- 1Department of Nursing, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
Background: The relationship between the blood urea nitrogen to creatinine ratio (BCR) and the risk of in-hospital mortality among intensive care unit (ICU) patients diagnosed with venous thromboembolism (VTE) remains unclear. This study aimed to assess the relationship between BCR upon admission to the ICU and in-hospital mortality in critically ill patients with VTE.
Methods: This retrospective cohort study included patients diagnosed with VTE from the Medical Information Mart for Intensive Care IV (MIMIC-IV) database. The primary endpoint was in-hospital mortality. Univariate and multivariate logistic regression analyses were conducted to evaluate the prognostic significance of the BCR. Receiver operating characteristic (ROC) curve analysis was utilized to determine the optimal cut-off value of BCR. Additionally, survival analysis using a Kaplan–Meier curve was performed.
Results: A total of 2,560 patients were included, with a median age of 64.5 years, and 55.5% were male. Overall, the in-hospital mortality rate was 14.6%. The optimal cut-off value of the BCR for predicting in-hospital mortality in critically ill VTE patients was 26.84. The rate of in-hospital mortality among patients categorized in the high BCR group was significantly higher compared to those in the low BCR group (22.6% vs. 12.2%, P < 0.001). The multivariable logistic regression analysis results indicated that, even after accounting for potential confounding factors, patients with elevated BCR demonstrated a notably increased in-hospital mortality rate compared to those with lower BCR levels (all P < 0.05), regardless of the model used. Patients in the high BCR group exhibited a 77.77% higher risk of in-hospital mortality than those in the low BCR group [hazard ratio (HR): 1.7777; 95% CI: 1.4016–2.2547].
Conclusion: An elevated BCR level was independently linked with an increased risk of in-hospital mortality among critically ill patients diagnosed with VTE. Given its widespread availability and ease of measurement, BCR could be a valuable tool for risk stratification and prognostic prediction in VTE patients.
1 Introduction
Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), is a potentially preventable complication in critically ill patients (1). It is often associated with morbidity, mortality, and financial burdens, making it one of the significant contributors to the global burden of disease (1–3). The risk of VTE is significantly higher in critically ill patients compared to those hospitalized for other medical conditions (2). Although therapeutic advancements for VTE have resulted in improved patient outcomes in recent years, mortality rates continue to be higher, especially among critically ill patients (4–7). Prior research has identified several prognostic factors linked to in-hospital mortality in VTE patients, including age, preexisting comorbidities, VTE type, severity of illness, time to diagnosis and treatment, anticoagulation type, bleeding complications, and various laboratory parameters (8–10). Given the risk of VTE, identifying non-invasive and inexpensive tests for prompt recognition of high-risk patients with an increased mortality risk is key for improving patient care and reducing the impact of this potentially fatal condition.
Blood urea nitrogen (BUN) and creatinine are nitrogenous terminal products that indicate human renal function. Nevertheless, growing evidence suggests a possible correlation between these indicators and neurohormonal activity (11, 12). Dysregulated cardiorenal function and increased neurohormonal activation may contribute to higher levels of BUN and creatinine, which have been associated with increased mortality in several diseases (12–14). Many factors, such as medications, protein intake, muscle mass, and dehydration, influence BUN and creatinine levels (15, 16). Therefore, the BUN to creatinine ratio (BCR) is more valuable than either BUN or creatinine alone, as it is less prone to fluctuations and better reflects kidney function. The BCR is a useful predictor of outcomes in various diseases, and a high BCR is linked to increasing in-hospital mortality in critically ill patients, including those with septic shock (17), acute myocardial infarction (18), cerebral infarction (19), acute respiratory distress syndrome (20), cardiogenic shock (21), and COVID-19 (22). In patients with VTE, cardiorenal function and neurohumoral regulation are impaired due to various factors such as systemic hypoxia, activation of chemoreflex, hypercapnia, and inflammatory state, leading to elevated BUN and creatinine levels (23–27).
Despite the evidence mentioned above, to the best of our knowledge, no prior research has investigated the potential association between BCR and mortality rates in critically ill patients diagnosed with VTE. Therefore, utilizing the Medical Information Mart for Intensive Care-IV (MIMIC-IV) database (28), we hypothesized that an elevated BUN/creatinine ratio could increase the risk of in-hospital mortality among ICU patients with VTE.
2 Material and methods
2.1 Data source
All data used in this retrospective cohort study were extracted from the Medical Information Mart for Intensive Care-IV (MIMIC-IV) version 1.0, which includes electronic health records of adult patients admitted to the intensive care unit at Beth Israel Deaconess Medical Center in Boston, Massachusetts, between 2008 and 2019. Access to the database is granted to individuals who have completed the “Protecting Human Research Participants” training. For this study, author AP obtained the necessary certification and extracted the relevant data from the database (certification number: 61239194). Our research complied with the ethical principles outlined in the Helsinki Declaration. This study was approved by the Institutional Review Boards of Beth Israel Deaconess Medical Center and the Massachusetts Institute of Technology (Cambridge, MA, USA). Informed consent was not required as patient health information in this database was anonymized (28).
2.2 Study population
The diagnosis of VTE was based on the International Classification of Diseases, Ninth Revision (ICD-9) code, and the Tenth Revision (ICD-10) code. The ICD codes used for identifying patients with VTE are presented in Supplementary Table S1. Patients aged 18 years or older diagnosed with VTE were included in this study. Patients with multiple ICU admissions, those under 18 years old, individuals with an ICU stay of less than 24 h, and patients with missing data on BUN or creatinine were excluded from this study.
2.3 Data extraction
The Structured Query Language (SQL) was utilized to extract data using script codes obtained from the GitHub repository (https://github.com/MIT-LCP/mimic-iv). The following variables were collected: age, gender, length of stay (LOS) at the hospital, length of stay at the ICU, hospital death sign, Charlson comorbidity index (CCI), Simplified Acute Physiology Score II (SAPS II), laboratory tests on the day one of admission including hemoglobin, white blood cells (WBC), red blood cells (RBC), platelets, glucose, mean corpuscular hemoglobin concentration (MCHC), red blood cell distribution width (RDW), hematocrit, blood urea nitrogen (BUN), creatinine, bicarbonate, international normalized ratio (INR), prothrombin time (PT), and partial prothrombin time (PTT). The extracted preexisting comorbidities included hypertension, diabetes, congestive heart failure, coronary artery disease, renal disease, severe liver disease, obesity, malignant cancer, cerebrovascular disease, and chronic obstructive pulmonary disease (COPD). The extracted data also encompasses vital signs, such as heart rate, respiratory rate, mean arterial pressure (MAP), and peripheral oxygen saturation (SpO2). Information on whether patients required mechanical ventilation, received renal replacement therapy (RRT), or used diuretics was also extracted. The average value was used if a variable was assessed multiple times on the first day of admission. To mitigate potential bias, variables with missing values >20% were excluded. For variables with less than 20% missing values, the random forest imputation method from the missForest package in R software was used for imputation (29).
2.4 Outcomes
The primary outcome in the present study was in-hospital mortality. Secondary outcomes included length of ICU stay and length of hospital stay.
2.5 Statistical analysis
The normality of the variables was assessed using the Kolmogorov-Smirnov test. Results were presented as mean ± standard deviation (SD) for normally distributed data, and the independent sample t-test was employed for comparison between the groups. Conversely, variables were described as median with interquartile range (IQR) for non-normally distributed data, and the Mann–Whitney test was used for comparisons. Categorical variables were presented as total numbers and percentages, and the chi-square test or Fisher's exact test was utilized for analyses.
The characteristics of the patients included in the study were compared between the survival group and the deceased group. The optimal threshold values for BCR associated with in-hospital mortality were determined using the maximum Youden index through receiver operating characteristic (ROC) curve analysis. Subsequently, all patients with VTE were divided into two groups based on these cut-off values: the high BCR group and the low BCR group. Binomial logistic regression analysis was conducted to evaluate the influence of BCR on in-hospital mortality among patients with VTE. Variables with a p-value <0.1 in the univariate analysis and potential confounders identified through clinical expertise were included in the multivariate analysis. The crude model did not incorporate any adjustments to variables. In the multivariable analysis, three models (Model I, Model II, Model III) were developed to examine the association between BCR and in-hospital mortality. Survival curves were constructed utilizing the Kaplan-Meier method, with the log-rank test employed to compare survival rates between the high BCR and low BCR groups. All statistical analyses were conducted using SPSS version 26.0 and MedCalc version 19.6 software. A p-value of less than 0.05 was considered statistically significant.
3 Results
3.1 Baseline characteristics of the participants
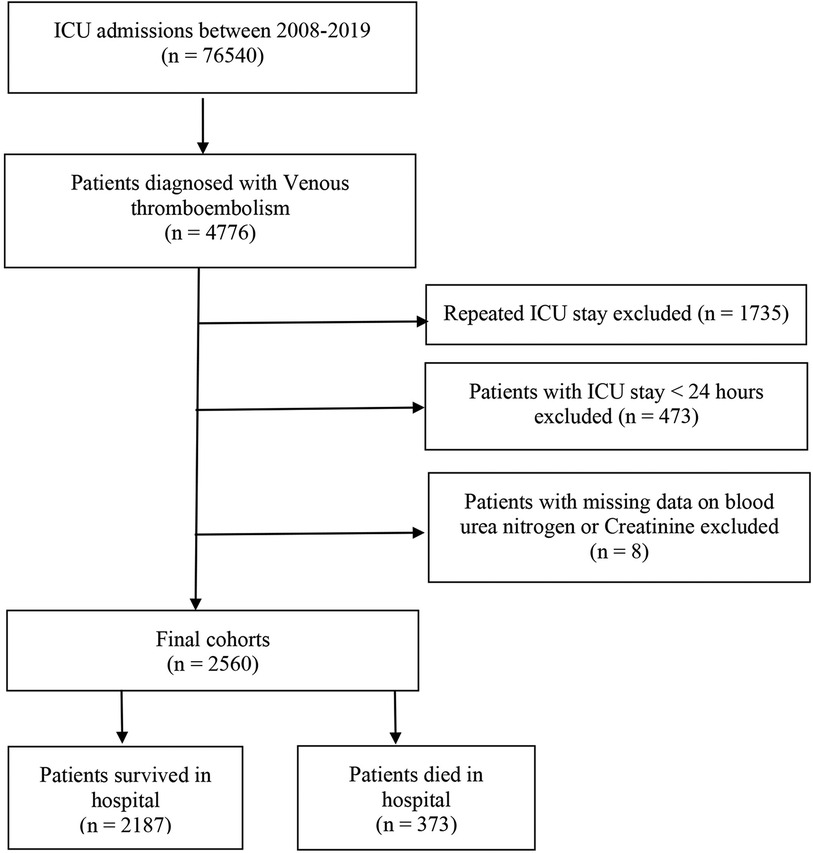
This study included a total of 2,560 critically ill patients diagnosed with VTE. The flowchart for patient screening is depicted in Figure 1. The median age of the participants with VTE was 64.5 years, and 55.5% were male. The in-hospital mortality rate was 14.6% (373/2,560). Table 1 summarizes the baseline characteristics of both the survival and deceased groups. Compared with patients in the survival group, the patients in the death group tended to be older. They had a higher proportion of diabetes, congestive heart failure, renal disease, severe liver disease, obesity, and cancer (all P < 0.05). Individuals in the deceased group exhibited elevated levels of creatinine, white blood cells (WBC), red cell distribution width (RDW), heart rate, and respiratory rate compared to those in the survival group (all p < 0.05). Conversely, they demonstrated lower mean arterial pressure, hemoglobin, red blood cell (RBC) count, platelet count, mean corpuscular hemoglobin concentration (MCHC), hematocrit, and bicarbonate levels (all p < 0.05). The CCI and SAPS II scores were higher in the deceased group, whereas the length of hospital stay was shorter compared to the survival group (all p < 0.05). Moreover, patients in the death group were more likely to receive RRT and diuretics, and they more frequently required mechanical ventilation during their hospital stay compared to the survival group (all p < 0.05). We found that patients in the death group had significantly higher levels of BUN, creatinine, and BCR when compared with the survival group (Table 1).
3.2 The prognostic significance of BCR
The ROC curve was generated for BCR to predict in-hospital mortality in critically ill patients with VTE, and the area under the ROC curve was 0.587 (95% CI: 0.568–0.607, P < 0.001) (Figure 2). The optimal cut-off value of BCR to predict survival status was 26.84, with a sensitivity of 35.93% and a specificity of 79.06%. Using this cut-off value, patients were categorized into two groups: the low BCR group (≤26.84, n = 1,966) and the high BCR group (>26.84, n = 594). Table 2 displays the baseline characteristics of the low BCR and high BCR groups. In comparison with patients in the low BCR group, those in the high BCR group exhibited a significantly higher in-hospital mortality rate (22.6% vs. 12.2%, P < 0.001). Patients with a higher BCR (>26.84) were more likely to have comorbid diseases, including congestive heart failure, coronary artery disease, severe liver disease, cancer, and COPD, as well as higher CCI, SAPS II scores, hemoglobin levels, platelet counts, RBC counts, MCHC, RDW, hematocrit, PT, and INR levels compared to those with a low BCR (≤26.84) (all P < 0.05). Additionally, individuals with a BCR >26.84 were prone to experiencing prolonged hospital and ICU stays (p < 0.05). They also exhibited a higher likelihood of receiving diuretic therapy, mechanical ventilation, and renal replacement therapy compared to those with a low BCR ≤26.84 (all P < 0.05) (Table 2).
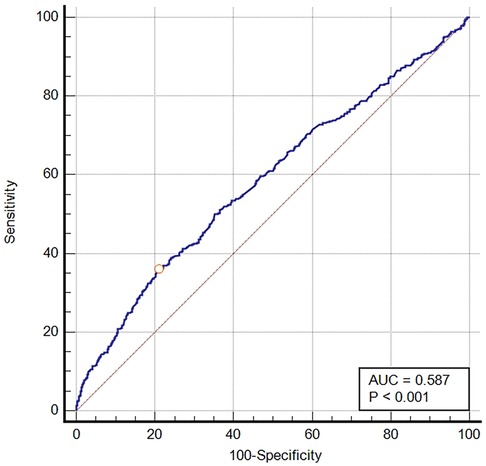
Figure 2. The ROC curve of predictive performance of BCR for in-hospital mortality. AUC, area under the curve; BCR, blood urea nitrogen to creatinine ratio; ROC, receiver operator characteristic curve.
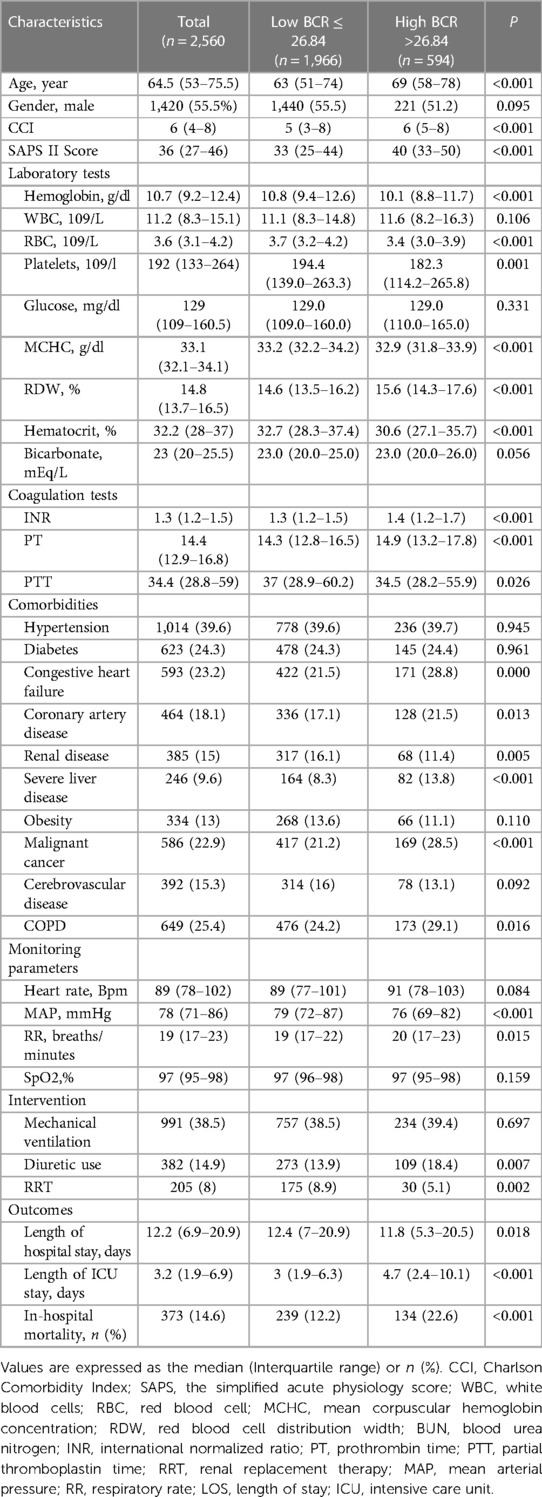
Table 2. Baseline characteristics of BUN/creatinine ratio groups in patients with venous thromboembolism.
3.3 Association between BCR and in-hospital mortality in patients with VTE
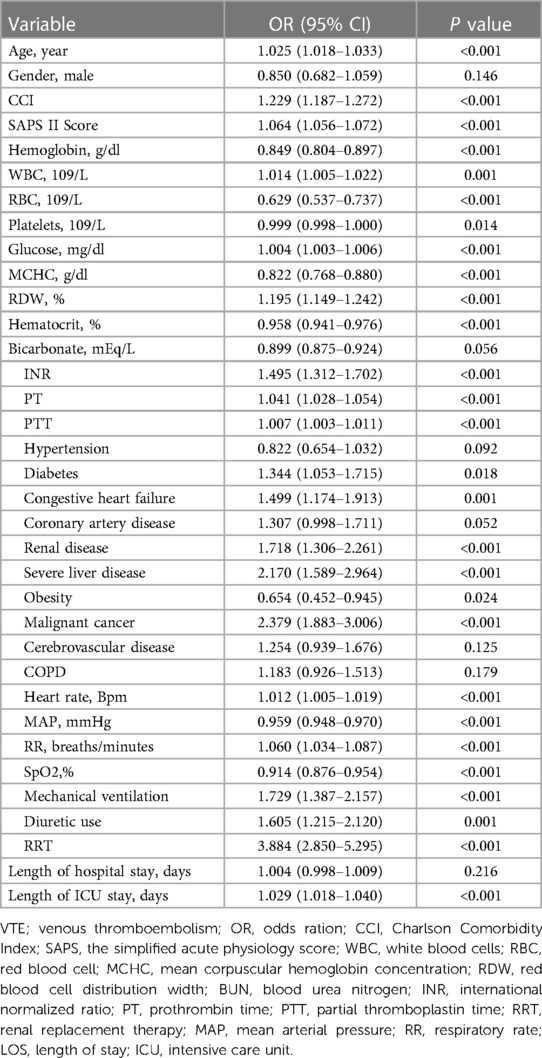
The results of the univariable logistic regression analysis are presented in Table 3. Additionally, Table 4 illustrates the unadjusted and multivariable-adjusted correlations between BCR and in-hospital mortality. The logistic regression model was employed to evaluate the impact of exposure variables on the outcome measures while adjusting for covariates. The crude model was not adjusted. In Model I, age and gender were incorporated as covariates to account for potential confounders. We adjusted for 11 variables in Model II, including hypertension, diabetes, congestive heart failure, coronary artery disease, renal disease, severe liver disease, obesity, malignant cancer, mechanical ventilation, diuretic use, and renal replacement therapy. Model III was adjusted for 18 variables, including CCI, SAPS II Score, hemoglobin, WBC, RBC, platelets, glucose, MCHC, RDW, hematocrit, bicarbonate, INR, PT, APTT, HR, MAP, RR, and SpO2. A statistically significant positive association was observed between the BCR (a continuous variable) and in-hospital mortality across all models: Crude Model: OR = 1.029, 95% CI: 1.020–1.038, P < 0.001; Model I: OR = 1.024, 95% CI: 1.014–1.033, P < 0.001; Model II: OR = 1.032, 95% CI: 1.023–1.042, P < 0.001; Model III: OR = 1.016, 95% CI: 1.006–1.026, P = 0.002). Additionally, compared to the low BCR (≤26.84) group, in-hospital mortality was significantly higher in the high BCR (>26.84) group across different models: Crude Model (OR = 2.105, 95% CI: 1.664–2.663, P < 0.001); Model I (OR = 1.874, 95% CI: 1.874–2.381, P < 0.001); Model II (OR = 2.091, 95% CI: 1.626–2.688, P < 0.001); and Model III (OR = 1.420, 95% CI: 1.086–1.857, P = 0.010) (Table 4).
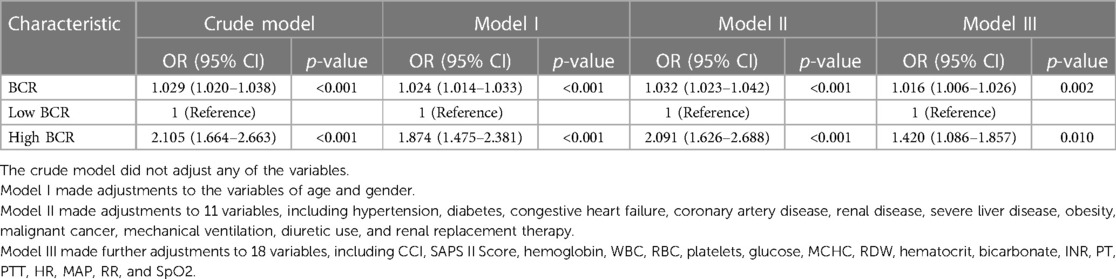
Table 4. Multivariable logistic regression analyses for in-hospital mortality in patients with venous thromboembolism.
3.4 Kaplan–Meier survival analysis of the cohort by BCR groups
A Kaplan-Meier curve was generated to demonstrate the survival outcomes of patients with VTE in both the high BCR and low BCR groups (Figure 3). The median survival time for the low BCR group was 86.976 days (95% CI: 77.704–377.026), whereas for the high BCR group, it was 45.477 days (95% CI: 38.876–80.425). This difference was statistically significant (log-rank test, P < 0.0001). In comparison to the low BCR group, the hazard ratio (HR) for the high BCR group was 1.7777 (95% CI: 1.4016–2.2547).
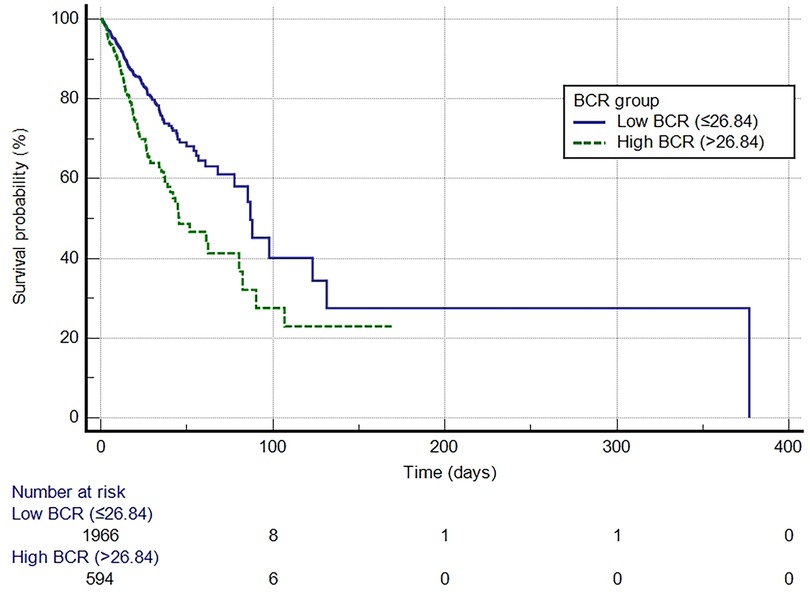
Figure 3. Kaplan-Meier survival curve for in-hospital mortality for the high and low BCR groups. BCR, blood urea nitrogen to creatinine ratio.
3.5 Subgroup analysis
Table 5 shows the results of the subgroup analysis. High BCR was consistently associated with the risk of in-hospital mortality among most subgroups (all P for interaction >0.05), except for age (interaction P = 0.042), diabetes (interaction P = 0.004), and severe liver disease (interaction P = 0.002).
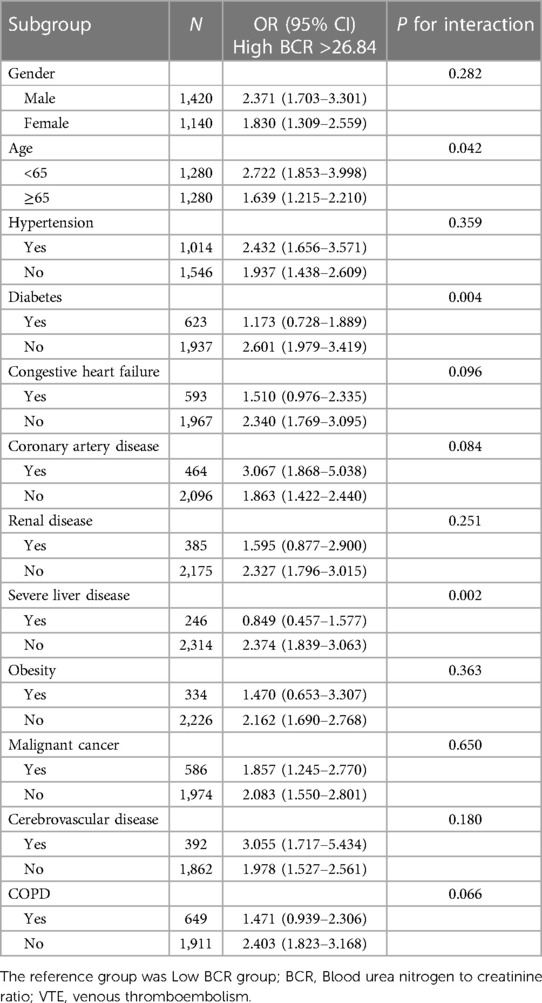
Table 5. Subgroup analysis of the association between BCR and in-hospital mortality in VTE patients.
3.6 Analysis based on types of VTE (DVT and PE)
All VTE patients were divided into groups based on their diagnosis of either DVT or PE. The results were then analyzed separately for each group. Table 6 summarizes the baseline demographic and clinical characteristics of patients with DVT and PE. For patients with DVT, the comparison between survival and deceased cohorts revealed significant differences. Deceased patients were significantly older with higher CCI and SAPS II scores (p < 0.001). Laboratory tests showed decreased hemoglobin, RBC, and platelet levels but increased WBC, glucose, RDW, BUN, creatinine, and BUN/Cr ratio in deceased individuals (all p < 0.05). Moreover, deceased individuals demonstrated lower bicarbonate levels and higher INR PT and APTT values (all p < 0.05). Comorbidities such as congestive heart failure, malignant cancer, severe liver disease, and renal disease were more prevalent among deceased DVT patients (p < 0.05). Deceased DVT patients more often received renal replacement therapy (p < 0.001). Furthermore, deceased DVT patients had longer ICU stays (p < 0.01). DVT patients in the high BCR group (BCR > 26.84) demonstrated a significantly higher mortality rate compared to those in the low BCR group (p < 0.001) (Table 6). In patients with DVT, the crude logistic regression analysis showed that those in the high BCR group have a significantly higher risk of in-hospital mortality compared to those in the low BCR group, with an OR of 2.170 (95% CI: 1.612–2.921, P < 0.001). Even after adjusting for potential confounders, the association remained significant, with an adjusted odds ratio (aOR) of 1.872 (95% CI: 1.330–2.634; p < 0.001) (Supplementary Table S2). In patients with DVT, the Kaplan-Meier survival analysis revealed that the median survival time for the high BCR group was 44.840 days (95% CI: 35.315–80.825), which is significantly shorter than the 86.976 days (95% CI: 68.013–377.026) for the low BCR group. The high BCR group had an 81% higher risk of in-hospital mortality (HR: 1.8145, 95% CI: 1.3432–2.4510). The log-rank test indicated a statistically significant difference in survival between the two groups (Logrank p < 0.0001) (Supplementary Figure S1).
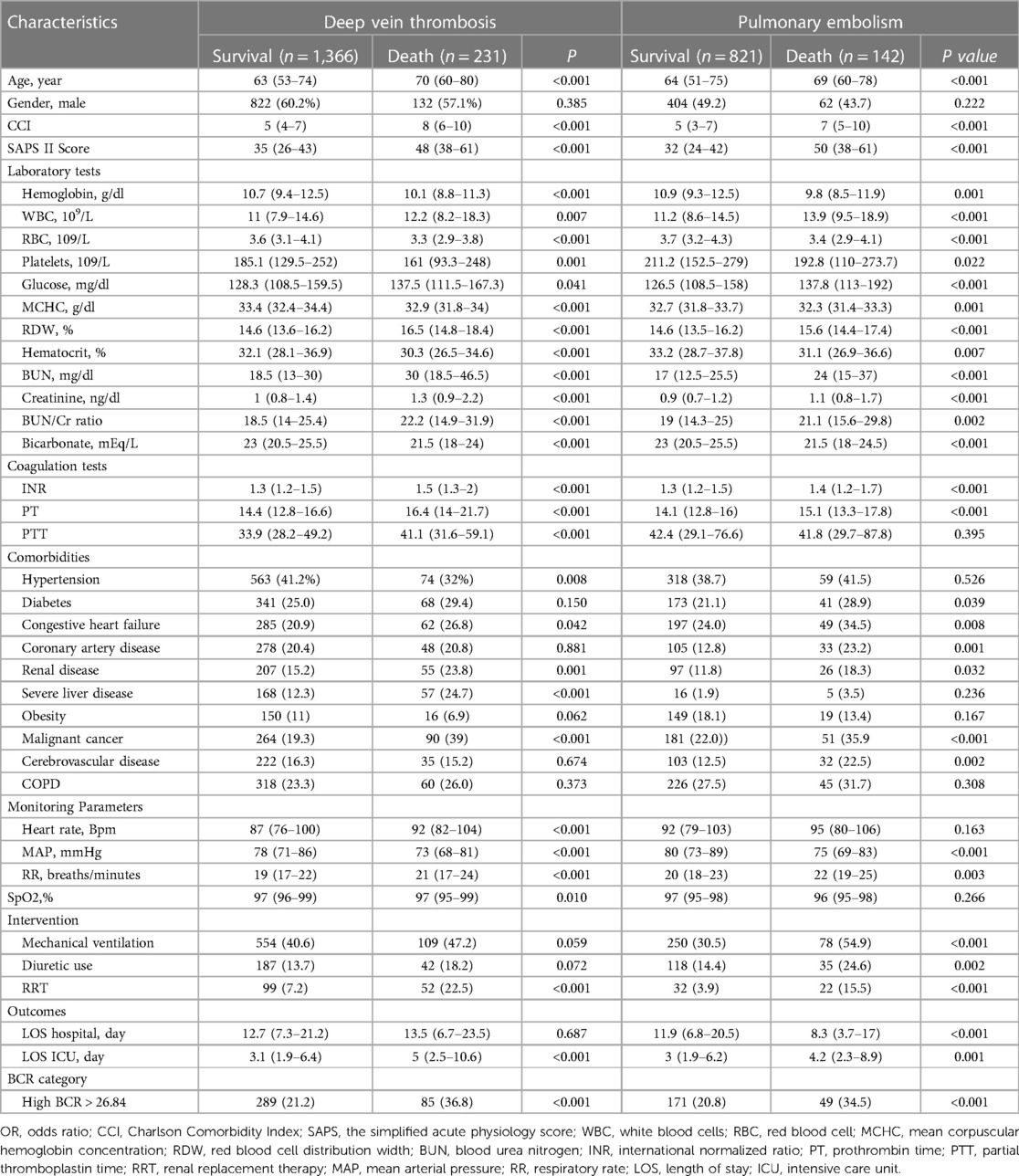
Table 6. Demographic and clinical characteristics of patients with deep vein thrombosis and pulmonary embolism.
The comparison between survival and deceased cohorts revealed significant differences for patients with PE. Deceased individuals were older with higher CCI and SAPS II scores (p < 0.001). Laboratory findings indicated lower hemoglobin, RBC, and platelet levels but higher WBC, glucose, RDW, BUN, creatinine, and BUN/Cr ratio in deceased patients (all p < 0.05). Furthermore, deceased patients exhibited lower bicarbonate levels and higher INR and PT values (all p < 0.05). Comorbidities such as diabetes, congestive heart failure, coronary artery disease, malignant cancer, renal disease, and cerebrovascular disease were more prevalent among deceased PE patients (all p < 0.05). Increased usage of mechanical ventilation, diuretics, and renal replacement therapy was observed in deceased PE patients (all p < 0.05). Furthermore, deceased PE patients experienced shorter hospital and ICU stays (all p < 0.05). PE patients with a BCR greater than 26.84 had a significantly higher mortality rate compared to those with a lower BCR (p < 0.001) (Table 6). In patients with PE, the crude logistic regression analysis indicated that those in the high BCR group had a significantly higher risk of in-hospital mortality compared to those in the low BCR group, with an odds ratio (OR = 2.003, 95% CI: 1.363–2.943, p < 0.001). The significant association persisted even after adjusting for potential confounders, with an aOR of 1.585 (95% CI: 1.024–2.45, p = 0.039) (Supplementary Table S2). In patients with PE, the Kaplan-Meier survival analysis showed that the median survival time for the high BCR group was 61.306 days (95% CI: 40.380–61.306), significantly shorter than the low BCR group's 97.894 days (95% CI: 85.482–97.894). The high BCR group had an 83% higher risk of in-hospital mortality (HR: 1.8343, 95% CI: 1.2450–2.7028). The log-rank test confirmed a statistically significant difference in survival between the groups (Logrank p = 0.0022) (Supplementary Figure S2).
4 Discussion
To our knowledge, this study is the first to explore the relationship between BCR and in-hospital mortality among critically ill patients with VTE. Our findings indicate that higher BCR was associated with increased in-hospital mortality among critically ill patients, even after adjusting for potential confounding variables. Furthermore, the baseline BCR levels of the individuals in the deceased cohort were significantly greater than those in the surviving group. Notably, among patients with VTE in critical condition, a BCR greater than 26.84 was found to be an independent risk factor for in-hospital death. Compared to the low BCR group, patients in the high BCR group had significantly shorter median survival times, indicating that BCR may be a prognostic factor for VTE patients, with high BCR indicating a poor prognosis. Moreover, the hazard ratio for the high BCR group was 1.7777, indicating a 77.77% higher risk of in-hospital death in comparison to the low BCR group. Analysis based on VTE types also revealed that patients with high BCR had a significantly increased risk of in-hospital mortality in both the DVT and PE groups. This association remained statistically significant even after adjusting for numerous potential confounders.
Although the kidneys filter both BUN and creatinine, only BUN is reabsorbed in both the proximal and distal renal tubules. The activation of the neurohormonal system, which comprises the sympathetic nervous system, the renin-angiotensin-aldosterone system (RAAS), and vasopressin, impacts the reabsorption of BUN (12). Neurohormonal activation, triggered by conditions like heart failure, VTE, liver cirrhosis, or dehydration, can lead to an elevated BCR even without significant renal dysfunction (30). The assessment of BUN and creatinine levels is a routine practice in the ICU. The BCR is a straightforward and readily obtainable measure, as it relies solely on venous blood samples in clinical settings. Previous studies have reported an association between the BCR and in-hospital mortality in various diseases (17–20). BCR, a biomarker of neurohormonal activity, has been associated with poor prognosis in patients with acute heart failure and is an independent predictor of all-cause mortality (31). Impaired blood flow and endothelial dysfunction in heart failure contribute to a prothrombotic state, increasing the risk of thromboembolic events like stroke, intracardiac thrombi, VTE, PE, and myocardial infarction (32). Chen et al. (33) examined the link between BCR levels and in-hospital mortality among patients with subarachnoid hemorrhage, utilizing the MIMIC-IV database. Their study revealed a significant association, indicating that elevated BCR levels (≥27.208) were correlated with an increased risk of in-hospital mortality in comparison to lower BCR levels (<27.208). Another study by Han et al. (17) examined the correlation between BCR and all-cause mortality in adult patients with septic shock. They demonstrated that even after adjusting for potential confounders, a high BCR (≥27.3 mg/dl) in patients with septic shock was significantly linked to all-cause mortality. Ok et al. (34) investigated the utility of the BUN/Cr ratio upon admission in predicting disease severity and survival rates for patients with COVID-19. Their investigation revealed that a higher BUN/Cr ratio served as an independent prognostic indicator for both the COVID-19 severity and patient outcomes related to survival. Ma et al. (20) conducted a recent investigation to elucidate the association between the BUN/Cr ratio and in-hospital mortality among patients diagnosed with trauma-induced acute respiratory distress syndrome. Their findings revealed a statistically significant correlation between elevated BUN/Cr ratios and an increased risk of death within the hospital setting for these patients. Additionally, the study demonstrated a superior predictive value of the BUN/Cr ratio in predicting in-hospital mortality compared to using BUN or creatinine levels alone. The aforementioned results suggest that a higher BCR is linked to more severe conditions compared to a lower BCR. Our results concur with the above findings. In this cohort study utilizing the MIMIC-IV database, we found that the optimal cut-off value of BCR to predict survival status among patients with VTE was 26.84. Moreover, our findings demonstrated that the BCR serves as an independent predictor of in-hospital death in critically ill VTE patients. Additionally, our results showed a strong association between low BCR levels and improved survival outcomes. Patients in the low BCR group exhibited a statistically significant longer median survival time (86.976 days) than those in the high BCR group (45.477 days). Notably, compared to those with lower BCR levels, a higher BCR level (>26.84) upon admission was correlated with an increased risk of in-hospital mortality in critically ill VTE patients. While additional research is required for a comprehensive understanding of the underlying mechanisms, the elevated BCR in VTE patients could potentially be associated with several mechanisms. Firstly, VTE has been associated with prolonged hypoxemia and hypercapnia, potentially leading to activation of the RAAS (35, 36). While the mechanism is not fully elucidated, this activation may contribute to increased BUN levels through tubular reabsorption of urea.. Secondly, patients with VTE often have concomitant cardiovascular comorbidities, including heart failure (37, 38). In the context of cardiovascular disease, a multifaceted neurohormonal response triggers activation of the renal sympathetic nervous system and the RAAS, potentially leading to altered urea reabsorption (12). Thirdly, VTE is associated with persistent neutrophilia and the subsequent release of proinflammatory mediators due to hypoxia (26). These mediators may disrupt cardiorenal function and neurohumoral regulation, potentially leading to increased BUN levels (30). Finally, VTE can indirectly lead to elevated BUN and creatinine levels by causing blood stasis and reducing blood flow to the kidneys (30).
In the present study, the overall in-hospital mortality of the study population was 14.6%. A study by Ambra et al. (39) in Qatar reported a 13.39% mortality rate for hospitalized patients with venous VTE. Another retrospective cohort study conducted at seven major hospitals in Saudi Arabia demonstrated that the mortality rate for patients with confirmed VTE was 14.3% (40). Using an extensive ICU database, Pisani et al. (41) assessed the risk of VTE in critically ill pneumonia patients and revealed that patients with VTE had a high mortality rate (20.6%). This wide variability in mortality rates among patients with VTE can be attributed to several factors, including the patient population, severity of VTE, comorbidities, and timeliness of diagnosis and treatment. Our findings emphasize the importance of carefully monitoring BCR in VTE patients admitted to ICUs. Since BCR is a simple parameter obtainable from routine clinical blood tests, it may enable clinicians to promptly identify VTE patients prone to adverse outcomes, necessitating early intensive care intervention. There are several notable strengths in this study. To our knowledge, it is the first to investigate the potential association between BCR and in-hospital mortality among VTE patients using a large dataset from a heterogeneous population in the ICU. Furthermore, even after adjusting for various confounding factors in three different models, the relationship between the BCR and mortality remained consistent, suggesting the robustness and stability of our results. The BCR is a readily available index for risk assessment in critically ill patients, as BUN and creatinine are part of routine blood tests. Furthermore, this research suggests clinicians can enhance prognostic accuracy for patients with VTE by utilizing this affordable and easily accessible biomarker.
This study has several limitations. Firstly, although multivariable analyses were employed, the findings of this study could still be affected by residual bias and unmeasured confounding factors due to its retrospective nature. Secondly, retrospective studies have inherent limitations, such as potential missing data and an inability to establish causality. These factors may impact the validity and interpretation of our findings. Thirdly, owing to constraints within the MIMIC-IV database, information regarding several factors affecting the BCR, such as the administration of corticosteroids and specific antibiotics, protein consumption, and muscle mass, were not evaluated in this study because they could not be extracted from the MIMIC-IV database. Fourthly, the ROC curve analysis was used to identify the optimal threshold value for the BCR, with the maximal Youden index serving as the predictor of survival status. Nevertheless, the area under the curve for BCR was smaller than expected. Finally, the MIMIC-IV database lacks detailed information regarding mortality causes and specific treatments such as anticoagulation and thrombolysis, which restricts our ability to assess potential differences in therapeutic approaches among VTE patients. Therefore, prospective cohort studies are warranted to validate these findings. Finally, this was a single-center study based on data obtained from the MIMIC-IV database, limiting our findings' generalizability.
5 Conclusions
Our findings demonstrate that the BCR serves as an independent predictor of mortality within the hospital setting for critically ill patients diagnosed with VTE. Patients presenting with elevated BCR levels exhibited a significantly increased risk of in-hospital mortality compared to those with lower BCR levels. BCR is a highly practical, readily available, and relatively inexpensive index that could be a valuable tool for clinicians in managing and stratifying the risk for critically ill VTE patients at an early stage.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: The Medical Information Mart for Intensive Care IV (MIMIC-IV) database at https://physionet.org/content/mimiciv/2.2/.
Ethics statement
The studies involving humans were approved by Institutional Review Boards (IRB) of the Massachusetts Institute of Technology (MIT, Cambridge, MA, USA) and Beth Israel Deaconess Medical Center. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
AP: Writing – review & editing, Writing – original draft, Visualization, Software, Resources, Methodology, Investigation, Funding acquisition, Formal Analysis, Data curation, Conceptualization. MG: Writing – review & editing, Writing – original draft, Validation, Software, Project administration, Methodology, Investigation, Funding acquisition, Formal Analysis, Data curation, Conceptualization. HH: Writing – review & editing, Writing – original draft, Visualization, Software, Project administration, Methodology, Investigation, Formal Analysis, Conceptualization. QZ: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal Analysis, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This study was supported by the Chongqing Science and Technology Bureau (Grant number: CSTC2021jscx-gksb-N0021).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1400915/full#supplementary-material
References
1. Wendelboe AM, Raskob GE. Global burden of thrombosis: epidemiologic aspects. Circ Res. (2016) 118:1340–7. doi: 10.1161/CIRCRESAHA.115.306841
2. Helms J, Carrier M, Klok FA. High-risk pulmonary embolism in the intensive care unit. Intensive Care Med. (2023) 49:579–82. doi: 10.1007/s00134-023-07011-0
3. Minet C, Potton L, Bonadona A, Hamidfar-Roy R, Somohano CA, Lugosi M, et al. Venous thromboembolism in the ICU: main characteristics, diagnosis and thromboprophylaxis. Crit Care. (2015) 19:287. doi: 10.1186/s13054-015-1003-9
4. Henke PK, Kahn SR, Pannucci CJ, Secemksy EA, Evans NS, Khorana AA, et al. Call to action to prevent venous thromboembolism in hospitalized patients: a policy statement from the American Heart Association. Circulation. (2020) 141:e914–31. doi: 10.1161/CIR.0000000000000769
5. Gao X, Zeng L, Wang H, Zeng S, Tian J, Chen L, et al. Prevalence of venous thromboembolism in intensive care units: a meta-analysis. J Clin Med. (2022) 11(22):6691. doi: 10.3390/jcm11226691
6. Zulkifly HH, Mansor NF, Zaki IAH, Kiok LC, Eng KS, Ravi T, et al. Venous thromboembolism (VTE) and mortality among COVID-19 patients in the intensive care unit (ICU): prevalence and risk factors. Int J Cardiol. (2022) 369:18–9. doi: 10.1016/j.ijcard.2022.10.064
7. Giorgio K, Walker RF, MacLehose RF, Adrianzen-Herrera D, Wang W, Alonso A, et al. Venous thromboembolism mortality and trends in older US adults, 2011–2019. Am J Hematol. (2023) 98:1364–73. doi: 10.1002/ajh.26996
8. Tran A, Fernando SM, Rochwerg B, Cook DJ, Crowther MA, Fowler RA, et al. Prognostic factors associated with development of venous thromboembolism in critically ill patients—a systematic review and meta-analysis. Crit Care Med. (2022) 50:e370–81. doi: 10.1097/CCM.0000000000005382
9. Lutsey PL, Zakai NA. Epidemiology and prevention of venous thromboembolism. Nat Rev Cardiol. (2023) 20:248–62. doi: 10.1038/s41569-022-00787-6
10. Guan C, Ma F, Chang S, Zhang J. Interpretable machine learning models for predicting venous thromboembolism in the intensive care unit: an analysis based on data from 207 centers. Crit Care. (2023) 27:406. doi: 10.1186/s13054-023-04683-4
11. Schrier RW. Blood urea nitrogen and serum creatinine: not married in heart failure. Circ Heart Fail. (2008) 1:2–5. doi: 10.1161/CIRCHEARTFAILURE.108.770834
12. Kazory A. Emergence of blood urea nitrogen as a biomarker of neurohormonal activation in heart failure. Am J Cardiol. (2010) 106:694–700. doi: 10.1016/j.amjcard.2010.04.024
13. Wang Y, Xu X, Shi S, Gao X, Li Y, Wu H, et al. Blood urea nitrogen to creatinine ratio and long-term survival in patients with chronic heart failure. Eur J Med Res. (2023) 28:343. doi: 10.1186/s40001-023-01066-x
14. Josiassen J, Frydland M, Holmvang L, Lerche Helgestad OK, Okkels Jensen L, Goetze JP, et al. Mortality in cardiogenic shock is stronger associated to clinical factors than contemporary biomarkers reflecting neurohormonal stress and inflammatory activation. Biomarkers. (2020) 25:506–12. doi: 10.1080/1354750X.2020.1795265
15. Haines RW, Zolfaghari P, Wan Y, Pearse RM, Puthucheary Z, Prowle JR. Elevated urea-to-creatinine ratio provides a biochemical signature of muscle catabolism and persistent critical illness after major trauma. Intensive Care Med. (2019) 45:1718–31. doi: 10.1007/s00134-019-05760-5
16. Sujino Y, Nakano S, Tanno J, Shiraishi Y, Goda A, Mizuno A, et al. Clinical implications of the blood urea nitrogen/creatinine ratio in heart failure and their association with haemoconcentration. ESC Hear Fail. (2019) 6:1274–82. doi: 10.1002/ehf2.12531
17. Han D, Zhang L, Zheng S, Xu F, Li C, Yang R, et al. Prognostic value of blood urea nitrogen/creatinine ratio for septic shock: an analysis of the MIMIC-III clinical database. Biomed Res Int. (2021) 2021:5595042. doi: 10.1155/2021/5595042
18. Huang S, Guo N, Duan X, Zhou Q, Zhang Z, Luo L, et al. Association between the blood urea nitrogen to creatinine ratio and in-hospital mortality among patients with acute myocardial infarction: a retrospective cohort study. Exp Ther Med. (2023) 25:36. doi: 10.3892/etm.2022.11735
19. Chen T, Li A-P, Gong Q, Zhou L, Zhao Y-X, Zhou Z-W, et al. The association of blood urea nitrogen to creatinine ratio and the prognosis of critically ill patients with cerebral infarction: a cohort study. Mediators Inflamm. (2022) 2022:2151840. doi: 10.1155/2022/2151840
20. Ma H, Lin S, Xie Y, Mo S, Huang Q, Ge H, et al. Association between BUN/creatinine ratio and the risk of in-hospital mortality in patients with trauma-related acute respiratory distress syndrome: a single-centre retrospective cohort from the MIMIC database. BMJ Open. (2023) 13:e069345. doi: 10.1136/bmjopen-2022-069345
21. Sun D, Wei C, Li Z. Blood urea nitrogen to creatinine ratio is associated with in-hospital mortality among critically ill patients with cardiogenic shock. BMC Cardiovasc Disord. (2022) 22:258. doi: 10.1186/s12872-022-02692-9
22. Liu Q, Wang Y, Zhao X, Wang L, Liu F, Wang T, et al. Diagnostic performance of a blood urea nitrogen to creatinine ratio-based nomogram for predicting in-hospital mortality in COVID-19 patients. Risk Manag Healthc Policy. (2021) 14:117–28. doi: 10.2147/RMHP.S278365
23. Ninivaggi M, de Laat M, Lancé MMD, Kicken CH, Pelkmans L, Bloemen S, et al. Hypoxia induces a prothrombotic state independently of the physical activity. PLoS One. (2015) 10:e0141797. doi: 10.1371/journal.pone.0141797
24. Gupta N, Sahu A, Prabhakar A, Chatterjee T, Tyagi T, Kumari B, et al. Activation of NLRP3 inflammasome complex potentiates venous thrombosis in response to hypoxia. Proc Natl Acad Sci U S A. (2017) 114:4763–8. doi: 10.1073/pnas.1620458114
25. Colombo PC, Ganda A, Lin J, Onat D, Harxhi A, Iyasere JE, et al. Inflammatory activation: cardiac, renal, and cardio-renal interactions in patients with the cardiorenal syndrome. Heart Fail Rev. (2012) 17:177–90. doi: 10.1007/s10741-011-9261-3
26. Potere N, Abbate A, Kanthi Y, Carrier M, Toldo S, Porreca E, et al. Inflammasome signaling, thromboinflammation, and venous thromboembolism. JACC Basic to Transl Sci. (2023) 8:1245–61. doi: 10.1016/j.jacbts.2023.03.017
27. Colling ME, Tourdot BE, Kanthi Y. Inflammation, infection and venous thromboembolism. Circ Res. (2021) 128:2017–36. doi: 10.1161/CIRCRESAHA.121.318225
28. Johnson AEW, Bulgarelli L, Shen L, Gayles A, Shammout A, Horng S, et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci Data. (2023) 10:1. doi: 10.1038/s41597-022-01899-x
29. Stekhoven DJ, Bühlmann P. Missforest–non-parametric missing value imputation for mixed-type data. Bioinformatics. (2012) 28:112–8. doi: 10.1093/bioinformatics/btr597
30. Colombo PC, Doran AC, Onat D, Wong KY, Ahmad M, Sabbah HN, et al. Venous congestion, endothelial and neurohormonal activation in acute decompensated heart failure: cause or effect? Curr Heart Fail Rep. (2015) 12:215–22. doi: 10.1007/s11897-015-0254-8
31. Zhu X, Cheang I, Liao S, Wang K, Yao W, Yin T, et al. Blood urea nitrogen to creatinine ratio and long-term mortality in patients with acute heart failure: a prospective cohort study and meta-analysis. Cardiorenal Med. (2020) 10:415–28. doi: 10.1159/000509834
32. Lin AY, Dinatolo E, Metra M, Sbolli M, Dasseni N, Butler J, et al. Thromboembolism in heart failure patients in Sinus rhythm: epidemiology, pathophysiology, clinical trials, and future direction. JACC Heart Fail. (2021) 9:243–53. doi: 10.1016/j.jchf.2021.01.009
33. Chen Z, Wang J, Yang H, Li H, Chen R, Yu J. Relationship between the blood urea nitrogen to creatinine ratio and in-hospital mortality in non-traumatic subarachnoid hemorrhage patients: based on propensity score matching method. J Clin Med. (2022) 11(23):7031. doi: 10.3390/jcm11237031
34. Ok F, Erdogan O, Durmus E, Carkci S, Canik A. Predictive values of blood urea nitrogen/creatinine ratio and other routine blood parameters on disease severity and survival of COVID-19 patients. J Med Virol. (2021) 93:786–93. doi: 10.1002/jmv.26300
35. Rajtik T, Galis P, Bartosova L, Paulis L, Goncalvesova E, Klimas J. Alternative RAS in various hypoxic conditions: from myocardial infarction to COVID-19. Int J Mol Sci. (2021) 22(23):12800. doi: 10.3390/ijms222312800
36. Garcia B, Zarbock A, Bellomo R, Legrand M. The alternative renin-angiotensin system in critically ill patients: pathophysiology and therapeutic implications. Crit Care. (2023) 27:453. doi: 10.1186/s13054-023-04739-5
37. Fanola CL, Norby FL, Shah AM, Chang PP, Lutsey PL, Rosamond WD, et al. Incident heart failure and long-term risk for venous thromboembolism. J Am Coll Cardiol. (2020) 75:148–58. doi: 10.1016/j.jacc.2019.10.058
38. Gregson J, Kaptoge S, Bolton T, Pennells L, Willeit P, Burgess S, et al. Cardiovascular risk factors associated with venous thromboembolism. JAMA Cardiol. (2019) 4:163–73. doi: 10.1001/jamacardio.2018.4537
39. Ambra N, Mohammad OH, Naushad VA, Purayil NK, Mohamedali MG, Elzouki AN, et al. Venous thromboembolism among hospitalized patients: incidence and adequacy of thromboprophylaxis—a retrospective study. Vasc Health Risk Manag. (2022) 18:575–87. doi: 10.2147/VHRM.S370344
40. Al-Hameed FM, Al-Dorzi HM, Qadhi AI, Shaker A, Al-Gahtani FH, Al-Jassir FF, et al. Thromboprophylaxis and mortality among patients who developed venous thromboembolism in seven major hospitals in Saudi Arabia. Ann Thorac Med. (2017) 12:282–9. doi: 10.4103/atm.ATM_101_17
Keywords: venous thromboembolism, blood urea nitrogen to creatinine ratio, intensive care unit, in-hospital mortality, critically ill
Citation: Puri A, Giri M, Huang H and Zhao Q (2024) Blood urea nitrogen to creatinine ratio is associated with in-hospital mortality in critically ill patients with venous thromboembolism: a retrospective cohort study. Front. Cardiovasc. Med. 11:1400915. doi: 10.3389/fcvm.2024.1400915
Received: 14 March 2024; Accepted: 3 June 2024;
Published: 13 June 2024.
Edited by:
Cristina Tudoran, Victor Babes University of Medicine and Pharmacy, RomaniaReviewed by:
Cristina Vacarescu, Victor Babes University of Medicine and Pharmacy, RomaniaLarisa Anghel, Institute of Cardiovascular Diseases, Romania
© 2024 Puri, Giri, Huang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qinghua Zhao, cWgyMDA2M0AxNjMuY29t
†These authors have contributed equally to this work
 Anju Puri
Anju Puri Mohan Giri
Mohan Giri Huanhuan Huang
Huanhuan Huang Qinghua Zhao1*
Qinghua Zhao1*