- 1Global Health, The Geneva Learning Foundation, Geneva, Switzerland
- 2Cardiovascular Medicine, Trinity College, Dublin, Ireland
Global progress in addressing cardiovascular diseases (CVD) has been insufficient to attain the nine WHO non-communicable disease (NCD) targets and the Sustainable Development Goal (SDG) target of reducing premature NCD mortality by one-third by 2030. Progress has been slowest in low- and middle-income countries (LMIC) where addressing the CVD burden is a foremost development imperative. This review examines the reasons for this situation to propose a way forward. First, we review policy instruments to address behavioral and metabolic risk factors of CVD and health system interventions to improve cardiovascular outcomes. Second, we illustrate the financial, health workforce, health system challenges, and weak national capacity that impede the implementation of these policy instruments. Third, we discuss how LMIC might move forward despite these challenges by (a) giving due consideration to contextual and other factors that determine the success of policy implementation (b) including affordable, high-impact interventions as the core of the universal health coverage health benefit package with primary health care as the foundation and (c) by taking note of the WHO guidance provided in the 2023–2030 implementation roadmap for the Global Action Plan for prevention and control of NCD.
Introduction
The impact of non-communicable diseases (NCD), including cardiovascular diseases (CVD), increased from causing 61% of global deaths in 2000 to 74% (or 41 million) of global deaths in 2019 and from causing 47% of global disability-adjusted life years (DALYs) in 2000 to causing 63% of DALYs in 2019 (1). In 2019, CVD, diabetes, cancer, and chronic respiratory disease were collectively responsible for 81% of NCD deaths. The highest number of NCD deaths was from CVD with 17.9 million deaths (1).
CVD are increasingly significant causes of premature morbidity and mortality, particularly in low- and middle-income countries (LMIC) (2, 3). They disproportionately affect individuals in lower socioeconomic classes (4). Worldwide, CVD impart a heavy economic burden on healthcare systems (5). Furthermore, ill health and premature mortality due to CVD reduce labor productivity and economic growth (6). In addition, seeking care for CVD in LMIC often results in high out-of-pocket spending by households and worsening of poverty (7).
LMIC have to grapple with the rising burden of NCD, including CVD, together with other competing health priorities such as mental health, maternal and child health, and communicable diseases. Consequences of climate change, conflicts, and humanitarian crises add to the workload. However, despite all these challenges, even low-income countries must accelerate national responses to address the CVD burden by adopting incremental and pragmatic approaches before it completely overshadows their growth and development prospects.
Methodology
Numerous peer-reviewed publications have summarized the scope and implementation challenges of population-based and individual-level cardiovascular policies. A knowledge synthesis was done of a body of literature which describes the current status of prevention and control of CVD while providing new insights on advancing the field of cardiovascular policy and program implementation. The purpose was not to provide a comprehensive review of the literature but to present a contextualized overview of broad CVD policy and program areas that are scalable and sustainable in resource-constrained settings. The article is a condensation of the current World Health Organization (WHO), World Bank, and United Nations (UN) reports (n = 49) and guidelines (n = 5) on this subject supported by relevant literature obtained from a Medline search. It includes publications pertaining to evidence synthesis (40 systematic and scoping reviews), implementation of cardiovascular policies, and programs with a special focus on LMIC (n = 29) and epidemiology of CVD (n = 17).
Policy instruments to address behavioral and metabolic risk factors for CVD
The substantial decline in CVD mortality in high-income countries over the last four decades has been attributed, among other things, to the success of public health policies to reduce population exposure to cardiovascular risk factors and health system strategies that improved medical care through early diagnosis and treatment (8–10).
Compelling evidence demonstrates that tobacco use, harmful use of alcohol, an unhealthy diet, physical inactivity, and air pollution are risk factors for CVD and their metabolic biomarkers (e.g., diabetes, hypertension, and hyperlipidemia) (11–18). A substantial body of evidence, mostly from developed countries, also shows that tobacco use, harmful use of alcohol, unhealthy diet, physical inactivity, and metabolic biomarkers of CVD are modifiable with appropriate policies and legislative and regulatory measures (19–36).
For example, various educational policies, including awareness programs, health warnings on tobacco and alcohol products, and food nutrition labeling, have been widely used to reduce the consumption of unhealthy commodities (19–24). Regulatory policies such as smoking bans in public places and restricting the availability of unhealthy products have also given positive results (19–22, 25–28). Furthermore, fiscal policies, in the form of higher prices and taxes on tobacco, alcohol, sugar-sweetened beverages (SSB), and junk foods, have been shown to promote healthy behaviors and positively impact health outcomes (19–22, 29–34). Notably, estimates suggest that if revenues from health taxes on tobacco, alcohol, and unhealthy food were invested in health systems, these could significantly close the revenue gap of LMIC in the short term (35). A recent systematic review of 33 studies indicates that population-level interventions to promote physical activity may effectively prevent CVD and diabetes when implemented with due consideration of local contextual factors (36).
Health system policies and interventions
There is a range of evidence-based health system interventions for preventing and treating CVD (37–63). They address cardiovascular risk (38–45), cardiovascular disease in ambulatory settings (46), acute coronary syndromes and stroke (47–52), heart failure (53, 54), prevention of rheumatic heart disease (55, 56), atrial fibrillation (57), revascularization interventions (58, 59), and rehabilitation of CVD (60). If primary health care (PHC) is used as a foundation, some of these interventions can be implemented very cost-effectively even in LMIC as core components of a universal health coverage (UHC) policy (61–63). An example is early detection and treatment of behavioral and metabolic risk factors of CVD in primary care using a total risk approach (20). In resource-constrained settings, diverting a high proportion of resources to less cost-effective high technology tertiary care interventions will tend to widen health inequities (6, 19, 20).
Inadequate national capacities for implementation of cardiovascular policies
National capacity is critical for the successful implementation of CVD policies and programs. The WHO has monitored national capacities periodically since 2001 using country capacity surveys. In the survey completed by the Ministry of Health, countries are requested to provide information on the following items relating to NCD: (i) public health infrastructure, partnerships, and multisectoral collaboration; (ii) policies, strategies, and action plans; (iii) health information systems and surveillance; and (iv) health system capacity for detection and treatment. All WHO Member States (n = 194) responded to the most recent survey conducted in 2021 (64). Although most countries (86%) have included NCD in their national health plans, only two-thirds (65%) had set NCD targets aligned with the nine voluntary global targets of the global NCD action plan (65) (Table 1). A national multisectoral mechanism to oversee policy coherence and accountability of sectors beyond health was present in only 46% of countries. Over half of countries (55%) reported that their policies were multisectoral and covered all four behavioral risk factors and all four main NCD, including CVD.

Table 1. Nine voluntary global NCD targets to be attained by 2030 (66).
Taxation on alcohol and tobacco was implemented by many countries (97% and 88%, respectively). Other fiscal measures, such as taxation on sugar-sweetened beverages (47% of countries) and food high in fats, sugar, or salt (13% of countries), remained underutilized. A third of countries had policies to reduce the intake of saturated fat (35%) or to eliminate trans fat (34%), and 53% of countries reported having a salt reduction policy in place. Only around a third of countries (38%) were implementing policies to reduce the impact of the marketing of unhealthy foods on children. Policies to promote physical activity were implemented by under half of countries. Overall, the four WHO country capacity surveys indicate that an increasing number of countries are committed to developing policies and plans to address NCD. However, the resources and structures needed for effective implementation long-term need to be improved in most LMIC (67).
Insufficient global progress despite evidence-based policies and interventions
Since 2010, the progress made in NCD prevention and control, as well as barriers to progress, has been captured in 11 reports by the WHO Director-General (68–78) and four reports by the Secretary-General of the United Nations (UN) (79–82). The Heads of State have made 66 commitments at three high-level meetings of the UN General Assembly in 2011, 2014, and 2018 to accelerate the global and national NCD responses (83–85). In addition, in 2019, at the UN high-level meeting on UHC, a commitment was also made to strengthen further efforts to address NCD, including CVD (86).
Despite an array of policies, interventions, and high-level commitments, the progress made by countries has been insufficient to attain the nine voluntary targets of the Global NCD Action Plan (65) and the Sustainable Development Goal (SDG) target of 3.4 (30% reduction of premature NCD deaths by 2030) (87, 88). Only 35 countries (for women) and 30 countries (for men) are on track to meet SDG target 3.4. Most of these are high-income countries. There has also not been a significant improvement in the global trends of CVD risk factors, except for tobacco, over the past decade (89). Furthermore, despite substantial advancements in our understanding of disease progression, heart attacks and strokes are increasingly diagnosed in younger age groups (90, 91), a distressing trend. It signals increased exposure of the younger generation to behavioral risk factors such as poor diet, tobacco and alcohol use, and physical inactivity.
Grave resource inequalities between countries
There appears to be an insufficient acknowledgement of the complexity of sustainable implementation of policies and programs in “real-world” settings in LMIC. Any initiative to accelerate prevention and control of CVD in LMIC must factor in resource inequalities between countries. In addition, deficiencies across all health system building blocks, including health financing, health workforce, governance, health information, access to supplies and medicines, referral pathways, and service delivery (19, 20), need to be given due consideration.
Only about 14% of those who die of NCD before the age of 70 years are from high-income countries. However, there is a stark contrast in the distribution of global health spending compared to needs. In 2022, high-income countries with 15% of the world's population accounted for about 80% of total global health spending of US$ 9.0 trillion (92). Upper-middle-income countries, with 33% of the world population, accounted for 16%; lower-middle-income countries, with 43% of the world's population, for just under 4%; and low-income countries, with 8% of the world population for approximately 0.2%.
Furthermore, about 11% of the world's population lives in countries that spend less than US$ 50 per person per year. Per capita health spending was US$ 39 in low-income countries, US$ 125 in lower-middle-income countries, US$ 515 in upper-middle-income countries, and US$ 3,708 in high-income countries. Health financing trends and worsening macro-fiscal conditions suggest that health spending gaps between low-income and high-income countries are unlikely to narrow in the next decade (93).
As of 2021, about half the world's population—4.5 billion people—was not covered by essential health services, and about two billion people experienced financial hardship due to out-of-pocket health spending, including 344 million people living in extreme poverty (94). Higher government spending on health in LMIC is essential to ensuring equitable access to health services and easing household financial hardship.
Globally, the needs-based shortage of healthcare workers in 2013 is estimated to be about 17.4 million, of which almost 2.6 million are doctors, nearly 9 million are nurses and midwives, and the remaining include other health worker cadres. The most significant shortages of health workers are in Southeast Asia, at 6.9 million, and Africa, at 4.2 million. The global needs-based shortage of healthcare workers is projected to decline only by about 17% by 2030 (95) (Table 2).
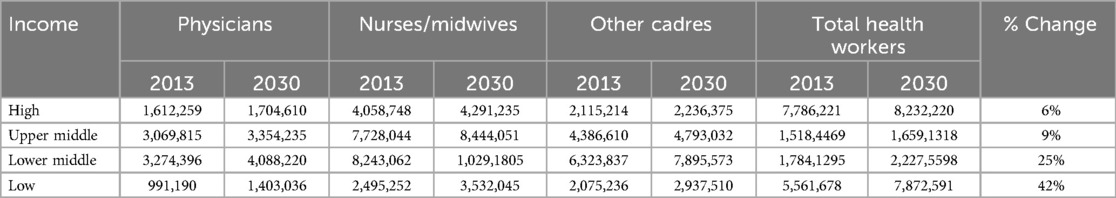
Table 2. Total numbers of health workers needed to reach the SDG threshold estimated for 2013 and forecasted for 2030 (by income group) (95).
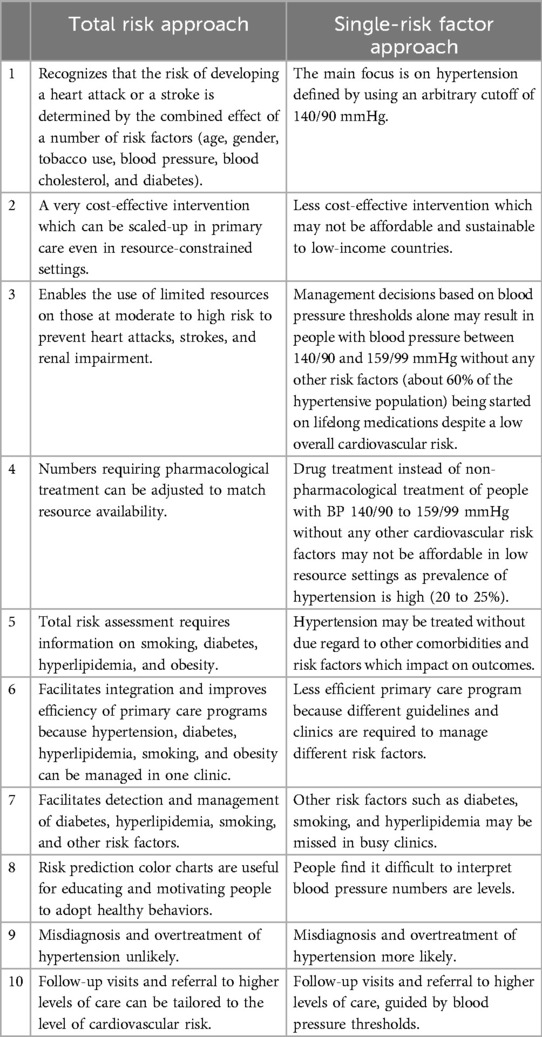
Given the above resource constraints, health system policies and programs that have been effective in high-income countries, such as single-risk factor programs on hypertension or diabetes, are unlikely to be scalable or sustainable in most LMIC. Single-risk factor hypertension control programs are resource-intensive, given that hypertension has a high prevalence in adults (20%–25%) and a very high prevalence in those above 60 years (higher than 50%). However, it is imperative that in these settings, all cardiovascular risk factors—hypertension, diabetes, hypercholesterolemia, and tobacco use—be addressed. This is feasible, provided hypertension, diabetes, and hyperlipidemia are addressed collectively in PHC using a more cost-effective total cardiovascular risk approach (61, 62, 96). This approach enables available resources to be targeted at those who are most vulnerable to developing heart attacks and strokes: individuals with established CVD or with moderate–high cardiovascular risk (20). This approach is particularly well-suited for LMIC (Table 3), and there are many successful examples of its implementation (20).
Key considerations for effective implementation of policies
Inadequate consideration of critical factors determining the successful implementation of policies and programs in “real-world” settings is an essential reason for slow progress in attaining global NCD targets (97). These factors include (i) context, (ii) sustainability, (iii) timeline, and (iv) unintended consequences.
Contextual factors, including intervention costs, feasibility, and scalability in specific settings, are essential for successful implementation. For example, healthcare budgets and organizational support (e.g., availability of health workers, staff workload, and primary care capacity) in LMIC are different from those in high-income countries. Political leadership, public health capacity, and multisectoral collaboration, identified as factors supporting public policy implementation, are also far from robust in most LMIC (98).
Sustainability is another critical consideration. For example, prioritizing only emergency care of myocardial infarction and stroke over implementation of WHO Best Buy policies and interventions is unlikely to be a sustainable approach to address the CVD burden in any LMIC. Sustainability could be strengthened by innovative financing strategies, such as taxation on unhealthy products (e.g., tobacco, alcohol, and sugary drinks) to fund NCD programs.
Vertical NCD programs initiated with short-term external aid may not be viable unless domestic funds are available for long-term implementation. Even public health policies (e.g., policies to control tobacco use, alcohol, unhealthy diet, physical inactivity, and air pollution) and PHC programs (screening, treatment, and follow-up) will need to be sustained over a long period of time to exert an impact. This requires uninterrupted resource allocation and attention to dynamic processes involved in successful implementation. Furthermore, unstable political contexts in most LMIC often make maintenance of implementation processes over time quite challenging (98, 99).
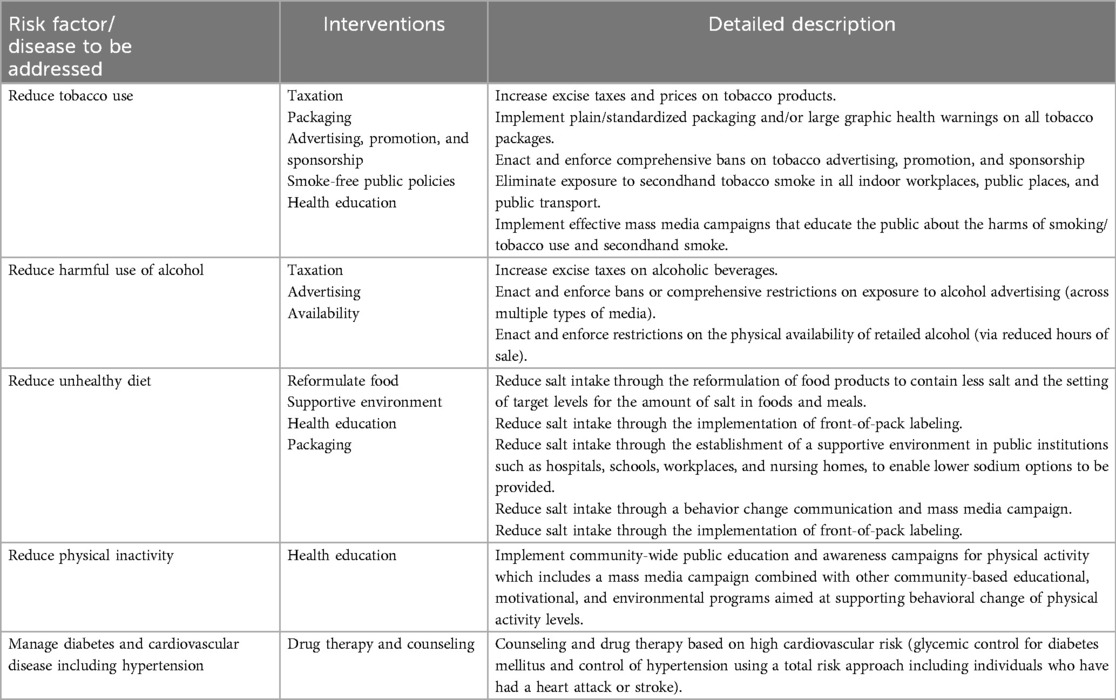
Another major challenge of policy and program implementation is the timeline. Adopting and implementing policies and programs must align with a favorable window of opportunity in the policy environment. For example, currently, as part of the global movement on UHC, many LMIC are legitimizing action for health system reforms. This is an opportunity to incorporate a core set of very cost-effective and high-impact CVD policies and interventions (e.g., WHO Best Buys, Table 4) in a health benefit package.
Unintended consequences of policies and programs should not be disregarded. In resource-constrained healthcare settings, where there is unmet demand, choosing to treat or care for one patient means a lost opportunity to treat or care for another patient (opportunity costs). For example, vertical single-cardiovascular risk factor programs, which are resource-intensive, can result in opportunity costs for maternal and child health programs. Furthermore, combining maternal and child health programs with NCD screening and treatment could mitigate the trade-offs between vertical and horizontal programs.
Paying attention to the unintended consequences of treatment policies is also essential. For instance, new hypertension treatment guidelines recommend a single-pill combination as the initial treatment for hypertension. Most single-pill combinations contain an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker, which should not be prescribed to women of childbearing age. Although the risk may be negligible in high-income country settings, in LMIC with limited access to antenatal health services, failure to discontinue these medications in pregnant women could cause foetal malformations (102), an eminently preventable risk. This example also highlights the importance of gender-sensitive health policies and the need of their adaptation to the specific needs and realities of women, particularly in limited resource settings.
Finally, before investing in implementation, in addition to the above country-specific factors, the Ministries of Health need to compare the cost-effectiveness, operational, and technical feasibility of different policies and programs to determine priorities. Through such priority setting, limited resources can be utilized to maximize coverage and impact (103–105). Many resources and tools including the WHO-CHOICE tool are available to guide this process (105–107).
Limitations
This review is based on an analysis of the current literature pertaining to the prevention and control of cardiovascular disease in “real-world” settings. It is of necessity a qualitative review because there are no relevant randomized control trials in this field, particularly in regard to the implementation of effective policies. A meta-analysis of the relevant literature would be desirable, but the heterogeneity of the sources would make this methodologically problematic and open to criticism.
Discussion: way forward
As underscored in objective one of the global NCD Action Plan, there is a continuing need to create awareness of the importance of tackling CVD, focusing more on cost-effective prevention and control policies and their roll-out (65). There are no quick fixes for complex implementation challenges which impede policy implementation for CVD prevention and control, particularly in LMIC. However, there are sustainable policy options appropriate for resource-constrained settings. The most promising pathway forward is embracing CVD policies and programs in progressive realization of UHC with PHC as the foundation. Advancing the service coverage dimension of UHC requires incremental expansion of essential health services to address CVD. Rapid scale-up of service coverage to comprehensively address CVD is unlikely to be feasible in LMIC, even with health financing reforms. A pragmatic approach would be to provide stepwise population coverage and full prepayment for a narrower scope of high-impact policies and interventions with a good return on investment, such as the WHO Best Buys (101, 108).
WHO Best Buys (Table 4) include 13 very cost-effective policy interventions to control tobacco and alcohol use, unhealthy diet, and physical inactivity. Population level services rely on strengthening multisectoral action as well as the health system. They can achieve significant health benefits and can be rapidly implemented at low cost, provided there is expertise and capacity in the Ministry of Health.
The WHO Best Buy portfolio's most cost-effective health system intervention is the total risk approach to address cardiovascular risk factors in individuals without and with CVD (primary and secondary prevention, respectively. Updated WHO risk charts, integrated protocols, and training material are available to deliver this PHC intervention even by non-physician health workers (62, 109). The protocols cover metabolic risk factors (hypertension, diabetes, hyperlipidemia) and behavioral risk factors (tobacco use, harmful use of alcohol, unhealthy diet, and physical inactivity) in individuals. This intervention is captured in global NCD targets 8 and 9 and is being implemented in many resource-constrained settings (110–127).
The global NCD target 9 is on the minimum requirements for implementing this intervention: basic diagnostics (blood pressure measurement device, a weighing scale, height measuring equipment, blood sugar and blood cholesterol measurement devices with strips, and urine strips for albumin assay) and essential medicines (aspirin, statin, angiotensin-converting enzyme inhibitor, thiazide diuretic, long-acting calcium-channel blocker, beta-blocker, metformin, and insulin).
LMIC need to leverage international development aid and innovative financing models to identify adequate resources for population coverage of this essential health service to maximize the impact and efficiency of health spending. Funds must be rationally allocated to primary care to ensure the availability of at least six basic diagnostics, eight essential medicines, and an adequately trained workforce. Even low-income countries could consider the best buys as a starting point in deliberations on a new health benefits package or refinement of an existing package.
Governments particularly in LMICs need to play a more proactive role in using fiscal and pricing policies, including taxes and subsidies to promote healthy diets and for tobacco control. Currently, 45 countries including 3 middle-income countries (Barbados, Mexico, and South Africa) have introduced SSB taxes. Implementing SSB taxes have resulted in higher SSB prices, a significant reduction in sales (15%) and an estimated decline in demand (18%) (128). While taxing non-essential energy-dense food in Mexico has resulted in increased prices and reduced sales of taxed products, fruit and vegetable subsidies are associated with a moderate increase in fruit and vegetable sales (129). Further research is required to understand the implications of food taxes and subsidies for population-level consumption, diet, and health outcomes. There is strong evidence that increased taxes that are passed on to tobacco users as higher prices reduce tobacco consumption and the health harm it causes (130). At present, except 15 countries, all others are applying some type of cigarette excise tax. It is critical to set taxes at a high level to reduce the affordability of tobacco products and make regular adjustments to increase the tax rate so that it keeps up with inflation and income growth in a country over time (131).
Since obesity is a major risk factor of cardiovascular disease and type 2 diabetes, it will not be possible to advance prevention and control of CVD even in LMIC, without tackling the growing burden of obesity simultaneously. At the 75th World Health Assembly in 2022, Member States adopted new recommendations for the prevention and management of obesity and endorsed the WHO Acceleration Plan to Stop Obesity (132). All countries need to implement multi-sector country-level action recommended in this plan.
When addressing policy gaps and system bottlenecks, the Ministries of Health in LMIC could benefit from lessons learned in other countries in implementing strategies to improve healthcare worker retention (133), governance models to improve health system efficiency and accountability (134), and programs that have enhanced access to affordable essential medicines (135, 136).
It should be underscored that while this paper focuses mainly on health system and financing issues, social determinants of health (poverty, education, urbanization, and food security) play a critical role in shaping cardiovascular outcomes. Integrating policies that address these determinants into national NCD strategies through multisectoral approaches involving the education sector, agriculture, and urban planning is critical (137). Policy action on social determinants of health as well as monitoring social determinants and health equity are essential for accelerating progress in prevention and control of CVD (138).
Actions recommended in the implementation roadmap to accelerate progress
The midpoint evaluation of the implementation of the Global Action Plan (67) noted the relatively slow progress of countries in achieving the nine global NCD targets. Based on this finding, in 2021, the 74th World Health Assembly requested that WHO develop an implementation roadmap to support the country implementation of the Global action plan for preventing and controlling noncommunicable diseases 2013–2030 (139). The implementation roadmap recommends that countries accelerate their national response to move into a sustainable path to attain global NCD targets and SDG target 3.4 to reduce premature mortality from NCD by one-third by 2030. Recommended actions include the following:
(1) identify the barriers and opportunities for scaling up, including through assessment of the status of domestic NCD responses against the nine global NCD targets and the SDG target on NCD;
(2) increase budgets for health and NCD prevention and control in a stepwise manner through domestic, bilateral, and multilateral channels, including from health taxes on unhealthy commodities (e.g., tobacco, alcohol, sugar-sweetened beverages, and unhealthy food);
(3) generate savings by cutting wasteful health spending on cost-ineffective interventions and identifying irrational use of high-cost items such as medicines and other health products that constitute a sizeable share of public sector budgets;
(4) reduce inequities in access to essential health services, including through health financing, PHC reforms, and basic benefit packages;
(5) strengthen health systems with a particular focus on PHC and include CVD interventions in UHC health benefit packages;
(6) prioritize and scale up the implementation of the most impactful and feasible interventions with a particular focus on WHO Best Buys;
(7) accelerate capacity for multisectoral and multistakeholder collaborations, including by engaging non-State actors, taking due consideration of their potential conflict of interest with public health goals;
(8) strengthen national monitoring and surveillance systems to ensure reliable national data on NCD risk factors, diseases, and mortality for data-driven policy actions and to strengthen accountability;
(9) promote engagement of people with lived experience of NCD to co-design, implementation, and accountability of health policy reforms;
(10) strengthen the national capacity for the governance of multistakeholder engagement, cross-sectoral collaboration, and result-oriented partnerships; and
(11) collaborate with international partners to support and strengthen research and innovation by working with academic partners and research institutions in countries.
Conclusion
All countries must improve CVD outcomes to reduce preventable morbidity and mortality. Every country still has options for achieving the global NCD targets, which can pave the way to attain the SDG target of reducing premature mortality from NCD by one-third by 2030. However, complex implementation challenges impede the national response in LMIC, and these are unlikely to ease out in the foreseeable future. To overcome these challenges, LMIC must adopt sustainable and resource-sensitive approaches. One such path which is affordable to all LMIC is to include the fourteen very cost-effective high-impact policies and interventions (WHO Best Buys that address the CVD burden) as a starting point of UHC with PHC as the foundation. When rolling out policies and programs, LMIC needs to be aware of the determinants of effective implementation: context, sustainability, timeline, and unintended consequences to maximize success. Increasing domestic investment in health and strengthening national capacity for ensuring action across government sectors will be indispensable for accelerating progress.
Author contributions
SM: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. IG: Conceptualization, Methodology, Validation, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organization. World Health Statistics: Monitoring Health for the SDGs, Sustainable Development Goals. Geneva: World Health Organization (2023).
2. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. (2020) 76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010
3. Leong D, McKee M, Anand SS, Schwalm JD, Teo K, Mente A, et al. Reducing the global burden of cardiovascular disease, part 1: the epidemiology and risk factors. Circ Res. (2017) 121(6):677–94. doi: 10.1161/CIRCRESAHA.117.308903
4. Williams J, Allen L, Wickramasinghe K, Mikkelsen B, Roberts N, Townsend N. A systematic review of associations between non-communicable diseases and socioeconomic status within low- and lower-middle-income countries. J Glob Health. (2018) 8(2):020409. doi: 10.7189/jogh.08.020409
5. Gheorghe A, Griffiths U, Murphy A, Legido-Quigley H, Lamptey P, Perel P. The economic burden of cardiovascular disease and hypertension in low- and middle-income countries: a systematic review. BMC Public Health. (2018) 18(1):975. doi: 10.1186/s12889-018-5806-x
6. World Health Organization. From Burden to Best Buys. Reducing the Economic Impact of Noncommunicable Diseases. Geneva: World Health Organization and World Economic Forum (2011).
7. Essue BM, Laba M, Knaul F, Chu A, Minh HV, Nguyen TKP, et al. Economic burden of chronic ill health and injuries for households in low- and middle-income countries. In: Jamison DT, Gelband H, Horton S, Jha P, Laxminarayan R, Mock CN, Nugent R, editors. Disease Control Priorities: Improving Health and Reducing Poverty. 3rd ed. Washington (DC): The International Bank for Reconstruction and Development/The World Bank (2017). Chapter 6, p. 121–46.
8. Di Cesare M, Bennett JE, Best N, Stevens GA, Danaei G, Ezzati M. The contributions of risk factor trends to cardiometabolic mortality decline in 26 industrialized countries. Int J Epidemiol. (2013) 42:838–48; 14. doi: 10.1093/ije/dyt063
9. Ezzati M, Obermeyer Z, Tzoulaki I, Mayosi BM, Elliott P, Leon DA. Contributions of risk factors and medical care to cardiovascular mortality trends. Nat Rev Cardiol. (2015) 12:508–30; 15. doi: 10.1038/nrcardio.2015.82
10. Mensah GA, Wei GS, Sorlie PD, Fine LJ, Rosenberg Y, Kaufmann PG, et al. Decline in cardiovascular mortality: possible causes and implications. Circ Res. (2017) 120(2):366–80. doi: 10.1161/CIRCRESAHA.116.309115
11. Sun K, Liu J, Ning G. Active smoking and risk of metabolic syndrome: a meta-analysis of prospective studies. PLoS One. (2012) 7(10):e47791. doi: 10.1371/journal.pone.0047791
12. Reynolds K, Lewis B, Nolen JD, Kinney GL, Sathya B, He J. Alcohol consumption and risk of stroke: a meta-analysis. JAMA. (2003) 289(5):579–88. doi: 10.1001/jama.289.5.579; Erratum in: JAMA. (2003) 289(21):2798. Lewis, Brian L [corrected to Lewis, Brian].12578491
13. Briasoulis A, Agarwal V, Messerli FH. Alcohol consumption and the risk of hypertension in men and women: a systematic review and meta-analysis. J Clin Hypertens. (2012) 14(11):792–8. doi: 10.1111/jch.12008
14. Zheng YL, Lian F, Shi Q, Zhang C, Chen YW, Zhou YH, et al. Alcohol intake and associated risk of major cardiovascular outcomes in women compared with men: a systematic review and meta-analysis of prospective observational studies. BMC Public Health. (2015) 15:773. doi: 10.1186/s12889-015-2081-y
15. Greenwood DC, Threapleton DE, Evans CE, Cleghorn CL, Nykjaer C, Woodhead C, et al. Association between sugar-sweetened and artificially sweetened soft drinks and type 2 diabetes: systematic review and dose-response meta-analysis of prospective studies. Br J Nutr. (2014) 112(5):725–34. doi: 10.1017/S0007114514001329
16. He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: cochrane systematic review and meta-analysis of randomised trials. Br Med J. (2013) 346:f1325. doi: 10.1136/bmj.f1325
17. Newman JD, Bhatt DL, Rajagopalan S, Balmes JR, Brauer M, Breysse PN, et al. Cardiopulmonary impact of particulate air pollution in high-risk populations: JACC state-of-the-art review. J Am Coll Cardiol. (2020) 76(24):2878–94. doi: 10.16/j.jacc.2020.10.020
18. World Health Organization. WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide. Geneva: World Health Organization (2021).
19. World Health Organization. Global Status Report on Noncommunicable Diseases. Geneva: World Health Organization (2010).
20. World Health Organization. Global Status Report on Noncommunicable Diseases. Geneva: World Health Organization (2014).
21. Wright A, Smith KE, Hellowell M. Policy lessons from health taxes: a systematic review of empirical studies. BMC Public Health. (2017) 17(1):583. doi: 10.1186/s12889-017-4497-z
22. Vellakkal S, Khan Z, Alavani H, Fledderjohann J, Stuckler D. Effects of public policies in the prevention of cardiovascular diseases: a systematic review of global literature. Public Health. (2022) 207:73–81. doi: 10.1016/j.puhe.2022.03.021
23. Noar SM, Francis DB, Bridges C, Sontag JM, Ribisl KM, Brewer NT. The impact of strengthening cigarette pack warnings: systematic review of longitudinal observational studies. Soc Sci Med. (2016) 164:118–29. doi: 10.1016/j.socscimed.2016.06.011
24. Campos S, Doxey J, Hammond D. Nutrition labels on pre-packaged foods: a systematic review. Public Health Nutr. (2011) 14(8):1496–506. doi: 10.1017/S1368980010003290
25. Meyers DG, Neuberger JS, He J. Cardiovascular effect of bans on smoking in public places: a systematic review and meta-analysis. J Am Coll Cardiol. (2009) 54(14):1249–55. doi: 10.1016/j.jacc.2009.07.022; Erratum in: J Am Coll Cardiol. (2009) 54(20):1902.19778665
26. Khuder SA, Milz S, Jordan T, Price J, Silvestri K, Butler P. The impact of a smoking ban on hospital admissions for coronary heart disease. Prev Med. (2007) 45(1):3–8. doi: 10.1016/j.ypmed.2007.03.011
27. Mackay DF, Irfan MO, Haw S, Pell JP. Meta-analysis of the effect of comprehensive smoke-free legislation on acute coronary events. Heart. (2010) 96(19):1525–30. doi: 10.1136/hrt.2010.199026
28. Tan CE, Glantz SA. Association between smoke-free legislation and hospitalizations for cardiac, cerebrovascular, and respiratory diseases: a meta-analysis. Circulation. (2012) 126(18):2177–83. doi: 10.1161/CIRCULATIONAHA.112.121301
29. Wagenaar AC, Tobler AL, Komro KA. Effects of alcohol tax and price policies on morbidity and mortality: a systematic review. Am J Public Health. (2010) 100(11):2270–8. doi: 10.2105/AJPH.2009.186007
30. Elliott LM, Dalglish SL, Topp SM. Health taxes on tobacco, alcohol, food and drinks in low- and middle-income countries: a scoping review of policy content, actors, process and context. Int J Health Policy Manag. (2022) 11(4):414–28. doi: 10.34172/ijhpm.2020.170
31. International Agency for Research on Cancer. Handbooks of Cancer Prevention,Tobacco Control, Vol.14: Effectiveness. Lyon: International Agency for Research on Cancer, World Health Organization (IARC) (2011).
32. Fletcher JM, Frisvold DE, Tefft N. The effects of soft drink taxes on child and adolescent consumption and weight outcomes. J Public Econ. (2010) 94(11–12):967–74. doi: 10.1016/j.jpubeco.2010.09.005
33. Powell LM, Chriqui JF, Khan T, Wada R, Chaloupka FJ. Assessing the potential effectiveness of food and beverage taxes and subsidies for improving public health: a systematic review of prices, demand and body weight outcomes. Obes Rev. (2013) 14(2):110–28. doi: 10.1111/obr.12002
34. Maniadakis N, Kapaki V, Damianidi L, Kourlaba G. A systematic review of the effectiveness of taxes on nonalcoholic beverages and high-in-fat foods as a means to prevent obesity trends. Clinicoecon Outcomes Res. (2013) 5:519–43. doi: 10.2147/CEOR.S49659
35. Lane C, Glassman A, Smitham E. Using Health Taxes to Support Revenue: An Action Agenda for the IMF and the World Bank. Centre for Global Development Policy paper 203. Centre for Global Development (2021). Available online at: https://www.cgdev.org/publication/using-health-taxes-support-revenue-action-agenda-imf-and-world-bank
36. Durão S, Burns J, Schmidt BM, Tumusiime D, Hohlfeld A, Pfadenhauer L, et al. Infrastructure, policy and regulatory interventions to increase physical activity to prevent cardiovascular diseases and diabetes: a systematic review. BMC Public Health. (2023) 23(1):112. doi: 10.1186/s12889-022-14841-y
37. Leong DP, Joseph PG, McKee M, Anand SS, Teo KK, Schwalm JD, et al. Reducing the global burden of cardiovascular disease, part 2: prevention and treatment of cardiovascular disease. Circ Res. (2017) 121(6):695–710. doi: 10.1161/CIRCRESAHA.117.311849
38. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. American College of Cardiology/American Heart Association task force on practice guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. (2014) 129:S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a
39. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. (2019) 140(11):e563–95. doi: 10.1161/CIR.0000000000000677; Erratum in: Circulation. (2019) 140(11):e647–8. Erratum in: Circulation. (2020) 141(4):e59. Erratum in: Circulation. (2020) 141(16):e773.30879339
40. Mills EJ, Wu P, Lockhart I, Thorlund K, Puhan M, Ebbert JO. Comparisons of high-dose and combination nicotine replacement therapy, varenicline, and bupropion for smoking cessation: a systematic review and multiple treatment meta-analysis. Ann Med. (2012) 44:588–97. doi: 10.3109/07853890.2012.705016
41. Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. (2016) 387:957–67. doi: 10.1016/S0140-6736(15)01225-8
42. Yusuf S, Lonn E, Pais P, Bosch J, López-Jaramillo P, Zhu J, et al. Blood-pressure and cholesterol lowering in persons without cardiovascular disease. N Engl J Med. (2016) 374:2032–43. doi: 10.1056/NEJMoa1600177
43. Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. (2012) 380:581–90. doi: 10.1016/S0140-6736(12)62027-3
44. Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. (2009) 373:1849–60. doi: 10.1016/S0140-6736(09)60503-1
45. Ray KK, Seshasai SR, Wijesuriya S, Sivakumaran R, Nethercott S, Preiss D, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. (2009) 373:1765–72. doi: 10.1016/S0140-6736(09)60697-8
46. Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. (2012) 126:e354–471. doi: 10.1161/CIR.0b013e318277d6a0
47. Bangalore S, Makani H, Radford M, Thakur K, Toklu B, Katz SD, et al. Clinical outcomes with β-blockers for myocardial infarction: a meta-analysis of randomized trials. Am J Med. (2014) 127:939–53. doi: 10.1016/j.amjmed.2014.05.032
48. Berkowitz AL, Westover MB, Bianchi MT, Chou SH. Aspirin for acute stroke of unknown etiology in resource-limited settings: a decision analysis. Neurology. (2014) 83:787–93. doi: 10.1212/WNL.0000000000000730
49. Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. (2014) 384:1929–35. doi: 10.1016/S0140-6736(14)60584-5
50. Chatterjee S, Chaudhuri D, Vedanthan R, Fuster V, Ibanez B, Bangalore S, et al. Early intravenous beta-blockers in patients with acute coronary syndrome–a meta-analysis of randomized trials. Int J Cardiol. (2013) 168:915–21. doi: 10.1016/j.ijcard.2012.10.050
51. Fanaroff AC, Hasselblad V, Roe MT, Bhatt DL, James SK, Steg PG, et al. Antithrombotic agents for secondary prevention after acute coronary syndromes: a systematic review and network meta-analysis. Int J Cardiol. (2017) 241:87–96. doi: 10.1016/j.ijcard.2017.03.046
52. Huynh T, Perron S, O’Loughlin J, Joseph L, Labrecque M, Tu JV, et al. Comparison of primary percutaneous coronary intervention and fibrinolytic therapy in ST-segment-elevation myocardial infarction: bayesian hierarchical meta-analyses of randomized controlled trials and observational studies. Circulation. (2009) 119:3101–9. doi: 10.1161/CIRCULATIONAHA.108.793745
53. Chatterjee S, Biondi-Zoccai G, Abbate A, D’Ascenzo F, Castagno D, Van Tassell B, et al. Benefits of β blockers in patients with heart failure and reduced ejection fraction: network meta-analysis. Br Med J. (2013) 346:f55. doi: 10.1136/bmj.f55
54. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized aldactone evaluation study investigators. N Engl J Med. (1999) 341:709–17. doi: 10.1056/NEJM199909023411001
55. Gerber MA, Baltimore RS, Eaton CB, Gewitz M, Rowley AH, Shulman ST, et al. Prevention of rheumatic fever and diagnosis and treatment of acute streptococcal pharyngitis: a scientific statement from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, the Interdisciplinary Council on Functional Genomics and Translational Biology, and the Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. (2009) 119:1541–51. doi: 10.1161/CIRCULATIONAHA.109.191959
56. Manyemba J, Mayosi BM. Penicillin for secondary prevention of rheumatic fever. Cochrane Database Syst Rev. (2002) 2002(3):CD002227. doi: 10.1002/14651858.CD002227
57. Investigators AWGotA, Connolly S, Pogue J, Hart R, Pfeffer M, Hohnloser S, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the atrial fibrillation clopidogrel trial with irbesartan for prevention of vascular events (ACTIVE W): a randomised controlled trial. Lancet. (2006) 367:1903–12. doi: 10.1016/S0140-6736(06)68845-4
58. Writing Committee Members; Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. (2022) 79(2):e21–129. doi: 10.1016/j.jacc.2021.09.006; Erratum in: J Am Coll Cardiol. (2022) 79(15):1547.34895950
59. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. (2016) 387:1723–31. doi: 10.1016/S0140-6736(16)00163-X
60. Chan DK, Cordato D, O’Rourke F, Chan DL, Pollack M, Middleton S, et al. Comprehensive stroke units: a review of comparative evidence and experience. Int J Stroke. (2013) 8:260–4. doi: 10.1111/j.1747-4949.2012.00850.x
61. World Health Organization. Prevention and Control of Noncommunicable Diseases: Guidelines for Primary Health Care in Low-Resource Settings. Geneva: World Health Organization (2012). https://pubmed.ncbi.nlm.nih.gov/23844451/
62. World Health Organization. Package of Essential Noncommunicable (PEN) Disease Interventions for Primary Health Care in Low-Resource Settings. Implementation Tools. Geneva: World Health Organization (2010). https://iris.who.int/handle/10665/133525
63. World Health Organization. Package of Essential Noncommunicable (PEN) Disease Interventions for Primary Health Care in Low-Resource Settings. Geneva: World Health Organization (2020). https://iris.who.int/handle/10665/334186
64. World Health Organization. Assessing National Capacity for the Prevention and Control of Noncommunicable Diseases: Report of the 2021 Global Survey. Geneva: World Health Organization (2023).
65. World Health Organization. Global action plan for the prevention and control of noncommunicable diseases 2013-2020. In: World Health Organization, editor. Follow-up to the Political Declaration of the High-Level Meeting of the General Assembly on the Prevention and Control of Noncommunicable Diseases. Geneva: World Health Organization (2013). p. 1–55.
66. World Health Organization. Advancing the Global Agenda on Prevention and Control of Noncommunicable Diseases 2000 to 2020: Looking Forwards to 2030. Geneva: World Health Organization (2023).
67. World Health Organization. Mid-Point Evaluation of the Implementation of the WHO Global Action Plan for the Prevention and Control of Noncommunicable Diseases. Geneva: World Health Organization (2020).
68. World Health Organization. Prevention and Control of Noncommunicable Diseases. WHO’s Role in the Preparation, Implementation and Follow-up to the High-Level Meeting of the United Nations General Assembly on the Prevention and Control of Noncommunicable Diseases (September 2011): Report by the Secretariat. Geneva: World Health Organization (2011). EB12/17; Available online at: https://apps.who.int/iris/handle/10665/3219
69. World Health Organization. Prevention and Control of Noncommunicable Diseases. Outcomes of the High-Level Meeting of the General Assembly on the Prevention and Control of Noncommunicable Diseases and the First Global Ministerial Conference on Healthy Lifestyles and Noncommunicable Disease Control. Geneva: World Health Organization (2011). EB130/6; Available online at: https://apps.who.int/iris/handle/10665/23733
70. Resolution WHA66.10. Follow-up to the political declaration of the high-level meeting of the general assembly on the prevention and control of non-communicable diseases. In: World Health Organization, editor. Sixty-sixth World Health Assembly, Geneva, 20–27 May 2013: Resolutions and Decisions. Geneva: World Health Organization (2013). p. 16–20. WHA66/2013/REC/1; Available online at: https://apps.who.int/iris/handle/10665/150207
71. World Health Organization. Prevention and Control of Noncommunicable Diseases: Report by the Secretariat. Sixty-Seventh World Health Assembly. Geneva: World Health Organization (2014). A67/14; Available online at: https://apps.who.int/iris/handle/10665/152571
72. World Health Organization. Follow-up to the 2014 High-level meeting of the United Nations General Assembly to Undertake a Comprehensive Review and Assessment of the Progress Achieved in the Prevention and Control of Noncommunicable Diseases: Report by the Director-General. Geneva: World Health Organization (2015). A68/11; Available online at: https://apps.who.int/iris/handle/10665/252839
73. World Health Organization. Prevention and Control of Noncommunicable Diseases: Responses to Specific Assignments in Preparation for the Third High-level meeting of the United Nations General Assembly on the Prevention and Control of non-communicable diseases in 2018. Geneva: World Health Organization (2016). EB138.R4; Available online at: https://apps.who.int/iris/handle/10665/250788
74. World Health Organization. Preparation for the Third High-level meeting of the General Assembly on the Prevention and Control of Non-communicable diseases, to be Held in 2018. Geneva: World Health Organization (2017). WHA70.11; Available online at: https://apps.who.int/iris/handle/10665/275670
75. World Health Organization. Preparation for the Third High-Level Meeting of the General Assembly on the Prevention and Control of Non-communicable Diseases, to be Held in 2018. Geneva: World Health Organization (2018). WHA71.2; Available online at: https://apps.who.int/iris/handle/10665/279458
76. World Health Organization. Follow-up to the High-level meetings of the United Nations General Assembly on Health-Related Issues: Prevention and Control of Noncommunicable Diseases: Report by the Director-General. Geneva; World Health Organization (2019). A72/19; Available online at: https://apps.who.int/iris/handle/10665/328648
77. World Health Organization. Follow-Up to the High-Level Meetings of the United Nations General Assembly on Health-Related Issues: Political Declaration of the Third High-Level Meeting of the General Assembly on the Prevention and Control of Non-communicable Diseases: Report by the Director-General. Geneva: World Health Organization (2020). EB146/7; Available online at: https://apps.who.int/iris/handle/10665/355903
78. World Health Organization. Political Declaration of the Third High-Level Meeting of the General Assembly on the Prevention and Control of Non-communicable Diseases. Mid-point Evaluation of the Implementation of the WHO Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020. Executive Summary. Report by the Secretariat. Geneva: World Health Organization (2021). A74/10 Add.1; Available online at: https://apps.who.int/iris/handle/10665/357858
79. World Health Organization. Progress on the Prevention and Control of Non-communicable Diseases: Report of the Secretary-General. New York: United Nations (2017). A/72/662; Available online at: https://digitallibrary.un.org/record/1474584?ln=en
80. World Health Organization. Note by the Secretary-General transmitting the Report by the Director-General of the World Health Organization on the Global Status of Non-communicable Diseases, with a Particular Focus on the Development Challenges Faced by Developing Countries. New York: United Nations (2010). A/65/362; Available online at: https://digitallibrary.un.org/record/691392?ln=en
81. World Health Organization. Prevention and Control of Non-communicable Diseases: Report of the Secretary-General. New York: United Nations (2011). A/66/83; Available online at: https://digitallibrary.un.org/record/704820?ln=en
82. World Health Organization. Note by the Secretary-General Transmitting the Report of the Director-General of the World Health Organization on the Prevention and Control of Non-communicable Diseases. New York: United Nations (2013). A/68/650; Available online at: https://digitallibrary.un.org/record/763111?ln=en
83. Resolution 66/2. Political Declaration of the High-Level Meeting of the General Assembly on the Prevention and Control of non-Communicable Diseases: Resolution Adopted by the General Assembly on 19 September 2011. New York: United Nations (2011). A/RES/66/2; Available online at: https://digitallibrary.un.org/record/720106?ln=en
84. United Nations. Outcome Document of the High-Level Meeting of the General Assembly on the Comprehensive Review and Assessment of the Progress Achieved in the Prevention and Control of Non-communicable Diseases. New York: United Nations (2014). A/68/L.53; Available online at: https://digitallibrary.un.org/record/774662?ln=en
85. Resolution 73/2. Political Declaration of the Third High-Level Meeting of the General Assembly on the Prevention and Control of non-Communicable Diseases. New York: United Nations (2018). A/RES/73.2; Available online at: https://digitallibrary.un.org/record/1648984?ln=en
86. Resolution 74/2. Political Declaration of the High-Level Meeting on Universal Health Coverage. New York: United Nations (2019). A/RES/74/2; Available online at: https://digitallibrary.un.org/record/3833350?ln=en
87. Transforming our world: the 2030 Agenda for Sustainable Development. Resolution Adopted by the General Assembly on 25 September 2015. New York: United Nations (2015). A/RES/70/1; Available online at: https://digitallibrary.un.org/record/3923923?ln=en
88. NCD Countdown 2030 collaborators. NCD Countdown 2030: worldwide trends in non-communicable disease mortality and progress towards sustainable development goal target 3.4. Lancet. (2018) 392(10152):1072–88. doi: 10.1016/S0140-6736(18)31992-5
89. World Health Organization. World Health Statistics 2020: Monitoring Health for the SDGs. Geneva: World Health Organization (2020). Available online at: https://apps.who.int/iris/handle/10665/332070
90. Li C, Baek J, Sanchez BN, Morgenstern LB, Lisabeth LD. Temporal trends in age at ischemic stroke onset by ethnicity. Ann Epidemiol. (2018) 28(10):686–690.e2. doi: 10.1016/j.annepidem.2018.07.010
91. Joundi RA, Smith EE, Yu AYX, Rashid M, Fang J, Kapral MK. Temporal and age-specific trends in acute stroke incidence: a 15-year population-based study of administrative data in Ontario, Canada. Can J Neurol Sci. (2021) 48(5):685–9. doi: 10.1017/cjn.2020.257
92. World Health Organization. Global Spending on Health: Rising to the Pandemic’s Challenges. Geneva: World Health Organization (2022).
93. Dieleman JL, Templin T, Sadat N, Reidy P, Chapin A, Foreman K, et al. National spending on health by source for 184 countries between 2013 and 2040. Lancet. (2016) 387(10037):2521–35. doi: 10.1016/S0140-6736(16)30167-2
94. World Health Organization. Tracking Universal Health Coverage: 2023 Global Monitoring Report. Geneva: World Health Organization and International Bank for Reconstruction and Development/The World Bank (2023).
95. World Health Organization. Health Workforce Requirements for Universal Health Coverage and the Sustainable Development Goals. Human Resources for Health Observer Series No. 17. Geneva: World Health Organization (2017). Available online at: http://apps.who.int/iris/bitstream/handle/10665/250330/9789241511407-eng.pdf?sequence=.
96. Moran A, Rasmussen P, Zhao R, Coxson PG, Guzman D, Pletcher M, et al. Should global cardiovascular risk guide treatment of stage one hypertension? A cost-effectiveness analysis. Circulation. (2012) 125:AMP019. doi: 10.1161/circ.125.suppl_10.AMP019
97. Yi SS, Lee M, Russo R, Li Y, Trinh-Shevrin C, Kwon SC. Dietary policies and programs: moving beyond efficacy and into “real-world” settings. Health Equity. (2021) 5(1):194–202. doi: 10.1089/heq.2020.0050
98. Nilsen P, Bernhardsson S. Context matters in implementation science: a scoping review of determinant frameworks that describe contextual determinants for implementation outcomes. BMC Health Serv Res. (2019) 19:189. doi: 10.1186/s12913-019-4015-3
99. Shelton RC, Lee M. Sustaining evidence-based interventions and policies: recent innovations and future directions in implementation science. Am J Public Health. (2019) 109(S2):S132–4. doi: 10.2105/AJPH.2018.304913
100. World Health Organization (WHO). ‘Best Buys’ and Other Recommended Interventions for the Prevention and Control of Noncommunicable Diseases. Updated (2017) Appendix 3 of the Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013-2020. Geneva: World Health Organization (2017).
101. World Health Organization. Saving Lives, Spending Less: A Strategic Response to Noncommunicable Diseases. Geneva: World Health Organization (2018). Available online at: https://iris.who.int/handle/10665/272534 License: CC BY-NC-SA 3.0 IGO.
102. Cooper WO, Hernandez-Diaz S, Arbogast PG, Dudley JA, Dyer S, Gideon PS, et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med. (2006) 354(23):2443–51. doi: 10.1056/NEJMoa055202
103. Tromp N, Baltussen R. Mapping of multiple criteria for priority setting of health interventions: an aid for decision makers. BMC Health Serv Res. (2012) 12:454. doi: 10.1186/1472-6963-12-454
104. Chalkidou K, Glassman A, Marten R, Vega J, Teerawattananon Y, Tritasavit N, et al. Priority-setting for achieving universal health coverage. Bull World Health Organ. (2016) 94(6):462–7. doi: 10.2471/BLT.15.155721
105. Watkins DA, Nugent RA. Setting priorities to address cardiovascular diseases through universal health coverage in low- and middle-income countries. Heart Asia. (2017) 9(1):54–8. doi: 10.1136/heartasia-2015-010690
106. Watkins DA, Jamison DT, Mills T, Atun T, Danforth K, Glassman A, et al. Universal health coverage and essential packages of care. In: Jamison DT, Gelband H, Horton S, Jha P, Laxminarayan R, Mock CN, Nugent R, editors. Disease Control Priorities: Improving Health and Reducing Poverty. 3rd ed. Washington (DC): The International Bank for Reconstruction and Development/The World Bank (2017). Chapter 3, p. 43–65.
107. Bertram MY, Chisholm D, Watts R, Waqanivalu T, Prasad V, Varghese C. Cost-Effectiveness of population level and individual level interventions to combat non-communicable disease in eastern sub-Saharan Africa and South East Asia: a WHO-CHOICE analysis. Int J Health Policy Manag. (2021) 10(11):724–33. doi: 10.34172/ijhpm.2021.37
108. Mendis S, Graham I, Narula J. Addressing the global burden of cardiovascular diseases; need for scalable and sustainable frameworks. Glob Heart. (2022) 17(1):48. doi: 10.5334/gh.1139
109. WHO CVD Risk Chart Working Group. World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Health. (2019) 7(10):e1332–45. doi: 10.1016/S2214-109X(19)30318-3
110. Mokhtari M, Khalil D, Farzadfar F, Daroudi R, Asadi-Lari M. The burden of cardiovascular disease attributable to modifiable risk factors and cost-effectiveness analysis of IraPEN program in the general population of Iran. Med J Islam Repub Iran. (2022) 36:73. doi: 10.47176/mjiri.36.73
111. Azmin M, Mohebi F, Yoosefi M, Ahmadi N, Shirazi S, Modirian M, et al. The incremental cost of implementing the world health organization package of essential non-communicable (PEN) diseases interventions in Iran. PLOS Glob Public Health. (2023) 3(2):e0000449. doi: 10.1371/journal.pgph.0000449
112. Gaziano TA, Abrahams-Gessel S, Denman CA, Montano CM, Khanam M, Puoane T, et al. An assessment of community health workers’ ability to screen for cardiovascular disease risk with a simple, non-invasive risk assessment instrument in Bangladesh, Guatemala, Mexico, and South Africa: an observational study. Lancet Glob Health. (2015) 3:e556–63. doi: 10.1016/S2214-109X(15)00143-6
113. Aye LL, Tripathy JP, Maung Maung T, Oo MM, Nwe ML, Thu HMM, et al. Experiences from the pilot implementation of the package of essential non-communicable disease interventions (PEN) in Myanmar, 2017–18: a mixed methods study. PLoS One. (2020) 15(2):e0229081. doi: 10.1371/journal.pone.0229081
114. Hyon CS, Nam KY, Sun HC, Garg R, Shrestha SM, Ok KU, et al. Package of essential noncommunicable disease (PEN) interventions in primary health-care settings in the democratic People’s Republic of Korea: a feasibility study. WHO South East Asia J Public Heal. (2017) 6:69–73. doi: 10.4103/2224-3151.213794
115. Wangchuk D, Virdi N, Garg R, Mendis S, Nair N, Wangchuk D, et al. Package of essential noncommunicable disease (PEN) interventions in primary health-care settings of Bhutan: a performance assessment study. WHO South East Asia J Public Heal. (2014) 3:154. doi: 10.4103/2224-3151.206731
116. Kontsevaya A, Farrington J. Implementation of a Package of Essential Noncommunicable (PEN) Disease Interventions in Kyrgyzstan: Evaluation of Effects and Costs in Bishkek After One Year. World Health Organization EURO (2017). Available online at: http://www.euro.who.int/__data/assets/pdf_file/0007/337498/PEN-report-2017-Kyrgyzstan-FINAL-ENG.pdf
117. Moh Thit WM, Myat Thein KM, Maung KT, Jat TR, Ko K, Lwin KS, et al. Integrating NCD Services into Primary Healthcare Through Package of Essential Non-communicable Disease Interventions in Myanmar. HelpAge International Myanmar (2017). Available online at: http://sphcmyanmar.org/policy-brief/
118. Nyarko KM, Ameme DK, Ocansey D, Commeh E, Markwei MT, Ohene S-A. Capacity assessment of selected health care facilities for the pilot implementation of package for essential non-communicable diseases (PEN) intervention in Ghana. Pan Afr Med J. (2016) 25:16. doi: 10.11604/pamj.supp.2016.25.1.6252
119. Praveen D, Peiris D, MacMahon S, Mogulluru K, Raghu A, Rodgers A, et al. Cardiovascular disease risk and comparison of different strategies for blood pressure management in rural India. BMC Public Health. (2018) 18:1264. doi: 10.1186/s12889-018-6142-x
120. Boudreaux C, Barango P, Adler A, Kabore P, McLaughlin A, Mohamed MOS, et al. Addressing severe chronic NCDs across Africa: measuring demand for the package of essential non-communicable disease interventions-plus (PEN-Plus). Health Policy Plan. (2022) 37(4):452–60. doi: 10.1093/heapol/czab142
121. Bollars C, Naseri T, Thomsen R, Varghese C, Sørensen K, de Vries N, et al. Adapting the WHO package of essential noncommunicable disease interventions. Samoa Bull World Health Organ. (2018) 96(8):578–83. doi: 10.2471/BLT.17.203695
122. Parashar A, Willeboordse M, Gupta AK, van Schayck OCP. Effect of brief interventions to promote behavior change on clinical outcomes of selected non-communicable diseases: the World Health Organization (WHO) package of essential non-communicable disease (PEN) interventions for primary health care settings—study protocol of a quasi-experimental study. Contemp Clin Trials. (2022) 113:106675. doi: 10.1016/j.cct.2022.106675
123. Martinez RE, Quintana R, Go JJ, Villones MS, Marquez MA. Use of the WHO package of essential noncommunicable disease interventions after typhoon haiyan. Western Pac Surveill Response J. (2015 6(1):18–20. doi: 10.5365/WPSAR.2015.6.3.HYN_024
124. Aryal BK, Daud M, Thapa A, Mahotra A, Ale Magar S, Malla CK. Assesssment of health facilities for implementation of non-communicable disease package. J Nepal Health Res Counc. (2018) 16(2):149–55. doi: 10.3126/jnhrc.v16i2.20301
125. Collins DRJ, Laatikainen T, Shoismatuloeva M, Mahmudzoha I, Rahimov Z, Sultonova D, et al. Evaluation and pilot implementation of essential interventions for the management of hypertension and prevention of cardiovascular diseases in primary health care in the republic of Tajikistan. F1000Res. (2019) 8:1639. doi: 10.12688/f1000research.20234.1
126. Ayat SA, Rostami S, Khadivi R. The incidence and mortality rates due to stroke and myocardial infarction following implementing the package of essential non-communicable diseases: a historical cohort study. J Cardiovasc Thorac Res. (2022) 14(3):191–6. doi: 10.34172/jcvtr.2022.32
127. Collins D, Inglin L, Laatikainen T, Ciobanu A, Curocichin G, Salaru V, et al. Implementing a package of noncommunicable disease interventions in the Republic of Moldova: two-year follow-up data. Prim Health Care Res Dev. (2020) 21:e39. doi: 10.1017/S1463423620000420
128. Andreyeva T, Marple K, Marinello S, MooreTE LP. Outcomes following taxation of sugar-sweetened beverages. A systematic review and meta-analysis. JAMA Network Open. (2022) 5(6):e2215276. doi: 10.1001/jamanetworkopen.2022.15276
129. Andreyeva T, Marple K, Moore TE, Powell LP. Evaluation of economic and health outcomes associated with food taxes and subsidies. A systematic review and meta-analysis. JAMA Network Open. (2022) 5(6):e2214371. doi: 10.1001/jamanetworkopen.2022.14371
130. World Health Organization. Implementing Fiscal and Pricing Policies to Promote Healthy Diets: A Review of Contextual Factors. Geneva: World Health Organization (2021).
131. WHO Technical Manual on Tobacco Tax Policy and Administration. Geneva: World Health Organization (2021).
132. World Health Organization. Acceleration Plan to Support Member States in Implementing the Recommendations for the Prevention and Management of Obesity Over the Life Course. Geneva: World Health Organization (2022). Available online at: https://apps.who.int/gb/ebwha/pdf_files/WHA75-REC1/A75_REC1_Interactive_en.pdf#page=105
133. Mbemba GI, Gagnon MP, Hamelin-Brabant L. Factors influencing recruitment and retention of healthcare workers in rural and remote areas in developed and developing countries: an overview. J Public Health Afr. (2016) 7(2):565. doi: 10.4081/jphia.2016.565
134. George J, Jack S, Gauld R, Colbourn T, Stokes T. Impact of health system governance on healthcare quality in low-income and middle-income countries: a scoping review. BMJ Open. (2023) 13(12):e073669. doi: 10.1136/bmjopen-2023-073669
135. Chattu VK, Singh B, Pattanshetty S, Reddy S. Access to medicines through global health diplomacy. Health Promot Perspect. (2023) 13(1):40–6. doi: 10.34172/hpp.2023.05
136. Tachkov K, Savova A, Manova M, Petrova G. Tackling reimbursement challenges to fair access to medicines—introduction to the topic. Expert Rev Pharmacoecon Outcomes Res. (2023) 23(6):597–606. doi: 10.1080/14737167.2023.2203384
137. Saunders M, Barr B, McHale P, Hamelmann C. Key Policies for Addressing the Social Determinants of Health and Health Inequities. Copenhagen: WHO Regional Office for Europe (2017). (Health Evidence Network (HEN) synthesis report 52).
138. World Health Organization. Operational Framework for Monitoring Social Determinants of Health Equity. Geneva: World Health Organization (2024).
139. World Health Organization. Implementation Road Map 2023–2030 for the Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2030. Geneva: World Health Organization (2022). https://apps.who.int/gb/ebwha/pdf_files/WHA75/A75_10Add8-en.pdf
Keywords: cardiovascular disease, inequities, low- and middle-income countries, health spending, health workforce availability
Citation: Mendis S and Graham I (2024) Prevention and control of cardiovascular disease in “real-world” settings: sustainable implementation of effective policies. Front. Cardiovasc. Med. 11:1380809. doi: 10.3389/fcvm.2024.1380809
Received: 2 February 2024; Accepted: 31 October 2024;
Published: 19 November 2024.
Edited by:
Stefanos Tyrovolas, Parc Sanitari Sant Joan de Déu, SpainReviewed by:
Edna J. Nava-Gonzalez, Autonomous University of Nuevo León, MexicoJaideep Menon, Amrita Vishwa Vidyapeetham University, India
Ibtihal Fadhil, NCD Alliance Eastern Mediterranean, Kuwait
Copyright: © 2024 Mendis and Graham. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shanthi Mendis, cHJvZi5zaGFudGhpLm1lbmRpc0BnbWFpbC5jb20=
 Shanthi Mendis
Shanthi Mendis Ian Graham
Ian Graham