- 1Department of Public Health, Federico II University, Naples, Italy
- 2Centro Interdipartimentale di Ricerca in Ipertensione Arteriosa e Patologie Associate, Federico II University of Naples, Naples, Italy
- 3Department of Advanced Biomedical Sciences, Federico II University, Naples, Italy
- 4CEINGE - Biotecnologie Avanzate, Naples, Italy
- 5Department of Clinical Medicine and Surgery, Federico II University, Naples, Italy
Fabry disease (FD), also known as Anderson-Fabry disease, is a hereditary disorder of glycosphingolipid metabolism, caused by a deficiency of the lysosomal alpha-galactosidase A enzyme. This causes a progressive accumulation of glycosphingolipids in tissues and organs which represents the main pathogenetic mechanism of FD. The disease is progressive and multisystemic and is characterized by early symptoms and late complications (renal, cardiac and neurological dysfunction). Fatigue and exercise intolerance are early common symptoms in FD patients but the specific causes are still to be defined. In this narrative review, we deal with the contribution of cardiac and pulmonary dysfunctions in determining fatigue and exercise intolerance in FD patients.
1 Introduction
Fabry disease (FD) is a rare and progressive genetic disorder characterized by the deficiency of α-galactosidase A enzyme, leading to the accumulation of glycosphingolipids in various tissues and organs. This multisystemic condition causes symptoms in many districts, including the skin, kidney, heart, and neurological system (1–5).
FD patients frequently experience fatigue, and numerous studies demonstrate that FD subjects have lower exercise tolerance than healthy controls, especially patients with peripheral neurological abnormalities (6). However, the precise causes of fatigue in FD and how it affects the effectiveness of exercise are still unknown (6).
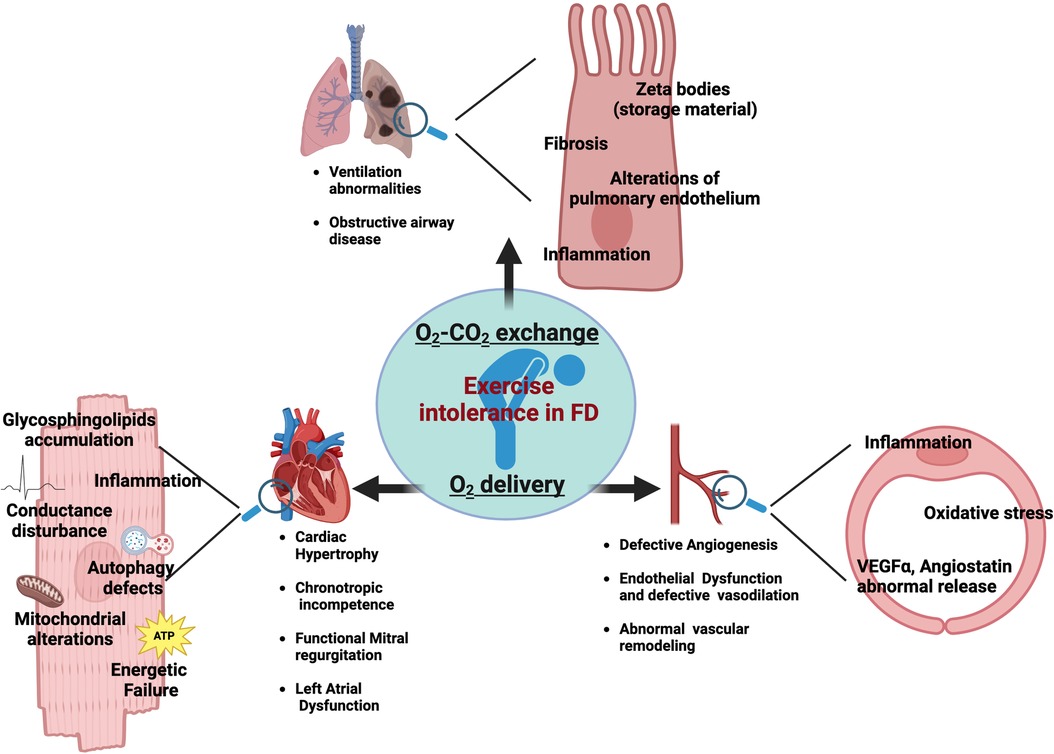
Fatigue is a complex phenomenon resulting from internal homeostasis breakdown in response to increased energetic demand by external stimuli. The mechanisms of fatigue are multifactorial as it can be influenced by a great variety of aspects. Some of these factors are modifiable, including lifestyle, while others are nonmodifiable, such as genetics, and sex. Indeed, fatigue susceptibility impacts men and women differently (7). In FD population, a wide spectrum of factors could synergically contribute to chronic fatigue. Primary muscle and metabolic alterations play an essential role in Fabry-related fatigue, alongside cardiac and respiratory dysfunctions which also have a strong impact on exercise tolerance and fatigability of FD patients. The detrimental impact of chronic fatigue on patients' quality of life highlights the need for further research to understand the underlying mechanisms and their synergic effects. In this issue, we specifically focus on the contribution of cardiac and pulmonary abnormalities in determining fatigue and exercise intolerance in FD patients (Figure 1). By a critical revision of the literature, we provide useful insights to diagnose and promptly manage fatigue in FD patients.
2 Exercise intolerance and fatigability in FD patients
An adequate exercise capacity is a complex response that requires the optimal interaction among heart, lung, vasculature, and skeletal muscle. Specifically, during physical effort the orchestration of the following processes is needed: (i) an adequate exchange of O2 and CO2 through pulmonary ventilation; (ii) an optimal function of the heart and vascular system to supply oxygenated blood at a sufficient flow rate to meet the metabolic demands of working muscles; (iii) efficient O2 diffusion, nutrients extraction and utilization in skeletal muscle (8). Alterations in any of these critical steps contribute to exercise intolerance and susceptibility to fatigue.
Fatigue and pain especially during effort is an emerging hallmark of FD (9). Commonly, this phenotype has an early onset and can precede FD-related organ damage (kidney, heart, lung) suggesting primary abnormalities of the motor system. However, the cardiovascular and pulmonary alterations also can contribute to exercise intolerance and fatigability in FD, especially in the late stage of the disease requiring specific prevention, management, and treatments. Indeed, although the exact underlying mechanisms are still unknown, it is certain that the reduced exercise capacity in FD is multifactorial and the specific vascular, cardiac and lung alterations described in FD patients could be potential contributors.
In the sections below we annotated the main cardiac and pulmonary morpho-functional alterations which can potentially contribute to exercise capacity limitation in FD.
2.1 Vascular determinants
Peripheral vascular function, especially endothelial homeostasis, is a key determinant of O2 delivery to muscle during exercise. Indeed, the impairment of endothelial dependent-vasodilation in response to aging and chronic illness is responsible for a reduction in systemic O2 delivery aggravating fatigue and dyspnea (10). Vascular tree alterations have been extensively described in FD patients. Specifically, endothelial and smooth muscle cells are among the main target of Gb3 storage (11), and drive the vascular manifestations in FD patients, including the basilar artery remodeling, increased intima-media thickness and decreased brachial flow–mediated dilation (12, 13). The vascular dysfunction strongly contributes to organ damage in FD exasperating kidney disease, cardiomyopathy, cerebral lesions and likely exercise capacity of skeletal muscle (9, 12, 14, 15). Systemic inflammation, oxidative stress and the abnormal release of angiogenic factors, including VEGFα and angiostatin, are considered the underling mechanisms of endothelial dysfunction in FD (16–18). Data about a direct correlation among endothelial function and exercise tolerance in FD patients are missing. However, already in the early stage of the disease, histological examination shows a significant presence in muscle vessels of glycosphingolipid accumulation (19). On this ground, we cannot exclude that the altered vessel homeostasis could affect O2 delivery, exacerbating muscle incompliance to physical effort. In this scenario, the well-established beneficial effects of physical activity on vessel homeostasis (20) further support the potential therapeutic power of an adapted physical activity program for FD patients.
2.2 Cardiac determinants
In health subjects, the increase of venous return during exercise is matched by an increased cardiac output through elevation in HR, contractility, and lusitropy and not in cardiac filling pressures (21). Specifically, the increased contractility in combination with vasodilation determines an enhanced end-diastolic volume and reduced end-systolic volume guaranteeing the match between systemic perfusion and muscle metabolic needs (8). An insufficient increase in cardiac output during effort leads to lactic acidosis and muscular fatigue limiting exercise and functional capacity (21, 22). In FD patients, cardiac alterations are not the exclusive but key determinants of exercise intolerance (8). More than 50% of FD patients show cardiac involvement including left ventricular hypertrophy, heart failure with preserved ejection fraction, chest pain, and arrhythmias (23, 24). FD register shows that cardiac damage is the first cause of death in FD patients and despite the late clinical onset, cardiac alterations start early in the life and progress sub-clinically (25). The buildup of glycosphingolipids and Gb3 occurs in all cardiac cell types including myocytes, endothelial and smooth muscle cells of intramyocardial vessels, endocardium, valvular fibroblasts, and conduction tissue probably culminating in inflammation, necrosis, fibrosis, and hypertrophic myocardial disarray (26, 27). However, Gb3 accumulation in the heart is per se not sufficient to explain the whole spectrum of cardiac manifestations and it has emerging the hypothesis that the primary enzymatic defect in FD triggers other processes that result in biochemical and functional alterations including autophagy alterations, mitochondrial defects, and energetic failure (25, 28). The energy depletion may activate pro-hypertrophic pathways, common to other hypertrophic cardiomyopathies, and may affect cardiac responsiveness to stress. Accordingly, the increase of energetic demand during physical effort represents a stress condition revealing the unsuitable energetic metabolism of the FD heart.
Chronotropic incompetence is another cardiac symptom frequently recorded in the FD population that could contribute to the exercise intolerance of FD patients. In healthy humans, during aerobic exercise, the VO2 increases approximately 4-fold, mainly through a significant increase in heart rate (2.2-fold) (29). Chronotropic incompetence, broadly defined as the inability of the heart to increase its rate in response to increased demand, is therefore, among the primary contributors of exercise intolerance (30). Approximately, 18%–20% of FD patients show chronotropic incompetence and/or sinus node dysfunction or severe atrioventricular block (31). In a cohort of 38 Australian patients with FD, 70% of subjects had resting bradycardia, with impaired ability to increase heart rate during exercise (32). Electrical alterations and conduction impairment could be involved in this phenomenon. Specifically, it has been proposed that glycosphingolipids accumulation may alter ion channel expression and/or cell membrane trafficking, affecting the electrical properties of cardiac cells (33). It should not be excluded that also energetic depletion can affect the functionality of ATP-dependent pumps, ionic homeostasis maintenance, and conductance capacity (34).
Functional mitral regurgitation as well is a key determinant of exercise intolerance in the general population. Despite the valve abnormalities are not the major limitations for cardiac function in the FD population, mild left ventricular valve regurgitations are commonly reported in FD patients (35). Indeed, the postmortem examination of Fabry hearts revealed that the greatest concentrations of glycosphingolipids were in the mitral valve (36). The most recent study revealed that in classic- FD, the prevalence of valvular disease, from moderate to severe, was 10%, with mitral and tricuspid regurgitation being the most common (37, 38). Beyond the glycosphingolipids accumulation specifically at valvular levels, other phenomenon could be involved in FD valve dysfunction, including thickening of the sub-valvular apparatus (35) geometric distortion of the atria, valvular annulus, or aortic root dilatation (37).
Emerging evidence implicates left atrial dysfunction as an important pathophysiologic mechanism of exercise intolerance (39). Specifically, it has been reported that LA stiffness is independently associated with impaired exercise tolerance and quality of life and may be an important therapeutic target in patients with heart failure with preserved ejection fraction. In FD population, studies of speckle-tracking echocardiography reveal that the left atrial reservoir, conduit, and contractile functions are all affected (40). Also, cardiac MRI studies show an impairment of left atrial function and morphological parameters already in the early stage of the disease (41), when effort tolerance is affected as well.
2.3 Lung determinants
While the main determinant in exercise tolerance is considered the heart, it is essential to recognize that limitation in lung function also contributes to the overall exercise capacity. Aerobic exercise increases oxygen and ventilation demands, leading to rapid and deep breathing, which can cause airway smooth muscle stretch, bronchodilation, and airway caliber maintenance (42). The multiorgan compromission in FD also includes the lung. Specifically, the pulmonary involvement in FD emerges as alterations of functional parameters, including the increase in resting dead space or ventilation abnormalities (43). Symptoms like coughing, wheezing, and shortness of breath are commonly reported in FD population, however, they could be also influenced by external factors such as smoking habits and age (44–46). Obstructive airway disease has been observed in a range of 27%–36% of FD patients across various cohorts, which is a higher prevalence compared to the general population (47).
The underlying mechanisms responsible for pulmonary function decrease in FD are still not fully elucidated. One hypothesis suggests that the accumulation of sphingolipids in the lung tissue, which in turn triggers an inflammatory response, may be responsible for mechanical damage and small airway disease (48). Rather, recent evidence suggests that pulmonary involvement in FD is indirect, linked to the lipid deposits occurring in vascular endothelium and bronchial smooth muscle, with subsequent obstruction of small airways. Also, in this case, the inflammatory process triggered by sphingolipids could serve as a crucial mechanism for mediating obstructive events (49). Even, Svensson et al. posited that the glycosphingolipids accumulation could activate a maladaptive remodeling of the bronchial tree with interstitial fibrosis and chronic airway limitation (27). Specifically, the obstructive events observed in FD patients could stem from either airway constriction due to smooth muscle hyperplasia or the accumulation of glycosphingolipids directly within bronchial cells (50, 51).
The electron microscopy analysis of sputum and lung biopsy samples from FD patients, revealed the presence of “myeloid-like” inclusions within ciliated cells (52). Additionally, lamellar inclusion bodies known as “Zebra bodies” were detected within the cytoplasm of ciliated bronchial epithelial cells (53). These inclusions were also found in bronchiolar/arteriolar smooth muscle cells and endothelium (54).
Overall, the pulmonary involvement in FD includes micro and macro-structural alterations producing a complex dysfunctional phenotype which still needs further research to be better characterized, as well as, the precise mechanisms responsible for lung function decline need to be delineated. Moreover, poor data are available on the effects of therapies on lung phenotype. Such studies including the report by Brier et al., showed an improvement in pulmonary function with ERT treatment (55), and the same results have also been shown in case reports with more critical situations (56).
2.4 The use of CPET to assess cardiopulmonary involvement in FD
The assessment of exercise tolerance is crucial to establish the overall health and fitness level of a subject. Cardiopulmonary exercise testing (CPET) is a comprehensive diagnostic test used to assess the integrated function of cardiovascular and respiratory systems during exercise. It involves incremental exercise, typically on a treadmill or stationary bike, while continuously monitoring various physiological parameters. These parameters include oxygen consumption, heart rate, blood pressure, ventilation, gas exchange (oxygen and carbon dioxide levels), and other relevant data (57–60). By measuring and integrating these data at different levels of physical effort, CPET provides valuable insights into an individual's exercise capacity, cardiorespiratory fitness, and any abnormalities or limitations in the cardiopulmonary system (61–64). Specifically, CPET provides parameters like heart rate, blood pressure, and cardiac output, allowing to identify alterations in anaerobic threshold and cardiac limitations. Contextually, Pulmonary limitations are detected through the analysis of oxygen uptake, ventilation, and gas exchange parameters, unveiling conditions like COPD or interstitial lung disease. Muscular fatigue could also be assessed by monitoring exercise capacity and an early onset of anaerobic metabolism. Overall, The CPET provides joint data analysis that allows complete assessment of the cardiovascular, respiratory, muscular and metabolic systems during exertion (57).
Therefore, the CPET is a useful tool to assess the impairment of cardiopulmonary homeostasis and function in FD patients. Specifically, the first consideration is that reduced exercise tolerance and fatigue are not only detectable in male patients where the clinical signs of FD are obvious (renal and cardiac dysfunction) but also in heterozygous female patients. Hence, CPET could unveil early and preclinical signs of cardiopulmonary dysfunction. For instance, Wang et al. investigated women with FD and recorded a reduced quality of life alongside fatigue in 58.5% and exercise intolerance in 82.5% of the participants. From this study, it emerges that a decrease in diastolic blood pressure greater than or equal to 10 mmHg was associated with exercise intolerance, while reduced maximal oxygen consumption correlated with fatigue. Moreover, during the stress test, the exercise intolerance reflected the decrease in maximal heart rate (65). A similar phenomenon was also described by Bierer et al. Their research revealed that approximately 46% of individuals with FD experienced a notable decline in diastolic blood pressure during exercise, especially among female patients (66).
To understand the relative involvement of heart and lung in reduced exercise tolerance, Spinelli et al. examined 16 patients with FD compared to control subjects, performing a radionuclide myocardial perfusion at rest and during exercise, tissue Doppler echocardiography, and magnetic resonance imaging (MRI) at rest. The participants were divided into two groups, according to their left ventricular mass and renal function parameters. The study revealed that patients with more severe organ damage exhibited abnormal stroke volume response to exercise, characterized by decreased end-diastolic volume and not reduced end-systolic volume. Moreover, compared with controls, FD patients had elevated plasma levels of NT-proBNP (a marker of cardiac stress), higher indexed left ventricular mass (LVMi), and altered parameters at tissue Doppler echocardiography, suggesting an advanced diastolic dysfunction. Overall, the study indicated that left ventricular hypertrophy and interstitial fibrosis play a significant role in affecting stroke volume response during exercise in FD, highlighting the impact of cardiac determinants in exercise intolerance of FD population (67).
The incompetence of the heart in supporting exercise-induced stress also emerged from the study by Réant and colleagues. The authors showed that FD subjects reach a lower mean peak oxygen consumption (VO2) and a higher VE/VCO2 slope compared to the general population, again confirming the reduction in cardiac output at peak exercise (68). Accordingly, Powell et al., by using CPET, showed a lower increase in heart rate at peak exercise in FD patients, alongside reduced indexed maximum oxygen consumption and indexed oxygen peak, with both maximal and submaximal testing criteria. The Authors also suggest the involvement of pulmonary circulation since a positive correlation between functional capacity and right ventricular volumes at cMRI was found. Indeed, although static imaging revealed normal systolic function, the impaired right ventricular stroke volume seemed to affect oxygen consumption (VO2) during exercise (69).
Overall, these findings indicate that CPET is a valuable tool to unveil cardiopulmonary alterations in FD even in the early stage of the disease, when the damage to cardiopulmonary systems is not yet full-blown or bland, for instance in women. The main results of all the studies evaluating exercise tolerance in Fabry patients are summarized in Table 1.
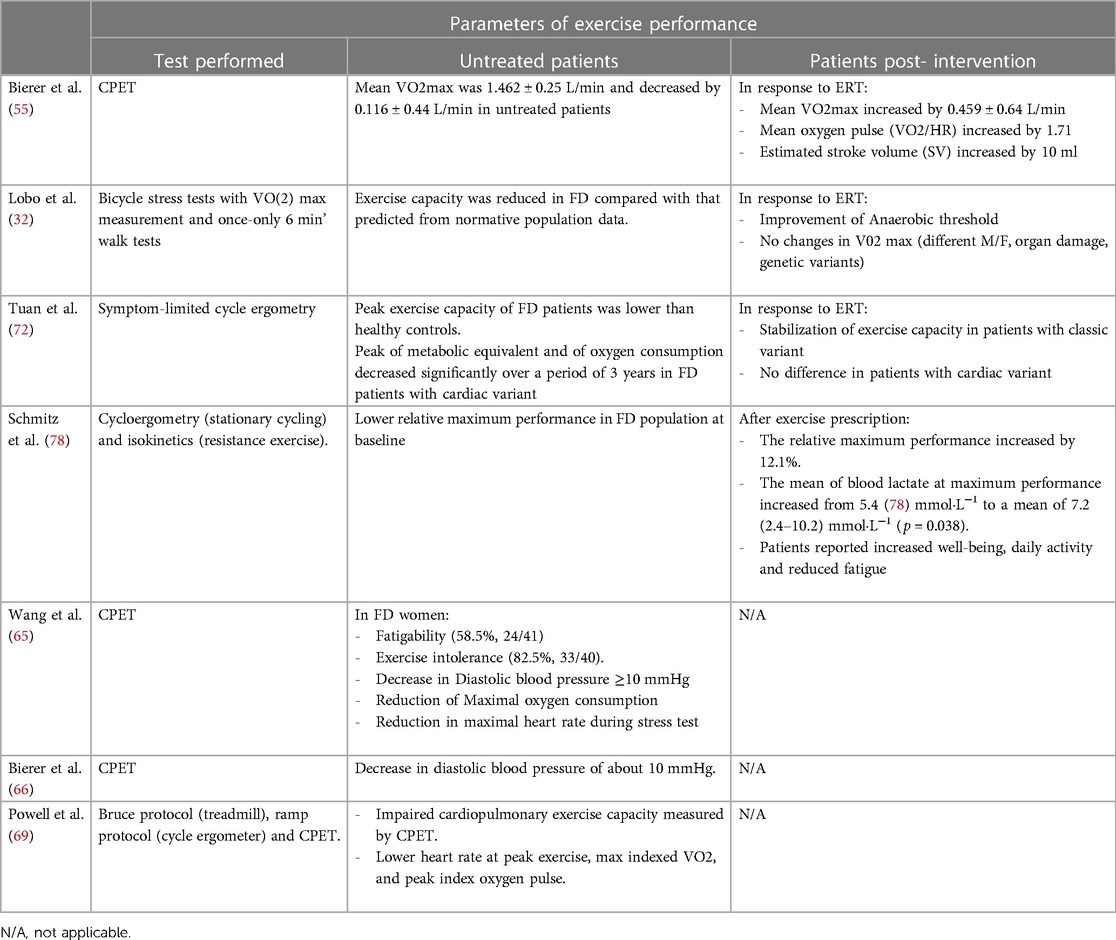
Table 1. Alterations of exercise performance parameters in FD patients and impact of interventions including ERT and exercise prescription.
2.5 Therapeutic strategies for impaired exercise tolerance and fatigue in FD
Enzyme Replacement Therapy has proven to be a life-changing treatment for Fabry disease, significantly improving the prognosis and quality of life for affected individuals. It addresses the underlying enzyme deficiency, slows disease progression, and mitigates symptoms and complications associated with the condition (1, 23, 70, 71). However, the impact of ERT on exercise tolerance in FD patients has been poorly addressed, and the few available data are controversial. Such studies suggest that ERT may positively influence the exercise capacity and cardiopulmonary performance of individuals with Fabry disease, in particular increasing the V02/HR ratio during physical activity (55). Conversely, from other studies, only a modest improvement in the anaerobic threshold emerges for FD patients under ERT, while the V02 max did not change at all (32). The conflicting results could be explained by differences among the study populations (male vs. female) and/or the entity of organ damage, and genetic variants. In a recent report, Tuan et al. assessed the peak exercise capacity of patients with classic vs. cardiac FD variant. The study revealed that patients with cardiac variant experienced a decrease in peak exercise capacity over time, while patients with classic variant-FD showed no significant changes in exercise capacity during the same period. Moreover, ERT appears to have potential benefits in stabilizing exercise capacity in patients with the classic variant and not for subjects with the cardiac variant (72). These findings confirm that the heart plays a critical role in determining exercise capacity in individuals with FD, and that the cardiac variant may have a more profound impact on the exercise tolerance of FD patients and on their response to ERT.
Non-conventional therapeutic strategies should also be employed to manage exercise intolerance and fatigability in FD patients. Exercise tolerance can be trained by exercise prescription. Indeed, exercise therapy is a widely recognized and evidence-based therapeutic approach that utilizes physical activities and exercises to prevent, manage, and rehabilitate various medical conditions. It plays a pivotal role in improving physical function, reducing pain, and enhancing overall well-being for individuals of all ages and fitness levels. It is utilized in various medical settings, including hospitals, clinics, and outpatient facilities, to optimize physical health and improve the quality of life for patients. Exercise therapy also plays a crucial role in managing chronic diseases such as diabetes (73), heart disease (74, 75), and chronic obstructive pulmonary disease (COPD) (76). Regular physical activity has been shown to improve symptoms, control disease progression, and enhance the overall quality of life for patients with these conditions (77). Only one pilot study evaluated the possibility of prescribing exercise in patients with FD. Over one year, the patients underwent an exercise protocol, and 58% of them reported decreased fatigability. Moreover, the study showed that physical performance improved among the patients, with an approximate load increase of 12% (78). This improvement in physical performance suggests that exercise therapy can be employed to enhance functional capacity and overall physical well-being in individuals with FD. However, further studies are needed to support the exercise training of FD patients and also to design a specific and adapted exercise program for this condition. The effects of intervention (ERT or training) on exercise tolerance in FD patients are reported in Table 1.
3 Conclusions
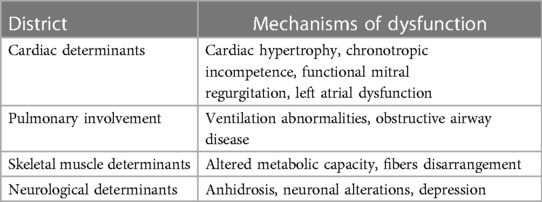
Exercise intolerance emerges as a phenotypic hallmark of FD. Even with different extensions, reduced exercise capacity, and fatigue are observed in FD patients with classic and cardiac variants as well, in males and females, in the presence or not of full-blown target organ damage. The exercise capacity is the result of a systemic engagement of different districts including heart, lung, vasculature, and skeletal muscle (Table 2). Likely, the alterations of their synergic work and dynamism occur early, preceding the single target organ abnormalities. Hence, the assessment of exercise capacity could be a precious tool to reveal the early signs of dysfunctions. The current challenge in FD management is the detection of the “silent alerts” which can guarantee a tempestive therapeutic decision, especially in female patients who are undertreated. In this scenario, we propose to employ the evaluation of exercise tolerance by CPET in the routine diagnostic and monitoring process of FD patients, including females. Another important gap that the research should overcome is the study of the effects of available therapies on exercise tolerance, even because fitness capacity is a key aspect of patient quality of life. Moreover, in the current report, we also point out the lack of data on the effects of exercise training in FD population. Hence, we underline the urgent need of research studies specifically focused on potential therapeutic effects of an adapted physical activity program in FD patients.
Author contributions
OD: Writing – review & editing, Writing – original draft, Conceptualization. JG: Writing – review & editing, Writing – original draft, Conceptualization. AB: Writing – original draft. AF: Writing – original draft. FC: Writing – original draft. AB: Writing – original draft. RA: Writing – original draft. IC: Writing – original draft. MA: Writing – original draft. TD: Writing – original draft. ER: Writing – original draft. LS: Writing – original draft. AP: Writing – original draft. GI: Writing – review & editing, Writing – original draft. DS: Writing – review & editing, Writing – original draft, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
DS is supported by Finanziamento della Ricerca di Ateneo of Federico II University (FRA 54 2020). JG is supported by PON “REACT-EU” IV.4 action 2014–2020. GI is supported by PRIN (2017HTKLRF), from the Italian Ministry of Research, and by co-funding from Next Generation EU, National Recovery and Resilience Plan, Investment PE8—Project Age-It: “Ageing Well in an Ageing Society”. In addition, GI is funded by Italian Ministry of Health, HubLife Science - Digital Health (LSH-DH), PNC-E3-2022-23683267 - DHEAL-COM.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pisani A, Visciano B, Roux GD, Sabbatini M, Porto C, Parenti G, et al. Enzyme replacement therapy in patients with Fabry disease: state of the art and review of the literature. Mol Genet Metab. (2012) 107:267–75. doi: 10.1016/j.ymgme.2012.08.003
2. Zarate YA, Hopkin RJ. Fabry’s disease. Lancet. (2008) 372:1427–35. doi: 10.1016/S0140-6736(08)61589-5
3. Di Risi T, Cuomo M, Vinciguerra R, Ferraro S, Della Monica R, Costabile D, et al. Methylome profiling in Fabry disease in clinical practice: a proof of concept. Int J Mol Sci. (2022) 23(20):12110. doi: 10.3390/ijms232012110
5. Germain DP, Altarescu G, Barriales-Villa R, Mignani R, Pawlaczyk K, Pieruzzi F, et al. An expert consensus on practical clinical recommendations and guidance for patients with classic Fabry disease. Mol Genet Metab. (2022) 137:49–61. doi: 10.1016/j.ymgme.2022.07.010
6. Ivleva A, Weith E, Mehta A, Hughes DA. The influence of patient-reported joint manifestations on quality of life in Fabry patients. JIMD Rep. (2018) 41:37–45. doi: 10.1007/8904_2017_84
7. Tornero-Aguilera JF, Jimenez-Morcillo J, Rubio-Zarapuz A, Clemente-Suárez VJ. Central and peripheral fatigue in physical exercise explained: a narrative review. Int J Environ Res Public Health. (2022) 19(7):3909. doi: 10.3390/ijerph19073909
8. Del Buono MG, Arena R, Borlaug BA, Carbone S, Canada JM, Kirkman DL, et al. Exercise intolerance in patients with heart failure: JACC state-of-the-art review. J Am Coll Cardiol. (2019) 73:2209–25. doi: 10.1016/j.jacc.2019.01.072
9. Gambardella J, Fiordelisi A, Cerasuolo FA, Buonaiuto A, Avvisato R, Viti A, et al. Experimental evidence and clinical implications of Warburg effect in the skeletal muscle of Fabry disease. iScience. (2023) 26:106074. doi: 10.1016/j.isci.2023.106074
10. Upadhya B, Haykowsky MJ, Eggebeen J, Kitzman DW. Exercise intolerance in heart failure with preserved ejection fraction: more than a heart problem. J Geriatr Cardiol. (2015) 12(3):294–304. doi: 10.11909/j.issn.1671-5411.2015.03.013
11. Park JL, Whitesall SE, D'Alecy LG, Shu L, Shayman JA. Vascular dysfunction in the alpha-galactosidase A-knockout mouse is an endothelial cell-, plasma membrane-based defect. Clin Exp Pharmacol Physiol. (2008) 35:1156–63. doi: 10.1111/j.1440-1681.2008.04984.x
12. Rombach SM, van den Bogaard B, de Groot E, Groener JE, Poorthuis BJ, Linthorst GE, et al. Vascular aspects of Fabry disease in relation to clinical manifestations and elevations in plasma globotriaosylsphingosine. Hypertension. (2012) 60:998–1005. doi: 10.1161/HYPERTENSIONAHA.112.195685
13. Manara R, Carlier RY, Righetto S, Citton V, Locatelli G, Colas F, et al. Basilar artery changes in Fabry disease. AJNR Am J Neuroradiol. (2017) 38:531–6. doi: 10.3174/ajnr.A5069
14. Kalliokoski RJ, Kalliokoski KK, Penttinen M, Kantola I, Leino A, Viikari JS, et al. Structural and functional changes in peripheral vasculature of Fabry patients. J Inherit Metab Dis. (2006) 29:660–6. doi: 10.1007/s10545-006-0340-x
15. Rombach SM, Twickler TB, Aerts JM, Linthorst GE, Wijburg FA, Hollak CE. Vasculopathy in patients with Fabry disease: current controversies and research directions. Mol Genet Metab. (2010) 99:99–108. doi: 10.1016/j.ymgme.2009.10.004
16. Bergmann M, Holz F, Kopitz J. Lysosomal stress and lipid peroxidation products induce VEGF-121 and VEGF-165 expression in ARPE-19 cells. Graefes Arch Clin Exp Ophthalmol. (2011) 249:1477–83. doi: 10.1007/s00417-011-1682-0
17. Shen JS, Meng XL, Moore DF, Quirk JM, Shayman JA, Schiffmann R, et al. Globotriaosylceramide induces oxidative stress and up-regulates cell adhesion molecule expression in Fabry disease endothelial cells. Mol Genet Metab. (2008) 95:163–8. doi: 10.1016/j.ymgme.2008.06.016
18. Namdar M, Gebhard C, Studiger R, Shi Y, Mocharla P, Schmied C, et al. Globotriaosylsphingosine accumulation and not alpha-galactosidase-A deficiency causes endothelial dysfunction in Fabry disease. PLoS One. (2012) 7:e36373. doi: 10.1371/journal.pone.0036373
19. Chimenti C, Padua L, Pazzaglia C, Morgante E, Centurion C, Antuzzi D, et al. Cardiac and skeletal myopathy in Fabry disease: a clinicopathologic correlative study. Hum Pathol. (2012) 43:1444–52. doi: 10.1016/j.humpath.2011.09.020
20. Konigstein K, Dipla K, Zafeiridis A. Training the vessels: molecular and clinical effects of exercise on vascular health-A narrative review. Cells. (2023) 12(21):2544. doi: 10.3390/cells12212544
21. Borlaug BA. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction. Circ J. (2014) 78:20–32. doi: 10.1253/circj.CJ-13-1103
22. Borlaug BA, Kane GC, Melenovsky V, Olson TP. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J. (2016) 37:3293–302. doi: 10.1093/eurheartj/ehw241
23. Ortiz A, Germain DP, Desnick RJ, Politei J, Mauer M, Burlina A, et al. Fabry disease revisited: management and treatment recommendations for adult patients. Mol Genet Metab. (2018) 123:416–27. doi: 10.1016/j.ymgme.2018.02.014
24. Ortiz A, Abiose A, Bichet DG, Cabrera G, Charrow J, Germain DP, et al. Time to treatment benefit for adult patients with Fabry disease receiving agalsidase beta: data from the Fabry registry. J Med Genet. (2016) 53:495–502. doi: 10.1136/jmedgenet-2015-103486
25. Pieroni M, Moon JC, Arbustini E, Barriales-Villa R, Camporeale A, Vujkovac AC, et al. Cardiac involvement in Fabry disease: JACC review topic of the week. J Am Coll Cardiol. (2021) 77:922–36. doi: 10.1016/j.jacc.2020.12.024
26. Messalli G, Imbriaco M, Avitabile G, Russo R, Iodice D, Spinelli L, et al. Role of cardiac MRI in evaluating patients with Anderson-Fabry disease: assessing cardiac effects of long-term enzyme replacement therapy. Radiol Med. (2012) 117:19–28. doi: 10.1007/s11547-011-0710-9
27. Azevedo O, Cordeiro F, Gago MF, Miltenberger-Miltenyi G, Ferreira C, Sousa N, et al. Fabry disease and the heart: a comprehensive review. Int J Mol Sci. (2021) 22(9):4434. doi: 10.3390/ijms22094434
28. Gambardella J, Riccio E, Bianco A, Fiordelisi A, Cerasuolo FA, Buonaiuto A, et al. Fatigue as hallmark of Fabry disease: role of bioenergetic alterations. Front Cardiovasc Med. (2024) 11:1341590. doi: 10.3389/fcvm.2024.1341590
29. Higginbotham MB, Morris KG, Williams RS, McHale PA, Coleman RE, Cobb FR. Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circ Res. (1986) 58:281–91. doi: 10.1161/01.RES.58.2.281
30. Brubaker PH, Kitzman DW. Chronotropic incompetence: causes, consequences, and management. Circulation. (2011) 123:1010–20. doi: 10.1161/CIRCULATIONAHA.110.940577
31. Hagege A, Reant P, Habib G, Damy T, Barone-Rochette G, Soulat G, et al. Fabry disease in cardiology practice: literature review and expert point of view. Arch Cardiovasc Dis. (2019) 112:278–87. doi: 10.1016/j.acvd.2019.01.002
32. Lobo T, Morgan J, Bjorksten A, Nicholls K, Grigg L, Centra E, et al. Cardiovascular testing in Fabry disease: exercise capacity reduction, chronotropic incompetence and improved anaerobic threshold after enzyme replacement. Intern Med J. (2008) 38:407–14. doi: 10.1111/j.1445-5994.2008.01669.x
33. Weissman D, Dudek J, Sequeira V, Maack C. Fabry disease: cardiac implications and molecular mechanisms. Curr Heart Fail Rep. (2024) 21(2):81–100. doi: 10.1007/s11897-024-00645-1
34. Singh S, Schwarz K, Horowitz J, Frenneaux M. Cardiac energetic impairment in heart disease and the potential role of metabolic modulators: a review for clinicians. Circ Cardiovasc Genet. (2014) 7:720–8. doi: 10.1161/CIRCGENETICS.114.000221
35. Weidemann F, Strotmann JM, Niemann M, Herrmann S, Wilke M, Beer M, et al. Heart valve involvement in Fabry cardiomyopathy. Ultrasound Med Biol. (2009) 35:730–5. doi: 10.1016/j.ultrasmedbio.2008.10.010
36. Desnick RJ, Blieden LC, Sharp HL, Hofschire PJ, Moller JH. Cardiac valvular anomalies in Fabry disease. Clinical, morphologic, and biochemical studies. Circulation. (1976) 54:818–25. doi: 10.1161/01.CIR.54.5.818
37. Yogasundaram H, Nikhanj A, Chatur S, Qi A, Hagen L, Bailey L, et al. Burden of valvular heart disease in patients with Fabry disease. J Am Soc Echocardiogr. (2022) 35:236–8. doi: 10.1016/j.echo.2021.09.013
38. Averbuch T, White JA, Fine NM. Anderson-Fabry disease cardiomyopathy: an update on epidemiology, diagnostic approach, management and monitoring strategies. Front Cardiovasc Med. (2023) 10:1152568. doi: 10.3389/fcvm.2023.1152568
39. Singleton MJ, Nelson MB, Samuel TJ, Kitzman DW, Brubaker P, Haykowsky MJ, et al. Left atrial stiffness index independently predicts exercise intolerance and quality of life in older, obese patients with heart failure with preserved ejection fraction. J Card Fail. (2022) 28:567–75. doi: 10.1016/j.cardfail.2021.10.010
40. Pichette M, Serri K, Page M, Di LZ, Bichet DG, Poulin F. Impaired left atrial function in Fabry disease: a longitudinal speckle-tracking echocardiography study. J Am Soc Echocardiogr. (2017) 30:170–9.e2. doi: 10.1016/j.echo.2016.10.014
41. Halfmann MC, Altmann S, Schoepf UJ, Reichardt C, Hennermann JB, Kreitner KF, et al. Left atrial strain correlates with severity of cardiac involvement in Anderson-Fabry disease. Eur Radiol. (2023) 33:2039–51. doi: 10.1007/s00330-022-09183-7
42. Lang JE. The impact of exercise on asthma. Curr Opin Allergy Clin Immunol. (2019) 19:118–25. doi: 10.1097/ACI.0000000000000510
43. Jezela-Stanek A. Pulmonary involvement in selected lysosomal storage diseases and the impact of enzyme replacement therapy: a state-of-the art review. Clin Respir J. (2020) 14(5):422–9. doi: 10.1111/crj.13150
44. Rosenberg DM, Ferrans VJ, Fulmer JD, Line BR, Barranger JA, Brady RO, et al. Chronic airflow obstruction in Fabry’s disease. Am J Med. (1980) 68:898–905. doi: 10.1016/0002-9343(80)90224-7
45. Brown LK, Miller A, Bhuptani A, Sloane MF, Zimmerman MI, Schilero G, et al. Pulmonary involvement in Fabry disease. Am J Respir Crit Care Med. (1997) 155:1004–10. doi: 10.1164/ajrccm.155.3.9116979
46. Aubert JD, Barbey F. Chapter 27. Pulmonary involvement in Fabry disease. In: Mehta A, Beck M, Sunder-Plassmann G, editors. Fabry Disease: Perspectives from 5 Years of FOS. Oxford: Oxford PharmaGenesis (2006). PMID: 21290686.
47. Faverio P, Stainer A, De Giacomi F, Gasperini S, Motta S, Canonico F, et al. Molecular pathways and respiratory involvement in lysosomal storage diseases. Int J Mol Sci. (2019) 20(2):327. doi: 10.3390/ijms20020327
48. Odler B, Cseh Á, Constantin T, Fekete G, Losonczy G, Tamási L, et al. Long time enzyme replacement therapy stabilizes obstructive lung disease and alters peripheral immune cell subsets in Fabry patients. Clin Respir J. (2017) 11:942–50. doi: 10.1111/crj.12446
49. Rozenfeld P, Feriozzi S. Contribution of inflammatory pathways to Fabry disease pathogenesis. Mol Genet Metab. (2017) 122:19–27. doi: 10.1016/j.ymgme.2017.09.004
50. Svensson CK, Feldt-Rasmussen U, Backer V. Fabry disease, respiratory symptoms, and airway limitation—a systematic review. Eur Clin Respir J. (2015) 2. doi: 10.3402/ecrj.v2.26721
51. Franzen D, Krayenbuehl PA, Lidove O, Aubert JD, Barbey F. Pulmonary involvement in Fabry disease: overview and perspectives. Eur J Intern Med. (2013) 24:707–13. doi: 10.1016/j.ejim.2013.05.003
52. Gaggl M, Kain R, Jaksch P, Haider D, Mundigler G, Voigtländer T, et al. A single lung transplant in a patient with Fabry disease: causality or far-fetched? A case report. Case Rep Transplant. (2013) 2013:905743. doi: 10.1155/2013/905743
53. Kelly MM, Leigh R, McKenzie R, Kamada D, Ramsdale EH, Hargreave FE. Induced sputum examination: diagnosis of pulmonary involvement in Fabry’s disease. Thorax. (2000) 55:720–1. doi: 10.1136/thorax.55.8.720
54. Wang RY, Abe JT, Cohen AH, Wilcox WR. Enzyme replacement therapy stabilizes obstructive pulmonary Fabry disease associated with respiratory globotriaosylceramide storage. J Inherit Metab Dis. (2008) 31(Suppl 2):S369–74. doi: 10.1007/s10545-008-0930-x
55. Bierer G, Balfe D, Wilcox WR, Mosenifar Z. Improvement in serial cardiopulmonary exercise testing following enzyme replacement therapy in Fabry disease. J Inherit Metab Dis. (2006) 29:572–9. doi: 10.1007/s10545-006-0361-5
56. Kim W, Pyeritz RE, Bernhardt BA, Casey M, Litt HI. Pulmonary manifestations of Fabry disease and positive response to enzyme replacement therapy. Am J Med Genet A. (2007) 143:377–81. doi: 10.1002/ajmg.a.31600
57. Herdy AH, Ritt LE, Stein R, Araújo CG, Milani M, Meneghelo RS, et al. Cardiopulmonary exercise test: background, applicability and interpretation. Arq Bras Cardiol. (2016) 107(5):467–81. doi: 10.5935/abc.20160171
59. Tran D. Cardiopulmonary exercise testing. Methods Mol Biol. (2018) 1735:285–95. doi: 10.1007/978-1-4939-7614-0_18
60. de Boer E, Petrache I, Mohning MP. Cardiopulmonary exercise testing. JAMA. (2022) 327:1284–5. doi: 10.1001/jama.2022.2037
61. Magrì D, Santolamazza C. Cardiopulmonary exercise test in hypertrophic cardiomyopathy. Ann Am Thorac Soc. (2017) 14:S102–9. doi: 10.1513/AnnalsATS.201611-884FR
62. Glaab T, Taube C. Practical guide to cardiopulmonary exercise testing in adults. Respir Res. (2022) 23:9. doi: 10.1186/s12931-021-01895-6
63. Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, et al. Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American heart association. Circulation. (2010) 122:191–225. doi: 10.1161/CIR.0b013e3181e52e69
64. Santoro C, Sorrentino R, Esposito R, Lembo M, Capone V, Rozza F, et al. Cardiopulmonary exercise testing and echocardiographic exam: an useful interaction. Cardiovasc Ultrasound. (2019) 17:29. doi: 10.1186/s12947-019-0180-0
65. Wang RY, Lelis A, Mirocha J, Wilcox WR. Heterozygous Fabry women are not just carriers, but have a significant burden of disease and impaired quality of life. Genet Med. (2007) 9:34–45. doi: 10.1097/GIM.0b013e31802d8321
66. Bierer G, Kamangar N, Balfe D, Wilcox WR, Mosenifar Z. Cardiopulmonary exercise testing in Fabry disease. Respiration. (2005) 72:504–11. doi: 10.1159/000087675
67. Spinelli L, Nicolai E, Acampa W, Imbriaco M, Pisani A, Rao MA, et al. Cardiac performance during exercise in patients with Fabry’s disease. Eur J Clin Invest. (2008) 38:910–7. doi: 10.1111/j.1365-2362.2008.02053.x
68. Réant P, Testet E, Reynaud A, Bourque C, Michaud M, Rooryck C, et al. Characterization of Fabry disease cardiac involvement according to longitudinal strain, cardiometabolic exercise test, and T1 mapping. Int J Cardiovasc Imaging. (2020) 36:1333–42. doi: 10.1007/s10554-020-01823-7
69. Powell AW, Jefferies JL, Hopkin RJ, Mays WA, Goa Z, Chin C. Cardiopulmonary fitness assessment on maximal and submaximal exercise testing in patients with Fabry disease. Am J Med Genet A. (2018) 176:1852–7. doi: 10.1002/ajmg.a.40369
70. Azevedo O, Gago MF, Miltenberger-Miltenyi G, Sousa N, Cunha D. Fabry disease therapy: state-of-the-art and current challenges. Int J Mol Sci. (2020) 22(1):206. doi: 10.3390/ijms22010206
71. Germain DP, Elliott PM, Falissard B, Fomin VV, Hilz MJ, Jovanovic A, et al. The effect of enzyme replacement therapy on clinical outcomes in male patients with Fabry disease: a systematic literature review by a European panel of experts. Mol Genet Metab Rep. (2019) 19:100454. doi: 10.1016/j.ymgmr.2019.100454
72. Tuan SH, Chiu PC, Liou IH, Lu WH, Huang HY, Wu SY, et al. Serial analysis of cardiopulmonary fitness and echocardiography in patients with Fabry disease undergoing enzyme replacement therapy. J Rehabil Med Clin Commun. (2020) 3:1000028. doi: 10.2340/20030711-1000028
73. Balducci S, Sacchetti M, Haxhi J, Orlando G, D'Errico V, Fallucca S, et al. Physical exercise as therapy for type 2 diabetes mellitus. Diabetes Metab Res Rev. (2014) 30(Suppl 1):13–23. doi: 10.1002/dmrr.2514
74. Bove AA. Exercise and heart disease. Methodist Debakey Cardiovasc J. (2016) 12:74–5. doi: 10.14797/mdcj-12-2-74
75. Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. American college of sports medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. (2011) 43:1334–59. doi: 10.1249/MSS.0b013e318213fefb
76. Fiorentino G, Esquinas AM, Annunziata A. Exercise and chronic obstructive pulmonary disease (COPD). Adv Exp Med Biol. (2020) 1228:355–68. doi: 10.1007/978-981-15-1792-1_24
77. Thompson PD, Arena R, Riebe D, Pescatello LS. ACSM’s new preparticipation health screening recommendations from ACSM’s guidelines for exercise testing and prescription, ninth edition. Curr Sports Med Rep. (2013) 12:215–7. doi: 10.1249/JSR.0b013e31829a68cf
Keywords: Fabry disease, fatigue, exercise intolerance, cardiac dysfunction, pulmonary dysfunction
Citation: De Marco O, Gambardella J, Bianco A, Fiordelisi A, Cerasuolo FA, Buonaiuto A, Avvisato R, Capuano I, Amicone M, Di Risi T, Riccio E, Spinelli L, Pisani A, Iaccarino G and Sorriento D (2024) Cardiopulmonary determinants of reduced exercise tolerance in Fabry disease. Front. Cardiovasc. Med. 11:1396996. doi: 10.3389/fcvm.2024.1396996
Received: 6 March 2024; Accepted: 10 April 2024;
Published: 2 May 2024.
Edited by:
Michele Ciccarelli, University of Salerno, ItalyReviewed by:
Daniele Masarone, Hospital of the Hills, ItalyAlessandro Maloberti, University of Milano Bicocca, Italy
© 2024 De Marco, Gambardella, Bianco, Fiordelisi, Cerasuolo, Buonaiuto, Avvisato, Capuano, Amicone, Di Risi, Riccio, Spinelli, Pisani, Iaccarino and Sorriento. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniela Sorriento ZGFuaWVsYS5zb3JyaWVudG9AdW5pbmEuaXQ=
 Oriana De Marco
Oriana De Marco Jessica Gambardella
Jessica Gambardella Antonio Bianco
Antonio Bianco Antonella Fiordelisi
Antonella Fiordelisi Federica Andrea Cerasuolo
Federica Andrea Cerasuolo Antonietta Buonaiuto
Antonietta Buonaiuto Roberta Avvisato
Roberta Avvisato Ivana Capuano
Ivana Capuano Maria Amicone
Maria Amicone Teodolinda Di Risi
Teodolinda Di Risi Eleonora Riccio
Eleonora Riccio Letizia Spinelli
Letizia Spinelli Antonio Pisani
Antonio Pisani Guido Iaccarino
Guido Iaccarino Daniela Sorriento
Daniela Sorriento