- 1Department of Anorectal, Chongqing Changshou Traditional Chinese Medicine Hospital, Chongqing, China
- 2Department of Cardiology, Traditional Chinese Medicine Hospital Dianjiang Chongqing, Chongqing, China
Objectives: The aim of this meta-analysis is to evaluate the effect of astragalus injection (AI) on left ventricular remodeling (LVR) in patients with heart failure with mildly reduced ejection fraction (HFmrEF).
Methods: The randomized controlled trials (RCTs) of AI in treating HFmrEF were retrieved from 8 major English and Chinese electronic databases, up until November 30, 2023. To evaluate the methodological quality of the included studies, the Cochrane bias risk tool and the Modified Jadad Scale were employed. Stata 17.0 software was utilized for statistical analysis, sensitivity analysis, and assessment of publication bias.
Results: Ten RCTs with 995 patients (562 males and 433 females) were identified. Meta-analysis indicated that compared to conventional treatment (CT), AI significantly improved LVR, specifically increasing left ventricular ejection fraction (LVEF, MD = 4.56, 95% CI: 3.68–5.44, p < 0.00001), decreasing left ventricular end-diastolic volume (LVEDV, MD = −7.89, 95% CI: −11.13 to −4.64, p < 0.00001), left ventricular end-diastolic diameter (LVEDD, MD = −4.18, 95% CI: −5.79 to −2.56, p < 0.00001), left ventricular end-systolic volume (LVESV, MD = −8.11, 95% CI: −11.79 to −4.43, p < 0.00001), and left ventricular end-systolic diameter (LVESD, MD = −3.42, 95% CI: −4.90 to −1.93, p < 0.00001). AI also improved clinical efficacy (RR = 4.62, 95% CI: 3.11–6.88, p < 0.00001), reduced N-terminal pro-brain natriuretic peptide (NT-pro BNP, MD = −27.94, 95% CI: −43.3 to −12.36) level, without increasing the incidence of adverse reactions (RR = 1.60, 95% CI: 0.59–4.29, p = 0.35). Sensitivity analysis confirmed the reliability of the merged results, and Begg's and Egger's tests showed no significant publication bias.
Conclusion: The systematic review and meta-analysis revealed that combining AI with CT improves LVR without increasing adverse events in HFmrEF patients. However, caution is needed in interpreting the results due to limited evidence. Future high-quality RCTs are needed to support these conclusions.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, PROSPERO [CRD42022347248].
1 Introduction
Heart failure (HF) is a global epidemic with increasing prevalence, affecting approximately 20 million patients worldwide (1). It is characterized by a poor prognosis and is the main cause of hospitalization for adults aged 65 and above, with a 1-year mortality rate of 10%–35% and a 5-year mortality rate greater than 50% (2). Among the various subtypes of HF, heart failure with mildly reduced ejection fraction (HFmrEF) is notable, accounting for 10%–20% of HF cases (3). HFmrEF is specifically identified by a left ventricular ejection fraction (LVEF) of 41%–49% and is closely associated with left ventricular remodeling (LVR), the pathophysiological core of HFmrEF, and a significant factor in its poor prognosis. LVR is marked by an increase in left ventricular volume and a decrease in contractile force (4, 5). Given these challenges, research targeting LVR, particularly in HFmrEF, is of paramount importance.
In recent years, with a deeper understanding of the pathophysiological mechanisms of HFmrEF, more studies have focused on treatment strategies targeting LVR. LVR refers to structural and functional changes in the left ventricle after chronic heart failure or myocardial infarction. These changes lead to decreased cardiac function, causing disease progression and poor outcomes in patients (6). Studies have shown that improving LVR can slow the progression of HF and improve patient prognosis (7). Traditional pharmacological treatments include beta-blockers, aldosterone antagonists, and angiotensin receptor-neprilysin inhibitors (ARNI). While these drugs have shown some efficacy in improving the prognosis of HFmrEF patients, significant limitations and inadequacies remain (8). For instance, beta-blockers can reduce cardiac load but may cause side effects such as fatigue and sexual dysfunction (9). Aldosterone antagonists are effective in reducing myocardial fibrosis but may lead to hyperkalemia (10). ARNIs show promise in improving prognosis, yet their long-term safety requires further investigation (11). Moreover, current treatments mainly focus on symptom control and do not fully address the fundamental issue of LVR (12). Therefore, finding new therapeutic methods that can effectively improve LVR has become a major research focus.
Astragalus membranaceus, a dried root of traditional Chinese medicine, has a long history of medicinal use for its various healing properties. It is renowned for its ability to nourish qi, promote blood circulation, unblock collaterals, induce diuresis, and reduce swelling (13). Astragalus injection (AI), derived from this root through water extraction and alcohol precipitation, contains active ingredients like flavonoids, saponins, polysaccharides, amino acids, and trace elements (14). These components endow AI with anti-inflammatory, antioxidant, and antiviral properties, and importantly, the potential to improve ventricular remodeling, a critical aspect of HFmrEF (13). Animal studies have shown that AI can mitigate LVR and cardiac damage in specific models, and a meta-analysis has revealed its benefits in immune regulation and inflammation reduction, particularly in viral myocarditis patients (15, 16). However, the specific impact of AI on LVR in HFmrEF patients remains an under-researched area.
To bridge this gap, our study aims to conduct a comprehensive meta-analysis of clinical randomized controlled trials (RCTs) focusing on the effects of AI on LVR in HFmrEF patients. This endeavor seeks to objectively evaluate AI's therapeutic potential in this context, thereby providing valuable insights for clinical application and guiding future research in managing HFmrEF.
2 Materials and methods
2.1 Study registration
This study adheres to established guidelines and maintains transparency through registration in the PROSPERO database (CRD42022347248). Consistent with the PRISMA guidelines, our report follows these rigorous standards for systematic reviews and meta-analysis (17).
2.2 Search strategy
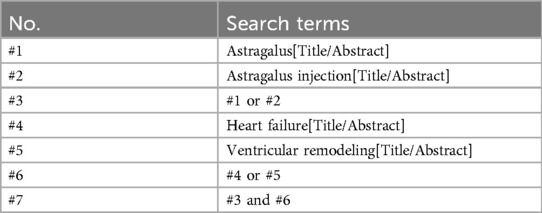
A comprehensive search across eight major databases, including PubMed, Embase, Web of Science, Cochrane Library, China Knowledge Infrastructure (CNKI), Wanfang Database, China Science Journal Database (VIP), and China Biomedical Database (CBM), was conducted to ensure the thoroughness and accuracy of our research retrieval. Additionally, we searched the Chinese Clinical Trial Registration Center. The search was carried out from the establishment of the databases until November 30, 2023. To ensure an effective search, we employed a combination of theme words and free words, including “astragalus”, “astragalus injection”, “heart failure”, and “ventricular remodeling”. Throughout the search process, we strictly followed the PRISMA guidelines to guarantee comprehensive and accurate retrieval of research articles. Table 1 presents the search strategy used for PubMed, while detailed search strategies for other databases can be found in the Supplementary File S1.
2.3 Type of studies
Inclusion criteria: (a) Study types: Randomized controlled trials (RCTs) published in English or Chinese. (b) Participants: Only patients diagnosed with HFmrEF (NYHA: II-IV), with a LVEF ranging from 41% to 49%. Patients with other types of HF (e.g., heart failure with preserved ejection fraction [HFpEF] or heart failure with reduced ejection fraction [HFrEF]) were excluded. (c) Interventions: The intervention group could include AI as a monotherapy or in combination with standard HF conventional treatment. The control group must receive either a placebo or standard HF conventional treatment. (d) Primary outcomes: Primary outcomes considered were LVEF, left ventricular end-diastolic volume (LVEDV), left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic volume (LVESV), and left ventricular end-systolic diameter (LVESD). (e) Secondary outcomes: Secondary outcomes included clinical efficacy and N-terminal pro-brain natriuretic peptide (NT-pro BNP) levels.
Exclusion criteria: (a) Non-RCTs. (b) Patients with unstable HF, with a LVEF < 40%. (c) Study on unconventional treatments, such as non-placebo control groups or unconfirmed alternative therapies. (d) Studies without primary outcomes. (e) Only the most comprehensive data from repeated published studies were selected. (f) Studies that could not be accessed online or through email.
2.4 Data extraction and quality assessment
In accordance with the inclusion and exclusion criteria, the title and abstract of the included studies were independently screened and retrieved by two reviewers (XH and LH). After excluding obviously unrelated studies, the full-texts of the remaining studies were thoroughly read to determine whether they met the criteria for inclusion. Subsequently, two other reviewers (GL and XM) independently extracted the data from the included studies, including the basic characteristics of the included studies and all outcome indicators.
To assess the risk of bias of the included studies, two reviewers (XH and LH) independently utilized the Cochrane Handbook (18). This tool allowed for the evaluation of potential biases, with assessments categorized as low, high, or unknown risk. To evaluate the quality of the study, the Modified Jadad Scale is used, which encompasses four aspects: random sequence production, allocation concealment, blinding method, withdrawal and dropout, with respective scores of 2, 2, 2, and 1. This scoring system categorizes the quality of RCTs: trials scoring between 1 and 3 are considered of low quality, whereas those scoring between 4 and 7 are deemed high quality. In the event of any discrepancies, the third reviewer (CC) was invited to participate in the discussion to resolve them.
2.5 Data analysis
Stata 17.0 software was employed for meta-analysis. For dichotomous data, we expressed the results as relative risk (RR) with 95% confidence interval (CI), while continuous data were presented as mean differences (MD) with 95% CI. I2 was utilized to assess heterogeneity between included studies. A fixed-effects model was applied when the heterogeneity between studies was minimal (p > 0.05, I2 < 50%). Conversely, a random-effects model was conducted. We also conducted sensitivity and subgroup analyses to identify potential sources of heterogeneity, along with Begg's and Egger's tests for publication bias assessment.
3 Results
3.1 Study identification
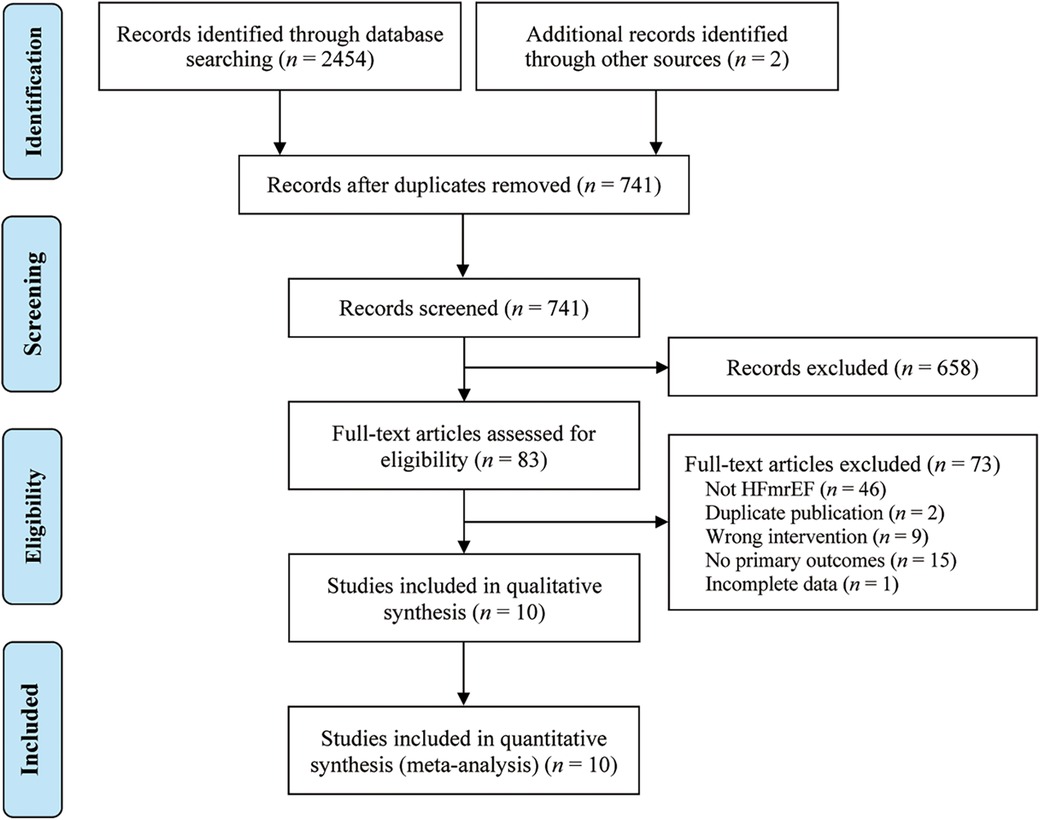
A systematic search was conducted on multiple databases, including PubMed (n = 58), Embase (n = 43), Cochrane Library (n = 30), Web of Science (n = 45), CNKI (n = 559), Wanfang Data (n = 532), VIP (n = 650), CBM (n = 537), and the Chinese Clinical Trial Registration Center (n = 2), retrieving 2,456 potential related original studies. Duplicate studies (n = 1,715) were then excluded using Endnote 20.5 software. After reviewing the titles and abstracts, 658 studies were further eliminated. The full texts of 83 studies were read, and ultimately, 73 studies were excluded based on specific criteria. Finally, 10 original studies (19–28) were included in the study. The research selection process is shown in Figure 1.
3.2 Included study characteristics
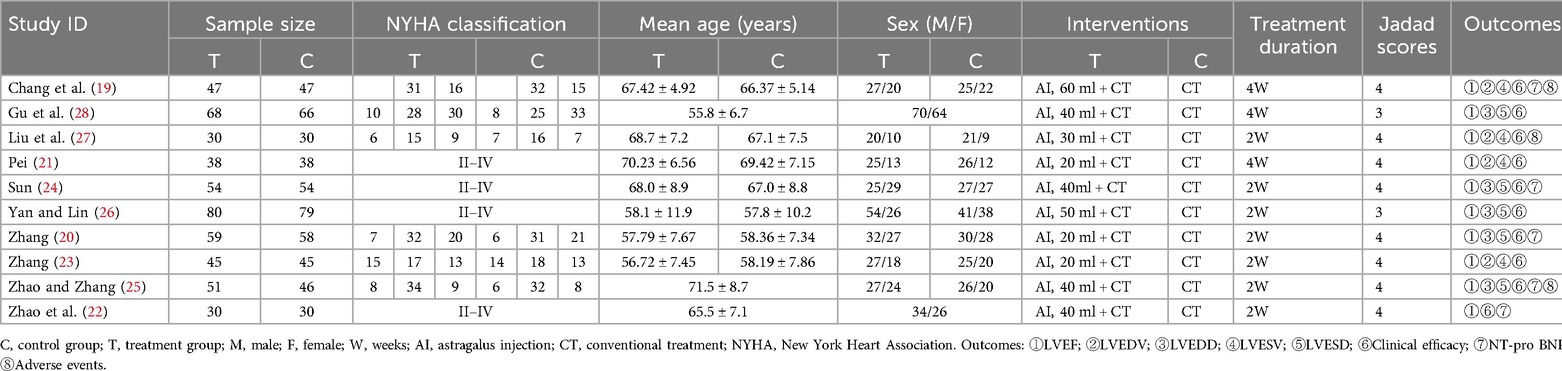
Table 2 presents the basic characteristics of the 10 included studies. A total of 995 patients (562 males and 433 females) were identified, with sample sizes ranging from 30 to 80. The duration of treatment varied from 2 weeks to 4 weeks. The control group received conventional treatment (CT) for HFmrEF according to the HF treatment guidelines. The intervention group received AI combined with CT. The included studies provided the following results: LVEF (19–28), LVEDV (19, 21, 23, 27), LVEDD (20, 24–26, 28), LVESV (19, 21, 23, 27), LVESD (20, 24–26, 28), clinical efficacy (19–28), and NT-pro BNP (19, 20, 22, 24, 25). Among these 10 studies, only 3 studies (19, 25, 27) reported adverse events.
3.3 Risk of bias assessment
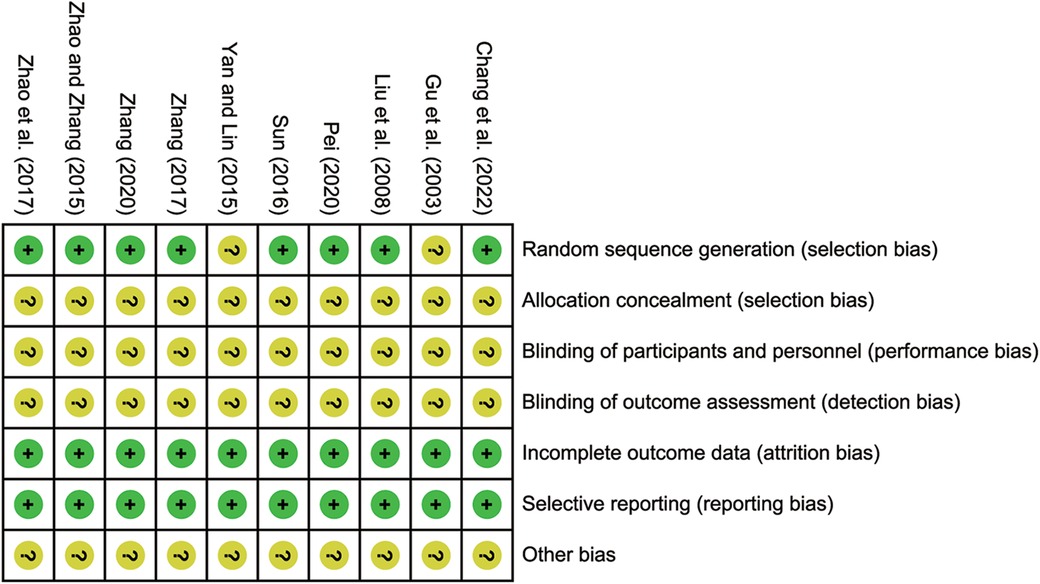
The Cochrane bias risk tool was employed to assess the risk of bias. 8 studies (19–25, 27) reported using a random number table method, which were identified as low risk. However, the remaining studies lacked a clear description of their randomization procedures, leading to unclear risk. None of the included studies reported allocation concealment and blinding, contributing to unclear risk. Nevertheless, in terms of selective reporting of incomplete outcome data, all included studies demonstrated no bias, resulting in low risk. The risk bias assessment and the quality assessment are detailed in Figure 2; Supplementary File S2.
3.4 Primary outcomes
3.4.1 LVEF
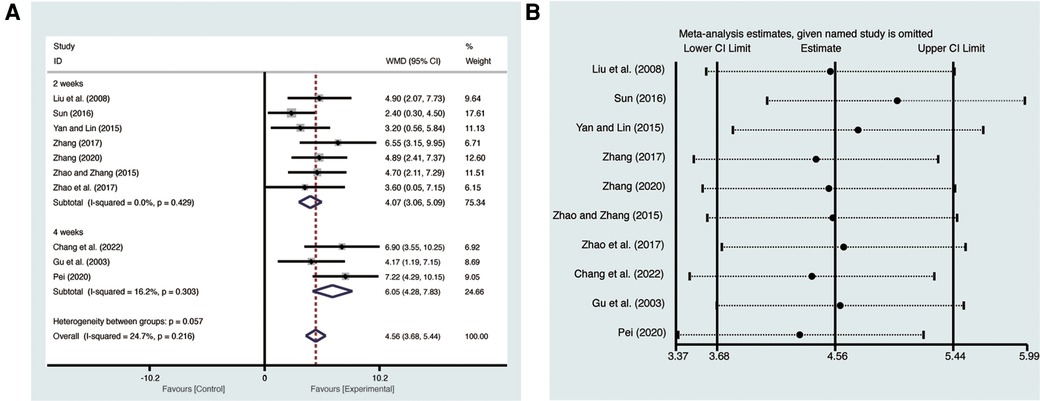
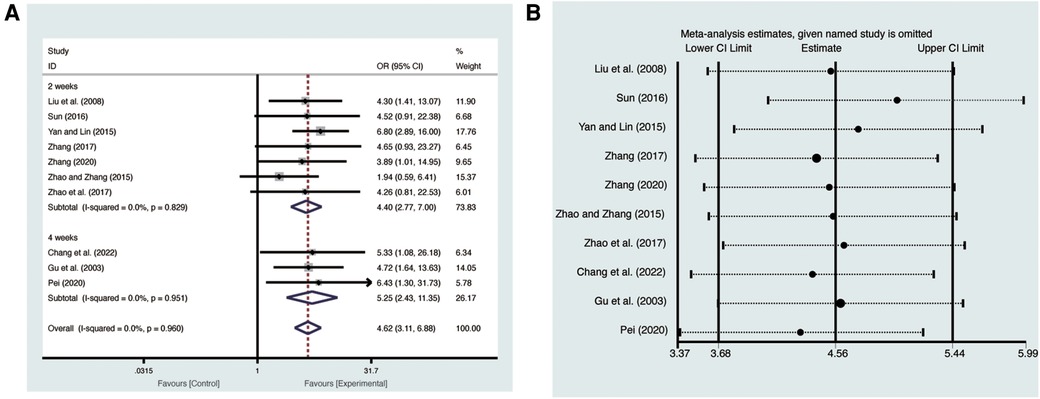
Ten studies (19–28) evaluated LVEF with low heterogeneity (I2 = 24.7%, p = 0.216) and were merged with a fixed-effects model. Results indicated that AI significantly improved LVEF (MD = 4.56, 95% CI: 3.68–5.44, p < 0.00001, Figure 3A). Subgroup analysis based on treatment duration of AI demonstrated significant distinctions between 2 weeks of AI (MD = 4.07, 95% CI: 3.06–5.09, p < 0.00001, Figure 3A), 4 weeks of AI (MD = 6.05, 95% CI: 4.28–7.83, p < 0.00001, Figure 3A), and CT. Sensitivity analysis confirmed the robustness and reliability of the merged results (Figure 3B).
3.4.2 LVEDV
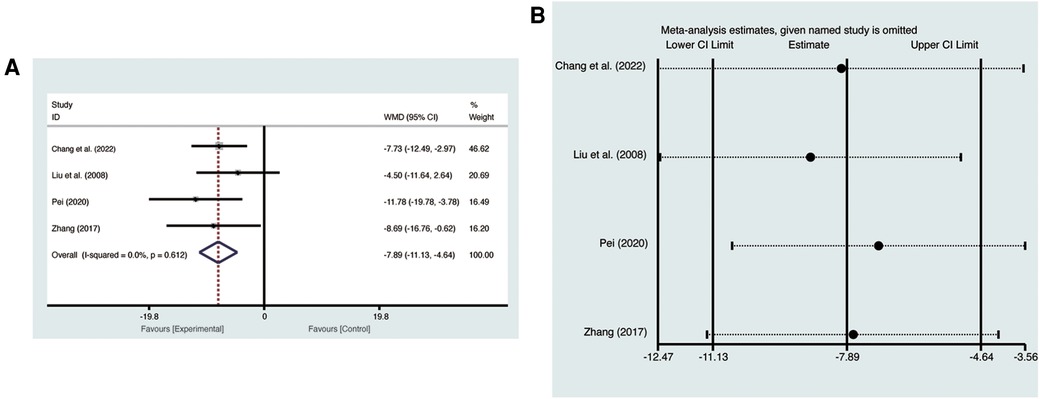
Four studies (19, 21, 23, 27) evaluated LVEDV with low heterogeneity (I2 = 0%, p = 0.612) and were merged with a fixed-effects model. Results indicated that AI significantly reduced LVEDV (MD = −7.89, 95% CI: −11.13 to −4.64, p < 0.00001, Figure 4A). Sensitivity analysis confirmed the robustness and reliability of the merged results (Figure 4B).
3.4.3 LVEDD
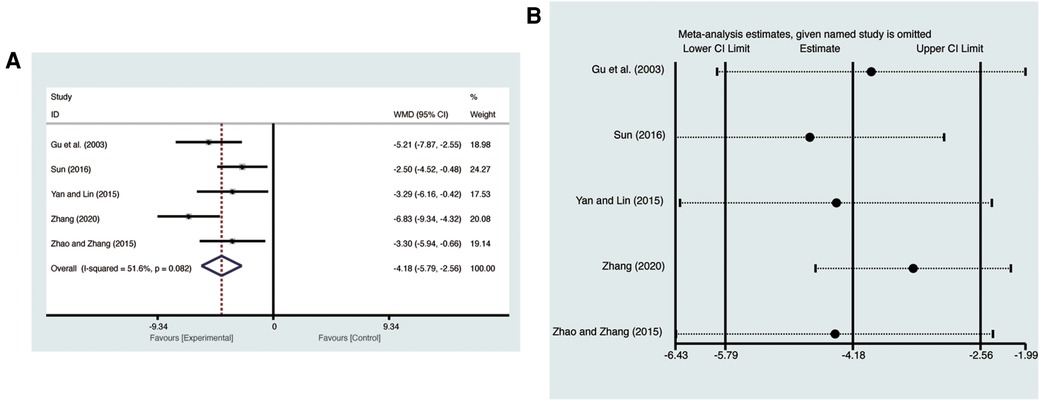
Five studies (20, 24–26, 28) evaluated LVEDD with high heterogeneity (I2 = 51.6%, p = 0.082) and were merged with a random-effects model. Results indicated that AI significantly reduced LVEDD (MD = −4.18, 95% CI: −5.79 to −2.56, p < 0.00001, Figure 5A). Sensitivity analysis confirmed the robustness and reliability of the merged results (Figure 5B).
3.4.4 LVESV
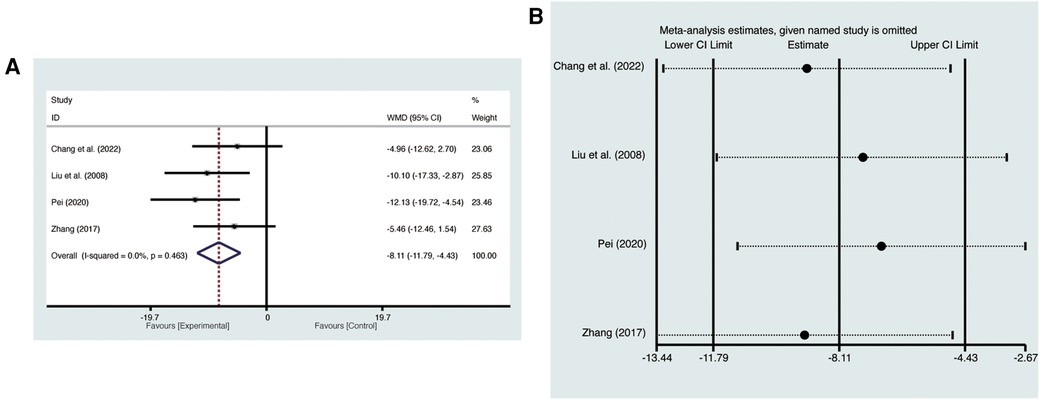
Four studies (19, 21, 23, 27) evaluated LVESV with low heterogeneity (I2 = 0%, p = 0.463) and were merged with a fixed-effects model. Results indicated that AI significantly reduced LVESV (MD = −8.11, 95% CI: −11.79 to −4.43, p < 0.00001, Figure 6A). Sensitivity analysis confirmed the robustness and reliability of the merged results (Figure 6B).
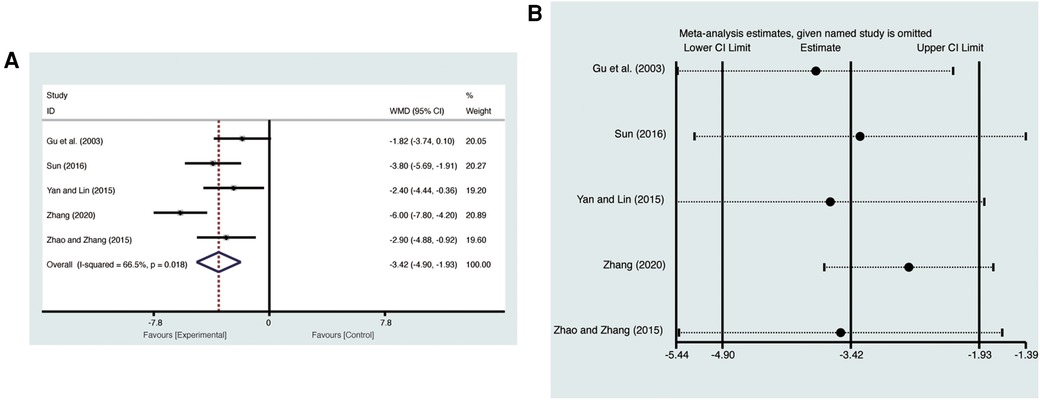
3.4.5 LVESD
Five studies (20, 24–26, 28) evaluated LVESD with high heterogeneity (I2 = 66.5%, p = 0.018) and were merged with a random-effects model. Results indicated that AI significantly reduced LVESD (MD = −3.42, 95% CI: −4.90 to −1.93, p < 0.00001, Figure 7A). Sensitivity analysis confirmed the robustness and reliability of the merged results (Figure 7B).
3.5 Secondary outcomes
3.5.1 Clinical efficacy
Ten studies (19–28) evaluated clinical efficacy with low heterogeneity (I2 = 0%, p = 0.960) and were merged with a fixed-effects model. Results indicated that AI significantly improved clinical efficacy (RR = 4.62, 95% CI: 3.11–6.88, p < 0.00001, Figure 8A). Subgroup analysis based on treatment duration of AI demonstrated significant distinctions between 2 weeks of AI (RR = 4.40, 95% CI: 2.77–7.00, p < 0.00001, Figure 8A), 4 weeks of AI (RR = 5.25, 95% CI: 2.43–11.35, p < 0.0001, Figure 8A), and CT. Sensitivity analysis confirmed the robustness and reliability of the merged results (Figure 8B).
3.5.2 NT-pro BNP
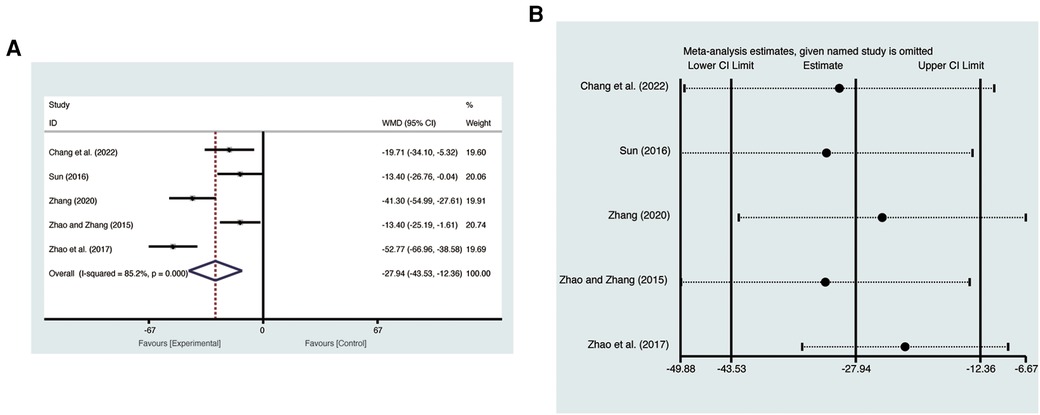
Five studies (19, 20, 22, 24, 25) evaluated NT-pro BNP with high heterogeneity (I2 = 85.2%, p = 0.000) and were merged with a random-effects model. Results indicated that AI significantly reduced NT-pro BNP (MD = −27.94, 95% CI: −43.3 to −12.36, p = 0.0004, Figure 9A). Sensitivity analysis confirmed the robustness and reliability of the merged results (Figure 9B).
3.5.3 Adverse events
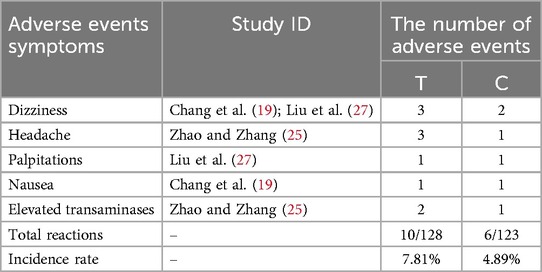
Only 3 (19, 25, 27) of the 10 included studies reported adverse events. These adverse events included dizziness, headache, palpitations, nausea, and elevated transaminases. However, the merged results indicated that there was no significant difference in adverse events between the two groups (RR = 1.60, 95% CI: 0.59–4.29, p = 0.35). Table 3 provided detailed adverse events.
3.6 Publication bias
Ten studies (19–28) that reported LVEF and clinical efficacy were evaluated for publication bias using Begg's and Egger's tests.The results of the Begg's test indicated no significant publication bias for LVEF (p = 0.474, Figure 10A) and clinical efficacy (p = 0.721, Figure 10C). Similarly, the Egger's test showed no significant publication bias between LVEF (p = 0.051, Figure 10B) and clinical efficacy (p = 0.571, Figure 10D).
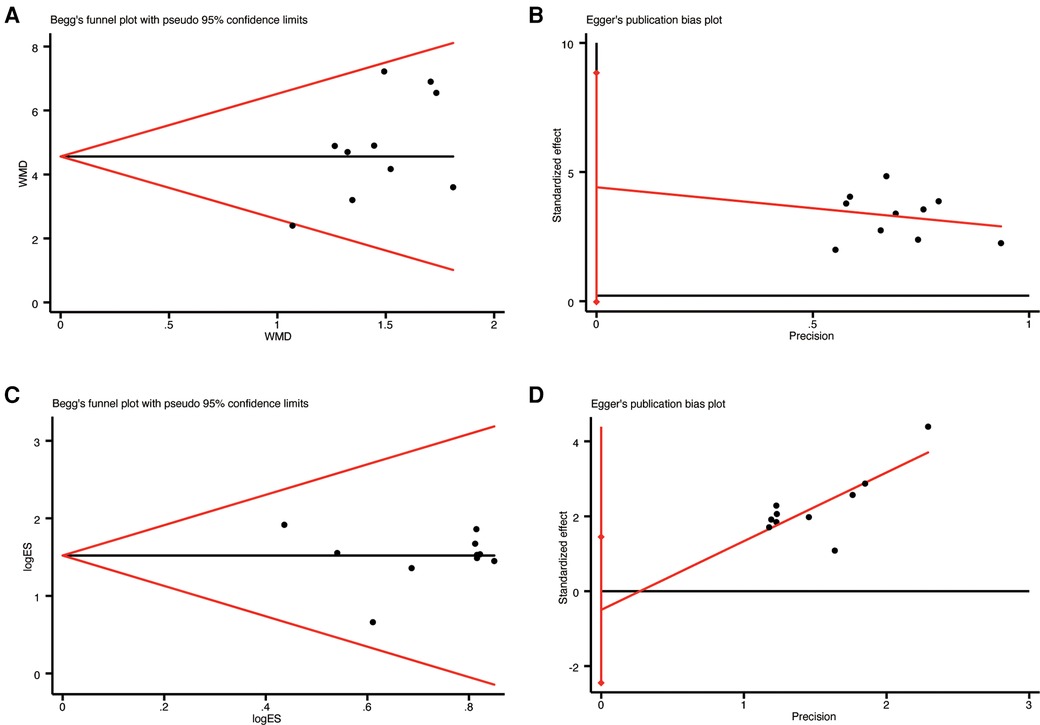
Figure 10. The results of publication bias: (A) begg's funnel plot of LVEF; (B) egger's funnel plot of LVEF; (C) begg's funnel plot of clinical efficacy; (D) egger's funnel plot of clinical efficac.
4 Discussion
HFmrEF, defined in the 2021 ESC guidelines as a subtype of HF, is characterized by left ventricular remodeling (LVR), which includes changes in ejection fraction and ventricular volumes (3). These pathological features have been shown to be associated with increased hospitalization and mortality risk, underscoring the need for effective treatment strategies (29). However, the majority of research on HF has focused on heart failure with reduced ejection fraction (HFrEF), with less attention given to HFmrEF (30, 31). While the 2021 ESC guidelines suggest that angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), diuretics, and β receptor blockers may have potential benefits for HFmrEF patients, the evidence supporting their effectiveness is relatively low (3). In light of this, there is an urgent need to explore alternative and complementary therapies to improve LVR in patients with HFmrEF.
Astragalus membranaceus is a medicinal and edible homologous species in China with a long history of application in food and clinical practice, commonly used to treat HF. AI mainly consists of extracts from Astragalus membranaceus, containing various active components such as saponins, polysaccharides, and flavonoids. These components exert anti-HF effects through multiple mechanisms, including anti-inflammatory, antioxidant, anti-fibrotic, regulation of cellular hypertrophy, and inhibition of senescence (32–34). Astragaloside IV (AS-IV) alleviates HF by inhibiting CCL2-mediated NF-κB signaling pathway activation, reducing LPS-induced myocardial hypertrophy and collagen deposition (35). Additionally, AS-IV mitigates ISO-induced myocardial fibrosis by inhibiting oxidative stress and regulating the P53 signaling pathway and cellular senescence (36). Astragalus polysaccharides (APS) reduce ISO-induced myocardial hypertrophy by regulating energy biogenesis mediated by the TNF-α/PGC-1α signaling pathway (37). Furthermore, APS improve doxorubicin-induced cardiotoxicity by regulating the PI3k/Akt and p38MAPK pathways to inhibit oxidative stress and apoptosis (38). However, despite multiple studies confirming its effectiveness in treating HFmrEF, there is a lack of evaluation regarding its impact on LVR. Since LVR plays a crucial role in the pathogenesis and progression of HFmrEF, this article aims to conduct a meta-analysis of randomized controlled trials (RCTs) to systematically evaluate the influence of AI on LVR in HFmrEF patients. The findings of this analysis aim to provide more reliable evidence for clinical decision-making in the treatment of HFmrEF.
4.1 Summary of findings
This meta-analysis is the first to investigate the effect of AI on LVR in patients with HFmrEF. The following findings were observed: (a) Compared to CT alone, the combination of AI and CT resulted in increased LVEF and decreased LVEDV, LVEDD, LVESV, LVESD, along with reduced NT-pro BNP levels. (b) Additionally, compared to CT alone, the combination of AI and CT exhibited increased clinical efficacy. (c) Importantly, the combination of AI and CT did not lead to an increase in adverse events. Based on these results, our meta-analysis suggests that AI may effectively improve LVR in patients with HFmrEF and enhance overall clinical efficacy.
The sensitivity analysis, which involved sequentially deleting individual studies, confirmed the robustness and reliability of the merged results. Additionally, to evaluate publication bias, STATA 17.0 software was used to conduct Begg's and Egg's tests. The analysis revealed no significant bias in the included studies.
4.2 Strengths and limitations
Currently, some researchers have conducted meta-analysis on the treatment of HF with AI (39). However, these studies have not considered the clinical heterogeneity among HF patients with different ejection fractions. It has been demonstrated that there may be significant differences in the pathological and physiological processes of HF with different ejection fraction types (40). Therefore, we believe that studying different types of HF is more meaningful for exploring personalized treatment plans. Furthermore, previous studies have focused primarily on the clinical efficacy and adverse reactions of AI, neglecting the crucial role of LVR in the progression of HF. In contrast, our study went beyond these limitations and evaluated the effect of AI on LVR in HFmrEF patients.
Additionally, there are several limitations to our study. First, the overall quality of the RCTs included is relatively low. Some of the included studies did not report randomized methods, blinding, and allocation concealment, which may lead to a serious risk of bias. Second, some of the RCTs included in this study are relatively small in scale, and therefore, the results should be interpreted with caution. Third, there are only three studies that reported adverse events; further investigation is needed to ensure the safety of AI. Fourth, none of the included studies reported follow-up time, indicating the necessity for further research to investigate the long-term effects of AI. Fifth, the study exclusively focused on patients with HFmrEF, thus excluding those with HFpEF and HFrEF. Therefore, the results may not be generalizable to all HF patients. Lastly, some of our results exhibit moderate to high heterogeneity, and conducting sensitivity analysis alone is not sufficient to draw clear conclusions. Therefore, further research is necessary to verify the reliability of our research findings.
4.3 Implication
In order to enhance the evidence strength of AI in treating HFmrEF, future clinical research should focus on the following aspects. Firstly, HF should be classified according to guidelines to reduce sources of bias and draw more accurate conclusions. Secondly, more high-quality RCTs should be conducted with a subject-centered approach, implementing strict randomization, allocation concealment, and blinding techniques. Thirdly, it is crucial to use a comprehensive standard reporting trial statement to report RCTs in a complete and comprehensive manner (41). This should include reporting medical history, readmission rate, quality of life, and follow-up time to analyze sources of heterogeneity and clarify the prognosis of HFmrEF. Fourthly, the included studies have relatively small sample sizes, future research should conduct large-scale, multicenter, prospective clinical trials and extend them to other populations to fully evaluate the effects of AI on HFmrEF. Fifthly, future research should also investigate the effects of AI on patients with HFpEF and HFrEF, which will help to understand the potential benefits and safety of AI across different HF populations. By addressing these aspects, future research can provide stronger evidence for the effectiveness of AI in treating HFmrEF, as well as HFpEF and HFrEF.
5 Conclusion
The systematic review and meta-analysis results indicate that combining AI with CT improves LVR in HFmrEF patients. This improvement is evidenced by increased LVEF, reduced LVEDV, LVEDD, LVESV, and LVESD, along with improved clinical efficacy and reduced NT-pro BNP levels without increasing adverse events. However, caution is needed in interpreting the results due to the low level of evidence. Future high-quality RCTs are needed to support these conclusions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
XH: Conceptualization, Data curation, Formal Analysis, Software, Writing – original draft. LH: Conceptualization, Data curation, Supervision, Writing – original draft. GL: Methodology, Writing – original draft. XM: Methodology, Supervision, Writing – original draft. CC: Conceptualization, Data curation, Formal Analysis, Methodology, Software, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1374114/full#supplementary-material
References
1. Roger VL. Epidemiology of heart failure: a contemporary perspective. Circ Res. (2021) 128(10):1421–34. doi: 10.1161/CIRCRESAHA.121.318172
2. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. (2014) 63(12):1123–33. doi: 10.1016/j.jacc.2013.11.053
3. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42(36):3599–726. doi: 10.1093/eurheartj/ehab368
4. Pezel T, Viallon M, Croisille P, Sebbag L, Bochaton T, Garot J, et al. Imaging interstitial fibrosis, left ventricular remodeling, and function in stage A and B heart failure. JACC Cardiovasc Imaging. (2021) 14(5):1038–52. doi: 10.1016/j.jcmg.2020.05.036
5. Aimo A, Gaggin HK, Barison A, Emdin M, Januzzi JL Jr. Imaging, biomarker, and clinical predictors of cardiac remodeling in heart failure with reduced ejection fraction. JACC Heart Fail. (2019) 7(9):782–94. doi: 10.1016/j.jchf.2019.06.004
6. Januzzi JL, Butler J, Fombu E, Maisel A, McCague K, Piña IL, et al. Rationale and methods of the prospective study of biomarkers, symptom improvement, and ventricular remodeling during sacubitril/valsartan therapy for heart failure (PROVE-HF). Am Heart J. (2018) 199:130–36. doi: 10.1016/j.ahj.2017.12.021
7. Lee MMY, Brooksbank KJM, Wetherall K, Mangion K, Roditi G, Campbell RT, et al. Effect of empagliflozin on left ventricular volumes in patients with type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction (SUGAR-DM-HF). Circulation. (2021) 143(6):516–25. doi: 10.1161/CIRCULATIONAHA.120.052186
8. Crea F. Fighting the pandemic of heart failure: better utilization of current treatments, new drugs, and new therapeutic targets. Eur Heart J. (2023) 44(44):4607–11. doi: 10.1093/eurheartj/ehad765
9. Lou IX, Chen J, Ali K, Chen Q. Relationship between hypertension, antihypertensive drugs and sexual dysfunction in men and women: a literature review. Vasc Health Risk Manag. (2023) 19:691–705. doi: 10.2147/VHRM.S439334
10. Desai R, Park H, Brown JD, Mohandas R, Pepine CJ, Smith SM. Comparative safety and effectiveness of aldosterone antagonists versus Beta-blockers as fourth agents in patients with apparent resistant hypertension. Hypertension. (2022) 79(10):2305–15. doi: 10.1161/HYPERTENSIONAHA.122.19280
11. Bhushan S, Huang X, Jiang F, Xiao Z. Impact of angiotensin receptor-neprilysin inhibition (ARNI) in improving ejection fraction and left and right ventricular remodeling in heart failure. Curr Probl Cardiol. (2024) 49(4):102464. doi: 10.1016/j.cpcardiol.2024.102464
12. Assmus B, Sossalla S. What we claim to do and what we really do—a discrepancy in heart failure treatment. Eur J Heart Fail. (2024) 26(6):1419–22. doi: 10.1002/ejhf.3279
13. Wang P, Wang Z, Zhang Z, Cao H, Kong L, Ma W, et al. A review of the botany, phytochemistry, traditional uses, pharmacology, toxicology, and quality control of the Astragalus memeranaceus. Front Pharmacol. (2023) 14:1242318. doi: 10.3389/fphar.2023.1242318
14. Guo XH. Pharmacological effects and research progress on the clinical application of Astragalus injection. Chin Phaf. (2015) 26(21):3018–21.
15. Dai H, Tao S, Guan Y, Zhang Y, Yang Z, Jia J, et al. Astragalus (Astragalus mongholicus) improves ventricular remodeling via ESR1 downregulation RhoA/ROCK pathway. Int Heart J. (2023) 64(6):1148–56. doi: 10.1536/ihj.23-265
16. Guo J, Zhao N, Jin P, Yin Y. Effect of Astragalus injection on inflammatory mediators in patients with viral myocarditis: a systematic review and meta-analysis. Phytomedicine. (2022) 107:154436. doi: 10.1016/j.phymed.2022.154436
17. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162(11):777–84. doi: 10.7326/M14-2385
18. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10(10):ed000142.31643080
19. Chang WN, Wang X, Chu PP, Ma KY. Clinical eficacy and influence on myocardial fibrosis of Astragalus injection combined with ivabradine in the refractory heart failure patients. J North China Univ Techno. (2022) 24(5):388–92.
20. Zhang XH. Effects of Astragalus injection on cardiac function and neuroendocrine function in patients with heart failure. Henan Med Res. (2020) 29(5):857–9.
21. Pei YY. Effects of rosuvastatin calcium tablets and Astragalus injection on elderly patients with heart failure, including atrial natriuretic peptide (ANP) and left ventricular remodeling. Clin Res. (2020) 28(3):113–4.
22. Zhao Y, Yan SH, Wang HD, Wang XY. The efficacy of Astragalus injection in the treatment of chronic heart failure and its effects on MDA, HO-1, and NO. Chin ArchTradit Chin Med. (2017) 35(8):2055–7.
23. Zhang ZB. Clinical observation of Astragalus injection combined with conventional western medicine for patients of chronic heart failure. J New Chin Med. (2017) 49(1):25–7.
24. Sun JJ. Clinical research on the treatment of chronic heart failure with Astragalus injection. J New Chin Med. (2016) 48(2):28–30.
25. Zhao FR, Zhang DB. The effects of Astragalus injection in the treatment of 51 cases of chronic heart failure and its impact on serum galectin-3 and B-type natriuretic peptide. Chin Pharm. (2015) 24(9):22–4.
26. Yan FY, Lin HZ. Observation of clinical effects of Astragalus injection in treatment of 80 patients with chronic heart failure. Jiangxi Trad China Med. (2015) 40(11):27–8.
27. Liu H, Lin DN, Lin YC, Zeng YP. Effects of combined Astragalus injection and valsartan on cardiac function and left ventricular function in patients with congestive heart failure. J New Chin Med. (2008) 40(8):33–4.
28. Gu X, Shang SZ, Guo R. Observation of clinical effects of Astragalus injection in treatment of 68 patients with chronic heart failure. Herald Med. (2003) 22(8):556–7.
29. Al-Jarallah M, Rajan R, Al-Zakwani I, Dashti R, Bulbanat B, Ridha M, et al. Mortality and morbidity in HFrEF, HFmrEF, and HFpEF patients with diabetes in the Middle East. Oman Med J. (2020) 35(1):e99. doi: 10.5001/omj.2020.17
30. Lavalle C, Di Lullo L, Jabbour JP, Palombi M, Trivigno S, Mariani MV, et al. New challenges in heart failure with reduced ejection fraction: managing worsening events. J Clin Med. (2023) 12(22):6956. doi: 10.3390/jcm12226956
31. Patel J, Rassekh N, Fonarow GC, Deedwania P, Sheikh FH, Ahmed A, et al. Guideline-directed medical therapy for the treatment of heart failure with reduced ejection fraction. Drugs. (2023) 83(9):747–59. doi: 10.1007/s40265-023-01887-4
32. Yang HY, Tu ZQ. Effects of Astragalus injection combined with torasemide on patients with renal insufficiency and heart failure, and its effects on scr, BNP, and VEGF. Mod J Integr Tradit Chin West Med. (2019) 28(11):1195–8.
33. Xian W. Influence of recombinant human BNP combined Astragalus injection on heart function and serum levels of inflammatory factors in AMI patients with post-PCI heart failure. Chin J Cardiovasc Rehabil Med. (2019) 28(4):424–8.
34. Gan PZ. Effects of Astragalus injection in the treatment of chronic heart failure and its effect on BNP and CA125. Chin Med Pharm. (2019) 9(9):48–51.
35. Chai CJ, Sun Y, Chi RF, Yang HY, Yang B, Li B. Astragaloside IV alleviates LPS-induced cardiomyocyte hypertrophy and collagen expression associated with CCL2-mediated activation of NF-κB signaling pathway. Biochem Biophys Res Commun. (2024) 693:149367. doi: 10.1016/j.bbrc.2023.149367
36. Shi L, Deng J, He J, Zhu F, Jin Y, Zhang X, et al. Integrative transcriptomics and proteomics analysis reveal the protection of astragaloside IV against myocardial fibrosis by regulating senescence. Eur J Pharmacol. (2024) 975:176632. doi: 10.1016/j.ejphar.2024.176632
37. Luan A, Tang F, Yang Y, Lu M, Wang H, Zhang Y. Astragalus polysaccharide attenuates isoproterenol-induced cardiac hypertrophy by regulating TNF-α/PGC-1α signaling mediated energy biosynthesis. Environ Toxicol Pharmacol. (2015) 39(3):1081–90. doi: 10.1016/j.etap.2015.03.014
38. Cao Y, Ruan Y, Shen T, Huang X, Li M, Yu W, et al. Astragalus polysaccharide suppresses doxorubicin-induced cardiotoxicity by regulating the PI3k/akt and p38MAPK pathways. Oxid Med Cell Longev. (2014) 2014:674219.25386226
39. Wu ZG, Zhou Q. Meta analysis of Astragalus injection in treating chronic heart failure. West J Chin Med. (2018) 31(2):54–8.
40. Cvijic M, Rib Y, Danojevic S, Radulescu CI, Nazghaidze N, Vardas P. Heart failure with mildly reduced ejection fraction: from diagnosis to treatment. Gaps and dilemmas in current clinical practice. Heart Fail Rev. (2023) 28(4):767–80. doi: 10.1007/s10741-022-10267-1
Keywords: astragalus injection, heart failure with mildly reduced ejection fraction, left ventricular remodeling, randomized controlled trials, systematic review, meta-analysis
Citation: Han X, Huang L, Li G, Mou X and Cheng C (2024) Effect of astragalus injection on left ventricular remodeling in HFmrEF: a systematic review and meta-analysis. Front. Cardiovasc. Med. 11:1374114. doi: 10.3389/fcvm.2024.1374114
Received: 2 February 2024; Accepted: 29 July 2024;
Published: 6 August 2024.
Edited by:
Marta Focardi, University of Siena, ItalyReviewed by:
Youhua Wang, Shanghai University of Traditional Chinese Medicine, ChinaThomas Hsueh, Taipei City Hospital, Taiwan
© 2024 Han, Huang, Li, Mou and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caihong Cheng, ODczOTc5NjAzQHFxLmNvbQ==
 Xu Han
Xu Han Lumei Huang2
Lumei Huang2