
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Cardiovasc. Med. , 02 May 2024
Sec. General Cardiovascular Medicine
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1376616
This article is part of the Research Topic New Insights of Cardiac Rehabilitation: from Basic to Translational and Clinical Research Vol. II View all 5 articles
Human and animal studies have demonstrated the mechanisms and benefits of aerobic exercise for both cardiovascular and neurovascular health. Aerobic exercise induces neuroplasticity and neurophysiologic reorganization of brain networks, improves cerebral blood flow, and increases whole-body VO2peak (peak oxygen consumption). The effectiveness of a structured cardiac rehabilitation (CR) program is well established and a vital part of the continuum of care for people with cardiovascular disease. Individuals post stroke exhibit decreased cardiovascular capacity which impacts their neurologic recovery and extends disability. Stroke survivors share the same risk factors as patients with cardiac disease and can therefore benefit significantly from a comprehensive CR program in addition to neurorehabilitation to address their cardiovascular health. The inclusion of individuals with stroke into a CR program, with appropriate adaptations, can significantly improve their cardiovascular health, promote functional recovery, and reduce future cardiovascular and cerebrovascular events thereby reducing the economic burden of stroke.
Vascular disease impacts not only the cardiovascular system but also the cerebrovascular system with ischemic heart disease and stroke being the top-ranked causes of disability (1). Individuals with stroke have significant atherosclerotic complications within their vascular system and a high prevalence of cardiovascular disease (2–4). Approximately 75% of stroke patients exhibit cardiovascular comorbidities, and the prevalence of coronary artery disease among stroke survivors is estimated to range from 32% to 65% (5, 6). Stroke leaves approximately one-third of survivors dependent, straining healthcare systems (7). The burden of stroke is especially high in individuals with cardiac disease (8), and treatment of stroke necessitates the modification of risk factors for cardiac disease, including changes in physical activity levels (2, 9). Physical activity is body movement that is produced by the contraction of skeletal muscle that substantially increases energy expenditure, whereas exercise is a type of physical activity that involves planned, structured bodily movement done to maintain or improve physical fitness (10). More specifically, aerobic exercise is a type of exercise that involves large muscle groups, is rhythmic and repetitive in nature, can be maintained continuously, and increases heart rate and oxygen consumption (11). Aerobic exercise has been shown to have many benefits in improving cardiovascular health in individuals with stroke (12–14). Hence, the American Heart Association (AHA) and American Stroke Association (ASA) guidelines recommend moderate-intensity aerobic exercise, lasting 20–60 min, performed 3–5 times a week for stroke survivors (15–17). However, a major challenge is that widespread implementation of these guidelines have proven very difficult (18–21). Studies have shown that individuals with stroke spend approximately 80% of their time in sedentary behaviors (22, 23). As a result of a significant reduction in their normal physical activity, stroke survivors are in fact more likely to experience the negative effects of deconditioning, including the increased risk of cardiovascular events and decreased cardiovascular health (24, 25). In the first 90 days post-stroke, 19% of survivors of stroke experience at least one serious cardiac adverse event, and cardiac mortality is the second leading cause of death during this critical time (26–28).
The recommendations for physical activity for secondary stroke prevention in individuals with deficits after stroke include supervision by a cardiac rehabilitation professional in addition to routine rehabilitation (29). Programs that use theoretical models of behavior change, proven techniques, and multidisciplinary support are needed to ensure that individuals with stroke receive the physical activity interventions required. Cardiac rehabilitation is a comprehensive, structured program that provides prescribed aerobic exercise in addition to medical evaluation, cardiac risk factor modification, education, and counseling (30). Traditional cardiac rehabilitation (CR) meets the recommended requirements by the AHA/ASA and if properly adapted can be implemented even in individuals with movement deficits after a stroke to improve cardiovascular health (31–35). However, more investigation is needed to determine the optimal intensity, timing, and long-term benefits of CR post-stroke. Importantly, CR provides a structure for physical activity in a motivational environment and risk factor education that will provide individuals recovering from stroke with a comprehensive program and opportunities to change risk factor behaviors similar to individuals with cardiac disease. This review synthesizes the available literature regarding the benefits and mechanisms of how CR, and its major component, aerobic exercise, affects both cardiovascular and neurovascular health.
Aerobic exercise challenges homeostasis and mediates substantial changes across the cardiovascular, pulmonary, musculoskeletal, neurovascular and metabolic systems, which occur in cells, tissues, and organs in direct response to the increased metabolic demand on the body (Figure 1) (36). The main mechanisms through which aerobic exercise impacts cardiovascular health include improved oxygen delivery, changes in vasculature and peripheral tissues, regulation of inflammation, and promotion of vasodilation and angiogenesis. Exercise upregulates the expression of hypoxia-inducible factor (HIF)1α and peroxisome proliferator-activated receptor γ co-activator 1α (PGC1α), which leads to the production of vascular endothelial growth factor (VEGF)—a critical factor for angiogenesis (37–39). Moreover, physical exercise increases mitochondrial biogenesis in skeletal muscle, myotubes, and cardiomyocytes (40, 41). Additionally, several studies have presented evidence indicating that exercise training induces changes in mitochondrial reactive oxygen species (ROS) production and mitochondrial permeability transition pore (mPTP) activation, ultimately reducing myocardial ischemia/reperfusion injury (42–44). Another benefit of exercise on cardiovascular health includes inducing a long-term anti-inflammatory effect on the body. This is inversely related to the increased inflammation typically seen in cardiovascular disease. Myokines, which are released from skeletal muscle during physical activity, play a pivotal role in mediating these anti-inflammatory effects and promote inter-tissue communication to mediate further cardiovascular benefits. Physical activity enhances myocardial perfusion and elevates high-density lipoprotein (HDL) cholesterol levels, collectively alleviating strain on the heart and enhancing cardiovascular function in both healthy individuals and those with underlying conditions (45–48).
It is important to underscore that aerobic exercise, in addition to major effects on cardiovascular health, also has a significant impact on the neurovascular system and brain function in both physiological and pathological conditions. Many clinical and pre-clinical studies demonstrate substantial structural and functional changes in the brain induced by aerobic exercise. Specifically, moderate to high-intensity aerobic exercise promotes neurogenesis and enhances synaptic plasticity, driven by elevated release of neurotrophic growth factors, such as brain-derived neurotrophic factor (BDNF), insulin-like growth factor-I (IGF-I), vascular endothelial growth factor (VEGF), and nerve growth factor (NGF) (49–52). Studies with animals and humans have shown that aerobic exercise over extended periods enhances brain activity and increases the size of various brain regions, such as the prefrontal, parietal, and temporal cortices (53–55). Furthermore, several clinical studies demonstrate the effects of aerobic exercise on improving cerebral blood flow, promoting neuroplasticity, and increasing whole-body VO2 peak oxygen consumption in patients with stroke (56–60). Additionally, engaging in high-intensity exercise has been shown to increase brain glutamate and gamma-amino-butyric acid (GABA) levels (61, 62).
The changes induced by aerobic exercise play a crucial role in post-stroke neurovascular health as they directly contribute to neural stem cell differentiation, neuronal plasticity, and have neuroprotective effects. For example, it was shown that increased VEGF levels are central to stimulating angiogenesis around the lesion and facilitating neurological recovery post-acute stroke (63, 64). Similarly, aerobic exercise significantly increases levels of BDNF and its primary receptor, tropomyosin receptor kinase B (TrkB), involved in neuroprotective effects during conditions like cerebral ischemia and neurotoxicity, as well as synaptotagmin, a synaptic protein crucial for learning and memory (65–69). Importantly, clinical studies have demonstrated the impact of aerobic exercise training on cortical excitability and alterations in neural circuits in individuals with stroke (70–74). Preclinical studies have unveiled additional mechanistic insights into the effects of aerobic exercise, including regulation of neuro-inflammation and mitochondrial biogenesis, as well as the repair of the blood-brain barrier (75–77). Overall, the current body of evidence strongly indicates that aerobic exercise is associated with changes in brain function and can potentiate neuroplasticity, which is vital following stroke.
The effects of CR, of which aerobic exercise is an integral component are multifaceted with numerous studies, both clinical and pre-clinical, demonstrating its multi-system benefits. CR is a well-established rehabilitation treatment for individuals recovering from cardiac disease. CR has evolved from exercise only to a comprehensive treatment program that includes: physician-prescribed exercise; cardiac risk factor modification, including behavioral and lifestyle health education (nutrition, smoking cessation, as well as lipid and blood pressure management), psychosocial counseling, behavioral intervention, outcomes assessment and physician supervision (78, 79). The specific exercise prescription includes intensity (dose), frequency, duration, and progression. CR traditionally consists of three phases, with the first two delivered in a supervised hospital/center-based setting (80). Phase I refers to inpatient rehabilitation during the index hospitalization. Phase II refers to physician-supervised, outpatient-monitored physical activity several months after discharge. Individuals participate in up to 36 sessions in a graduated exercise program. Phase III consists of continuing to an unmonitored outpatient exercise program. The Centers for Medicare & Medicaid Services (CMS) has determined that the evidence is sufficient to support that cardiac rehabilitation is reasonable and necessary following acute myocardial infarction within the preceding 12 months, coronary artery bypass graft (CABG), stable angina pectoris, heart valve repair or replacement, percutaneous transluminal coronary angioplasty (PTCA) or coronary stenting, heart or heart-lung transplant and stable chronic systolic heart failure (79). Multimodal rehabilitative interventions including exercise training, lifestyle modification, and psychological intervention, has proven to be an effective strategy to improve cardiovascular health and overall well-being after cardiovascular disease (81).
The numerous benefits of CR after cardiac disease led to the adoption of recommendations for CR as standard of care after cardiovascular disease. Recent systematic review and meta-analysis of exercise-based CR in people with existing coronary heart disease shows a reduction in cardiovascular mortality (relative risk: 0.74; 95% confidence interval: 0.64–0.86) and a reduction in the risk of hospital admissions (relative risk: 0.82; 95% confidence interval: 0.70–0.96). Most studies (70%) showed higher levels of health-related quality of life in 1 or more domains following exercise-based CR compared with control subjects (80). Another systematic review and meta-analysis showed that CR reduces cardiovascular mortality, recurrent cardiac events, hospitalizations and improves health-related quality of life, highlighting the cost-effectiveness of CR (82). As such, the American Heart Association (AHA) and American College of Cardiology (ACC) consider CR a Class I indication for several cardiac conditions (83). Similarly, for patients with peripheral artery disease (84), the AHA/ACC has given Class I, Level A support for a supervised exercise program similar to cardiac rehabilitation. Based on the strength of existing evidence-based research, clinical practice guidelines approve cardiac rehabilitation as an effective adjunct to medical management to improve outcomes in patients after cardiac and vascular disease.
In the United States, stroke survivors benefit from aerobic programs with similar dosing to cardiac rehabilitation. Individuals with stroke have many of the same risk factors as individuals with cardiac disease (Figure 2). Current practice guidelines after stroke recommend structured aerobic exercise for at least 3–5 days per week, for a minimum of 20 min per session, with at least 5 min warm-up and cool-down periods (15, 85, 86). Preliminary evidence demonstrates that high-intensity interval training is associated with improvements in functional, cardiovascular, and neuroplastic outcomes post-stroke (87). Exercise programs that include aerobic exercise can improve aerobic capacity, walking ability, vascular health, and quality of life of stroke survivors. Specifically, exercise programs comprised of moderate intensity, 3 days per week, for 20 weeks should be considered for greater effect on cardiorespiratory fitness, muscle strength, and walking capacity in stroke patients (88). Exercise is also able to positively affect cognitive performance and neurovascular health in patients with known vascular disease, with a potential dose-response relationship (89). Beyond aerobic exercise alone, studies have acknowledged the feasibility and effectiveness of adapting exercise-based cardiac rehabilitation interventions for individuals with stroke who have mild to moderate disability (32, 33, 90, 91). Furthermore, comprehensive models like CR that integrate exercise, lifestyle modification, and medication management are also beneficial for individuals after TIA or stroke (18, 34, 92). Stroke survivors who participated in a comprehensive stroke recovery program incorporating modified cardiac rehabilitation had decreased all-cause mortality, improved overall function, improved cardiovascular performance (31, 93), and showed a 22% reduction in acute care hospital readmissions (94). Comprehensive programs that show clinical promise include secondary prevention strategies that integrate exercise interventions into a comprehensive risk-reduction program for stroke survivors (15, 95, 96).
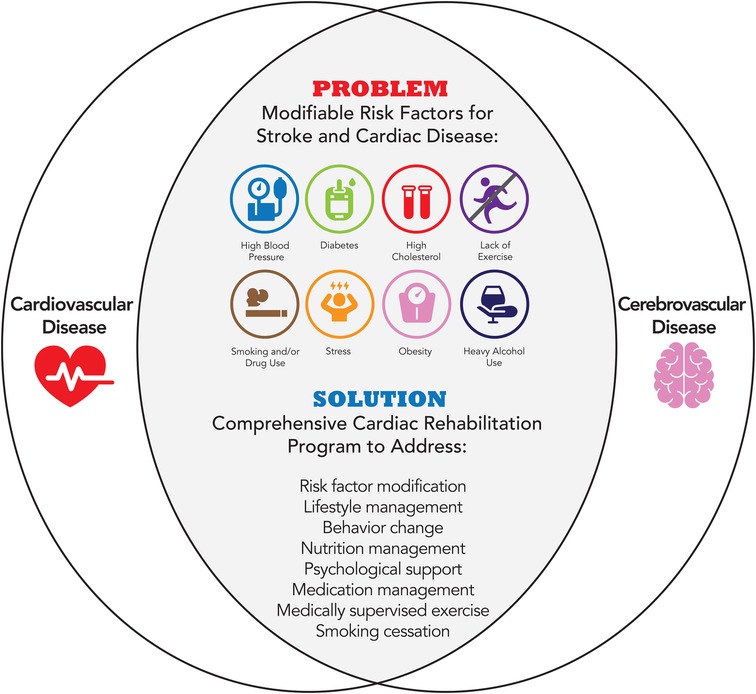
Figure 2. Modifiable risk factors for cardiovascular and cerebrovascular disease addressed by cardiac rehabilitation.
In addition to recommendations that all eligible stroke survivors receive an inpatient rehabilitation stay for comprehensive interprofessional post-stroke care (Class 1, Level B) (16), in patients with deficits after stroke that impair their ability to exercise, supervision of an exercise program by a health care professional such as a physical therapist or cardiac rehabilitation professional, can be beneficial for secondary stroke prevention (Class 2a, Level-Expert Opinion) (29).
Several of the benefits of CR are a result of supervised and progressive exercise training to promote sustained physical activity. In 2010, the American Heart Association defined a novel construct of cardiovascular health to promote a paradigm shift from a focus solely on disease treatment to one inclusive of positive health promotion and preservation across the life course in populations and individuals (97). More recently, the elements of this construct were updated, and the American Heart Association issued a presidential advisory introducing an enhanced approach to assessing cardiovascular health: Life's Essential 8. The components of Life's Essential 8 include diet, physical activity, nicotine exposure, sleep health, body mass index, blood lipids, blood glucose, and blood pressure (98). Of these components, physical activity appears to have an effect on all the other components. Interventions that include physical activity have a greater effect on adherence to recommended diets than interventions that do not include physical activity, including in individuals with obesity (99–101). Aerobic exercise can assist with smoking cessation (102), and improve sleep health (103–105). Supervised aerobic exercise training was effective in reducing fasting plasma glucose (9.38 mg/dl lower), total cholesterol (20.24 mg/dl lower), triacylglycerol (19.34 mg/dl lower), and low-density lipoprotein cholesterol (11.88 mg/dl lower) (106, 107). Aerobic exercise reduces blood pressure in both hypertensive and normotensive persons (108). Exercise training increases VO2max, along with cardiovascular capacity and endurance (78). In addition. exercise training has multiple other beneficial effects including improving endothelial function, and cardiac mitochondrial function (78, 109, 110). Thus, the promotion of a healthy lifestyle including aerobic exercise as prescribed in cardiac rehabilitation plays a critical role in optimizing cardiometabolic health in patients with vascular diseases (e.g., cardiovascular disease, cerebrovascular disease, and peripheral vascular disease) (111).
The benefits of aerobic activity extend beyond the vascular system. In addition to cerebrovascular and cardiovascular diseases (e.g., stroke, coronary artery disease, chronic heart failure, and peripheral vascular disease), aerobic activity is recommended for the treatment of various conditions including chronic kidney disease, Parkinson's disease, Alzheimer's disease, chronic obstructive pulmonary disease, low back pain, osteoporosis, osteoarthritis, obesity, depression, anxiety disorders, and several cancers (e.g., colon cancer, prostate cancer, lung cancer) (112). In a study of 750,302 U.S. veterans aged 30–95 years, cardiorespiratory fitness was measured using peak METs achieved during a standardized exercise treadmill test, and they were followed for a median of 10.2 years. Cardiorespiratory fitness was inversely associated with all-cause mortality and graded across the age spectrum, sex, and race. The mortality risk for the least fit individuals (20th percentile) was 4-fold higher compared with extremely fit individuals, and being unfit carried a greater risk than any of the other risk factors examined, including smoking, diabetes, cardiovascular disease, and hypertension (113). These data suggest that it is imperative to facilitate physical fitness through aerobic exercise, particularly in individuals who are at increased risk for cardiovascular and cerebrovascular disease.
Despite published guidelines and robust evidence on the benefits of aerobic exercise for cardiovascular and cerebrovascular health, the majority of the US population does not meet current recommendations. It is therefore critical to effectively motivate and initiate behavior change, especially in clinical populations. A recent science advisory from the American Heart Association presented a framework, the 5A Model (assess, advise, agree, assist, and arrange) and strategies to promote efficient lifestyle-related behavior change counseling for patients with cardiovascular disease (114).
For patients with stroke, these arrangements should ideally include CR which has been found to be safe and effective in this population. However, presently, stroke survivors are excluded from standard cardiovascular conditioning programs as part of the standard of care. There is mounting evidence showing that exercise therapies or other rehabilitation strategies delivered during the early stages of recovery post-stroke (subacute phase; 1 week to 6 months) amplify spontaneous recovery and enhance the biological recovery process (115–117). Decreased cardiovascular capacity is one of the main reasons for limited activity and a major contributor to excess morbidity, hospitalization, mortality, and poor quality of life post-stroke. Currently, 50%–60% of stroke patients are readmitted to the hospital within the first year post their stroke event (118, 119). Hence, it is critical to prioritize improvement in cardiovascular health in the stroke survivor population.
A limitation of this narrative review is that it does not provide a quantitative assessment of the effects of CR for stroke survivors. Future meta-analysis to evaluate the benefits of CR may compare the timing of the initiation of a CR program, the aerobic exercise prescription (dose, duration, and intensity), as well as type of stroke.
Structured, medically supervised programs like CR, which address physical activity, aerobic exercise, risk factor education, and behavior change, definitively improve the overall health of cardiac patients (80, 120) and reduce hospital readmissions, secondary cardiovascular events, and mortality (121, 122). Since stroke survivors have many of the same risk factors as cardiac patients (e.g., hypertension, diabetes, hyperlipidemia, and obesity), a comprehensive program such as CR could potentially improve their quality of life and vascular health, as well as reduce hospital readmission and mortality (31, 94). Future studies should demonstrate the value of adding cardiac rehabilitation to standard neurorehabilitation in reducing disability, and all-cause mortality for individuals with stroke.
SC: Writing – review & editing, Writing – original draft. TF: Writing – review & editing, Writing – original draft. HP: Writing – review & editing, Writing – original draft. DH: Writing – review & editing. PR: Writing – review & editing, Writing – original draft.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Diseases GBD, Injuries C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396(10258):1204–22. doi: 10.1016/S0140-6736(20)30925-9
2. Banerjee C, Chimowitz MI. Stroke caused by atherosclerosis of the Major intracranial arteries. Circ Res. (2017) 120(3):502–13. doi: 10.1161/CIRCRESAHA.116.308441
3. Adams RJ, Chimowitz MI, Alpert JS, Awad IA, Cerqueria MD, Fayad P, et al. Coronary risk evaluation in patients with transient ischemic attack and ischemic stroke: a scientific statement for healthcare professionals from the stroke council and the council on clinical cardiology of the American heart association/American stroke association. Stroke. (2003) 34(9):2310–22. doi: 10.1161/01.STR.0000090125.28466.E2
4. Touze E, Varenne O, Chatellier G, Peyrard S, Rothwell PM, Mas JL. Risk of myocardial infarction and vascular death after transient ischemic attack and ischemic stroke: a systematic review and meta-analysis. Stroke. (2005) 36(12):2748–55. doi: 10.1161/01.STR.0000190118.02275.33
5. Roth EJ. Heart disease in patients with stroke: incidence, impact, and implications for rehabilitation. Part 1: classification and prevalence. Arch Phys Med Rehabil. (1993) 74(7):752–60. doi: 10.1016/0003-9993(93)90038-C
6. Black-Schaffer RM, Kirsteins AE, Harvey RL. Stroke rehabilitation. 2. Co-morbidities and complications. Arch Phys Med Rehabil. (1999) 80(5 Suppl 1):S8–16. doi: 10.1016/S0003-9993(99)90096-5
7. Lucas-Noll J, Clua-Espuny JL, Lleixa-Fortuno M, Gavalda-Espelta E, Queralt-Tomas L, Panisello-Tafalla A, et al. The costs associated with stroke care continuum: a systematic review. Health Econ Rev. (2023) 13(1):32. doi: 10.1186/s13561-023-00439-6
8. Robinson K, Katzenellenbogen JM, Kleinig TJ, Kim J, Budgeon CA, Thrift AG, et al. Large burden of stroke incidence in people with cardiac disease: a linked data cohort study. Clin Epidemiol. (2023) 15:203–11. doi: 10.2147/CLEP.S390146
9. Turan TN, Nizam A, Lynn MJ, Egan BM, Le NA, Lopes-Virella MF, et al. Relationship between risk factor control and vascular events in the SAMMPRIS trial. Neurology. (2017) 88(4):379–85. doi: 10.1212/WNL.0000000000003534
10. National Health Interview Survey, Centers for Disease Control and Prevention, National Center for Health Statistics. Available online at: https://www.cdc.gov/nchs/nhis/physical_activity/pa_glossary.htm (Accessed March 18, 2024).
11. Patel H, Alkhawam H, Madanieh R, Shah N, Kosmas CE, Vittorio TJ. Aerobic vs anaerobic exercise training effects on the cardiovascular system. World J Cardiol. (2017) 9(2):134–8. doi: 10.4330/wjc.v9.i2.134
12. Potempa K, Braun LT, Tinknell T, Popovich J. Benefits of aerobic exercise after stroke. Sports Med. (1996) 21(5):337–46. doi: 10.2165/00007256-199621050-00003
13. Ivey FM, Macko RF, Ryan AS, Hafer-Macko CE. Cardiovascular health and fitness after stroke. Top Stroke Rehabil. (2005) 12(1):1–16. doi: 10.1310/GEEU-YRUY-VJ72-LEAR
14. Billinger SA, Mattlage AE, Ashenden AL, Lentz AA, Harter G, Rippee MA. Aerobic exercise in subacute stroke improves cardiovascular health and physical performance. J Neurol Phys Ther. (2012) 36(4):159–65. doi: 10.1097/NPT.0b013e318274d082
15. Billinger SA, Arena R, Bernhardt J, Eng JJ, Franklin BA, Johnson CM, et al. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American heart association/American stroke association. Stroke. (2014) 45(8):2532–53. doi: 10.1161/STR.0000000000000022
16. Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. (2016) 47(6):e98–e169. doi: 10.1161/STR.0000000000000098
17. Stein J, Katz DI, Black Schaffer RM, Cramer SC, Deutsch AF, Harvey RL, et al. Clinical performance measures for stroke rehabilitation: performance measures from the American heart association/American stroke association. Stroke. (2021) 52(10):e675–700. doi: 10.1161/STR.0000000000000388
18. Tang A, Closson V, Marzolini S, Oh P, McIlroy W, Brooks D. Cardiac rehabilitation after stroke-need and opportunity. J Cardiopulm Rehabil Prev. (2009) 29(2):97–104. doi: 10.1097/HCR.0b013e31819a00d4
19. Marzolini S. Including patients with stroke in cardiac rehabilitation: barriers and facilitators. J Cardiopulm Rehabil Prev. (2020) 40(5):294–301. doi: 10.1097/HCR.0000000000000540
20. Toma J, Hammond B, Chan V, Peacocke A, Salehi B, Jhingan P, et al. Inclusion of people poststroke in cardiac rehabilitation programs in Canada: a missed opportunity for referral. CJC Open. (2020) 2(4):195–206. doi: 10.1016/j.cjco.2020.01.007
21. Howes T, Mahenderan N, Freene N. Cardiac rehabilitation: are people with stroke or transient ischaemic attack being included? A cross-sectional survey. Heart Lung Circ. (2020) 29(3):483–90. doi: 10.1016/j.hlc.2019.03.018
22. Tieges Z, Mead G, Allerhand M, Duncan F, van Wijck F, Fitzsimons C, et al. Sedentary behavior in the first year after stroke: a longitudinal cohort study with objective measures. Arch Phys Med Rehabil. (2015) 96(1):15–23. doi: 10.1016/j.apmr.2014.08.015
23. Fini NA, Holland AE, Keating J, Simek J, Bernhardt J. How physically active are people following stroke? Systematic review and quantitative synthesis. Phys Ther. (2017) 97(7):707–17. doi: 10.1093/ptj/pzx038
24. Fini NA, Bernhardt J, Churilov L, Clark R, Holland AE. A 2-year longitudinal study of physical activity and cardiovascular risk in survivors of stroke. Phys Ther. (2021) 101(2):1–9. doi: 10.1093/ptj/pzaa205
25. MacKay-Lyons MJ, Howlett J. Exercise capacity and cardiovascular adaptations to aerobic training early after stroke. Top Stroke Rehabil. (2005) 12(1):31–44. doi: 10.1310/RDQM-JTGL-WHAA-XYBW
26. Prosser J, MacGregor L, Lees KR, Diener HC, Hacke W, Davis S, et al. Predictors of early cardiac morbidity and mortality after ischemic stroke. Stroke. (2007) 38(8):2295–302. doi: 10.1161/STROKEAHA.106.471813
27. Sposato LA, Lam M, Allen B, Shariff SZ, Saposnik G, Group PS. First-ever ischemic stroke and incident Major adverse cardiovascular events in 93 627 older women and men. Stroke. (2020) 51(2):387–94. doi: 10.1161/STROKEAHA.119.028066
28. Chen Z, Venkat P, Seyfried D, Chopp M, Yan T, Chen J. Brain-heart interaction: cardiac complications after stroke. Circ Res. (2017) 121(4):451–68. doi: 10.1161/CIRCRESAHA.117.311170
29. Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American heart association/American stroke association. Stroke. (2021) 52(7):e364–467. doi: 10.1161/STR.0000000000000375
30. National Coverage Determination, Cardiac Rehabilitation Programs 20.10.: Centers for Medicare and Medicaid Services. Available online at: https://www.cms.gov/medicare-coverage-database/view/ncd.aspx?ncdid=36&ncdver=2&doctype=all&year=2023&sortBy=title&bc=14#:∼:text=General,modification%2C%20education%2C%20and%20counseling (Accessed March 18, 2024).
31. Cuccurullo SJ, Fleming TK, Zinonos S, Cosgrove NM, Cabrera J, Kostis JB, et al. Stroke recovery program with modified cardiac rehabilitation improves mortality, functional & cardiovascular performance. J Stroke Cerebrovasc Dis. (2022) 31(5):106322. doi: 10.1016/j.jstrokecerebrovasdis.2022.106322
32. Reynolds H, Steinfort S, Tillyard J, Ellis S, Hayes A, Hanson ED, et al. Feasibility and adherence to moderate intensity cardiovascular fitness training following stroke: a pilot randomized controlled trial. BMC Neurol. (2021) 21(1):132. doi: 10.1186/s12883-021-02052-8
33. Tang A, Marzolini S, Oh P, McIlroy WE, Brooks D. Feasibility and effects of adapted cardiac rehabilitation after stroke: a prospective trial. BMC Neurol. (2010) 10:40. doi: 10.1186/1471-2377-10-40
34. Prior PL, Hachinski V, Unsworth K, Chan R, Mytka S, O'Callaghan C, et al. Comprehensive cardiac rehabilitation for secondary prevention after transient ischemic attack or mild stroke: I: feasibility and risk factors. Stroke. (2011) 42(11):3207–13. doi: 10.1161/STROKEAHA.111.620187
35. Kirk H, Kersten P, Crawford P, Keens A, Ashburn A, Conway J. The cardiac model of rehabilitation for reducing cardiovascular risk factors post transient ischaemic attack and stroke: a randomized controlled trial. Clin Rehabil. (2014) 28(4):339–49. doi: 10.1177/0269215513502211
36. Hawley JA, Hargreaves M, Joyner MJ, Zierath JR. Integrative biology of exercise. Cell. (2014) 159(4):738–49. doi: 10.1016/j.cell.2014.10.029
37. Pinckard K, Baskin KK, Stanford KI. Effects of exercise to improve cardiovascular health. Front Cardiovasc Med. (2019) 6:69. doi: 10.3389/fcvm.2019.00069
38. Arany Z. PGC-1 coactivators and skeletal muscle adaptations in health and disease. Curr Opin Genet Dev. (2008) 18(5):426–34. doi: 10.1016/j.gde.2008.07.018
39. Tao L, Bei Y, Zhang H, Xiao J, Li X. Exercise for the heart: signaling pathways. Oncotarget. (2015) 6(25):20773–84. doi: 10.18632/oncotarget.4770
40. Vega RB, Konhilas JP, Kelly DP, Leinwand LA. Molecular mechanisms underlying cardiac adaptation to exercise. Cell Metab. (2017) 25(5):1012–26. doi: 10.1016/j.cmet.2017.04.025
41. Riehle C, Wende AR, Zhu Y, Oliveira KJ, Pereira RO, Jaishy BP, et al. Insulin receptor substrates are essential for the bioenergetic and hypertrophic response of the heart to exercise training. Mol Cell Biol. (2014) 34(18):3450–60. doi: 10.1128/MCB.00426-14
42. Boulghobra D, Dubois M, Alpha-Bazin B, Coste F, Olmos M, Gayrard S, et al. Increased protein S-nitrosylation in mitochondria: a key mechanism of exercise-induced cardioprotection. Basic Res Cardiol. (2021) 116(1):66. doi: 10.1007/s00395-021-00906-3
43. French JP, Hamilton KL, Quindry JC, Lee Y, Upchurch PA, Powers SK. Exercise-induced protection against myocardial apoptosis and necrosis: mnSOD, calcium-handling proteins, and calpain. FASEB J. (2008) 22(8):2862–71. doi: 10.1096/fj.07-102541
44. Powers SK, Quindry JC, Kavazis AN. Exercise-induced cardioprotection against myocardial ischemia-reperfusion injury. Free Radic Biol Med. (2008) 44(2):193–201. doi: 10.1016/j.freeradbiomed.2007.02.006
45. Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev. (2008) 88(3):1009–86. doi: 10.1152/physrev.00045.2006
46. Fontana L. Interventions to promote cardiometabolic health and slow cardiovascular ageing. Nat Rev Cardiol. (2018) 15(9):566–77. doi: 10.1038/s41569-018-0026-8
47. Che L, Li D. The effects of exercise on cardiovascular biomarkers: new insights, recent data, and applications. Adv Exp Med Biol. (2017) 999:43–53. doi: 10.1007/978-981-10-4307-9_3
48. Laughlin MH, Bowles DK, Duncker DJ. The coronary circulation in exercise training. Am J Physiol Heart Circ Physiol. (2012) 302(1):H10–23. doi: 10.1152/ajpheart.00574.2011
49. van Praag H. Exercise and the brain: something to chew on. Trends Neurosci. (2009) 32(5):283–90. doi: 10.1016/j.tins.2008.12.007
50. Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. (2008) 9(1):58–65. doi: 10.1038/nrn2298
51. Lauretta G, Ravalli S, Maugeri G, D'Agata V, Rosa MD, Musumeci G. The impact of physical exercise on the hippocampus in physiological condition and ageing-related decline: current evidence from animal and human studies. Curr Pharm Biotechnol. (2022) 23(2):180–9. doi: 10.2174/1389201022666210405142611
52. Boyne P, Meyrose C, Westover J, Whitesel D, Hatter K, Reisman DS, et al. Effects of exercise intensity on acute circulating molecular responses poststroke. Neurorehabil Neural Repair. (2020) 34(3):222–34. doi: 10.1177/1545968319899915
53. Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. (2004) 101(9):3316–21. doi: 10.1073/pnas.0400266101
54. Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, et al. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. (2003) 58(2):176–80. doi: 10.1093/gerona/58.2.M176
55. Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. (2006) 61(11):1166–70. doi: 10.1093/gerona/61.11.1166
56. Luo L, Meng H, Wang Z, Zhu S, Yuan S, Wang Y, et al. Effect of high-intensity exercise on cardiorespiratory fitness in stroke survivors: a systematic review and meta-analysis. Ann Phys Rehabil Med. (2020) 63(1):59–68. doi: 10.1016/j.rehab.2019.07.006
57. Saunders DH, Sanderson M, Hayes S, Johnson L, Kramer S, Carter DD, et al. Physical fitness training for stroke patients. Cochrane Database Syst Rev. (2020) 3(3):CD003316. doi: 10.1002/14651858.CD003316.pub7
58. Saunders B, Gobbi N. Location location location: muscle glycogen content and endurance exercise. J Physiol. (2021) 599(1):19–21. doi: 10.1113/JP280808
59. Luft AR, Macko RF, Forrester LW, Villagra F, Ivey F, Sorkin JD, et al. Treadmill exercise activates subcortical neural networks and improves walking after stroke: a randomized controlled trial. Stroke. (2008) 39(12):3341–50. doi: 10.1161/STROKEAHA.108.527531
60. Macko RF, Ivey FM, Forrester LW, Hanley D, Sorkin JD, Katzel LI, et al. Treadmill exercise rehabilitation improves ambulatory function and cardiovascular fitness in patients with chronic stroke: a randomized, controlled trial. Stroke. (2005) 36(10):2206–11. doi: 10.1161/01.STR.0000181076.91805.89
61. Coxon JP, Cash RFH, Hendrikse JJ, Rogasch NC, Stavrinos E, Suo C, et al. GABA concentration in sensorimotor cortex following high-intensity exercise and relationship to lactate levels. J Physiol. (2018) 596(4):691–702. doi: 10.1113/JP274660
62. Maddock RJ, Casazza GA, Fernandez DH, Maddock MI. Acute modulation of cortical glutamate and GABA content by physical activity. J Neurosci. (2016) 36(8):2449–57. doi: 10.1523/JNEUROSCI.3455-15.2016
63. Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. (2000) 106(7):829–38. doi: 10.1172/JCI9369
64. Yang JP, Liu HJ, Liu XF. VEGF promotes angiogenesis and functional recovery in stroke rats. J Invest Surg. (2010) 23(3):149–55. doi: 10.3109/08941930903469482
65. Liu YF, Chen HI, Yu L, Kuo YM, Wu FS, Chuang JI, et al. Upregulation of hippocampal TrkB and synaptotagmin is involved in treadmill exercise-enhanced aversive memory in mice. Neurobiol Learn Mem. (2008) 90(1):81–9. doi: 10.1016/j.nlm.2008.02.005
66. Soule J, Messaoudi E, Bramham CR. Brain-derived neurotrophic factor and control of synaptic consolidation in the adult brain. Biochem Soc Trans. (2006) 34(Pt 4):600–4. doi: 10.1042/BST0340600
67. Bathina S, Das UN. Brain-derived neurotrophic factor and its clinical implications. Arch Med Sci. (2015) 11(6):1164–78. doi: 10.5114/aoms.2015.56342
68. Chen J, Qin J, Su Q, Liu Z, Yang J. Treadmill rehabilitation treatment enhanced BDNF-TrkB but not NGF-TrkA signaling in a mouse intracerebral hemorrhage model. Neurosci Lett. (2012) 529(1):28–32. doi: 10.1016/j.neulet.2012.09.021
69. Binder DK. The role of BDNF in epilepsy and other diseases of the mature nervous system. Adv Exp Med Biol. (2004) 548:34–56. doi: 10.1007/978-1-4757-6376-8_3
70. Sivaramakrishnan A, Subramanian SK. A systematic review on the effects of acute aerobic exercise on neurophysiological, molecular, and behavioral measures in chronic stroke. Neurorehabil Neural Repair. (2023) 37(2-3):151–64. doi: 10.1177/15459683221146996
71. Penna LG, Pinheiro JP, Ramalho SHR, Ribeiro CF. Effects of aerobic physical exercise on neuroplasticity after stroke: systematic review. Arq Neuropsiquiatr. (2021) 79(9):832–43. doi: 10.1590/0004-282x-anp-2020-0551
72. Li X, Charalambous CC, Reisman DS, Morton SM. A short bout of high-intensity exercise alters ipsilesional motor cortical excitability post-stroke. Top Stroke Rehabil. (2019) 26(6):405–11. doi: 10.1080/10749357.2019.1623458
73. Madhavan S, Stinear JW, Kanekar N. Effects of a single session of high intensity interval treadmill training on corticomotor excitability following stroke: implications for therapy. Neural Plast. (2016) 2016:1686414. doi: 10.1155/2016/1686414
74. Murdoch K, Buckley JD, McDonnell MN. The effect of aerobic exercise on neuroplasticity within the motor cortex following stroke. PLoS One. (2016) 11(3):e0152377. doi: 10.1371/journal.pone.0152377
75. Chen C, Nakagawa S. Physical activity for cognitive health promotion: an overview of the underlying neurobiological mechanisms. Ageing Res Rev. (2023) 86:101868. doi: 10.1016/j.arr.2023.101868
76. Zhang Y, Liu Y, Bi X, Hu C, Ding F, Ding W. Therapeutic approaches in mitochondrial dysfunction, inflammation, and autophagy in uremic cachexia: role of aerobic exercise. Mediators Inflamm. (2019) 2019:2789014. doi: 10.1155/2019/2789014
77. Sakakima H, Khan M, Dhammu TS, Shunmugavel A, Yoshida Y, Singh I, et al. Stimulation of functional recovery via the mechanisms of neurorepair by S-nitrosoglutathione and motor exercise in a rat model of transient cerebral ischemia and reperfusion. Restor Neurol Neurosci. (2012) 30(5):383–96. doi: 10.3233/RNN-2012-110209
78. McMahon SR, Ades PA, Thompson PD. The role of cardiac rehabilitation in patients with heart disease. Trends Cardiovasc Med. (2017) 27(6):420–5. doi: 10.1016/j.tcm.2017.02.005
79. memo Cd. (2014). Available online at: https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&NCAId=270 (Accessed January 21, 2024).
80. Anderson L, Oldridge N, Thompson DR, Zwisler AD, Rees K, Martin N, et al. Exercise-based cardiac rehabilitation for coronary heart disease: cochrane systematic review and meta-analysis. J Am Coll Cardiol. (2016) 67(1):1–12. doi: 10.1016/j.jacc.2015.10.044
81. Bellmann B, Lin T, Greissinger K, Rottner L, Rillig A, Zimmerling S. The beneficial effects of cardiac rehabilitation. Cardiol Ther. (2020) 9(1):35–44. doi: 10.1007/s40119-020-00164-9
82. Dibben GO, Faulkner J, Oldridge N, Rees K, Thompson DR, Zwisler AD, et al. Exercise-based cardiac rehabilitation for coronary heart disease: a meta-analysis. Eur Heart J. (2023) 44(6):452–69. doi: 10.1093/eurheartj/ehac747
83. Bracewell NJ, Plasschaert J, Conti CR, Keeley EC, Conti JB. Cardiac rehabilitation: effective yet underutilized in patients with cardiovascular disease. Clin Cardiol. (2022) 45(11):1128–34. doi: 10.1002/clc.23911
84. Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. (2017) 135(12):e726–e79. doi: 10.1161/CIR.0000000000000470
85. MacKay-Lyons M, Billinger SA, Eng JJ, Dromerick A, Giacomantonio N, Hafer-Macko C, et al. Aerobic exercise recommendations to optimize best practices in care after stroke: AEROBICS 2019 update. Phys Ther. (2020) 100(1):149–56. doi: 10.1093/ptj/pzz153
86. Mead GE, Sposato LA, Sampaio Silva G, Yperzeele L, Wu S, Kutlubaev M, et al. A systematic review and synthesis of global stroke guidelines on behalf of the world stroke organization. Int J Stroke. (2023) 18(5):499–531. doi: 10.1177/17474930231156753
87. Crozier J, Roig M, Eng JJ, MacKay-Lyons M, Fung J, Ploughman M, et al. High-intensity interval training after stroke: an opportunity to promote functional recovery, cardiovascular health, and neuroplasticity. Neurorehabil Neural Repair. (2018) 32(6-7):543–56. doi: 10.1177/1545968318766663
88. Lee J, Stone AJ. Combined aerobic and resistance training for cardiorespiratory fitness, muscle strength, and walking capacity after stroke: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. (2020) 29(1):104498. doi: 10.1016/j.jstrokecerebrovasdis.2019.104498
89. Brunt A, Albines D, Hopkins-Rosseel D. The effectiveness of exercise on cognitive performance in individuals with known vascular disease: a systematic review. J Clin Med. (2019) 8(3):294. doi: 10.3390/jcm8030294
90. Lennon O, Carey A, Gaffney N, Stephenson J, Blake C. A pilot randomized controlled trial to evaluate the benefit of the cardiac rehabilitation paradigm for the non-acute ischaemic stroke population. Clin Rehabil. (2008) 22(2):125–33. doi: 10.1177/0269215507081580
91. Smith AC, Saunders DH, Mead G. Cardiorespiratory fitness after stroke: a systematic review. Int J Stroke. (2012) 7(6):499–510. doi: 10.1111/j.1747-4949.2012.00791.x
92. Lennon O, Crystal A, Kwan M, Tierney C, Gallagher A, Murphy S. Perspectives and experiences of cardiac rehabilitation after stroke-a qualitative study. Healthcare (Basel). (2022) 10(8):1579. doi: 10.3390/healthcare10081579
93. Cuccurullo SJ, Fleming TK, Kostis WJ, Greiss C, Gizzi MS, Eckert A, et al. Impact of a stroke recovery program integrating modified cardiac rehabilitation on all-cause mortality, cardiovascular performance and functional performance. Am J Phys Med Rehabil. (2019) 98(11):953–63. doi: 10.1097/PHM.0000000000001214
94. Cuccurullo SJ, Fleming TK, Kostis JB, Greiss C, Eckert A, Ray AR, et al. Impact of modified cardiac rehabilitation within a stroke recovery program on all-cause hospital readmissions. Am J Phys Med Rehabil. (2022) 101(1):40–7. doi: 10.1097/PHM.0000000000001738
95. Marzolini S. Integrating individuals with stroke into cardiac rehabilitation following traditional stroke rehabilitation: promoting a Continuum of care. Can J Cardiol. (2018) 34(10 Suppl 2):S240–S6. doi: 10.1016/j.cjca.2018.06.017
96. Buckley BJR, Harrison SL, Fazio-Eynullayeva E, Underhill P, Lane DA, Thijssen DHJ, et al. Exercise-based cardiac rehabilitation associates with lower major adverse cardiovascular events in people with stroke. Cerebrovasc Dis. (2022) 51(4):488–92. doi: 10.1159/000521025
97. Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American heart association’s strategic impact goal through 2020 and beyond. Circulation. (2010) 121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703
98. Lloyd-Jones DM, Allen NB, Anderson CAM, Black T, Brewer LC, Foraker RE, et al. Life’s essential 8: updating and enhancing the American heart association’s construct of cardiovascular health: a presidential advisory from the American heart association. Circulation. (2022) 146(5):e18–43. doi: 10.1161/CIR.0000000000001078
99. Serra MC, Treuth MS, Ryan AS. Dietary prescription adherence and non-structured physical activity following weight loss with and without aerobic exercise. J Nutr Health Aging. (2014) 18(10):888–93. doi: 10.1007/s12603-014-0481-9
100. Recio-Rodriguez JI, Garcia-Ortiz L, Garcia-Yu IA, Lugones-Sanchez C, Olmo EZ, Bolibar B, et al. Effectiveness of a multiple health-behaviour-change intervention in increasing adherence to the Mediterranean diet in adults (EIRA study): a randomized controlled hybrid trial. BMC Public Health. (2022) 22(1):2127. doi: 10.1186/s12889-022-14590-y
101. O'Donoghue G, Blake C, Cunningham C, Lennon O, Perrotta C. What exercise prescription is optimal to improve body composition and cardiorespiratory fitness in adults living with obesity? A network meta-analysis. Obes Rev. (2021) 22(2):e13137. doi: 10.1111/obr.13137
102. Santos CP, Proenca M, Gouveia TDS, Soares de Oliveira CB, Tacao GY, Trevisan IB, et al. Effectiveness of aerobic exercise on smoking cessation in adults: a systematic review and meta-analysis. J Phys Act Health. (2021) 18(2):230–42. doi: 10.1123/jpah.2019-0339
103. Dobrosielski DA, Papandreou C, Patil SP, Salas-Salvado J. Diet and exercise in the management of obstructive sleep apnoea and cardiovascular disease risk. Eur Respir Rev. (2017) 26(144):160110. doi: 10.1183/16000617.0110-2016
104. Kanagasabai T, Riddell MC, Ardern CI. Physical activity contributes to several sleep-cardiometabolic health relationships. Metab Syndr Relat Disord. (2017) 15(1):44–51. doi: 10.1089/met.2016.0103
105. Gezer H, Karaahmet OZ, Gurcay E, Dulgeroglu D, Cakci A. The effect of aerobic exercise on stroke rehabilitation. Ir J Med Sci. (2019) 188(2):469–73. doi: 10.1007/s11845-018-1848-4
106. Pan B, Ge L, Xun YQ, Chen YJ, Gao CY, Han X, et al. Exercise training modalities in patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. Int J Behav Nutr Phys Act. (2018) 15(1):72. doi: 10.1186/s12966-018-0703-3
107. D'Isabella NT, Shkredova DA, Richardson JA, Tang A. Effects of exercise on cardiovascular risk factors following stroke or transient ischemic attack: a systematic review and meta-analysis. Clin Rehabil. (2017) 31(12):1561–72. doi: 10.1177/0269215517709051
108. Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med. (2002) 136(7):493–503. doi: 10.7326/0003-4819-136-7-200204020-00006
109. Tao X, Chen Y, Zhen K, Ren S, Lv Y, Yu L. Effect of continuous aerobic exercise on endothelial function: a systematic review and meta-analysis of randomized controlled trials. Front Physiol. (2023) 14:1043108. doi: 10.3389/fphys.2023.1043108
110. Viloria MAD, Li Q, Lu W, Nhu NT, Liu Y, Cui ZY, et al. Effect of exercise training on cardiac mitochondrial respiration, biogenesis, dynamics, and mitophagy in ischemic heart disease. Front Cardiovasc Med. (2022) 9:949744. doi: 10.3389/fcvm.2022.949744
111. Mattioni Maturana F, Martus P, Zipfel S, NI AM. Effectiveness of HIIE versus MICT in improving cardiometabolic risk factors in health and disease: a meta-analysis. Med Sci Sports Exerc. (2021) 53(3):559–73. doi: 10.1249/MSS.0000000000002506
112. Luan X, Tian X, Zhang H, Huang R, Li N, Chen P, et al. Exercise as a prescription for patients with various diseases. J Sport Health Sci. (2019) 8(5):422–41. doi: 10.1016/j.jshs.2019.04.002
113. Kokkinos P, Faselis C, Samuel IBH, Pittaras A, Doumas M, Murphy R, et al. Cardiorespiratory fitness and mortality risk across the Spectra of age, race, and sex. J Am Coll Cardiol. (2022) 80(6):598–609. doi: 10.1016/j.jacc.2022.05.031
114. Kris-Etherton PM, Petersen KS, Despres JP, Anderson CAM, Deedwania P, Furie KL, et al. Strategies for promotion of a healthy lifestyle in clinical settings: pillars of ideal cardiovascular health: a science advisory from the American heart association. Circulation. (2021) 144(24):e495–514. doi: 10.1161/CIR.0000000000001018
115. Schroder J, Truijen S, Van Criekinge T, Saeys W. Feasibility and effectiveness of repetitive gait training early after stroke: a systematic review and meta-analysis. J Rehabil Med. (2019) 51(2):78–88. doi: 10.2340/16501977-2505
116. Askim T, Bernhardt J, Salvesen O, Indredavik B. Physical activity early after stroke and its association to functional outcome 3 months later. J Stroke Cerebrovasc Dis. (2014) 23(5):e305–12. doi: 10.1016/j.jstrokecerebrovasdis.2013.12.011
117. Marzolini S, Robertson AD, Oh P, Goodman JM, Corbett D, Du X, et al. Aerobic training and mobilization early post-stroke: cautions and considerations. Front Neurol. (2019) 10:1187. doi: 10.3389/fneur.2019.01187
118. Zhong W, Geng N, Wang P, Li Z, Cao L. Prevalence, causes and risk factors of hospital readmissions after acute stroke and transient ischemic attack: a systematic review and meta-analysis. Neurol Sci. (2016) 37(8):1195–202. doi: 10.1007/s10072-016-2570-5
119. Fonarow GC, Smith EE, Reeves MJ, Pan W, Olson D, Hernandez AF, et al. Hospital-level variation in mortality and rehospitalization for medicare beneficiaries with acute ischemic stroke. Stroke. (2011) 42(1):159–66. doi: 10.1161/STROKEAHA.110.601831
120. Taylor RS, Sagar VA, Davies EJ, Briscoe S, Coats AJ, Dalal H, et al. Exercise-based rehabilitation for heart failure. Cochrane Database Syst Rev. (2014) 2014(4):CD003331. doi: 10.1002/14651858.CD003331.pub4
121. Lawler PR, Filion KB, Eisenberg MJ. Efficacy of exercise-based cardiac rehabilitation post-myocardial infarction: a systematic review and meta-analysis of randomized controlled trials. Am Heart J. (2011) 162(4):571–84.e2. doi: 10.1016/j.ahj.2011.07.017
Keywords: stroke, cerebrovascular accident, stroke rehabilitation, cardiac rehabilitation, exercise, physical activity, neurorehabilitation, stroke recovery
Citation: Cuccurullo SJ, Fleming TK, Petrosyan H, Hanley DF and Raghavan P (2024) Mechanisms and benefits of cardiac rehabilitation in individuals with stroke: emerging role of its impact on improving cardiovascular and neurovascular health. Front. Cardiovasc. Med. 11:1376616. doi: 10.3389/fcvm.2024.1376616
Received: 25 January 2024; Accepted: 17 April 2024;
Published: 2 May 2024.
Edited by:
Herbert F. Jelinek, Khalifa University, United Arab Emirates© 2024 Cuccurullo, Fleming, Petrosyan, Hanley and Raghavan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sara J. Cuccurullo c2FyYS5jdWNjdXJ1bGxvQGhtaG4ub3Jn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.