
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 12 April 2024
Sec. Heart Valve Disease
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1372792
This article is part of the Research Topic Reviews in Transcatheter Aortic Valve Implantation View all 6 articles
Background: Neurocognitive changes occurring after a surgical aortic valve replacement (SAVR) or transcatheter aortic valve implantation (TAVI) procedure for the correction of severe aortic stenosis (AS) have not been widely addressed and, if addressed, have produced conflicting results. The purpose of this study is to identify the pre-procedural neurocognitive pattern and its determinants in a setting of elderly (>65 years) patients with severe AS undergoing SAVR or TAVI and the changes occurring at a 2–3 month follow-up.
Methods: This was a prospective cohort study included in the Italian Registry on Outcomes in Aortic Stenosis Treatment in Elderly Patients. Patients were assessed both before and after (2–3 months) the procedure using the Montreal Cognitive Assessment (MoCA) test. Data on periprocedural demographics, clinical factors, and outcome measures were collected.
Results: Before the procedure, 70% of the patients demonstrated a MoCA score <23 points, which was indicative of cognitive dysfunction. The factors associated with neurocognitive dysfunction were age, functional capacity, chronic heart failure, and hemoglobin levels. After the procedure, there was an overall improvement in the MoCA score of the patients, but 28% of the patients showed a reliable worsening of their condition. The factors associated with MoCA worsening were platelet transfusions and the amount of red blood cell units transfused.
Conclusion: The correction of severe AS leads to an improvement in neurocognitive function after 2–3 months. This improvement does not differentiate between SAVR and TAVI after matching for pre-procedural factors. The only modifiable factor associated with pre-procedural neurocognitive function is anemia, and anemia correction with red blood cell transfusions is associated with a worsening of neurocognitive function. This leads to the hypothesis that anemia correction before the procedure (with iron and/or erythropoietin) may limit the risk of a post-procedural worsening of neurocognitive function.
Aortic valve stenosis (AS) is the most common heart valve disease in the elderly. Patients >75 years have a prevalence rate of AS of 12.4% and that of severe AS of 3.4% (1). Presently, the main therapeutic options for severe AS are surgical aortic valve replacement (SAVR) and transcatheter aortic valve implantation (TAVI). Many studies have compared the clinical outcomes of both techniques in terms of mortality and complications after the procedures. Conversely, a neurocognitive assessment after the procedures on those with severe AS has been done only in a few studies, often with a limited patient population (2–6).
So far, the results produced have either remained limited or conflicting in nature, and there is a gap in knowledge with respect to the factors that may influence the changes in neurocognitive function after the procedures. One certain factor is that elderly (>70 years) patients referred to AS procedures have a pre-procedural impairment of neurocognitive function. When tested with a Montreal Cognitive Assessment (MoCA), 70% of the patients have a MoCA score <26, which is considered the cutoff value for normal cognitive function (7). This pattern finds different causative factors such as comorbidities, previous neurological events, dementia, and other neurological diseases; however, inadequate cerebral blood flow may be a contributor, especially in elderly subjects (8). As a matter of fact, both TAVI and SAVR imply a number of mechanisms that can affect neurocognitive function. Restoring adequate cerebral blood flow by removing valvular obstruction may, per se, determine an improvement in neurocognitive function. However, other factors may deteriorate neurocognitive function. In SAVR, the use of cardiopulmonary bypass induces an inflammatory reaction, and the open heart chamber procedure inevitably leads to air micro-emboli and possibly solid particle embolism (9). TAVI is associated with calcium embolism at the time of the aortic valve deployment and with patterns of solid particle embolization that correlate to aortic annulus calcification (10).
This multifactorial impact of procedures correcting severe AS is probably the major determinant of the changes observed in neurocognitive function.
The OUTcomes evaluation of current therapeutic STrategies for severe Aortic valve steNosis and the ageING population in ITALY (OUTSTANDING ITALY) is a registry study performed by the Cardiologic Network of the Italian Clinical Research Hospitals (IRCCS). Within this registry, there are different branches exploring a number of outcome variables in procedures correcting severe AS. The neurocognitive branch explores both pre- and post-procedural changes in neurocognitive function in patients aged 65–80 years. The present study addresses the pre-procedural pattern of neurocognitive function, the effects of the procedure on neurocognitive changes, and the factors associated with these changes.
All patients were prospectively enrolled in the OUTSTANDING ITALY registry, which is funded by the Italian Ministry of Health. The study was approved by the local ethics committee of San Raffaele Hospital (OSR 14/12/2017 protocol number 298/2017) and subsequently amended to include a neuropsychological function assessment. All patients provided written informed consent for participation.
All patients were enrolled at the IRCCS Policlinico San Donato. The minimum age for participation in the study was fixed at 66 years. The data collection process included the pre-procedural, procedural, and post-procedural factors incorporated in the OUTSTANDING ITALY registry. Neuropsychological function was assessed the day before the procedure and at the follow-up visit 2–3 months after the procedure. Professional psychologists (LR and LB) provided the cognitive assessment, which was based on the MoCA test.
The MoCA is a screening test designed to evaluate cognitive function and the presence of mild cognitive impairment (MCI) (11, 12). It consists of 30 items that assess multiple cognitive domains: memory (immediate and delayed recall), visuospatial abilities, executive functions, attention, concentration, language, and spatial and temporal orientation. The MoCA test yields a total score of 30, with a cutoff value of 26, which is indicative of normal functioning. However, the literature suggests a second cutoff of 23, reporting greater specificity (95%) and sensitivity (96%) for detecting cognitive impairment (13).
The MoCA-MIS (Memory Index Score) represents a subscore of the delayed recall memory test, which is able to discriminate between coding and recalling difficulties. It is also a useful predictor of the possible evolution of mild and neurocognitive disorders (14). The MIS score ranges from 0 to 15 points, with the cutoff set at 7 points: 3 points are assigned for each spontaneously recalled word (5 in total), 2 points if the recall occurs by semantic cues, 1, if it occurs by multiple choice cues, and 0 points, are assigned in the absence of a recall.
A total of 206 patients were enrolled in the pre-procedural phase, 99 of whom completed the post-procedural neurocognitive screening 2–3 months later.
Data are expressed as the mean (standard deviation), the median (interquartile range), or the number (%). Differences between binary variables were assessed by performing a univariate analysis using Pearson's chi-square test. Differences between continuous variables were assessed using paired (within-group differences) and unpaired (between-group differences) Student's t-tests. Regression analysis was used to correlate continuous variables. A sensitivity analysis comparing SAVR and TAVI patients was performed through propensity matching.
The primary outcome measure of the study was the difference between pre-procedural and follow-up MoCA parameters. To identify patients with a clinically relevant decrease in neurocognitive function, the pre-/post- procedural changes in the MoCA score were expressed as a categorical variable (improved, unchanged, and worsened). This categorization was performed using the Reliable Change Index (RCI). Briefly, the RCI is calculated as [(X2 − X1) − (M2 − M1)]/SED, where X1 = observed first test score, X2 = observed second test score, M1 = group mean first test score, M2 = group mean second test score, and SED = standard error of the difference corrected for the test–retest correlation coefficient r (5, 15). The RCI describes a confidence interval around the mean differences in scores. For all the RCIs, a reliable change was calculated by setting the alpha value to 0.05 (two-tailed). Therefore, a “reliable improvement” was adjudicated for an RCI exceeding + 1.96 and a “reliable worsening” for an RCI below −1.96.
All statistical analyses were performed by using computerized packages (SPSS 20.0, IBM, Chicago, IL, USA; GraphPad, GraphPad Software, Inc, San Diego, CA, USA; and MedCalc, MedCalc Software, Ostend, Belgium). A two-tailed P-value<0.05 was considered significant for all the statistical tests.
The study period was between September 2022 and December 2023. During this period, 420 patients received either a TAVI or a SAVR for severe aortic stenosis. A total of 206 patients completed the pre-procedure evaluation. The median follow-up time was 66 days (interquartile range 51–89), and 99 patients completed the evaluation at follow-up. Figure 1 shows the determinants of this 50% dropout. The general characteristics of the patient population are reported in Table 1. The patients’ age ranged from 66 to 95 years. A total of 42 (20.4%) patients received a TAVI procedure and 164 (79.6%) a SAVR procedure. A number of comorbidities were present, the most frequent being diabetes, stable angina, syncope, and chronic heart failure. Data on pre-procedural neurocognitive function are reported in Table 2. Overall, the great majority of the patients (91%) had MoCA scores below the limit of normality of 26 points and 70% below the cutoff of 23 points. Univariate analysis demonstrated that a limited number of pre-procedural factors were associated with the MoCA score; these were age (correlation coefficient −0.312, P = 0.001), hemoglobin (correlation coefficient 0.152, P = 0.026), New York Heart Association (NYHA) class (correlation coefficient −0.184, P = 0.029), and chronic heart failure. When pooled together in a linear multivariable regression analysis, only age remained independently associated with the MoCA score (regression coefficient −0.182, P = 0.001).
Table 3 reports the neurocognitive changes that occur 2–3 months after the procedure. In total, 99 patients completed the follow-up; of them, 22 received a SAVR, and 77 received a TAVI. The total MoCA score improved significantly (P = 0.001); all its components improved with the exception of orientation, but the improvement was statistically significant only for visuospatial and memory components. The MIS improved significantly. The distribution of patients according to the RCI is reported in Figure 2. Overall, the largest changes were observed with respect to the memory components; 27 patients (27.3%) showed an improvement in terms of the RCI, 28 (28.3%) experienced worsening, and 44 (44.4%) experienced no changes.
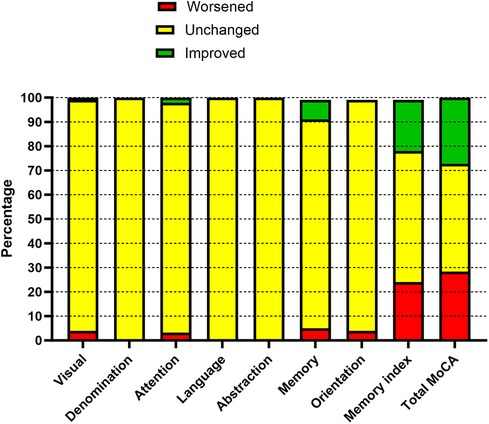
Figure 2. Reliable changes in cognitive function before and after the procedure (2–3-month follow-up).
Worsening of the RCI was defined as neurocognitive deterioration and tested for an association with pre-procedure factors and with the outcome data (Table 4). The only factors that were associated with neurocognitive failure were the total amount of red blood cells transfused (units) and the need for platelet transfusions (yes/no). Patients receiving platelet transfusions experienced significantly (P = 0.038) higher periprocedural bleeding (650 ± 707 ml/12 h) compared with those who did not (290 ± 207 ml/12 h). No pre-procedural factor was associated with neurocognitive failure. Patients who experienced a pre-procedural stroke (11 patients) had a neurocognitive failure in 4 (36.4%) cases, those who did not (88 patients) had a neurocognitive failure in 24 (27.3%) cases (P = 0.528).
In a subanalysis, the 22 patients undergoing SAVR were cross-matched with 22 TAVI patients using a propensity score based on age and pre-procedural MoCA. The pre-/post-procedural changes in the MoCA in this subgroup are reported in Table 5. The two groups were comparable for the pre-procedural factors associated with the MoCA score (age, NYHA class, hemoglobin, and chronic heart failure) and for the different components of the MoCA. At follow-up, the total MoCA score and its components did not significantly differ between the two groups.
In this study, a large cohort of patients with severe AS, referred to as either TAVI or SAVR, was tested for cognitive function. A total of 206 patients took a MoCA test immediately after the procedure, and 99 (48%) underwent the same evaluation after a median follow-up of 2 months. The rate of patients lost to follow-up is common in this kind of studies. Knipp et al. (16), in a series of 64 patients receiving transapical TAVI with a mean age of 73 years, reported an 80% rate of patients available for a 3-month follow-up. In an older (80-year-old) patient population undergoing TAVI, Auffret et al. (5) reported a follow-up failure rate of 50%, which was similar to that in our series for a similar mean age.
The 206 patients who underwent a pre-procedural test had a mean MoCA score of 20.3 and a rate of cognitive impairment (cutoff of 23/30) of 70%. This pattern was more severe than that reported by other authors (MoCA at baseline 22–25 points), but similar to that reported by a few others (3–5), all of whom included only TAVI patients. We are not aware of any study that assesses the determinants of the pre-procedural MoCA score, even if preoperative cognitive performance predicts clinical stroke and mortality after SAVR (17). In our series, the factors negatively associated with the MoCA score were age, hemoglobin, functional capacity, and chronic heart failure. Within these factors, the only modifiable component is the hemoglobin value. In the setting of chronic heart failure and cardiac surgery, treatment with iron supplementation and/or erythropoietin has been suggested as an effective measure to improve the outcome (18). There are no studies in the specific setting of a SAVR or TAVI procedure for severe AS; our findings generate the hypothesis that pre-procedural anemia correction may be beneficial in improving the cognitive status before and after the procedure. Low values of hemoglobin induce a decrease in the arterial oxygen content and oxygen delivery to the brain, which may justify poor levels of neurocognitive function.
Overall, the MoCA test result showed a significant improvement in the condition of patients at follow-up. This was in agreement with that of other studies using the MoCA or other cognitive function tests in the TAVI procedure (4, 5, 16, 19), but not with that of other studies where the cognitive function remained unchanged at 1–6 months of follow-up (3, 20). A meta-analysis demonstrated that cognitive function significantly improved 1 month after TAVI but not after 3 or 6 months (21). Considering our median time of follow-up, our data seem to confirm this finding. Of course, it must be noted that patients with TAVI are generally older than 80 years and that a worsening of neurocognitive function after 6 months may be justified simply by the well-known effects of aging. Limited data exist for cognitive function changes occurring after SAVR. De Rui et al. (7) showed a significant worsening of the patients’ condition in the MoCA test performed 1 year after SAVR; however, this finding is not confirmed in our series. Basically, our data support the concept that the removal of aortic valve obstruction is beneficial in neurocognitive terms, regardless of the procedure.
Even if the mean neurocognitive function improves, there are a non-trivial number of patients who show a reliable worsening of their condition in the MoCA test (28%). Of note, this pattern is not associated with pre-procedural factors, whereas some outcome factors (for example, the total amount of RBC transfused and the need for platelet transfusion) are significantly more prevalent in patients who suffer a worsening of cognitive function. This may be a marker for a more complex post-procedural outcome and/or procedural bleeding, especially in SAVR. However, a direct effect of RBC transfusions on cognitive function cannot be excluded, as suggested by other studies in cardiac surgery (22, 23) and other surgeries (24). In addition, RBC transfusions may be a marker of low levels of hemoglobin, which, in turn, are determinants of pre-procedural low MoCA scores. This again leads to the hypothesis that a pre-procedural optimization of hemoglobin values may be an important factor in reducing the risk of post-procedural cognitive decline. The role of platelet transfusions could be interpreted as a direct negative effect or as a surrogate for a bleeding episode, with both being expressions of a complicated post-procedural course. In this study, the patients who received platelet transfusions experienced a significantly high amount of bleeding.
An important finding of our study is that the memory domain plays a major role both as a determinant of improved neurocognitive function and as the main factor determining worsening.
Memory is a complex and multifaceted cognitive domain that is most commonly affected in cases of MCI (25). Gathering or assimilating new information not only requires the ability to store but also the use of strategies aimed at facilitating the memorization process. The underlying mechanisms are influenced by other cognitive abilities, such as executive functions and attentional abilities, and are therefore indicative of global functioning. Shin et al. (26) showed how an improvement in memory and executive functions in patients with MCI leads to an increase in global cognitive function; our data seem to confirm these results. Our research suggests an increase in autonomous recall abilities after aortic procedures (SAVR or TAVI), as well as in cue recall. The MoCA-MIS score enhancement indicates a better ability to encode information in the post-procedural phase compared with the pre-procedural phase, with a lower propensity to forgetfulness and slipping into an amnesiac state (27). Memory improvements may be the result of the restoration of cardiac output and improved hemodynamic status following TAVI or surgical procedures (28). This can lead to an increase in cerebral blood flow and oxygenation in the brain areas responsible for these processes (29).
Furthermore, cerebral hypoperfusion and low cardiac output have been shown to be primarily linked to cognitive decline, especially in the domains of attention and executive function (28). Hypothetically, the restoration of cerebral blood flow could be responsible for the improvement of these cognitive functions, as revealed in our data (28, 30).
Finally, the literature shows that patients with severe AS typically present a high prevalence of vascular risk factors associated with the possible development of vascular cognitive impairment affecting the frontal lobes. Delayed recall memory, attention, and executive function are domains dependent on these brain structures. For this reason, they may see improvement because of hemodynamic changes, in addition to worsening cases of adverse events such as microembolic lesions (28).
This study is not a targeted one and is certainly underpowered to detect differences in neurocognitive outcomes in SAVR vs. TAVI. However, in a propensity-matched subanalysis, no differences were detected between the two procedures. In a series of 100 patients undergoing transfemoral TAVI vs. SAVR, no differences in early (3 days) post-procedural cognitive function were detected, with both groups showing a worsening (2). A prospective trial of neurocognitive outcomes in patients undergoing TAVI vs. SAVR is ongoing, but the results are not available yet (31).
In conclusion, our study demonstrates that the correction of severe AS determines a significant improvement in neurocognitive function at a 2–3-month follow-up; a worsening of neurocognitive function (for example in the memory domain) affects a non-trivial rate (28%) of patients. The main factors associated with low MoCA scores before and after the procedure are related to anemia and its correction with RBC transfusions. This highlights the role of pre-procedural anemia correction in reducing the risk of neurocognitive deterioration.
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
The studies involving humans were approved by the Ethics Committee of the IRCCS San Raffaele Hospital. The studies were conducted in accordance with local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
LR: Investigation, Writing – review & editing. LB: Investigation, Writing – review & editing. VM: Data curation, Writing – review & editing. MA: Data curation, Writing – review & editing. LM: Conceptualization, Funding acquisition, Writing – review & editing. FB: Writing – review & editing. MR: Conceptualization, Methodology, Writing – original draft.
The authors declare financial support was received for the research, authorship, and/or publication of this article.
This study was funded by the Italian Ministry of Health through the Cardiac Network of the IRCCS.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declared that they were editorial board members of Frontiers at the time of submission. This had no impact on the peer review process or the final decision.
The reviewer AP declared a past co-authorship with the author FB to the handling editor.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Osnabrugge RL, Mylotte D, Head SJ, Van Mieghem NM, Nkomo VT, LeReun CM, et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol. (2013) 62(11):1002–12. doi: 10.1016/j.jacc.2013.05.015
2. Holinski S, Staebe P, Geyer T, Neumann K, Uebelhack R, Konertz W. Transfemoral versus conventional aortic valve implantation—early postoperative cognitive outcome. Ann Thorac Cardiovasc Surg. (2013) 19(3):195–200. doi: 10.5761/atcs.oa.11.01872
3. Lansky AJ, Brown D, Pena C, Pietras CG, Parise H, Ng VG, et al. Neurologic complications of unprotected transcatheter aortic valve implantation (from the neuro-TAVI trial). Am J Cardiol. (2016) 118(10):1519–26. doi: 10.1016/j.amjcard.2016.08.013
4. Fanning JP, Wesley AJ, Walters DL, Eeles EM, Barnett AG, Platts DG, et al. Neurological injury in intermediate-risk transcatheter aortic valve implantation. J Am Heart Assoc. (2016) 5(11):e004203. doi: 10.1161/JAHA.116.004203
5. Auffret V, Campelo-Parada F, Regueiro A, Del Trigo M, Chiche O, Chamandi C, et al. Serial changes in cognitive function following transcatheter aortic valve replacement. J Am Coll Cardiol. (2016) 68(20):2129–41. doi: 10.1016/j.jacc.2016.08.046
6. Aroney N, Patterson T, Allen C, Redwood S, Prendergast B. Neurocognitive status after aortic valve replacement: differences between TAVI and surgery. J Clin Med. (2021) 10(8):1789. doi: 10.3390/jcm10081789
7. De Rui M, Tarzia V, Mazzochin M, Bertocco A, Ceolin C, Trevisan C, et al. Surgical aortic valve replacement in elderly patients: effects on physical performance, cognitive function and health-related quality of life. Aging Clin Exp Res. (2022) 34(3):643–52. doi: 10.1007/s40520-021-01969-x
8. Krishnamurthy V, Paredes Spir I, Mammino KM, Nocera JR, McGregor KM, Crosson BA, et al. The relationship between resting cerebral blood flow, neurometabolites, cardio-respiratory fitness and aging-related cognitive decline. Front Psychiatry. (2022) 13:923076. doi: 10.3389/fpsyt.2022.923076
9. Lombard FW, Mathew JP. Neurocognitive dysfunction following cardiac surgery. Semin Cardiothorac Vasc Anesth. (2010) 14(2):102–10. doi: 10.1177/1089253210371519
10. Aggarwal SK, Delahunty N, Menezes LJ, Perry R, Wong B, Reinthaler M, et al. Patterns of solid particle embolization during transcatheter aortic valve implantation and correlation with aortic valve calcification. J Interv Cardiol. (2018) 31(5):648–54. doi: 10.1111/joic.12526
11. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53(4):695–9. doi: 10.1111/j.1532-5415.2005.53221.x
12. Aiello EN, Gramegna C, Esposito A, Gazzaniga V, Zago S, Difonzo T, et al. The Montreal Cognitive Assessment (MoCA): updated norms and psychometric insights into adaptive testing from healthy individuals in Northern Italy. Aging Clin Exp Res. (2022) 34(2):375–82. doi: 10.1007/s40520-021-01943-7
13. Carson N, Leach L, Murphy KJ. A re-examination of Montreal Cognitive Assessment (MoCA) cutoff scores. Int J Geriatr Psychiatry. (2018) 33(2):379–88. doi: 10.1002/gps.4756
14. Julayanont P, Brousseau M, Chertkow H, Phillips N, Nasreddine ZS. Montreal Cognitive Assessment Memory Index Score (MoCA-MIS) as a predictor of conversion from mild cognitive impairment to Alzheimer’s disease. J Am Geriatr Soc. (2014) 62(4):679–84. doi: 10.1111/jgs.12742
15. Frerichs RJ, Tuokko HA. A comparison of methods for measuring cognitive change in older adults. Arch Clin Neuropsychol. (2005) 20(3):321–33. doi: 10.1016/j.acn.2004.08.002
16. Knipp SC, Kahlert P, Jokisch D, Schlamann M, Wendt D, Weimar C, et al. Cognitive function after transapical aortic valve implantation: a single-centre study with 3-month follow-up. Interact Cardiovasc Thorac Surg. (2013) 16(2):116–22. doi: 10.1093/icvts/ivs461
17. Simone SM, Price CC, Floyd TF, Fanning M, Messé SR, Drabick DAG, et al. Preoperative cognition predicts clinical stroke/TIA and mortality after surgical aortic valve replacement in older adults. J Clin Exp Neuropsychol. (2022) 44(8):550–61. doi: 10.1080/13803395.2022.2142526
18. Okonko DO, Grzeslo A, Witkowski T, Mandal AK, Slater RM, Roughton M, et al. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC-HF: a randomized, controlled, observer-blinded trial. J Am Coll Cardiol. (2008) 51(2):103–12. doi: 10.1016/j.jacc.2007.09.036
19. Kahlert P, Al-Rashid F, Döttger P, Mori K, Plicht B, Wendt D, et al. Cerebral embolization during transcatheter aortic valve implantation: a transcranial Doppler study. Circulation. (2012) 126(10):1245–55. doi: 10.1161/CIRCULATIONAHA.112.092544
20. Kahlert P, Knipp SC, Schlamann M, Thielmann M, Al-Rashid F, Weber M, et al. Silent and apparent cerebral ischemia after percutaneous transfemoral aortic valve implantation: a diffusion-weighted magnetic resonance imaging study. Circulation. (2010) 121(7):870–8. doi: 10.1161/CIRCULATIONAHA.109.855866
21. Khan MM, Herrmann N, Gallagher D, Gandell D, Fremes SE, Wijeysundera HC, et al. Cognitive outcomes after transcatheter aortic valve implantation: a metaanalysis. J Am Geriatr Soc. (2018) 66(2):254–62. doi: 10.1111/jgs.15123
22. Stanley ME, Kant S, Raker C, Sabe S, Sodha NR, Ehsan A, et al. Effect of patient sex on neurocognitive decline after cardiac surgery. J Am Coll Surg. (2023) 236(6):1112–24. doi: 10.1097/XCS.0000000000000574
23. Soliman R, Saad D, Abukhudair W, Abdeldayem S. The neurocognitive outcomes of hemodilution in adult patients undergoing coronary artery bypass grafting using cardiopulmonary bypass. Ann Card Anaesth. (2022) 25(2):133–40. doi: 10.4103/aca.aca_206_20
24. Zhu SH, Ji MH, Gao DP, Li WY, Yang JJ. Association between perioperative blood transfusion and early postoperative cognitive dysfunction in aged patients following total hip replacement surgery. Ups J Med Sci. (2014) 119(3):262–7. doi: 10.3109/03009734.2013.873502
25. Villeneuve S, Massoud F, Bocti C, Gauthier S, Belleville S. The nature of episodic memory deficits in MCI with and without vascular burden. Neuropsychologia. (2011) 49(11):3027–35. doi: 10.1016/j.neuropsychologia.2011.07.001
26. Shin M, Lee A, Cho AY, Son M, Kim YH. Effects of process-based cognitive training on memory in the healthy elderly and patients with mild cognitive impairment: a randomized controlled trial. Psychiatry Investig. (2020) 17(8):751–61. doi: 10.30773/pi.2019.0225
27. Kaur A, Edland SD, Peavy GM. The MoCA-memory index score: an efficient alternative to paragraph recall for the detection of amnestic mild cognitive impairment. Alzheimer Dis Assoc Disord. (2018) 32(2):120–4. doi: 10.1097/WAD.0000000000000240
28. Lai KS, Herrmann N, Saleem M, Lanctôt KL. Cognitive outcomes following transcatheter aortic valve implantation: a systematic review. Cardiovasc Psychiatry Neurol. (2015) 2015:209569. doi: 10.1097/WAD.0000000000000240
29. Tsuchiya S, Matsumoto Y, Suzuki H, Takanami K, Kikuchi Y, Takahashi J, et al. Transcatheter aortic valve implantation and cognitive function in elderly patients with severe aortic stenosis. EuroIntervention. (2020) 15(18):e1580–7. doi: 10.4244/EIJ-D-19-00489
30. Leeuwis AE, Smith LA, Melbourne A, Hughes AD, Richards M, Prins ND, et al. Cerebral blood flow and cognitive functioning in a community-based, multi-ethnic cohort: the SABRE study. Front Aging Neurosci. (2018) 10:279. doi: 10.3389/fnagi.2018.00279
31. Gomis M, Fernández C, Dacosta-Aguayo R, Carrillo X, Martínez S, Guijosa CM, et al. Aortic valve Replacement compared to Transcatheter Implant and its relationship with COgnitive impairment (ARTICO) evaluated with neuropsychological and advanced neuroimaging: a longitudinal cohort study. BMC Neurol. (2023) 23(1):310. doi: 10.1186/s12883-023-03362-9
Keywords: aortic valve stenosis, TAVI, SAVR, neurocognitive function, transfusions
Citation: Ranucci L, Brischigiaro L, Mazzotta V, Anguissola M, Menicanti L, Bedogni F and Ranucci M (2024) Neurocognitive function in procedures correcting severe aortic valve stenosis: patterns and determinants. Front. Cardiovasc. Med. 11:1372792. doi: 10.3389/fcvm.2024.1372792
Received: 18 January 2024; Accepted: 26 March 2024;
Published: 12 April 2024.
Edited by:
Elisabetta Ricottini, Campus Bio-Medico University, ItalyReviewed by:
Anna Olasinska-Wisniewska, Poznan University of Medical Sciences, Poland© 2024 Ranucci, Brischigiaro, Mazzotta, Anguissola, Menicanti, Bedogni and Ranucci. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. Ranucci Y2FyZGlvYW5lc3Rlc2lhQHZpcmdpbGlvLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.