
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 13 May 2024
Sec. Pediatric Cardiology
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1404432
Objective: Soluble suppression of tumorigenicity 2 (sST2) is associated with the prognosis of some cardiac diseases, but studies on sST2 and the prognosis of patients with myocarditis are rare. This study investigated the relationship between major adverse cardiovascular events (MACEs) and sST2 during hospitalization in pediatric patients with myocarditis.
Methods: This was a single-center retrospective cohort study. A total of 252 patients aged ≤14 years diagnosed with myocarditis were enrolled. Events during the hospitalization were defined as MACEs (all-cause death > new heart failure > ventricular arrhythmia).
Results: A total of 25 people had MACEs during their hospital stay. The mortality during hospitalization was 6/23 (26%) in patients with heart failure and 3/10 (30%) in patients with ventricular arrhythmias. After including these risk factors in a multivariate logistic regression analysis, NT-proBNP (OR 4.323; 95% CI, 2.433–7.679; p < 0.001) and sST2 (OR 1.020; 95% CI, 1.003–1.037; p = 0.022) remained statistically significant and were independent risk factors for MACEs during hospitalization in pediatric myocarditis patients.
Conclusions: Elevated levels of NT-proBNP and sST2 were independently associated with major adverse cardiovascular events during hospitalization in children with myocarditis, and both showed good predictive efficacy.
Myocarditis is an inflammatory disease that causes necrosis or degeneration of cardiomyocytes (1), and it is a common cause of morbidity and mortality in pediatric patients (2). A national registry study from Europe showed that the incidence of acute myocarditis in children is as high as 1.95 per 100,000 per year and is still rising (3, 4). Previous studies have shown that the mortality rate in children with myocarditis is between 7% and 17%, and the rate of need for mechanical circulatory support or heart transplantation can reach 30% (2). In children hospitalized for myocarditis, congestive heart failure and ventricular arrhythmias are common complications of myocarditis and are associated with increased mortality (5, 6). Therefore, exploring more predictors for major adverse cardiovascular events (MACEs) in children with myocarditis would be valuable.
It is well known that the development of myocarditis is closely related to inflammation (7, 8). Soluble suppression of tumorigenicity 2 (sST2) acts as a decoy receptor that promotes the inflammatory response by binding to interleukin (IL)-33 and inhibits the cardioprotective effects of IL-33 (9–12). sST2 is now considered a valuable prognostic and monitoring tool, and it was included in the American Heart Association's updated heart failure guidelines (13). Indeed, sST2 is prognostically relevant in a variety of diseases. Van Vark et al. showed an association between sST2 and all-cause mortality in a 1-year follow-up of 496 patients with acute heart failure (14). In patients with acute myocardial infarction, sST2 levels within 24 h were considered an early marker of prognosis (15). In patients with acute stroke, sST2 was an independent predictor of poor prognosis and all-cause mortality within the first 12 months (16). In a recent study, Coronado MJ et al. found higher sST2 concentrations in patients with myocarditis and that sST2 levels were associated with exacerbating heart failure symptoms (17). However, no studies on sST2 and prognosis in children with myocarditis have been reported. This study investigated the relationship between MACEs and sST2 during hospitalization in pediatric patients with myocarditis.
This was a single-center retrospective cohort study. We consecutively enrolled pediatric patients (aged ≤14 years) diagnosed with myocarditis (18) at the Xuzhou Children's Hospital from December 2020 to December 2023, and all patients were perfected sST2 detection. The study protocol was verified by the Xuzhou Children's Hospital Ethics Committee, and the written informed consent was exempted due to low risk to patients according to the relevant IRB regulatory guidelines. All patient details have been de-identified. The reporting of this study conforms to STROBE guidelines (19). Patients with known congenital or acquired heart disease before admission were excluded.
All patients were collected clinical baseline data, including gender, age, and weight. Venous blood samples were collected on admission for laboratory analysis. High-sensitivity C-reactive protein (hs-CRP), N-terminal B-type natriuretic peptide (NT-proBNP), creatine kinase isoenzyme MB (CKMB), neutrophil/lymphocyte ratio (NLR), creatinine, urea, uric acid, alanine transaminase (ALT) and aspartate transaminase (AST) were detected. Left ventricular ejection fraction (LVEF) and left ventricular end-diastolic diameter (LVED) were obtained by cardiac ultrasound. The concentration of sST2 in blood samples was determined using an enzyme-linked immunosorbent assay kit (ELISA) (ElabScience Biotechnology, China).
Events during the hospitalization were major adverse cardiovascular events (MACEs, all-cause death > new heart failure > ventricular arrhythmia). New congestive heart failure was identified as the first episode of cardiac decompensation requiring intravenous diuretic therapy (20). Ventricular arrhythmias include ventricular tachycardia and ventricular fibrillation. Ventricular tachycardia was defined as a ventricular tachycardia lasting ≥30 s, or, although less than 30 s, the patient is hemodynamically unstable, requiring immediate termination of the tachycardia (21).
SPSS 24.0 software was used for statistical analysis. Data conforming to a normal distribution were expressed as mean ± standard deviation (SD) and analyzed by an unpaired t-test. Not normally distributed data were expressed as median (interquartile range, IQR) and analyzed by a non-parametric test (Mann-Whiney U-test). Categorical variables were analyzed by the chi-square test. Univariate and multivariate regression analyses were used to identify risk factors for MACEs. Receiver operating characteristic (ROC) was used to assess the sensitivity and specificity of risk factors for MACEs. P < 0.05 was considered to be statistically significant.
A total of 252 patients were included in this study, with a male-to-female ratio of 1.2:1. A total of 25 people had MACEs during their hospital stay. Of these, 7 were all-cause deaths, 17 were heart failure, and 1 was ventricular arrhythmia. The mortality rate during hospitalization was 6/23 (26%) in patients with heart failure and 3/10 (30%) in patients with ventricular arrhythmias (Figure 1).
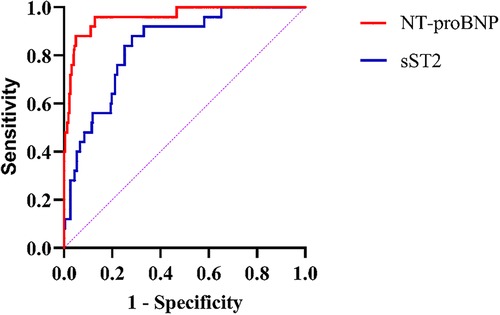
Figure 1. Mortality during hospitalization in patients combined with heart failure or ventricular arrhythmias.
Compared to the No MACEs group, patients in the MACEs group had significantly higher creatinine [32 (26, 39) vs. 42 (34, 50.5) umol/L; p < 0.001], urea [4.19 (3.37, 5.22) vs. 5.85 (4.91, 7.95) mmol/L; p < 0.001], AST [38 (27, 56) vs. 90 (62, 394) U/L; p < 0.001], ALT [18 (12, 28) vs. 38 (18, 133) U/L; p < 0.001], hs-CRP [1 (0.44, 3.86) vs. 5.67 (2.90, 17.24) mg/dl; p < 0.001], NLR [1.38 (0.82, 2.6) vs. 4.22 (2.09, 5.77); p < 0.001], NT-proBNP [141 (80, 460) vs. 4,710 (1,820, 12,200) pg/ml; p < 0.001], CKMB [45 (25, 89) vs. 61 (38.5, 131) ng/ml; p = 0.018], length of stay [6 (5, 7) vs. 10 (3, 14.5) days; p = 0.017], LVED [59 (56, 62) vs. 48 (35, 59.5) %; p < 0.001] and sST2 [19.69 (14.22, 27.22) vs. 47.18 (37, 82.13) ng/ml; p < 0.001], while LVEF [3.8 (3.4, 4.1) vs. 4.1 (3.6, 4.45) cm; p = 0.019] was significantly lower (p < 0.05) (Table 1).
Univariate analysis found that creatinine, AST, ALT, hs-CRP, NT-proBNP, length of stay, LVED, sST2, and LVEF were associated with MACEs in pediatric myocarditis patients. After including these risk factors in a multivariate logistic regression analysis using a stepwise forward method, NT-proBNP (OR 4.323; 95% CI, 2.433–7.679; p < 0.001) and sST2 (OR 1.020; 95% CI, 1.003–1.037; p = 0.022) remained statistically significant and were independent risk factors for MACEs during hospitalization in pediatric myocarditis patients (Table 2).
NT-proBNP and sST2 were included in the ROC analysis. ROC analysis for NT-proBNP found an area under the curve (AUC) of 0.96, 95% CI of 0.922–0.998, a cut-off value of 745.5 pg/ml, sensitivity of 0.96, specificity of 0.872, and Youden index of 0.832. ROC analysis for sST2 found an AUC of 0.839, 95% CI of 0.767–0.911, cut-off value of 31.6 ng/ml, sensitivity of 0.88, specificity of 0.718, and Youden index of 0.598. Both NT-proBNP and sST2 showed good predictive efficacy for MACEs (Table 3, Figure 2).
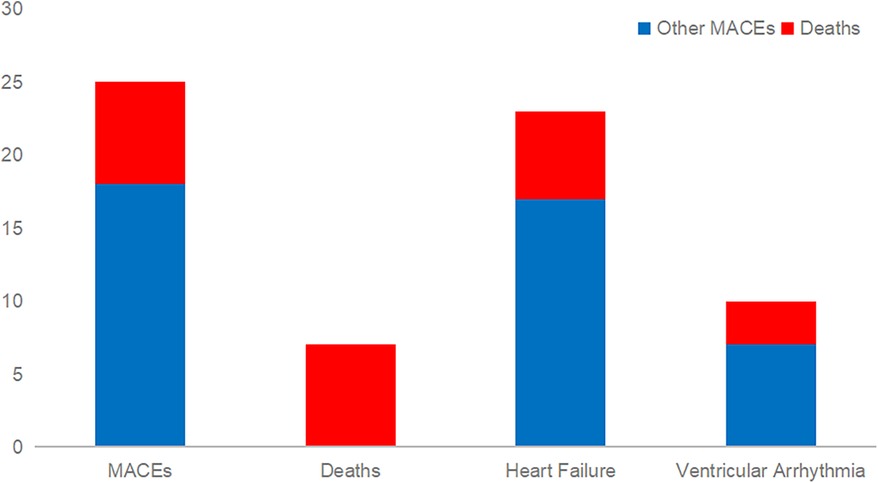
Figure 2. ROC curve analysis association of NT-proBNP and sST2 with the risk of MACEs. NT-proBNP N terminal pro-B-type natriuretic peptide; sST2 Soluble suppression of tumorigenicity 2.
To our knowledge, this study was the first to investigate the relationship between MACEs and sST2 during hospitalization in pediatric patients with myocarditis. The main finding of this study was that NT-proBNP and sST2 were independent risk factors for MACEs during hospitalization in pediatric myocarditis patients, and both showed good predictive power.
Inflammation can lead to arrhythmogenesis by altering ion channels, ventricular tachycardia (VT) and ventricular fibrillation (VF) are common complications of myocarditis in children and are usually associated with significant hemodynamic impairment (22, 23). In our study, the mortality rate in patients with ventricular arrhythmias was 30%, and patients with ventricular arrhythmias were ten times more likely to die during hospitalization than those without ventricular arrhythmias. This is a similar result to previous studies (24).
Considering the rapidly changing and progressive nature of myocarditis in children, a reliable rapid marker to early identify those at risk would be essential. Previous studies have shown that myocarditis and the inflammatory response are closely linked in adults and children (7, 8). sST2 is an interleukin (IL) receptor family member and can be expressed in various circulating immune cells and cardiac myocytes. sST2 acts as a detrimental “decoy receptor” for circulating IL-33, minimizing the protective effect of IL33 on the cardiovascular system (9, 25). Coronado MJ et al. found that serum sST2 levels were associated with cardiac inflammation in adult patients with myocarditis. Elevated serum sST2 was associated with an increased risk of heart failure in men ≤50 years of age (17). In this study, we found that creatinine, urea, AST, ALT, hs-CRP, NLR, NT-proBNP, CKMB, length of stay, LVED, and sST2 were significantly higher in the MACEs group compared to the No MACEs group, while LVEF was significantly lower. This suggests that patients with myocarditis in combination with MACEs may have a more severe inflammatory response and impairment of organ function. After adjusting for possible risk factors, multifactorial regression analysis showed that NT-proBNP and sST2 were independent risk factors for MACEs during hospitalization in children with myocarditis. Recent studies indicate that sST2, unlike NT-proBNP, does not appear to be affected by renal function or residual diuresis (26, 27). So, the correlation analysis of ST2 with creatinine was conducted (Supplementary Figure S1). Consistent with previous findings, this study discovered no significant correlation between sST2 and renal function, and sST2 was associated with prognosis in children with myocarditis independent of renal function. A previous study has shown that sST2 levels at admission in patients with ICM (inflammatory cardiomyopathy) correlate with the degree of functional left ventricular impairment. In addition, ICM patients with elevated baseline sST2 levels were at higher risk of developing NYHA class III/IV at 12-month follow-up compared to ICM patients with lower baseline sST2 levels (28). In patients with acute myocardial infarction, higher sST2 levels are associated with an increased risk of cardiovascular death and heart failure within 30 days (15). sST2 was a predictor of all-cause and cardiovascular event-related death in patients with chronic heart failure in a meta-analysis by Aimo et al. (29).
In a previous study, Amer, Eslam et al. included 60 children with congestive heart failure as a patient group and sixty age- and sex-matched healthy children as a control group. The results showed that sST2 is a good diagnostic and predictive biomarker for children with congestive heart failure (30). As a traditional cardiac biomarker, NT-proBNP has been extensively reported to be significantly elevated in patients with myocarditis or animal models of myocarditis and is a risk factor for poor prognosis in children with fulminant myocarditis (31–34). An observational study of suspected myocarditis found that higher BNP levels were associated with higher mortality and that elevated BNP levels were independently associated with poor patient prognosis (35). Although CKMB and LVED were elevated and LVEF was reduced in the MACEs group compared to the No MACEs group, they were not independent risk factors for MACEs during hospitalization in children with myocarditis, which is similar to the results of the previous study (35). However, Schultz et al. showed different results regarding LVEF (36). This is controversial and may need to be explored in more studies in the future. Including NT-proBNP and sST2 in the ROC analysis showed that both had reasonable specificity and sensitivity for MACEs during hospitalization in pediatric patients with myocarditis. Previous studies have shown that baseline sST2 is comparable to NT-proBNP in predicting MACEs in ICM cohorts (28). Braunwald E showed that BNP levels were superior to endothelin-1 or norepinephrine as a predictor of death (37). This study found that NT-proBNP appeared to perform more superiorly, which may be related to the predominance of heart failure in the MACEs in this study. More studies should be conducted in the future to explore the superiority of the two biomarkers. Myocarditis in children is an important challenge for clinicians. Our study suggests that sST2 may be a useful marker for the development of in-hospital MACEs in pediatric myocarditis patients. In contrast to NT-proBNP, sST2 is not significantly affected by renal function and may be uniquely valuable in patients with renal insufficiency. Early identification of these high-risk patients by sST2 will perhaps help optimize risk stratification, guide clinical decision-making, and improve prognosis.
This was a single-center retrospective cohort study, and some bias may exist. Second, this study focused on the MACEs during hospitalization, and there is insufficient data on these patients' long-term prognosis. Third, this study only detected the baseline sST2 levels at admission and was not continuously monitored dynamically.
Elevated levels of NT-proBNP and sST2 were independently associated with major adverse cardiovascular events during hospitalization in children with myocarditis, and both showed good predictive efficacy.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the study protocol was verified by the Xuzhou Children's Hospital Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin. Written informed consent was exempted due to low risk to the patients according to the relevant IRB regulatory guidelines.
TS: Writing – original draft. JG: Writing – original draft. SL: Writing – original draft. YZ: Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1404432/full#supplementary-material
Supplementary Figure S1
The correlation analysis of sST2 with creatinine.
1. Feldman AM, McNamara D. Myocarditis. N Engl J Med. (2000) 343(19):1388–98. doi: 10.1056/NEJM200011093431908
2. Abe T, Tsuda E, Miyazaki A, Ishibashi-Ueda H, Yamada O. Clinical characteristics and long-term outcome of acute myocarditis in children. Heart Vessels. (2013) 28(5):632–8. doi: 10.1007/s00380-012-0296-8
3. Vasudeva R, Bhatt P, Lilje C, Desai P, Amponsah J, Umscheid J, et al. Trends in acute myocarditis related pediatric hospitalizations in the United States, 2007–2016. Am J Cardiol. (2021) 149:95–102. doi: 10.1016/j.amjcard.2021.03.019
4. Arola A, Pikkarainen E, Sipila JO, Pykari J, Rautava P, Kyto V. Occurrence and features of childhood myocarditis: a nationwide study in Finland. J Am Heart Assoc. (2017) 6(11):e005306. doi: 10.1161/JAHA.116.005306
5. Cofer BR, Warner BW, Stallion A, Ryckman FC. Extracorporeal membrane oxygenation in the management of cardiac failure secondary to myocarditis. J Pediatr Surg. (1993) 28(5):669–72. doi: 10.1016/0022-3468(93)90028-J
6. Drucker NA, Colan SD, Lewis AB, Beiser AS, Wessel DL, Takahashi M, et al. Gamma-globulin treatment of acute myocarditis in the pediatric population. Circulation. (1994) 89(1):252–7. doi: 10.1161/01.CIR.89.1.252
7. Caforio AL, Marcolongo R, Jahns R, Fu M, Felix SB, Iliceto S. Immune-mediated and autoimmune myocarditis: clinical presentation, diagnosis and management. Heart Fail Rev. (2013) 18(6):715–32. doi: 10.1007/s10741-012-9364-5
8. Kociol RD, Cooper LT, Fang JC, Moslehi JJ, Pang PS, Sabe MA, et al. Recognition and initial management of fulminant myocarditis: a scientific statement from the American heart association. Circulation. (2020) 141(6):e69–92. doi: 10.1161/CIR.0000000000000745
9. Griesenauer B, Paczesny S. The ST2/IL-33 axis in immune cells during inflammatory diseases. Front Immunol. (2017) 8:475. doi: 10.3389/fimmu.2017.00475
10. Weinberg EO, Shimpo M, De Keulenaer GW, MacGillivray C, Tominaga S, Solomon SD, et al. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. (2002) 106(23):2961–6. doi: 10.1161/01.CIR.0000038705.69871.D9
11. Fairlie-Clarke K, Barbour M, Wilson C, Hridi SU, Allan D, Jiang HR. Expression and function of IL-33/ST2 axis in the central nervous system under normal and diseased conditions. Front Immunol. (2018) 9:2596. doi: 10.3389/fimmu.2018.02596
12. Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. (2008) 7(10):827–40. doi: 10.1038/nrd2660
13. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart failure society of America. J Am Coll Cardiol. (2017) 70(6):776–803. doi: 10.1016/j.jacc.2017.04.025
14. van Vark LC, Lesman-Leegte I, Baart SJ, Postmus D, Pinto YM, Orsel JG, et al. Prognostic value of serial ST2 measurements in patients with acute heart failure. J Am Coll Cardiol. (2017) 70(19):2378–88. doi: 10.1016/j.jacc.2017.09.026
15. Shimpo M, Morrow DA, Weinberg EO, Sabatine MS, Murphy SA, Antman EM, et al. Serum levels of the interleukin-1 receptor family member ST2 predict mortality and clinical outcome in acute myocardial infarction. Circulation. (2004) 109(18):2186–90. doi: 10.1161/01.CIR.0000127958.21003.5A
16. Mechtouff L, Paccalet A, Da Silva CC, Buisson M, Mewton N, Amaz C, et al. Prognosis value of serum soluble ST2 level in acute ischemic stroke and STEMI patients in the era of mechanical reperfusion therapy. J Neurol. (2022) 269(5):2641–8. doi: 10.1007/s00415-021-10865-3
17. Coronado MJ, Bruno KA, Blauwet LA, Tschope C, Cunningham MW, Pankuweit S, et al. Elevated sera sST2 is associated with heart failure in men ≤50 years old with myocarditis. J Am Heart Assoc. (2019) 8(2):e008968. doi: 10.1161/JAHA.118.008968
18. Law YM, Lal AK, Chen S, Cihakova D, Cooper LT Jr., Deshpande S, et al. Diagnosis and management of myocarditis in children: a scientific statement from the American heart association. Circulation. (2021) 144(6):e123–35. doi: 10.1161/CIR.0000000000001001
19. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. (2007) 147(8):573–7. doi: 10.7326/0003-4819-147-8-200710160-00010
20. Reindl M, Reinstadler SJ, Feistritzer HJ, Theurl M, Basic D, Eigler C, et al. Relation of low-density lipoprotein cholesterol with microvascular injury and clinical outcome in revascularized ST-elevation myocardial infarction. J Am Heart Assoc. (2017) 6(10):e006957. doi: 10.1161/JAHA.117.006957
21. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. Circulation. (2018) 138(13):e272–391. doi: 10.1016/j.jacc.2017.10.054
22. Saito J, Niwano S, Niwano H, Inomata T, Yumoto Y, Ikeda K, et al. Electrical remodeling of the ventricular myocardium in myocarditis: studies of rat experimental autoimmune myocarditis. Circ J. (2002) 66(1):97–103. doi: 10.1253/circj.66.97
23. Sharma JR, Sathanandam S, Rao SP, Acharya S, Flood V. Ventricular tachycardia in acute fulminant myocarditis: medical management and follow-up. Pediatr Cardiol. (2008) 29(2):416–9. doi: 10.1007/s00246-007-9044-8
24. Othman HF, Byrnes J, Elsamny E, Hamzah M. Impact of ventricular arrhythmias on outcomes in children with myocarditis. Eur J Pediatr. (2020) 179(11):1779–86. doi: 10.1007/s00431-020-03687-4
25. Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. (2007) 117(6):1538–49. doi: 10.1172/JCI30634
26. Bayes-Genis A, Zamora E, de Antonio M, Galan A, Vila J, Urrutia A, et al. Soluble ST2 serum concentration and renal function in heart failure. J Card Fail. (2013) 19(11):768–75. doi: 10.1016/j.cardfail.2013.09.005
27. Zhang R, Zhang Y, An T, Guo X, Yin S, Wang Y, et al. Prognostic value of sST2 and galectin-3 for death relative to renal function in patients hospitalized for heart failure. Biomark Med. (2015) 9(5):433–41. doi: 10.2217/bmm.15.12
28. Obradovic DM, Buttner P, Rommel KP, Blazek S, Loncar G, von Haehling S, et al. Soluble ST2 receptor: biomarker of left ventricular impairment and functional Status in patients with inflammatory cardiomyopathy. Cells. (2022) 11(3):414. doi: 10.3390/cells11030414
29. Aimo A, Vergaro G, Passino C, Ripoli A, Ky B, Miller WL, et al. Prognostic value of soluble suppression of tumorigenicity-2 in chronic heart failure: a meta-analysis. JACC Heart Fail. (2017) 5(4):280–6. doi: 10.1016/j.jchf.2016.09.010
30. Amer E, El Amrousy D, Hazaa S, Zoair A. Serum-soluble suppression of tumourigenicity-2 as a biomarker in children with congestive heart failure. Cardiol Young. (2023) 33(12):2481–6. doi: 10.1017/S1047951123000240
31. Grabowski M, Karpinski G KJF, Rdzanek A, Pietrasik A, Wretowski D, Rudowski R, et al. Diagnostic value of BNP in suspected perimyocarditis–a preliminary report. Kardiol Pol. (2004) 61(11):451–8, discussion 459–460.15883593
32. Ogawa T, Veinot JP, Kuroski de Bold ML, Georgalis T, de Bold AJ. Angiotensin II receptor antagonism reverts the selective cardiac BNP upregulation and secretion observed in myocarditis. Am J Physiol Heart Circ Physiol. (2008) 294(6):H2596–603. doi: 10.1152/ajpheart.00215.2008
33. Tanaka K, Ito M, Kodama M, Tomita M, Kimura S, Hoyano M, et al. Sulfated polysaccharide fucoidan ameliorates experimental autoimmune myocarditis in rats. J Cardiovasc Pharmacol Ther. (2011) 16(1):79–86. doi: 10.1177/1074248410378751
34. Casadonte JR, Mazwi ML, Gambetta KE, Palac HL, McBride ME, Eltayeb OM, et al. Risk factors for cardiac arrest or mechanical circulatory support in children with fulminant myocarditis. Pediatr Cardiol. (2017) 38(1):128–34. doi: 10.1007/s00246-016-1493-5
35. Abrar S, Ansari MJ, Mittal M, Kushwaha KP. Predictors of mortality in paediatric myocarditis. J Clin Diagn Res. (2016) 10(6):SC12–16. doi: 10.7860/JCDR/2016/19856.7967
36. Schultz JC, Hilliard AA, Cooper LT Jr., Rihal CS. Diagnosis and treatment of viral myocarditis. Mayo Clin Proc. (2009) 84(11):1001–9. doi: 10.1016/S0025-6196(11)60670-8
Keywords: myocarditis, soluble suppression of tumorigenicity 2, children, MACEs, biomarker
Citation: Shi T, Ge J, Li S and Zhang Y (2024) Soluble suppression of tumorigenicity 2 associated with major adverse cardiac events in children with myocarditis. Front. Cardiovasc. Med. 11:1404432. doi: 10.3389/fcvm.2024.1404432
Received: 21 March 2024; Accepted: 29 April 2024;
Published: 13 May 2024.
Edited by:
Nazmi Narin, Izmir Katip Celebi University, TürkiyeReviewed by:
Derya Karpuz, Mersin University, Türkiye© 2024 Shi, Ge, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yali Zhang, bGl6aTcyNzJAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.