- 1Department of Pharmacy, Affiliated Dongyang Hospital, Wenzhou Medical University, Dongyang, Zhejiang, China
- 2Department of Biomedical Sciences Laboratory, Affiliated Dongyang Hospital, Wenzhou Medical University, Dongyang, Zhejiang, China
Objective: This study aimed to develop a predictive model for assessing bleeding risk in dual antiplatelet therapy (DAPT) patients.
Methods: A total of 18,408 DAPT patients were included. Data on patients’ demographics, clinical features, underlying diseases, past history, and laboratory examinations were collected from Affiliated Dongyang Hospital of Wenzhou Medical University. The patients were randomly divided into two groups in a proportion of 7:3, with the most used for model development and the remaining for internal validation. LASSO regression, multivariate logistic regression, and six machine learning models, including random forest (RF), k-nearest neighbor imputing (KNN), decision tree (DT), extreme gradient boosting (XGBoost), light gradient boosting machine (LGBM), and Support Vector Machine (SVM), were used to develop prediction models. Model prediction performance was evaluated using area under the curve (AUC), calibration curves, decision curve analysis (DCA), clinical impact curve (CIC), and net reduction curve (NRC).
Results: The XGBoost model demonstrated the highest AUC. The model features were comprised of seven clinical variables, including: HGB, PLT, previous bleeding, cerebral infarction, sex, Surgical history, and hypertension. A nomogram was developed based on seven variables. The AUC of the model was 0.861 (95% CI 0.847–0.875) in the development cohort and 0.877 (95% CI 0.856–0.898) in the validation cohort, indicating that the model had good differential performance. The results of calibration curve analysis showed that the calibration curve of this nomogram model was close to the ideal curve. The clinical decision curve also showed good clinical net benefit of the nomogram model.
Conclusions: This study successfully developed a predictive model for estimating bleeding risk in DAPT patients. It has the potential to optimize treatment planning, improve patient outcomes, and enhance resource utilization.
1 Introduction
Dual antiplatelet therapy (DAPT) is a commonly used treatment for patients at risk of cardiovascular events (1). However, one significant challenge associated with DAPT is an increased risk of bleeding complications in some patients (2). One major hurdle is striking the delicate balance between reducing the risk of cardiovascular events and minimizing bleeding risks (3). Overall, although DAPT has proven effective in preventing cardiovascular events, addressing the associated bleeding risk and optimizing patient management remain ongoing challenges in clinical practice.
Several bleeding risk scores have been created to aid in selecting the appropriate treatment regimen and duration. These studies have explored different aspects of bleeding risk assessment, offering valuable insights for clinical practice (4). The ACC/AHA guidelines on DAPT duration were issued prior to the development and validation of the PRECISE-DAPT risk score, a 5-item scoring system, was developed to predict bleeding risk in patients undergoing DAPT (2). It has received a Class IIB recommendation for identifying high-risk patients prone to bleeding. The DAPT score effectively stratifies bleeding and ischemic risk in various study populations, providing the advantage of benefit-risk difference stratification consistently (5). The ESC and EACTS guidelines emphasized a personalized approach to balancing bleeding and ischemic risks, moving away from a generalized strategy in the context of DAPT (6). While American and European guidelines primarily recommend DAPT and PRECISE-DAPT scores, their limitations arise from variations in patient cohorts (7, 8). Recently, new clinical models have been developed and validated in diverse clinical scenarios to improve the prediction of hemorrhagic events. Commonly used scoring systems including PARIS (9), CRUSADE (10), ARC-HBR (11), ACUITY-HORIZONS (12), BleeMACS (13), TIMI risk score (14), HAS-Bled score (15), GRACE score (10), and CHA2DS2-VASC score (16) are extensively employed in clinical practice. These scores assess various clinical characteristics such as coronary anatomy, surgical procedures, genotyping, lifestyle factors, and adherence to treatment (17). Each score has its advantages and limitations based on the characteristics of the patient cohorts used for development and validation, making them applicable to specific patients, clinical contexts, and timeframes (17). such as the TIMI risk score, which is primarily used for assessing risk in patients with non-ST elevation myocardial infarction, and the PARIS score, which is specifically designed for evaluating bleeding risk after coronary interventions. While these tools have broad applicability in their respective domains, they may not fully account for certain unique clinical variables and patient characteristics present in our specific population.
Therefore, our research aims to develop a novel prediction model that addresses this gap. With a growing trend towards individualized bleeding risk stratification for patients receiving DAPT, alternative risk scores validated in large patient cohorts could be applied in specific clinical scenarios that closely resemble those studied.
Further studies on bleeding risk scores are necessary to establish the correlation between bleeding events and the use of these clinical tools. The aim of this study was to create novel bleeding risk scores and evaluate their predictive performance.
2 Methods
2.1 Study population
Participants in the study were recruited from Affiliated Dongyang Hospital of Wenzhou Medical University. Inclusion criteria for participants were: (1) age over 18 years; (2) The documented utilization of dual antiplatelet therapy (aspirin and clopidogrel) was extracted from hospital electronic medical records (EMRs) spanning the period from January 2008 to December 2017. Exclusion Criteria: (1) Individuals younger than 18 years; (2) Pregnant or lactating women; (3) Patients with incomplete medical histories or examination test results; (4) Patients with missing data on dual antiplatelet therapy (DAPT) or lacking relevant bleeding records; (5) Individuals who died during hospitalization. The study protocol received ethics approval from the Ethics Committee of Affiliated Dongyang Hospital of Wenzhou Medical University (approval #2023-YX-408). Informed consent was waived for this study. Prior to conducting the analysis, all patient medical information was anonymized and de-identified.
2.2 Outcome definition
The primary endpoint of this study was defined as the occurrence of any documented bleeding incidents, including gastrointestinal bleeding, intracranial hemorrhage, and other bleeding events such as urinary bleeding, oral bleeding, and ophthalmic hemorrhage (18), within five years following the initiation of dual antiplatelet therapy (DAPT), as recorded in the hospital EMRs at the time of patient discharge. For the purposes of this analysis, the presence of any bleeding event was classified as a positive outcome, while the absence of such events was categorized as a negative outcome.
2.3 Risk factors
We obtained the following information from the EMRs of the subjects in our hospital: sex, age, height, weight, BMI, and past medical history, including smoking, drinking, diabetes, hypertension, surgical history, previous bleeding episodes, presence of tumors, acute myocardial infarction, Percutaneous Coronary Interventions (PCI), gastric ulcers, use of gastric protective drugs, cerebral infarction, portal hypertension, anticoagulant usage, and various clinical test indicators such as cardiac ejection fraction (EF), white blood cell count (WBC), platelet count (PLT), peripheral hemoglobin (HGB), and glomerular filtration rate (GFR). For the research parameter, we considered the lowest clinical test indicators within one month prior to commencing DAPT. Other past medical histories were recorded if they occurred before the initiation of DAPT.
2.4 Data pre-processing
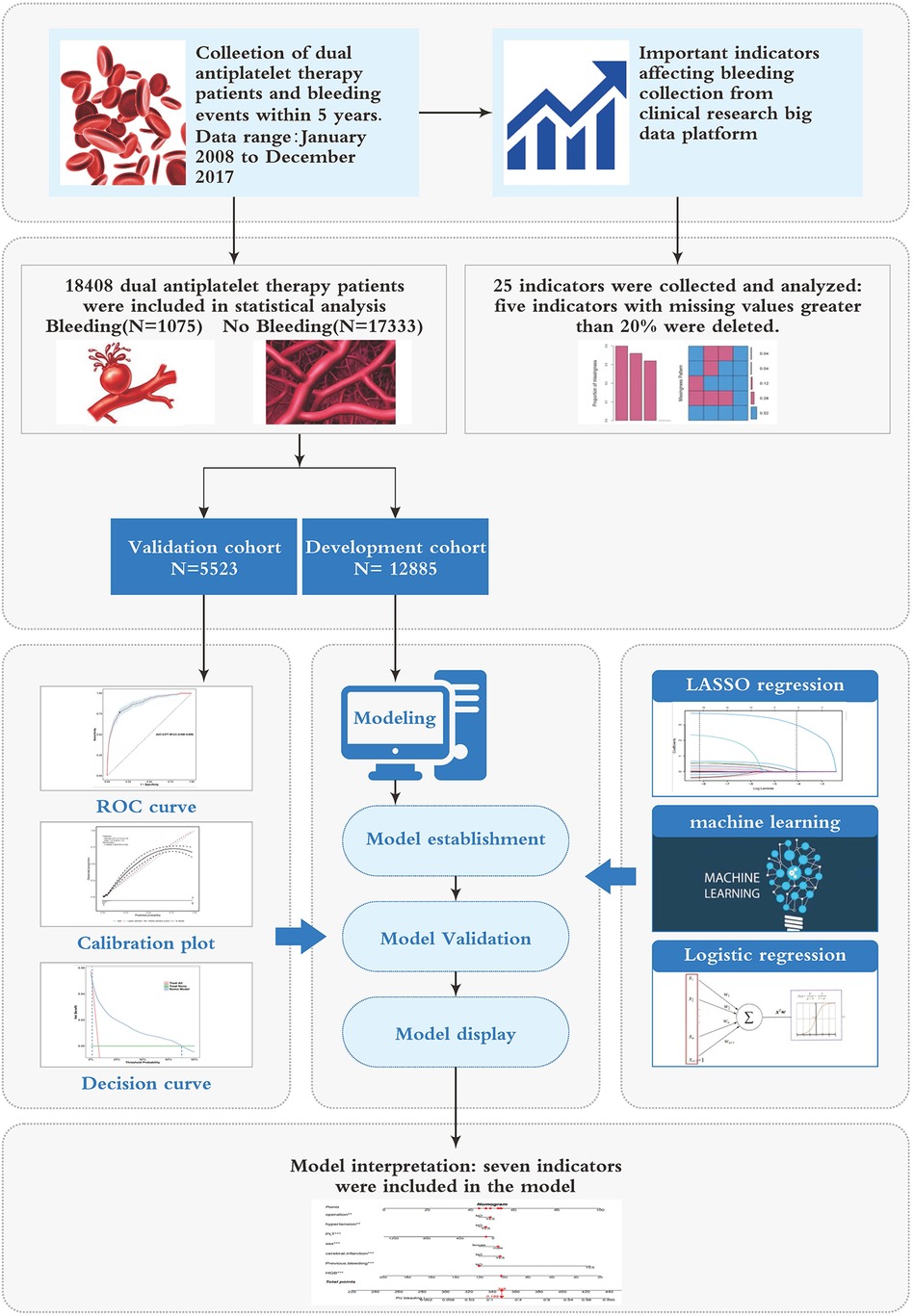
The data obtained from the clinical research big data platform underwent effective cleaning processes, including the removal of extreme values and imputation of missing values. Indicators with missing values exceeding 20%, such as height, weight, BMI, EF, and GFR, were excluded from the analysis. For the remaining missing predictor values, multiple imputation techniques were employed. To perform model development and evaluation, the data was split into a development cohort comprising 70% of the data and a validation cohort containing the remaining portion. The classification model was trained using the development cohort, while the validation cohort was utilized to assess the model's performance.
2.5 Model building
LASSO regression (19) and six machine learning algorithms were utilized to identify the optimal predictive features. Machine learning algorithms employed included random forest (RF), k-nearest neighbor imputing (KNN), decision tree (DT), extreme gradient boosting (XGBoost), light gradient boosting machine (LGBM), and Support Vector Machine (SVM). Shapley additive explanation (SHAP) values were used to identify feature importance. Logistic regression modeling was performed on the 5 or 10 most significant parameters from the best machine learning model, as well as the parameters selected by LASSO regression. These parameters were categorized into three models. To compare the performance of these three models, metrics such as the area under the receiver operating characteristic (ROC) curve (AUC), Net Reclassification Improvement (NRI), and Integrated Discrimination Improvement (IDI) were evaluated. Based on the assessment of the performance metrics, the best model was selected. Using this model, a nomogram for predicting bleeding was established.
2.6 Model evaluation
The sensitivity and specificity of the model were assessed using the AUC of the ROC curve, evaluating its discrimination performance. Calibration was evaluated by analyzing calibration curves. The clinical efficacy of the identified risk factors in predicting bleeding risk was verified through decision curve analysis (DCA), clinical impact curve (CIC), and net reduction curve (NRC). These analyses considered the net benefit under varying risk thresholds for patients. Additionally, the model was validated by comparing it to individual indicators in terms of discrimination and clinical utility. Figure 1 illustrates the flowchart outlining the process of model construction and validation.
2.7 Statistical methods
Statistical analysis and plotting were conducted using R4.2.1 software for Windows. Categorical variables were presented as frequencies with percentages and compared using χ2 test or Fisher's exact test. Continuous variables were expressed as means with standard deviations or medians with interquartile ranges and compared using Student's t-test or Mann-Whitney U test. Multiple imputation techniques using the “mice” package. Baseline description and differences analysis utilized the “comparegroups” package. LASSO regression employed the “glmnet” package, while multivariable logistic regression used the “glm” package. Discrimination analysis was performed using the “pROC,” “ggROC,” and “fbroc” packages. Calibration assessment utilized the “rms” and “riskregression” packages. DCA and CIC were conducted using the “rmda,” “dca.R,” and “dcurves” packages. The nomogram was created using the “regplot” package, and NRI calculations employed the “nricens” package. IDI analysis was performed using the “PredictABEL” package. Comparisons of multiple models for ROC analysis were conducted using the “ROCR” package, while DCA comparisons were carried out using the “Dcurves” package. Diagnostic evaluation utilized the “reportROC” package. All statistical tests were 2-sided, and a significance level of P < 0.05 was considered statistically significant.
3 Results
3.1 Study population characteristics
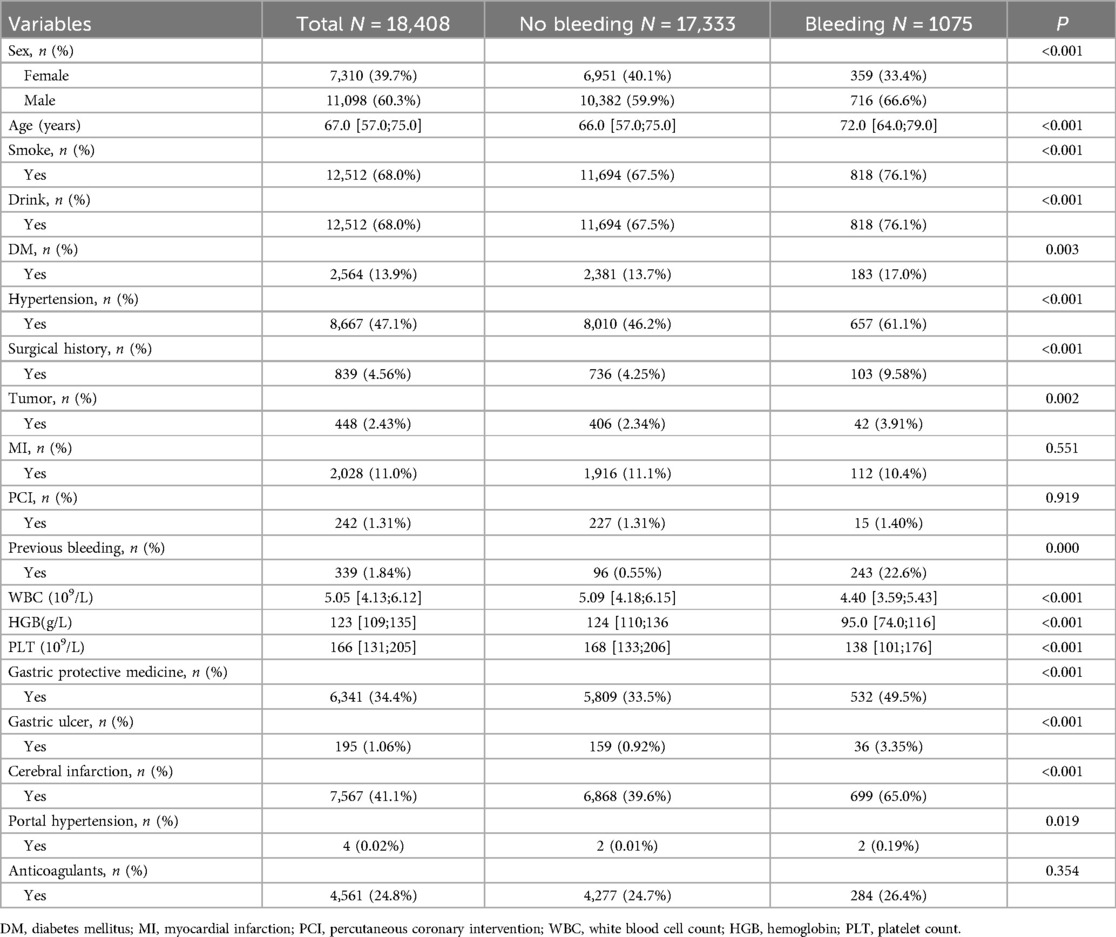
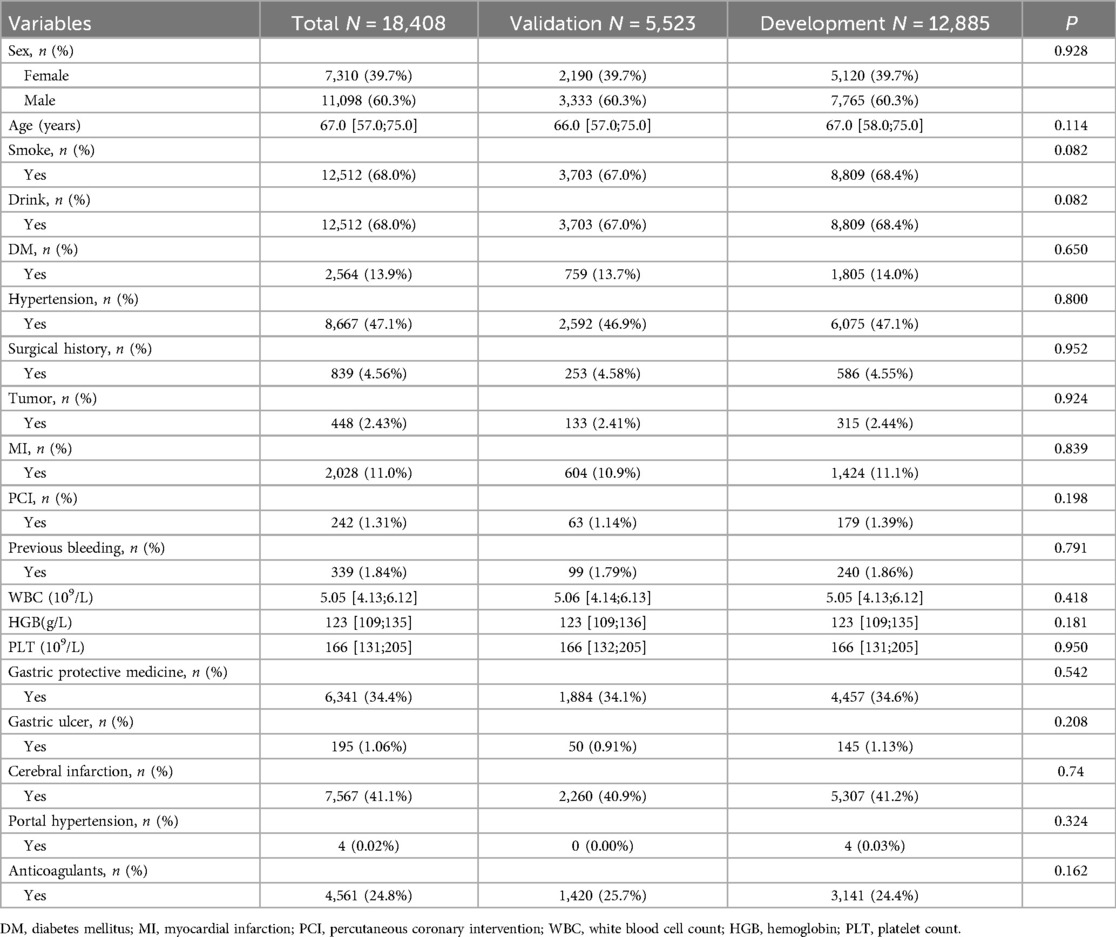
A total of 18,408 DAPT patients (clopidogrel and aspirin) were included in this study, among whom 1,075 experienced bleeding (Figure 1). Out of the 25 variables examined, only WBC, HGB, PLT, Height, Weight, BMI, EF, and GFR were continuous variables. We excluded five variables (Height, Weight, BMI, EF, and GFR) with missing information in more than 20% of patients, resulting in 20 variables with missing data in less than 20% of patients (details provided in Supplementary Material S1). There were no significant differences observed in terms of myocardial infarction and PCI between the cohorts with bleeding and without bleeding. Table 1 presents the baseline characteristics of the DAPT patients. A random 7:3 division was performed to allocate patients into the development cohort (n = 12,885) and the validation cohort (n = 5,523). Table 2 displays the baseline characteristics of patients in both cohorts, revealing no significant differences in each indicator between the two cohorts.
3.2 Selected predictors and construction model
After conducting LASSO regression with ten-fold cross-validation, three variables (previous bleeding, HGB, and cerebral infarction) were selected for inclusion in the model based on the criteria of “family = binomial” and lambda.1SE. The pathway of variable shrinkage and cross-validation is illustrated in Figures 2A,B, respectively. Additionally, six machine learning models were used to identify the best predictive feature. Among these models, the XGBoost model demonstrated the highest AUC [0.877 (95% CI: 0.856–0.897), Figure 2C], supporting its selection as the optimal model for assessing bleeding risk in DAPT patients. The XGBoost model was further used to explain feature importance using SHAP. Figures 3A,B display important variables based on two methods of variable importance, Gain and Cover, respectively. We selected the five most important indicators, the ten most important indicators in SHAP, and three indicators selected by LASSO regression to perform separate logistic regression modeling. After multivariate logistic regression with the “backward” process, several variables were included in the final models (Table 3). Model 3 included three indicators, while Model 7 included seven indicators.
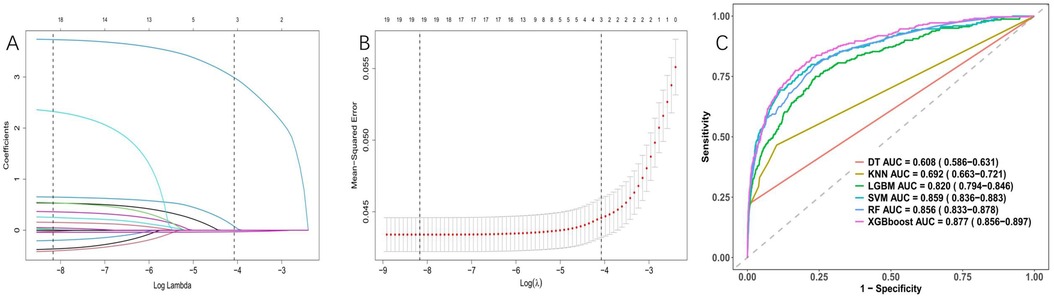
Figure 2. Variable selection was performed using LASSO binary logistic regression and optimal machine learning model selection. (A) Coefficient profile plots were generated against the log(lambda) sequence to visualize the variable selection process and identify nonzero coefficient variables based on the optimal lambda value. (B) Dotted vertical lines represent optimal values determined using the 1 standard error of the minimum criteria (lambda.1se). (C) The right plots display the AUC values of various machine learning models.
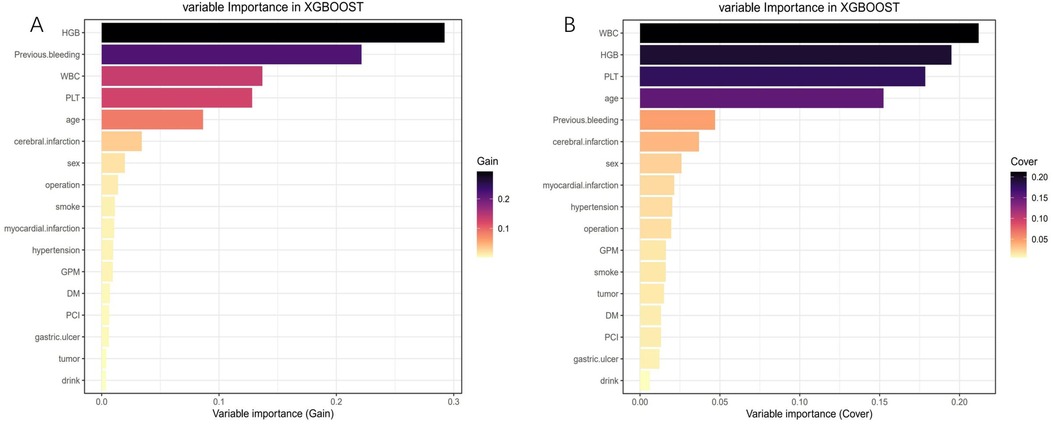
Figure 3. Feature importance analysis using the shapley additive explanation (SHAP). (A) Variable importance (Gain); (B) Variable importance (Cover).
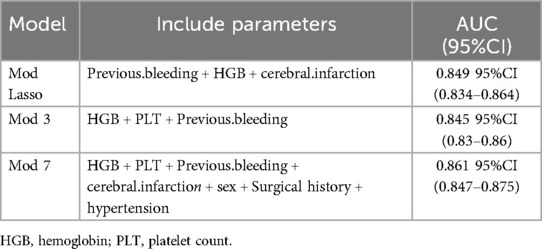
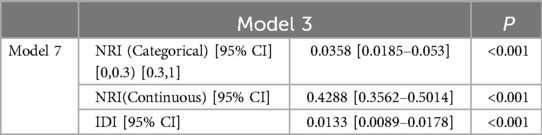
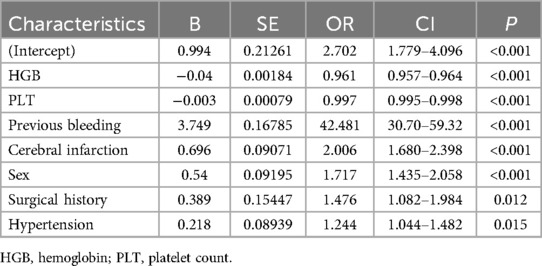
The AUC of the ROC curves for LASSO model, Model 3, and Model 7 in the development and validation cohorts are presented in Figure 4. These results indicate that the discrimination of Model 7 was significantly superior to the other two models according to DeLong's test (p < 0.001). Moreover, a comparison of Model 7 and Model 3 performance using NRI and IDI showed that Model 7 exhibited significantly higher values, indicating improved efficacy compared to Model 3 (Table 4). Finally, a nomogram model (Table 5) was constructed using seven variables: HGB, PLT, previous bleeding, cerebral infarction, sex, Surgical history, and hypertension.
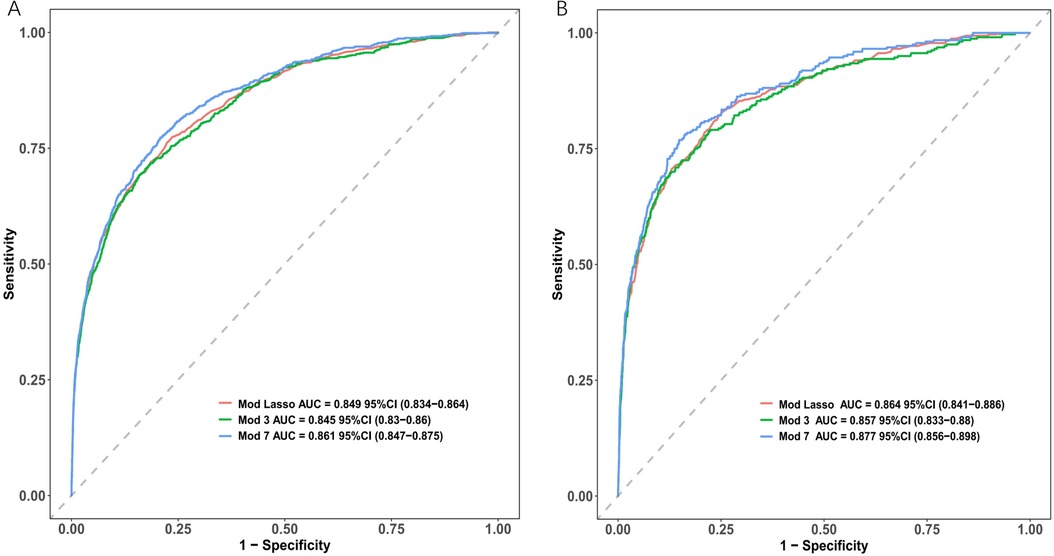
Figure 4. Receiver operating characteristic curves (ROC) of different model distinguishing bleeding from non-bleeding. (A) in the development cohort; (B) in the validation cohort.
3.3 Model visualization
The nomogram in Figure 5 provides a visual representation of the logistic regression analysis results, enabling the prediction of bleeding risk in DAPT patients. By locating the value of each risk factor on the respective vertical line, points can be obtained. The total points on the nomogram are calculated by summing up the points from each risk factor. To determine the bleeding prediction for a specific DAPT patient, a vertical line is drawn from the total points axis, intersecting with the corresponding probability on the nomogram. For instance, if a male DAPT patient has undergone surgery, has hypertension, developed a cerebral infarction, had previous bleeding, a platelet counts of 86 × 109/L, and an HGB level of 102 g/L, the total score would be 348. Drawing a vertical line from the total score of 348 intersects the probability axis at approximately 0.189, indicating an estimated probability of bleeding of 18.9%.
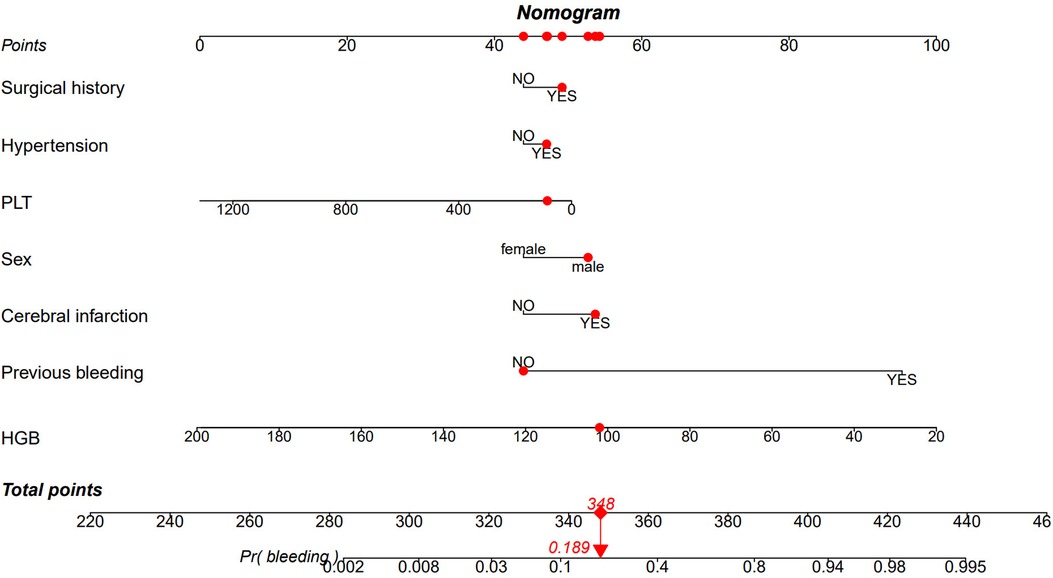
Figure 5. Nomogram based on the combination of seven indicators was developed using logistic regression analysis. If a patient's total score is 348, the corresponding probability of bleeding is 0.189 (highlighted in red). HGB, Hemoglobin; PLT, platelet count.
3.4 Model validation
Model discrimination was assessed by calculating the AUC of the ROC curve. In Figure 6A, the AUC of the development cohort was 0.861 (95% CI 0.847–0.875), while in Figure 6B, the AUC of the validation cohort was 0.877 (95% CI 0.856–0.898). Calibration curves Figures 6C,D illustrate the excellent concordance between the predicted probability of bleeding and the actual observations in the development and validation cohort. The Hosmer and Lemeshow goodness of fit (GOF) test also showed good consistency (p = 0.1285). Figure 7 displays the DCA, CIC, and NRC results for the model developed in this study. The DCA results indicate that the model has a favorable net benefit in predicting bleeding risk among DAPT patients. The threshold probability ranges were 1.0%–71% for the development cohort (Figure 7A) and 1.5%–72% for the validation cohort (Figure 7D). A lower risk threshold corresponds to a higher net benefit. However, the CIC analysis revealed that as the risk threshold decreases, there is an increase in the false positive rate and unnecessary interventions (Figures 7,E). Therefore, when making final decisions, it is crucial to consider both DCA and CIC results to strike the optimal balance between high net benefit and low false positive rate. Figures 7C,F provide NRC plots, demonstrating a good fit for the development and validation cohorts of the model.
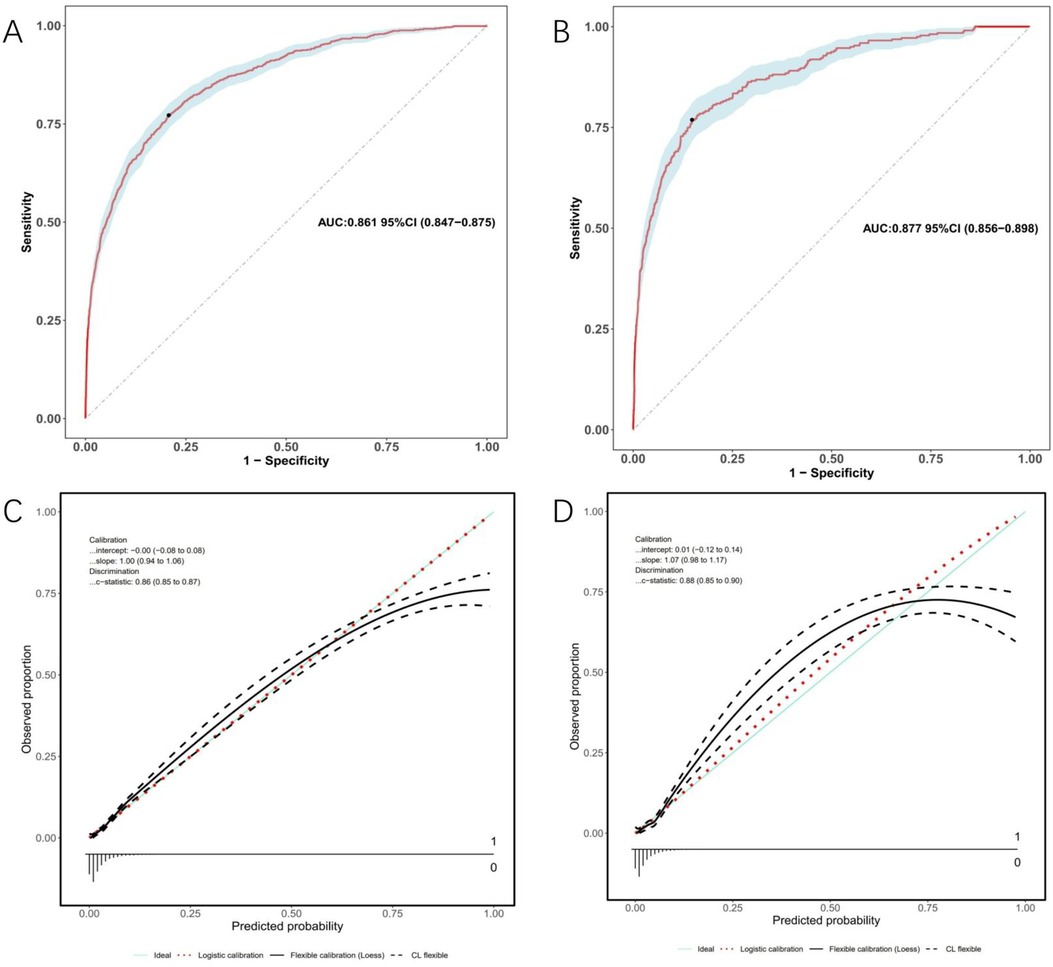
Figure 6. ROC curves (upper) and calibration curves (lower) of the nomogram. (A) ROC curves in the development cohort; (B) in the validation cohort. (C) Calibration curves in the development cohort; (D) in the validation cohort. Calibration curves: The y-axis represents the actual diagnosed cases of bleeding, while the x-axis represents the predicted risk of bleeding. Diagonal dotted lines represent perfect predictions by an ideal model (blue line), and the red dashed line represents the performance of the development cohort (left) and validation cohort (right). A closer alignment between the red dashed line and diagonal dotted lines indicates better prediction performance.
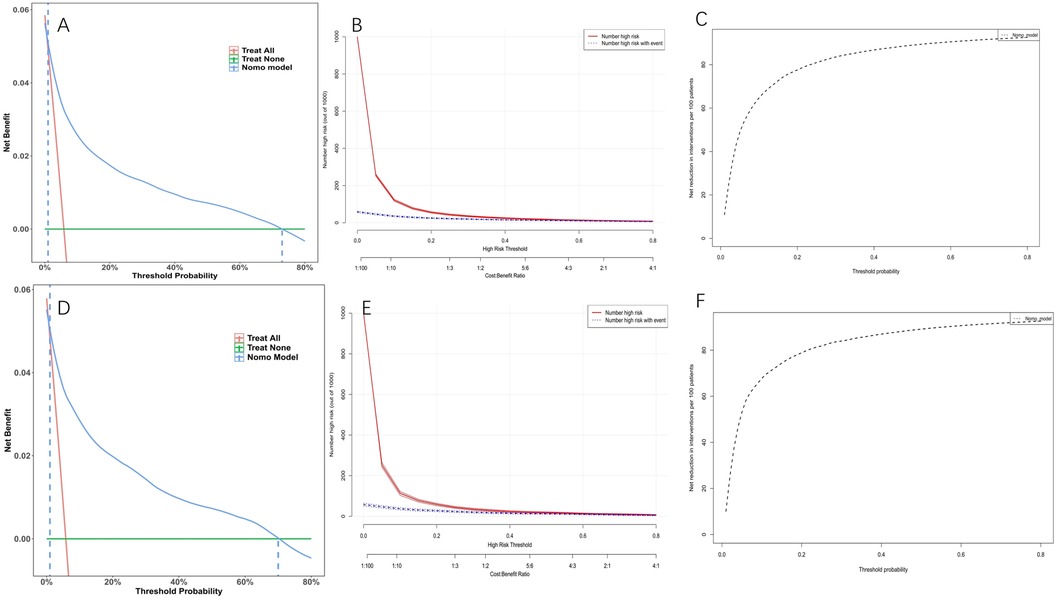
Figure 7. Decision curve analysis (DCA), clinical impact curves (CIC) and the net reduction curves (NRC) of the nomogram. (A) DCA in the development cohort; (B) CIC in the development cohort; (C) NRC in the development cohort; (D) DCA in the validation cohort; (E) CIC in the validation cohort; (F) NRC in the validation cohort. In the DCAs, the y-axis represents the net benefit. The horizontal lines labeled “None” represent the assumption that no participant experienced bleeding. The lines labeled “All” represent the assumption that all participants had bleeding. The lines labeled “nomogram model” represent the predictive model developed in this study. In CICs, the red curve represents the number of individuals classified as positive (high risk) by the model at each threshold probability, indicating the number of high-risk individuals. The blue curve represents the number of true positives (individuals with the outcome) at each threshold probability. In NRCs, the values on the y-axis represent the number of patients that could be reduced under the same effect size by utilizing a specific threshold probability of diagnosis, indicated by the value on the x-axis.
3.5 Model compare with single indicator
We compared the discriminative ability and clinical decision-making ability of our constructed model (nomogram) with that of a single indicator. Figure 8 demonstrates that our model surpasses a single indicator in terms of both discriminative ability and clinical decision-making ability.
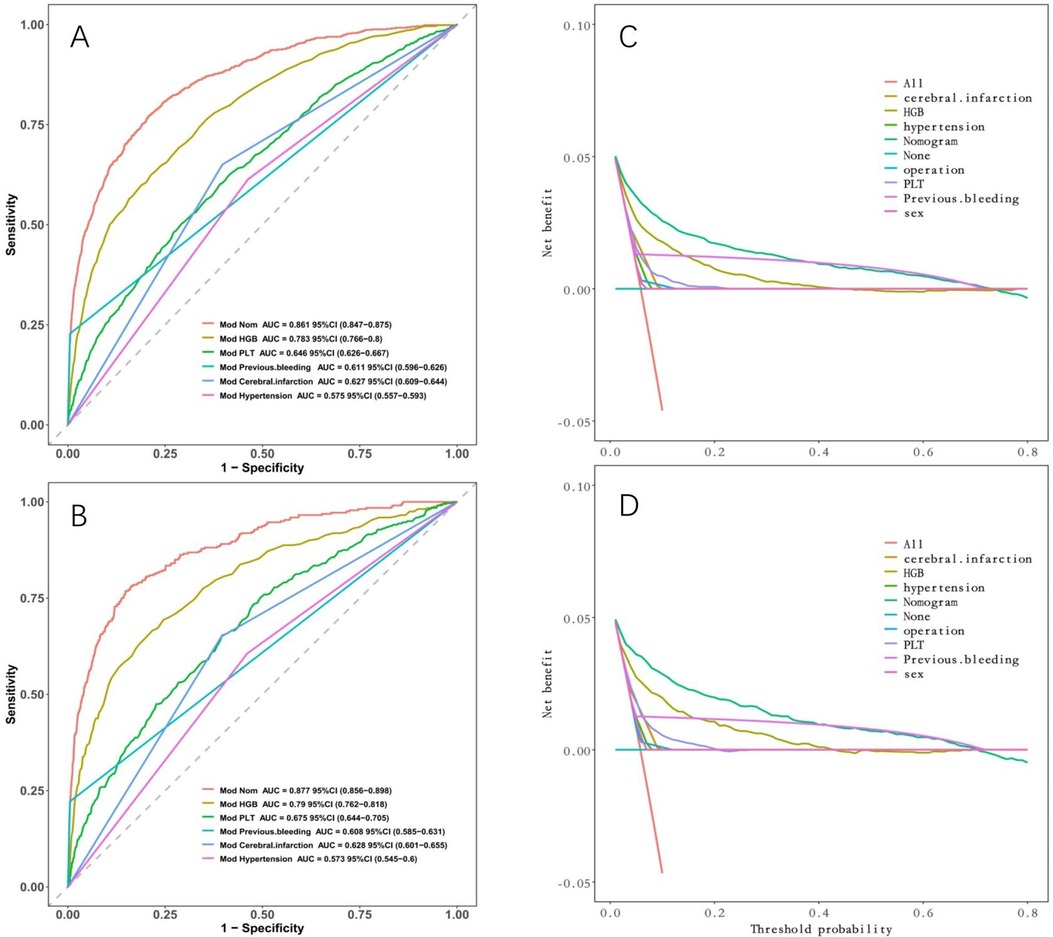
Figure 8. Comparison between nomogram and individual indicators. (A) ROC curves in the development cohort; (B) in the validation cohort. (C) DCA in the development cohort; (D) DCA in the validation cohort. Nomogram has the maximum AUC and maximum clinical net benefit.
4 Discussion
In this study, we created and validated a machine learning derived model to assess bleeding risk in DAPT patients. Among the six machine learning models and LASSO regression, the XGBoost model demonstrated the highest discriminatory ability. After analyzing feature importance, we constructed the final model with seven indicators: HGB, PLT, previous bleeding, cerebral infarction, sex, surgical history, and hypertension. The derived nomogram from this model provides a visual representation that enables clinicians to estimate the bleeding risk for individual patients receiving DAPT.
Several factors have been reported to influence bleeding in DAPT patients, including HGB (20), PLT (21), previous bleeding (20), cerebral infarction (22), sex (23), Surgical history (24), and hypertension (25). Therefore, we aimed to include comprehensive information in our risk models for predicting bleeding. Previous studies have indicated that a decrease in HGB levels is a strong predictor of major bleeding (26), Low baseline HGB levels were linked to higher bleeding rates (27). Our study reaffirmed the importance of HGB as a critical indicator for assessing bleeding risk. We also identified PLT levels as another significant predictor, with lower levels indicating higher bleeding risk and poorer prognosis (28). Our predictive model aligned with previous studies’ findings. In addition, we recognize the importance of genetic components influencing platelet responses, such as the F2rl3 SNP and alterations in clopidogrel metabolism (29, 30). These genetic factors can significantly impact platelet function and response to antiplatelet therapy, potentially affecting patient outcomes. While our current study did not specifically analyze these genetic influences due to its focus on clinical and demographic data. Previous bleeding has been established as a crucial factor in guiding treatment plans for DAPT patients (20). Our risk model showed that previous bleeding increased bleeding risk in DAPT patients. Artery occlusion cerebral infarction was associated with an elevated risk of hemorrhage transformation (31), consistent with our results and potentially linked to increased antithrombotic medication usage. Moreover, male sex was independently associated with bleeding risk, in line with previous research (32). In addition to the factors mentioned above, Surgical history has been recognized as a potential predictor of bleeding (33), Our findings indicated that Surgical history is a significant factor that elevates the risk of bleeding. Consistent with prior research findings (34), our study uncovered a substantial increase in bleeding risk associated with hypertension. This elevated risk may be attributed to structural changes in blood vessels caused by hypertension, rendering them more susceptible to rupture and ultimately increasing the likelihood of bleeding (35).
Tailored management strategies are essential for DAPT patients with varying bleeding risks. High-risk patients require comprehensive evaluation and optimization. Developing an accurate predictive model for bleeding in DAPT patients holds significant clinical implications. This includes managing comorbidities, optimizing blood parameters (e.g., HGB, PLT), considering clinical features (e.g., age, sex), addressing underlying diseases (e.g., hypertension, diabetes), and evaluating medication factors to enhance coagulation function. Our developed predictive model incorporates key variables such as HGB, PLT, previous bleeding, cerebral infarction, sex, Surgical history, and hypertension using machine learning techniques like LASSO regression and XGBoost. The model's output is visualized through a user-friendly nomogram, enabling clinicians to estimate individual bleeding risk and support personalized treatment decisions. Evaluation metrics, including clinical decision curve, clinical impact curve, and net reduction curve analyses, demonstrate a high net clinical benefit, suggesting the potential for improved patient outcomes and reduced healthcare costs. We acknowledge that its performance may vary in more diverse populations. Factors such as genetic differences, variations in healthcare access, and demographic disparities can influence the effectiveness of predictive models.
Despite our significant findings, there are limitations to consider. Firstly, our study was retrospective, limiting the establishment of causal relationships. Due to the retrospective nature of this real-world study, we did not analyze data regarding BMI, estimated glomerular filtration rate, statin use, contraceptive drug use, or the influence of inflammatory markers such as CRP on our prediction model. The absence of these data limits our ability to assess their potential impacts on patient outcomes and bleeding risk. Prospective studies are needed to further validate the predictive models developed in this research. Secondly, the missing data for certain variables may have introduced bias, although we mitigated this by excluding variables with missing information in over 20% of patients. Additionally, our study focused on DAPT patients, and the generalizability of our findings to other patient populations requires further investigation.
Overall, our model offers valuable clinical insights and aids in decision-making for managing bleeding in DAPT patients. It has the potential to optimize treatment planning, improve patient outcomes, and enhance resource utilization. In future research, external validation of our models in different patient cohorts is warranted to confirm their reliability and effectiveness in routine clinical practice. Moreover, the integration of additional variables and the exploration of different machine learning algorithms could further improve the accuracy of bleeding risk prediction. Finally, prospective studies evaluating the impact of utilizing our predictive models in clinical decision-making would provide valuable insights into their potential benefits and limitations.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of the Affiliated Dongyang Hospital of Wenzhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because This study received approval from the Medical Ethics Committee of the Affiliated Dongyang Hospital of Wenzhou Medical University (approval #2023-YX-408). Informed consent was waived, and patient records/information were anonymized and deidentified before analysis.
Author contributions
YQ: Data curation, Writing – original draft. LW: Formal Analysis, Methodology, Writing – original draft. WM: Conceptualization, Formal Analysis, Funding acquisition, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by Zhejiang Provincial Natural Science Foundation of China under Grant No. LTGY23H200002.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1402672/full#supplementary-material
References
1. Costa F, van Klaveren D, James S, Heg D, Räber L, Feres F, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet. (2017) 389(10073):1025–34. doi: 10.1016/S0140-6736(17)30397-5
2. Munafò AR, Montalto C, Franzino M, Pistelli L, Di Bella G, Ferlini M, et al. External validity of the PRECISE-DAPT score in patients undergoing PCI: a systematic review and meta-analysis. Eur Heart J Cardiovasc Pharmacother. (2023) 9(8):709–21. doi: 10.1093/ehjcvp/pvad063
3. Lv M, Zheng X, Wu T, Chen W, Jiang S, Zhang H, et al. A new score for predicting acute gastrointestinal bleeding in patients administered oral antiplatelet drugs. Front Pharmacol. (2020) 11:571605. doi: 10.3389/fphar.2020.571605
4. Mihatov N, Kirtane AJ, Stoler R, Feldman R, Neumann FJ, Boutis L, et al. Bleeding and ischemic risk prediction in patients with high bleeding risk (an EVOLVE short DAPT analysis). Am J Cardiol. (2023) 207:370–9. doi: 10.1016/j.amjcard.2023.06.036
5. Zhao X, Li J, Liu F, Zhu P, Jiang L, Tang X, et al. The PRECISE-DAPT score and 5-year outcomes after percutaneous coronary intervention: a large-scale, real-world study from China. Eur Heart J Qual Care Clin Outcomes. (2022) 8(8):812–20. doi: 10.1093/ehjqcco/qcab068
6. Capodanno D, Alfonso F, Levine GN, Valgimigli M, Angiolillo DJ. ACC/AHA versus ESC guidelines on dual antiplatelet therapy: jACC guideline comparison. J Am Coll Cardiol. (2018) 72(23 Pt A):2915–31. doi: 10.1016/j.jacc.2018.09.057
7. Mihatov N, Secemsky EA, Kereiakes DJ, Steg G, Serruys PW, Chichareon P, et al. Utility of the dual antiplatelet therapy score to guide antiplatelet therapy: a systematic review and meta-analysis. Catheter Cardiovasc Interv. (2021) 97(4):569–78. doi: 10.1002/ccd.29352
8. Vries MJ, van der Meijden PE, Henskens YM, ten Cate-Hoek AJ, ten Cate H. Assessment of bleeding risk in patients with coronary artery disease on dual antiplatelet therapy. A systematic review. Thromb Haemost. (2016) 115(1):7–24. doi: 10.1160/TH15-04-0355
9. Sorrentino S, Sartori S, Baber U, Claessen BE, Giustino G, Chandrasekhar J, et al. Bleeding risk, dual antiplatelet therapy cessation, and adverse events after percutaneous coronary intervention: the PARIS registry. Circ Cardiovasc Interv. (2020) 13(4):e008226. doi: 10.1161/CIRCINTERVENTIONS.119.008226
10. Choi SY, Kim MH, Serebruany V. Comparison of ACUITY, CRUSADE, and GRACE risk scales for predicting clinical outcomes in patients treated with dual-antiplatelet therapy. TH Open. (2018) 2(4):e399–406. doi: 10.1055/s-0038-1675576
11. Kadiyala V, Long S, Has P, Lima FV, Sherrod C, Heinl R, et al. PRECISE-DAPT and ARC-HBR predict in-hospital outcomes in patients who underwent percutaneous coronary intervention. Am J Cardiol. (2023) 191:43–50. doi: 10.1016/j.amjcard.2022.12.004
12. Sim DS, Jeong MH, Kim HS, Gwon HC, Seung KB, Rha SW, et al. Utility of GRACE and ACUITY-HORIZONS risk scores to guide dual antiplatelet therapy in Korean patients with acute myocardial infarction undergoing drug-eluting stenting. J Cardiol. (2018) 72(5):411–9. doi: 10.1016/j.jjcc.2018.04.006
13. Grodecki K, Huczek Z, Scisło P, Kowara M, Raposeiras-Roubín S, D'Ascenzo F, et al. Gender-related differences in post-discharge bleeding among patients with acute coronary syndrome on dual antiplatelet therapy: a BleeMACS sub-study. Thromb Res. (2018) 168:156–63. doi: 10.1016/j.thromres.2018.06.022
14. Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. (2015) 372(19):1791–800. doi: 10.1056/NEJMoa1500857
15. Costa F, Tijssen JG, Ariotti S, Giatti S, Moscarella E, Guastaroba P, et al. Incremental value of the CRUSADE, ACUITY, and HAS-BLED risk scores for the prediction of hemorrhagic events after coronary stent implantation in patients undergoing long or short duration of dual antiplatelet therapy. J Am Heart Assoc. (2015) 4(12):e002524. doi: 10.1161/JAHA.115.002524
16. Capodanno D, Rossini R, Musumeci G, Lettieri C, Senni M, Valsecchi O, et al. Predictive accuracy of CHA2DS2-VASc and HAS-BLED scores in patients without atrial fibrillation undergoing percutaneous coronary intervention and discharged on dual antiplatelet therapy. Int J Cardiol. (2015) 199:319–25. doi: 10.1016/j.ijcard.2015.07.064
17. Brinza C, Burlacu A, Tinica G, Covic A, Macovei L. A systematic review on bleeding risk scores’ accuracy after percutaneous coronary interventions in acute and elective settings. Healthcare (Basel). (2021) 9(2):148. doi: 10.3390/healthcare9020148
18. Xu D, Zhou H, Zhang T, Gong W, Zhong J, Yu H, et al. Safety of antiplatelet therapy in noncardioembolic ischemic stroke with thrombocytopenia: the CASE II study. J Am Heart Assoc. (2024) 13(16):e032327. doi: 10.1161/JAHA.123.032327
19. Jianling Q, Lulu J, Liuyi Q, Lanfang F, Xu M, Wenchen L, et al. A nomogram for predicting the risk of pulmonary embolism in neurology department suspected PE patients: a 10-year retrospective analysis. Front Neurol. (2023) 14:1139598. doi: 10.3389/fneur.2023.1139598
20. Kamran H, Jneid H, Kayani WT, Virani SS, Levine GN, Nambi V, et al. Oral antiplatelet therapy after acute coronary syndrome: a review. JAMA. (2021) 325(15):1545–55. doi: 10.1001/jama.2021.0716
21. Morici N, Tavecchia GA, Antolini L, Caporale MR, Cantoni S, Bertuccio P, et al. Use of PRECISE-DAPT score and admission platelet count to predict mortality risk in patients with acute coronary syndrome. Angiology. (2019) 70(9):867–77. doi: 10.1177/0003319719848547
22. Huang J, Liao F, Tang J, Shu X. Risk factors for gastrointestinal bleeding in patients with cerebral infarction after dual antiplatelet therapy. Clin Neurol Neurosurg. (2023) 231:107802. doi: 10.1016/j.clineuro.2023.107802
23. Landi A, Alasnag M, Heg D, Frigoli E, Malik FTN, Gomez-Blazquez I, et al. Abbreviated or standard dual antiplatelet therapy by sex in patients at high bleeding risk: a prespecified secondary analysis of a randomized clinical trial. JAMA Cardiol. (2024) 9(1):35–44. doi: 10.1001/jamacardio.2023.4316
24. Premji AM, Blegen MB, Corley AM, Ulloa J, Booth MS, Begashaw M, et al. Dual antiplatelet management in the perioperative period: updated and expanded systematic review. Syst Rev. (2023) 12(1):197. doi: 10.1186/s13643-023-02360-9
25. Deng T, Zhang T, Lu H, Chen J, Liu X, He W, et al. Evaluation and subgroup analysis of the efficacy and safety of intensive rosuvastatin therapy combined with dual antiplatelet therapy in patients with acute ischemic stroke. Eur J Clin Pharmacol. (2023) 79(3):389–97. doi: 10.1007/s00228-022-03442-8
26. Ng AK, Ng PY, Ip A, Ling IW, Lam LT, Siu CW. Incidence, prediction, and outcomes of Major bleeding after percutaneous coronary intervention in Chinese patients. JACC Asia. (2022) 2(3):341–50. doi: 10.1016/j.jacasi.2021.12.009
27. Chilbert MR, Reidy SE, Clark CM, Guszkowski M, Gargala E, Woodruff AE. Comparison of bleeding rates between oral anticoagulants in combination with dual antiplatelet therapy (triple therapy) in a real-world cohort. Hosp Pharm. (2022) 57(2):253–9. doi: 10.1177/00185787211024602
28. Lambert C, Maitland H, Ghanima W. Risk-based and individualised management of bleeding and thrombotic events in adults with primary immune thrombocytopenia (ITP). Eur J Haematol. (2023) 112(4):504–15. doi: 10.1111/ejh.14154
29. Whitley MJ, Henke DM, Ghazi A, Nieman M, Stoller M, Simon LM, et al. The protease-activated receptor 4 Ala120Thr variant alters platelet responsiveness to low-dose thrombin and protease-activated receptor 4 desensitization, and is blocked by non-competitive P2Y(12) inhibition. J Thromb Haemost. (2018) 16(12):2501–14. doi: 10.1111/jth.14318
30. Morikawa Y, Kato H, Kashiwagi H, Nishiura N, Akuta K, Honda S, et al. Protease-activated receptor-4 (PAR4) variant influences on platelet reactivity induced by PAR4-activating peptide through altered ca(2+) mobilization and ERK phosphorylation in healthy Japanese subjects. Thromb Res. (2018) 162:44–52. doi: 10.1016/j.thromres.2017.12.014
31. Kumazawa R, Jo T, Matsui H, Fushimi K, Yasunaga H. Direct oral anticoagulants versus warfarin for secondary prevention of cerebral infarction and bleeding in older adults with atrial fibrillation. J Am Geriatr Soc. (2022) 70(7):2029–39. doi: 10.1111/jgs.17770
32. Qian J, Zan J, Kuang L, Che L, Yu Y, Shen T, et al. A predictive nomogram of bleeding risk in patients with atrial fibrillation after drug-eluting stent implantation. Ann Transl Med. (2021) 9(3):193. doi: 10.21037/atm-20-3971
33. Li X, Zhang X, Jin Q, Xue Y, Lu W, Ge J, et al. Clinical efficacy and safety comparison of rivaroxaban and dabigatran for nonvalvular atrial fibrillation patients undergoing percutaneous left atrial appendage closure operation. Front Pharmacol. (2021) 12:614762. doi: 10.3389/fphar.2021.614762
34. Hao J, Dang P, Quan X, Chen Z, Zhang G, Liu H, et al. Risk factors, prediction model, and prognosis analysis of myocardial injury after acute upper gastrointestinal bleeding. Front Cardiovasc Med. (2022) 9:1041062. doi: 10.3389/fcvm.2022.1041062
Keywords: dual antiplatelet therapy, bleeding, machine learning, predictive model, nomogram, risk factor
Citation: Qian Y, Wanlin L and Maofeng W (2024) Machine learning derived model for the prediction of bleeding in dual antiplatelet therapy patients. Front. Cardiovasc. Med. 11:1402672. doi: 10.3389/fcvm.2024.1402672
Received: 3 July 2024; Accepted: 19 September 2024;
Published: 2 October 2024.
Edited by:
DeLisa Fairweather, Mayo Clinic Florida, United StatesReviewed by:
Silvio Antoniak, University of North Carolina at Chapel Hill, United StatesJun Qian, Tongji Hospital Affiliated to Tongji University, China
Copyright: © 2024 Qian, Wanlin and Maofeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wang Maofeng, d3ptY3dtZkB3bXUuZWR1LmNu
†These authors have contributed equally to this work
 Yang Qian
Yang Qian Lei Wanlin
Lei Wanlin Wang Maofeng
Wang Maofeng