- 1Department of Anaesthesiology, HongHui Hospital, Xi'an JiaoTong University, Xi’an, Shaanxi, China
- 2Department of Anaesthesiology, Binzhou Medical College Affiliated Hospital, Binzhou, Shandong, China
- 3Emergency Department, Linfen Hospital Affiliated to Shanxi Medical University, Linfen, Shanxi, China
Objective: This study aimed to determine the associated risk factors for proximal deep vein thrombosis (DVT) in patients with lower extremity and pelvic-acetabular fractures.
Methods: The medical records of 4,056 patients with lower extremity and pelvic-acetabular fractures were retrospectively reviewed. The patients were classified into proximal or non-proximal DVT groups. Logistic regression models were used to determine the independent risk variables for proximal DVT. The predictive value of the related risk factors was further analyzed using receiver operating characteristic curves.
Results: The prevalence of proximal DVT was 3.16%. Sex, body mass index (BMI), fracture site, injury mechanism, diabetes, coronary heart disease (CHD), injury-to-admission interval, hematocrit, platelet counts, and D-dimer levels differed significantly between the two groups. BMI ≥ 24.0 kg/m2, femoral shaft fractures, high-energy injury, diabetes, injury-to-admission interval >24 h were independent risk factors for proximal DVT. CHD decreased the risk of proximal DVT. The platelet and D-dimer had high negative predictive value for predicting proximal DVT formation, with cut-off values of 174 × 109/L and 2.18 mg/L, respectively.
Conclusion: BMI ≥ 24.0 kg/m2, femoral shaft fractures, high-energy injury, diabetes, injury-to-admission interval >24 h were independent risk factors for proximal DVT in patients with lower extremity and pelvic-acetabular fractures. Platelet count and D-dimer level were effective indicators for excluding proximal DVT occurrence. CHD decreased the risk of proximal DVT.
Introduction
Venous thromboembolism (VTE) is a common complication after traumatic injury (1–3). Pulmonary embolism (PE) is a potentially fatal condition in patients with fractures and can occur within 72 h after a trauma (4). The prevalence of early PE after trauma can be as high as 10%–42% (5). Studies have confirmed that VTE is a major risk factor for PE (6). Therefore, being able to predict deep vein thrombosis (DVT) in patients with traumatic fracture at the time of admission for implementing appropriate interventions is of considerable clinical importance.
Clinically, lower extremity DVT is classified as distal DVT (isolated calf vein thrombosis) or proximal DVT (thrombosis involving the popliteal vein and above) (7). Studies have shown that patients with distal DVT only are less likely to have PE (8, 9). Compared with distal DVT, proximal DVT is considered to be more prone to PE (10). Therefore, exploring and analyzing the prevalence and associated risk factors of proximal DVT at admission in patients with traumatic fractures to achieve early detection, early diagnosis, and early treatment, and prevent PE and death are important.
However, to date, there are no reports on the risk factors of proximal thrombosis at admission in patients with trauma. To address this knowledge gap, we conducted a multicenter retrospective study. In this study, we reviewed the medical records of individuals with lower extremity and pelvic-acetabular fractures admitted to three hospitals in China between February 2018 and March 2023, and analyzed the prevalence and associated risk factors for proximal DVT at admission.
Materials and methods
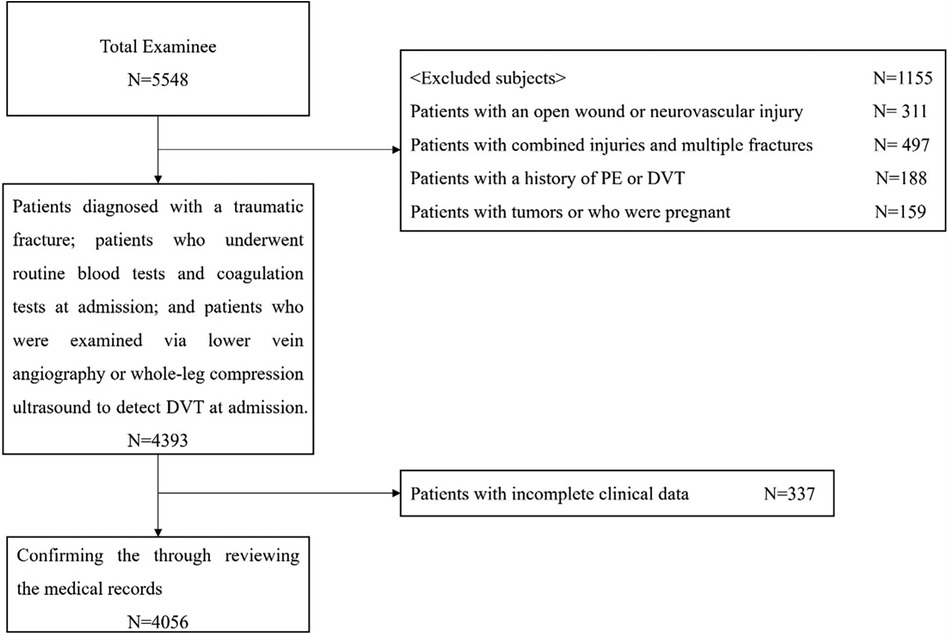
This study was approved by our institutional review board. We reviewed the medical records of individuals with lower extremity and pelvic-acetabular fractures admitted to three hospitals in China from February 2018 to March 2023 and analyzed 4,056 patients who met the inclusion and exclusion criteria. The inclusion and exclusion criteria and the selection process are illustrated in Figure 1.
Data collection
Patient data, including age, sex, height, weight, fracture site, injury mechanism, complications, smoking status, interval between injury and admission (h), results of routine blood tests and coagulation function tests at admission, and results of venography or ultrasonography of the lower extremities at admission, were collected. The fracture sites were classified as ankle-foot, tibia-fibula, peri-knee, femoral shaft, peri-hip, and pelvic-acetabular fractures. Injury mechanisms were classified as high- and low-energy injuries. The patients were classified as having proximal or non-proximal DVT based on venography or ultrasonography results of the lower extremities. Proximal DVT was defined as thrombosis involving popliteal vein and above, while non-proximal DVT was defined as isolated calf vein thrombosis (7).
Statistical analysis
Statistical analyses were performed using SPSS (version 21.0; SPSS, Chicago, Illinois, USA). Measurement data are expressed as mean ± standard deviation (SD) and compared using two independent sample t-tests. Count data are reported as numbers (percentages) and were compared using the χ2 test. A multivariate logistic regression model was used to identify independent risk factors for proximal DVT. Moreover, the predictive value of the related risk factors for proximal DVT was further analyzed using a receiver operating characteristic (ROC) curve. The cutoff points of platelet count and D-dimer level were selected according to the maximum Youden index. Sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) for DVT diagnosis were also determined. Statistical significance was set at P < 0.05.
Results
Demographic characteristics of all patients
A total of 4,056 patients were evaluated, including 1,920 females (47.34%) and 2,136 males (52.66%) with a mean age of 54.32 years (SD, 19.46; range, 17–96 years). The mean body mass index (BMI) was 21.26 kg/m2 (SD, 3.58; range, 14.27–39.51 kg/m2). Of the 4,056 patients, 776 had ankle-foot fractures, 464 had tibia-fibula fractures, 616 had peri-knee fractures, 248 had femoral shaft fractures, 1,280 had peri-hip fractures, and 672 had pelvic-acetabular fractures. There were 3,383 cases and 673 cases of low- and high-energy injuries, respectively. The mean time from injury to admission was 23.65 h (SD, 19.66; range, 1.00–68.00 h).
Prevalence of proximal DVT
The prevalence of proximal DVT was 3.16% (128/4,056). In patients with ankle-foot, tibia-fibula, peri-knee, femoral shaft, peri-hip, and pelvic-acetabular fractures, the prevalence rates of proximal DVT were 1.03% (8/776), 1.72% (8/464), 2.60% (16/616), 19.35% (48/248), 1.25% (16/1,280), and 4.76% (32/672), respectively (Figure 2). Except for pelvic fractures, proximal DVT was located in the injured lower extremities. Among patients with pelvic fractures, 77.27% (17/22) of proximal DVTs were located in the right lower extremity. PE was diagnosed based on computed tomography pulmonary angiography (CTPA) within 72 h of admission in 31 (24.22%) patients; all of them were proximal DVT cases (nine with femoral shaft fractures, 14 with femoral neck fractures, and eight with pelvic-acetabular fractures).
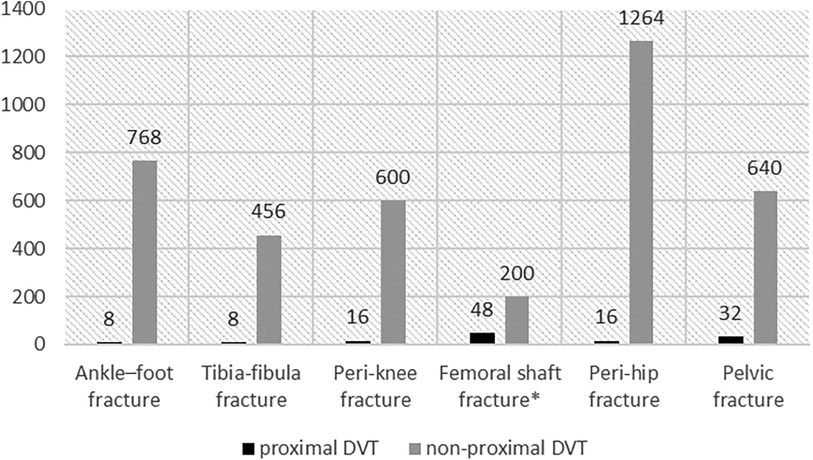
Figure 2. The distribution of DVT in different fracture sites. Femoral shaft fracture*: Patients with femoral shaft fractures had the highest prevalence at 19.35%.
Univariate analysis of risk factors
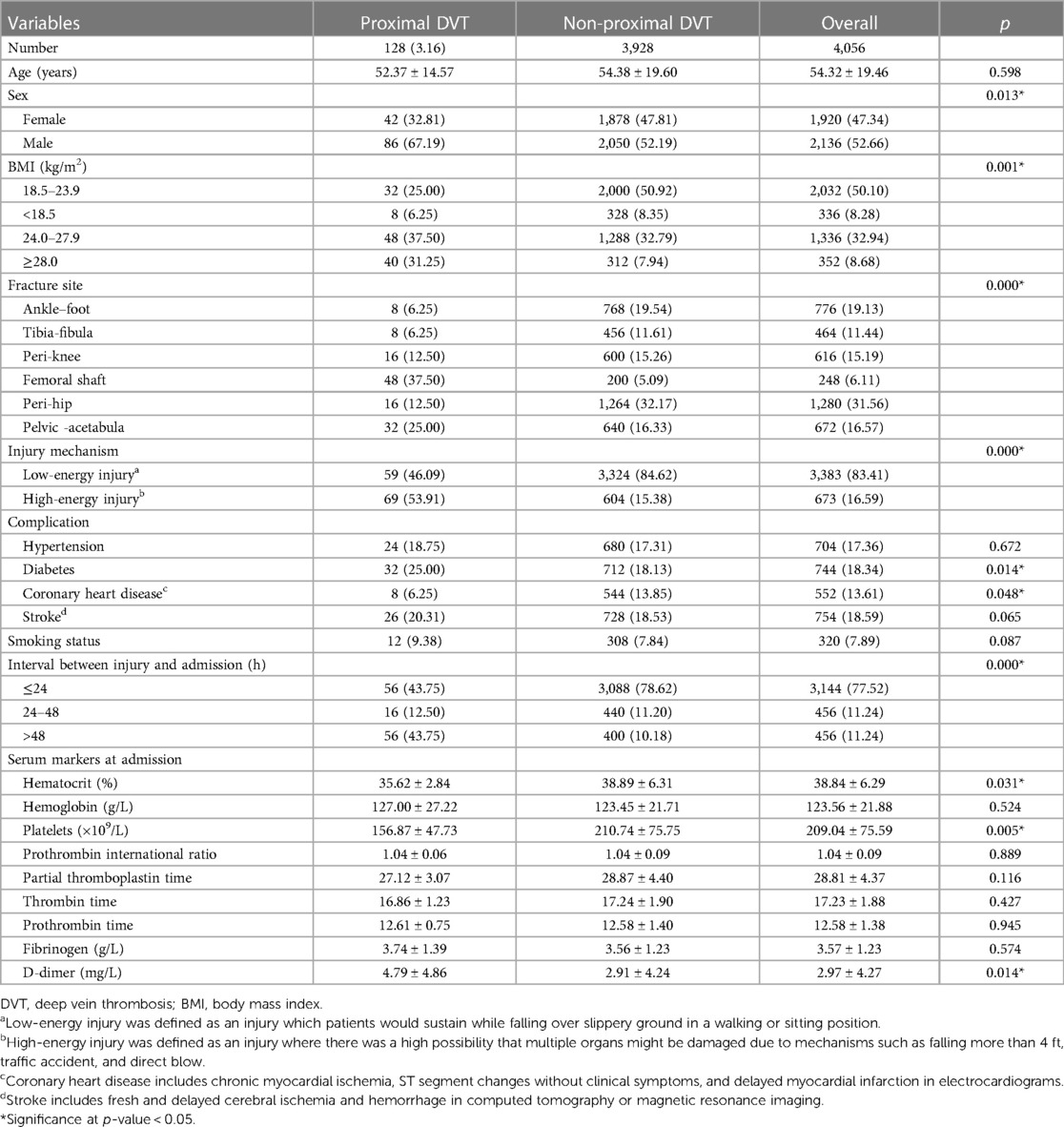
Males had a higher prevalence of proximal DVT than females (4.03% vs. 2.19%, P = 0.013). There were significant differences in BMI, fracture site, and interval between injury and admission between the two groups (P < 0.05). Patients with high-energy injuries had a higher incidence of proximal DVT than those with low-energy injuries (10.25% vs. 1.74%, P < 0.001). Patients with proximal DVT had a higher prevalence of diabetes (25.00% vs. 18.13%, P = 0.014) and a lower prevalence of coronary heart disease (CHD) (6.25% vs. 13.85%, P = 0.048) than those without proximal DVT. Patients with proximal DVT had a lower hematocrit level (35.62 ± 2.84% vs. 38.89 ± 6.31%, P = 0.031) and platelet count (156.87 ± 47.73 × 109/L vs. 210.74 ± 75.75 × 109/L, P = 0.005), and a higher D-dimer level (4.79 ± 4.86 mg/L vs. 2.91 ± 4.24 mg/L, P = 0.0141) (Table 1).
There were no significant differences in age, hypertension, stroke, smoking status, hemoglobin level, prothrombin international ratio, partial thromboplastin time, thrombin time, prothrombin time, or fibrinogen levels between the two groups (P > 0.05) (Table 1).
Multivariate analysis of risk factors
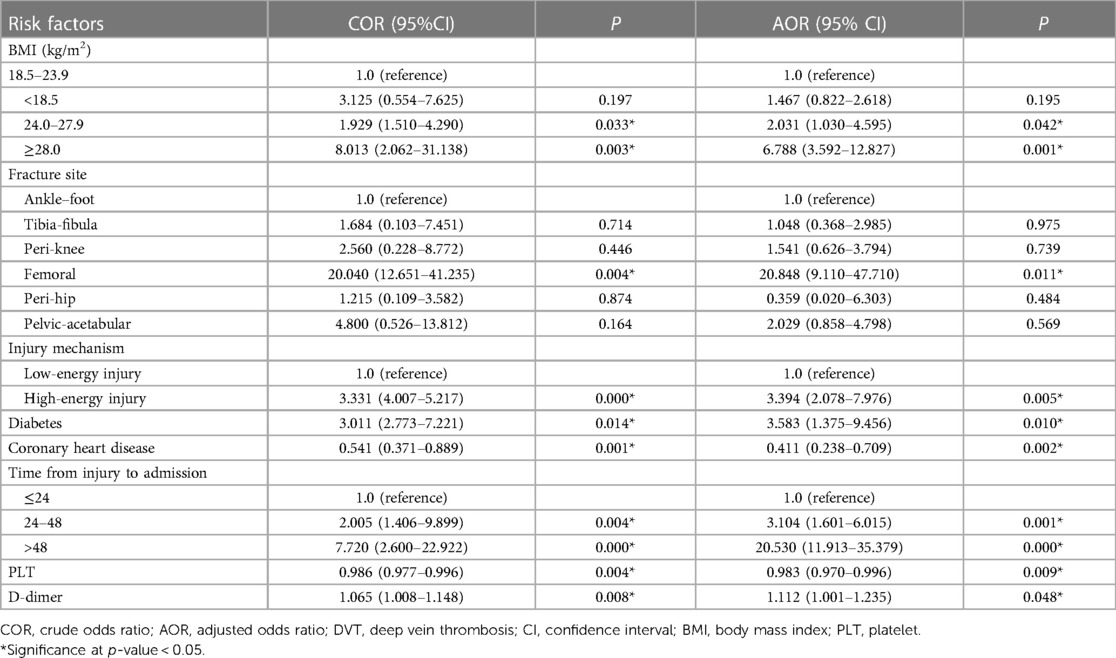
BMI ≥ 24.0 kg/m2 (BMI: 24.0–27.9, odds ratio [OR] = 2.031, 95% confidence interval [CI] 1.030–4.595, P = 0.042; BMI: ≥28, OR = 6.788, 95% CI: 3.592–12.827, P = 0.001), femoral shaft fractures (OR = 20.848, 95% CI: 9.110–47.710, P = 0.011), high-energy injury (OR = 3.394, 95% CI: 2.078–7.976, P = 0.005), diabetes (OR = 3.583, 95% CI: 1.375–9.456, P = 0.010), and interval between injury and admission >24 h (interval between injury and admission: 24–48 h, OR = 3.104, 95% CI: 1.601–6.015, P = 0.001; interval between injury and admission: >48 h, OR = 20.530, 95% CI: 11.913–35.379, P < 0.001) were independent risk factors for proximal DVT. Patients with CHD (OR = 0.411, 95% CI: 0.238–0.709, P = 0.002) had a decreased risk of admission for proximal DVT (Table 2).
In addition, a lower platelet count (OR = 0.989, 95% CI: 0.970–0.996, P = 0.009) and high D-dimer levels (OR = 1.112, 95% CI: 1.001–1.235, P = 0.048) increased the risk of proximal DVT at admission (Table 2).
ROC curve analysis for platelet and D-dimer value
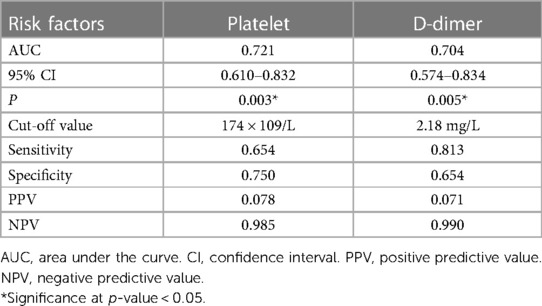
ROC curve analysis was performed to determine the predictive values of platelet count and D-dimer level for proximal DVT (Figures 3A,B), and the detailed results are listed in Table 3. The area under the curve (AUC) was 0.721 for platelets and 0.704 for D-dimers. The cut-off points were 174 × 109/L (sensitivity, 0.654; specificity, 0.750) and 2.18 mg/L (sensitivity, 0.813; specificity, 0.654) for platelet counts and D-dimer levels, respectively. The PPV and NPV were 0.078 and 0.985 for platelet counts and 0.071 and 0.990 for D-dimer level, respectively.
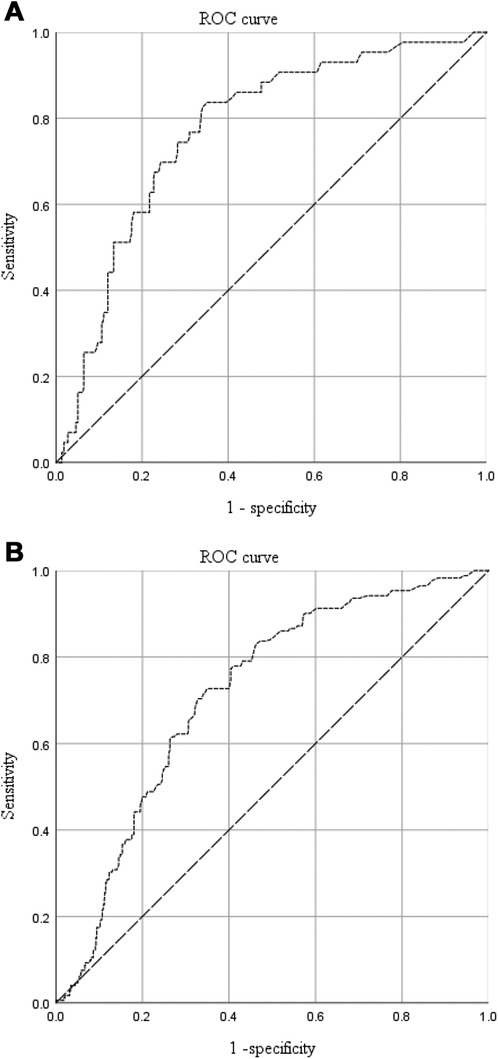
Figure 3. The predictive value of platelet counts (A) and D-dimer levels (B) for proximal DVT in patients with traumatic fractures.
Discussion
Prevalence of proximal DVT
The prevalence of proximal DVT was 3.16%, similar to that reported by Hao et al. In their report, the incidence was 3.69% (59/1,596) (11). Of note, in this study, 24.22% of all patients with proximal DVT were diagnosed with PE within 72 h after admission. Girard et al. reported a higher incidence of PE in patients with proximal thrombosis (40%–50%) (12). Research suggests that the case fatality rate of PE is close to 50% (5). Therefore, for patients with proximal DVT, vital signs should be closely monitored, retrievable inferior vena cava filters should be implanted when necessary, and pulmonary angiography should be performed in patients with suspected PE to prevent fatal PE. Previous studies suggest that DVT is more likely to occur on the left side (13, 14). Due to the anatomical position, the left common iliac vein between the left common iliac artery and sacrum is prone to compression, which leads to slow blood flow in the left vein and thrombosis occurrence (15). However, we found that in patients with pelvic fractures, 77.27% of proximal DVT was located in the right lower extremity. This may be because in pelvic fractures, the right side of the body is the most commonly affected side; this results in the right vascular endothelium being more susceptible to injury (16). More importantly, Hou et al. found that patients with proximal acute lower extremity VTE were more likely to develop PE than those with distal VTE. Furthermore, patients with right-sided acute lower extremity VTE were at higher risk of symptomatic PE than were those with left-sided acute lower extremity VTE (17). Therefore, in patients with pelvic fracture, screening for right proximal DVT should be strengthened.
Risk factors of admission DVT
Our study showed that age was not associated with proximal DVT occurrence, which is consistent with the findings of Nathan et al. They found that age was not a risk factor for proximal thrombosis (18). Studies have shown that female sex is an independent risk factor for DVT (19, 20). First, platelet reactivity is significantly higher in women than in men (21), and second, the common iliac vein (CIV) is more likely to be compressed in women (22, 23), leading to a higher incidence of DVT. This study showed that men had a higher incidence of proximal DVT. However, this was not an independent risk factor. It is speculated that this may be related to differences in trauma mechanisms between male and female patients. Regarding trauma, males are more likely to have high-energy fractures (24, 25). Previous studies have confirmed that high-energy injury is an independent risk factor for DVT (20, 26). In the present study, we obtained the same results.
Obesity is closely associated with the formation of DVT (27–29). Kornblith et al. (30) demonstrated that obese patients are more likely to have a hypercoagulable state after injury. Additionally, obese patients underwent less functional exercise and activity than non-obese patients, which increased the risk of abnormal venous valve pressure and hemodynamics (31). In our study, overweight and obesity were found to be independent risk factors for proximal DVT. The incidence of DVT is closely related to injury severity (32). Patients with overweight and obese tend to experience more severe injuries during the trauma process, which may be another reason why they are prone to proximal DVT. Ryb et al. (33) found that patients with (but not those with obesity) experienced more severe injuries, and Durgun et al. (34) found that the injury severity score (ISS) increased in proportion to increases in BMI.
The prevalence of DVT in trauma patients is related to the fracture sites. Additionally, Wang et al. found that femoral shaft fractures were associated with the highest incidence of proximal DVT (35). Yang et al. found that the incidence rates of proximal DVT at admission were as high as 14.81% (64/432) in patients with femoral shaft fractures (36). In our study, a higher incidence (19.35%) of proximal DVT was also found in patients with femoral shaft fractures; femoral shaft fractures were independent risk factors for proximal DVT.
Similar to most studies (37, 38), we found that diabetes was an independent risk factor for proximal DVT. Some studies have reported that patients with CHD are prone to DVT, which is related to the hypercoagulable state of the blood in patients with CHD (39, 40). However, we found that patients with CHD had a decreased risk of proximal DVT, this result may be attributed to long-term anticoagulation therapy. Platelets play an essential role in the pathogenesis of acute coronary syndromes, therefore an important part of the treatment of acute coronary syndromes, and of primary and secondary preventive measures in coronary heart disease, consists of antiplatelet treatment (41). Aspirin is now a commonly used antiplatelet agent in patients with coronary artery disease (42). Numerous studies have confirmed that aspirin significantly reduces the incidence of DVT (43–45). Bala et al. found that patients taking aspirin had the lowest incidence of deep vein thrombosis compared with those using other antiplatelet agents, including factor Xa inhibitors, enoxaparin, and warfarin (46).
A large number of studies have shown that the delay from injury to admission is an important factor leading to the high incidence of DVT in patients with lower extremity fractures (11, 20, 39). Our study showed that an interval between injury and admission of >24 h was an independent risk factor for proximal DVT in patients with lower extremity and pelvic-acetabular fractures. Hypercoagulability occurs 24 h after trauma (47), which may be the physiological basis of proximal DVT in patients with fractures. In addition, delayed anticoagulation owing to delayed hospital admission in patients with trauma may contribute to the development of proximal DVT. Wu et al. and Xia et al. found that delayed anticoagulation 24 h after trauma was positively correlated with the occurrence of VTE (48, 49).
Some previous studies have shown that patients with DVT have a significantly higher platelet count (50, 51). However, Sevuk et al. found that platelet counts were lower in patients with acute proximal DVT (52). A potential mechanism for low platelet count status in patients with proximal DVT is increased platelet consumption during the evolution of thrombosis (53). In the study, we found that platelet counts had a high NPV for proximal DVT formation in patients with traumatic fractures. In addition, the risk of proximal DVT was low in patients with a low clinical probability and a platelet count of not less than 174 × 109/L. There is much accumulated evidence that DVT can be safely ruled out in patients with a low or intermediate clinical probability and a negative D dimer (<0.5 mg/L) without performing additional examinations. In this study, D-dimer was found to have a high NPV for proximal DVT. However, the cutoff value was as high as 2.18 mg/L. This was due to the fact that, except for venous thrombosis, elevated levels of D-dimer are also found in patients in whom coagulation and fibrinolysis are co-activated, such as those with recent trauma or surgery and those with severe sepsis (54).
Therefore, in patients with traumatic fractures, special attention should be paid to those with BMI ≥ 24.0 kg/m2, femoral shaft fractures, high-energy injury, diabetes and interval between injury and admission >24 h, and early prophylaxis and treatment plans should be formulated to prevent proximal thrombosis extension and acute PE. During thrombosis screening, DVT could be safely ruled out in patients with a low clinical probability and a platelet count >174 × 109/L or D-dimer level <2.18 mg/L without performing additional examinations.
Limitations of this study
This study has four limitations. First, the retrospective design has its inherent limitation of accuracy in data collection. Second, the diagnostic value of duplex ultrasonography for DVT remains controversial; most patients in this study only underwent ultrasound examination without venography, which may have led to underdiagnosis. Although venography is the gold standard for diagnosing lower extremity thrombosis, it is an invasive procedure that requires a specific work scenario. Therefore, venography is not routinely used to screen for lower extremity thrombosis. In addition, Cavaye et al. found that duplex scanning produced sufficiently accurate data on the diagnosis of lower limb DVT to warrant its clinical use (55). Canakci et al. found that point-of-care ultrasound had high specificity and sensitivity for the examination of the popliteal and femoral veins by an emergency physician to evaluate patients with a preliminary diagnosis of DVT (56). Barrellier et al. found that the prevalence of duplex-ultrasonography-detected venous thrombosis in patients with suspected or proven PE was equivalent to the rates reported in phlebography and autopsy series (57). Third, we excluded patients with combined injuries and multiple fractures, which might limit wider application of the findings. Wu et al. (49) and Shi et al. (58) found that patients with multiple trauma had a higher risk of DVT. Additionally, Song et al. (59) found that combined cranial trauma was an independent risk factor for preoperative DVT. Therefore, more attention should be paid to patients with combined injuries or multiple fractures. Fourth, this study only identified the associated risk factors for proximal DVT in patients with lower extremity and pelvic-acetabular fractures. However, external validation studies were still lacking, which was a drawback of this study.
Conclusions
The prevalence of proximal DVT upon admission in patients with lower extremity and pelvic-acetabular fractures was 3.16%. BMI ≥ 24.0 kg/m2, femoral shaft fractures, high-energy injury, diabetes, and interval between injury and admission >24 h were independent risk factors for proximal DVT. However, the presence of CHD decreased the risk of proximal DVT. Platelet counts and D-dimer levels were effective indicators for excluding proximal DVT occurrence, with cut-off values of 174 × 109/L and 2.18 mg/L, respectively. These epidemiologic data are helpful in the assessment and risk stratification of admission proximal DVT, and supporting the formulation of an early prophylaxis and treatment plan for DVT.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional review board of Hong Hui Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
XL: Data curation, Formal Analysis, Investigation, Methodology, Software, Writing – original draft. PP: Data curation, Investigation, Software, Supervision, Writing – original draft. ZL: Data curation, Investigation, Validation, Writing – review & editing. WC: Conceptualization, Data curation, Investigation, Writing – review & editing. WL: Data curation, Investigation, Writing – original draft. JH: Data curation, Funding acquisition, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article.
This work was funded by the Key Research and Development Project of Shaanxi Province (NO. 2023-YBSF-069).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Shin WC, Woo SH, Lee SJ, Lee JS, Kim C, Suh KT. Preoperative prevalence of and risk factors for venous thromboembolism in patients with a hip fracture: an indirect multidetector CT venography study. J Bone Joint Surg Am. (2016) 98(24):2089–95. doi: 10.2106/JBJS.15.01329
2. Karcutskie CA, Meizoso JP, Ray JJ, Horkan D, Ruiz XD, Schulman CI, et al. Association of mechanism of injury with risk for venous thromboembolism after trauma. JAMA Surg. (2017) 152(1):35–40. doi: 10.1001/jamasurg.2016.3116
3. Geerts WH, Code KI, Jay RM, Chen E, Szalai JP. A prospective study of venous thromboembolism after major trauma. New Engl J Med. (1994) 331(24):1601–6. doi: 10.1056/NEJM199412153312401
4. Coleman JJ, Zarzaur BL, Katona CW, Plummer ZJ, Johnson LS, Fecher A, et al. Factors associated with pulmonary embolism within 72 h of admission after trauma: a multicenter study. J Am Coll Surg. (2015) 220(4):731–6. doi: 10.1016/j.jamcollsurg.2014.12.032
5. Bahloul M, Dlela M, Bouchaala K, Kallel H, Ben Hamida C, Chelly H, et al. Post traumatic pulmonary embolism: incidence, physiopathology, risk factors of early occurrence, and impact outcome. A narrative review. Am J Cardiovasc Dis. (2020) 10(4):432–43.33224594
6. Keller K, Wöllner J, Schmitt VH, Ostad MA, Sagoschen I, Münzel T, et al. Risk factors for pulmonary embolism in patients with paralysis and deep venous thrombosis. J Clin Med. (2021) 10 (22):5412. doi: 10.3390/jcm10225412
7. Jenkins JS, Michael P. Deep venous thrombosis: an interventionalist’s approach. Ochsner J. (2014) 14(4):633–40.25598728
8. Lagerstedt CI, Olsson CG, Fagher BO, Oqvist BW, Albrechtsson U. Need for long-term anticoagulant treatment in symptomatic calf-vein thrombosis. Lancet. (1985) 2(8454):515–8. doi: 10.1016/S0140-6736(85)90459-3
9. Hirsh J, Hoak J. Management of deep vein thrombosis and pulmonary embolism. A statement for healthcare professionals. Council on thrombosis (in consultation with the council on cardiovascular radiology), American heart association. Circulation. (1996) 93(12):2212–45. doi: 10.1161/01.CIR.93.12.2212
10. Galanaud JP, Sevestre-Pietri MA, Bosson JL, Laroche JP, Righini M, Brisot D, et al. Comparative study on risk factors and early outcome of symptomatic distal versus proximal deep vein thrombosis: results from the OPTIMEV study. Thromb Haemost. (2009) 102 (3):493–500. doi: 10.1160/TH09-01-0053
11. Zhang L, Liu X, Pang P, Luo Z, Cai W, Li W, et al. Incidence and risk factors of admission deep vein thrombosis in patients with traumatic fracture: a multicenter retrospective study. Clin Appl Thromb Hemost. (2023) 29:10760296231167143. doi: 10.1177/10760296231167143
12. Girard P, Decousus M, Laporte S, Buchmuller A, Hervé P, Lamer C, et al. Diagnosis of pulmonary embolism in patients with proximal deep vein thrombosis: specificity of symptoms and perfusion defects at baseline and during anticoagulant therapy. Am J Respir Crit Care Med. (2001) 164(6):1033–7. doi: 10.1164/ajrccm.164.6.2101045
13. Voke J, Keidan J, Pavord S, Spencer NH, Hunt BJ. The management of antenatal venous thromboembolism in the UK and Ireland: a prospective multicentre observational survey. Br J Haematol. (2007) 139(4):545–58. doi: 10.1111/j.1365-2141.2007.06826.x
14. Chan WS, Spencer FA, Ginsberg JS. Anatomic distribution of deep vein thrombosis in pregnancy. CMAJ. (2010) 182(7):657–60. doi: 10.1503/cmaj.091692
15. Ouriel K, Green RM, Greenberg RK, Clair DG. The anatomy of deep venous thrombosis of the lower extremity. J Vasc Surg. (2000) 31(5):895–900. doi: 10.1067/mva.2000.105956
16. Pereira GJC, Damasceno ER, Dinhane DI, Bueno FM, Leite JBR, Ancheschi BDC. Epidemiology of pelvic ring fractures and injuries. Rev Bras Ortop. (2017) 52(3):260–9. doi: 10.1016/j.rbo.2016.07.021
17. Hou J, Wang W, Cai H, Chen J, Chen B, Shen Z, et al. Patients with right lower extremity deep vein thrombosis have a higher risk of symptomatic pulmonary embolism: a retrospective study of 1585 patients. Ann Vasc Surg. (2022) 81:240–8. doi: 10.1016/j.avsg.2021.08.049
18. Nathan S, Aleem MA, Thiagarajan P, Das De S. The incidence of proximal deep vein thrombosis following total knee arthroplasty in an Asian population: a Doppler ultrasound study. J Orthop Surg (Hong Kong). (2003) 11(2):184–9. doi: 10.1177/230949900301100214
19. Tao J, Lou F, Liu Y. The role of vitamin D in the relationship between gender and deep vein thrombosis among stroke patients. Front Nutr. (2021) 8:755883. doi: 10.3389/fnut.2021.755883
20. Fan J, Zhou F, Xu X, Zhang Z, Tian Y, Ji H, et al. Clinical predictors for deep vein thrombosis on admission in patients with intertrochanteric fractures: a retrospective study. BMC Musculoskelet Disord. (2021) 22(1):328. doi: 10.1186/s12891-021-04196-7
21. Ranucci M, Aloisio T, Di Dedda U, Menicanti L, de Vincentiis C, Baryshnikova E. Gender-based differences in platelet function and platelet reactivity to P2Y12 inhibitors. PLoS One. (2019) 14(11): e0225771. doi: 10.1371/journal.pone.0225771
22. Larkin TA, Hovav O, Dwight K, Villalba L. Common iliac vein obstruction in a symptomatic population is associated with previous deep venous thrombosis, and with chronic pelvic pain in females. J Vasc Surg Venous Lymphat Disord. (2020) 8(6):961–9. doi: 10.1016/j.jvsv.2020.02.011
23. Nazzal M, El-Fedaly M, Kazan V, Qu W, Renno AW, Al-Natour M, et al. Incidence and clinical significance of iliac vein compression. Vascular. (2015) 23(4):337–43. doi: 10.1177/1708538114551194
24. Almigdad A, Mustafa A, Alazaydeh S, Alshawish M, Bani Mustafa M, Alfukaha H. Bone fracture patterns and distributions according to trauma energy. Adv Orthop. (2022) 2022:8695916. doi: 10.1155/2022/8695916
25. Cantore M, Candela V, Sessa P, Giannicola G, Gumina S. Epidemiology of isolated olecranon fractures: a detailed survey on a large sample of patients in a suburban area. JSES Int. (2022) 6(2):309–14. doi: 10.1016/j.jseint.2021.11.015
26. Park JS, Jang JH, Park KY, Moon NH. High energy injury is a risk factor for preoperative venous thromboembolism in the patients with hip fractures: a prospective observational study. Injury. (2018) 49(6):1155–61. doi: 10.1016/j.injury.2018.04.026
27. Dong Y, Zhong S, Ren Y, Zheng Z. Analysis on risk factors for deep vein thrombosis in patients with traumatic fractures. Chin J Orthop. (2015)35:1077–83. doi:doi: 10.3760/CMA.J.ISSN.0253-2352.2015.11.001
28. Tan JZJ, Meng Y, Zhang L, Tang F. Pre-operative incidence of deep venous thrombosis and its risk factors in the elderly with hip fracture. Chin J Mult Organ Dis Elderly. (2016) 15:373–6.
29. Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. (2003) 107:9–16. doi: 10.1161/01.CIR.0000078469.07362.E6
30. Kornblith LZ, Howard B, Kunitake R, Redick B, Callcut R. Obesity and clotting: body mass index independently contributes to hypercoagulability after injury. J Trauma Acute Care Surg. (2015) 78:30–8. doi: 10.1097/TA.0000000000000490
31. El-Menyar A, Asim M, Al-Thani H. Obesity paradox in patients with deep venous thrombosis. Clin Appl Thromb Hemost. (2018) 24:986–92. doi: 10.1177/1076029617727858
32. Stawicki SP, Grossman MD, Cipolla J, Hoff WS, Hoey BA, Wainwright G, et al. Deep venous thrombosis and pulmonary embolism in trauma patients: an overstatement of the problem? Am Surg. (2005) 71(5):387–91. doi: 10.1177/000313480507100504
33. Ryb GE, Dischinger PC. Injury severity and outcome of overweight and obese patients after vehicular trauma: a crash injury research and engineering network (CIREN) study. J Trauma. (2008) 64(2):406–11. doi: 10.1097/TA.0b013e31802beff9
34. Durgun HM, Dursun R, Zengin Y, Özhasenekler A, Orak M, Üstündağ M, et al. The effect of body mass index on trauma severity and prognosis in trauma patients. Ulus Travma Acil Cerrahi Derg. (2016) 22(5):457–65. doi: 10.5505/tjtes.2016.93385
35. Wang H, Kandemir U, Liu P, Zhang H, Wang PF, Zhang BF, et al. Perioperative incidence and locations of deep vein thrombosis following specific isolated lower extremity fractures. Injury. (2018) 49 (7):1353–7. doi: 10.1016/j.injury.2018.05.018
36. Yang W, Wei Q, Wang H, Ding K, Li M, Li C, et al. Preoperative incidence and risk factors of deep venous thrombosis in patients with isolated femoral shaft fracture. BMC Surg. (2022) 22(1):83. doi: 10.1186/s12893-022-01534-x
37. Chung WS, Lin CL, Kao CH. Diabetes increases the risk of deep-vein thrombosis and pulmonary embolism. A population-based cohort study. Thromb Haemost. (2015) 114(4):812–8. doi: 10.1160/TH14-10-0868
38. Chang W, Wang B, Li Q, Zhang Y, Xie W. Study on the risk factors of preoperative deep vein thrombosis (DVT) in patients with lower extremity fracture. Clin Appl Thromb Hemost. (2021) 27:10760296211002900. doi: 10.1177/10760296211002900
39. Zhang BF, Wei X, Huang H, Wang PF, Liu P, Qu SW, et al. Deep vein thrombosis in bilateral lower extremities after hip fracture: a retrospective study of 463 patients. Clin Interv Aging. (2018) 13:681–9. doi: 10.2147/CIA.S161191
40. Zheng H, Ma HP, Chen L, Zhan HT, Guo H. Prethrombotic state and cardiac events in patients with coronary heart disease during noncardiac surgery. Clin Appl Thromb Hemost. (2014) 20(1):84–90. doi: 10.1177/1076029612470489
41. Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (2). N Engl J Med. (1992) 326(5):310–8. doi: 10.1056/NEJM199201303260506
42. Clappers N, Brouwer MA, Verheugt FW. Antiplatelet treatment for coronary heart disease. Heart. (2007) 93(2):258–65. doi: 10.1136/hrt.2005.071209
43. DeDea L. DVT prophylaxis with aspirin in orthopedic surgery patients. JAAPA. (2012) 25(4):21. doi: 10.1097/01720610-201204000-00002
44. Kim YH, Choi IY, Park MR, Park TS, Cho JL. Prophylaxis for deep vein thrombosis with aspirin or low molecular weight dextran in Korean patients undergoing total hip replacement. A randomized controlled trial. Int Orthop. (1998) 22(1):6–10. doi: 10.1007/s002640050199
45. Li P, Ning Y, Li M, Cai P, Siddiqui AD, Liu EY, et al. Aspirin is associated with reduced rates of venous thromboembolism in older patients with cancer. J Cardiovasc Pharmacol Ther. (2020) 25(5):456–65. doi: 10.1177/1074248420925021
46. Bala A, Huddleston JI 3rd, Goodman SB, Maloney WJ, Amanatullah DF. Venous thromboembolism prophylaxis after TKA: aspirin, warfarin, enoxaparin, or factor Xa inhibitors? Clin Orthop Relat Res. (2017) 475(9):2205–13. doi: 10.1007/s11999-017-5394-6
47. Moore EE, Moore HB, Kornblith LZ, Neal MD, Hoffman M, Mutch NJ, et al. Trauma-induced coagulopathy. Nat Rev Dis Primers. (2021) 7(1):30. doi: 10.1038/s41572-021-00264-3
48. Xia ZN, Xiao K, Zhu W, Feng B, Zhang BZ, Lin J, et al. Risk assessment and management of preoperative venous thromboembolism following femoral neck fracture. J Orthop Surg Res. (2018) 13(1):291. doi: 10.1186/s13018-018-0998-4
49. Wu L, Cheng B. Analysis of perioperative risk factors for deep vein thrombosis in patients with femoral and pelvic fractures. J Orthop Surg Res. (2020) 15(1):597. doi: 10.1186/s13018-020-02131-5
50. Zhang L, He M, Jia W, Xie W, Song Y, Wang H, et al. Analysis of high-risk factors for preoperative DVT in elderly patients with simple hip fractures and construction of a nomogram prediction model. BMC Musculoskelet Disord. (2022) 23(1):441. doi: 10.1186/s12891-022-05377-8
51. Niu S, Pei Y, Hu X, Ding D, Jiang G. Relationship between the neutrophil-to-lymphocyte ratio or platelet-to-lymphocyte ratio and deep venous thrombosis (DVT) following femoral neck fractures in the elderly. Front Surg. (2022) 9:1001432. doi: 10.3389/fsurg.2022.1001432
52. Sevuk U, Bahadir MV, Altindag R, Baysal E, Yaylak B, Ay N, et al. Value of serial platelet indices measurements for the prediction of pulmonary embolism in patients with deep venous thrombosis. Ther Clin Risk Manag. (2015) 11:1243–9. doi: 10.2147/TCRM.S89355
53. Schwein A, Magnus L, Markovits J, Chinnadurai P, Autry K, Jenkins L, et al. Endovascular porcine model of iliocaval venous thrombosis. Eur J Vasc Endovasc Surg. (2022) 63(4):623–30. doi: 10.1016/j.ejvs.2021.12.022
54. Tang N, Pan Y, Xu C, Li D. Characteristics of emergency patients with markedly elevated D-dimer levels. Sci Rep. (2020) 10(1):7784. doi: 10.1038/s41598-020-64853-0
55. Cavaye D, Kelly AT, Graham JC, Appleberg M, Briggs GM. Duplex ultrasound diagnosis of lower limb deep venous thrombosis. Aust N Z J Surg. (1990) 60(4):283–8. doi: 10.1111/j.1445-2197.1990.tb07368.x
56. Canakci ME, Acar N, Bilgin M, Kuas C. Diagnostic value of point-of-care ultrasound in deep vein thrombosis in the emergency department. J Clin Ultrasound. (2020) 48(9):527–31. doi: 10.1002/jcu.22892
57. Barrellier MT, Lezin B, Landy S, Le Hello C. Prevalence of duplex ultrasonography detectable venous thrombosis in patients with suspected or acute pulmonary embolism. J Mal Vasc. (2001) 26(1):23–30.11240526
58. Shi D, Bao B, Zheng X, Wei H, Zhu T, Zhang Y, et al. Risk factors for deep vein thrombosis in patients with pelvic or lower-extremity fractures in the emergency intensive care unit. Front Surg. (2023) 10:1115920. doi: 10.3389/fsurg.2023.1115920
Keywords: lower extremity fracture, pelvic-acetabular fracture, proximal venous thromboembolism, admission, risk factor
Citation: Liu X, Pang P, Luo Z, Cai W, Li W and Hao J (2024) Prevalence and risk factors for proximal deep vein thrombosis at admission in patients with traumatic fractures: a multicenter retrospective study. Front. Cardiovasc. Med. 11:1372268. doi: 10.3389/fcvm.2024.1372268
Received: 31 January 2024; Accepted: 10 April 2024;
Published: 25 April 2024.
Edited by:
Nicola Mumoli, ASST Ovest Milanese, ItalyReviewed by:
Mingxing Lei, Chinese PLA General Hospital, ChinaEmilia Antonucci, Fondazione Arianna Anticoagulazione, Italy
© 2024 Liu, Pang, Luo, Cai, Li and Hao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianhong Hao aGFvamlhbmhvbmc3MjJAMTYzLmNvbQ==
†ORCID Jianhong Hao orcid.org/0000-0001-5753-9910
 Xiaobing Liu1
Xiaobing Liu1 Jianhong Hao
Jianhong Hao