
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 09 October 2024
Sec. Cardiovascular Epidemiology and Prevention
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1367704
This article is part of the Research TopicBlood Biomarkers in Cardiovascular DiseasesView all 8 articles
 Xinxin Yin1,2,3,4
Xinxin Yin1,2,3,4 Xin Pan1,2,3,4
Xin Pan1,2,3,4 Jingyu Zhang1,2,3,4
Jingyu Zhang1,2,3,4 Shuo Wu1,2,3,4
Shuo Wu1,2,3,4 Weikai Cui1,2,3,4
Weikai Cui1,2,3,4 Yuting Wang1,2,3,4
Yuting Wang1,2,3,4 Chuanbao Li1,2,3,4
Chuanbao Li1,2,3,4 Jiali Wang1,2,3,4*
Jiali Wang1,2,3,4* Yuguo Chen1,2,3,4*
Yuguo Chen1,2,3,4*
Objective: Although the association between admission glucose (AG) and major adverse cardiac events (MACE) is well-documented, its relationship with 30-day MACE in patients presenting with cardiac chest pain remains unclarified. In light of this, this study aims to examine the correlation between AG levels and the incidence of MACE in patients with chest pain in an emergency setting.
Materials and methods: We consecutively enrolled patients who presented to the emergency department for chest pain symptoms within 24 h from the EMPACT cohort in Eastern China (clinicaltrials.gov, Identifier: NCT02536677). The primary outcome was 30-day MACE, including all-cause death, recurrent myocardial infarction, urgent target vessel revascularization, stroke, cardiogenic shock, and cardiac arrest (CA). The associations of AG levels with 30-day MACE were analyzed using Kaplan–Meier analysis and Cox regression models.
Results: Among 1,705 patients who were included in this study, 154 (9.03%) patients met the primary outcome at 30 days. The average age of the patients was 65.23 ± 12.66 years, with 1,028 (60.29%) being male and 500 (29.33%) having diabetes. The median AG levels were 7.60 mmol/L (interquartile range: 6.30–10.20). Kaplan–Meier survival analysis revealed significant differences in the 30-day MACE risk (P < 0.001 according to the log-rank test). We found that the highest AG level (Q4) was associated with increased MACE risk compared with the lowest AG level [adjusted hazard radio (aHR): 2.14; 95% confidence interval (CI): 1.2–3.815; P = 0.010]. In addition, Q4 level was also associated with increased all-cause death risk (aHR: 3.825; 95% CI: 1.613–9.07; P = 0.002) and increased CA risk (aHR: 3.14; 95% CI: 1.251–7.884; P = 0.015).
Conclusions: An elevated AG level significantly correlates with a higher incidence of 30-day MACE in patients with acute chest pain. The findings reveal the importance of managing AG levels to potentially reduce the risk of adverse cardiac events.
Chest pain is a common clinical complaint in emergency departments (EDs) across the world. Studies indicate that approximately 5%–10% of ED visits are due to chest pain, with tertiary hospitals in China reporting rates of over 20% (1–3). Various cardiovascular conditions, such as acute myocardial infarction (AMI), aortic dissection, pulmonary embolism, and others, significantly endanger patient wellbeing and survival (1, 4–6). Research has identified a 13.1% risk of major adverse cardiac events (MACE) within 30 days for patients presenting with chest pain, highlighting a considerable MACE risk in this demographic (7). Identifying individuals at high risk for MACE among those presenting with acute chest pain is therefore crucial. Diabetes mellitus is a recognized significant risk factor for cardiac chest pain (8, 9), yet the presence of diabetes alone does not sufficiently predict the likelihood of admission hyperglycemia, which can affect both diabetic and non-diabetic individuals alike.
The incidence of stress hyperglycemia is notably frequent among patients in the ED. Admission glucose (AG) is identified as the initial random blood glucose measurement taken upon hospital admission (10), serving as a stress response that can affect anyone under significant stress, especially those with critical cardiovascular conditions, trauma, or multiorgan dysfunction or failure (11). Previous research has shown its predictive value for adverse outcomes across a broad spectrum of diseases (12–14). However, the specific relationship between AG levels and prognosis in patients with acute cardiac chest pain remains unclear.
Therefore, this paper endeavors to explore the association between AG and MACE in patients experiencing acute cardiac chest pain, aiming to enhance our understanding and management of such cases.
For examining the correlation between 30-day MACE of chest pain patients and AG, we utilized data from individuals presenting with chest pain symptoms from the regional Evaluation and Management of Patients with Acute Chest Pain in China (EMPACT) cohort (NCT02536677). EMPACT was a multicenter prospective registry, which enrolled patients presenting to the ED with acute chest pain from 22 representative public hospitals in Shandong province, China (15). This registry contained details about clinical baseline, initial evaluation, further diagnostic testing, treatment, and other hospitalization information. Patients were tracked for MACE through medical records and telephone follow-up at 30 days after their enrollment. The final diagnoses of patients and all MACE were independently adjudicated by a clinical events committee. Finally, we excluded one hospital with a small number of AG tests from four regional grade III-A urban hospitals and ultimately selected three hospitals for inclusion in the analysis.
Patient inclusion spanned from January 2016 to October 2017 and required individuals to be over 18 years of age. These patients presented to the ED for chest pain symptoms suspected to be of cardiac origin, occurring within 24 h of pain onset. In addition, patients exhibiting atypical symptoms (such as sweating, faintness, and back pain) followed by chest pain were also considered for inclusion. Exclusions were made for patients transferred from another hospital, those previously recruited in an earlier presentation, or individuals who declined to provide informed consent.
As soon as a patient was identified as eligible for inclusion, research assistants collected demographic data and data on risk factors and medical history through a direct interview, as well as information from hospital records about investigations and management during hospitalization. The patient's personal information is first recorded on a standardized case report form (CRF) and then entered into the electronic data capture (EDC) system (Likangtimes Corporation, Beijing, China). The clinical data collected included those on demographics, risk factors, medical history, presenting symptoms, electrocardiograms (ECGs), medications, and in-hospital procedures.
This study adhered to the guidelines of the Declaration of Helsinki and received approval from the research ethics committee of Qilu Hospital. To ensure patient confidentiality, all identifiable personal information was anonymized before analysis. Unique identifiers were assigned to each participant to replace personal data, ensuring that no individual could be identified from the data set. Access to raw data was restricted to the primary research team, and data were stored in secure, password-protected databases. The data handling procedures were designed to comply with ethical standards and institutional guidelines. Only aggregated data were reported, ensuring that individual patient information remained confidential.
After the initial screening process, a random blood sample was collected from each patient, and it was centrifuged immediately after collection. These plasma samples were analyzed at three reputed medical centers using the Johnson VITROS 5600 Integrated System, which employs dry chemical technology. We also provided unified training for the laboratory technicians of the three hospitals. The quality of sample collection and testing in each hospital is controlled by the monitoring system. In quality control, monitoring systems consist of three critical elements. First, algorithms will check for missing or implausible data; second, investigators at participating sites will verify the accuracy of all CRFs and medical records. Third, the coordinating center monitors the data online on a daily basis to confirm that they meet the project requirements.
The endpoint for this study was MACE within 30 days of patient presentation, encompassing recurrent MI, stroke, all-cause death, cardiogenic shock, urgent target vessel revascularization, and cardiac arrest (CA). The definition of recurrent MI is a new MI occurring within 30 days postadmission, defined by clinical or ECG evidence of myocardial ischemia coupled with a cardiac troponin increase and/or decrease, with at least one value exceeding the 99th percentile upper reference limit (URL), as per the fourth universal definition. Stroke is identified by acute focal neurological deficits of vascular origin, lasting ≥24 h or until death, substantiated by brain imaging (such as CT or MRI) and neurologic/neurosurgical assessments. Cardiogenic shock is defined as systolic blood pressure (SBP) <90 mmHg and/or cardiac index <2.2 L/(min m2) due to persistent (>30 min) cardiac dysfunction, accompanied by tissue and organ hypoperfusion. Urgent target vessel revascularization is identified by coronary revascularization for 30 days, which includes the two main types of procedures: coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI). CA is the ejection of the heart, which suddenly stops and aortic pulsation disappears.
A descriptive analysis of the characteristics of patients with AG measurements was performed by the AG quartile group. Continuous variables were represented as means and standard deviations (SDs), and categorical variables were represented using numbers and percentages. For continuous variables, one-way ANOVA or Student's t-test was used, and for categorical variables, chi-square or Fisher's exact test was used. For categorical variables, the trends were calculated with the Cochran–Armitage trend test.
The dissimilarities between the quartiles in 30-day MACE were illustrated by Kaplan–Meier curves and evaluated using the log-rank test. In addition, we calculated MACE hazard ratios with 95% confidence intervals (CI) using Cox regression models. These models treated AG as either a continuous or a quartile-based categorical variable and were adjusted for potential confounders, including age, sex, body mass index (BMI), heart rate at admission, systolic blood pressure, smoking status, and past medical history (covering myocardial infarction, coronary artery disease (CAD), hypertension, diabetes mellitus, and chronic renal insufficiency), as well as diagnosis. Moreover, we also used a Chi-square test to evaluate in-hospital MACE, and this test result is included in the Supplementary Material.
Based on previous studies and results of baseline characteristics analysis, subgroup analyses were conducted across various demographics and medical histories, including age, sex, history of diabetes, hypertension, dyslipidemia, prior CAD, and the type of presenting condition (cardiac diseases and non-cardiac diseases). In this analysis, cardiac diseases included AMI, unstable angina, and stable angina, while non-cardiac diseases included arrhythmias, digestive diseases, respiratory diseases, neurodynia, and mental system diseases. Models with interaction terms were tested between AG and subgroup variables by using a likelihood ratio test, adjusting for the aforementioned covariates, unless the variable was used as a subgroup variable. A p-value <0.05 on a two-tailed test was considered statistically significant. Statistical analyses were conducted with SAS version 9.4 (SAS Institute, Cary, NC, USA).
In this study, 1,705 patients from three hospitals (Qilu Hospital, Jinan, China; Zibo Central Hospital, Zibo, China; the Affiliated Hospital of Jining Medical College, Jining, China) met the inclusion criteria. The cohort comprised 1,705 individuals presenting with chest pain, with an average age of 65.23 ± 12.66 years. Of these patients, 1,028 (60.29%) were male, and 500 (29.33%) had a history of diabetes. The median AG levels were 7.60 mmol/L, with an interquartile range of 6.30–10.20 mmol/L. We divided the AG levels into quartiles: Q1, 5.7 (5.3, 6) mmol/L; Q2, 6.8 (6.6, 7.1) mmol/L; Q3, 8.5 (7.9, 9.2) mmol/L; Q4, 13.2 (11.5, 15.8) mmol/L.
All included patients with chest pain comprised 1,213 patients with cardiac diseases (802 cases of AMI, 379 cases of unstable angina, and 32 cases of stable angina) and 492 patients with non-cardiac diseases (56 cases of arrhythmias, 33 cases of digestive disease, 19 cases of disease of respiratory diseases, 46 cases of artery diseases, 19 cases of neuromuscular diseases, 12 cases of mental system diseases, 204 cases of chest pain with unknown reason, and 103 cases of others).
Notably, we observed distinct differences in baseline characteristics among the quartiles, including variations in age (P < 0.001), sex (P = 0.009), heart rate at admission (P < 0.001), history of hypertension (P < 0.001), history of diabetes (P < 0.001), history of dyslipidemia (P = 0.047) and disease classification (P < 0.001) (cardiac vs. non-cardiac diseases). Moreover, the use of ADP receptor antagonists also varied between these groups (P < 0.001) (Table 1). In addition, an increasing trend was evident in the parameters of age (P < 0.001), heart rate at admission (P < 0.001), prevalence of prior diabetes (P < 0.001), and disease classification (P = 0.003) as AG levels rose.
During the follow-up, MACE occurred in 154 patients, accounting for 9.03% of all patients (Table 2). Notably, the Kaplan–Meier curves depicted in Figure 1 demonstrate that there is a significant difference in the risk of MACE among the quartile groups (P < 0.001 according to the log-rank test). The shadow area in this figure represents the survival interval of the Kaplan–Meier curve. Furthermore, there were significant differences in the risk of all-cause mortality (P < 0.001), CA (P = 0.008), cardiogenic shock (P = 0.014), and stroke (P = 0.032) among patients in the quartile groupings, while no differences were found in the risk of MI (P = 0.370) and urgent target vessel revascularization (P = 0.580) (Supplementary Figure S1).
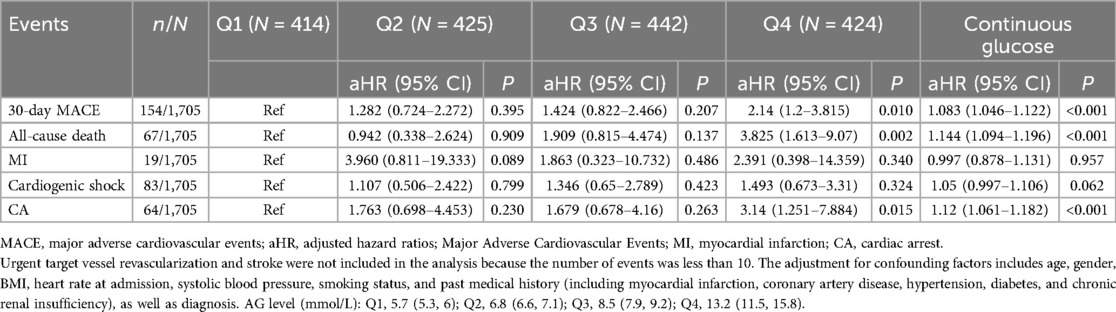
Table 2. Adjusted hazard ratios (95% CI) of 30-day MACE according to quartiles of admission glycemia levels in patients with chest pain.

Figure 1. A Kaplan–Meier survival analysis of 30-day MACE according to admission glucose levels in patients with chest pain. The shadow area represents the survival interval of the Kaplan–Meier curve. MACE, major adverse cardiac events.
After performing Cox regression analysis, we found that the Q4 AG levels were significantly associated with an increased risk of MACE [adjusted hazard radio (aHR): 2.14; 95% CI: 1.2–3.815; P = 0.010]. Furthermore, the Q4 AG levels also correlated with an elevated risk of all-cause death (aHR: 3.825; 95% CI: 1.613–9.07; P = 0.002) and CA (aHR: 3.14; 95% CI: 1.251–7.884; P = 0.015). However, no statistically significant association was observed for the 30-day risk of recurrent MI and cardiogenic shock between the groups (aHR: 2.391; 95% CI: 0.398–14.359; P = 0.340; aHR: 1.493; 95% CI: 0.673–3.31; P = 0.324, respectively). In addition, AG as a continuous variable was associated with increased risks of 30-day MACE (aHR: 1.083; 95% CI: 1.046–1.122; P < 0.001), all-cause death (aHR: 1.144; 95% CI: 1.094–1.196; P < 0.001), and CA (aHR: 1.12; 95% CI: 1.061–1.182; P < 0.001).
We also analyzed AG levels and MACE during ED admission and hospitalization. MACE during ED admission and hospitalization also represents MACE in the emergency phase. In our analysis, a total of 129 patients experienced MACE in the ED and during hospitalization. The median length of stay for patients in both the ED and the hospital was 10 days (range: 7–14 days). Finally, we found that patients with high AG levels had a higher likelihood of experiencing MACE, all-cause death, MI, cardiogenic shock, CA, and stroke during their ED visits and hospital stays (Supplementary Table S1).
Subgroup analysis was conducted based on age (over 65 years old), sex, previous medical history (diabetes, hypertension, dyslipidemia, and CAD), and the type of diagnosis. The results indicated that AG levels were significantly correlated with 30-day MACE, regardless of the patient's age, history of diabetes, hypertension, or CAD (Table 3). In addition, AG was significantly associated with 30-day MACE in male (aHR: 1.09; 95% CI: 1.045–1.137; P < 0.001) normolipidemic patients (aHR: 1.082; 95% CI: 1.043–1.122; P < 0.001) and cardiac disease patients (aHR: 1.081; 95% CI: 1.039–1.124; P < 0.001). Intriguingly, no notable interaction was found between each subgroup and AG levels in the overall cohort analysis.
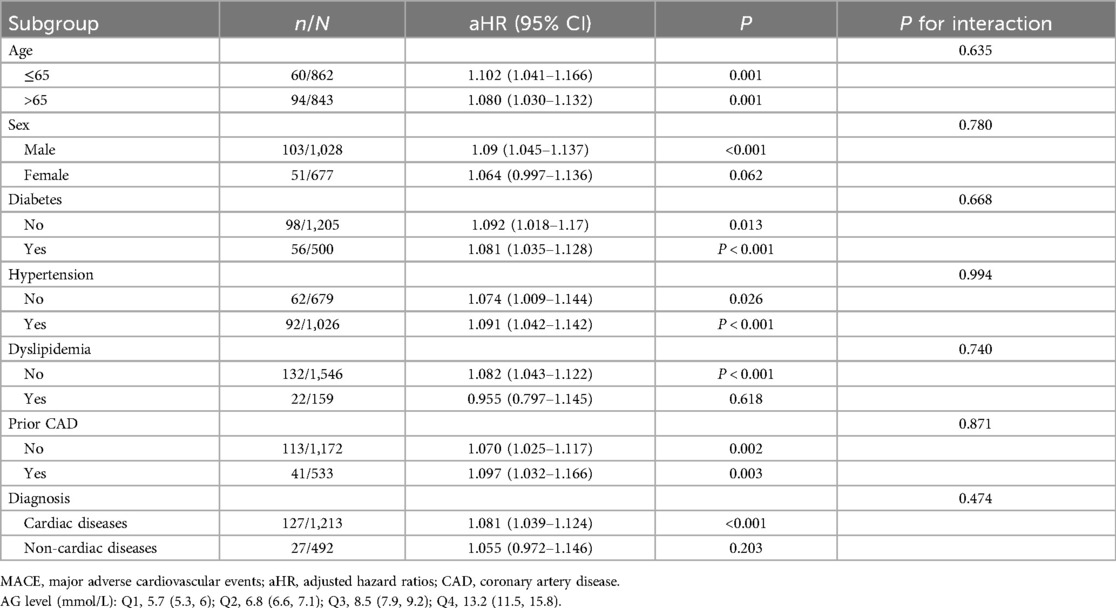
Table 3. A subgroup analysis between admission glucose levels and 30-day MACE in patients with chest pain.
In this registry-based cohort study, 1,705 patients discharged after treatment for acute cardiac chest pain from three tertiary hospitals in Eastern China were enrolled. The rate of incidence of 30-day MACE was 9.03%. The study's findings suggest that patients with elevated AG levels have a higher risk of experiencing 30-day MACE compared with those with lower levels of AG. Moreover, Cox regression analysis, adjusted for confounding factors, confirmed that AG levels were significantly associated with 30-day MACE. Particularly, the risk of MACE was notably higher among male patients who were diagnosed with cardiac diseases. The findings also indicate that regardless of whether a patient is over 65 years old, and has comorbidities such as hypertension, CAD, and diabetes, AG levels are significantly associated with 30-day MACE. However, no significant interaction between AG levels and each subgroup with regard to the risk of MACE was observed.
Despite previous research exploring the correlation between AG and various specific diseases, such as AMI (16, 17) and other critical diseases (18–21), the role of AG in patients with undifferentiated chest pain remains unknown. Considering the importance of managing patients with chest pain in emergency settings, clarifying the predictive role of AG would have clinical significance. Previous studies have found that AG levels are significantly correlated with both long-term and short-term prognoses of acute critical illnesses (17, 21), while this observational study examined the relationship between AG and 30-day MACE of patients with acute chest pain. As an indication of stress-induced hyperglycemia, our findings demonstrate that AG serves as a strong prognostic indicator for short-term outcomes in individuals experiencing chest pain. Numerous factors contribute to the development of stress hyperglycemia, such as inflammation, heightened release of counterregulatory hormones, and the suppression of pancreatic beta cells, as well as interventions like the administration of glucocorticoids or parenteral nutrition (22, 23). Moderate stress hyperglycemia has been found to enhance cell survival factors and decrease apoptosis, leading to a potential reduction in infarct size and improvement in systolic function in cardiogenic diseases (24). However, excessive stress hyperglycemia is associated with oxidative stress, inflammatory responses, cell damage to coronary microcirculation, and significantly impaired cardioprotective responses. In addition, the aggregation of platelets in response to ADP triggers the production of plasma catecholamine, which further exacerbates microcirculation dysfunction and thrombogenesis (25–27).
In examining the relationship between AG levels and 30-day MACE, we further carried out subgroup analysis. We found that AG levels were significantly correlated with 30-day MACE, regardless of the patient's age, history of diabetes, hypertension, or CAD. In the case of other diseases, many researchers have studied the relationship between AG levels and outcomes in males or females, but the results are varied (28–30). Takada et al. (30) found that the rate of mortality was higher in hyperglycemic men compared with lower blood glucose male and female groups, but there were no differences between women groups in respect to glycemia after adjustment for coronary risk factors, which is consistent with our findings. We found that AG levels were associated with 30-day MACE in men but not in women. In addition, we found a significant association between AG levels and outcomes in patients over 65 years, which is consistent with the findings of previous studies of AMI (31). Mamadjanov et al. (31) further grouped patients over the age of 65 and found that AG was significantly associated with 28-day case fatality in patients with AMI who were aged 65–74 years but not 75–84 years. Furthermore, in our subgroup analysis, non-diabetic patients with elevated AG levels exhibited a higher aHR compared with diabetic patients (aHR: 1.87; 95% CI: 0.932–3.752; P = 0.078 for non-diabetics; aHR: 1.443; 95% CI: 0.335–6.22; P = 0.623 for diabetics), despite p-values exceeding 0.05. This could be attributed to a significant proportion of non-diabetic patients having underlying insulin resistance, thereby increasing their mortality risk (32, 33).
In this analysis, there were only 492 patients with non-cardiac diseases, among whom 27 patients experienced 30-day MACE. Ultimately, we found no correlation between AG levels and 30-day MACE in patients with non-cardiac diseases. However, previous studies have shown that patients with pulmonary embolism in the fourth AG quartile, regardless of their diabetes history, had a significantly higher all-cause and PE-cause in-hospital mortality compared with those in the first quartile (34). In addition, among patients with diabetes and pulmonary embolism, those with higher AG levels tend to have a higher proportion of massive and submassive pulmonary embolism and higher pro-BNP levels (35). Furthermore, in other non-cardiac diseases such as aortic dissection, AG levels are also associated with poor patient outcomes (36). Therefore, we feel that larger sample size studies are needed in the future to validate the correlation between AG levels and 30-day MACE in patients with non-cardiac diseases.
Moreover, extensive previous research has highlighted the advantages of strict blood glucose control (37, 38). Our study underscores the importance of considering AG as a critical predictor in the ED. Physicians should particularly focus on chest pain patients with elevated AG levels, initiating treatment for early control of stress hyperglycemia. In addition to the above, previous studies did not evaluate how the relationship between AG levels and outcomes varies between different medical history subgroups in other diseases.
This study has several limitations. First, it did not investigate the issue of glycemic management of patients postadmission, nor could it elaborate on the correlation between in-hospital glucose levels and prognosis. Future research could further explore this aspect and the relationship between various tests and patients with chest pain. Second, the study included patients only from three hospitals in Eastern China, and therefore, it may not be applicable to other regions. Third, although we included diabetes history as a confounder for adjusted hazard ratios and performed a subgroup analysis of diabetes history, we did not collect the test values of glycated hemoglobin A1c (HbA1c). Last, being an observational study, the potential for the presence of unmeasured confounders remains, despite our efforts to adjust for known confounders.
The present study investigates the relationship between AG levels and the incidence of MACE within a 30-day period in patients presenting with cardiac chest pain in an emergency setting. The results demonstrate that patients with elevated AG levels face an increased risk of MACE within the aforementioned time frame. Therefore, it is imperative for emergency physicians to prioritize the monitoring and management of glucose levels in patients with chest pain, especially those presenting with high AG levels, as early as possible.
The original contributions presented in the study are included in the article/Supplementary Materials, and further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the research ethics committee of Qilu Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
XY: Visualization, Writing – original draft. XP: Investigation, Writing – review & editing. JZ: Investigation, Writing – review & editing. SW: Data curation, Formal Analysis, Validation, Writing – review & editing. WC: Investigation, Writing – review & editing. YW: Investigation, Writing – review & editing. CL: Project administration, Writing – review & editing. JW: Conceptualization, Funding acquisition, Methodology, Project administration, Writing – review & editing. YC: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by the National Key R&D Program of China (2020YFC1512700, 2020YFC1512705, 2020YFC1512703), National S&T Fundamental Resources Investigation Project (2018FY100600, 2018FY100602), National Natural Science Foundation of China (82172178), Key R&D Program of Shandong Province (2021ZLGX02, 2021SFGC0503), Taishan Pandeng Scholar Program of Shandong Province (tspd20181220), Taishan Young Scholar Program of Shandong Province (tsqn201812129, tsqn20161065), Youth Top-Talent Project of National Ten Thousand Talents Plan, and Qilu Young Scholar Program.
We thank the Home for Researchers team for carrying out language polishing of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1367704/full#supplementary-material
1. Hsia RY, Hale Z, Tabas JA. A national study of the prevalence of life-threatening diagnoses in patients with chest pain. JAMA Intern Med. (2016) 176:1029–32. doi: 10.1001/jamainternmed.2016.2498
2. Pedersen CK, Stengaard C, Friesgaard K, Dodt KK, Søndergaard HM, Terkelsen CJ, et al. Chest pain in the ambulance; prevalence, causes and outcome—a retrospective cohort study. Scand J Trauma Resusc Emerg Med. (2019) 27(1):84. doi: 10.1186/s13049-019-0659-6
3. Dawson LP, Smith K, Cullen L, Nehme Z, Lefkovits J, Taylor AJ, et al. Care models for acute chest pain that improve outcomes and efficiency: JACC state-of-the-art review. J Am Coll Cardiol. (2022) 79:2333–48. doi: 10.1016/j.jacc.2022.03.380
4. Lenfant C. Chest pain of cardiac and noncardiac origin. Metab Clin Exp. (2010) 59(Suppl 1):S41–6. doi: 10.1016/j.metabol.2010.07.014
5. Atluri DK, Chandar AK, Fass R, Falck-Ytter Y. Systematic review with meta-analysis: selective serotonin reuptake inhibitors for noncardiac chest pain. Aliment Pharmacol Ther. (2015) 41(2):167–76. doi: 10.1111/apt.13015
7. Greenslade JH, Nayer R, Parsonage W, Doig S, Young J, Pickering JW, et al. Validating the Manchester acute coronary syndromes (MACS) and troponin-only Manchester acute coronary syndromes (T-MACS) rules for the prediction of acute myocardial infarction in patients presenting to the emergency department with chest pain. Emerg Med J. (2017) 34(8):517–23. doi: 10.1136/emermed-2016-206366
8. Than M, Aldous S, Lord SJ, Goodacre S, Frampton CM, Troughton R, et al. A 2-hour diagnostic protocol for possible cardiac chest pain in the emergency department: a randomized clinical trial. JAMA Intern Med. (2014) 174(1):51–8. doi: 10.1001/jamainternmed.2013.11362
9. Sanchis J, Bodí V, Llácer A, Núñez J, Consuegra L, Bosch MJ, et al. Risk stratification of patients with acute chest pain and normal troponin concentrations. Heart. (2015) 91(8):1013–8. doi: 10.1136/hrt.2004.041673
10. Lazarus G, Audrey J, Wangsaputra VK, Tamara A, Tahapary DL. High admission blood glucose independently predicts poor prognosis in COVID-19 patients: a systematic review and dose-response meta-analysis. Diabetes Res Clin Pract. (2021) 171:108561. doi: 10.1016/j.diabres.2020.108561
11. Wang X, Cheng FTF, Lam TYT, Liu Y, Huang D, Liu X, et al. Stress hyperglycemia is associated with an increased risk of subsequent development of diabetes among bacteremic and nonbacteremic patients. Diabetes Care. (2022) 45(6):1438–44. doi: 10.2337/dc21-1682
12. Rinkel LA, Nguyen TTM, Guglielmi V, Groot AE, Posthuma L, Roos YBWEM, et al. High admission glucose is associated with poor outcome after endovascular treatment for ischemic stroke. Stroke. (2020) 51(11):3215–23. doi: 10.1161/STROKEAHA.120.029944
13. Lin S, He W, Zeng M. Association of diabetes and admission blood glucose levels with short-term outcomes in patients with critical illnesses. J Inflamm Res. (2020) 13:1151–66. doi: 10.2147/JIR.S287510
14. Jiang H, Li Y, Mo J, Chen X, Li M, Lin P, et al. Comparison of outcomes in emergency department patients with suspected cardiac chest pain: two-centre prospective observational study in southern China. BMC Cardiovasc Disord. (2018) 18(1):95. doi: 10.1186/s12872-018-0814-4
15. Zheng W, Wang J, Xu F, Zheng J, Zhang H, Ma J, et al. Evaluation and management of patients with acute chest pain in China (EMPACT): protocol for a prospective, multicentre registry study. BMJ Open. (2018) 8:e017872. doi: 10.1136/bmjopen-2017-017872
16. Cui K, Fu R, Yang J, Xu H, Yin D, Song W, et al. Admission blood glucose and 2-year mortality after acute myocardial infarction in patients with different glucose metabolism status: a prospective, nationwide, and multicenter registry. Front Endocrinol (Lausanne). (2022) 13:898384. doi: 10.3389/fendo.2022.898384
17. Liu L, Qian J, Yan W, Liu L, Zhao Y, Che L. Relationship between hyperglycaemia at admission and prognosis in patients with acute myocardial infarction: a retrospective cohort study. Postgrad Med J. (2022) 99:736–43. doi: 10.1136/pmj-2021-141454
18. Arnold M, Mattle S, Galimanis A, Kappeler L, Fischer U, Jung S, et al. Impact of admission glucose and diabetes on recanalization and outcome after intra-arterial thrombolysis for ischaemic stroke. Int J Stroke. (2014) 9(8):985–91. doi: 10.1111/j.1747-4949.2012.00879.x
19. Rajaratnam SG, Martin IG. Admission serum glucose level: an accurate predictor of outcome in gallstone pancreatitis. Pancreas. (2006) 33(1):27–30. doi: 10.1097/01.mpa.0000222315.36490.9b
20. Yang X, Shi N, Yao L, He W, Zhu P, Li S, et al. Impact of admission and early persistent stress hyperglycemia on clinical outcomes in acute pancreatitis. Front Endocrinol (Lausanne). (2022) 13:998499. doi: 10.3389/fendo.2022.998499
21. Long C, Fan W, Liu Y, Hong K. Stress hyperglycemia is associated with poor outcome in critically ill patients with pulmonary hypertension. Front Endocrinol (Lausanne). (2024) 15:1302537. doi: 10.3389/fendo.2024.1302537
22. Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet. (2009) 373(9677):1798–807. doi: 10.1016/S0140-6736(09)60553-537
23. Deane AM, Horowitz M. Dysglycaemia in the critically ill-significance and management. Diabetes Obes Metab. (2013) 15(9):792–801. doi: 10.1111/dom.1207
24. Wang M, Su W, Cao N, Chen H, Li H. Prognostic implication of stress hyperglycemia in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Cardiovasc Diabetol. (2023) 22(1):63. doi: 10.1186/s12933-023-01790-y
25. Fu R, Cui K, Yang J, Xu H, Yin D, Song W, et al. Fasting stress hyperglycemia ratio and in-hospital mortality after acute myocardial infarction in patients with different glucose metabolism status: results from China acute myocardial infarction registry. Diabetes Res Clin Pract. (2023) 196:110241. doi: 10.1016/j.diabres.2023.110241
26. Chen G, Li M, Wen X, Wang R, Zhou Y, Xue L, et al. Association between stress hyperglycemia ratio and in-hospital outcomes in elderly patients with acute myocardial infarction. Front Cardiovasc Med. (2021) 8:698725. doi: 10.3389/fcvm.2021.698725
27. Yang J, Zheng Y, Li C, Gao J, Meng X, Zhang K, et al. The impact of the stress hyperglycemia ratio on short-term and long-term poor prognosis in patients with acute coronary syndrome: insight from a large cohort study in Asia. Diabetes Care. (2022) 45(4):947–56. doi: 10.2337/dc21-1526
28. Huang ZX, Huang Y, Zeng J, Hao H, Petroski GF, Lu H, et al. Admission glucose levels may increase the risk for early neurological deterioration in females with acute ischemic stroke. Front Neurol. (2020) 11:548892. doi: 10.3389/fneur.2020.548892
29. Zindrou D, Taylor KM, Bagger JP. Admission plasma glucose: an independent risk factor in nondiabetic women after coronary artery bypass grafting. Diabetes Care. (2001) 24(9):1634–9. doi: 10.2337/diacare.24.9.1634
30. Takada JY, Ramos RB, Roza LC, Avakian SD, Ramires JAF, Mansur ADP. In-hospital death in acute coronary syndrome was related to admission glucose in men but not in women. Cardiovasc Diabetol. (2012) 17(11):47. doi: 10.1186/1475-2840-11-47
31. Mamadjanov T, Volaklis K, Heier M, Freuer D, Amann U, Peters A, et al. Admission glucose level and short-term mortality in older patients with acute myocardial infarction: results from the KORA myocardial infarction registry. BMJ Open. (2021) 11(6):e046641. doi: 10.1136/bmjopen-2020-046641
32. Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. (2000) 355(9206):773–8. doi: 10.1016/S0140-6736(99)08415-9
33. Mehta SR, Yusuf S, Díaz R, Zhu J, Pais P, Xavier D, et al. Effect of glucose-insulin-potassium infusion on mortality in patients with acute ST-segment elevation myocardial infarction: the CREATE-ECLA randomized controlled trial. JAMA. (2005) 293(4):437–46. doi: 10.1001/jama.293.4.437
34. Ljiljana J, Milena R, Vesna S, Bojana S, Boris D, Jovan M, et al. Predictive value of admission glycemia in diabetics with pulmonary embolism compared to non-diabetic patients. Acta Diabetol. (2022) 59(5):653–9. doi: 10.1007/s00592-021-01843-2
35. Gülru P, Mutlu OG, Özer Ö, Merve AT, Damla SU, Dursun T. The association between glycemia and clinical outcomes in patients with diabetes mellitus and pulmonary thromboembolism. Arch Endocrinol Metab. (2023) 67(3):341–7. doi: 10.20945/2359-3997000000544
36. Liu Z, Huang WQ. Effect of stress-induced hyperglycemia on long-term mortality in non-diabetic patients with acute type A aortic dissection: a retrospective analysis. Scand Cardiovasc J. (2024) 58(1):2373099. doi: 10.1080/14017431.2024.2373099
37. Ceriello A, Zarich SW, Testa R. Lowering glucose to prevent adverse cardiovascular outcomes in a critical care setting. J Am Coll Cardiol. (2009) 53:S9–13. doi: 10.1016/j.jacc.2008.09.054
Keywords: acute chest pain, admission glucose, MACE, emergency department, prognosis
Citation: Yin X, Pan X, Zhang J, Wu S, Cui W, Wang Y, Li C, Wang J and Chen Y (2024) Impact of admission glucose and 30-day major adverse cardiovascular events on patients with chest pain in an emergency setting: insights from the China EMPACT registry. Front. Cardiovasc. Med. 11:1367704. doi: 10.3389/fcvm.2024.1367704
Received: 9 January 2024; Accepted: 23 September 2024;
Published: 9 October 2024.
Edited by:
Wen-Jun Tu, Capital Medical University, ChinaReviewed by:
Zhi Qi, Nankai University, ChinaCopyright: © 2024 Yin, Pan, Zhang, Wu, Cui, Wang, Li, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiali Wang, d2FuZ2ppYWxpXzIwMDBAMTI2LmNvbQ==; Yuguo Chen, Y2hlbjkxOTA4NUBzZHUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.