
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 12 November 2024
Sec. Hypertension
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1391282
 Hongxiang Ji1
Hongxiang Ji1 Hualin Sun2
Hualin Sun2 Yinghui Zhang3
Yinghui Zhang3 Ziyi Zhao4
Ziyi Zhao4 Xin Gao1
Xin Gao1 Chunhe Wang1
Chunhe Wang1 Yang Yang5
Yang Yang5 Xiaodong Zhang5
Xiaodong Zhang5 Jianyong Gao5
Jianyong Gao5 Dequan Man5
Dequan Man5 Qian Yang5
Qian Yang5 Ying Yang5
Ying Yang5 Chengbin Yue5
Chengbin Yue5 Changjiang Chen5
Changjiang Chen5 Xiaoheng Ding4*
Xiaoheng Ding4* Tongshang Ni5,6*
Tongshang Ni5,6*
Aim: To evaluate the real-life effectiveness and safety of hydrogen inhalation (HI) therapy as an additional treatment in Chinese adults with hypertension.
Methods: This observational, retrospective clinical study included hypertensive patients receiving routine antihypertensives with or without HI initiation from 2018 to 2023. Participants were assigned to the HI group or non-HI group (control group) after propensity score matching. The changes in mean systolic blood pressure (SBP) level during the 24-week follow-up period in different groups were examined primarily. The secondary outcome was the changes in diastolic blood pressure (DBP) and blood pressure (BP) control rate during the study. Several subgroup and sensitivity analyses were performed to confirm the robustness of our main findings. Adverse event (AE) was also assessed in patients of both groups.
Results: In total, we selected 2,364 patients into the analysis. Both mean SBP and DBP levels significantly decreased in the HI group compared to control group at each follow-up visit with the between group difference of −4.63 mm Hg (95% CI, −6.51 to −2.74) at week 8, −6.69 mm Hg (95% CI, −8.54 to −4.85) at week 16, −7.81 mm Hg (95% CI, −9.57 to −6.04) at week 24 for SBP, and −1.83 mm Hg (95% CI, −3.21 to −0.45) at week 8, −2.57 mm Hg (95% CI, −3.97 to −1.17) at week 16, −2.89 mm Hg (95% CI, −4.24 to −1.54) at week 24 for DBP. Patients in the HI group were more likely to attain controlled BP at the follow-up period with odds ratio of 1.44 (95% CI, 1.21–1.72) at week 8, 1.90 (95% CI, 1.59–2.27) at week 16, and 2.24 (95% CI, 1.87–2.68) at the end. The trends of subgroup and sensitivity analyses were mostly consistent with the main analysis. The incidences of AEs were similar between the HI group and control group with all p-value >0.05.
Conclusion: The HI therapy is related to significant amelioration in BP levels with acceptable safety profile in Chinese hypertensive adults after 24 weeks of treatment, building a clinical ground for further research to evaluate the antihypertensive effect of HI therapy.
It is estimated that the number of hypertensive patients worldwide will reach 1.56 billion by 2025, and the Chinese morbidity was 23.2%–44.7% in 2015 (1, 2). However, under the current medical care, the hypertension control rates are usually low in populations of low- and middle-income countries, with merely 10% patients having the capacity of gaining controlled blood pressure (BP) (3, 4). Based on a study of 1.7 million adults between 2014 and 2017, hypertensive patients achieving control merely accounted for 7.2% in China (5). Therefore, further developing effective therapy for hypertension is rather essential.
Molecular hydrogen, as an emerging medical gas with comprehensive effects of rapid diffuse, anti-apoptotic, anti-inflammatory, and antioxidant, has shown favorable effects to various diseases (6, 7). Numerous studies have reported that the application of hydrogen is therapeutic for neurodegeneration (8, 9), cerebral infarction (10, 11), chronic obstructive pulmonary disease (12, 13), metabolic syndrome (14, 15), non-alcoholic fatty liver disease (16, 17), cancer (18, 19). Furthermore, hydrogen has demonstrated beneficial effect on cardiovascular system. In animal models, it was reported that 4-week hydrogen inhalation (HI) led to therapeutic effect for hypertensive rats (20). The left ventricular hypertrophy was alleviated by hydrogen-rich saline injection or HI in rats with various hypertension (21, 22). The renal injury was also improved by hydrogen-rich water (HRW) intake in hypertensive rats (23). In clinical studies, the BP control was ameliorated by hydrogen-rich dialysate in patients with chronic dialysis (24). Recently, a randomized, placebo-controlled trial has revealed that maintaining HI therapy 4 h per day for 2 weeks demonstrates marked BP lowering effect in 60 Chinese hypertensive patients (25).
The hydrogen medicine is receiving extensive attention globally, especially in east Asia countries such as China and Japan. Ever-growing number of Chinese patients with metabolic disorders has acknowledged the therapeutic effects of molecular hydrogen. There are substantial Chinese hypertensive patients applying HI therapy in order to improve their BP levels.
Despite randomized controlled trial (RCT) is widely recognized as the golden standard, the relatively small sample size and short follow-up period of previous studies greatly limit their findings to examine the antihypertensive effect of HI treatment. Therefore, we performed a retrospective study to assess whether the 24-week HI therapy is effective and safe as an additional treatment for Chinese patients with hypertension in real life clinical practice.
Herein, a multicenter, retrospective, double-arm study evaluated the effectiveness and safety of HI in Chinese hypertensive patients in real-life setting. This study was performed on the basis of a medical records database which had been previously described in detail (26). In short, the health records database documents and stores clinical data via electronic medical record, which contains basic demographics, vitals, laboratory values, diagnostic reports, pharmacy dispenses, etc., from several hospitals and health examination centers in Qingdao, China. Study numbers were utilized to de-identify eligible participants.
Hypertensive patients who initiated HI therapy or received routine hypotensive treatment for at least 6 months from January 1, 2018 to January 1, 2023 were eligible for this study. Subjects were divided into two groups: the HI group or non-HI group (control group), as per former treatment details. All subjects were treated with routine therapeutic standard according to their attending physicians’ discretion, while patients in the HI group additionally initiated and continuously maintained HI therapy. The initiation of HI therapy was defined as the absence of molecular hydrogen intervention by any means in the previous 52 weeks. Participants’ medical data extracted from electronic medical records and their HI conditions during the study were connected by individualized identification.
The inclusion criteria were: (1) ≥18 years old. (2) BP ≥140/90 mm Hg or receiving antihypertensive treatment simultaneously. (3) Without change in recorded antihypertensive medication during the 6-month follow-up period. (4) ≥1 clinical measurement at baseline period and one of the visit points at follow-up period. (5) Maintaining HI duration ≥15 h per week for subjects in HI group. Patients were excluded if they were: (1) secondary hypertension. (2) Pregnant or breastfeeding female. (3) Serious disorders with a poor prognosis, including advanced renal dysfunction or severe liver dysfunction. (4) Entering hospice at the follow-up period. (5) Concurrently participation in any investigational research demanding interventions apart from routine therapeutic practice.
Since the therapeutic effects of hydrogen therapy are widely recognized by Chinese public, hypertensive patients received HI therapy voluntarily in the specific hydrogen treatment departments. Experienced health professionals of hydrogen medicine provided specialized knowledge (the benefit and mechanism of hydrogen molecule, detailed operation procedures and points for attention of HI, etc.) to each participant, ensuring the valid implementation of hydrogen therapy. Individuals were treated with hydrogen gas therapy at their convenience without specific time point. Health technicians were assigned to monitor the entire process of HI and record personally identifiable information (name, resident identity card number, medical insurance card number, etc.) together with hydrogen gas intake conditions (each duration of HI, flow rate, etc.) for all patients. All health workers and researchers were well trained and tested systematically each year to remain the professionalism. No other intervention (diet suggestion, exercise management, etc.) was provided for individuals receiving HI.
Subjects utilized nasal tube to inhale pure hydrogen gas at the flow rate of 2.0 L/min, which was provided by hydrogen-producing machine (Qingdao Haizhisheng Corp., Ltd., Qingdao, China). Although specialists in hydrogen medicine would make individualized recommendation of HI duration for each patient, the time length of each inhalation and the frequency were based on individual decision, in view of the high safety profile of hydrogen gas (27). The flow rate of 2.0 L/min was chosen as it was most frequently applied by hypertensive subjects, as well as other studies (13, 16, 18, 28–31).
The demographic and clinical data were taken at 4 visit points in 24-week follow-up period: V1, i.e., baseline (prior to index date), V2 (8 ± 2 weeks post index date), V3 (16 ± 2 weeks post index date), V4 (24 ± 2 weeks post index date). The timepoint of HI therapy initiation was defined as index date.
Baseline characteristics including sex, age, body mass index (BMI), heart rate, smoking and drinking status, family history of disease, creatinine, estimated glomerular filtration rate (eGFR), medical comorbidity, and concomitant medications were analyzed at baseline. The concomitant antihypertensives, BP measurements, and application status of HI were assessed at each visit point. BMI was calculated via dividing weight (kg) by height squared (m2). The eGFR was determined by the creatinine equation of Chronic Kidney Disease Epidemiology Collaboration (32). BP was taken by electronic sphygmomanometer in seated patients after ≥5 min of rest. To maintain accuracy, BP response underwent manual inspection if the value was ≥2 standard deviation (SD) from the mean. The average value was applied if BP was measured more than once on the same date, and clinical data most proximal to baseline or each visit point were evaluated.
The incidence of adverse event (AE) including no. of events, no. of patients, and incidence rate during 24-week follow-up period was screened and selected from physicians’ recordings to evaluate the safety outcome of HI.
The primary endpoint of this study was the changes in systolic blood pressure (SBP) levels from baseline to follow-up visits. The secondary endpoint was the changes in diastolic blood pressure (DBP) and BP control rate up to 24 weeks after the index date. The BP control rate was defined as the proportion of patients maintaining BP <140/90 mm Hg.
The Ethics Committee of Hengxing University approved this study. Written informed consents were collected from all the patients. The study was executed in line with the revised Declaration of Helsinki.
Due to the absence of prospective randomization, massive differences of baseline characteristics appeared in subjects between the HI group and control group. Thus, the propensity score matching (PSM) was performed to minimize selective bias and balance the cohorts. We established a logistic regression model according to all baseline variables in Table 1, evaluating the propensity scores of each treatment cohort. PSM was applied with a nearest neighbor 1:1 matched approach (without replacement) and a caliper of 0.10 SD (33).
Continuous data and categorical data were expressed as mean (SD) and frequency (percentage) separately. The changes of clinical values from baseline to different visit point in same group were assessed by paired t-tests. Meanwhile, the differences in changes of clinical parameters between different cohorts at same time point were evaluated by independent t-tests. Odds ratio (OR) with 95% CI was calculated to compare the proportion of patients maintaining controlled BP between two groups. χ2 test was performed to analyze the safety outcome of HI therapy. We performed these analyses on the available-case basis, excluding missing data.
Subgroup analysis was conducted based on several baseline characteristics including age (stratified by <65 and ≥65), sex (male vs. female), BMI (stratified by <25 kg/m2 and ≥25 kg/m2), diabetes mellitus (yes vs. no), SBP (stratified by <140 mm Hg and ≥140 mm Hg), antihypertension medication usage (≤1 vs. >1).
A series of sensitivity analysis were adopted to confirm the robustness of study findings: First, we settled the missing data at each visit point during the follow-up period by using a multiple-imputation method (34) (N = 305 in HI group and 287 in control group). Second, analyses were re-applied in all patients without performance of PSM (N = 1,734 in HI group and 2,392 in control group). Third, we performed a patient selection criterion with less restriction: the inclusion and exclusion criteria remained unchanged with the exception that participants with recording of change in antihypertensives during the study period were allowed (N = 1,773 in HI group and control group after PSM).
It required a sample size of 1,005 participants in each group to demonstrate statistically significant between-cohort difference of 2 mm Hg in SBP reduction with SD of 16.0 mm Hg, power of 80%, and in 0.05 two-sided significant level.
We used SAS 9.2 (SAS Institute) to conduct all analyses, and considered 2-sided P < 0.05 as statistically significant.
Based on the inclusion and exclusion criteria, a total of 4,126 patients were considered eligible for this study. 2,364 participants were selected for final analysis after PSM with 1,182 in HI group and 1,182 in control group (Figure 1). As shown in Table 1, patients’ baseline characteristics were similar between two groups after matching with mean age of 63.71 (11.86) vs. 63.27 (11.40) years and 51.9% vs. 49.3% female, separately. The mean BP was well balanced between HI group and control group with SBP: 144.49 (16.49) vs. 144.39 (17.16) mm Hg; DBP: 90.42 (12.17) vs. 89.58 (12.53) mm Hg; BP control rate: 20.0% vs. 20.6%. Other characteristics, such as BMI, heart rate, serum creatinine, eGFR, concomitant medications, and comorbidities were also comparable between two groups with all p-value >0.05.
Despite the antihypertensives remained invariant for all participants during the study, the alternations of dose were recorded (14.9% in HI group and 21.3% in control group) in 24-week follow-up period.
In HI group, the mean time period of HI per week was 21.3 (2.2) h during the study. Patients inhaled hydrogen gas for 18.6 (1.1) h on average at week 1 and it reached 23.1 (1.6) h at week 24. Time period of HI per week demonstrated an increasing trend throughout the study (Figure 2).
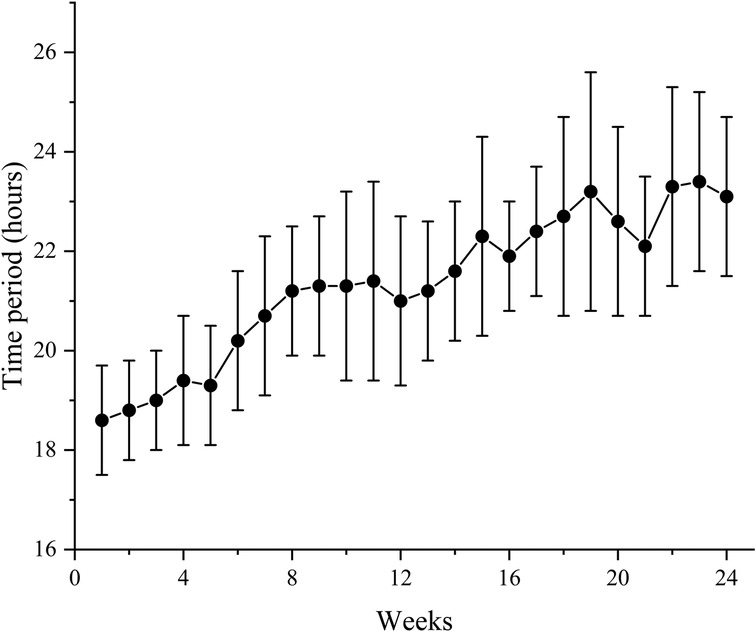
Figure 2. Mean time period of HI per week for all patients in HI group during the study. Data are shown as mean ± SD. HI, hydrogen inhalation.
The SBP levels decreased in HI group (−5.93 mm Hg; 95% CI, −7.30 to −4.57) and control group (−1.31 mm Hg; 95% CI, −2.61 to −0.00) at week 8 with the mean between-group difference of −4.63 mm Hg (95% CI, −6.51 to −2.74). At week 16, the mean SBP had reduced by −9.00 mm Hg (95% CI, −10.30 to −7.71) in HI group and −2.31 mm Hg (95% CI, −3.63 to −0.99) in control group. Meanwhile, the HI therapy demonstrated significantly favorable effect with between group difference of −6.69 mm Hg (95% CI, −8.54 to −4.85). Both the HI therapy and routine hypotensive treatment led to reduction in SBP (−11.54 mm Hg; 95% CI, −12.76 to −10.31 and −3.73 mm Hg; 95% CI, −5.00 to −2.46 respectively) by the end of week 24 and greater reduction was observed in HI group (−7.81 mm Hg; 95% CI, −9.57 to −6.04). The between-group difference in SBP increased during the study (Figures 3A, 4A).
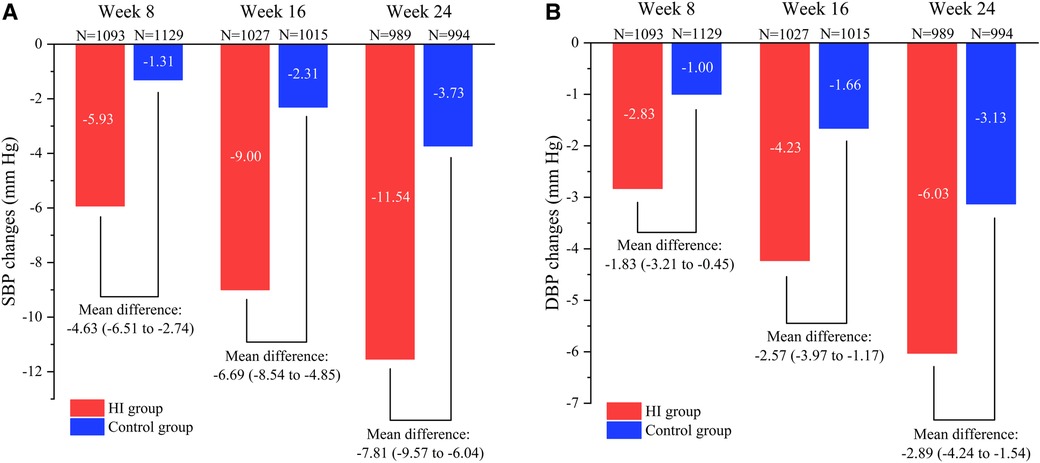
Figure 3. Comparison of reduction in SBP (A) and DBP (B) between HI group and control group from baseline to different visit point during the follow-up period. Data are shown as mean (95% CI). SBP, systolic blood pressure; DBP, diastolic blood pressure; HI, hydrogen inhalation.
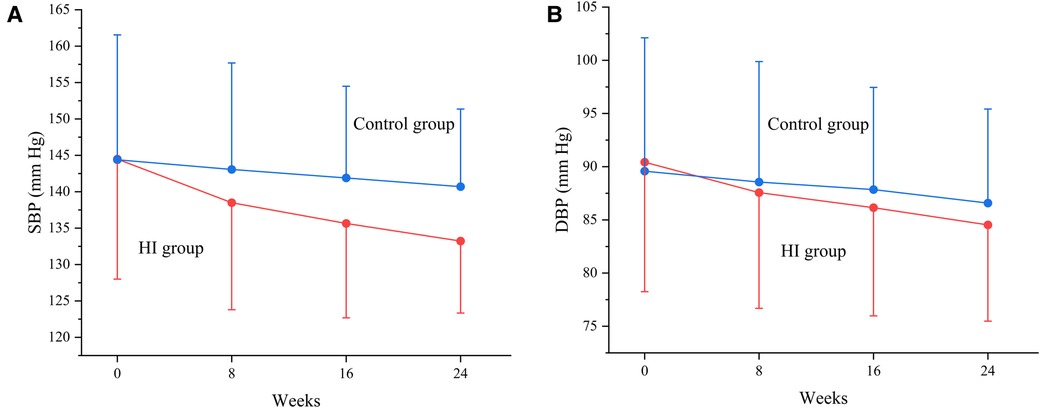
Figure 4. Change in SBP (A) and DBP (B) between HI and control groups from baseline to each visit point. Data are shown as mean ± SD. SBP, systolic blood pressure; DBP, diastolic blood pressure; HI, hydrogen inhalation.
At week 8, it demonstrated reductions of mean DBP level both in participants treated with HI therapy (−2.83 mm Hg; 95% CI, −3.83 to −1.84) and routine antihypertensives (−1.00 mm Hg; 95% CI, −1.97 to −0.04) with between-group difference of −1.83 mm Hg (95% CI, −3.21 to −0.45). The DBP had reduced by −4.23 mm Hg (95% CI, −5.21 to −3.24) in HI group and −1.66 mm Hg (95% CI, −2.65 to −0.66) in control group at week 16, while the HI therapy showed greater effect compared to control group (−2.57 mm Hg; 95% CI, −3.97 to −1.17). At week 24, the DBP levels decreased in HI group (−6.03 mm Hg; 95% CI, −6.98 to −5.08) and control group (−3.13 mm Hg; 95% CI, −4.09 to −2.17) with the mean between-group difference of −2.89 mm Hg (95% CI, −4.24 to −1.54). The between-group difference in DBP had increased as well during the study (Figures 3B, 4B).
At baseline, the BP control rate was 20.0% in HI group and 20.6% in control group. The BP control rate reached 38.2% (95% CI, 35.4–41.1) at week 8, 51.1% (95% CI, 48.1–54.2) at week 16, and 60.4% (95% CI, 57.3–63.4) at week 24 in HI group. In control group, it increased to 30.0% (95% CI, 27.3–32.7) at week 8, 35.5% (95% CI, 32.5–38.4) at week 16, and 40.4% (95% CI, 37.4–43.5) at week 24. The likelihoods of attaining controlled BP were higher in HI group with OR of 1.44 (95% CI, 1.21–1.72) at week 8, 1.90 (95% CI, 1.59–2.27) at week 16, and 2.24 (95% CI, 1.87–2.68) by the end of week 24. The BP control rate demonstrated an increasing trend throughout the study for both groups (Figures 5, 6).
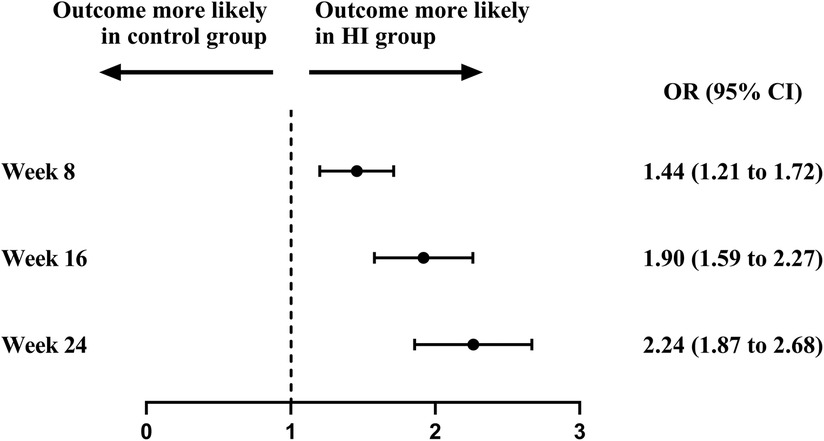
Figure 5. Attainment of controlled blood pressure between HI group and control group during follow-up period. Controlled blood pressure was defined as the blood pressure <140/90 mm Hg. HI, hydrogen inhalation; OR, odds ratio.
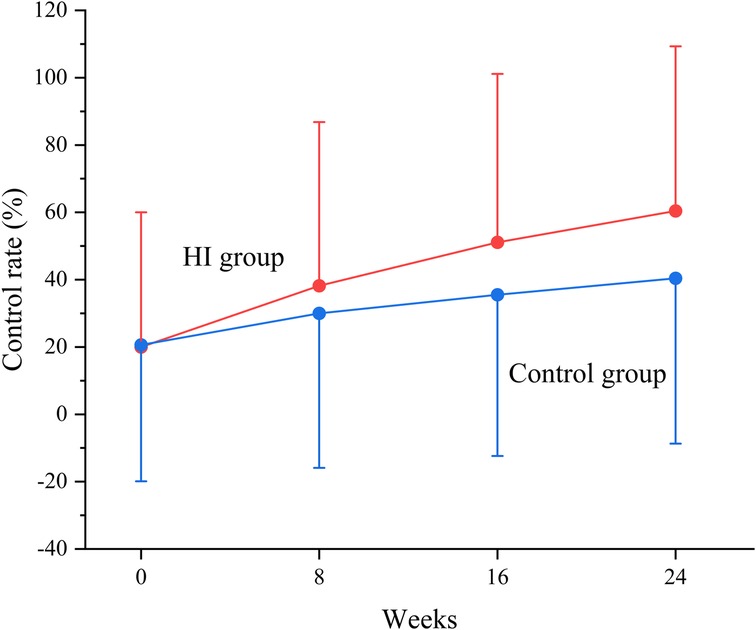
Figure 6. Change in blood pressure control rate between HI and control groups from baseline to each visit point. Data are shown as mean ± SD and controlled blood pressure was defined as the blood pressure <140/90 mm Hg. HI, hydrogen inhalation.
The results of subgroup and sensitivity analysis were consistent with the overall findings. Similar trends were found in prespecified subgroups including age, sex, BMI, diabetes mellitus status, SBP, and antihypertension medication usage (Supplementary Table S1). However, the between-group difference of reduction in DBP was insignificant at week 8 in patients who were male or with BMI ≥25 kg/m2 at baseline. Compared to control group, HI therapy did not provide beneficial effect on mean DBP levels at week 8 and week 16 for participants aged <65 years, with diabetes mellitus history, or taking >1 antihypertensives. Notably, there was no significant between-group difference of reduction in DBP during 24 weeks follow-up period for subjects with baseline SBP <140 mm Hg. The results of settling missing follow-up data by multiple-imputation method did not differ from the main analysis (Supplementary Table S2). The findings remained mostly consistent after re-applying analysis in all subjects with the absence of PSM (Supplementary Table S3). Similarly, using less restrictive criteria of patient selection led to inappreciable changes (Supplementary Table S4).
Generally, 47 (4.0%) patients in HI group experienced 68 AEs and 61 (5.2%) patients in control group experienced 79 AEs with no significant difference (p = 0.168). The most common AE in both groups was dizziness with 15 events occurring in 15 (1.3%) participants in HI group and 25 events occurring in 24 (2.0%) participants in control group. The incidences of all AEs (dizziness, headache, palpitations, asthenia, edema, flushing, cough, etc.) were similar between two groups with all p-value >0.05. More details of AE are shown at Table 2.
This multicenter, observational, retrospective, real world study aims at examining the effectiveness and safety of HI as an additional therapy in Chinese hypertensive patients. We found that the HI is related to greater improvement in mean BP and amelioration of BP control rate with comparable incidence of AE during 24 weeks compared to routine antihypertensive medication.
Recently, a clinical trial reported that compared to placebo group, the intervention of a low-dose 66% H2/33% O2 mixture in 60 patients with hypertension for 2 weeks significantly reduced right arm SBP by 4.8 mm Hg and improved nighttime DBP by 2.7 mm Hg, while other BP remained no marked change (25). In current study, both the mean SBP and DBP levels remain decreasing and the BP control rates keep increasing for HI group and control group during 24-week follow-up period. Meanwhile, patients treated with HI therapy receive more favorable effects in BP amelioration and attainment of controlled BP compared to participants treated with routine hypertensive medication for each visit point. The results of subgroup and sensitivity analysis are mostly the same as the main analysis, which confirms the robustness of our findings. Despite the primary outcome of our study is consistent with previous RCT, the different population of patients, follow-up duration, and intervention strategy of molecular hydrogen may account for the distinct results.
The most common AE is dizziness both in HI group and control group, and the incidences of all AEs are similar between two groups during follow-up period, which indicts that the HI therapy has high safety profile. Our findings are consistent with many previous RCTs, which reported no AE during HI intervention (16, 28, 29, 35). Lacking of cytotoxicity even at high concentration, hydrogen played as a role in the gas mixture for prevention of decompression sickness in extremely deep diving (36–39). 98.87% H2 and 1.13% O2 at 19.1 atm also showed no toxic effect (40). Meanwhile, as our intestinal bacteria ferment indigestible carbohydrates, our blood and breath naturally contain hydrogen gas (41). Thus, patients in HI group could apply hydrogen therapy freely without strict limitation of frequency or duration.
It is hard to directly assess the compliance of patients in this retrospective study. Nevertheless, the time period of HI per week has an increasing trend for participants receiving hydrogen therapy during 24-week follow-up period, which might reflect the favorable compliance of patients for HI treatment.
Although HI therapy has been proved to be an effective BP lowering strategy, the underlaying mechanism remains vague. First, the magnitude of antioxidant intervention in hypertension management has been highlighted in several clinical studies (42). Acting as an essential antioxidant enzyme to adjust reactive oxygen species (ROS) levels, superoxide dismutase (SOD) has lower activity for participants with hypertension (43). Therefore, oxidative stress appears to be generally existing in hypertension. Ohsawa et al. (44) had reported that the hydrogen presented therapeutic antioxidant effect by selectively eliminating ROS. The oxidative stress of several animal models with high BP was alleviated by hydrogen therapy (22, 45). Therefore, molecular hydrogen may ameliorate hypertension via reducing oxidative stress. Second, The renin–angiotensin–aldosterone system (RAAS) consists of several relevant hormones which function together to modulate BP (46). The angiotensin II, formed by the interaction of renin and angiotensin converting enzyme, leads to vasoconstriction, and further triggers elevation of BP. Meanwhile, it induces aldosterone secretion, which raises body fluid volume and BP level by promoting kidney to reabsorb sodium and water (47, 48). In addition, Whitworth et al. (49) reported that the incidence of hypertension and cardiovascular events was related to increased stress hormone cortisol. Previous clinical study found that HI therapy was associated with significant reduction in the plasma angiotensin II, aldosterone, aldosterone-to-renin ratio, and cortisol levels, which at least partly accounts for antihypertensive effect of molecular hydrogen (25). Third, inflammation acts as an essential part in hypertensive pathophysiology. It was reported that several new targets were developed for hypertension, such as specific immune cell types, toll-like receptors, and cytokines (50). The inflammatory status and BP level were improved by using hydrogen-rich hemodialysis solutions for 21 hemodialysis patients (51). The pro-inflammatory cytokines level was ameliorated by hydrogen-rich saline in rat with pulmonary hypertension (52). These findings indict that molecular hydrogen alleviates inflammatory reaction in hypertension. Thus, the therapeutic effect of HI for hypertension might be due to its anti-inflammatory feature.
In current clinical trials and real-life application, the most frequent methods of intaking molecular hydrogen are drinking HRW and hydrogen gas inhalation. However, it was reported that 6-month intervention of HRW or HI had an different influence upon 13 serum biochemical parameters in rats (53). In addition, different concentrations of hydrogen gas resulted in distinct therapeutic effects in rats with ischemia–reperfusion injury (54). Thus, further study is demanded to assess the optimal concentration and method for molecular hydrogen therapy.
Hypertension usually require long-term medication intervention continuously. A systematic review indicated that the BP of three quarters of patients who withdrew antihypertensive treatment would return to the level requiring reuse of therapy (55). Although HI therapy demonstrates therapeutic effect for hypertension in this study, its persistence after discontinuation still demands further assessment in future researches.
This study contains several limitations: First, the unmeasured confounding and sample bias are inevitable in this retrospective real-world study even after the application of PSM, due to the lack of randomization. Second, the incidence of AE may be underestimated in this study, as the evaluation is based on the medical records. Third, this study solely selects Chinese subjects with hypertension, and the results could hardly be fully generalizable to populations in other countries. Fourth, several essential factors related to treatment are unmeasured in current research, such as the blood concentration of H2 and O2, and the persistence status of antihypertensive effect after HI discontinuation. Finally, the optimal strategy and dosage of hydrogen intervention for hypertension require deeper investigation. Nevertheless, we evaluated the beneficial effects and safety of HI therapy in hypertensive patients in greater follow-up period and larger sample size, enriching the evidence base of hydrogen medicine in hypertension.
In conclusion, our study indicts that the 24-week HI therapy significantly ameliorates BP level and increases the BP control rate with similar incidence of AE compared with control group in Chinese hypertensive patients. It demonstrates an effective and possible antihypertensive therapy for current clinical practice.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethics Committee of Hengxing University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
HJ: Conceptualization, Methodology, Software, Writing – original draft. HS: Methodology, Writing – original draft. YZ: Formal Analysis, Writing – original draft. ZZ: Software, Writing – original draft. XG: Methodology, Writing – original draft. CW: Formal Analysis, Writing – original draft. YY: Visualization, Writing – original draft. XZ: Visualization, Writing – original draft. JG: Software, Supervision, Writing – original draft. DM: Supervision, Writing – original draft. QY: Methodology, Writing – original draft. YY: Formal Analysis, Writing – original draft. CY: Visualization, Writing – original draft. CC: Methodology, Writing – original draft. XD: Conceptualization, Supervision, Validation, Writing – original draft, Writing – review & editing. TN: Conceptualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1391282/full#supplementary-material
1. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. (2005) 365(9455):217–23. doi: 10.1016/s0140-6736(05)17741-1
2. Wang ZW, Chen Z, Zhang LF, Wang X, Hao G, Zhang ZG, et al. Status of hypertension in China results from the China hypertension survey, 2012–2015. Circulation. (2018) 137(22):2344–56. doi: 10.1161/circulationaha.117.032380
3. Sudharsanan N, Theilmann M, Kirschbaum TK, Manne-Goehler J, Azadnajafabad S, Bovet P, et al. Variation in the proportion of adults in need of blood pressure-lowering medications by hypertension care guideline in low- and middle-income countries a cross-sectional study of 1 037 215 individuals from 50 nationally representative surveys. Circulation. (2021) 143(10):991–1001. doi: 10.1161/circulationaha.120.051620
4. Geldsetzer P, Manne-Goehler J, Marcus ME, Ebert C, Zhumadilov Z, Wesseh CS, et al. The state of hypertension care in 44 low-income and middle-income countries: a cross-sectional study of nationally representative individual-level data from 1.1 million adults. Lancet. (2019) 394(10199):652–62. doi: 10.1016/s0140-6736(19)30955-9
5. Lu JP, Lu Y, Wang XC, Li XY, Linderman GC, Wu CQ, et al. Prevalence, awareness, treatment, and control of hypertension in China: data from 1.7 million adults in a population-based screening study (China peace million persons project). Lancet. (2017) 390(10112):2549–58. doi: 10.1016/s0140-6736(17)32478-9
6. Ohta S. Molecular hydrogen as a preventive and therapeutic medical gas: initiation, development and potential of hydrogen medicine. Pharmacol Ther. (2014) 144(1):1–11. doi: 10.1016/j.pharmthera.2014.04.006
7. Huang C-S, Kawamura T, Toyoda Y, Nakao A. Recent advances in hydrogen research as a therapeutic medical gas. Free Radical Res. (2010) 44(9):971–82. doi: 10.3109/10715762.2010.500328
8. Yoritaka A, Takanashi M, Hirayama M, Nakahara T, Ohta S, Hattori N. Pilot study of H-2 therapy in Parkinson’s disease: a randomized double-blind placebo-controlled trial. Mov Disord. (2013) 28(6):836–9. doi: 10.1002/mds.25375
9. Li J, Wang C, Zhang JH, Cai J-M, Cao Y-P, Sun X-J. Hydrogen-rich saline improves memory function in a rat model of amyloid-beta-induced Alzheimer’s disease by reduction of oxidative stress. Brain Res. (2010) 1328:152–61. doi: 10.1016/j.brainres.2010.02.046
10. Yao WJ, Shen LK, Ma ZX, Zhao J, Liu L, Wang ZF, et al. Efficacy and safety of hydrogen gas versus standard therapy in Chinese patients with cerebral infarction: a pilot study. Trop J Pharm Res. (2022) 21(3):665–71. doi: 10.4314/tjpr.v21i3.29
11. Ono H, Nishijima Y, Ohta S, Sakamoto M, Kinone K, Horikosi T, et al. Hydrogen gas inhalation treatment in acute cerebral infarction: a randomized controlled clinical study on safety and neuroprotection. J Stroke Cerebrovasc Dis. (2017) 26(11):2587–94. doi: 10.1016/j.jstrokecerebrovasdis.2017.06.012
12. Wang ST, Bao C, He Y, Tian X, Yang Y, Zhang T, et al. Hydrogen gas (XEN) inhalation ameliorates airway inflammation in asthma and COPD patients. QJM. (2020) 113(12):870–5. doi: 10.1093/qjmed/hcaa164
13. Zheng ZG, Sun WZ, Hu JY, Jie ZJ, Xu JF, Cao J, et al. Hydrogen/oxygen therapy for the treatment of an acute exacerbation of chronic obstructive pulmonary disease: results of a multicenter, randomized, double-blind, parallel-group controlled trial. Respir Res. (2021) 22(1). doi: 10.1186/s12931-021-01740-w
14. LeBaron TW, Singh RB, Fatima G, Kartikey K, Sharma JP, Ostojic SM, et al. The effects of 24-week, high-concentration hydrogen-rich water on body composition, blood lipid profiles and inflammation biomarkers in men and women with metabolic syndrome: a randomized controlled trial. Diabetes Metab Syndr Obes. (2020) 13:889–96. doi: 10.2147/dmso.S240122
15. Chiu SH, Douglas FL, Chung JR, Wang KY, Chu CF, Chou HY, et al. Evaluation of the safety and potential lipid-lowering effects of oral hydrogen-rich coral calcium (HRCC) capsules in patients with metabolic syndrome: a prospective case series study. Front Nutr. (2023) 10:1198524. doi: 10.3389/fnut.2023.1198524
16. Tao GR, Zhang GJ, Chen W, Yang C, Xue YZ, Song GH, et al. A randomized, placebo-controlled clinical trial of hydrogen/oxygen inhalation for non-alcoholic fatty liver disease. J Cell Mol Med. (2022). doi: 10.1111/jcmm.17456
17. Korovljev D, Stajer V, Ostojic J, LeBaron TW, Ostojic SM. Hydrogen-rich water reduces liver fat accumulation and improves liver enzyme profiles in patients with non-alcoholic fatty liver disease: a randomized controlled pilot trial. Clin Res Hepatol Gastroenterol. (2019) 43(6):688–93. doi: 10.1016/j.clinre.2019.03.008
18. Chen J-B, Kong X-F, Mu F, Lu T-Y, Lu Y-Y, Xu K-C. Hydrogen therapy can be used to control tumor progression and alleviate the adverse events of medications in patients with advanced non-small cell lung cancer. Med Gas Res. (2020) 10(2):75–80. doi: 10.4103/2045-9912.285560
19. Akagi J, Baba H. Hydrogen gas activates coenzyme Q10 to restore exhausted Cd8(+) T cells, especially Pd-1(+)Tim3(+)Terminal Cd8(+) T cells, leading to better nivolumab outcomes in patients with lung cancer. Oncol Lett (2020) 20(5). doi: 10.3892/ol.2020.12121
20. Sugai K, Tamura T, Sano M, Uemura S, Fujisawa M, Katsumata Y, et al. Daily inhalation of hydrogen gas has a blood pressure-lowering effect in a rat model of hypertension. Sci Rep. (2020) 10(1). doi: 10.1038/s41598-020-77349-8
21. Matsuoka H, Miyata S, Okumura N, Watanabe T, Hashimoto K, Nagahara M, et al. Hydrogen gas improves left ventricular hypertrophy in Dahl rat of salt-sensitive hypertension. Clin Exp Hypertens. (2019) 41(4):307–11. doi: 10.1080/10641963.2018.1481419
22. Yu Y-S, Zheng H. Chronic hydrogen-rich saline treatment reduces oxidative stress and attenuates left ventricular hypertrophy in spontaneous hypertensive rats. Mol Cell Biochem. (2012) 365(1–2):233–42. doi: 10.1007/s11010-012-1264-4
23. Xin HG, Zhang BB, Wu ZQ, Hang XF, Xu WS, Ni W, et al. Consumption of hydrogen-rich water alleviates renal injury in spontaneous hypertensive rats. Mol Cell Biochem. (2014) 392(1-2):117–24. doi: 10.1007/s11010-014-2024-4
24. Nakayama M, Itami N, Suzuki H, Hamada H, Yamamoto R, Tsunoda K, et al. Novel haemodialysis (HD) treatment employing molecular hydrogen (H-2)-enriched dialysis solution improves prognosis of chronic dialysis patients: a prospective observational study. Sci Rep. (2018) 8. doi: 10.1038/s41598-017-18537-x
25. Liu BY, Jiang X, Xie YB, Jia XB, Zhang JS, Xue YZ, et al. The effect of a low dose hydrogen-oxygen mixture inhalation in midlife/older adults with hypertension: a randomized, placebo-controlled trial. Front Pharmacol. (2022) 13. doi: 10.3389/fphar.2022.1025487
26. Zhao ZY, Ji HX, Zhao YS, Liu ZY, Sun RT, Li YQ, et al. Effectiveness and safety of hydrogen inhalation as an adjunct treatment in Chinese type 2 diabetes patients: a retrospective, observational, double-arm, real-life clinical study. Front Endocrinol (Lausanne). (2023) 13. doi: 10.3389/fendo.2022.1114221
27. LeBaron TW, Laher I, Kura B, Slezak J. Hydrogen gas: from clinical medicine to an emerging ergogenic molecule for sports athletes. Can J Physiol Pharmacol. (2019) 97(9):797–807. doi: 10.1139/cjpp-2019-0067
28. Okada M, Ogawa H, Takagi T, Nishihara E, Yoshida T, Hyodo J, et al. A double-blinded, randomized controlled clinical trial of hydrogen inhalation therapy for idiopathic sudden sensorineural hearing loss. Front Neurosci. (2022) 16. doi: 10.3389/fnins.2022.1024634
29. Kong XF, Lu TY, Lu YY, Yin ZA, Xu KC. Effect of hydrogen inhalation therapy on hearing loss of patients with nasopharyngeal carcinoma after radiotherapy. Front Med (Lausanne). (2022) 9. doi: 10.3389/fmed.2022.828370
30. Chen JB, Kong XF, Qian W, Mu F, Lu TY, Lu YY, et al. Two weeks of hydrogen inhalation can significantly reverse adaptive and innate immune system senescence patients with advanced non-small cell lung cancer: a self-controlled study. Med Gas Res. (2020) 10(4):149–54. doi: 10.4103/2045-9912.304221
31. Chen JB, Kong XF, Mu F, Lu TY, Du DM, Xu KC. Hydrogen-oxygen therapy can alleviate radiotherapy-induced hearing loss in patients with nasopharyngeal cancer. Ann Palliat Med. (2019) 8(5):746–51. doi: 10.21037/apm.2019.11.18
32. Stevens LA, Claybon MA, Schmid CH, Chen J, Horio M, Imai E, et al. Evaluation of the chronic kidney disease epidemiology collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int. (2011) 79(5):555–62. doi: 10.1038/ki.2010.462
33. Seeger JD, Williams PL, Walker AM. An application of propensity score matching using claims data. Pharmacoepidemiol Drug Saf. (2005) 14(7):465–76. doi: 10.1002/pds.1062
34. Yucel RM. Random covariances and mixed-effects models for imputing multivariate multilevel continuous data. Stat Modelling. (2011) 11(4):351–70. 10.1177/1471082×100110040422271079
35. Yoritaka A, Kobayashi Y, Hayashi T, Saiki S, Hattori N. Randomized double-blind placebo-controlled trial of hydrogen inhalation for Parkinson’s disease: a pilot study. Neurol Sci. (2021) 42(11):4767–70. doi: 10.1007/s10072-021-05489-4
36. Abraini JH, Gardettechauffour MC, Martinez E, Rostain JC, Lemaire C. Psychophysiological reactions in humans during an open sea dive to 500 M with a hydrogen-helium-oxygen mixture. J Appl Physiol. (1994) 76(3):1113–8. doi: 10.1152/jappl.1994.76.3.1113
37. Lillo RS, Parker EC, Porter WR. Decompression comparison of helium and hydrogen in rats. J Appl Physiol. (1997) 82(3):892–901. doi: 10.1152/jappl.1997.82.3.892
38. Fontanari P, Badier M, Guillot C, Tomei C, Burnet H, Gardette B, et al. Changes in maximal performance of inspiratory and skeletal muscles during and after the 7.1-Mpa hydra 10 record human dive. Eur J Appl Physiol. (2000) 81(4):325–8. doi: 10.1007/s004210050050
39. Lillo RS, Parker EC. Mixed-gas model for predicting decompression sickness in rats. J Appl Physiol. (2000) 89(6):2107–16. doi: 10.1152/jappl.2000.89.6.2107
40. Friess SL, Hudak WV, Boyer RD. Toxicology of hydrogen-containing diving environments.1. Antagonism of acute Co2 effects in the rat by elevated partial pressures of H-2 gas. Toxicol Appl Pharmacol. (1978) 46(3):717–25. doi: 10.1016/0041-008x(78)90317-4
41. Strocchi A, Levitt MD. Maintaining intestinal H-2 balance - credit the colonic bacteria. Gastroenterology. (1992) 102(4):1424–6. doi: 10.1016/0016-5085(92)90790-6
42. Ahmad KA, Yuan DY, Nawaz W, Ze H, Zhuo CX, Talal B, et al. Antioxidant therapy for management of oxidative stress induced hypertension. Free Radical Res. (2017) 51(4):428–38. doi: 10.1080/10715762.2017.1322205
43. Ahmad A, Singhal U, Hossain MM, Islam N, Rizvi I. The role of the endogenous antioxidant enzymes and malondialdehyde in essential hypertension. J Clin Diagn Res. (2013) 7(6):987–90. doi: 10.7860/jcdr/2013/5829.3091
44. Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. (2007) 13(6):688–94. doi: 10.1038/nm1577
45. Guan P, Lin X-M, Yang S-C, Guo Y-J, Li W-Y, Zhao Y-S, et al. Hydrogen gas reduces chronic intermittent hypoxia-induced hypertension by inhibiting sympathetic nerve activity and increasing vasodilator responses via the antioxidation. J Cell Biochem. (2019) 120(3):3998–4008. doi: 10.1002/jcb.27684
46. Mentz RJ, Bakris GL, Waeber B, McMurray JJV, Gheorghiade M, Ruilope LM, et al. The past, present and future of renin-angiotensin aldosterone system inhibition. Int J Cardiol. (2013) 167(5):1677–87. doi: 10.1016/j.ijcard.2012.10.007
47. Atlas SA. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm. (2007) 13(8):S9–S20. doi: 10.18553/jmcp.2007.13.s8-b.9
48. Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. (2007) 59(3):251–87. doi: 10.1124/pr.59.3.3
49. Whitworth JA, Mangos GJ, Kelly JJ. Cushing, cortisol, and cardiovascular disease. Hypertension. (2000) 36(5):912–6. doi: 10.1161/01.Hyp.36.5.912
50. De Miguel C, Rudemiller NP, Abais JM, Mattson DL. Inflammation and hypertension: new understandings and potential therapeutic targets. Curr Hypertens Rep. (2015) 17(1). doi: 10.1007/s11906-014-0507-z
51. Nakayama M, Nakano H, Hamada H, Itami N, Nakazawa R, Ito S. A novel bioactive haemodialysis system using dissolved dihydrogen (H-2) produced by water electrolysis: a clinical trial. Nephrol Dial Transplant. (2010) 25(9):3026–33. doi: 10.1093/ndt/gfq196
52. Wang Y, Jing L, Zhao X-M, Han J-J, Xia Z-L, Qin S-C, et al. Protective effects of hydrogen-rich saline on monocrotaline-induced pulmonary hypertension in a rat model. Respir Res. (2011) 12. doi: 10.1186/1465-9921-12-26
53. Xun Z-m, Zhao Q-h, Zhang Y, Ju F-d, He J, Yao T-t, et al. Effects of long-term hydrogen intervention on the physiological function of rats. Sci Rep. (2020) 10(1):18509. doi: 10.1038/s41598-020-75492-w
54. Hayashida K, Sano M, Ohsawa I, Shinmura K, Tamaki K, Kimura K, et al. Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury. Biochem Biophys Res Commun. (2008) 373(1):30–5. doi: 10.1016/j.bbrc.2008.05.165
Keywords: hypertension, observational study, hydrogen inhalation, blood pressure, real-world study
Citation: Ji H, Sun H, Zhang Y, Zhao Z, Gao X, Wang C, Yang Y, Zhang X, Gao J, Man D, Yang Q, Yang Y, Yue C, Chen C, Ding X and Ni T (2024) Effectiveness and safety of hydrogen inhalation therapy as an additional treatment for hypertension in real-world practice: a retrospective, observational study in China. Front. Cardiovasc. Med. 11:1391282. doi: 10.3389/fcvm.2024.1391282
Received: 25 February 2024; Accepted: 21 October 2024;
Published: 12 November 2024.
Edited by:
Claudio de Lucia, Local Health Authority Naples 1 Center, ItalyReviewed by:
Shigeo Ohta, Juntendo University, JapanCopyright: © 2024 Ji, Sun, Zhang, Zhao, Gao, Wang, Yang, Zhang, Gao, Man, Yang, Yang, Yue, Chen, Ding and Ni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoheng Ding, ZGluZ3hpYW9oZW5nQHFkdS5lZHUuY24=; Tongshang Ni, bmVldG9uZ3NoYW5nQDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.