- Department of Emergency Medicine, Hangzhou Third People’s Hospital, Hangzhou, China
Background: Lactate dehydrogenase (LDH) and albumin (ALB) were found to be significantly correlated with mortality in pulmonary embolism (PE) patients. However, data regarding the LDH/ALB ratio (LAR) in patients with acute PE are scanty. Therefore, the aim of this study was to investigate the association between LAR and the risk of mortality in patients with acute PE.
Methods: A retrospective cohort study was conducted on patients with acute PE represented in the Medical Information Mart for Intensive Care IV (MIMIC-IV). A receiver operating characteristic (ROC) curve analysis and calibration curve were used to assess the accuracy of the LAR for predicting mortality in patients with acute PE. We utilized Cox regression analysis to determine adjusted hazard ratios (HR) and 95% confidence interval (CI). Survival curves were used to evaluate a connection between the LAR and prognosis in patients with acute PE.
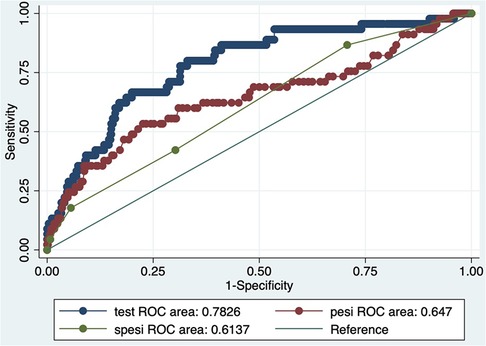
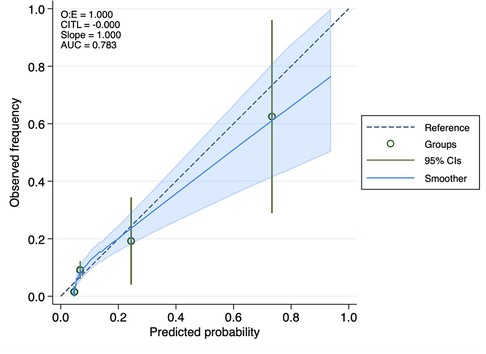
Results: The study comprised 581 patients, and the 30-day all-cause mortality rate was 7.7%. We observed a higher LAR in the non-survival group compared to the surviving group (21.24 ± 21.22 vs. 8.99 ± 7.86, p < 0.0001). The Kaplan–Meier analysis showed that patients with an elevated LAR had a significantly lower likelihood of surviving the 30-day mortality compared to those with a low LAR. Cox regression analysis showed that LAR (HR = 1.04, 95% CI: 1.03–1.05) might have associations with 30-day mortality in patients with acute PE. This result was supported by sensitivity analyses. According to the results of the ROC curve analysis, the LAR's prediction of 30-day mortality in patients with acute PE yielded an area under the ROC curve of 0.73. A calibration curve showed LAR is well calibrated.
Conclusion: Our research suggests LAR monitoring may be promising as a prognostic marker among patients with acute PE.
Introduction
Pulmonary embolism (PE) is a serious disease, exhibiting high morbidity and mortality rates, and stands as the third most fatal cardiovascular disease, surpassed only by acute myocardial infarction (MI) and stroke (1–3). It impacts millions of individuals worldwide, representing a substantial contributor to morbidity, mortality, and escalating healthcare expenditure globally. Despite considerable progress in the diagnosis and treatment of acute pulmonary embolism (acute PE) in recent years, the incidence of this condition continues to rise year on year (3). In addition, despite a global trend toward decreasing fatality rates of PE, the associated mortality proportions continue to appear high (4). In the acute phase, a wide clinical spectrum is observed, ranging from asymptomatic PE to cardiovascular collapse and mortality. Therefore, it is essential to conduct accurate assessments and risk stratification of patients with acute PE to assist clinicians in making optimal treatment decisions. According to relevant studies, the Pulmonary Embolism Severity Index (PESI) score is the most commonly used method for predicting short-term mortality in patients with acute PE. However, the PESI score involves numerous variables with varying weights and is complex to calculate, making it less widely accepted among emergency medical personnel (5). Hence, the simplified Pulmonary Embolism Severity Index (sPESI) has been developed to predict the risk of death in patients with acute PE (6). Furthermore, the strengths of both the PESI and sPESI primarily lie in identifying patients with low- to moderate-risk acute PE, but their ability to identify patients with moderate- to high-risk acute PE is limited (5). Therefore, there is an urgent need for simple and reliable methods to assist clinicians in the early assessment of patients with acute PE in the intensive care unit (ICU) and to enhance the accuracy of existing prognosis predictions for critically ill patients.
Venous thrombosis (VTE) primarily involves three elements: stagnant blood flow, vascular injury, and hypercoagulability of the blood (7). Studies have shown that immune response and inflammation play an indispensable role in the above pathophysiological processes (8). Vascular endothelial cells (VECs) play essential roles in the pathogenesis of VTE. Previous studies have documented that VTE induces VEC injury, the accumulation of inflammatory substances, blood hypercoagulation, and worsened thromboembolism, resulting in severe health (9–11). Lactate dehydrogenase (LDH) is regarded as a biomarker of disease activity and tissue damage. In some previous studies, an elevated LDH level was identified to be independently associated with VTE (12–15). Moreover, in recent years, a wealth of research has suggested that inflammation plays a crucial role in the pathophysiological processes of VTE (16). Although the relationship between inflammation and clinical venous thrombosis is not clearly known, it is speculated that inflammation of the vessel wall initiates thrombus formation in an intact vein and that inflammation and coagulation systems are coupled by a common activation pathway (17). Serum albumin (ALB) is an acute-phase protein primarily synthesized in the liver and serves as a reliable indicator of nutritional status (18). ALB performs several crucial physiological functions, including exerting antithrombotic effects, inhibiting platelet aggregation, and promoting anticoagulation (19). Furthermore, ALB can influence the thrombotic triad and regulate abnormal coagulation through various mechanisms (20). Research indicates that low ALB levels serve as an indicator of poor prognosis in hospitalized patients (21, 22). Furthermore, certain studies have identified ALB as an independent predictor of mortality associated with PE (23–27). One hypothesis is that elevated LDH and reduced albumin might be indicative of a hyperinflammatory or hypercoagulable state (20, 23, 28, 29). Recently, the LAR (LDH/ALB ratio) has been found to be significantly correlated with the mortality rates of sepsis and sepsis-associated acute kidney injury (30, 31). Therefore, it is essential to investigate the correlation between LAR and acute PE.
However, data regarding LAR in acute PE patients are scanty. We hypothesized that an increased LAR could be associated with a poor prognosis in patients with acute PE. Accordingly, we sought to explore the relationship between LAR and acute PE in critically ill patients.
Materials and methods
Data source
Based on primary data obtained from the comprehensive Medical Information Mart for Intensive Care IV (MIMIC-IV) database (version 2.2), this retrospective observational study was conducted (32). The MIMIC-IV database includes data for each medical record pertaining to patients admitted to the ICU or emergency room at the Beth Israel Deaconess Medical Center between 2008 and 2019. Acknowledged as one of the most voluminous and frequently engaged databases in intensive care medicine, it offers crucial resources for research and analytic purposes. The establishment of the database was approved by the institutional review boards of the Massachusetts Institute of Technology (Cambridge, MA, USA) and Beth Israel Deaconess Medical Center (Boston, MA, USA). To maintain ethical standards and protect patient privacy, the data utilized in this study underwent de-identification, with all necessary precautions implemented to safeguard patient confidentiality. The first author (Jingjing Hu, certification ID: 52583254) was authorized to use the MIMIC-IV database after completing the National Institutes of Health's online education program. The requirement for informed consent was waived by the ethical committee at Beth Israel Deaconess Medical Center, given the de-identified nature of the data.
Study population
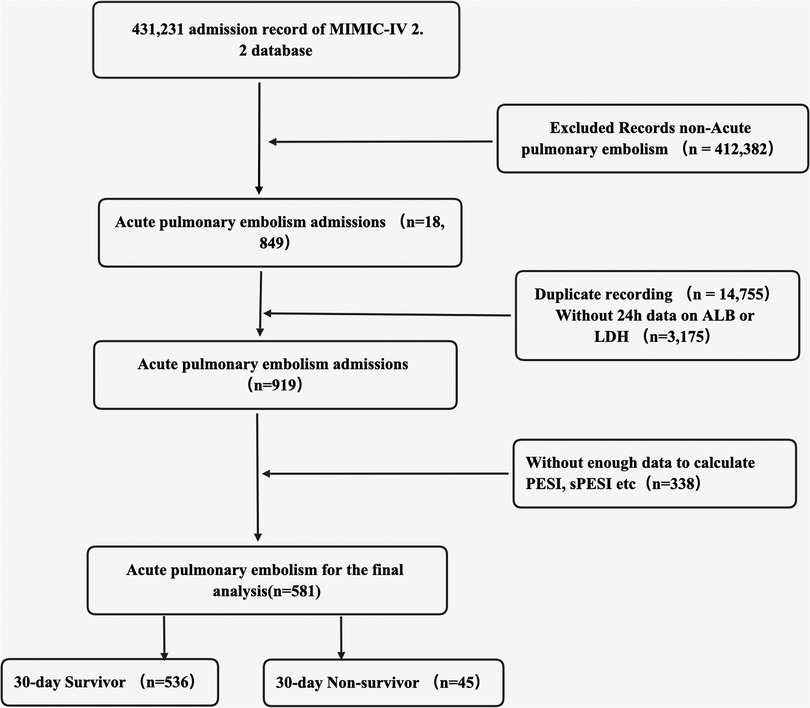
All eligible patients diagnosed with acute PE based on ICD codes (ICD-9: 41511, 41512, 41519, 67380–67384; ICD-10 code: I26, I260, I2601, I2609, I269, I2690, I2693, I2699) from the MIMIC-IV database were included as participants in this study. The inclusion criteria comprised adult patients (aged ≥18 years) diagnosed with acute PE. For individuals with repeated admissions, only data from their first admission were considered. The exclusion criteria included ICU readmission, individuals lacking data on ALB or LDH within 24 h, missing data for calculating important metrics such as PESI and sPESI, and those with insufficient follow-up information. As a result, this study comprised only 581 patients.
Data extraction
Structured Query Language data extraction tool (pgAdmin 4) was used to extract data in the first 24 h of admission: (1) demographic variables: age, sex, race, insurance status, and initial admission unit; (2) vital signs: temperature, respiratory rate (RR), heart rate, systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), and oxygen saturation levels (SpO2) were recorded on the first day of admission; (3) comorbidities: MI, heart failure (HF), chronic pulmonary disease, diabetes mellitus (DM), renal disease, and liver disease; (4) laboratory tests were performed within the initial 24 h after admission, including hematocrit level, white blood cell (WBC) count, platelet count, serum potassium level, serum sodium level, hemoglobin level, blood glucose level, serum bicarbonate level, serum calcium level, serum chloride level, serum urea nitrogen level (BUN), serum creatinine level (SCR), anion gap, prothrombin time (PT), partial thromboplastin time (PTT), international normalized ratio (INR), LDH, ALB, and other laboratory markers. If a variable was measured multiple times within the previous 24 h, the mean value was used; (5) Simplified Acute Physiology Score II (SAPS II) score, PESI score, sPESI score, and Sequential Organ Failure Assessment (SOFA) score were used to assess the severity of illness upon admission; (6) the use of vasoactive drugs, continuous renal replacement therapy (CRRT) and mechanical ventilation (MV) during hospitalization; (7) the duration of ICU stays, overall length of hospital stay, and cases of 30-day death during hospitalization. LAR was calculated from the LDH (U/L)/ALB (g/L) ratio.
Endpoints
The endpoint was 30-day all-cause mortality.
Statistical analysis
The basic clinical characteristics of patients were analyzed according to the death and survival groups. Normal distribution was assessed using the Shapiro–Wilk test. Categorical variables were presented as numbers and percentages (%) and were compared using the chi-square test. Continuous variables were presented as mean ± standard deviation (SD) for variables with normal distribution or as median [interquartile range (IQR)] for variables without normal distribution and compared using either the Student t-test or Mann–Whitney U test, respectively. Survival curves were generated using the Kaplan–Meier method and compared using the log-rank test.
Cox proportional hazard models were constructed to test the associations between 30-day mortality, with results expressed as hazard ratios (HR) with 95% confidence intervals (CIs). We used multivariate Cox regression models to estimate the relationship between LAR and 30-day mortality in patients with acute PE. Model I adjusted for nothing. Model II adjusted for age, sex, race, and insurance. Model III adjusted for use of vasoactive drugs, use of CRRT, use of MV, and ICU management at admission. Model IV adjusted for SAPS II score and PESI score. A time-dependent receiver operating characteristic (ROC) curve analysis was used to assess the predictive ability of LAR, PESI score, and sPESI score for 30-day mortality. Through this analysis, the sensitivity and specificity of each index and the area under the ROC curve (AUC) were computed. The optimal cut-off value of LAR was ascertained by the Youden index. The calibration curve was used to evaluate the agreement between the number of observed and expected deaths predicted by LAR. A calibration curve close to the ideal y = x line indicates good calibration.
Finally, because non-ICU patients lacked crucial data, our final analysis only included patients admitted to the ICU. Therefore, we conducted a sensitivity analysis. This analysis included 338 non-ICU patients with acute PE with LDH and ALB data available within 24 h. Sensitivity analyses were also conducted on 338 patients with acute PE who were not admitted to the ICU. Furthermore, because active tumors have a significant impact on patient survival, we excluded this subset of patients and conducted sensitivity analyses (n = 393). We used Cox regression models to estimate the relationship between LAR and 30-day mortality in patients with acute PE. Data analyses were conducted using Stata 16.0. Statistical significance was defined as a two-tailed p-value <0.05.
Results
Study population and basic clinical characteristics
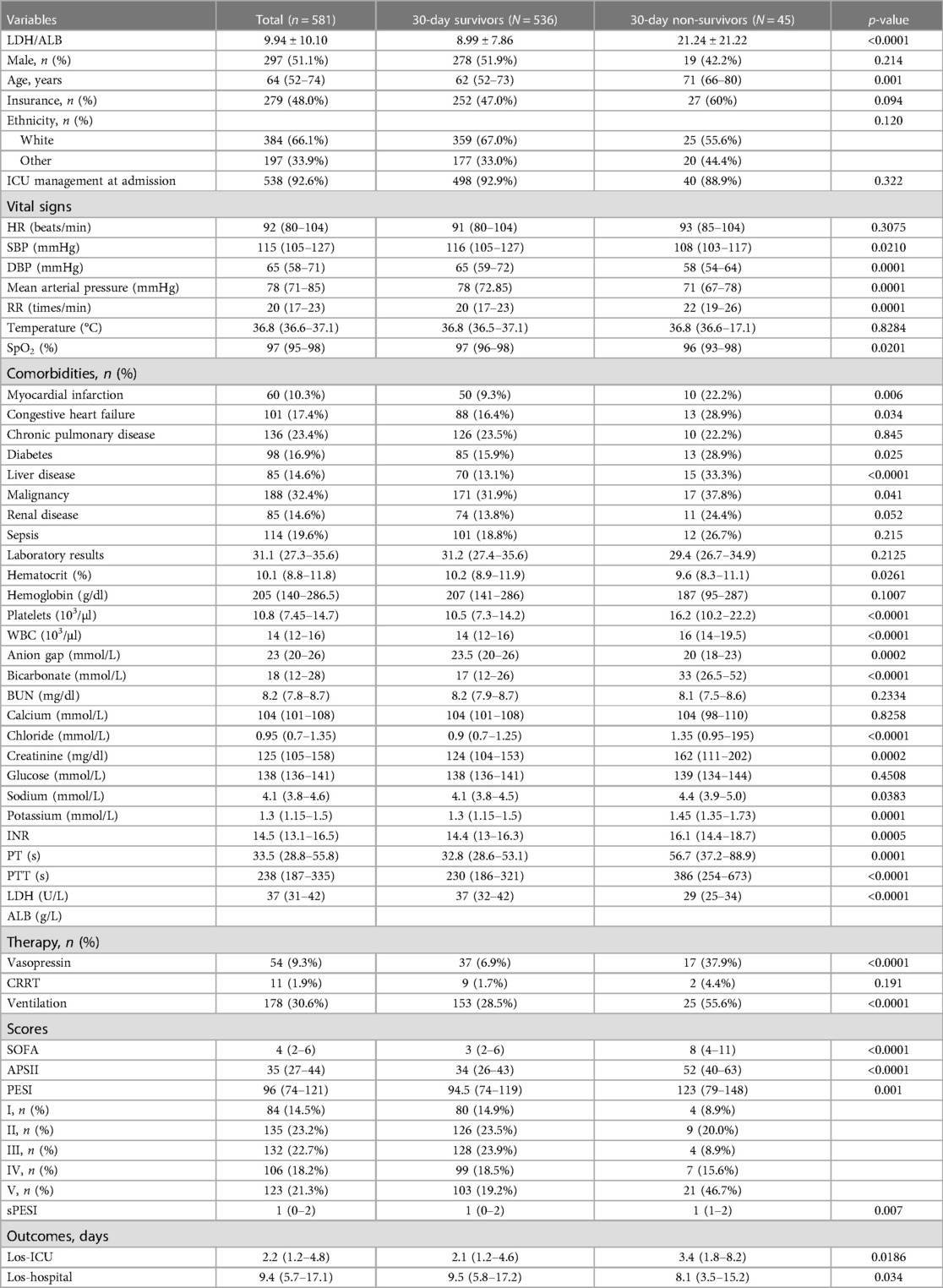
Overall, a total of 581 participants were included in the final study according to the inclusion and exclusion criteria (Figure 1). The basic clinical characteristics of all participants were shown in Table 1. The 30-day mortality for patients with acute PE in this study was 7.7%. Patients in the non-survival group had higher LAR (21.24 ± 21.22 vs. 8.99 ± 7.86, p < 0.0001) and age (71 vs. 62 years, p = 0.001). The patients in the non-survival group were more likely to have a history of MI (22.2% vs. 9.3%, p = 0.006), HF (28.9% vs. 6.4%, p = 0.034), DM (28.9% vs. 15.9%, p = 0.025), liver disease (33.3% vs. 13.1%, p < 0.0001), and cancer (37.8% vs. 31.9%, p = 0.041). However, no statistical differences were found in the history of chronic pulmonary disease, renal disease, and sepsis (all p > 0.05). Patients in the non-survival group had a higher APSII score, PESI score, sPESI score, and SOFA score (all p < 0.05). Patients in the non-survival group were more likely to use vasopressin (37.9% vs. 6.9%, p < 0.0001) and MV (55.6% vs. 28.5%, p < 0.0001), while no difference was observed in CRRT (4.4% vs. 1.7%, p = 0.191).
Relationship between LAR and 30-day all-cause mortality
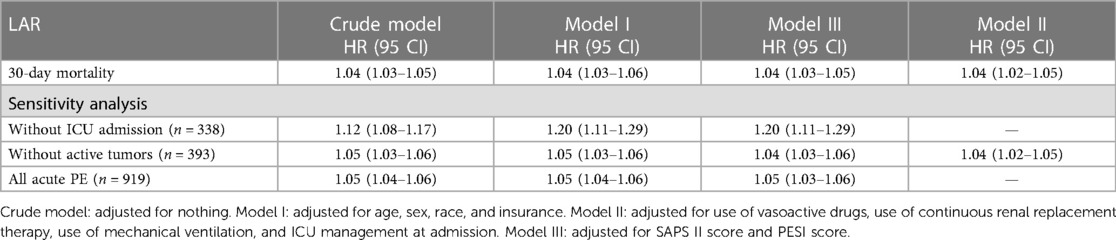
When analyzed as a continuous variable, LAR was associated with 30-day mortality. As exhibited in Table 2, univariate analysis revealed that LAR (HR = 1.04, 95% CI: 1.03–1.05) might have associations with 30-day mortality in patients with acute PE. After adjusting for confounders including age, sex, race, and insurance, the LAR level was associated with an increased risk of 30-day mortality in patients with acute PE (HR = 1.04, 95% CI: 1.03–1.06). Model III adjusted for use of vasoactive drugs, use of CRRT, use of MV, and ICU management at admission, and the LAR level was associated with an increased risk of 30-day mortality in patients with acute PE (HR = 1.04, 95% CI: 1.03–1.05). Model IV adjusted for SAPS II score and PESI score, and the LAR level was associated with an increased risk of 30-day mortality in patients with acute PE (HR = 1.04, 95% CI: 1.02–1.05).
Analysis of ROC curves and calibration curve
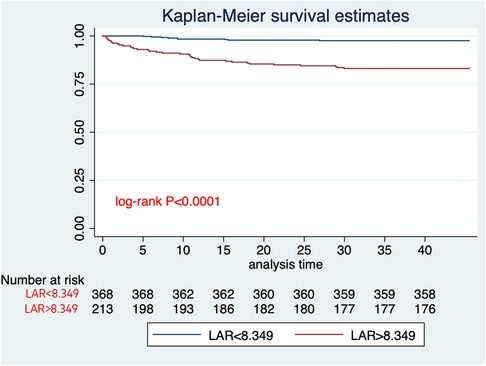
The analysis of ROC curves demonstrated that LAR exhibited a significant predictive ability for 30-day mortality, with an optimal threshold of 8.349, a sensitivity of 80%, and a specificity of 67%. It is noteworthy that the predictive performance of LAR closely resembled that of PESI score, and sPESI score (PESI score AUC = 0.65; sPESI score AUC = 0.56). LAR is well calibrated; however, at higher score ranges, mortality may be underestimated (Figures 2–4).
Sensitivity analysis
We conducted a sensitivity analysis, including 338 non-ICU patients. A total of 919 patients were included in the analysis, among which the overall mortality rate was 5.5% (51 deaths), with a mortality rate of 7.7% (45 deaths) among patients admitted to the ICU and 1.8% (6 deaths) among patients not admitted to the ICU. Furthermore, we conducted sensitivity analyses on patients with acute PE (n = 393) who were managed in the ICU without active tumors and on patients who were managed outside the ICU without active tumors (n = 338). The sensitivity analysis supported our conclusion that LAR is associated with 30-day mortality in patients with acute PE (Table 2).
Discussion
In this study, we investigated the prognostic values of LAR for the 30-day mortality of patients with acute PE using data from the MIMIC-IV database. To the best of our knowledge, our study is the first to investigate the association between LAR and all-cause mortality in patients with acute PE. In this retrospective study, we analyzed 581 patients with acute PE from the MIMIC-IV database and found that the non-survival group had significantly higher serum LAR levels than the survival group. Serum LAR levels were independently positively associated with 30-day mortality in patients with acute PE. The AUC values of LAR for diagnosing the risk of 30-day mortality in acute PE were 0.73. The findings suggested that LAR monitoring may be promising as a prognostic marker among patients with acute PE. However, our conclusions still need to be verified in multicenter, prospective cohort studies.
Despite advancements in diagnostic and treatment, PE persists as a global public health concern, with annual incidence rates continuing to rise over time (3). While the global fatality rates of PE tend to decrease, the mortality proportions associated with PE still appear to be high, accounting for up to 0.2%–0.4% of all deaths (4). The international Registro Informatizado Enfermedad Tromboembólica (RIETE) registry study revealed that the all-cause mortality rate for patients with PE was 4.9%–6.6% at 30 days (33). In our study, the 30-day mortality of patients with acute PE was 7.7%. A 30-year nationwide population-based cohort study involving 128,223 patients found that the mortality risk for patients with acute PE within the first 30 days was 31% (34). On the other hand, a study conducted at Mayo Clinic reported a similar 30-day mortality of 7.7%, consistent with our findings (35). Mortality among patients with acute PE vary across studies. This variability may be attributed to differences in population characteristics, available medical resources, and the severity of PE among the various studies.
LDH is regarded as a biomarker of disease activity and tissue damage. Alkindi et al. found a higher LDH level in patients with VTE compared with the controls (p < 0.05) (15). Abdel-Razeq et al. conducted a retrospectively study and found that a high LDH level was an important factor that may increase the risk of VTE [Odds Ratio (OR) = 5.82] (12). Similarly, Chen et al. discover that a high LDH level was significantly associated with VTE (OR = 2.441). The pathophysiological mechanism of how LDH causes VTE has not been fully elucidated. VECs serve key roles in the pathogenesis of VTE. Previous studies have documented that VTE induces VEC injury, the accumulation of inflammatory substances, blood hypercoagulation, and worsened thromboembolism, resulting in severe health consequences (9–11). The impairment of VECs may compromise the integrity of blood vessels, potentially leading to bleeding, which can impact the anticoagulant therapy in VTE (36). Some studies showed lactate (LA) may serve an important role in VEC damage (37, 38). VECs uptake glucose from peripheral blood and, through the catalytic activity of various enzymes such as glycogen and pyruvate kinase, convert glucose to tricarboxylic acid. This process serves as an energy source for cells. Apart from this, pyruvate can also be converted to LA by lactic acid dehydrogenase A (LDHA) (39–42). However, LA does not directly provide energy to cells; it must first be converted by lactic acid dehydrogenase B (LDHB) to pyruvate (43). Some studies suggest that elevated LA levels contribute to VEC aging, potentially associated with an increased conversion of pyruvate to LA mediated by LDHA (44). In addition, VTE can result in glucose and oxygen deficiency in local VECs, leading to changes in the levels of metabolic substances, including LA, which plays an important role in VTE (45, 46). Therefore, LDH may affect VEC supply capability by influencing lactic acid metabolism. This, in turn, could impact the prognosis of patients with VTE. However, the specific mechanism by which LDH is involved in the occurrence and adverse prognosis of VTE remains unclear. Further research is needed to elucidate this mechanism.
ALB is an acute-phase protein primarily synthesized in the liver and serves as a reliable indicator of nutritional status (18). It is the most abundant circulating protein endowed with multiple functions, including transport of molecules, maintenance of colloidal osmotic pressure, anti-inflammatory and antioxidant properties, and inhibitory activity toward the clotting system and platelet activation (19). A low ALB concentration is considered an integrative index for inflammation, hypercoagulability, or disease states that predispose patients to thrombosis (47). The mechanisms by which low ALB is associated with VTE may include the following aspects: first, ALB is considered an acute-phase inflammatory response protein with crucial physiological functions in endothelin stabilization and inflammatory pathways (48). Inflammation may result in endothelial damage in the venous wall, exposure of collagen, and ultimately thrombosis. This inflammatory response in the venous wall can further stimulate inflammation (49). In addition, inflammation can increase vascular permeability, leading to additional loss of ALB. Second, low albumin levels can contribute to a hypercoagulable state in the blood. Reduced ALB has been associated with higher fibrinogen and factor VIII (FVIII) levels, as well as a shorter activated partial thromboplastin time (aPTT) (20). This suggests that low ALB may indicate a tendency toward hypercoagulation. ALB plays a crucial role in maintaining plasma colloid osmotic pressure. As ALB levels decrease, water leaks out of blood vessels, causing increased blood viscosity, slowed flow rate, elevated shear force between blood and vessel endothelium, and increased susceptibility of vessel endothelium to injury, ultimately leading to thrombosis (50). Third, ALB significantly contributes to binding anticoagulant factors, inhibiting platelet accumulation. It exerts anticoagulant effects by inhibiting fibrin polymerization and platelet aggregation. ALB also has heparin-like effects by enhancing the activity of antithrombin III (51–53). Moreover, ALB demonstrates antithrombotic effects by binding nitric oxide (NO) at the Cys-34 site, forming nitroso-albumin. This compound appears to prevent the rapid inactivation of NO, thereby prolonging its vasodilating and anti-aggregation effects on platelets (54, 55). Finally, low albumin levels result in increased bioavailability of arachidonic acid and elevated thromboxane A2, favoring platelet aggregation and hyperactivity (52). Jellinge et al. (21) and Thongprayoon et al. (22) found hypoalbuminemia to be associated with short- and long-term mortality in hospitalized patients. In a population-based prospective cohort study including 109 patients with VTE, ALB was found to potentially influence the risk of future VTE (25). Furthermore, a study analyzing 552 patients with acute PE found that ALB was associated with acute PE severity and that patients with hypoalbuminemia had an increased risk of VTE (20). Olson et al. (23) and Kunutsor et al. (25) found the same result. In addition, Königsbrügge et al. carried out a prospective observational cohort study and found that decreased serum albumin levels in patients with cancer were significantly associated with an increased risk of VTE and mortality (24). Gök et al. conducted a retrospective study and found that low ALB levels (OR = 0.049, 95% CI: 0.006–0.383; p = 0.049) were associated with 30-day mortality in patients with acute PE (26). Hoskin et al. found that hypoalbuminemia independently predicted both 30-day (OR = 2.57, 95% CI: 1.03–6.41) and 90-day (HR: 2.42 95% CI: 1.38–4.22) mortality. In our study, we found a significant association between LAR and 30-day mortality in patients with acute PE, even after adjusting for multiple factors.
While this study presents compelling evidence supporting the predictive value of LAR for 30-day all-cause mortality in patients with acute PE using a comprehensive database, it is important to acknowledge its limitations. First, the use of ICD-9 and ICD-10 codes for identifying acute PE or other comorbidities introduces a potential source of partial error. Second, the single-center, retrospective nature of our study may lead to selection bias. Due to a large amount of missing data, this study ultimately only included patients with acute PE patients in the ICU, where a high prevalence of comorbidities was observed. This may have had some impact on the study's conclusions. However, we conducted a sensitivity analysis, including non-ICU-managed individuals, and the results remained robust. Furthermore, given our study's limited sample size and the inability to classify the cause of death, we solely explored in-hospital mortality. This might result in an overestimation of the overall outcome. Future research with a multicenter, prospective design is crucial to validate our findings. Third, the absence of some crucial data, such as echocardiography, D-dimer, troponin, and N-terminal pro-BNP, which are vital indicators for assessing acute PE, poses a limitation. Further randomized controlled trials are necessary for validation in the future. Fourth, despite our efforts to adjust for known confounding factors using multivariate analysis, the presence of residual confounding by unknown factors cannot be ruled out. Finally, we did not explore the impact of LAR on long-term mortality due to the lack of long-term mortality data in the included studies.
Conclusion
This study evaluated the predictive values of LAR for 30-day mortality of patients with acute PE. We found that LAR was a potential prognostic biomarker for predicting 30-day mortality in patients with acute PE, which might help clinicians enhance risk stratification, design individual treatments, and determine follow-up schedules for these patients. This study provides some suggestions for the risk stratification of patients with acute PE.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center. The studies were conducted in accordance with the local legislation and institutional requirements. The Ethics Committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this was a study using the MIMIC database. The institutional review boards of the Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center approved the establishment of the database.
Author contributions
JH: Writing – original draft. YZ: Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fleitas Sosa D, Lehr AL, Zhao H, Roth S, Lakhther V, Bashir R, et al. Impact of pulmonary embolism response teams on acute pulmonary embolism: a systematic review and meta-analysis. Eur Respir Rev. (2022) 31(165):220023. doi: 10.1183/16000617.0023-2022
2. Wendelboe AM, Raskob GE. Global burden of thrombosis: epidemiologic aspects. Circ Res. (2016) 118(9):1340–7. doi: 10.1161/circresaha.115.306841
3. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European respiratory society (ERS). Eur Heart J. (2020) 41(4):543–603. doi: 10.1093/eurheartj/ehz405
5. Elias A, Mallett S, Daoud-Elias M, Poggi JN, Clarke M. Prognostic models in acute pulmonary embolism: a systematic review and meta-analysis. BMJ Open. (2016) 6(4):e010324. doi: 10.1136/bmjopen-2015-010324
6. Venetz C, Jiménez D, Mean M, Aujesky D. A comparison of the original and simplified pulmonary embolism severity Index. Thromb Haemost. (2011) 106(3):423–8. doi: 10.1160/th11-04-0263
7. Bagot CN, Arya R. Virchow and his triad: a question of attribution. Br J Haematol. (2008) 143(2):180–90. doi: 10.1111/j.1365-2141.2008.07323.x
8. Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. (2013) 13(1):34–45. doi: 10.1038/nri3345
9. Torres C, Matos R, Morais S, Campos M, Lima M. Soluble endothelial cell molecules and circulating endothelial cells in patients with venous thromboembolism. Blood Coagul Fibrinolysis. (2017) 28(8):589–95. doi: 10.1097/mbc.0000000000000650
10. Pilard M, Ollivier EL, Gourdou-Latyszenok V, Couturaud F, Lemarié CA. Endothelial cell phenotype, a major determinant of venous thrombo-inflammation. Front Cardiovasc Med. (2022) 9:864735. doi: 10.3389/fcvm.2022.864735
11. Poredos P, Jezovnik MK. The role of inflammation in venous thromboembolism and the link between arterial and venous thrombosis. Int Angiol. (2007) 26(4):306–11.18091697
12. Abdel-Razeq H, Tamimi F, Abdel-Razeq R, Salah S, Omari Z, Salama O, et al. Predictors of venous thromboembolism in patients with testicular germ cell tumors: a retrospective study. Clin Appl Thromb Hemost. (2021) 27:10760296211024756. doi: 10.1177/10760296211024756
13. Ding K, Wang H, Jia Y, Zhao Y, Yang W, Chen W, et al. Incidence and risk factors associated with preoperative deep venous thrombosis in the young and middle-aged patients after hip fracture. J Orthop Surg Res. (2022) 17(1):15. doi: 10.1186/s13018-021-02902-8
14. Chen S, Sun W, Dan M, Sun Y, Jiang Y. A nomogram for predicting the risk of venous thromboembolism in patients with solid cancers. J Thromb Thrombolysis. (2023) 56(3):414–22. doi: 10.1007/s11239-023-02856-0
15. Alkindi S, Al-Ghadani AR, Al-Zeheimi SR, Alkindi SY, Fawaz N, Ballas SK, et al. Predicting risk factors for thromboembolic complications in patients with sickle cell anaemia—lessons learned for prophylaxis. J Int Med Res. (2021) 49(12):3000605211055385. doi: 10.1177/03000605211055385
16. Saghazadeh A, Hafizi S, Rezaei N. Inflammation in venous thromboembolism: cause or consequence? Int Immunopharmacol. (2015) 28(1):655–65. doi: 10.1016/j.intimp.2015.07.044
17. Branchford BR, Carpenter SL. The role of inflammation in venous thromboembolism. Front Pediatr. (2018) 6:142. doi: 10.3389/fped.2018.00142
18. Quinlan GJ, Martin GS, Evans TW. Albumin: biochemical properties and therapeutic potential. Hepatology. (2005) 41(6):1211–9. doi: 10.1002/hep.20720
19. Fanali G, di Masi A, Trezza V, Marino M, Fasano M, Ascenzi P. Human serum albumin: from bench to bedside. Mol Aspects Med. (2012) 33(3):209–90. doi: 10.1016/j.mam.2011.12.002
20. Folsom AR, Lutsey PL, Heckbert SR, Cushman M. Serum albumin and risk of venous thromboembolism. Thromb Haemost. (2010) 104(1):100–4. doi: 10.1160/th09-12-0856
21. Jellinge ME, Henriksen DP, Hallas P, Brabrand M. Hypoalbuminemia is a strong predictor of 30-day all-cause mortality in acutely admitted medical patients: a prospective, observational, cohort study. PLoS One. (2014) 9(8):e105983. doi: 10.1371/journal.pone.0105983
22. Thongprayoon C, Cheungpasitporn W, Chewcharat A, Mao MA, Thirunavukkarasu S, Kashani KB. Impacts of admission serum albumin levels on short-term and long-term mortality in hospitalized patients. QJM. (2020) 113(6):393–8. doi: 10.1093/qjmed/hcz305
23. Olson NC, Cushman M, Lutsey PL, McClure LA, Judd S, Tracy RP, et al. Inflammation markers and incident venous thromboembolism: the REasons for geographic and racial differences in stroke (REGARDS) cohort. J Thromb Haemost. (2014) 12(12):1993–2001. doi: 10.1111/jth.12742
24. Königsbrügge O, Posch F, Riedl J, Reitter EM, Zielinski C, Pabinger I, et al. Association between decreased serum albumin with risk of venous thromboembolism and mortality in cancer patients. Oncologist. (2016) 21(2):252–7. doi: 10.1634/theoncologist.2015-0284
25. Kunutsor SK, Seidu S, Katechia DT, Laukkanen JA. Inverse association between serum albumin and future risk of venous thromboembolism: interrelationship with high sensitivity C-reactive protein. Ann Med. (2018) 50(3):240–8. doi: 10.1080/07853890.2018.1441537
26. Gök G, Karadağ M, Çinar T, Nurkalem Z, Duman D. In-hospital and short-term predictors of mortality in patients with intermediate-high risk pulmonary embolism. J Cardiovasc Thorac Res. (2020) 12(4):321–7. doi: 10.34172/jcvtr.2020.51
27. Hoskin S, Chow V, Kritharides L, Ng ACC. Incidence and impact of hypoalbuminaemia on outcomes following acute pulmonary embolism. Heart Lung Circ. (2020) 29(2):280–7. doi: 10.1016/j.hlc.2019.01.007
28. Nichetti F, Ligorio F, Montelatici G, Porcu L, Zattarin E, Provenzano L, et al. Risk assessment of thromboembolic events in hospitalized cancer patients. Sci Rep. (2021) 11(1):18200. doi: 10.1038/s41598-021-97659-9
29. Falanga A, Marchetti M, Russo L. The mechanisms of cancer-associated thrombosis. Thromb Res. (2015) 135(Suppl 1):S8–11. doi: 10.1016/s0049-3848(15)50432-5
30. Liu Z, Liu F, Jie Y, Chen X. Association between lactate dehydrogenase to albumin ratio and 28-day mortality in patients with sepsis: a retrospective cohort study. Clin Lab. (2024) 70(1). doi: 10.7754/Clin.Lab.2023.230509
31. Liang M, Ren X, Huang D, Ruan Z, Chen X, Qiu Z. The association between lactate dehydrogenase to serum albumin ratio and the 28-day mortality in patients with sepsis-associated acute kidney injury in intensive care: a retrospective cohort study. Ren Fail. (2023) 45(1):2212080. doi: 10.1080/0886022x.2023.2212080
32. Johnson AEW, Bulgarelli L, Shen L, Gayles A, Shammout A, Horng S, et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci Data. (2023) 10(1):1. doi: 10.1038/s41597-022-01899-x
33. Jiménez D, de Miguel-Díez J, Guijarro R, Trujillo-Santos J, Otero R, Barba R, et al. Trends in the management and outcomes of acute pulmonary embolism: analysis from the RIETE registry. J Am Coll Cardiol. (2016) 67(2):162–70. doi: 10.1016/j.jacc.2015.10.060
34. Søgaard KK, Schmidt M, Pedersen L, Horváth-Puhó E, Sørensen HT. 30-year mortality after venous thromboembolism: a population-based cohort study. Circulation. (2014) 130(10):829–36. doi: 10.1161/circulationaha.114.009107
35. Smith SB, Geske JB, Maguire JM, Zane NA, Carter RE, Morgenthaler TI. Early anticoagulation is associated with reduced mortality for acute pulmonary embolism. Chest. (2010) 137(6):1382–90. doi: 10.1378/chest.09-0959
36. Molina-Ortiz P, Orban T, Martin M, Habets A, Dequiedt F, Schurmans S. Rasa3 controls turnover of endothelial cell adhesion and vascular lumen integrity by a Rap1-dependent mechanism. PLoS Genet. (2018) 14(1):e1007195. doi: 10.1371/journal.pgen.1007195
37. Yang K, Fan M, Wang X, Xu J, Wang Y, Gill PS, et al. Lactate induces vascular permeability via disruption of VE-cadherin in endothelial cells during sepsis. Sci Adv. (2022) 8(17):eabm8965. doi: 10.1126/sciadv.abm8965
38. Yang K, Holt M, Fan M, Lam V, Yang Y, Ha T, et al. Cardiovascular dysfunction in COVID-19: association between endothelial cell injury and lactate. Front Immunol. (2022) 13:868679. doi: 10.3389/fimmu.2022.868679
39. Mamun AA, Hayashi H, Yamamura A, Nayeem MJ, Sato M. Hypoxia induces the translocation of glucose transporter 1 to the plasma membrane in vascular endothelial cells. J Physiol Sci. (2020) 70(1):44. doi: 10.1186/s12576-020-00773-y
40. Shao H, Li S. A new perspective on HIV: effects of HIV on brain-heart axis. Front Cardiovasc Med. (2023) 10:1226782. doi: 10.3389/fcvm.2023.1226782
41. Edwards YH, Povey S, LeVan KM, Driscoll CE, Millan JL, Goldberg E. Locus determining the human sperm-specific lactate dehydrogenase, LDHC, is syntenic with LDHA. Dev Genet. (1987) 8(4):219–32. doi: 10.1002/dvg.1020080406
42. Mdluli K, Booth MP, Brady RL, Rumsby G. A preliminary account of the properties of recombinant human glyoxylate reductase (GRHPR), LDHA and LDHB with glyoxylate, and their potential roles in its metabolism. Biochim Biophys Acta. (2005) 1753(2):209–16. doi: 10.1016/j.bbapap.2005.08.004
43. Koukourakis MI, Giatromanolaki A, Sivridis E, Bougioukas G, Didilis V, Gatter KC, et al. Lactate dehydrogenase-5 (LDH-5) overexpression in non-small-cell lung cancer tissues is linked to tumour hypoxia, angiogenic factor production and poor prognosis. Br J Cancer. (2003) 89(5):877–85. doi: 10.1038/sj.bjc.6601205
44. Stabenow LK, Zibrova D, Ender C, Helbing DL, Spengler K, Marx C, et al. Oxidative glucose metabolism promotes senescence in vascular endothelial cells. Cells. (2022) 11(14):2213. doi: 10.3390/cells11142213
45. Franczyk B, Gluba-Brzózka A, Ławiński J, Rysz-Górzyńska M, Rysz J. Metabolomic profile in venous thromboembolism (VTE). Metabolites. (2021) 11(8):495. doi: 10.3390/metabo11080495
46. Lim CS, Kiriakidis S, Sandison A, Paleolog EM, Davies AH. Hypoxia-inducible factor pathway and diseases of the vascular wall. J Vasc Surg. (2013) 58(1):219–30. doi: 10.1016/j.jvs.2013.02.240
47. Chi G, Gibson CM, Liu Y, Hernandez AF, Hull RD, Cohen AT, et al. Inverse relationship of serum albumin to the risk of venous thromboembolism among acutely ill hospitalized patients: analysis from the APEX trial. Am J Hematol. (2019) 94(1):21–8. doi: 10.1002/ajh.25296
48. Tanık VO, Çınar T, Karabağ Y, Şimşek B, Burak C, Çağdaş M, et al. The prognostic value of the serum albumin level for long-term prognosis in patients with acute pulmonary embolism. Clin Respir J. (2020) 14(6):578–85. doi: 10.1111/crj.13176
49. Wakefield TW, Myers DD, Henke PK. Mechanisms of venous thrombosis and resolution. Arterioscler Thromb Vasc Biol. (2008) 28(3):387–91. doi: 10.1161/atvbaha.108.162289
50. Garcia-Martinez R, Caraceni P, Bernardi M, Gines P, Arroyo V, Jalan R. Albumin: pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology. (2013) 58(5):1836–46. doi: 10.1002/hep.26338
51. Galanakis DK. Anticoagulant albumin fragments that bind to fibrinogen/fibrin: possible implications. Semin Thromb Hemost. (1992) 18(1):44–52. doi: 10.1055/s-2007-1002409
52. Mikhailidis DP, Ganotakis ES. Plasma albumin and platelet function: relevance to atherogenesis and thrombosis. Platelets. (1996) 7(3):125–37. doi: 10.3109/09537109609023571
53. Jøorgensen KA, Stoffersen E. Heparin like activity of albumin. Thromb Res. (1979) 16(3–4):569–74. doi: 10.1016/0049-3848(79)90105-1
54. Kim SB, Chi HS, Park JS, Hong CD, Yang WS. Effect of increasing serum albumin on plasma D-dimer, von willebrand factor, and platelet aggregation in CAPD patients. Am J Kidney Dis. (1999) 33(2):312–7. doi: 10.1016/s0272-6386(99)70306-9
Keywords: lactate dehydrogenase, albumin, pulmonary embolism, venous thrombosis, acute pulmonary embolism
Citation: Hu J and Zhou Y (2024) The association between lactate dehydrogenase to serum albumin ratio and in-hospital mortality in patients with pulmonary embolism: a retrospective analysis of the MIMIC-IV database. Front. Cardiovasc. Med. 11:1398614. doi: 10.3389/fcvm.2024.1398614
Received: 10 March 2024; Accepted: 27 May 2024;
Published: 19 June 2024.
Edited by:
Emanuele Valeriani, Umberto 1 Hospital, ItalyReviewed by:
Paolo Santini, Agostino Gemelli University Policlinic, ItalyDanilo Menichelli, Sapienza University of Rome, Italy
Ludovica Anna Cimini, University of Perugia, Italy
© 2024 Hu and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yidan Zhou, 929348691@qq.com
 Jingjing Hu
Jingjing Hu Yidan Zhou*
Yidan Zhou*