
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Cardiovasc. Med., 02 July 2024
Sec. Structural Interventional Cardiology
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1367515
 Eustaquio M. Onorato1*
Eustaquio M. Onorato1* Francesco Alamanni2
Francesco Alamanni2 Giovanni Monizzi1
Giovanni Monizzi1 Angelo Mastrangelo1
Angelo Mastrangelo1 Antonio Luca Bartorelli1,3
Antonio Luca Bartorelli1,3
Background: Even though the optimal management of a moderate or large residual shunt following patent foramen ovale (PFO) closure is open to question, recent data confirmed that it is associated with an increased risk of stroke recurrence.
Case summary: A 48-year-old woman, a migraineur with visual aura, was diagnosed with a PFO associated with a huge multifenestrated atrial septal aneurysm (mfASA) and a moderate right-to-left shunt, detectable only after a Valsalva maneuver on contrast-transthoracic echocardiography. Brain magnetic resonance imaging showed a 1-mm silent white matter lesion in the right frontal lobe. Although the indication was not supported by guidelines, a transcatheter PFO closure was performed at another center with implantation of a large, equally sized, double-disc device (Figulla UNI 33/33 mm). At 6-month follow-up, a 2D/3D transesophageal echocardiography (TEE) color Doppler showed incorrect orientation of the device, which was not parallel to the interatrial septum, with two discs failing to capture the aortic muscular rim and partially protruding in the right atrium; furthermore, a 4 mm × 7 mm ASA fenestration was documented with a residual bidirectional shunt. Thereafter, the same team performed a minimally invasive cardiac surgery under femoro-femoral cardiopulmonary bypass; however, the procedure proved ineffective and was complicated by postoperative pericarditis with pericardial effusion, requiring further rehospitalization 1 month later due to persistent pericarditis, bilateral pleuritis, phrenic nerve palsy, and atrial flutter, which was treated with amiodarone. The patient asked for a second opinion, and our multidisciplinary heart team decided to offer a percutaneous redo intervention. An uneventful implantation of a regular PFO occluder (Figulla Flex II 16/18 mm) across the septal defect was performed successfully. Twelve-month follow-up with 2D TTE color Doppler and contrast transcranial Doppler showed correct position and good interaction between the two devices, with no residual shunt.
Discussion: In addition to the incorrect indication for PFO closure and the failure of minimally invasive surgery, the procedural mishap in this case could have been due to the inappropriate implantation of the first large device within the tunnel. It would have been better to deploy the same large device in the most central fenestration, covering the PFO and a greater part of the remaining mfASA at the same time.
Transcatheter patent foramen ovale (PFO) closure is a safe, effective, and highly successful procedure, associated with a low incidence of in-hospital complications and a low frequency of recurrent ischemic events at long-term follow-up; the risk of recurrence increases with the grade of the residual shunt (1–3). Complications related to the procedure and the device have been very low and generally transient (4). Furthermore, the selection of appropriate patients and devices suitable for the corresponding anatomical features of the PFO is essential for effective closure (5).
Moderate-to-severe residual right-to-left shunt (RLS) after PFO closure has been reported in approximately 10% of patients (6, 7) and has been associated with an increased risk of recurrent stroke or transient ischemic attack in long-term follow-up.
Until now, no agreement has been reached regarding the best management of a persisting residual shunt. Despite the lack of long-term data, a second transcatheter procedure appears to be technically feasible and safe, avoiding a more invasive surgical procedure. Several reports in the literature describe the implantation of a second device achieving complete closure (8–10).
We describe a case of persistent residual shunt after percutaneous PFO closure, followed by a minimal invasive surgical failure in a patient for whom a third procedure using a second device was successful, resulting in good clinical outcomes thereafter.
We present a case of a 48-year-old woman, a nurse and sportswoman, in whom contrast 2D transesophageal echocardiography (TTE) color Doppler demonstrated the presence of a tunnel-like PFO associated with huge mfASA and moderate RLS, visible only after the Valsalva maneuver. Brain magnetic resonance imaging (MRI) showed a 1-mm, silent, non-specific white matter lesion in the right frontal lobe. Her past medical history was unremarkable, except for episodic migraines with a visual aura that were responsive to ibuprofen. No thrombophilic disorders and atrial fibrillation were reported.
Although the indication was not supported by the guidelines (11, 12), transcatheter PFO closure was performed at another center with the implantation of a large, equally sized, double-disc device, Figulla UNI 33/33 mm (Occlutech International AB, Helsinborg, Sweden). The patient was discharged home on dual antiplatelet therapy (aspirin 100 mg and clopidogrel 75 mg daily) for 6 months. At 6 months follow-up, 2D/3D transesophageal echocardiography (TEE) color Doppler showed incorrect orientation of the device, not parallel to the interatrial septum, with the two discs not capturing the aortic muscular rim and partially protruding in the right atrium (Figures 1A,B); furthermore, a 4 mm × 7 mm ASA fenestration (septal defect) far from the UNI device was also identified (Figures 1C,D), with a residual bidirectional shunt that persisted unaltered on subsequent controls. Eight months later, the same team performed a minimally invasive cardiac surgery (MICS) procedure on the patient using a right parasternal approach under femoro-femoral cardiopulmonary bypass, which failed to close the residual shunt with interrupted sutures, ultimately leaving the inappropriately oriented device in situ. Regrettably, surgery proved not only ineffective but also complicated by postoperative pericarditis with pericardial effusion, which prolonged hospital stay. One month later, the patient was re-hospitalized due to persistent pericarditis, bilateral pleuritis, phrenic nerve palsy, and atrial flutter and was treated with amiodarone. Thereafter, colchicine and high doses of prednisone were used to treat and prevent recurrent pericarditis.
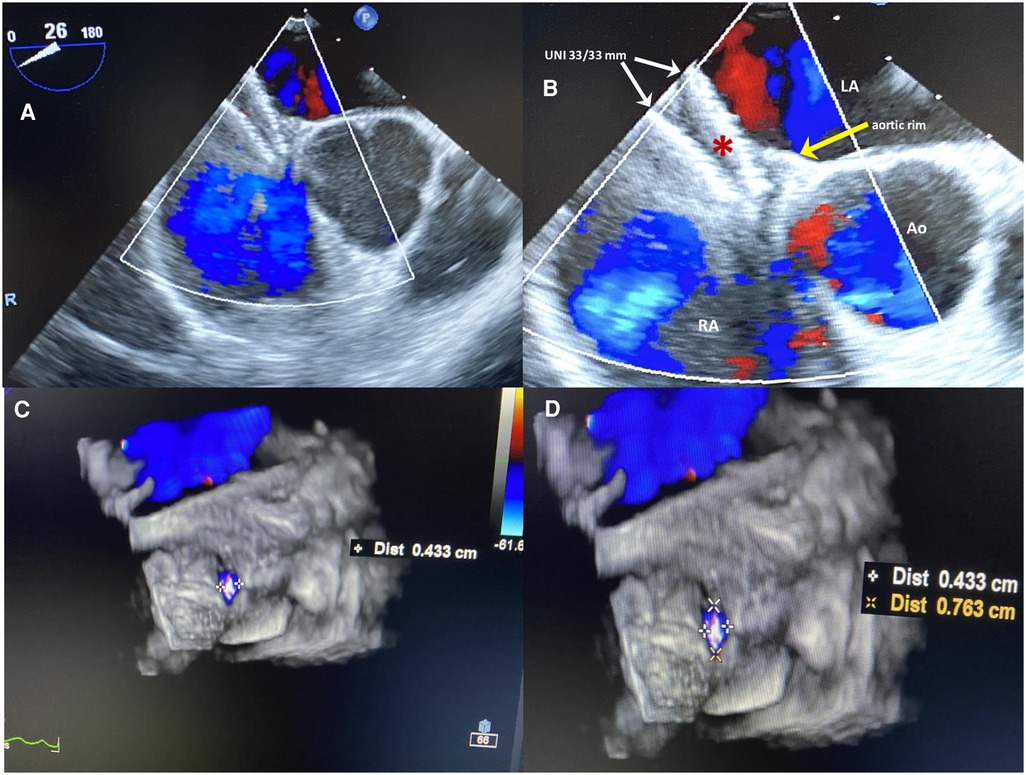
Figure 1 (A,B) Six-months 2D TEE color Doppler in the short view after the first percutaneous PFO closure procedure. The large equally sized double-disc UNI 33/33 mm device (red asterisk), is not parallel to the interatrial septum, with two discs diverging on one side (white arrows) and on the other side not capturing the aortic muscular rim (yellow arrow); the device is partially protruding in the right atrium with inappropriate orientation. (C,D) 3D TEE color Doppler showing a 4 mm × 7 mm ASA fenestration (septal defect) far from the UNI device.
The patient continued to complain of malaise, asthenia, and palpitations. Therefore, ergometric testing and stress echocardiography were performed, which showed normal results; no arrhythmias were recorded on the ECG Holter. Multidetector computed tomography angiography ruled out pulmonary thromboembolism. Moderate lung function impairment was detected, characterized by a reduction in forced expiratory volume (FEV1) and forced vital capacity (FVC). Meantime, the patient was unable to resume her nursing responsibilities and sporting activities due to persistent dyspnea on exertion and tiredness. She finally decided to attend our Heart and Brain Clinic a year later for a second opinion; after evaluation, our multidisciplinary heart team decided to offer a percutaneous redo intervention due to deteriorating clinical conditions and the persistent significant residual shunt, which was confirmed by contrast 2D TTE (Supplementary Figure S1). Written informed consent for a redo procedure was obtained from the patient. During her third hospitalization, the electrocardiogram (ECG) showed an incomplete right bundle branch block, and continuous ECG monitoring ruled out atrial fibrillation. A chest x-ray revealed no signs of increased pulmonary flow. The procedure was carried out under local anesthesia, fluoroscopic guidance, and continuous rotational intracardiac echocardiography by an Ultra ICE (EP Technologies, Boston Scientific Corporation, San Jose, CA, USA), as previously described (13), using two standardized sections: a transverse one on the aortic valve plane and a longitudinal section on the four-chamber plane (14).
Access to the right and left femoral veins was obtained using 8-Fr short introducer sheaths. Thereafter, the septal fenestration, apart from the previously implanted device, was crossed by a 6-Fr multipurpose catheter. After successful placement of a 260-cm exchange guidewire in the upper left pulmonary vein, the dedicated 9-Fr delivery sheath was advanced over the wire into the left atrium. The distal disc of the PFO Figulla Flex II 16/18 mm (Occlutech International AB, Sweden) was then opened in the left atrium and pulled back against the septum at the edge of the previously implanted UNI 33/33 mm device. While maintaining tension on the delivery cable, the proximal disc was then opened in the right atrium and pushed forward to the septal fenestration. After a meticulous “push-and-pull” maneuver, the device was successfully released, and fluoroscopic and rotational intracardiac ultrasound guidance imaging showed the two devices in profile without interferences or malapposition/dislocations between them (Figures 2,3). The patient was discharged home the following day in good clinical condition. Dual antiplatelet therapy (aspirin 100 mg/daily and clopidogrel 75 mg/daily) was recommended for the first 2 months, followed by single antiplatelet therapy (aspirin 100 mg/daily) up to 6 months. Antibiotic prophylaxis for infective endocarditis was also suggested. Follow-up with 2D TTE color Doppler and contrast transcranial Doppler (cTCD) at 6 and 12 months confirmed the correct position and good interaction between the two devices, without any residual shunt (Figure 4, Supplementary Figure S2). Currently, clinical improvement has been achieved, providing a better quality of life.
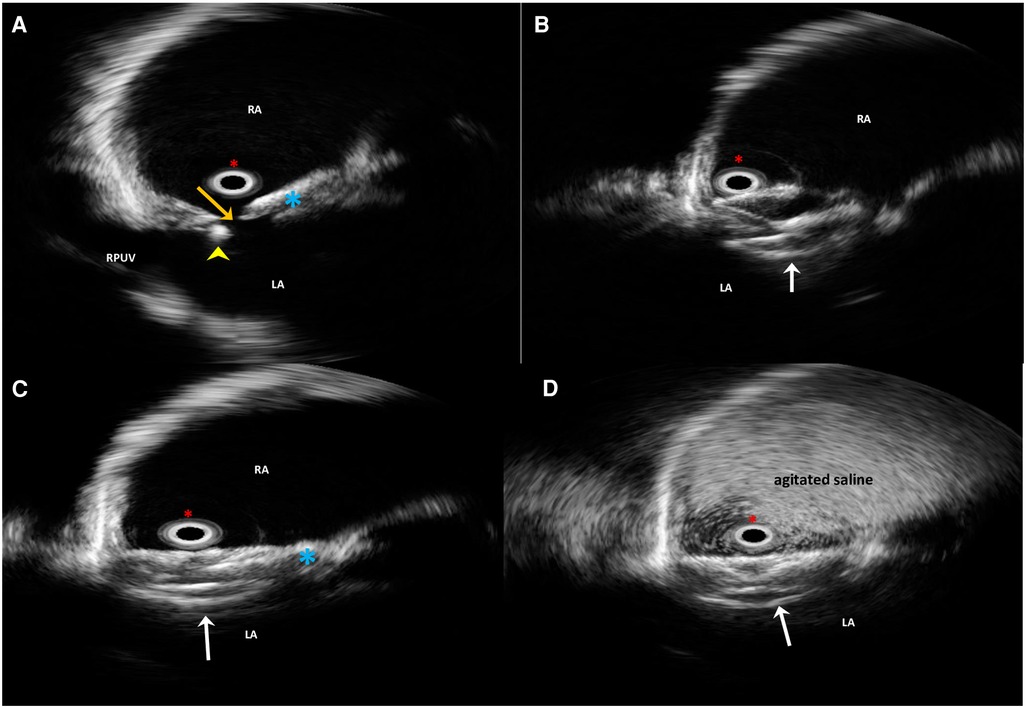
Figure 2 Long-axis four-chamber plane intraprocedural rotational intracardiac echocardiography by Ultra ICE (mechanical 9F/9 MHz 360° scan probe) procedural steps. The guidewire (yellow arrowhead) is across the residual septal defect (orange arrow) apart from the previously implanted device (light blue asterisk) (A). Successful implantation steps of the Flex II PFO 16/18-mm device (white arrows) (B,C). Contrast-enhanced Ultra ICE image confirming the abolition of the residual shunt (D). LA, left atrium; RA, right atrium; RUPV, right upper pulmonary vein. Red asterisk, the Ultra ICE-9F-9 MHz catheter located at the center of the image.
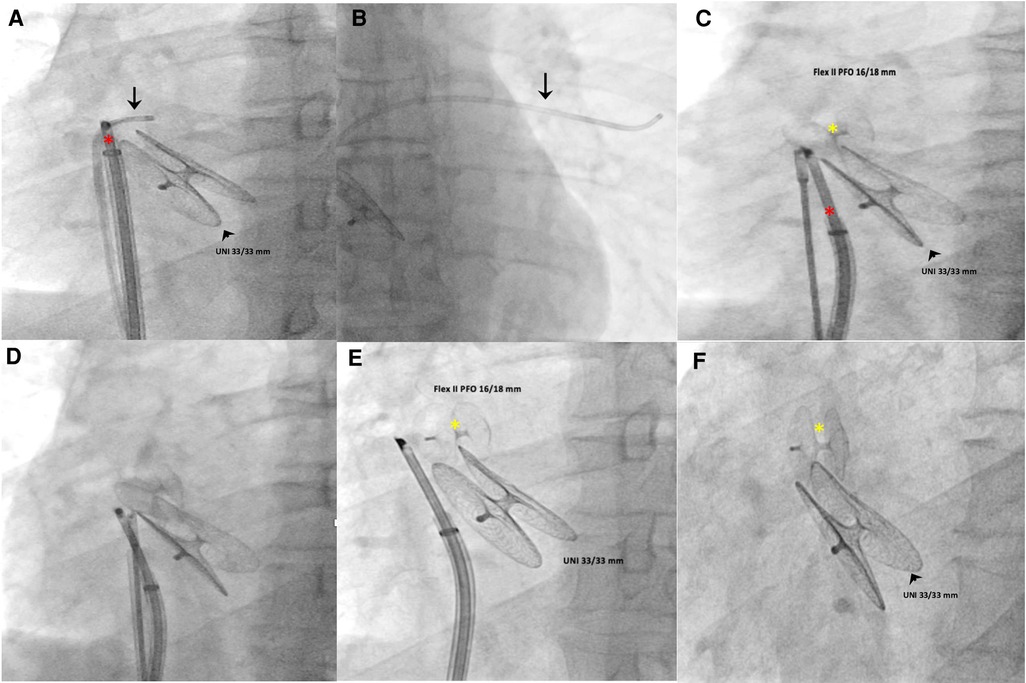
Figure 3 Intraprocedural fluoroscopic procedural steps (left anterior oblique 30° view). A multipurpose catheter (black arrow) crossed the septal fenestration and was then positioned in the upper left pulmonary vein (A,B). Using a dedicated 9-Fr-long sheath, a PFO occluder (Figulla Flex II 16/18 mm) was advanced across the septal fenestration and deployed in the appropriate orientation without impinging the previously implanted device (C–F). The red asterisk indicates the Ultra ICE-9F-9 MHz catheter; black arrowhead, UNI 33/33 mm; yellow asterisk, Figulla Flex II 16/18 mm.
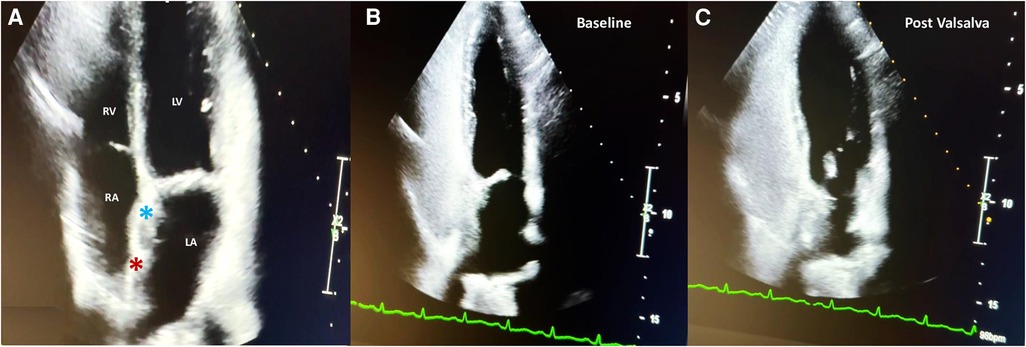
Figure 4 Two-year follow-up 2D TTE color Doppler in the apical four-chamber view showing the correct interaction between the UNI 33/33-mm device (light blue asterisk) and the Figulla Flex II 16/18-mm device (red asterisk) (A); contrast 2D TTE confirming abolition of the residual shunt at baseline (B) and after the Valsalva maneuver (C).
Among the different types of residual shunts after PFO closure, the most common is the in-tunnel shunt between the devices’ discs. Another type of residual shunt may be due to iatrogenic erosion at the edge of device rims, resulting in a small atrial septal defect that can be responsible for left-to-right or bidirectional shunts, with or without hemodynamic impairment (15, 16).
Furthermore, accessory undetected multiple fenestrations of ASA associated with PFO, as in our case, are another frequent but avoidable cause of a significant residual shunt after PFO closure; their presence should be ruled out at during baseline echocardiographic evaluation or at the time of the procedure (17).
Some anatomical conditions are proven independent predictors of residual shunt after percutaneous PFO closure (18–21). Among them, ASA, defined as an excursion ≥10 mm with a base diameter ≥15 mm of the septum primum, is considered one of the key features of complex PFO anatomies. In the majority of cases, it is frequently associated with moderate-to-severe baseline RLS and a fourfold higher risk of paradoxical events (22).
Implantation of larger devices to cover the vast majority of the aneurysm is a controversial strategy still adopted in many centers, resulting in additional risk factor for the occurrence of a residual shunt (9). Indeed, a bigger device does not always guarantee the effective closure of the multifenestrations, and the choice of implanting a single larger device through the largest fenestration or, alternatively, two smaller occluders instead of a single larger one may prove successful for this purpose.
In fact, the presence of a residual shunt after PFO closure has been a topic of ongoing debate for many years, primarily due to the controversy regarding its association with an increased risk of recurrent ischemic events (23–25).
Recently, a prospective cohort study aimed at comparing the recurrence of ischemic cerebral events in those with and without a residual shunt has been published, confirming that residual shunt was associated with a significant increased risk of recurrent event and the risk was heightened in patients with higher grades of the residual shunt (26). Moreover, the 2021 American Guidelines for the Prevention of Stroke reached the same conclusion, confirming that residual shunt is associated with an increased risk of stroke recurrence (27).
Even though the optimal management of residual moderate or large residual shunt after PFO closure remains open to question (20, 28), the literature on long-term results after the implantation of a second device is increasing, and retrospective evaluations are encouraging (8–10, 29, 30).
The paradigm of the modern era of cardiac surgery is changing with the use of MICS, which allows performing a wide variety of complex operations (valve repair, valve replacement, and coronary artery bypass graft surgery) through right or left mini-thoracotomy (sternotomy-free) and, in some cases, without cardiopulmonary bypass (31–34). Nevertheless, the risks associated with minimally invasive heart surgery are similar to those of open-heart surgery, including bleeding, infection, stroke, pericarditis, pleuritis, and phrenic nerve traction injury. Furthermore, these procedures may be more expensive and take longer to perform.
Although performed entirely through a mini-thoracotomy in the right intercostal space, minimizing surgical trauma, allowing quick recovery, and offering excellent cosmetic results, MICS may not be the best choice for treating residual shunts. In fact, in our patient, it was not only ineffective but also complicated by pericarditis, pleuritis, and phrenic nerve palsy. Therefore, a second percutaneous closure procedure, less invasive than surgery, should be strongly considered for patients with moderate-to-large residual shunts and appears to be technically feasible, effective, and safe. Moreover, to improve procedural success, a better understanding of the anatomic and device-related factors associated with closure efficacy is needed (17, 18).
In addition to the incorrect PFO closure indication and the ineffective and risky minimally invasive surgery, procedural failure in this case could have been due to the inappropriate implantation of the first large device within the PFO tunnel. Given the complex morphology of the PFO associated with mfASA, it would have been better to deploy the same large device in the most central fenestration, covering the PFO and a great part of the remaining mfASA at the same time.
Moderate-to-large residual shunts after PFO closure represent technical- or procedure-related failures that may be due to complex, unsuitable PFO anatomies and inaccurate selection of devices in the majority of cases. This complication needs further treatment, percutaneously or surgically, due to the risk of recurrent embolic events. The present case confirms that a second transcatheter closure of a residual shunt should be the first-line treatment option because it is technically feasible, effective, safe, and less invasive than surgery.
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
The studies involving humans were approved by I.R.C.C.S. Ospedale Galeazzi-Sant’Ambrogio, GSD, Milan, Italy. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
EO: Conceptualization, Data curation, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. FA: Conceptualization, Methodology, Supervision, Writing – review & editing. GM: Data curation, Investigation, Methodology, Software, Writing – original draft. AM: Conceptualization, Investigation, Software, Writing – original draft. AB: Conceptualization, Data curation, Methodology, Supervision, Validation, Visualization, Writing – original draft.
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declared that they were an editorial board member of Frontiers at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1367515/full#supplementary-material
Supplementary Figure S1
Contrast 2D TTE in the apical four-chamber view showing a moderate right-to-left shunt at baseline (A) that increases significantly after the Valsalva maneuver (B).
Supplementary Figure S2
Two-year follow-up contrast-enhanced transcranial Doppler at baseline (A) and after the decisive Valsalva maneuver (B) showing no RLS at all.
1. Shah R, Nayyar M, Jovin IS, Rashid A, Bondy BR, Fan TM, et al. Device closure versus medical therapy alone for patent foramen ovale in patients with cryptogenic stroke: a systematic review and meta-analysis. Ann Intern Med. (2018) 168:335–42. doi: 10.7326/M17-2679
2. Carroll AM, Carroll JD. Device closure of patent foramen ovale for cryptogenic stroke: patient selection and outcomes according to new randomized trials. Curr Cardiol Rep. (2019) 21(6):48. doi: 10.1007/s11886-019-1137-5
3. Caputi L, Butera G, Parati E, Sangiorgi G, Onorato E, Anzola GP, et al. New trends in neurosonology and cerebral hemodynamics—an update. Perspect Med. (2012) 1:236–40. doi: 10.1016/j.jstrokecerebrovasdis.2012.12.002
4. Caputi L, Butera G, Anzola GP, Carminati M, Carriero MR, Chessa M, et al. Residual shunt after patent foramen ovale closure: preliminary results from Italian Patent Foramen Ovale Survey. J Stroke Cerebrovasc Dis. (2013) 22(7):e219–26. doi: 10.1016/j.jstrokecerebrovasdis.2012.12.002
5. Matsumura K, Gevorgyan R, Mangels D, Masoomi R, Mojadidi MK, Tobis J. Comparison of residual shunt rates in five devices used to treat patent foramen ovale. Catheter Cardiovasc Interv. (2014) 84:455–63. doi: 10.1002/ccd.25453
6. Schwerzmann M, Windecker S, Wahl A, Nedeltchev K, Mattle HP, Seiler C, et al. Implantation of a second closure device in patients with residual shunt after percutaneous closure of patent foramen ovale. Catheter Cardiovasc Interv. (2004) 63:490–5. doi: 10.1002/ccd.20221
7. Diaz T, Cubeddu RJ, Rengifo-Moreno PA, Cruz-Gonzalez I, Solis-Martin J, Buonanno FS, et al. Management of residual shunts after initial percutaneous patent foramen ovale closure: a single center experience with immediate and long-term follow-up. Catheter Cardiovasc Interv. (2010) 76:145–50. doi: 10.1002/ccd.22475
8. Majunke N, Wallenborn J, Baranowski A, Wunderlich N, Sievert H. Device closure of residual shunt after percutaneous closure of patent foramen ovale. EuroIntervention. (2010) 5:833–7. doi: 10.4244/eijv5i7a139
9. Susuri N, Obeid S, Ulmi M, Siontis GCM, Wahl A, Windecker S, et al. Second transcatheter closure for residual shunt following percutaneous closure of patent foramen ovale. EuroIntervention. (2017) 13:858–66. doi: 10.4244/EIJ-D-17-00061
10. Butera G, Sarabia JF, Saracino A, Chessa M, Piazza L, Carminati M. Residual shunting after percutaneous PFO closure: how to manage and how to close. Catheter Cardiovasc Interv. (2013) 82:950–8. doi: 10.1002/ccd.25097
11. Pristipino C, Sievert H, D'Ascenzo F, Louis Mas J, Meier B, Scacciatella P, et al. European Position paper on the management of patients with patent foramen ovale. General approach and left circulation thromboembolism. Eur Heart J. (2019) 40(38):3182–95. doi: 10.1093/eurheartj/ehy649
12. Kavinsky CJ, Szerlip M, Goldsweig A, Amin Z, Boudoulas KD, Carroll JD, et al. SCAI guidelines for the management of patent foramen ovale. J Soc Cardiovasc Angiograph Interv. (2022) 1(4):100039. doi: 10.1016/j.jscai.2022.100039
13. Zanchetta M, Rigatelli G, Pedon L, Zennaro M, Onorato E, Maiolino P. Intracardiac echocardiography during catheter-based procedures: ultrasound system, examination technique, and image presentation. Echocardiography. (2002) 19:501–7. doi: 10.1046/j.1540-8175.2002.00501.x
14. Zanchetta M, Onorato E, Rigatelli G, Pedon L, Zennaro M, Carrozza A, et al. Intracardiac echocardiography-guided transcatheter closure of secundum atrial septal defect: a new efficient device selection method. J Am Coll Cardiol. (2003) 42(9):1677–82. doi: 10.1016/j.jacc.2003.08.023
15. Meier B. Iatrogenic atrial septal defect, erosion of the septum primum after device closure of a patent foramen ovale as a new medical entity. Catheter Cardiovasc Interv. (2006) 68(1):165–8. doi: 10.1002/ccd.20584
16. Scacciatella P, Biava LM, Marra S. Iatrogenic erosion of the septum primum resulting in an atrial septal defect with left-to-right shunt: a rare pitfall of patent foramen ovale percutaneous closure. Catheter Cardiovasc Interv. (2014) 84:494–6. doi: 10.1002/ccd.25333
17. Vitarelli A, Mangieri E, Capotosto L, Tanzilli G, D’Angeli I, Toni D, et al. Echocardiographic findings in simple and complex patent foramen ovale before and after transcatheter closure. Eur Heart J Cardiovasc Imaging. (2014) 15:1377–85. doi: 10.1093/ehjci/jeu143
18. Marchese N, Pacilli MA, Inchingolo V, Fanelli R, Loperfido F, Vigna C. Residual shunt after percutaneous closure of patent foramen ovale with AMPLATZER occluder devices—influence of anatomic features: a transcranial Doppler and intracardiac echocardiography study. EuroIntervention. (2013) 9(3):382–8. doi: 10.4244/EIJV9I3A61
19. He L, Cheng G, Du Y, Zhang Y. Importance of persistent right-to-left shunt after patent foramen ovale closure in cryptogenic stroke patients. Tex Heart Inst J. (2020) 47(4):244–9. doi: 10.14503/THIJ-17-6582
20. Hammerstingl C, Bauriedel B, Stüsser C, Momcilovic D, Tuleta I, Nickenig G, et al. Risk and fate of residual interatrial shunting after transcatheter closure of patent foramen ovale: a long-term follow-up study. Eur J Med Res. (2011) 16(1):13–9. doi: 10.1186/2047-783x-16-1-13
21. Anzola GP, Morandi E, Casilli F, Onorato E. Does transcatheter closure of patent foramen ovale really “shut the door?” A prospective study with transcranial Doppler. Stroke. (2004) 35(9):2140–4. doi: 10.1161/01.STR.0000137764.07815.de
22. von Bardeleben RS, Richter C, Otto J, Himmrich L, Schnabel R, Kampmann C, et al. Long-term follow up after percutaneous closure of PFO in 357 patients with paradoxical embolism: difference in occlusion systems and influence of atrial septum aneurysm. Int J Cardiol. (2009) 134(1):33–41. doi: 10.1016/j.ijcard.2008.02.031
23. Wallenborn J, Bertog SC, Franke J, Steinberg DH, Majunke N, Wilson N, et al. Recurrent events after percutaneous closure of patent foramen ovale. Catheter Cardiovasc Interv. (2013) 82(4):541–6. doi: 10.1002/ccd.24511
24. Moon J, Kim M, Oh PC, Shin DH, Park HM, Jo YY, et al. Residual shunt after patent foramen ovale device closure in patients with cryptogenic stroke: serial bubble contrast transesophageal echocardiography data. J Stroke Cerebrovasc Dis. (2019) 28(2):347–53. doi: 10.1016/j.jstrokecerebrovasdis.2018.10.006
25. Liu TT, Jiao RH, Chen T, Jiang ZA, Bai WL. A systematic review and meta-analysis of the association between residual shunts after patent foramen ovale closure and long-term cerebrovascular events. Cerebrovasc Dis. (2023) 52(4):387–92. doi: 10.1159/000527457
26. Deng W, McMullin D, Inglessis-Azuaje I, Locascio JJ, Palacios IF, Buonanno FS, et al. Effect of patent foramen ovale closure after stroke on circulatory biomarkers. Neurology. (2021) 97(2):e203–14. doi: 10.1212/WNL.0000000000012188
27. Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. (2021) 52(7):e364–467. doi: 10.1161/STR.0000000000000375
28. Hung J, Landzberg MJ, Jenkins KJ, King ME, Lock JE, Palacios IF, et al. Closure of patent foramen ovale for paradoxical emboli: intermediate-term risk of recurrent neurological events following transcatheter device placement. J Am Coll Cardiol. (2000) 35(5):1311–2316. doi: 10.1016/s0735-1097(00)00514-3
29. Taggart NW, Reeder GS, Lennon RJ, Slusser JP, Freund MA, Cabalka AK, et al. Long-term follow-up after PFO device closure: outcomes and complications in a single-center experience. Catheter Cardiovasc Interv. (2017) 89(1):124–33. doi: 10.1002/ccd.26518
30. Messé SR, Gronseth GS, Kent DM, Kizer JR, Homma S, Rosterman L, et al. Practice advisory update summary: patent foramen ovale and secondary stroke prevention: report of the guideline subcommittee of the American Academy of Neurology. Neurology. (2020) 94(20):876–85. doi: 10.1212/WNL.0000000000009443
31. Jeanmart H, Casselman FP, De Grieck Y, Bakir I, Coddens J, Foubert L, et al. Avoiding vascular complications during minimally invasive, totally endoscopic intracardiac surgery. J Thorac Cardiovasc Surg. (2007) 133(4):1066–70. doi: 10.1016/j.jtcvs.2006.12.002
32. Marin Cuartas M, Davierwala PM. Minimally invasive mitral valve repair. Indian J Thorac Cardiovasc Surg. (2020) 36(Suppl 1):44–52. doi: 10.1007/s12055-019-00843-w
33. Akowuah E, Burdett C, Khan K, Goodwin A, Lage IB, El-Saegh M, et al. Early and late outcomes after minimally invasive mitral valve repair surgery. J Heart Valve Dis. (2015) 24:470–7. PMID: 26897819.26897819
Keywords: patent foramen ovale, transcatheter closure, atrial septal aneurysm, multifenestrated atrial septum aneurysm, residual shunt, minimally invasive cardiac surgery
Citation: Onorato EM, Alamanni F, Monizzi G, Mastrangelo A and Bartorelli AL (2024) Case Report: Persistent residual shunt after a first percutaneous PFO closure followed by minimally invasive surgical failure: third time is a charm. Front. Cardiovasc. Med. 11:1367515. doi: 10.3389/fcvm.2024.1367515
Received: 8 January 2024; Accepted: 12 June 2024;
Published: 2 July 2024.
Edited by:
Marcel Weber, University Hospital Bonn, GermanyReviewed by:
Tiziana Attisano, Ospedali Riuniti San Giovanni di Dio e Ruggi d'Aragona, Italy© 2024 Onorato, Alamanni, Monizzi, Mastrangelo and Bartorelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eustaquio M. Onorato, ZXVzdGFxdWlvLm9ub3JhdG9AZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.