- 1School of Clinical Medicine, Ningxia Medical University, Yinchuan, China
- 2Department of Gynaecology and Obstetrics, People’s Hospital of Ningxia Hui Autonomous Region, Yinchuan, China
- 3Heart Centre & Department of Cardiovascular Diseases, General Hospital of Ningxia Medical University, Yinchuan, China
- 4Department of Cardiology, Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, China
- 5Institute of Medical Sciences, General Hospital of Ningxia Medical University, Yinchuan, China
Background: The relationship between the triglyceride–glucose (TyG) index and no-reflow phenomenon after percutaneous coronary intervention (PCI) in patients with type 2 diabetes mellitus (T2DM) and acute ST-segment elevation myocardial infarction (STEMI) remains unclear. This study aimed to investigate the relationship between baseline TyG index and no-reflow phenomenon in STEMI patients with T2DM after PCI.
Methods: This study enrolled 695 patients with T2DM and STEMI from the General Hospital of Ningxia Medical University (2014–2019). Patients were divided into tertiles according to the TyG index levels. The incidence of no-reflow phenomenon was recorded. A multivariate regression model was developed to analyze the association between the baseline TyG index and no-reflow phenomenon. The linear association between the baseline TyG index and no-reflow phenomenon was explored using smooth curve fitting with parallel subgroup analyses. Receiver operating characteristic (ROC) curves were generated to determine the predictive power of the TyG index.
Results: A multivariate logistic regression model revealed that the TyG index was an independent risk factor of no-reflow phenomenon [OR = 3.23, 95%CI: 2.15–4.86, P < 0.001], and the occurrence of no-reflow phenomenon increased gradually with the increase of TyG index tertile interval (P < 0.001). Smooth curve fitting showed that the TyG index was linearly related to the risk of no-reflow. Subgroup analysis showed that they participated in this positive correlation. The area under the ROC curve (AUC) of the TyG index for evaluating the occurrence of no-reflow was 0.710 (95% CI: 0.640–0.780; P < 0.01).
Conclusions: The TyG index is independently associated with no-reflow phenomenon, suggesting that the simple index of the TyG index can be used for risk assessment of no-reflow phenomenon after PCI in STEMI patients with T2DM.
Background
Myocardial perfusion should be re-established as soon as possible in patients with acute ST-elevation myocardial infarction (STEMI) (1). Primary percutaneous coronary intervention (PCI) is the modality of choice for restoring myocardial blood flow, which significantly prevents further necrosis of cardiomyocytes and markedly improves the quality of life of STEMI patients (2). However, even after the culprit vessel is reopened, there may be suboptimal coronary reperfusion, with slow, incomplete, or no coronary flow in the affected coronary arteries based on a thrombolysis in myocardial infarction (TIMI) score of <3 (3). This phenomenon, known as “no-reflow” (NR), occurs in 2%–60% of patients and can complicate up to 60% of STEMI cases (4). No-reflow is associated with an increased incidence of rehospitalization, adverse ventricular remodeling, malignant arrhythmias, and heart failure and is an independent predictor of cardiovascular mortality (5). This risk is particularly high in patients with type 2 diabetes mellitus (T2DM), which accounts for approximately 37% of acute myocardial infarction (AMI) cases in China and is considered a high-risk group for recurrent cardiovascular mortality (6). A previous study has shown that T2DM is significantly associated with more complex coronary artery lesions and a higher incidence of no-reflow in STEMI patients (7). Therefore, early identification of residual risk factors in STEMI patients with T2DM is essential for better clinical management to reduce the incidence of future no-reflow.
Insulin resistance (IR) is a state of reduced sensitivity and responsiveness of the body to insulin, usually occurring in the prediabetic and diabetic stages (8). The hyperinsulinemic–euglycemic clamp is the gold standard test for assessing IR, but it is not widely used in clinical care and large population studies due to the complexity and cost of the test procedure (9). The Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) is a method used to quantify IR and beta-cell function, which is calculated using fasting glucose and insulin levels, providing an estimate of IR that is crucial in understanding the metabolic profile of patients. The triglyceride–glucose (TyG) index, a composite index calculated as Ln fasting triglycerides (mg/dl) × fasting plasma glucose (FPG) (mg/dl)/2, is not only inexpensive and readily available but also performs consistently with or better than HOMA-IR in the assessment of IR (10). In recent studies, the TyG index has been widely used as a marker of IR. It has been shown that a high TyG index was associated with an increased risk of major adverse cardiac and cerebrovascular events in STEMI patients undergoing PCI (11) and the risk of ischemic stroke was associated with a proportional and linear increase in TyG index (12). However, no previous study has specifically examined the association of the TyG index with the no-reflow after PCI in STEMI patients with T2DM. Our study was designed to fill this knowledge gap.
Methods
Study population
The study subjects were screened from the database of the cardiovascular center of the General Hospital of Ningxia Medical University. The patient flowchart is presented in Figure 1. A total of 8,525 consecutive patients were diagnosed with acute coronary syndrome (ACS) from January 2014 to December 2019. Of these 8,525 patients, 1,471 were diagnosed with STEMI and T2DM. A total of 776 patients were excluded according to the exclusion criteria, including (1) without primary PCI; (2) with acute infectious disease, rheumatic disease, or hematological disease; (3) with severe heart valve diseases or cardiomyopathy; and (4) lacking clinical data. Finally, 695 patients were included in this analysis. According to the TyG index level, 695 patients were stratified into four groups (Q1 group: 6.25 < TyG index <8.15, n = 174; Q2 group: 8.15 ≤ TyG index <8.68, n = 173; Q3 group:8.68 ≤ TyG index <9.29, n = 174; Q4 group:9.29 ≤ TyG index <11.34, n = 174).
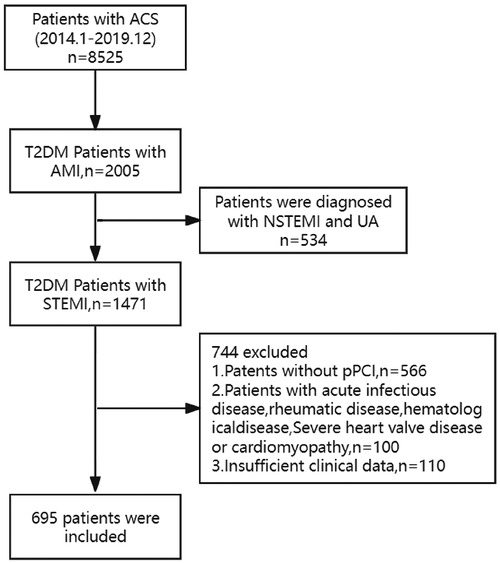
Figure 1. The flowchart of the research subject. ACS, acute coronary syndrome; AMI, acute myocardial infarction; STEMI, acute ST-elevation myocardial infarction; PCI, primary percutaneous coronary intervention; T2DM, type 2 diabetes mellitus.
PCI procedure and angiographic analysis
We used the standard PCI procedure: a thorough preoperative evaluation was carried out, with an episodic dose of 300 mg clopidogrel, 180 mg ticagrelor, and 300 mg aspirin and total heparinization. Selective coronary angiography and infarct-related arteriography (IRA) were performed after puncture of the right radial artery using the conventional Seldinger technique. The optimal interventional transport device was selected to deliver the stent and the angiogram was reviewed. Success criteria for PCI treatment: TIMI grade 3 under direct vision. The decision to administer intracoronary adenosine, intravenous tirofiban, and other drugs is made by the interventionalist based on the patient's specific coronary artery lesions.
Data collections and definitions
The data collection process was approved by the Institutional Review Board of the General Hospital of Ningxia Medical University and was in accordance with the Regulations on the Review of Medical Institutions.
Patient demographics, medical history, laboratory findings, and echocardiographic and angiographic assessments were collected and validated from our hospital's electronic medical record system. The concentrations of triglyceride (TG) and fasting plasma glucose (FPG) in the first fasting blood sample during hospitalization were determined in the central laboratory of the General Hospital of Ningxia Medical University. The TyG index was calculated as ln[fasting TG (mg/dl) × FPG (mg/dl)/2] (13). The single-point insulin sensitivity estimator (SPISE) has been proven to be an effective surrogate index for insulin sensitivity, so it is included in the baseline clinical characteristics. The novel formula for SPISE was computed as follows: .
Definition of the no-reflow phenomenon: For the purpose of this study, the diagnosis of the no-reflow phenomenon was by angiography during the PCI procedure by two independently experienced interventionalists. We defined no-reflow as a TIMI grade flow of ≤2 after coronary reopening.
Criteria for T2DM included: (1) a previous diagnosis of T2DM and being treated with antidiabetic medication and (2) FPG ≥7.0 mmol/L and/or random blood glucose (RBG) ≥11.1 mmol/L and/or blood glucose ≥11.1 mmol/L 2 h after an oral glucose tolerance test (OGTT) as a typical symptom of DM.
Statistical analyses
Empower software (http://www.empowerstats.com) and R version 4.1.0 (http://www.R-project.org) were employed for all analyses. Study participants were categorized into four groups based on quartiles (Q1–Q4) of the TyG index. Non-parametric Kruskal–Wallis ANOVA was used to compare the sample means of the four groups in fully randomized experimental design data when the study data population was not normally distributed and/or the variance was not homogeneous. For continuous variable data in the randomized area group design, the Friedman rank sum test was used when the experimental groups were not normally distributed. Once a significant difference was found, a post hoc test was performed to determine which specific treatment groups differed from each other, using the Dunnett validated randomization test. A multivariate logistic regression model was used to analyze the correlation between the TyG index and no-reflow. The correlation between the TyG index and no-reflow was further evaluated by smooth curve fitting. Stratified analyses were conducted based on gender, age (<65 or ≥65 years), hypertension, dyslipidemia, current smoker, BMI (<25.00 or ≥25.00), and eGFR (<60 or ≥60 ml/min/1.73 m2). Receiver operating characteristic (ROC) curves were generated to determine the cutoff values and predictive power of the TyG index, HbA1c, and the SPISE index. All statistical tests were two-tailed, and a significance level of P < 0.05 was considered statistically significant.
Results
Baseline characteristics of study participants
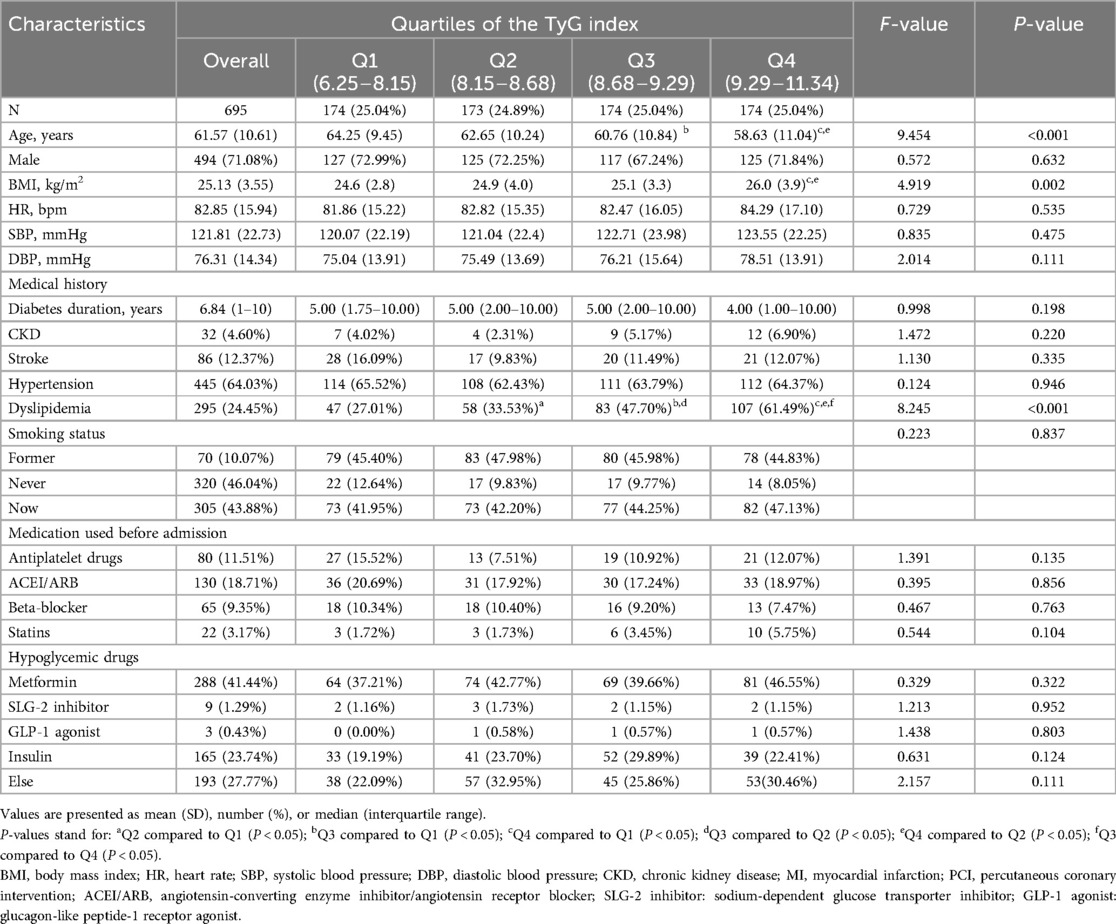
Table 1 shows the baseline characteristics of the patients (n = 695) stratified by TyG index quartiles. The average age of the patients was 61.57 years, and 71.08% of them were male. The average TyG index in the enrolled patients was 8.71 ± 0.83. Compared with the lowest quartile, the participants with a higher TyG index were more likely to be younger and showed a higher body mass index (BMI) and higher percentage of previous dyslipidemia (all P < 0.05). Additionally, the laboratory and angiographic characteristics at baseline are shown in Table 2 according to the TyG index quartiles. The participants in the highest quartile showed significantly higher levels of TyG index, hemoglobin A1c (HbA1c), total cholesterol(TC), TG, FPG, and albumin compared with those of the participants in the first quartile. However, the SPISE index and HDL cholesterol and hemoglobin levels of the participants in the highest quartile were lower than those of the participants in the first quartile (all P < 0.05). Angiographically, the participants in the highest quartile had a significantly higher percentage of no-reflow than that of the participants in the lowest quartile (P < 0.001).
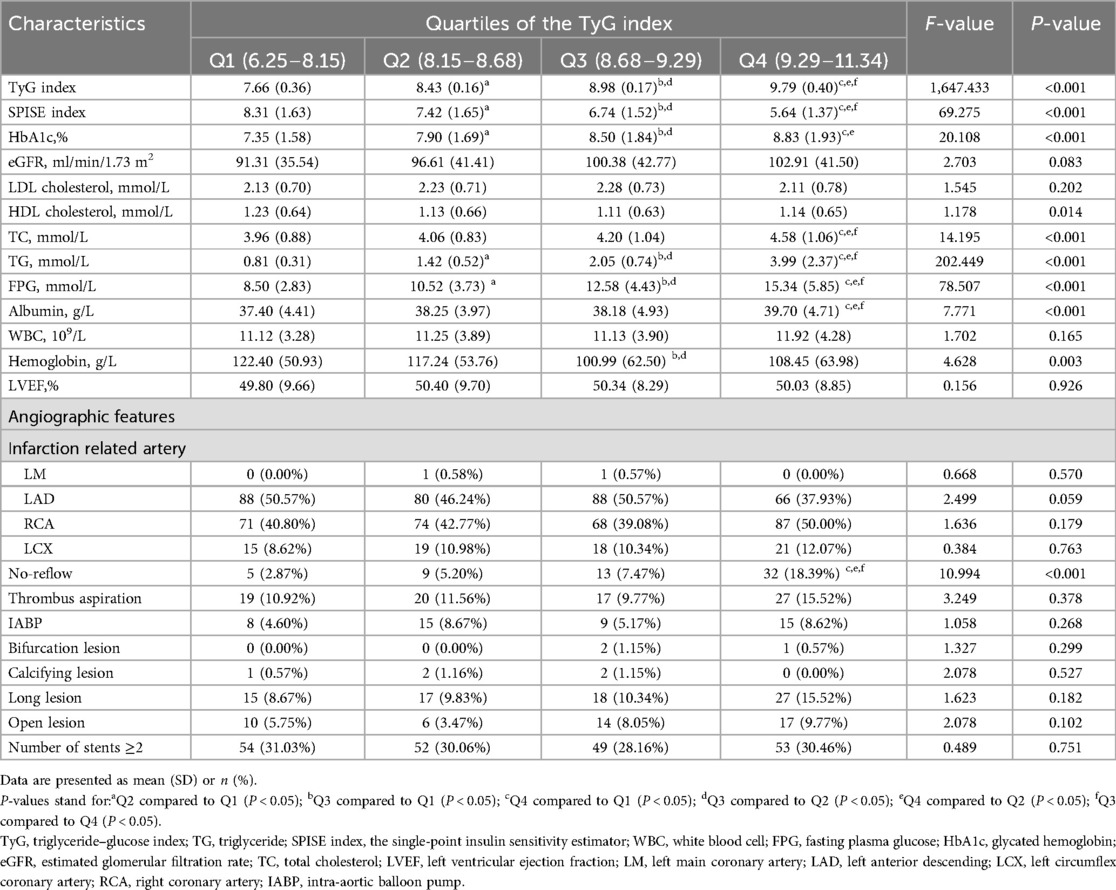
Table 2. Baseline levels of laboratory and angiographic characteristics according to the TyG index quartiles.
Relationships between TyG index and no-reflow
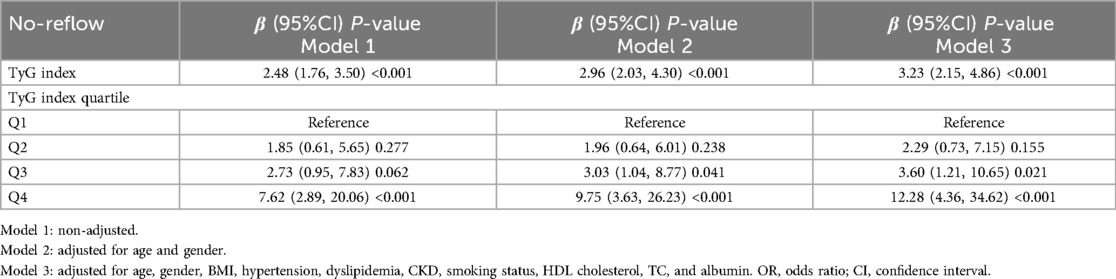
Table 3 presents the occurrence of no-reflow in 59 patients during emergency PCI. We constructed multivariate regression models to investigate the independent association between the level of TyG index and no-reflow phenomenon. Our findings suggested that a higher TyG index was associated with increased no-reflow. The correlation between TyG index and no-reflow was significant in both our crude model (OR = 2.48, 95% CI: 1.76–3.50), P < 0.001) and the minimally adjusted model (OR = 2.96, 95% CI: 2.03–4.3, P < 0.001). The positive association between TyG index and no-reflow remained stable in the fully adjusted model (OR = 3.23, 95% CI: 2.15–4.86, P < 0.001), which indicated that each unit increase in TyG index was associated with a 2.23-fold increase in the risk of no-reflow.
When the authors considered TyG index as tertiles, the participants in the highest tertile had an 11.28-fold increased risk of no-reflow compared to that of the participants in the first tertile (OR = 13.28, 95% CI: 4.44–39.71, P < 0.001). Additionally, the smooth curve fitting showed that TyG index is linearly related to the risk of no-reflow (Figure 2A). After adjusting for age, gender, hypertension, dyslipidemia, chronic kidney disease (CKD), smoking status, BMI, HDL cholesterol, TC, and albumin, the weighted smooth curve fitting also showed that TyG index was still linearly related to the risk of no-reflow (Figure 2B).

Figure 2. Analysis of the relationship between the TyG index and no-reflow by smooth curve fitting. The red line in the middle represented the fit line, and the line formed by the blue circles on both sides represented CI. (A) Smooth curve fitting analysis of the relationship between the TyG index and no-reflow did not correct any factors. (B) After adjusting for age, gender, hypertension, dyslipidemia, CKD, smoking status, BMI, HDL cholesterol, TC, and albumin, the relationship between the TyG index and no-reflow was analyzed by smooth curve fitting.
Independent association of TyG index with no-reflow
A subgroup analysis was performed according to age, sex, BMI, current smoker, hypertension, dyslipidemia, and eGFR (Figure 3). We found that the predictive effect of TyG index on no-reflow is effective in most subgroups.

Figure 3. Forest plot of no-reflow according to different subgroups. The adjusted model included age, gender, hypertension, dyslipidemia, CKD, smoking status, BMI, HDL cholesterol, TC, and albumin.
Receiver operating characteristic (ROC) curve analyses to evaluate no-reflow
The area under the ROC curves (AUCs) of the TyG index for evaluating the occurrence of no-reflow was 0.710 (95% CI: 0.640–0.780; P < 0.01). The cutoff value of the TyG index to evaluate no-reflow was 8.99, the sensitivity was 0.712, and the specificity was 0.665. The AUCs of HbA1c for evaluating the occurrence of no-reflow was 0.490 (95% CI: 0.392–0.589; P = 0.845). The cutoff value of the SPISE index to evaluate no-reflow was 0. 423 (95% CI: 0.323–0.524; P = 0.117) (Figure 4).
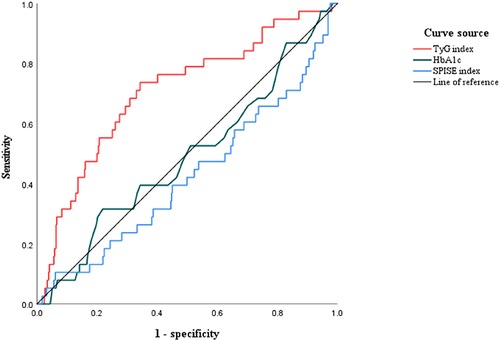
Figure 4. The receiver operating characteristic (ROC) curves of the TyG index, HbA1c, and the SPISE index to evaluate no-reflow in STEMI patients with T2DM. The area under the ROC curve (AUC) of the TyG index for evaluating the occurrence of no-reflow was 0.710 (95% CI: 0.633–0.771; P < 0.01).
Discussion
To the best of our knowledge, this is the first study to explore the association between the TyG index and no-reflow in STEMI patients with T2DM after PCI. Our main findings include the following: (1) The incidence of no-reflow increased significantly as the TyG index increased; (2) the TyG index was a univariate risk factor of no-reflow; (3) the association of the TyG index with no-reflow was mainly reflected in most subgroups; and (4) the AUC of the TyG index for evaluating the occurrence of no-reflow was 0.710 with a cutoff value of 8.99. Based on the present study, we found that the TyG index was linearly associated with no-reflow. Most importantly, this study suggests a simple method of estimating the association of IR and the risk of no-reflow after PCI in STEMI patients with T2DM.
IR is the decrease in the efficiency of insulin to stimulate the uptake and use of glucose, and the body responds by overproducing insulin, causing hyperinsulinemia, to maintain stable blood glucose levels. IR contributes to the development of cardiovascular diseases by inducing imbalances in glucose metabolism, generation of oxygen free radicals, reduction of nitric oxide production in endothelial cells, and alteration of systemic lipid metabolism, among other mechanisms (14). Several previous studies have found that IR is a significant risk factor for cardiovascular diseases and poor clinical outcomes (15, 16). At present, the complexity and cost of using the hyperinsulinemic–euglycemic clamp and HOMA-IR to detect IR make them inappropriate for widespread clinical use. To address this clinical challenge, researchers have identified the TyG index as a practical and effective alternative indicator of IR from numerous studies (17, 18). Therefore, the TyG index can be used in clinical practice to identify IR if the hyperinsulinemia–glucose clamp test and HOMA-IR are not measurable.
A large number of previous studies have provided strong evidence for the predictive role of the TyG index in diabetes and cardiovascular disease and for a poor prognosis. Zhao et al. (19) showed that an elevated TyG index is positively associated with a higher risk of atherosclerosis and renal microvascular damage. A study has also shown that the cumulative risk of developing T2DM increases with an increase in the TyG index (20). Mao et al. (21) first demonstrated that a higher TyG index was independently associated with SYNTAX score [OR (95% CI): 6.06 (2.92, 12.58), P < 0.001] and major adverse cardiovascular events [HR (95% CI): 1.79 (1.05, 3.07), P = 0.034] in the NSTE-ACS population. Additionally, a present study indicated that an increased TyG index is associated with an increased risk of major adverse cardiovascular events in STEMI patients undergoing PCI (11), and the risk of ischemic stroke is associated with a proportional linear increase in the TyG index (12). As almost one-third of patients with AMI have T2DM, these patients have more complex coronary disease, higher rates of recurrent adverse events, and a worse prognosis. To date, studies on the TyG index for the prediction of adverse cardiovascular events in patients with AMI in combination with T2DM have been published. A study by Ma and colleagues involving 776 patients with T2DM and ACS treated with PCI found that the TyG index was consistently associated with adverse cardiovascular outcomes, including total mortality, non-fatal stroke, non-fatal MI, and unplanned repeat revascularization (22). Furthermore, a study of 798 NSTE-ACS patients with T2DM showed a 2.208-fold increased risk of recurrent all-cause death, non-fatal MI, and ischemia-driven revascularization per one unit increase in TyG index [HR (95%CI): 3.208 (2.40–4.29), P < 0.001] and found that adding the TyG index to the baseline risk model had an incremental effect on the predictive value of poor cardiovascular prognosis (AUC: baseline risk model, 0.800 vs. baseline risk model + TyG index, 0.856, P < 0.001) (23). However, the association of the TyG index on no-reflow in STEMI patients with T2DM remains unclear.
In STEMI, acute hyperglycemia is a predictor of the occurrence of no-reflow and usually occurs as a result of a sudden increase in catecholamine levels. However, stress hyperglycemia may not be representative of a true acute glycemic state as it is also influenced by chronic blood glucose levels, particularly in diabetic patients (24). Recent studies (25) have shown that metrics combining acute and chronic blood glucose levels are more truly predictive of no-reflow than stress hyperglycemia. HbA1c is an important marker used in the diagnosis and treatment modalities of diabetes mellitus and is closely related to its complications and prognoses. HbA1c indicates an individual's blood sugar regulation in the last 3 months. Only a few studies have shown that HbA1c may be associated with no-reflow (26). Compared to stress hyperglycemia and HbA1c, the TyG index, consisting of lipid-related factors, glucose-related factors, and inflammation-related factors, may be a more reliable relevance of no-reflow in terms of the mechanism of no-reflow. In this study, we investigated for the first time the association of the TyG index with no-reflow after PCI in STEMI patients with T2DM. To gain a better understanding of the association of the TyG index with no-reflow, we analyzed and compared the correlation between different levels of the TyG index and no-reflow, which has not been attempted in other studies. In addition, we have also conducted the association of the TyG index with no-reflow in different subgroups, including age, gender, BMI, smoker, hypertension, eGFR, and dyslipidemia. We found that the TyG index has a favorable evaluating value for no-reflow in most subgroups.
Recent studies have shown that SGLT2 inhibitors (SGLT2i) reduce atherosclerotic plaque thickness, macrophage infiltration, and lipid arc, enhancing plaque stability in diabetic patients (27). SGLT2i also reduces intra-stent restenosis post-AMI, improving outcomes after PCI through glycemic control and anti-inflammatory effects, potentially lowering no-reflow incidence (28). Combining SGLT2i with GLP1 receptor agonists (GLP1-RA) enhances cardiac function, reduces hospitalizations, and improves survival in post-AMI patients (29). Given the link between no-reflow, inflammation, and vascular dysfunction, these drugs may reduce no-reflow. While our study did not include SGLT2i or GLP1-RA, their potential to improve no-reflow outcomes is acknowledged. Future research should explore their effects on myocardial perfusion and cardiovascular outcomes, underscoring the need for comprehensive management of T2DM patients undergoing PCI for STEMI.
There are limitations to our study. First, the generalization of the findings should be made with caution because this was a single-center study with a small sample size. Second, the laboratory parameters were only measured for the first time and lacked dynamic monitoring. This may introduce potential bias due to measurement error. Third, the TyG index for assessing the correlation with no-reflow after PCI in patients with STEMI combined with T2DM could not be directly compared with experimental methods such as HOMA-IR. Moreover, because our study did not have follow-up results, we could not draw conclusions about the prognostic relationship between TyG and clinical outcomes in STEMI patients with T2DM. Finally, as the TyG index is derived from fasting triglyceride and glucose levels, this parameter is not available for patients before PCI. Therefore, it can not predict the risk of no-reflow in non-fasting patients. Prospective cohort studies are needed to confirm our findings.
Conclusions
Our results indicate that the TyG index is closely associated with the risk of no-reflow after PCI in patients with STEMI and T2DM and that the relationship between the TyG index and no-reflow incidence is linear. Therefore, the measurement of the TyG index may be useful in the assessment of risk and in the evaluation of prognosis in this patient population.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of the General Hospital of Ningxia Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
PJ: Funding acquisition, Methodology, Writing – review & editing. JM: Data curation, Formal Analysis, Writing – original draft. PW: Data curation, Formal Analysis, Writing – original draft. SM: Data curation, Writing – review & editing. XM: Funding acquisition, Supervision, Writing – review & editing. SJ: Funding acquisition, Methodology, Writing – review & editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (Grant number 82260086); the Key Research and Development Projects of Ningxia, China (Grant number 2020BFG02002); the Natural Science Foundation of Ningxia, China (Grant number 2023AAC02069); Open competition mechanism to select the best candidates for key research projects of Ningxia Medical University (grant number: XJKF230205); Special Project for Central Government-Guided Local Science and Technology Development in Ningxia Hui Autonomous Region (grant number: 2022FRD05046) and the Natural Science Foundation of Shaanxi Province, China (Grant number 2022JQ-787).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Byrne RA, Rossello X, Coughlan JJ, Barbato E, Berry C, Chieffo A, et al. 2023 ESC guidelines for the management of acute coronary syndromes. Eur Heart J. (2023) 44(38):3720–826. doi: 10.1093/eurheartj/ehad191
2. Kunamalla A, Schaer GL. Advances in our understanding and treatment of the no-reflow phenomenon after PCI for STEMI. Cardiovasc Revasc Med. (2022) 37:102–4. doi: 10.1016/j.carrev.2022.01.018
3. Ibánez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Rev Esp Cardiol (Engl Ed). (2017) 70(12):1082. doi: 10.1016/j.rec.2017.11.010
4. Ashraf T, Khan MN, Afaque SM, Aamir KF, Kumar M, Saghir T, et al. Clinical and procedural predictors and short-term survival of the patients with no reflow phenomenon after primary percutaneous coronary intervention. Int J Cardiol. (2019) 294:27–31. doi: 10.1016/j.ijcard.2019.07.067
5. Rezkalla SH, Stankowski RV, Hanna J, Kloner RA. Management of no-reflow phenomenon in the catheterization laboratory. JACC Cardiovasc Interv. (2017) 10(3):215–23. doi: 10.1016/j.jcin.2016.11.059
6. Zhou M, Liu J, Hao Y, Liu J, Huo Y, Smith SC Jr, et al. Prevalence and in-hospital outcomes of diabetes among patients with acute coronary syndrome in China: findings from the Improving Care for Cardiovascular Disease in China-acute coronary syndrome project. Cardiovasc Diabetol. (2018) 17(1):147. doi: 10.1186/s12933-018-0793-x
7. Kelsey MD, Nelson AJ, Green JB, Granger CB, Peterson ED, McGuire DK, et al. Guidelines for cardiovascular risk reduction in patients with type 2 diabetes: JACC guideline comparison. J Am Coll Cardiol. (2022) 79(18):1849–57. doi: 10.1016/j.jacc.2022.02.046
8. Zhang Q, Xiao S, Jiao X, Shen Y. The triglyceride-glucose index is a predictor for cardiovascular and all-cause mortality in CVD patients with diabetes or pre-diabetes: evidence from NHANES 2001–2018. Cardiovasc Diabetol. (2023) 22(1):279. doi: 10.1186/s12933-023-02030-z
9. Lee SB, Ahn CW, Lee BK, Kang S, Nam JS, You JH, et al. Association between triglyceride glucose index and arterial stiffness in Korean adults. Cardiovasc Diabetol. (2018) 17(1):41. doi: 10.1186/s12933-018-0692-1
10. Park HM, Lee HS, Lee YJ, Lee JH. The triglyceride-glucose index is a more powerful surrogate marker for predicting the prevalence and incidence of type 2 diabetes mellitus than the homeostatic model assessment of insulin resistance. Diabetes Res Clin Pract. (2021) 180:109042. doi: 10.1016/j.diabres.2021.109042
11. Luo E, Wang D, Yan G, Qiao Y, Liu B, Hou J, et al. High triglyceride-glucose index is associated with poor prognosis in patients with acute ST-elevation myocardial infarction after percutaneous coronary intervention. Cardiovasc Diabetol. (2019) 18(1):150. doi: 10.1186/s12933-019-0957-3
12. Shi W, Xing L, Jing L, Tian Y, Yan H, Sun Q, et al. Value of triglyceride-glucose index for the estimation of ischemic stroke risk: insights from a general population. Nutr Metab Cardiovasc Dis. (2020) 30(2):245–53. doi: 10.1016/j.numecd.2019.09.015
13. Park K, Ahn CW, Lee SB, Kang S, Nam JS, Lee BK, et al. Elevated TyG index predicts progression of coronary artery calcification. Diabetes Care. (2019) 42(8):1569–73. doi: 10.2337/dc18-1920
14. Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. (2018) 17(1):122. doi: 10.1186/s12933-018-0762-4
15. Kosmas CE, Bousvarou MD, Kostara CE, Papakonstantinou EJ, Salamou E, Guzman E. Insulin resistance and cardiovascular disease. J Int Med Res. (2023) 51(3):3000605231164548. doi: 10.1177/03000605231164548
16. Lambie M, Bonomini M, Davies SJ, Accili D, Arduini A, Zammit V. Insulin resistance in cardiovascular disease, uremia, and peritoneal dialysis. Trends Endocrinol Metab. (2021) 32(9):721–30. doi: 10.1016/j.tem.2021.06.001
17. Taheri E, Pourhoseingholi MA, Moslem A, Hassani AH, Mousavi Jarrahi A, Asadzadeh Aghdaei H, et al. The triglyceride-glucose index as a clinical useful marker for metabolic associated fatty liver disease (MAFLD): a population-based study among Iranian adults. J Diabetes Metab Disord. (2022) 21(1):97–107. doi: 10.1007/s40200-021-00941-w
18. Li Y, Gui J, Liu H, Guo LL, Li J, Lei Y, et al. Predicting metabolic syndrome by obesity- and lipid-related indices in mid-aged and elderly Chinese: a population-based cross-sectional study. Front Endocrinol (Lausanne). (2023) 14:1201132. doi: 10.3389/fendo.2023.1201132
19. Zhao S, Yu S, Chi C, Fan X, Tang J, Ji H, et al. Association between macro- and microvascular damage and the triglyceride glucose index in community-dwelling elderly individuals: the northern Shanghai study. Cardiovasc Diabetol. (2019) 18(1):95. doi: 10.1186/s12933-019-0898-x
20. Chamroonkiadtikun P, Ananchaisarp T, Wanichanon W. The triglyceride-glucose index, a predictor of type 2 diabetes development: a retrospective cohort study. Prim Care Diabetes. (2020) 14(2):161–7. doi: 10.1016/j.pcd.2019.08.004
21. Mao Q, Zhou D, Li Y, Wang Y, Xu SC, Zhao XH. The triglyceride-glucose index predicts coronary artery disease severity and cardiovascular outcomes in patients with non-ST-segment elevation acute coronary syndrome. Dis Markers. (2019) 2019:6891537. doi: 10.1155/2019/6891537
22. Ma X, Dong L, Shao Q, Cheng Y, Lv S, Sun Y, et al. Triglyceride glucose index for predicting cardiovascular outcomes after percutaneous coronary intervention in patients with type 2 diabetes mellitus and acute coronary syndrome. Cardiovasc Diabetol. (2020) 19(1):31. doi: 10.1186/s12933-020-01006-7
23. Zhao Q, Zhang TY, Cheng YJ, Ma Y, Xu YK, Yang JQ, et al. Impacts of triglyceride-glucose index on prognosis of patients with type 2 diabetes mellitus and non-ST-segment elevation acute coronary syndrome: results from an observational cohort study in China. Cardiovasc Diabetol. (2020) 19(1):108. doi: 10.1186/s12933-020-01086-5
24. Demir ÖF, Sensoy NÖ, Akpinar E, Demir G. The stress hyperglycemic ratio can predict the no-reflow phenomenon following saphenous vein graft intervention in patients with acute coronary syndrome. Acta Diabetol. (2024) 61(3):333–41. doi: 10.1007/s00592-023-02201-0
25. Şimşek B, Çınar T, Tanık VO, Inan D, Zeren G, Avci II, et al. The association of acute–to–chronic glycemic ratio with no-reflow in patients with ST–segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Kardiol Pol. (2021) 79(2):170–8. doi: 10.33963/KP.15736
26. Toprak K, Kaplangoray M, Memioğlu T, Inanir M, Omar B, Ermis MF, et al. The HbA1c/C-peptide ratio is associated with the no-reflow phenomenon in patients with ST-elevation myocardial infarction. Angiology. (2023). doi: 10.1177/00033197231213166
27. Sardu C, Trotta MC, Sasso FC, Sacra C, Carpinella G, Mauro C, et al. SGLT2-inhibitors effects on the coronary fibrous cap thickness and MACEs in diabetic patients with inducible myocardial ischemia and multi vessels non-obstructive coronary artery stenosis. Cardiovasc Diabetol. (2023) 22(1):80. doi: 10.1186/s12933-023-01814-7
28. Marfella R, Sardu C, D’Onofrio N, Fumagalli C, Scisciola L, Sasso FC, et al. SGLT-2 inhibitors and in-stent restenosis-related events after acute myocardial infarction: an observational study in patients with type 2 diabetes. BMC Med. (2023) 21(1):71. doi: 10.1186/s12916-023-02781-2
29. Marfella R, Prattichizzo F, Sardu C, Rambaldi PF, Fumagalli C, Marfella LV, et al. GLP-1 receptor agonists-SGLT-2 inhibitors combination therapy and cardiovascular events after acute myocardial infarction: an observational study in patients with type 2 diabetes. Cardiovasc Diabetol. (2024) 23(1):10. doi: 10.1186/s12933-023-02118-6
Keywords: insulin resistance (IR), triglyceride–glucose index (TyG index), type 2 diabetes mellitus (T2DM), ST-segment elevation myocardial infarction (STEMI), no-reflow phenomenon (NRP)
Citation: Ma J, Wu P, Ma S, Ma X, Jin P and Jia S (2024) The triglyceride–glucose index is associated with no-reflow phenomenon in STEMI patients with type 2 diabetes after percutaneous coronary intervention. Front. Cardiovasc. Med. 11:1386318. doi: 10.3389/fcvm.2024.1386318
Received: 15 February 2024; Accepted: 26 August 2024;
Published: 12 September 2024.
Edited by:
Istvan Szokodi, University of Pécs, HungaryReviewed by:
Richard Daniel Rainbow, University of Liverpool, United KingdomHesham Refaat, Zagazig University, Egypt
Celestino Sardu, University of Campania Luigi Vanvitelli, Italy
Vjekoslav Tomulic, Clinical Hospital Centre Rijeka, Croatia
Copyright: © 2024 Ma, Wu, Ma, Ma, Jin and Jia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Jin, bnhqaW5waW5nMTk5MEAxNjMuY29t; Shaobin Jia, anNieG5AMTYzLmNvbQ==
 Juan Ma
Juan Ma Peng Wu
Peng Wu Shengzong Ma2
Shengzong Ma2 Ping Jin
Ping Jin Shaobin Jia
Shaobin Jia