- 1Department of Cardiology, Zhangzhou Affiliated Hospital of Fujian Medical University, Zhangzhou, Fujian, China
- 2Department of Endocrinology, Shengli Clinical Medical College of Fujian Medical University, Fujian Provincial Hospital, Fuzhou University Affiliated Provincial Hospital, Fuzhou, China
Background: In the ICU, patients with acute myocardial infarction and cardiogenic shock (AMI-CS) often face high mortality rates, making timely and precise mortality risk prediction crucial for clinical decision-making. Despite existing models, machine learning algorithms hold the potential for improved predictive accuracy.
Methods: In this study, a predictive model was developed using the MIMIC-IV database, with external validation performed on the eICU-CRD database. We included ICU patients diagnosed with AMI-CS. Feature selection was conducted using the Boruta algorithm, followed by the construction and comparison of four machine learning models: Logistic Regression (LR), eXtreme Gradient Boosting (XGBoost), Adaptive Boosting (AdaBoost), and Gaussian Naive Bayes (GNB). Model performance was evaluated based on metrics such as AUC (Area Under the Curve), accuracy, sensitivity, specificity, and so on. The SHAP method was employed to visualize and interpret the importance of model features. Finally, we constructed an online prediction model and conducted external validation in the eICU-CRD database.
Results: In this study, a total of 570 and 391 patients with AMI-CS were included from the MIMIC-IV and eICU-CRD databases, respectively. Among all machine learning algorithms evaluated, LR exhibited the best performance with a validation set AUC of 0.841(XGBoost: 0.835, AdaBoost: 0.839, GNB: 0.826). The model incorporated five variables: prothrombin time, blood urea nitrogen, age, beta-blockers and Angiotensin-Converting Enzyme Inhibitors or Angiotensin II Receptor Blockers. SHAP plots are employed to visualize the importance of model features and to interpret the results. An online prediction tool was developed, externally validated with the eICU-CRD database, achieving an AUC of 0.755.
Conclusion: Employing the LR algorithm, we developed a predictive model for assessing the mortality risk among AMI-CS patients in the ICU setting. Through model predictions, this facilitates early detection of high-risk individuals, ensures judicious allocation of healthcare resources.
1 Introduction
Acute myocardial infarction (AMI) with cardiogenic shock (CS) presents as a critical syndrome marked by a rapid decline in cardiac pump function, leading to systemic circulatory failure and multi-organ dysfunction (1). Despite a downward trend in the incidence and mortality of AMI (2, 3), patients presenting with concurrent CS continue to have a dire prognosis (4). In patients with AMI, the incidence of CS is approximately 5%–10% (5–7). This often leads to multi-organ dysfunction, including acute kidney injury, respiratory failure, and neurological complications (8). The 30-day mortality rate for AMI-CS approaches 40%, with the one-year mortality reaching up to 50%, indicating its exceedingly high lethality (7, 9, 10). AMI-CS patients frequently require emergency coronary interventions and mechanical support, increasing treatment complexity and cost (7). Accurately predicting mortality risk in AMI-CS patients is vital for guiding clinical decisions, enabling personalized treatment, and optimizing resources (11).
Currently, predictive models for in-hospital mortality among AMI-CS patients are primarily constructed using logistic regression methods (12–14). Commonly used severity scoring systems such as APACHE II, APACHE III, SAPS II, and SOFA often exhibit limited predictive performance (13, 14). The IABP-SHOCK II score and CardShock score model exhibit satisfactory performance in predicting mortality among CS patients, yet there are certain limitations regarding the size of the model construction cohort and external validation (15, 16). With the rapid progress of precision medicine, machine learning is increasingly utilized in healthcare for outcome prediction, diagnosis, medical image interpretation, and treatment (17–19). Machine learning exhibits superior clinical prediction accuracy and performance compared to traditional statistical methods, with the added advantage of faster processing speeds (20). With the advent of interpretable techniques like SHAP, users can gain a better understanding of the predictive outcomes generated by machine learning models (21). Currently, interpretable machine learning (ML) models for predicting in-hospital mortality in patients with AMI-CS have not been established.
Our study aims to utilize diverse machine learning algorithms to construct predictive models for assessing in-hospital mortality risk among AMI-CS patients. We identified machine learning models with superior predictive performance and clinical relevance, established an online predictive system, and conducted external validation. Furthermore, we employed the SHAP methodology to identify key clinical predictive factors and interpret the model outcomes.
2 Materials and methods
2.1 Data sources
This study utilized patient data from two databases: the Medical Information Mart for Intensive Care IV (MIMIC-IV) database and the eICU Collaborative Research Database dataset (eICU-CRD). MIMIC-IV (version 2.2) is an extensive critical care database, encompassing detailed records of over 190,000 ICU patients from 2008 to 2019. This dataset aggregates a wealth of clinical information, including patients’ demographic profiles, laboratory test results, medication histories, and additional comprehensive data sets (22). The eICU Collaborative Research Database (eICU-CRD) pools detailed data from more than 200,000 patients in various U.S. intensive care units, collected between 2014 and 2015, making it an essential tool for advancing research in critical care medicine (23).
2.2 Study population
This study focuses on patients with AMI-CS, and obtains the relevant patient data from two databases using ICD-9 and ICD-10 codes. The exclusion criteria for the study population are as follows: (1) under the age of 18, and (2) no ICU experience or an ICU stay less than 24 h. For patients with multiple admissions or a history of ICU stays, only the first ICU experience during their first admission is included. In our research, cardiogenic shock (CS) was defined based on several clinical parameters, primarily including the following: ① Systolic blood pressure (SBP) < 90 mmHg or the need for vasopressor support to maintain SBP ≥ 90 mmHg; ② Signs of reduced cardiac output leading to poor organ perfusion (e.g., low urine output, cold and clammy skin, altered mental status); ③ Exclusion of other causes of shock, such as hypovolemic or septic shock.
2.3 Data extraction and preprocessing
In this study, we included ICU patients diagnosed with AMI-CS, from whom we extracted the following data: (1) Demographics: age, gender, height, and weight; (2) Vital Signs: temperature (T), heart rate (HR), respiratory rate (R), systolic blood pressure (SBP), diastolic blood pressure (DBP), mean blood pressure (MBP), and peripheral oxygen saturation (SpO2); (3) Laboratory Indicators: complete blood count, liver and kidney function tests, electrolytes, lipid profile, blood gases, coagulation profile, and cardiac enzymes; (4) Comorbidities: hypertension, diabetes mellitus, hyperlipidemia, chronic obstructive pulmonary disease (COPD), pneumonia, chronic kidney disease (CKD), and atrial fibrillation (AF); (5) Surgical Indicators: coronary angiography (CAG), percutaneous coronary intervention (PCI), percutaneous transluminal coronary angioplasty (PTCA), and intra-aortic balloon pump (IABP); (6) Medication data: angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers (aceiorarb), beta Blockers, furosemide, spironolactone, dobutamine, dopamine, epinephrine, milrinone, norepinephrine, and phenylephrine; and (7) Other Indicators such as Mechanical Ventilation and APACHE II score. The primary outcome was 28-day all-cause mortality, defined as death from any cause within 28 days starting from admission to the ICU.
The raw data is refined through advanced calculations. This involves computing Body Mass Index (BMI) from height and weight, and assessing in-hospital mortality within 28 days using hospitalization duration and survival status. In both the MIMIC-IV and eICU-CRD datasets, we rigorously addressed missing data by eliminating entries with missing rates exceeding 30%. Subsequently, we employed the K-Nearest Neighbors (KNN) imputation method to handle remaining missing values. KNN imputation leverages sample similarity, utilizing observed values from the K nearest samples to predict and fill missing values effectively. Exploration of variable relationships was conducted using Spearman correlation coefficients, visually represented through heatmaps. Multicollinearity among variables was meticulously assessed using Variance Inflation Factor (VIF) values. To optimize predictive performance and prevent overfitting, we selectively pre-screened predictive variables with high correlations or VIF exceeding 5.
2.4 Model construction and evaluation
After preprocessing, we initially included 38 predictive factors to construct a model for predicting in-hospital mortality within 28 days for patients with AMI-CS. To improve the stability of the predictive model, all continuous variables were standardized, scaled to have a mean distribution of 0 and a standard deviation of 1. The Boruta algorithm was used for feature selection. Its principle is to determine the most relevant features in the dataset based on the Random Forest by comparing with randomly generated “shadow” features. This method has the advantage of not requiring assumptions, which enhances the robustness of the model and simplifies the feature selection process (24). The XGBoost method was employed to rank the selected features based on their importance in a professional manner. The MIMIC dataset was used to construct the model, employing a 10-fold cross-validation method to generate training and validation sets. Four machine learning models were established and validated, including Logistic Regression (LR), eXtreme Gradient Boosting (XGBoost), Adaptive Boosting (AdaBoost), and Gaussian Naive Bayes (GNB). Model comparisons are conducted on the validation set. The Area Under the Receiver Operating Characteristic (ROC) curve (AUC) is utilized to assess the model's discriminative ability, while calibration curves and Brier scores are employed to evaluate model accuracy. Additionally, Decision Curve Analysis (DCA) curves are utilized to assess clinical utility. Additionally, confusion matrix metrics were included for evaluation, such as accuracy, sensitivity, specificity, positive predictive value, negative predictive value, and F1 score. We also compared the predictive performance of our model with commonly used severity scoring systems such as SOFA, APSIII, SAPSII, and OASIS.
2.5 Model interpretation
The SHAP (SHapley Additive exPlanations) method is an approach for explaining machine learning model predictions (25). It leverages Shapley values to decompose the impact of each feature on the model output, providing insights into the prediction process. By considering interactions between features, SHAP offers intuitive explanations for individual predictions, aiding in the identification of key features and prediction sources. In SHAP plots, red and blue points represent the SHAP values of each sample, with red indicating higher feature values and blue indicating lower feature values. Observing the distribution of red and blue points helps understand the contribution and direction of each feature towards the model output.
2.6 Statistical analysis
Patients were categorized into two groups based on their 28-day mortality status. Continuous variables were summarized using means and standard deviations and compared using t-tests (or Wilcoxon rank-sum tests). Categorical variables were presented as percentages of the total and compared using chi-square tests (or Fisher's exact tests). A P-value < 0.05 was considered statistically significant. Statistical analyses were conducted using R version 4.2.3 and Python version 3.11.4.
3 Results
3.1 Baseline characteristics
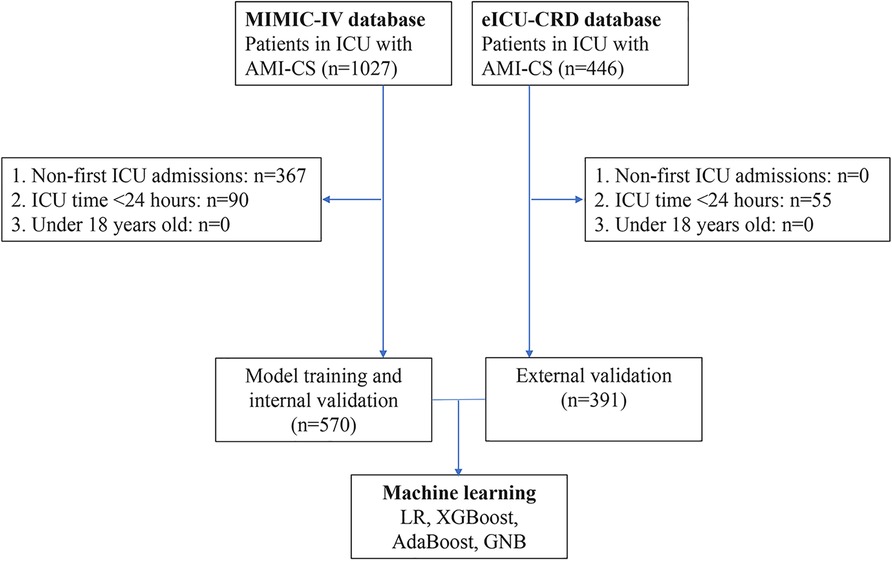
According to the inclusion and exclusion criteria, a total of 961 patients with AMI-CS were enrolled in this study, including 570 in the MIMIC-IV database and 391 in the eICU-CRD database. The screening process is illustrated in Figure 1. In the MIMIC-IV database, 215 cases of AMI-CS patients died within 28 days (mortality rate: 37.7%), compared to 102 cases in the eICU-CRD (mortality rate: 26.1%). Differences in baseline characteristics are summarized in Table 1. In both the MIMIC-IV and eICU-CRD databases, patients who died had higher levels of age, blood urea nitrogen (BUN), prothrombin time (PT), creatinine (Cr), international normalized ratio (INR), respiratory rate (R), and APACHE III score, as well as lower levels of serum albumin (ALB) and pH (p < 0.05). Furthermore, differences were observed in the use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (aceiorarb), beta-blockers, spironolactone, epinephrine, and ventilation (p < 0.05) between the survival and mortality groups. However, there were no significant differences in comorbidities such as hypertension, diabetes, and chronic obstructive pulmonary disease (COPD) between the two groups.
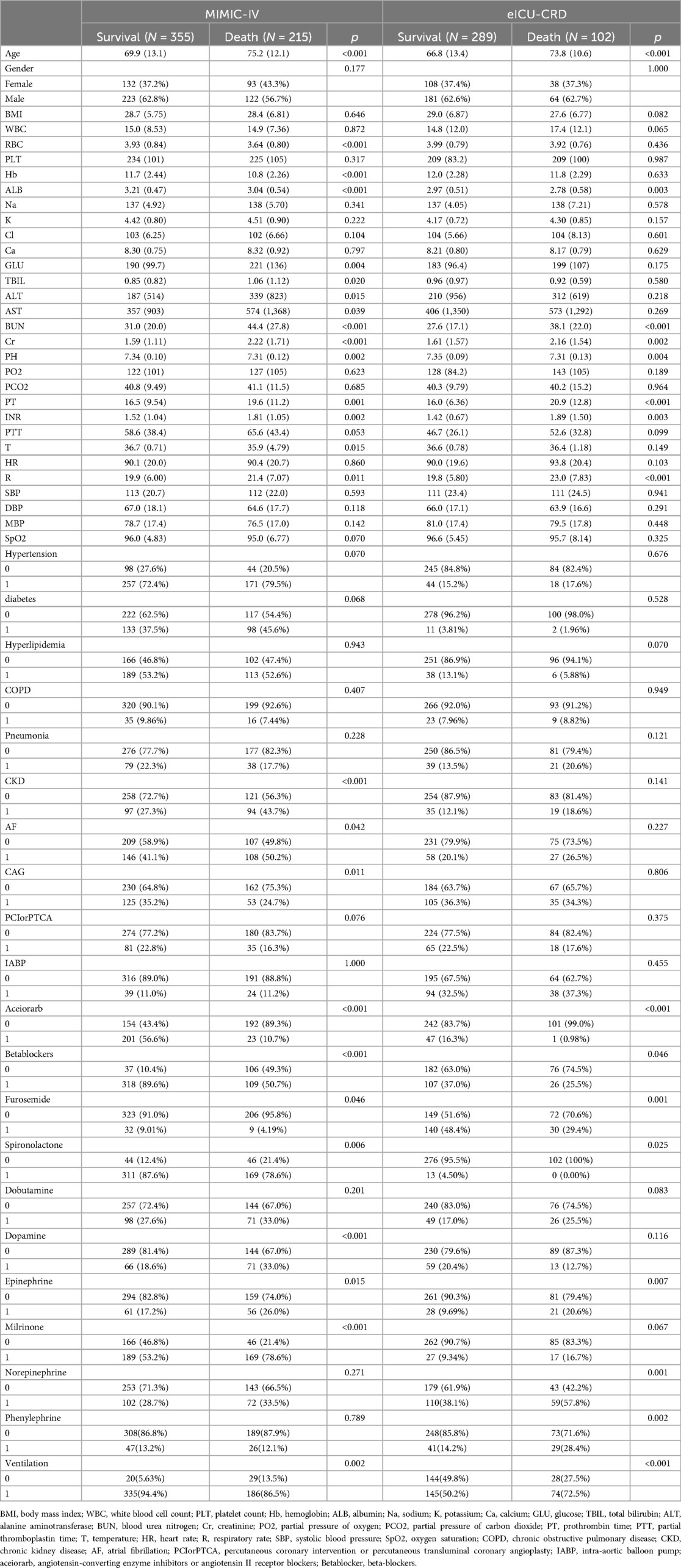
Table 1. The baseline characteristics of the MIMIC-IV and eICU-CRD databases, categorized by survival and death groups.
We removed features with a missing rate exceeding 30%, as shown in the Supplementary Figure S1. Additionally, we illustrated the correlation heat map for features with correlations exceeding 0.5, along with the results of features with VIF exceeding 5, as depicted in the Supplementary Figure S2 and Supplementary Figure S3. For features exhibiting high correlation and VIF, we conducted screening before model construction and excluded the following features: red blood Cell count (RBC), coronary angiography (CAG), aspartate aminotransferase (AST), mean blood pressure (MBP), diastolic blood pressure (DBP), international normalized ratio (INR).
3.2 Feature selection
We employed the Boruta algorithm for feature selection and generated a plot, shown in Figure 2. Boruta assesses feature importance by creating shadow features (derived from shuffling original feature values) and training them alongside original features in a random forest. In the plot, green denotes important features, enhancing model prediction and thus included. Red represents unimportant features excluded from the model, while yellow signifies uncertain importance, necessitating further investigation. Blue indicates shadow features used for comparison but not in model training. Through Boruta selection, we identified 13 features for inclusion: angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers (aceiorarb), beta blockers (betablockers), furosemide, dobutamine, norepinephrine, age, albumin (ALB), glucose (GLU), alanine aminotransferase (ALT), blood urea nitrogen (BUN), creatinine (Cr), prothrombin time (PT), and temperature (T), along with two potential features: hemoglobin (Hb) and sodium (Na).
We utilized the XGBoost method to rank the importance of features, as depicted in Supplementary Figure S4. The top 10 variables, ranked from highest to lowest importance, are: “aceiorarb”, “betablockers”, “PT”, “age”, “BUN”, “GLU”, “T”, “Na”, “dobutamine”, and “Hb”.
3.3 Model construction
This study employed four binary classification machine learning algorithms: Logistic Regression (LR), eXtreme Gradient Boosting (XGBoost), Adaptive Boosting (AdaBoost), and Gaussian Naive Bayes (GNB). Utilizing the MIMIC database, we employed a 10-fold cross-validation technique to divide the dataset into training and validation subsets, and evaluated the model performance on the validation subsets. Figure 3A and Table 2 describe the performance of these predictive models, with results indicating that the LR model exhibits better discriminative ability, achieving an AUC of 0.841 in the test queue, compared to other ML models (AUC: XGBoost = 0.835; AdaBoost = 0.839, GNB = 0.826). Furthermore, LR's calibration curve closely approximated the ideal line (Figure 3B), exhibiting the lowest Brier score, indicative of superior calibration. Decision Curve Analysis (DCA) illustrated in Figure 3C revealed LR's highest net benefit within the 0%–80% threshold range. As shown in the PR curve (Figure 3D), the LR model maintains high precision while capturing more positive samples, demonstrating superior performance. Hence, LR was selected for model development, incorporating five predictive variables: betablockers, aceiorarb, PT, age, and BUN. Model parameter optimization through hyperparameter tuning and grid search yielded the following settings: tol (convergence criterion) = 0.0001, penalty (regularization type) = l2, max_iter (maximum number of iterations) = 100, and C (regularization factor) = 10.0.

Figure 3. Summary plot of machine learning performance evaluation. (A) ROC curve, (B) calibration plot, (C) DCA curve, (D) PR curve.
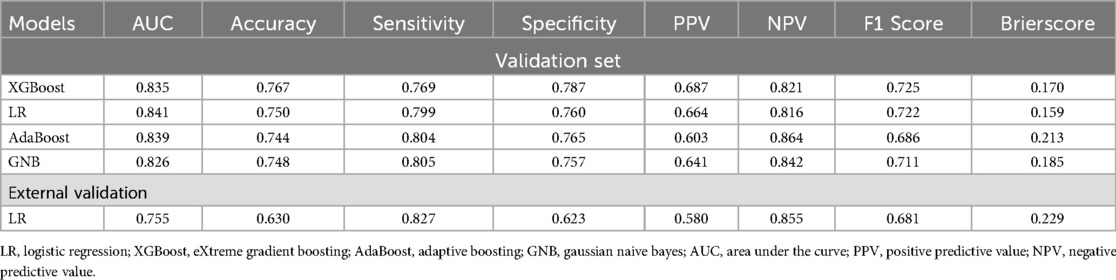
Table 2. Model performance compariso: AUC, accuracy, sensitivity, specificity; PPV, NPV, F1 score, brier score.
3.4 Model interpretation
We utilize the SHAP method to interpret the model results. For global interpretation, the absolute Shapley values are averaged across all instances in the data, yielding importance values for each feature. The results are visualized as SHAP summary plots, where the Y-axis represents the features, and the X-axis indicates the magnitude of the feature's impact on the outcome. Each point represents a sample, with red points indicating high-risk values and blue points indicating low-risk values. As shown in SHAP summary plot (Figure 4A), the feature importance from top to bottom is: aceiorarb, betablockers, age, BUN, PT. Patients using aceiorarb (red points) have a reduced risk of death, while patients not using betablockers (blue points) have an increased risk of death. Similarly, higher values of age, BUN, and PT are associated with higher risk of death.
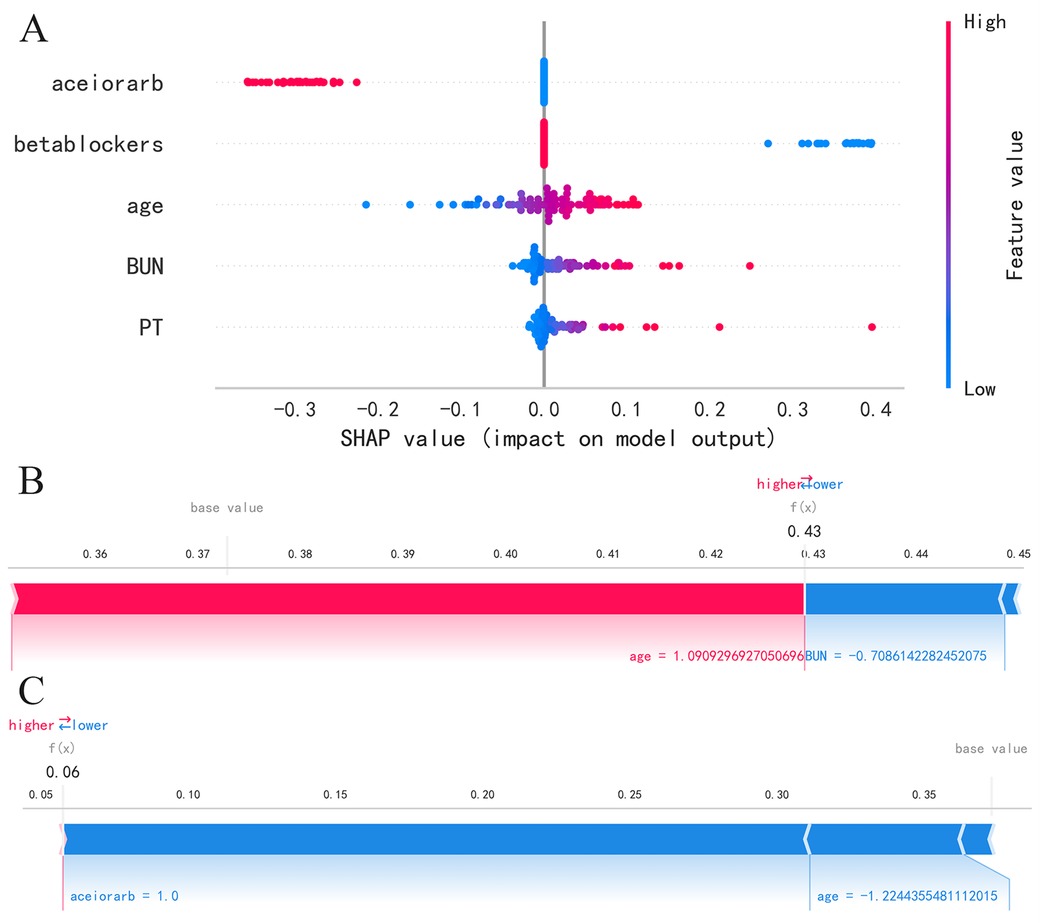
Figure 4. SHAP summary plot and SHAP force plot, (A) SHAP summary plot, (B) SHAP force plots for patient 1, (C) SHAP force plots for patient 2.
For local interpretation, each observation has its own set of Shapley values, which can be utilized to explain the contribution of each sample and feature to the prediction. The results are visualized as SHAP force plots, where each Shapley value is represented as an arrow that can either push the prediction up (positive value) or down (negative value). Due to the standardization of numerical variables, the age, BUN, and PT values in the plot do not represent the actual values of individuals. As depicted in Figure 4B for patient 1, as age increases, the predicted mortality risk rises, while a decrease in BUN value corresponds to a decrease in predicted mortality risk. As depicted in Figure 4C for patient 2, a decrease in age and the use of ACEI or ARB drugs are associated with a reduction in predicted mortality risk.
3.5 Model deployment and external validation
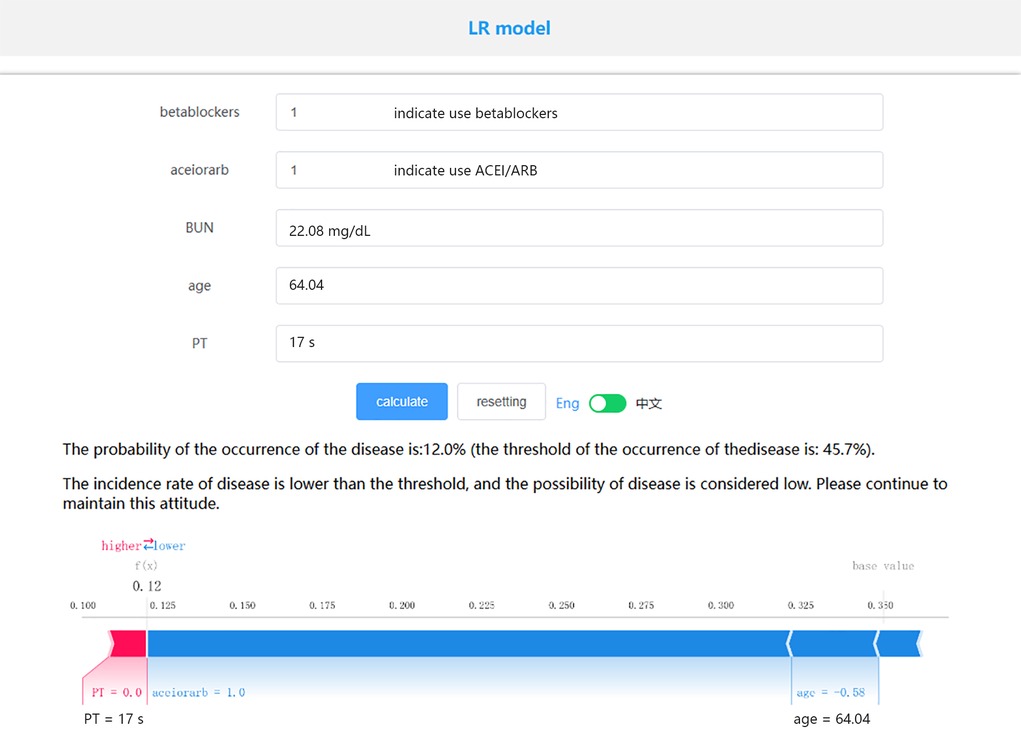
Based on the LR model, which exhibited the best performance, we established an online prediction model. As shown in Figure 5, we present the survival prediction for actual patients using the online website. The patient is 64 years old, with PT of 17s, BUN of 22.08 mg/dl, has used ACEI/ARB, and has used beta-blockers. The probability of the occurrence of the disease is: 12.0% (the threshold of the occurrence of the disease is: 45.7%). To facilitate understanding, we randomly selected four survivors and four deceased patients from the MIMIC-IV and eICU databases in the past. We had used the online prediction model to estimate their mortality risk. According to the LR model, when the predicted mortality risk was less than 50%, the patient was inferred to have survived beyond 28 days, and when it was greater than 50%, the patient was inferred to have died within 28 days. The final results, which were presented in Supplementary Table S3, demonstrated a 100% accuracy in predicting the outcomes for these eight patients. We extracted 391 cases of AMI-CS patients from the eICU-CRD database as an external validation dataset. The data characteristics are outlined in Table 2. The 28-day in-hospital mortality rate in the eICU-CRD database was 26.1%, lower than that in the MIMIC database. Prior to external validation, we processed the eICU-CRD data using the same methods as the MIMIC data. Due to the substantial imbalance between survival and death cases, we applied Synthetic Minority Over-sampling Technique (SMOTE) to balance the dataset. The results of external validation revealed an AUC of 0.755 and a Brier score of 0.229. Other metrics for external validation, such as accuracy, sensitivity, etc., are available in Table 2. Additionally, an analysis of commonly used severity scores using the MIMIC-IV dataset revealed model AUC values, including SOFA (AUC = 0.620), APSIII (AUC = 0.710), SAPSII (AUC = 0.660), and OASIS (AUC = 0.640).
4 Discussion
In this paper, we introduce for the first time an interpretable machine learning algorithm for predicting in-hospital mortality in patients with AMI-CS. This novel model outperforms traditional prediction tools by effectively managing complex datasets and identifying intricate nonlinear relationships. It demonstrates superior accuracy in differentiating between survival and death outcomes of patients, and shows commendable performance in terms of calibration and clinical utility. The high interpretability of the model facilitates ease of understanding and application of its results. Moreover, the model utilizes a minimal number of easily accessible predictive variables, which enhances its practicality in clinical settings.
Several retrospective studies have utilized critical illness scoring systems, including APACHE II, APACHE III, SAPS II, and SOFA, to predict the in-hospital mortality risk of patients with AMI-CS (13, 14). However, the discriminative ability of these models only falls within an AUC range of 0.67 to 0.79. Additionally, the models have included a sample size of fewer than 100 individuals and lack validation cohorts. The IABP-SHOCK II scoring system, derived from multicenter, randomized, controlled trial data, is designed to assess the short-term mortality risk in patients with AMI-CS (16). It effectively categorizes patients into low, moderate, and high-risk groups. However, the model is based on a limited cohort study (n = 480) and has only been validated in two small sample sizes of 137 and 98 patients, respectively. Consequently, this model offers a valuable tool for physicians to more precisely evaluate the risk profiles of AMI-CS patients and implement timely interventions to improve their prognoses. CardShock scoring model exhibits good discriminative ability in predicting short-term mortality risk in CS (AUC = 0.85). However, in external cohorts, the AUC of the CardShock scoring model drops to 0.71, suggesting potential overfitting (26). Our model outperforms common critical illness scoring systems. Additionally, with a larger cohort of AMI-CS patients, it demonstrates strong performance in both training and validation sets and shows good generalization ability in external validation.
Our study shows that the use of ACEI/ARB and beta-blockers are significant prognostic factors for mortality in AMI-CS patients. However, these results should be interpreted with caution, as they do not imply a causal relationship between the use of these medications and the prognosis of AMI-CS patients. It is likely that patients who were able to use these medications were in better overall condition, which could explain their better outcomes. These medications are identified as the two most important predictive factors. ACEI/ARB can dilate blood vessels by inhibiting the renin-angiotensin system, reducing cardiac load, and improving myocardial remodeling. Potential mechanisms may involve early inhibition of neurohormonal activation and reduction in infarct size, as well as an increase in regional wall motion and collateral coronary flow (27, 28). Beta-blockers reduce myocardial oxygen demand by attenuating sympathetic nervous system activity, leading to improved cardiac function and prognosis by suppressing ventricular arrhythmias (29). A retrospective study of 4,478 AMI patients with SBP < 100 mmHg showed that early use of ACEI/ARB significantly reduced MACE occurrence compared to non-users (1.67% vs. 3.66%) (30). The timing of initiating ACEIs/ARBs and beta-blockers in AMI patients with low blood pressure is controversial in clinical practice due to their hypotensive effects. The expert consensus recommends that in coronary heart disease patients, if hypotension (systolic blood pressure <90mmHg) occurs during ACEI treatment and the patient is asymptomatic, ACEI should be continued (31). Beta-blockers are recommended as one of the first-line medications for improving mortality rates in patients with chronic heart failure (32, 33). As is well known, beta-blockers are contraindicated in overt heart failure or low-output states due to their negative inotropic effects. Research has indicated that reducing the dosage or discontinuing beta-blockers in heart failure patients previously treated with them may lead to adverse outcomes (34). However, a meta-analysis found that although beta-blockers reduce recurrent myocardial infarction, they increase the risk of cardiogenic shock, without providing a mortality benefit in treating AMI (35). Similarly, a large randomized controlled trial indicated that early use of beta-blockers in acute myocardial infarction reduces the risk of recurrent infarction and ventricular fibrillation but increases the risk of cardiogenic shock (36). Hence, the general prudent approach is to initiate beta-blocker therapy in the hospital setting only after achieving hemodynamic stability following myocardial infarction.
Our study findings indicate that older age is associated with a higher predicted risk of mortality, consistent with the CardShock risk score (26). As age increases, there's a decline in cardiac and organ function. Elderly individuals are more likely to have multiple chronic diseases, reducing their ability to cope with and recover from cardiogenic shock, increasing mortality risk. Cinar et al. found that age remains an important risk factor for mortality in AMI-CS patients (37). Ming-Lung et al.'s study finds a significant positive correlation between age and risk, showing that STEMI patients aged 85 and above have a 3.42 times higher short-term risk compared to those under 55 years old (38). The study suggests that higher PT levels may be associated with poorer risk prediction. Similarly, in mortality prediction models for acute myocardial infarction, PT was observed as one of the top 8 predictors for predicting death in AMI patients (39). Prolonged PT in these patients may indicate a higher risk of mortality as it reflects disruptions in the coagulation system, possibly leading to severe complications. Additionally, it may serve as a marker of systemic illness severity and multi-organ dysfunction, correlating with elevated mortality risk.
Our study indicates that higher BUN levels are associated with an increased risk of mortality in AMI-CS patients, while creatinine clearance rate is not a significant predictor. Yuan et al.'s study of 218 AMI-CS patients showed that higher admission BUN levels, particularly those exceeding 8.95 mmol/L, were independently associated with a greater risk of 30-day mortality (40). Some research suggests that kidney function plays a crucial role in predicting the prognosis of AMI-CS patients. In a subgroup analysis of the IABP-SHOCK II trial, serum creatinine levels were found to be a significant independent predictor of one-year mortality (41). In the TRIUMPH multicenter trial, higher creatinine clearance rates were associated with lower 30-day mortality in univariate analysis (odds ratio 0.77) (42). In heart failure patients, BUN has been observed to have the highest predictive efficacy for predicting 30-day mortality among BUN, creatinine, BUN-to-creatinine ratio, and GFR (43–45). Creatinine is filtered in the renal glomeruli and not reabsorbed, while urea is reabsorbed in the renal tubules. A decrease in urine flow in AMI-CS patients can lead to increased urea reabsorption, resulting in higher BUN levels, which may be a more sensitive indicator.
This study has several limitations. Firstly, being a retrospective study, despite conducting internal and external validation using two databases, it's challenging to completely avoid selection bias. Further multicenter and large-scale clinical studies are warranted. Secondly, the clinical information collected is limited. Future research should delve into and integrate imaging modalities such as echocardiography, lung CT, and coronary imaging results to enhance prediction accuracy. Lastly, our data originates from ICU patients, potentially differing from those in other cardiovascular units. Therefore, additional validation is necessary to ascertain its applicability to other cardiovascular units.
5 Conclusion
This article uses machine learning algorithms to construct models that can accurately predict the in-hospital mortality risk of patients with AMI-CS in the ICU. The LR algorithm demonstrates the best predictive performance, clear result interpretation, and the predictive variables are easily accessible, offering valuable guidance for clinical practice.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
QZ: Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Writing – original draft, Writing – review & editing, Conceptualization, Project administration, Resources, Supervision, Visualization. LX: Conceptualization, Data curation, Investigation, Project administration, Resources, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing. ZX: Data curation, Investigation, Software, Writing – original draft, Writing – review & editing, Formal Analysis, Methodology, Validation. WH: Data curation, Formal Analysis, Methodology, Conceptualization, Project administration, Writing – original draft, Writing – review & editing. XH: Conceptualization, Investigation, Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to acknowledge the contributions of specific colleagues and institutions that aided the efforts of the authors in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1402503/full#supplementary-material
References
1. Shah AH, Puri R, Kalra A. Management of cardiogenic shock complicating acute myocardial infarction: a review. Clin Cardiol. (2019) 42(4):484–93. doi: 10.1002/clc.23168
2. Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. (2010) 362(23):2155–65. doi: 10.1056/nejmoa0908610
3. Chi GC, Kanter MH, Li BH, Qian L, Reading SR, Harrison TN, et al. Trends in acute myocardial infarction by race and ethnicity. J Am Heart Assoc. (2020) 9(5):e013542. doi: 10.1161/jaha.119.013542
4. Dalzell JR. Review of cardiogenic shock after acute myocardial infarction. Jama. (2022) 327(9):878. doi: 10.1001/jama.2021.25175
5. Fox KA, Steg PG, Eagle KA, Goodman SG, Anderson FA Jr, Granger CB, et al. Decline in rates of death and heart failure in acute coronary syndromes, 1999–2006. JAMA. (2007) 297(17):1892–900. doi: 10.1001/jama.297.17.1892
6. Samsky MD, Krucoff MW, Morrow DA, Abraham WT, Aguel F, Althouse AD, et al. Cardiac safety research consortium “shock II” think tank report: advancing practical approaches to generating evidence for the treatment of cardiogenic shock. Am Heart J. (2020) 230:93–7. doi: 10.1016/j.ahj.2020.09.015
7. Thiele H, Ohman EM, de Waha-Thiele S, Zeymer U, Desch S. Management of cardiogenic shock complicating myocardial infarction: an update 2019. Eur Heart J. (2019) 40(32):2671–83. doi: 10.1093/eurheartj/ehz363
8. Samsky MD, Morrow DA, Proudfoot AG, Hochman JS, Thiele H, Rao SV. Cardiogenic shock after acute myocardial infarction: a review. JAMA. (2021) 326(18):1840–50. doi: 10.1001/jama.2021.18323
9. Thiele H, Akin I, Sandri M, de Waha-Thiele S, Meyer-Saraei R, Fuernau G, et al. One-Year outcomes after PCI strategies in cardiogenic shock. N Engl J Med. (2018) 379(18):1699–710. doi: 10.1056/NEJMoa1808788
10. Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. (2012) 367(14):1287–96. doi: 10.1056/NEJMoa1208410
11. Auffret V, Cottin Y, Leurent G, Gilard M, Beer JC, Zabalawi A, et al. Predicting the development of in-hospital cardiogenic shock in patients with ST-segment elevation myocardial infarction treated by primary percutaneous coronary intervention: the ORBI risk score. Eur Heart J. (2018) 39(22):2090–102. doi: 10.1093/eurheartj/ehy127
12. Wang J, Shen B, Feng X, Zhang Z, Liu J, Wang Y. A review of prognosis model associated with cardiogenic shock after acute myocardial infarction. Front Cardiovasc Med. (2021) 8:754303. doi: 10.3389/fcvm.2021.754303
13. Ranard LS, Guber K, Fried J, Takeda K, Kaku Y, Karmpaliotis D, et al. Comparison of risk models in the prediction of 30-day mortality in acute myocardial infarction-associated cardiogenic shock. Struct Heart. (2022) 6(6):100116. doi: 10.1016/j.shj.2022.100116
14. Kellner P, Prondzinsky R, Pallmann L, Siegmann S, Unverzagt S, Lemm H, et al. Predictive value of outcome scores in patients suffering from cardiogenic shock complicating AMI: aPACHE II, APACHE III, elebute-stoner, SOFA, and SAPS II. Med Klin Intensivmed Notfmed. (2013) 108(8):666–74. doi: 10.1007/s00063-013-0234-2
15. Pöss J, Köster J, Fuernau G, Eitel I, de Waha S, Ouarrak T, et al. Risk stratification for patients in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. (2017) 69(15):1913–20. doi: 10.1016/j.jacc.2017.02.027
16. Thiele H, Zeymer U, Thelemann N, Neumann FJ, Hausleiter J, Abdel-Wahab M, et al. Intraaortic balloon pump in cardiogenic shock complicating acute myocardial infarction: long-term 6-year outcome of the randomized IABP-SHOCK II trial. Circulation. (2019) 139(3):395–403. doi: 10.1161/circulationaha.118.038201
17. Holmes JH, Sacchi L, Bellazzi R, Peek N. Artificial intelligence in medicine AIME 2015. Artif Intell Med. (2017) 81:1–2. doi: 10.1016/j.artmed.2017.06.011
18. Jiang F, Jiang Y, Zhi H, Dong Y, Li H, Ma S, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. (2017) 2(4):230–43. doi: 10.1136/svn-2017-000101
19. Deo RC. Machine learning in medicine. Circulation. (2015) 132(20):1920–30. doi: 10.1161/circulationaha.115.001593
20. Lee A, Taylor P, Kalpathy-Cramer J, Tufail A. Machine learning has arrived!. Ophthalmology. (2017) 124(12):1726–8. doi: 10.1016/j.ophtha.2017.08.046
21. Lubo-Robles D, Devegowda D, Jayaram V, Bedle H, Marfurt KJ, Pranter MJ. Machine Learning Model Interpretability Using SHAP Values: Application to a Seismic Facies Classification Task. SEG International Exposition and Annual Meeting. Tulsa, Oklahoma: SEG (2020).
23. Pollard TJ, Johnson AEW, Raffa JD, Celi LA, Mark RG, Badawi O. The eICU collaborative research database, a freely available multi-center database for critical care research. Sci Data. (2018) 5:180178. doi: 10.1038/sdata.2018.178
24. Degenhardt F, Seifert S, Szymczak S. Evaluation of variable selection methods for random forests and omics data sets. Brief Bioinformatics. (2019) 20(2):492–503. doi: 10.1093/bib/bbx124
25. Lundberg SM, Lee S-I. A unified approach to interpreting model predictions. In: Advances in Neural Information Processing Systems. (2017) p. 30.
26. Harjola VP, Lassus J, Sionis A, Køber L, Tarvasmäki T, Spinar J, et al. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur J Heart Fail. (2015) 17(5):501–9. doi: 10.1002/ejhf.260
27. Dai W, Kloner RA. Potential role of renin-angiotensin system blockade for preventing myocardial ischemia/reperfusion injury and remodeling after myocardial infarction. Postgrad Med. (2011) 123(2):49–55. doi: 10.3810/pgm.2011.03.2263
28. Lévy BI, Mourad JJ. Renin angiotensin blockers and cardiac protection: from basis to clinical trials. Am J Hypertens. (2022) 35(4):293–302. doi: 10.1093/ajh/hpab108
29. Harari R, Bangalore S. Beta-blockers after acute myocardial infarction: an old drug in urgent need of new evidence!. Eur Heart J. (2020) 41(37):3530–2. doi: 10.1093/eurheartj/ehaa436
30. Zhao X, Zhao G, Zhou M, Wang G, Ma C, Smith SC Jr, et al. Early ACEI/ARB use and in-hospital outcomes of acute myocardial infarction patients with systolic blood pressure <100 mmHg and undergoing percutaneous coronary intervention: findings from the CCC-ACS project. Front Cardiovasc Med. (2022) 9:1003442. doi: 10.3389/fcvm.2022.1003442
31. Chinese Society of Cardiology of Chinese Medical Association, Editorial Board of Chinese Journal of Cardiology. Chinese expert consensus document on angiotensin converting enzyme inhibitors in cardiovascular diseases. Zhonghua xin xue Guan Bing za zhi. (2007) 35(2):97–106.17445398
32. Writing Committee Members, ACC/AHA Joint Committee Members. 2022 AHA/ACC/HFSA guideline for the management of heart failure. J Card Fail. (2022) 28(5):e1–e167. doi: 10.1016/j.cardfail.2022.02.010
33. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42(36):3599–726. doi: 10.1093/eurheartj/ehab368
34. Prins KW, Neill JM, Tyler JO, Eckman PM, Duval S. Effects of Beta-blocker withdrawal in acute decompensated heart failure: a systematic review and meta-analysis. JACC Heart Fail. (2015) 3(8):647–53. doi: 10.1016/j.jchf.2015.03.008
35. Bangalore S, Makani H, Radford M, Thakur K, Toklu B, Katz SD, et al. Clinical outcomes with β-blockers for myocardial infarction: a meta-analysis of randomized trials. Am J Med. (2014) 127(10):939–53. doi: 10.1016/j.amjmed.2014.05.032
36. Chen ZM, Pan HC, Chen YP, Peto R, Collins R, Jiang LX, et al. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet (London, England). (2005) 366(9497):1622–32. doi: 10.1016/s0140-6736(05)67661-1
37. Çinar T, Hayiroğlu M, Şeker M, Doğan S, Çiçek V, Öz A, et al. The predictive value of age, creatinine, ejection fraction score for in-hospital mortality in patients with cardiogenic shock. Coron Artery Dis. (2019) 30(8):569–74. doi: 10.1097/mca.0000000000000776
38. Tsai ML, Hsieh MJ, Chen CC, Wu VC, Lan WC, Huang YT, et al. Prognosis of patients with cardiogenic shock following acute myocardial infarction: the difference between ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction. Medicine (Baltimore). (2022) 101(36):e30426. doi: 10.1097/md.0000000000030426
39. Zhu X, Xie B, Chen Y, Zeng H, Hu J. Machine learning in the prediction of in-hospital mortality in patients with first acute myocardial infarction. Clin Chim Acta. (2024) 554:117776. doi: 10.1016/j.cca.2024.117776
40. Zhu Y, Sasmita BR, Hu X, Xue Y, Gan H, Xiang Z, et al. Blood urea nitrogen for short-term prognosis in patients with cardiogenic shock complicating acute myocardial infarction. Int J Clin Pract. (2022) 2022:9396088. doi: 10.1155/2022/9396088
41. Fuernau G, Poenisch C, Eitel I, Denks D, De Waha S, Pöss J, et al. Prognostic impact of established and novel renal function biomarkers in myocardial infarction with cardiogenic shock: a biomarker substudy of the IABP-SHOCK II-trial. Int J Cardiol. (2015) 191:159–66. doi: 10.1016/j.ijcard.2015.04.242
42. Katz JN, Stebbins AL, Alexander JH, Reynolds HR, Pieper KS, Ruzyllo W, et al. Predictors of 30-day mortality in patients with refractory cardiogenic shock following acute myocardial infarction despite a patent infarct artery. Am Heart J. (2009) 158(4):680–7. doi: 10.1016/j.ahj.2009.08.005
43. Aronson D, Mittleman MA, Burger AJ. Elevated blood urea nitrogen level as a predictor of mortality in patients admitted for decompensated heart failure. Am J Med. (2004) 116(7):466–73. doi: 10.1016/j.amjmed.2003.11.014
44. Khoury J, Bahouth F, Stabholz Y, Elias A, Mashiach T, Aronson D, et al. Blood urea nitrogen variation upon admission and at discharge in patients with heart failure. ESC Heart Fail. (2019) 6(4):809–16. doi: 10.1002/ehf2.12471
Keywords: MIMIC-IV, eICU-CRD, acute myocardial infarction, cardiogenic shock, machine learning, hospital mortality
Citation: Zhang Q, Xu L, Xie Z, He W and Huang X (2024) Machine learning-based prediction of mortality in acute myocardial infarction with cardiogenic shock. Front. Cardiovasc. Med. 11:1402503. doi: 10.3389/fcvm.2024.1402503
Received: 4 April 2024; Accepted: 20 September 2024;
Published: 14 October 2024.
Edited by:
Ioanna Koniari, Manchester University NHS Foundation Trust, United KingdomReviewed by:
Lan Shen, Shanghai Jiao Tong University, ChinaTikal Kansara, Aultman Hospital, United States
Copyright: © 2024 Zhang, Xu, Xie, He and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohong Huang, MjQzMDg3NDY1N0BxcS5jb20=
†These authors share first authorship
 Qitian Zhang
Qitian Zhang Lizhen Xu2,†
Lizhen Xu2,†