14. Izumi C, Kitai T, Kume T, Onishi T, Yuda S, Hirata K, et al. Effect of left ventricular reverse remodeling on long-term outcomes after aortic valve replacement. Am J Cardiol. (2019) 124:105–12. doi: 10.1016/j.amjcard.2019.04.010
15. Harrington CM, Sorour N, Gottbrecht M, Nagy A, Kovell LC, Truong V, et al. Effect of transaortic valve intervention for aortic stenosis on myocardial mechanics. Am J Cardiol. (2021) 146:56–61. doi: 10.1016/j.amjcard.2021.01.021
16. Merdler I, Loewenstein I, Hochstadt A, Morgan S, Schwarzbard S, Sadeh B, et al. Effectiveness and safety of transcatheter aortic valve implantation in patients with aortic stenosis and variable ejection fractions (<40%, 40%–49%, and >50%). Am J Cardiol. (2020) 125:583–8. doi: 10.1016/j.amjcard.2019.10.059
17. Martinovic I, Farah I, Everlien M, Lindemann S, Knez I, Wittlinger T, et al. Eight-year results after aortic valve replacement with the Cryolife-O’Brien stentless aortic porcine bioprosthesis. J Thorac Cardiovasc Surg. (2005) 130:777–82. doi: 10.1016/j.jtcvs.2005.05.011
18. Al-Rashid F, Totzeck M, Saur N, Jánosi RA, Lind A, Mahabadi AA, et al. Global longitudinal strain is associated with better outcomes in transcatheter aortic valve replacement. BMC Cardiovasc Disord. (2020) 20:267. doi: 10.1186/s12872-020-01556-4
19. Thomson H. Haemodynamics and left ventricular mass regression: a comparison of the stentless, stented and mechanical aortic valve replacement. Eur J Cardiothorac Surg. (1998) 13:572–5. doi: 10.1016/S1010-7940(98)00058-X
20. Ewe SH, Muratori M, Delgado V, Pepi M, Tamborini G, Fusini L, et al. Hemodynamic and clinical impact of prosthesis-patient mismatch after transcatheter aortic valve implantation. J Am Coll Cardiol. (2011) 58:1910–8. doi: 10.1016/j.jacc.2011.08.027
21. Douglas PS, Hahn RT, Pibarot P, Weissman NJ, Stewart WJ, Xu K, et al. Hemodynamic outcomes of transcatheter aortic valve replacement and medical management in severe, inoperable aortic stenosis: a longitudinal echocardiographic study of cohort B of the PARTNER trial. J Am Soc Echocardiogr. (2015) 28:210–7. doi: 10.1016/j.echo.2014.10.009
22. Ledwoch J, Fröhlich C, Olbrich I, Poch F, Thalmann R, Fellner C, et al. Impact of sinus rhythm versus atrial fibrillation on left ventricular remodeling after transcatheter aortic valve replacement. Clin Res Cardiol. (2021) 110:689–98. doi: 10.1007/s00392-021-01810-5
23. Al-Hijji MA, Zack CJ, Nkomo VT, Pislaru SV, Pellikka PA, Reeder GS, et al. Left ventricular remodeling and function after transapical versus transfemoral transcatheter aortic valve replacement. Catheter Cardiovasc Interv. (2019) 94:738–44. doi: 10.1002/ccd.28074
24. Weber A, Büttner AL, Rellecke P, Petrov G, Albert A, Sixt SU, et al. Osteopontin as novel biomarker for reversibility of pressure overload induced left ventricular hypertrophy. Biomark Med. (2020) 14:513–23. doi: 10.2217/bmm-2019-0410
25. Fuster RG, Argudo JAM, Albarova OG, Sos FH, López SC, Codoñer MB, et al. Patient-prosthesis mismatch in aortic valve replacement: really tolerable? Eur J Cardiothorac Surg. (2005) 27:441–9. doi: 10.1016/j.ejcts.2004.11.022
26. Theron A, Ravis E, Grisoli D, Jaussaud N, Morera P, Candolfi P, et al. Rapid-deployment aortic valve replacement for severe aortic stenosis: 1-year outcomes in 150 patients. Interact Cardiovasc Thorac Surg. (2017) 25:68–74. doi: 10.1093/icvts/ivx050
27. Chau KH, Douglas PS, Pibarot P, Hahn RT, Khalique OK, Jaber WA, et al. Regression of left ventricular mass after transcatheter aortic valve replacement. J Am Coll Cardiol. (2020) 75:2446–58. doi: 10.1016/j.jacc.2020.03.042
28. Ochiai T, Saito S, Yamanaka F, Shishido K, Tanaka Y, Yamabe T, et al. Renin–angiotensin system blockade therapy after transcatheter aortic valve implantation. Heart. (2018) 104:644–51. doi: 10.1136/heartjnl-2017-311738
29. Little SH, Oh JK, Gillam L, Sengupta PP, Orsinelli DA, Cavalcante JL, et al. Self-expanding transcatheter aortic valve replacement versus surgical valve replacement in patients at high risk for surgery. Circ Cardiovasc Interv. (2016) 9(6):e003426. doi: 10.1161/CIRCINTERVENTIONS.115.003426
30. Ninomiya R, Orii M, Fujiwara J, Yoshizawa M, Nakajima Y, Ishikawa Y, et al. Sex-related differences in cardiac remodeling and reverse remodeling after transcatheter aortic valve implantation in patients with severe aortic stenosis in a Japanese population. Int Heart J. (2020) 61:961–9. doi: 10.1536/ihj.20-154
31. Iliopoulos DC, Deveja AR, Androutsopoulou V, Filias V, Kastelanos E, Satratzemis V, et al. Single-center experience using the Freedom SOLO aortic bioprosthesis. J Thorac Cardiovasc Surg. (2013) 146:96–102. doi: 10.1016/j.jtcvs.2012.06.041
32. Beholz S, Repossini A, Livi U, Schepens M, Gabry ME, Matschke K, et al. The Freedom SOLO valve for aortic valve replacement: clinical and hemodynamic results from a prospective multicenter trial. J Heart Valve Dis. (2010) 19:115–23. WE—Science Citation Index Expanded (SCI). 20329497.20329497
33. Fischlein T, Meuris B, Hakim-Meibodi K, Misfeld M, Carrel T, Zembala M, et al. The sutureless aortic valve at 1 year: a large multicenter cohort study. J Thorac Cardiovasc Surg. (2016) 151:1617–26. doi: 10.1016/j.jtcvs.2015.12.064
34. Medvedofsky D, Koifman E, Miyoshi T, Rogers T, Wang Z, Goldstein SA, et al. Usefulness of longitudinal strain to assess remodeling of right and left cardiac chambers following transcatheter aortic valve implantation. Am J Cardiol. (2019) 124:253–61. doi: 10.1016/j.amjcard.2019.04.029
35. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing (2023).
36. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. (2019) 22:153–60. doi: 10.1136/ebmental-2019-300117
37. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n7133782057
38. Mehdipoor G, Chen S, Chatterjee S, Torkian P, Ben-Yehuda O, Leon MB, et al. Cardiac structural changes after transcatheter aortic valve replacement: systematic review and meta-analysis of cardiovascular magnetic resonance studies. J Cardiovasc Magn Reson. (2020) 22:41. doi: 10.1186/s12968-020-00629-9
39. Wilde NG, Mauri V, Piayda K, Al-Kassou B, Shamekhi J, Maier O, et al. Left ventricular reverse remodeling after transcatheter aortic valve implantation in patients with low-flow low-gradient aortic stenosis. Hellenic J Cardiol. (2023) 74:1–7. doi: 10.1016/j.hjc.2023.04.009
40. Rigolli M, Anandabaskaran S, Christiansen JP, Whalley GA. Bias associated with left ventricular quantification by multimodality imaging: a systematic review and meta-analysis. Open Heart. (2016) 3:e000388. doi: 10.1136/openhrt-2015-000388
41. Malik SB, Chen N. Transthoracic echocardiography: pitfalls and limitations as delineated at cardiac CT and MR imaging. Radiographics. (2017) 37:383–406. doi: 10.1148/rg.2017160105
42. Aurich M, André F, Keller M, Greiner S, Hess A, Buss SJ, et al. Assessment of left ventricular volumes with echocardiography and cardiac magnetic resonance imaging: real-life evaluation of standard versus new semiautomatic methods. J Am Soc Echocardiogr. (2014) 27:1017–24. doi: 10.1016/j.echo.2014.07.006
Keywords: aortic stenosis, transcatheter aortic valve implantation (TAVI), surgical aortic valve replacement (SAVR), reverse left ventricle remodeling, echocardiography
Citation: Sousa Nunes F, Amaral Marques C, Isabel Pinho A, Sousa-Pinto B, Beco A, Ricardo Silva J, Saraiva F, Macedo F, Leite-Moreira A and Sousa C (2024) Reverse left ventricular remodeling after aortic valve replacement for aortic stenosis: a systematic review and meta-analysis. Front. Cardiovasc. Med. 11:1407566. doi: 10.3389/fcvm.2024.1407566
Received: 26 March 2024; Accepted: 6 June 2024;
Published: 4 July 2024.
Edited by:
Giulia Elena Mandoli, University of Siena, ItalyReviewed by:
Yohann Bohbot, Centre Hospitalier Universitaire (CHU) d’Amiens, FranceMaria Martin Fernandez, Central University Hospital of Asturias, Spain
© 2024 Sousa Nunes, Amaral Marques, Isabel Pinho, Sousa-Pinto, Beco, Ricardo Silva, Saraiva, Macedo, Leite-Moreira and Sousa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: C. Amaral Marques, catmarques@med.up.pt
†These authors share co-first authorship
 F. Sousa Nunes
F. Sousa Nunes