
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cardiovasc. Med. , 18 July 2024
Sec. Heart Failure and Transplantation
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1397907
The triglyceride–glucose (TyG) index, proven to be a crucial insulin resistance biomarker (better than the Homeostasis Model Assessment for Insulin Resistance), is simple and non-invasive. Recently, indisputable evidence has shown that the TyG index is strongly associated with cardiovascular disease [CVD, including atherosclerosis, heart failure (HF), and hypertension] prognosis and mortality. Nevertheless, the value of the TyG index in HF patients treated with sodium–glucose cotransporter 2 inhibitors (SGLT2is) has not been systematically evaluated. Therefore, in this review, we summarized the value of the TyG index and its related parameters as markers of CVD, especially HF. Furthermore, we addressed the use of SGLT2is and GLP-1 receptor antagonists in HF patients. Finally, we summarized the mechanism of the “obesity paradox.”
Heart failure (HF), including HF-related symptoms and signs, is a complex clinical syndrome that results from certain functional or structural damage to the ventricles, impairing their ability to fill with or eject blood (1). Approximately 40 million people worldwide suffer from HF, which can be caused by many factors, such as hypertension, coronary artery disease (CAD), smoking, obesity, and genetic factors (2). HF is a leading cause of hospitalizations in patients aged >65 years, and 1%–2% of all hospitalizations in the Western world are attributed to HF (3). In addition, a study conducted in China indicated that HF poses a substantial burden on health systems, and people may face more serious challenges in the future. In the coming years, the epidemic of HF is possibly to worsen, mostly on account of the millions of patients with increasing risk factors, such as coronary heart disease, hypertension, and diabetes. In addition, the figures of this study show that the prevalence and incidence of HF increase with age. With the trend of an aging population, the total number of HF patients is growing rapidly, especially in China, which has the largest population worldwide (4). HF can result in many wasted resources and other types of losses—premature death, lost productivity, disability, loss of quality of life, and racial health disparities (5); thus, there is an urgent need to address this problem. Therefore, to better stratify and cope with the devastating risks of HF, a multitude of biomarkers that can be obtained with non-invasive methods have been identified. For instance, B-type natriuretic peptide (BNP) and N-terminal proBNP (NT-proBNP) have worldwide applicability and high diagnostic and therapeutic validity for the treatment of HF (6). Molvin et al. reported that mid-regional pro-adrenomedullin (MR-proADM) and NT-proBNP concentrations are positively correlated with the risk of experiencing postdischarge mortality, with NT-proBNP being the exclusive biomarker for predicting cardiac hospitalizations (7). It remains unclear which is the best indicator for HF or which combination of several indices is better. NT-proBNP is stable in circulation, while BNP is affected by renal function and age. Interestingly, in clinical practice, BNP is prone to more frequent testing due to its shorter half-life, and it is a widely recognized biomarker.
However, with ongoing research, more novel biomarkers have been utilized as predictors in clinical practice. The triglyceride–glucose (TyG) index, an indicator of insulin resistance (IR), calculated as TyG index = ln [fasting triglyceride (mg/dl) × fasting glucose (mg/dl)]/2, was independently associated with a higher incidence of acute kidney injury (8), CAD, and atherosclerotic cardiovascular diseases, such as myocardial infarction (MI) and stroke, in a meta-analysis involving 5,731,294 participants (9). Moreover, the TyG index has been shown to correlate with several cardiovascular outcomes, such as cardiac arrest and peripheral artery disease (10, 11) (Figure 1). Hence, according to recent studies, this index is strongly associated with the risk of developing cardiovascular disease (CVD), especially HF. As a promising prognostic marker of HF with preserved ejection fraction (HFpEF), a high TyG index is closely associated with an increased risk of mortality and rehospitalization in patients with HFpEF (12). In addition, the TyG index, a valuable tool for use in individuals with prediabetes or diabetes, was associated with the risk of experiencing all-cause and CVD mortality (13) and the risk of experiencing subclinical HFpEF in patients with type 2 diabetes (T2DM) (14). The underlying mechanism is that microRNA 181c, which targets cardiac fibroblasts, is elevated in frail elderly adults with diabetes (15). For patients with HFpEF, insulin resistance and the TyG index are important, as a study revealed that insulin resistance is associated with myocardial dysfunction in HFpEF patients (16). Concurrently, the term “metabolic cardiomyopathy,” referring to the condition under which metabolic inflammatory molecules damage myocardial function in patients with insulin resistance and diabetes, has been gradually used in the literature on HFpEF (17). In most studies, the TyG index is usually measured at admission to better predict the incidence or prognosis of HF. However, a study also collected data in the outpatient setting (18). It was also reported that the TyG-BMI (body mass index), a parameter related to the TyG index and connected with IR, is significantly associated with the risk of developing hypertension (19). The above-mentioned studies show that the TyG index is closely associated with CVD incidence and mortality; thus, it is reasonable to focus on the relationships between HF incidence and the TyG index and its related parameters. Recently, as part of the effort to cure HF, several new drugs have been developed. Sodium–glucose cotransporter 2 inhibitors (SGLT2is) and GLP-1 receptor agonists (GLP-1 RAs) are known to diminish cardiovascular risks in patients with T2DM (20). These popular drugs were initially targeted at treating diabetes, but various studies have shown that they have unexpected effects on HF and have gradually been confirmed to reduce HF-related mortality.
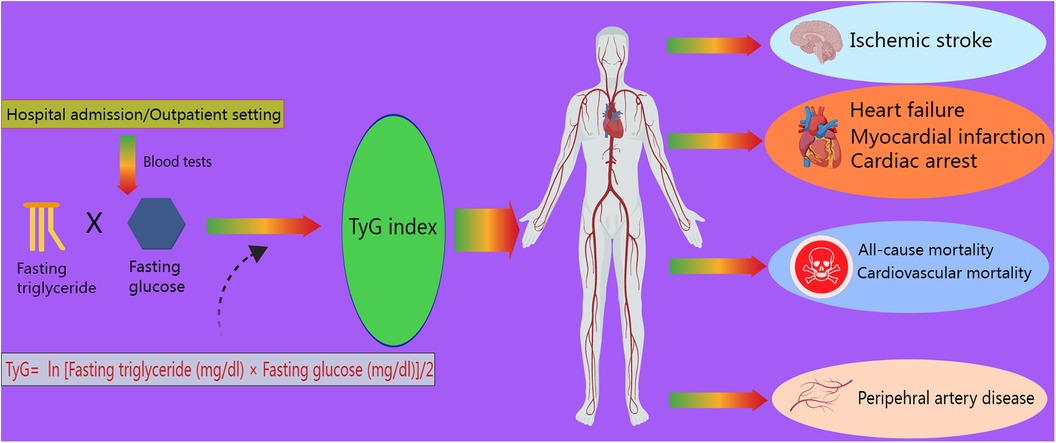
Figure 1 Role of the TyG index in cardiovascular outcomes. The TyG index, defined as ln [fasting triglyceride (mg/dl) × fasting glucose (mg/dl)]/2, is measured during admission or outpatient visits. It is associated with ischemic stroke, myocardial infarction, heart failure, cardiac arrest, peripheral artery disease, cardiovascular mortality, and all-cause mortality.
Hence, in this review, we aimed to discuss the usefulness of the TyG index compared to that of the Homeostasis Model Assessment for Insulin Resistance (HOMA-IR) for identifying IR. In addition, we reviewed the published literature that highlights the application value of NT-proBNP, MR-proADM, the TyG index, and related parameters in HF. Furthermore, we explored the effects of the increasingly popular SGLT2is on HF and their correlation with the TyG index.
HOMA-IR, determined as [fasting insulin (μIU/ml) × fasting glucose (mmol/L)]/22.5, is a marker that reflects IR and can be simultaneously quantified by the TyG index in some metabolic diseases that increase the risk of cardiovascular disease (21). When comparing the TyG index with the HOMA-IR, the former was found to be more valuable for identifying IR in Chinese T2DM patients with a BMI less than 35 kg/m2 (22). A study enrolling 3,185 patients with T2DM showed that each one-unit increase in the TyG index was accompanied by a 1.40-fold increase in the incidence of progressive arterial stiffness. Nonetheless, there was no notable association between HOMA-IR and the incidence of progressive arterial stiffness (23). Moreover, Park et al. analyzed data from 9,730 adults and reported that the TyG index was better able to predict the incidence of T2DM than HOMA-IR, with an area under the curve (AUC) of 0.640 (0.628–0.652) for the incidence of T2DM predicted by the TyG index and 0.531 (0.521–0.541) for the incidence of T2DM predicted by HOMA-IR (24). Moreover, Son et al. (25) conducted a study of 6,091 adults without metabolic syndrome (MetS) and demonstrated that HOMA-IR had a less powerful ability to predict the prevalence of MetS than the TyG index. The results of the Coronary Artery Risk Development in Young Adults investigation, which involved 4,992 participants, suggested that the TyG index can function as a surrogate biomarker for IR to predict the risk of chronic HF (CHF), potentially replacing HOMA-IR (26). As Wang et al. stated, the TyG index presented a stronger connection with intracranial atherosclerosis than HOMA-IR. The authors reported that the TyG index reflects insulin resistance not only in hepatic tissues but also in peripheral tissues, while the HOMA-IR index reflects insulin resistance in only hepatic tissues (27). In addition, the TyG index accounts for both lipids and glucose, making it more appropriate than HOMA-IR for predicting atherosclerosis. Beyond atherosclerosis, an inverse association was observed between HOMA-IR and cognitive function in older patients with hypertension and prediabetes (28). Previous studies have proven an association between renal damage and cognitive function in frail, hypertensive older adults with diabetes (29). In this way, insulin resistance may correlate with cognitive impairment in populations with diabetes and have potential effects on renal function. Similarly, the TyG index has been proven to correlate with low cognitive function in non-diabetic elderly patients (30). The strength of these studies lies in the elucidation of the relationship between cardiovascular damage and cognitive impairment. However, no study has compared the two metabolic parameters in the investigation of cognitive function. In short, the TyG index is more useful than HOMA-IR for the diagnosis of CVD and MetS. Notably, many authors have ascertained the usefulness of the TyG index in numerous studies, but few have noted its ability to predict disease. The TyG index is a relatively comprehensive index since it is a composite of lipids and glucose, but HOMA-IR still has a unique value. In future studies, more diseases, including dementia, cancer, and mental disease, could be used for further comparison of these two parameters.
The TyG index is not superior to HOMA-IR in every aspect. For example, Liu et al. showed that the ability of the TyG index to predict testosterone deficiency was actually inferior to that of HOMA-IR (31). In this way, HOMA-IR may have unexpected impacts on conditions other than cardiovascular disease, warranting further studies. However, overall, the TyG index is more effective than the HOMA-IR index in diverse aspects if applied pertinently. Further exploration into the strengths or shortcomings of the TyG index is expected in future research.
Several studies (13, 32, 33) concluded that the TyG index was independently associated with both all-cause mortality and cardiovascular mortality. Cai et al. (34) reported that an elevated TyG index was significantly associated with hospital mortality and ICU mortality. Similarly, a study involving 3,026 patients (35) revealed that the TyG index independently predicted the risk of hospital death and ICU mortality, with each one-unit increase in the TyG index accompanied by a 1.19-fold increase in the risk of death during hospitalization. Zhang et al. (36) reported that the TyG index was independently associated with an increased risk of ICU mortality and hospital mortality. Indeed, in view of the general population, a positive relationship was clearly found between the TyG index and cardiovascular mortality in people under 65 years old (37). That meta-analysis presented a shortcoming in that the TyG index measurement methods and study designs were different, which might have led to heterogeneity. Obviously, the TyG index plays a potential role in predicting the mortality rate according to all convincing studies that have carefully considered different mortality types. Apart from the above mortality-related findings, Yang et al. emphasized in a meta-analysis (38) that among ischemic stroke patients, a higher TyG index was associated with a higher risk of experiencing stroke recurrence or mortality. This study revealed that the TyG index might have tremendous predictive ability for various vascular diseases throughout the body, a condition referred to as “panvascular disease.” Glucose in the blood can trigger inflammation in vessels, leading to stenosis and stiffness and increasing the susceptibility of patients with diabetes to CVD and cardiovascular mortality. Panvascular disease refers to atherosclerosis that usually occurs in large vessels and microvessels, including renal, cerebral, peripheral, and cardiac vessels, in patients with diabetes (39). Hence, when we determine the function of the TyG index in CVD mortality, it is suitable to apply it to other vascular diseases, especially under conditions of impaired glucose tolerance. However, the available literature mostly focuses on the association between CVD mortality and a single vascular lesion and barely concerns the multifactorial atherosclerosis burden; thus, researchers should further explore these associations in patients with panvascular disease. Moreover, most current studies are retrospective and cannot be used to determine causality in the association between the TyG index and adverse CVD outcomes. The URRAH cohort, a prospective study including 16,649 participants, indicated that a higher risk of all-cause and CV mortality was associated with an increased TyG index (40). The strengths of this research were the long follow-up of 144 months and the large and homogeneous general population. In a state of insulin resistance, the activation of oxidative stress and the inflammatory response may damage cardiomyocytes, and hyperglycemia and inflammation can subsequently lead to and predict the occurrence of adverse cardiovascular events (41). Hence, this prospective study strongly demonstrated that a higher TyG index reflecting anomalies of lipid and glucose metabolism is a risk factor for predicting adverse CVD outcomes in a causal relationship.
Despite the positive association between the TyG index and mortality reported in numerous remarkable studies, the TyG index seemingly does not correlate with cardiovascular mortality (3 studies) or all-cause mortality (4 studies), according to a meta-analysis of a collection of 12 cohort studies (42). Moreover, a contradictory study (32) argued that at the 1-year follow-up, there was no difference in the TyG index among patients with or without major adverse cardiovascular events (MACEs) [8.73 (8.36–9.08) vs. 8.81 (8.5–9.17); P = 0.09]. Therefore, the authors concluded that the use of the TyG index to predict MACEs and overall mortality in non-diabetic individuals with MI was not advisable. The reasons for these inconsistent results lie in the limited sample sizes and differences in ethnicity since meta-analyses combine many cohort studies and might amplify the likelihood of statistical significance, whereas a single study may fail to achieve these results. In addition, many studies did not provide sufficient follow-up time, and the TyG index was measured only once, which might also slightly affect the statistical power.
NT-proBNP plays a key role in the development of HF. In patients with HF with mildly reduced ejection fraction (HFmrEF) or HFpEF, a much greater risk of experiencing cardiovascular events was consistently associated with increased NT-proBNP (43). With respect to the risk of obesity, increasing NT-proBNP was independently associated with a greater risk of cardiac death in all BMI groups except for people who were severely obese (BMI ≥ 40 kg/m2) (22). The authors of that study wisely adjusted for obesity status, which is a crucial cause of CVD-related death, and promoted the reliable prognostic value of NT-proBNP. In a US population trial, Ciardullo et al. (44) reported that participants with NT-proBNP levels ≥100 pg/ml suffered a greater incidence of both cardiovascular mortality and all-cause mortality. These results verified that NT-proBNP is indispensable for predicting incidence and mortality in HF patients. Of course, in clinical practice, NT-proBNP levels less than 100 pg/ml cannot be arbitrarily used to exclude HF without considering symptoms or other tests.
In addition to mortality, NT-proBNP is also beneficial for assessing treatment effects. Based on what Ezekowitz et al. explained, compared to that of placebo, the impact of vericiguat on the endpoint was more pronounced in patients with NT-proBNP levels less than 8,000 pg/ml, and this effect was further amplified in patients with NT-proBNP levels less than 4,000 pg/ml (45). In turn, Armstrong et al. reported that compared to those taking a placebo, patients treated with vericiguat experienced more significant decreases and fewer increases in sequential measures of NT-proBNP, and these interesting alterations were seemingly related to the advantages of vericiguat therapy (46). Therefore, the causality of the relationship between vericiguat treatment and the level of NT-proBNP remains unclear because of the limitations of retrospective studies. Medicinally controlled or lower NT-proBNP is sensitive to treatment. Notably, in an important study, Cunningham and Myhre (47) argued that NT-proBNP is an imperfect surrogate to assess the response to HF therapies because a convincing sensitivity analysis, which exclusively included patients with an ejection fraction of less than 30%, revealed no relationship between treatment effects and NT-proBNP concentrations.
In a different study, researchers aimed to evaluate the effects of combinations of indices. The combined use of MR-proADM and NT-proBNP measurements had additional predictive value mortality and new readmission for acute HF after discharge at 30 days (48). Surprisingly, based on a randomized controlled trial (49) involving 214 patients, Klip et al. reported that MR-proADM (AUC = 0.81) demonstrated greater predictive strength for mortality than BNP (AUC = 0.66) and NT-proBNP (AUC = 0.67). Furthermore, compared with both BNP and NT-proBNP concentrations, MR-proADM concentrations markedly improved mortality risk prediction. This finding suggested that NT-proBNP is not the best predictor of HF since there are other biomarkers that provide better precision and accuracy. Fraty et al. concluded that MR-proADM serves as a prognostic biomarker for HF in people with T2DM; however, compared with NT-proBNP, it does not provide significant complementary information on the prediction of HF without T2DM (50). An apparent shortcoming of this study was its failure to establish a solid history of CHF and a lack of cardiac ultrasound information, which included important baseline left ventricular ejection fractions. The authors thought that diabetes status would affect outcomes in some situations, but echocardiography was not easily available for patients without diabetes. Hence, this study lacked abundant information on non-diabetic control groups.
Five different types of failure—respiratory system, coagulation system, cardiovascular system, neurological system, and kidney organ failure—were independently predicted by MR-proADM (51). In clinical practice, MR-proADM is not widely used by doctors as a standard predictor of HF since this index is still immature or unreliable, but its value can be exploited. A study enrolling 1,088 patients with CHF (52) indicated that patients with elevated MR-proADM levels tended to be older, have a higher New York Heart Association (NYHA) class, be frequently afflicted with lower limb edema, and have a greater incidence of chronic diseases such as hypertension, diabetes, and atrial fibrillation. However, MR-proADM has a poor ability to confirm or exclude HF (53). MR-proADM is not commonly used to evaluate HF, so further prospective studies are warranted. Clearly, with the growing need to reduce the mortality of HF patients, more prognostic and predictive biomarkers need to be explored and updated.
In HF patients, the TyG index is reportedly related to mortality Table 1 (1, 12, 33, 35, 54–57). Among patients with HFpEF, a high TyG index, as a study enrolling 823 patients claimed, is associated with an elevated risk of mortality and rehospitalization (12, 14). The TyG index not only predicts HFpEF but also acute decompensated HF (ADHF) due to the evidence from a paper elaborating that an elevated TyG index is independently linked to an unfavorable prognosis, making it a valuable factor for stratifying the risk of patients with ADHF (37). Similar results were reported by Guo et al. regarding CHF (58). Zheng et al. also reported in a prospective study that an elevated risk of HF is associated with a high cumulative TyG index (59). In China, a study reported that in groups in which the highest and lowest TyG index tertiles were compared, the hazard ratios (HRs) for all-cause and CV-related deaths were 1.84 and 1.94, respectively (33). In addition, a significant positive association existed between HF incidence and the TyG index, which concurrently was proven to be connected with an elevated incidence of coronary heart disease, dyslipidemia, and hypertension (60).
According to the findings from Sun et al., an independent and positive correlation between the TyG index and the risk of experiencing MACEs was detected among patients with HF undergoing percutaneous coronary intervention (57). Hao et al. concluded that the TyG index is closely associated with the risk of experiencing HF and is likely a valuable indicator for predicting long-term patient prognosis in individuals with acute MI (AMI) (61). Notably, Huang et al. reported that a 0.1-unit increase in the TyG index was associated with a 1.05-fold increase in the risk of experiencing MACEs and a 1.07-fold increase in the risk of worsening HF (62). Confirmed by evidence from two large cohorts (63), Li et al. explained that, a higher TyG index, serving as an independent as well as causal risk factor, contributed to incident HF in the general population. This study included two large-sample-size general population cohorts and utilized Mendelian analysis. In addition, this study clarified the causal relationship between the TyG index and the incidence of HF. However, it is important to note that the Mendelian analysis was limited by its exclusive use of data from patients of European descent, which makes it difficult to extrapolate the results to other populations.
No matter how valuable this index is, there are always limitations and failures. For example, Li et al. reported no meaningful association between the TyG index and the risk of experiencing HF in individuals with a BMI of less than 30 kg/m2 (60). In fact, the outcome was triggered by numerous factors. First, the study excluded patients whose HF was not officially diagnosed, and the selection population may have bias. Second, some patients without the TyG index and HF data were excluded. Third, because of the lack of generalizability, this study failed to maintain accuracy in other regions. In the same study, Li et al. concluded that an elevated risk of stroke was not significantly associated with the TyG index, while Zhao et al. (64) showed that the TyG index can independently predict the onset of ischemic stroke in the general population. This discrepancy resulted from the fact that Zhao et al. enrolled only people older than 40 years in rural China in their prospective cohort study, so the range and ethnicity were disparate.
Not until guideline-directed medical therapy (GDMT) for HF with reduced ejection fraction (HFrEF) recommended four medication classes, including SGLT2is, did people widely apply SGLT2is in clinical practice (1). Through many retrospective and prospective studies, SGLT2is have been recognized as safe and absolutely effective in the treatment of HF in many situations and have emerged as a miraculous crossover drug. According to a meta-analysis of 8,474 patients (65), SGLT2is contributed to a greater than 25% reduction in the combined risk of cardiovascular death or initial hospitalization for HF. In addition, they decreased the risk by 25% in the composite of recurrent hospitalizations for HF. In addition, it was reported that among patients with HF, HFpEF, and HFmrEF, dapagliflozin decreased the combined risk of worsening HF and cardiovascular death (66). Three studies (67–69) focused on one famous trial, the EMPEROR-Preserved Trial involving 5,988 patients, which suggested that empagliflozin reduced the risk and severity of a large range of worsening HF events, whether in or out of the hospital, and decreased the risk of HF consequences irrespective of diabetes status. Consistently, Butler et al. asserted that compared with placebo treatment, empagliflozin treatment resulted in a comparable reduction in the risk of cardiovascular death or hospitalization for HF in both sexes (70). Overall, the above-mentioned results elucidated the meaningful use of SGLT2is in HF and can guide clinical therapy to effectively minimize HF risk and mortality. Paradoxically, a multicenter, double-blinded trial (71) showed that dapagliflozin and ertugliflozin were completely non-inferior to placebo for reducing the risk of experiencing MACEs. However, the authors failed to explain the outcomes robustly and suspected that the trial may have been influenced to a greater extent by the evolving long-term changes in more intensive secondary preventive therapies in recent years compared to earlier trials.
Based on certain studies, the TyG index is used as an indicator to assess the effects of SGLT2is in some respects. Zhu et al. (72) reported that dapagliflozin was strongly associated with a decreased risk of experiencing MACEs and that the TyG index decreased with dapagliflozin administration. This study suggested that dapagliflozin affects both lipid metabolism and glucose metabolism, which is comprehensively reflected by the TyG index. This finding provided enough evidence that the TyG index can be utilized as an easily accessible indicator in clinical practice to evaluate the curative effects of dapagliflozin, and it is not just an index to predict mortality. Nonetheless, missing measured factors at discharge and longer follow-up were shortcomings of that study. As indicated by various plasma atherogenic biomarkers, both empagliflozin and dapagliflozin treatment have been demonstrated to result in substantial alterations in the TyG index regardless of the administration of statin treatment (73, 74). From this perspective, the usefulness of the TyG index extends beyond the indication of HF, as it can also be used to evaluate some medical effects, which is another pleasant surprise for physicians. In conclusion, after receiving more research attention, the TyG index will be fully applied in clinical work. There are a dozen indices that can be used to assess therapeutic effects, but the TyG index is simple and quick.
GLP-1 RAs are new drugs that are characterized by amazing glucose-lowering effects. Initially, people often applied them for diabetes treatment and blood glucose control; however, spectacular unexpected effects on the heart have been investigated recently. In a meta-analysis (75) involving 60,080 patients, GLP-1 RAs reduced the risk of experiencing all-cause mortality by 12% and hospital admission for HF by 11%, indicating that GLP-1 RAs are cardioprotective, similar to SGLT2is. Moreover, Zhao et al. reported that compared with placebo, GLP-1 RAs may significantly lower the risk of experiencing MACEs in individuals with HF (76). Apart from the respective contributions of SGLT2is and GLP-1 RAs, researchers have tested whether their combination yields better results. Compared with other combination regimens, a 57% lower odds of HF was significantly associated with the combination of SGLT2is and GLP-1RA regimens (77). However, the mechanism of action of GLP-1 RAs is not completely clear. Recently, Ussher et al. reported that by targeting the central nervous system, people taking GLP-1 RAs tend to have a decreased appetite, leading to weight loss and improved cardiovascular outcomes. In addition, to reduce hepatic steatosis, GLP-1 RAs indirectly improve circulating lipid profiles. GLP-1 RAs might also affect blood vessels, kidneys, and the heart to prevent atherosclerosis and ameliorate cardiac function (78). In addition, a lower risk for hospitalization for HF was associated with SGLT-2 inhibitors than with GLP-1 RAs (79). Interestingly, that meta-analysis included 23 cardiovascular outcome trials, with a considerable sample size and minimal risk of bias. However, no trial has directly compared antidiabetic drug classes, so the meta-analysis failed to interpret the data based on direct comparisons. The above studies highlighted that GLP-1 RAs are extraordinary novel drugs for the treatment of HF, albeit inferior to SGLT2is.
However, Merza et al. noted that compared with a placebo, the controversial medicine GLP-1 RAs did not increase the risk of experiencing MACEs (80). However, in his study, Merza et al. assembled only 9 randomized controlled trials (RCTs) containing only 871 participants, which is small for a meta-analysis. Moreover, RCTs are too limited to produce accurate outcomes in contrast with real-world data; thus, more prospective studies are needed in authentic situations in the future.
Some derivative indices, such as the waist-to-height ratio (TyG-WHtR), the waist-to-height ratio (TyG-BMI), and the waist circumference (TyG-WC), have also attracted attention for their ability to predict CVD (81) in certain ways since they are more comprehensive. These related indices contribute to the prognosis of HF or any other CVD, such as the TyG index. Among them, the TyG-BMI is widely studied because weight and height are essential for assessing the risk of CVD. For example, a lower TyG-BMI was linked to a lower incidence of HF in both prediabetes mellitus and diabetes mellitus patients (82). In addition to the TyG-BMI, Dang et al. reported that the TyG-WC and TyG-WHtR are correlated with congestive HF, which proved the significance of these two indices (83). From this perspective, the TyG index seems to have immense potential to indicate CVD and its combination indices also display vivid potential.
Regarding mortality, a study enrolling 423 patients with HF confirmed that the population with a higher TyG-BMI had a markedly greater 1-year survival rate (84). In addition, Chen et al. reported a negative correlation between the TyG-BMI and early-onset HF, whose incidence rates were explicitly lower in males than in females (14.8% vs. 29%) (85). Obviously, the finding that the TyG-BMI is positively related to the incidence but negatively related to the mortality of HF is confusing. A meta-analysis reported that HF mortality displays a U-shaped curve with a nadir at 30.0–34.9 kg/m2, indicating that compared with lean patients, higher-BMI patients have a survival benefit (86). Indeed, the “obesity paradox” may be a gorgeous explanation concerning the catabolic state, hemodynamic properties, adipokine protection, and muscle wasting (84). First, an “energy-starved” heart is produced by metabolic deficits in both fatty acid oxidation and glucose metabolism in HF, and this condition is primed to use alternative adenosine triphosphate (ATP)-generating fuels. Under these circumstances, individuals choose to switch to ketone fuel utilization since in HF ketone oxidation can bypass not only the complex dysregulation of the β-oxidation pathway but also the pyruvate dehydrogenase complex (87), causing malnutrition and a worse prognosis. Second, HF with a deficiency of left ventricular function usually results in lower blood pressure, and a systolic blood pressure less than 130 mmHg is associated with poor outcomes among hospitalized patients with HFrEF (88). The hemodynamics of obesity accompanied by elevated blood pressure counteract weak cardiac function and spare more space for drugs to target HF. Third, in patients with HF, cachexia can cause the generation of tumor necrosis factor (TNF), which is detrimental to the myocardium and induces inflammation. More molecules and adipokines, provided by increased levels of cholesterol and serum lipids or hyperlipidemia, bind to endotoxins and remove them from blood circulation to prevent the inflammatory response (89). Fourth, the factors involved in muscle wasting are intricate and include disuse, malnutrition, neurohumoral factor activation, and HF medications (90). In an animal trial in lean septic mice, muscle wasting and weakness were confirmed to be protected against by increased lipid availability (91). Given that adipose tissue protects muscle, underweight individuals suffer from muscle wasting, which is an independent predictor of death in patients with HF (92). Accordingly, Ohori et al. asserted that in patients with muscle wasting, the incidence of NYHA functional class III was greater (93) (Figure 2). Not surprisingly, the underlying mechanism of the “obesity paradox” is controversial and unclear because of the different outcomes reported by Benn et al. (94). Both HF incidence and mortality are causally increased by high BMI. The debates on this topic are ongoing because of many confounding factors, such as age, comorbidities, and pharmacotherapy. Multiple studies have confirmed the association between high BMI and mortality in HF patients. According to a meta-analysis involving 5,819 patients with chronic HF, the outcomes of all-cause mortality or HF hospitalization were poorer in the lower-BMI groups (95). In addition, Jones et al. emphasized that compared with people with normal weight, those with higher weight were at elevated risk of mortality (96). In the future, more accurate and comprehensive studies are required to prove this theory to better understand the mechanism of HF and develop corresponding therapies.
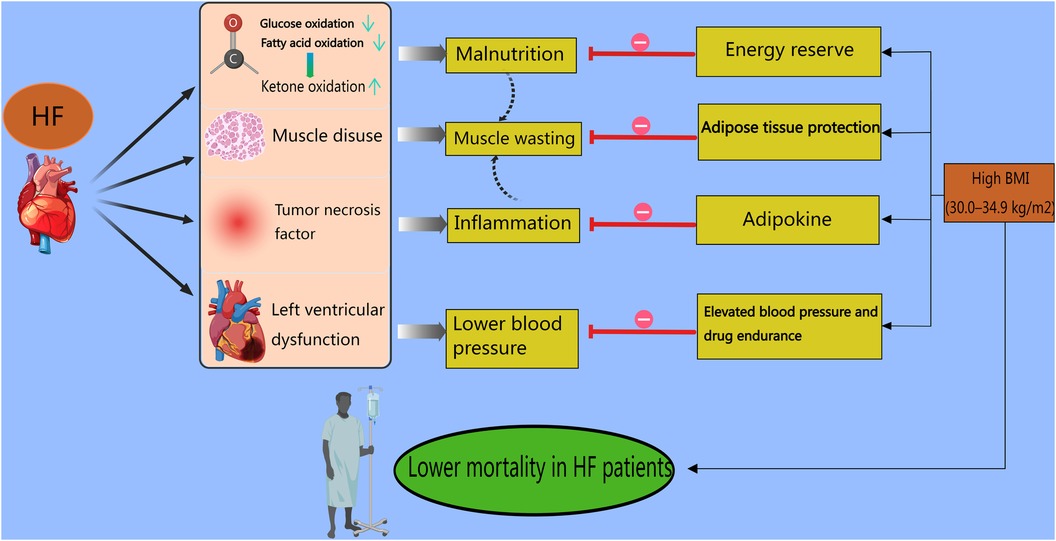
Figure 2 Obesity paradox in heart failure patients. In HF patients, first, fatty acid oxidation and glucose oxidation are suppressed, leading the bodies to switch to ketone fuel utilization in an energy-starved state. Second, HF patients with a deficiency of left ventricular function usually experience lower blood pressure. Third, HF patients generate tumor necrosis factor, which causes inflammation in the myocardium. Finally, muscle disuse, malnutrition, neurohumoral factor activation, and HF medications contribute to muscle wasting. The above-mentioned factors result in higher mortality rates. However, for higher-BMI (30.0–34.9 kg/m2) patients with HF, stored adipose tissue provides abundant energy to avoid malnutrition. In addition, obese patients present with elevated blood pressure and spare more space for drugs to target HF. Also, molecules and adipokines, provided by increased levels of cholesterol and serum lipids or hyperlipidemia, prevent the inflammatory response. Moreover, adipose tissue protects muscle. All these points counteract the damage caused by lower BMI in HF patients and result in higher mortality rates.
Overall, several indices can be used to predict HF, which was previously diagnosed using BNP and NT-proBNP, with positive feedback from clinicians. Gradually, physicians have attached immense importance to the TyG index, an indicator of IR, to comprehensively estimate the incidence or mortality of CVD and the progression of CAD. With the prevalent administration of SGLT2is in HF treatment, the TyG index, which includes glucose and triglycerides, shows a strong association with therapeutic effects as a non-invasive and convenient indicator. However, further studies should elucidate the underlying biological mechanism of the TyG index in different HF types. Given that the TyG index is a useful predictor and tool for stratifying the risk for stroke, fatty liver, and acute kidney injury, more studies are warranted to explore the associations of the TyG index with other targeted organs with or without HF. In addition, large cohort studies covering the general population are required to validate previously published findings. Current studies rarely consider lifestyle factors (smoking, drinking, and diet), medication treatment, and many other confounding factors, indicating the need for a full-scale study design.
YF: Writing – original draft, Writing – review & editing. JS: Writing – review & editing. LL: Writing – review & editing.
The authors declare financial support was received for the research, authorship, and/or publication of this article.
This study was supported by the Zhejiang Medical Association Clinical Medicine Special Fund project (2023ZYC-Z29).
The figures were provided by Medpeer.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. (2022) 145(18):e895–1032. doi: 10.1161/CIR.0000000000001063
3. Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats AJS. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. (2023) 118(17):3272–87. doi: 10.1093/cvr/cvac013
4. Wang H, Chai K, Du M, Wang S, Cai J-P, Li Y, et al. Prevalence and incidence of heart failure among urban patients in China: a national population-based analysis. Circ Heart Fail. (2021) 14(10):e008406. doi: 10.1161/CIRCHEARTFAILURE.121.008406
5. Van Nuys KE, Xie Z, Tysinger B, Hlatky MA, Goldman DP. Innovation in heart failure treatment: life expectancy, disability, and health disparities. JACC Heart Fail. (2018) 6(5):401–9. doi: 10.1016/j.jchf.2017.12.006
6. Tsutsui H, Albert NM, Coats AJS, Anker SD, Bayes-Genis A, Butler J, et al. Natriuretic peptides: role in the diagnosis and management of heart failure: a scientific statement from the Heart Failure Association of the European Society of Cardiology, Heart Failure Society of America and Japanese Heart Failure Society. Eur J Heart Fail. (2023) 25(5):616–31. doi: 10.1002/ejhf.2848
7. Molvin J, Jujic A, Bachus E, Gallo W, Tasevska-Dinevska G, Holm H, et al. Cardiovascular biomarkers predict post-discharge re-hospitalization risk and mortality among Swedish heart failure patients. ESC Heart Fail. (2019) 6(5):992–9. doi: 10.1002/ehf2.12486
8. Yang Z, Gong H, Kan F, Ji N. Association between the triglyceride glucose (TyG) index and the risk of acute kidney injury in critically ill patients with heart failure: analysis of the MIMIC-IV database. Cardiovasc Diabetol. (2023) 22(1):232. doi: 10.1186/s12933-023-01971-9
9. Ding X, Wang X, Wu J, Zhang M, Cui M. Triglyceride–glucose index and the incidence of atherosclerotic cardiovascular diseases: a meta-analysis of cohort studies. Cardiovasc Diabetol. (2021) 20(1):76. doi: 10.1186/s12933-021-01268-9
10. Boshen Y, Yuankang Z, Xinjie Z, Taixi L, Kaifan N, Zhixiang W, et al. Triglyceride-glucose index is associated with the occurrence and prognosis of cardiac arrest: a multicenter retrospective observational study. Cardiovasc Diabetol. (2023) 22(1):190. doi: 10.1186/s12933-023-01918-0
11. Duran Karaduman B, Ayhan H, Keleş T, Bozkurt E. The triglyceride-glucose index predicts peripheral artery disease complexity. Turk J Med Sci. (2020) 50(5):1217–22. doi: 10.3906/sag-2006-180
12. Zhou Q, Yang J, Tang H, Guo Z, Dong W, Wang Y, et al. High triglyceride-glucose (TyG) index is associated with poor prognosis of heart failure with preserved ejection fraction. Cardiovasc Diabetol. (2023) 22(1):263. doi: 10.1186/s12933-023-02001-4
13. Zhang Q, Xiao S, Jiao X, Shen Y. The triglyceride-glucose index is a predictor for cardiovascular and all-cause mortality in CVD patients with diabetes or pre-diabetes: evidence from NHANES 2001–2018. Cardiovasc Diabetol. (2023) 22(1):279. doi: 10.1186/s12933-023-02030-z
14. Wang T, Xu J, Zhang H, Tao L, Huang X. Triglyceride-glucose index for the detection of subclinical heart failure with preserved ejection fraction in patients with type 2 diabetes. Front Cardiovasc Med. (2023) 10:1086978. doi: 10.3389/fcvm.2023.1086978
15. Jankauskas SS, Mone P, Avvisato R, Varzideh F, De Gennaro S, Salemme L, et al. miR-181c targets Parkin and SMAD7 in human cardiac fibroblasts: validation of differential microRNA expression in patients with diabetes and heart failure with preserved ejection fraction. Mech Ageing Dev. (2023) 212:111818. doi: 10.1016/j.mad.2023.111818
16. Gudenkauf B, Shaya G, Mukherjee M, Michos ED, Madrazo J, Mathews L, et al. Insulin resistance is associated with subclinical myocardial dysfunction and reduced functional capacity in heart failure with preserved ejection fraction. J Cardiol. (2024) 83(2):100–4. doi: 10.1016/j.jjcc.2023.06.008
17. Wenzl FA, Ambrosini S, Mohammed SA, Kraler S, Lüscher TF, Costantino S, et al. Inflammation in metabolic cardiomyopathy. Front Cardiovasc Med. (2021) 8:742178. doi: 10.3389/fcvm.2021.742178
18. Pan X, Yue L, Ren L, Ban J, Chen S. Triglyceride-glucose index and cervical vascular function: outpatient-based cohort study. BMC Endocr Disord. (2023) 23(1):191. doi: 10.1186/s12902-023-01449-5
19. Li Y, You A, Tomlinson B, Yue L, Zhao K, Fan H, et al. Insulin resistance surrogates predict hypertension plus hyperuricemia. J Diabetes Investig. (2021) 12(11):2046–53. doi: 10.1111/jdi.13573
20. Palmer SC, Tendal B, Mustafa RA, Vandvik PO, Li S, Hao Q, et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. Br Med J. (2021) 372:m4573. doi: 10.1136/bmj.m4573
21. Tahapary DL, Pratisthita LB, Fitri NA, Marcella C, Wafa S, Kurniawan F, et al. Challenges in the diagnosis of insulin resistance: focusing on the role of HOMA-IR and tryglyceride/glucose index. Diabetes Metab Syndr. (2022) 16(8):102581. doi: 10.1016/j.dsx.2022.102581
22. Luo P, Cao Y, Li P, Li W, Song Z, Fu Z, et al. TyG index performs better than HOMA-IR in Chinese type 2 diabetes mellitus with a BMI <35kg/m2: a hyperglycemic clamp validated study. Medicina (Kaunas). (2022) 58(7):876. doi: 10.3390/medicina58070876
23. Wang S, Shi J, Peng Y, Fang Q, Mu Q, Gu W, et al. Stronger association of triglyceride glucose index than the HOMA-IR with arterial stiffness in patients with type 2 diabetes: a real-world single-centre study. Cardiovasc Diabetol. (2021) 20(1):82. doi: 10.1186/s12933-021-01274-x
24. Park HM, Lee HS, Lee Y-J, Lee J-H. The triglyceride–glucose index is a more powerful surrogate marker for predicting the prevalence and incidence of type 2 diabetes mellitus than the homeostatic model assessment of insulin resistance. Diabetes Res Clin Pract. (2021) 180:109042. doi: 10.1016/j.diabres.2021.109042
25. Son D-H, Lee HS, Lee Y-J, Lee J-H, Han J-H. Comparison of triglyceride-glucose index and HOMA-IR for predicting prevalence and incidence of metabolic syndrome. Nutr Metab Cardiovasc Dis. (2022) 32(3):596–604. doi: 10.1016/j.numecd.2021.11.017
26. Zeng X, Han D, Zhou H, Xue Y, Wang X, Zhan Q, et al. Triglyceride-glucose index and homeostasis model assessment-insulin resistance in young adulthood and risk of incident congestive heart failure in midlife: the coronary artery risk development in young adults study. Front Cardiovasc Med. (2022) 9:944258. doi: 10.3389/fcvm.2022.944258
27. Wang M, Mei L, Jin A, Cai X, Jing J, Wang S, et al. Association between triglyceride glucose index and atherosclerotic plaques and burden: findings from a community-based study. Cardiovasc Diabetol. (2022) 21(1):204. doi: 10.1186/s12933-022-01638-x
28. Mone P, De Gennaro S, Moriello D, Frullone S, D'Amelio R, Ferrante MNV, et al. Insulin resistance drives cognitive impairment in hypertensive pre-diabetic frail elders: the CENTENNIAL study. Eur J Prev Cardiol. (2023) 30(12):1283–8. doi: 10.1093/eurjpc/zwad173
29. Santulli G, Visco V, Ciccarelli M, Ferrante MNV, De Masi P, Pansini A, et al. Frail hypertensive older adults with prediabetes and chronic kidney disease: insights on organ damage and cognitive performance—preliminary results from the CARYATID study. Cardiovasc Diabetol. (2024) 23(1):125. doi: 10.1186/s12933-024-02218-x
30. Wei B, Dong Q, Ma J, Zhang A. The association between triglyceride-glucose index and cognitive function in nondiabetic elderly: NHANES 2011–2014. Lipids Health Dis. (2023) 22(1):188. doi: 10.1186/s12944-023-01959-0
31. Liu N, Luo X, Li P, Xiong W. The triglycerides and glucose index is not superior to HOMA-IR in predicting testosterone deficiency among adult males. Andrology. (2023) 11(2):215–24. doi: 10.1111/andr.13207
32. Wen J, Pan Q, Du L-L, Song J-J, Liu Y-P, Meng X-B, et al. Association of triglyceride-glucose index with atherosclerotic cardiovascular disease and mortality among familial hypercholesterolemia patients. Diabetol Metab Syndr. (2023) 15(1):39. doi: 10.1186/s13098-023-01009-w
33. Zhou Y, Wang C, Che H, Cheng L, Zhu D, Rao C, et al. Association between the triglyceride–glucose index and the risk of mortality among patients with chronic heart failure: results from a retrospective cohort study in China. Cardiovasc Diabetol. (2023) 22(1):171. doi: 10.1186/s12933-023-01895-4
34. Cai W, Xu J, Wu X, Chen Z, Zeng L, Song X, et al. Association between triglyceride-glucose index and all-cause mortality in critically ill patients with ischemic stroke: analysis of the MIMIC-IV database. Cardiovasc Diabetol. (2023) 22(1):138. doi: 10.1186/s12933-023-01864-x
35. Liao Y, Zhang R, Shi S, Zhao Y, He Y, Liao L, et al. Triglyceride-glucose index linked to all-cause mortality in critically ill patients: a cohort of 3026 patients. Cardiovasc Diabetol. (2022) 21(1):128. doi: 10.1186/s12933-022-01563-z
36. Zhang R, Shi S, Chen W, Wang Y, Lin X, Zhao Y, et al. Independent effects of the triglyceride-glucose index on all-cause mortality in critically ill patients with coronary heart disease: analysis of the MIMIC-III database. Cardiovasc Diabetol. (2023) 22(1):10. doi: 10.1186/s12933-023-01737-3
37. Chen J, Wu K, Lin Y, Huang M, Xie S. Association of triglyceride glucose index with all-cause and cardiovascular mortality in the general population. Cardiovasc Diabetol. (2023) 22(1):320. doi: 10.1186/s12933-023-02054-5
38. Yang Y, Huang X, Wang Y, Leng L, Xu J, Feng L, et al. The impact of triglyceride-glucose index on ischemic stroke: a systematic review and meta-analysis. Cardiovasc Diabetol. (2023) 22(1):2. doi: 10.1186/s12933-022-01732-0
39. Li Y, Liu Y, Liu S, Gao M, Wang W, Chen K, et al. Diabetic vascular diseases: molecular mechanisms and therapeutic strategies. Signal Transduct Target Ther. (2023) 8(1):152. doi: 10.1038/s41392-023-01400-z
40. D'Elia L, Masulli M, Virdis A, Casiglia E, Tikhonoff V, Angeli F, et al. Triglyceride-glucose index and mortality in a large regional-based Italian database (URRAH project). J Clin Endocrinol Metab. (2024):1–8. doi: 10.1210/clinem/dgae170
41. Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. (2018) 17(1):122. doi: 10.1186/s12933-018-0762-4
42. Liu X, Tan Z, Huang Y, Zhao H, Liu M, Yu P, et al. Relationship between the triglyceride-glucose index and risk of cardiovascular diseases and mortality in the general population: a systematic review and meta-analysis. Cardiovasc Diabetol. (2022) 21(1):124. doi: 10.1186/s12933-022-01546-0
43. Myhre PL, Vaduganathan M, Claggett BL, Miao ZM, Jhund PS, de Boer RA, et al. Influence of NT-proBNP on efficacy of dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. JACC Heart Fail. (2022) 10(12):902–13. doi: 10.1016/j.jchf.2022.08.007
44. Ciardullo S, Rea F, Cannistraci R, Muraca E, Perra S, Zerbini F, et al. NT-ProBNP and mortality across the spectrum of glucose tolerance in the general US population. Cardiovasc Diabetol. (2022) 21(1):236. doi: 10.1186/s12933-022-01671-w
45. Ezekowitz JA, O'Connor CM, Troughton RW, Alemayehu WG, Westerhout CM, Voors AA, et al. N-terminal pro-B-type natriuretic peptide and clinical outcomes. JACC: Heart Failure. (2020) 8(11):931–9. doi: 10.1016/j.jchf.2020.08.008
46. Armstrong PW, Zheng Y, Troughton RW, Lund LH, Zhang J, Lam CSP, et al. Sequential evaluation of NT-proBNP in heart failure: insights into clinical outcomes and efficacy of vericiguat. JACC Heart Fail. (2022) 10(9):677–88. doi: 10.1016/j.jchf.2022.04.015
47. Cunningham JW, Myhre PL. NT-proBNP response to heart failure therapies. J Am Coll Cardiol. (2021) 78(13):1333–6. doi: 10.1016/j.jacc.2021.07.045
48. Spoto S, Argemi J, Di Costanzo R, Gavira Gomez JJ, Salterain Gonzales N, Basili S, et al. Mid-regional pro-adrenomedullin and N-terminal pro-B-type natriuretic peptide measurement: a multimarker approach to diagnosis and prognosis in acute heart failure. J Pers Med. (2023) 13(7):1155. doi: 10.3390/jpm13071155
49. Klip IT, Voors AA, Anker SD, Hillege HL, Struck J, Squire I, et al. Prognostic value of mid-regional pro-adrenomedullin in patients with heart failure after an acute myocardial infarction. Heart. (2011) 97(11):892–8. doi: 10.1136/hrt.2010.210948
50. Fraty M, Velho G, Gand E, Fumeron F, Ragot S, Sosner P, et al. Prognostic value of plasma MR-proADM vs NT-proBNP for heart failure in people with type 2 diabetes: the SURDIAGENE prospective study. Diabetologia. (2018) 61(12):2643–53. doi: 10.1007/s00125-018-4727-7
51. Andrés C, Andaluz-Ojeda D, Cicuendez R, Nogales L, Martín S, Martin-Fernandez M, et al. MR-proADM to detect specific types of organ failure in infection. Eur J Clin Invest. (2020) 50(6):e13246. doi: 10.1111/eci.13246
52. Špinarová M, Špinar J, Špinarová L, Krejčí J, Goldbergová-Pávková M, Pařenica J, et al. Relation between mid-regional pro-adrenomedullin in patients with chronic heart failure and the dose of diuretics in 2-year follow-up-data from FAR NHL registry. Medicina (Kaunas). (2022) 58(10):1477. doi: 10.3390/medicina58101477
53. Huang Z, Zhong J, Ling Y, Zhang Y, Lin W, Tang L, et al. Diagnostic value of novel biomarkers for heart failure: a meta-analysis. Herz. (2020) 45(1):65–78. doi: 10.1007/s00059-018-4702-6
54. Han S, Wang C, Tong F, Li Y, Li Z, Sun Z, et al. Triglyceride glucose index and its combination with the get with the guidelines-heart failure score in predicting the prognosis in patients with heart failure. Front Nutr. (2022) 9:950338. doi: 10.3389/fnut.2022.950338
55. Cheng H, Huang W, Huang X, Miao W, Huang Y, Hu Y. The triglyceride glucose index predicts short-term mortality in non-diabetic patients with acute heart failure. Adv Clin Exp Med. (2024) 33(2):103–10. doi: 10.17219/acem/166043
56. Özcan KS, Hayıroğlu MI, Çınar T. Admission triglyceride-glucose index is predictor of long-term mortality and appropriate implantable cardiac defibrillator therapy in patients with heart failure. Biomark Med. (2023) 17(10):487–96. doi: 10.2217/bmm-2023-0113
57. Sun T, Huang X, Zhang B, Ma M, Chen Z, Zhao Z, et al. Prognostic significance of the triglyceride-glucose index for patients with ischemic heart failure after percutaneous coronary intervention. Front Endocrinol (Lausanne). (2023) 14:1100399. doi: 10.3389/fendo.2023.1100399
58. Guo W, Zhao L, Mo F, Peng C, Li L, Xu Y, et al. The prognostic value of the triglyceride glucose index in patients with chronic heart failure and type 2 diabetes: a retrospective cohort study. Diabetes Res Clin Pract. (2021) 177:108786. doi: 10.1016/j.diabres.2021.108786
59. Zheng H, Chen G, Wu K, Wu W, Huang Z, Wang X, et al. Relationship between cumulative exposure to triglyceride-glucose index and heart failure: a prospective cohort study. Cardiovasc Diabetol. (2023) 22(1):239. doi: 10.1186/s12933-023-01967-5
60. Li X, Wang J, Niu L, Tan Z, Ma J, He L, et al. Prevalence estimates of the insulin resistance and associated prevalence of heart failure among united Status adults. BMC Cardiovasc Disord. (2023) 23(1):294. doi: 10.1186/s12872-023-03294-9
61. Hao Q, Yuanyuan Z, Lijuan C. The prognostic value of the triglyceride glucose Index in patients with acute myocardial infarction. J Cardiovasc Pharmacol Ther. (2023) 28:10742484231181846. doi: 10.1177/10742484231181846
62. Huang H, Li Q, Liu J, Qiao L, Chen S, Lai W, et al. Association between triglyceride glucose index and worsening heart failure in significant secondary mitral regurgitation following percutaneous coronary intervention. Cardiovasc Diabetol. (2022) 21(1):260. doi: 10.1186/s12933-022-01680-9
63. Li X, Chan JSK, Guan B, Peng S, Wu X, Lu X, et al. Triglyceride-glucose index and the risk of heart failure: evidence from two large cohorts and a Mendelian randomization analysis. Cardiovasc Diabetol. (2022) 21(1):229. doi: 10.1186/s12933-022-01658-7
64. Zhao Y, Sun H, Zhang W, Xi Y, Shi X, Yang Y, et al. Elevated triglyceride-glucose index predicts risk of incident ischaemic stroke: the rural Chinese cohort study. Diabetes Metab. (2021) 47(4):101246. doi: 10.1016/j.diabet.2021.101246
65. Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-reduced and DAPA-HF trials. Lancet. (2020) 396(10254):819–29. doi: 10.1016/S0140-6736(20)31824-9
66. Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. (2022) 387(12):1089–98. doi: 10.1056/NEJMoa2206286
67. Packer M, Butler J, Zannad F, Filippatos G, Ferreira JP, Pocock SJ, et al. Effect of empagliflozin on worsening heart failure events in patients with heart failure and preserved ejection fraction: EMPEROR-preserved trial. Circulation. (2021) 144(16):1284–94. doi: 10.1161/CIRCULATIONAHA.121.056824
68. Filippatos G, Butler J, Farmakis D, Zannad F, Ofstad AP, Ferreira JP, et al. Empagliflozin for heart failure with preserved left ventricular ejection fraction with and without diabetes. Circulation. (2022) 146(9):676–86. doi: 10.1161/CIRCULATIONAHA.122.059785
69. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. (2021) 385(16):1451–61. doi: 10.1056/NEJMoa2107038
70. Butler J, Filippatos G, Siddiqi TJ, Ferreira JP, Brueckmann M, Bocchi E, et al. Effects of empagliflozin in women and men with heart failure and preserved ejection fraction. Circulation. (2022) 146(14):1046–55. doi: 10.1161/CIRCULATIONAHA.122.059755
71. Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. (2020) 383(15):1425–35. doi: 10.1056/NEJMoa2004967
72. Zhu Y, Zhang J-L, Yan X-J, Sun L, Ji Y, Wang F-F. Effect of dapagliflozin on the prognosis of patients with acute myocardial infarction undergoing percutaneous coronary intervention. Cardiovasc Diabetol. (2022) 21(1):186. doi: 10.1186/s12933-022-01627-0
73. Imre E, Gunhan HG, Erel P, Ustay O. SGLT2 inhibitors improve plasma atherogenic biomarkers in patients with type 2 diabetes: a real-world retrospective observational study. Minerva Endocrinol (Torino). (2023) 48(3):295–304. doi: 10.23736/S2724-6507.21.03465-5
74. Radellini S, Vigneri E, Guarnotta V, Panto F, Giordano C. One year of dapaglifozin add-on therapy ameliorates surrogate indexes of insulin resistance and adiposity in patients with type 2 diabetes mellitus. Diabetes Ther. (2021) 12(6):1677–88. doi: 10.1007/s13300-021-01056-4
75. Sattar N, Lee MMY, Kristensen SL, Branch KRH, Del Prato S, Khurmi NS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. (2021) 9(10):653–62. doi: 10.1016/S2213-8587(21)00203-5
76. Zhao H, Liu Y, Liu M, Xu Y, Ling Q, Lin W, et al. Clinical outcomes with GLP-1 receptor agonists in patients with heart failure: a systematic review and meta-analysis of randomized controlled trials. Drugs. (2023) 83(14):1293–307. doi: 10.1007/s40265-023-01932-2
77. Wright AK, Carr MJ, Kontopantelis E, Leelarathna L, Thabit H, Emsley R, et al. Primary prevention of cardiovascular and heart failure events with SGLT2 inhibitors, GLP-1 receptor agonists, and their combination in type 2 diabetes. Diabetes Care. (2022) 45(4):909–18. doi: 10.2337/dc21-1113
78. Ussher JR, Drucker DJ. Glucagon-like peptide 1 receptor agonists: cardiovascular benefits and mechanisms of action. Nat Rev Cardiol. (2023) 20(7):463–74. doi: 10.1038/s41569-023-00849-3
79. Giugliano D, Longo M, Signoriello S, Maiorino MI, Solerte B, Chiodini P, et al. The effect of DPP-4 inhibitors, GLP-1 receptor agonists and SGLT-2 inhibitors on cardiorenal outcomes: a network meta-analysis of 23 CVOTs. Cardiovasc Diabetol. (2022) 21(1):42. doi: 10.1186/s12933-022-01474-z
80. Merza N, Akram M, Mengal A, Rashid AM, Mahboob A, Faryad M, et al. The safety and efficacy of GLP-1 receptor agonists in heart failure patients: a systematic review and meta-analysis. Curr Probl Cardiol. (2023) 48(5):101602. doi: 10.1016/j.cpcardiol.2023.101602
81. Miao H, Zhou Z, Yang S, Zhang Y. The association of triglyceride-glucose index and related parameters with hypertension and cardiovascular risk: a cross-sectional study. Hypertens Res. (2023) 47(4):877–86. doi: 10.1038/s41440-023-01502-9
82. Yang S, Shi X, Liu W, Wang Z, Li R, Xu X, et al. Association between triglyceride glucose-body mass index and heart failure in subjects with diabetes mellitus or prediabetes mellitus: a cross-sectional study. Front Endocrinol (Lausanne). (2023) 14:1294909. doi: 10.3389/fendo.2023.1294909
83. Dang K, Wang X, Hu J, Zhang Y, Cheng L, Qi X, et al. The association between triglyceride-glucose index and its combination with obesity indicators and cardiovascular disease: NHANES 2003–2018. Cardiovasc Diabetol. (2024) 23(1):8. doi: 10.1186/s12933-023-02115-9
84. Dou J, Guo C, Wang Y, Peng Z, Wu R, Li Q, et al. Association between triglyceride glucose-body mass and one-year all-cause mortality of patients with heart failure: a retrospective study utilizing the MIMIC-IV database. Cardiovasc Diabetol. (2023) 22(1):309. doi: 10.1186/s12933-023-02047-4
85. Chen N, Xu Y, Xu C, Duan J, Zhou Y, Jin M, et al. Effects of triglyceride glucose (TyG) and TyG-body mass index on sex-based differences in the early-onset heart failure of ST-elevation myocardial infarction. Nutr Metab Cardiovasc Dis. (2023) 34(3):590–7. doi: 10.1016/j.numecd.2023.09.027
86. Padwal R, McAlister FA, McMurray JJV, Cowie MR, Rich M, Pocock S, et al. The obesity paradox in heart failure patients with preserved versus reduced ejection fraction: a meta-analysis of individual patient data. Int J Obes (Lond). (2014) 38(8):1110–4. doi: 10.1038/ijo.2013.203
87. Selvaraj S, Kelly DP, Margulies KB. Implications of altered ketone metabolism and therapeutic ketosis in heart failure. Circulation. (2020) 141(22):1800–12. doi: 10.1161/CIRCULATIONAHA.119.045033
88. Arundel C, Lam PH, Gill GS, Patel S, Panjrath G, Faselis C, et al. Systolic blood pressure and outcomes in patients with heart failure with reduced ejection fraction. J Am Coll Cardiol. (2019) 73(24):3054–63. doi: 10.1016/j.jacc.2019.04.022
89. Femminò S, Pagliaro P, Penna C. Obesity and cardioprotection. Curr Med Chem. (2020) 27(2):230–9. doi: 10.2174/0929867326666190325094453
90. Izumiya Y. Heart failure medication and muscle wasting. Circ J. (2023) 88(1):20–1. doi: 10.1253/circj.CJ-23-0774
91. Goossens C, Weckx R, Derde S, Dufour T, Vander Perre S, Pauwels L, et al. Adipose tissue protects against sepsis-induced muscle weakness in mice: from lipolysis to ketones. Crit Care. (2019) 23(1):236. doi: 10.1186/s13054-019-2506-6
92. von Haehling S, Garfias Macedo T, Valentova M, Anker MS, Ebner N, Bekfani T, et al. Muscle wasting as an independent predictor of survival in patients with chronic heart failure. J Cachexia Sarcopenia Muscle. (2020) 11(5):1242–9. doi: 10.1002/jcsm.12603
93. Ohori K, Yano T, Katano S, Kouzu H, Honma S, Shimomura K, et al. High percent body fat mass predicts lower risk of cardiac events in patients with heart failure: an explanation of the obesity paradox. BMC Geriatr. (2021) 21(1):16. doi: 10.1186/s12877-020-01950-9
94. Benn M, Marott SCW, Tybjærg-Hansen A, Nordestgaard BG. Obesity increases heart failure incidence and mortality: observational and Mendelian randomization studies totalling over 1 million individuals. Cardiovasc Res. (2023) 118(18):3576–85. doi: 10.1093/cvr/cvab368
95. Marcks N, Aimo A, Januzzi JL, Vergaro G, Clerico A, Latini R, et al. Re-appraisal of the obesity paradox in heart failure: a meta-analysis of individual data. Clin Res Cardiol. (2021) 110(8):1280–91. doi: 10.1007/s00392-021-01822-1
Keywords: TyG index, heart failure, obesity paradox, SGLT-2 inhibitors, HOMA-IR
Citation: Fang Y, Shen J and Lyu L (2024) Value of the triglyceride–glucose index and related parameters in heart failure patients. Front. Cardiovasc. Med. 11: 1397907. doi: 10.3389/fcvm.2024.1397907
Received: 8 March 2024; Accepted: 27 June 2024;
Published: 18 July 2024.
Edited by:
Cristiano Amarelli, Azienda dei Colli, ItalyReviewed by:
Aneesh Dhore-Patil, Houston Methodist Hospital, United States© 2024 Fang, Shen and Lyu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lingchun Lyu, bHZsaW5nY2h1bkBtZWRtYWlsLmNvbS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.