- Department of Geriatrics, Fujian Key Laboratory of Vascular Aging, Fujian Institute of Geriatrics, Fujian Medical University Union Hospital, Fuzhou, Fujian, China
Background: High pulse pressure (PP) and aortic root diameter (AoD) are hallmarks of arterial stiffness or vascular aging and they are considered as risk factors for age-related cardiovascular disease, including heart failure (HF). However, the relationship between PP and AoD in patients with heart failure (HF) is uncertain. This study aimed to evaluate the relationship between PP and AoD in the middle-aged and the elderly with HF.
Methods: A total of 1,027 Chinese middle-aged and elderly patients with HF, including HF with reduced ejection fraction (HFrEF), HF with mid-range EF (HFmrEF), and HF with preserved EF (HFpEF) were included in this study. Pearson correlation analysis was used to evaluate the relationship between PP and AoD in the three types of HF. Multiple linear regression analysis was performed to assess the factors that affected AoD. Multivariate logistic regression was performed to determine the association between the PP level quartiles and AoD. The results were validated in an independent dataset included a total of 378 consecutive patients with HFrEF hospitalized at the Pingtan Branch of Fujian Medical University Union Hospital (Fujian, China).
Results: There was a positive correlation between PP and AoD in the middle-aged and the elderly with HFrEF. Multiple linear regression analysis revealed that PP, age, and body mass index (BMI) were independently correlated with AoD in HFrEF patients. In multivariate logistic regression analysis, an increased risk of aortic root dilation was observed in the highest quartile of the PP level compared with the lowest quartile. Age significantly interacted with PP (p = 0.047). A significant association between PP levels and AoD was only observed in patients ≥ 65 years old, but not in patients < 65 years old. In the validation dataset, PP was independently related to AoD in patients with HFrEF (β = 0.205, p = 0.001).
Conclusions: PP level was independently and positively associated with AoD, especially in the elderly with HFrEF, but not in patients with HFmrEF and HFpEF. Arterial stiffening or vascular aging may play a certain role in the elderly HFrEF patients.
1 Introduction
Millions of adults worldwide suffer from heart failure (HF), which is associated with higher mortality rates, morbidity, and healthcare costs in the world (1, 2). Clinically, three types of HF are recognized: HF with reduced ejection fraction (HFrEF), HF with mid-range ejection fraction (HFmrEF), and HF with preserved ejection fraction (HFpEF) (3). Previous studies demonstrated that pulse pressure (PP) could be used to predict left ventricular hypertrophy and cardiovascular events (4–9). Wei et al. found that high PP predicted all-cause death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization in patients with HF (10).
Several factors influence aortic root dilation, including age, gender, height, weight, body surface area (BSA), body mass index (BMI), and diseases such as hypertension, valvular heart disease, congenital heart disease, cardiomyopathy and ischemic cardiomyopathy (11). Recently, we and others have found that aortic root dilation also is one of the hall-mark of vascular aging or arterial stiffness (12, 13) which can lead to isolated systolic hypertension (14–16) and result in left ventricular remodeling, dysfunction, and heart failure (17, 18). A community-based cohort of Afro-American study found that aortic root diameter (AoD) was associated with an increased risk of cardiovascular events (19). The Framingham Heart Study indicated that the risk of incident HF increased with greater AoD at baseline and an increase in AoD over 8 years (20). AoD may be useful as a predictor of cardiovascular events even in the absence of aneurysmatic alterasions (21).
The precise relationship between PP and AoD is debatable (22–25) and there has yet to be a study looking into the association between them in the three types of HF. Furthermore, the relationship between PP and AoD in the middle-aged and the elderly with HF remains to be elucidated. Therefore, we aimed to investigate the relationship between PP and AoD in patients with HF in the present study.
2 Methods
2.1 Patients
We investigated the medical records of 1027 consecutive HF patients (age ≥ 45 years old) were hospitalized at the Fujian Medical University Union Hospital (Fujian, China) between January 2015 and December 2018. The patients' medical histories and relevant clinical characteristics were recorded. Patients were excluded from this study if they presented with any of the following conditions: acute myocardial infarction, acute myocarditis, significant valvular heart disease, congenital heart disease, renal failure [estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73 m2], malignancy, and chronic inflammatory disease.
The validation dataset included a total of 378 consecutive patients with HFrEF hospitalized at the Pingtan Branch of Fujian Medical University Union Hospital (Fujian, China) between January 2015 and December 2018. The inclusion criteria included the HFrEF patients who underwent Echocardiography. The exclusion criteria included patients lacking echocardiography test, patients ≤ 45 years of age, and other confounding conditions, such as acute myocardial infarction, acute myocarditis, significant valvular heart disease, congenital heart disease, renal failure [estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73 m2], malignancy, and chronic inflammatory disease.
2.2 Ethics statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Medical Faculty of Fujian Medical University Union Hospital Ethics Committee (No. 2019KY004).
2.3 Anthropometric and hemodynamic variables
Clinical information, including age, BMI, white blood cell count, red blood cell count, blood lipid, serum creatinine, blood pressure, echocardiographic parameters, medical history of hypertension, diabetes and HF, and use of cardiovascular drugs, was extracted from medical records. BMI was calculated using the following formula: BMI (kg/m2) = weight (kg)/height2 (m2). Brachial BP determinations were performed in the supine position after a 15-min rest in the hospital by traditional mercury sphygmomanometry, with the first and the fifth Korotkoff sounds for SBP and DBP measurements, respectively. The average of the last 3 consecutive BP measurements was used for data analysis (26). PP was calculated using the following formula: PP = systolic blood pressure (SBP)—diastolic blood pressure (DBP). The eGFR was calculated using the Recommended equations for GFR estimation (27). The AoD was measured from the M-mode tracing as the maximal distance between the leading edge to the leading edge (L-L) convention of anterior and posterior aortic root wall at the maximal level of the sinuses of Valsalva, as recommended by the American Society of Echocardiography guidelines (28). Left ventricular dimensions were measured based on the American Society of Echocardiography recommendations. Aortic root dilation was defined as an AoD of ≥34 mm and ≥30 mm in males and females, respectively.
Patients were classified into non-smokers and current smokers (continuously smoking one or more cigarettes a day for at least six months) based on their smoking status (29). Hypertension was diagnosed as SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg and/or taking antihypertensive medications according to the European Society of Cardiology (ESC) guidelines (30).
2.4 Definition and type of HF
The diagnosis of HF was based on the symptoms or signs, electrocardiograms (ECG), chest radiographs, and echocardiography (31). HF was categorized as HFpEF if the left ventricular ejection fraction (LVEF) was ≥50%, HFmrEF if LVEF was 40%–49%, and HFrEF if LVEF was <40%. Chronic kidney disease (CKD) was diagnosed when eGFR was <90 ml/min/1.73 m2. Coronary heart disease (CHD) was defined as a history of coronary stent implantation or coronary artery bypass graft and myocardial ischemia on ECG, with symptoms typical of angina or myocardial infarction. Stroke was defined as the presence of a definite history of stroke or signs of cerebral infarction on computed tomography or magnetic resonance imaging.
2.5 Statistical analysis
The normality of the data was assessed by the Kolmogorov–Smirnov test. Normally distributed variables are presented as mean ± standard deviation and compared via student t test. Non-normally distributed variables are expressed as the median and interquartile range (IQR) and analyzed by Mann–Whitney U test. Categorical variables are expressed as numbers and percentages (%) and compared using the χ2 test or Fisher's exact test (if theoretical frequency T < 5). 1-way ANOVA followed by the Bonferroni post test for multiple comparisons. Linear correlations between variables were assessed using Pearson's correlation analysis. Non-linear associations between variables were assessed using restricted cubic spline analysis. Multiple linear regression analyses were performed to assess the independent determinants of AoD/BSA. The association between AoD/BSA and clinical parameters including patients' age, BMI, blood test indicators, hemodynamic parameters and echocardiographic indicators, was analyzed based on stepwise linear regression. This analysis was conducted by considering AoD/BSA as independent variables, and relevant clinical characteristics were set as dependent variables. Using the logistic regression analysis, PP was grouped by quartiles when analyzing the relationship between PP and aortic dilation risk using logistic regression analysis. The interactions between PP and aortic dilation risk were assessed by introducing a cross-product term into the regression models. Statistical significance was set at p < 0.05. All data were analyzed using the SPSS (version 19.0, IL, USA) software.
3 Results
3.1 Clinical characteristics of patients with HF
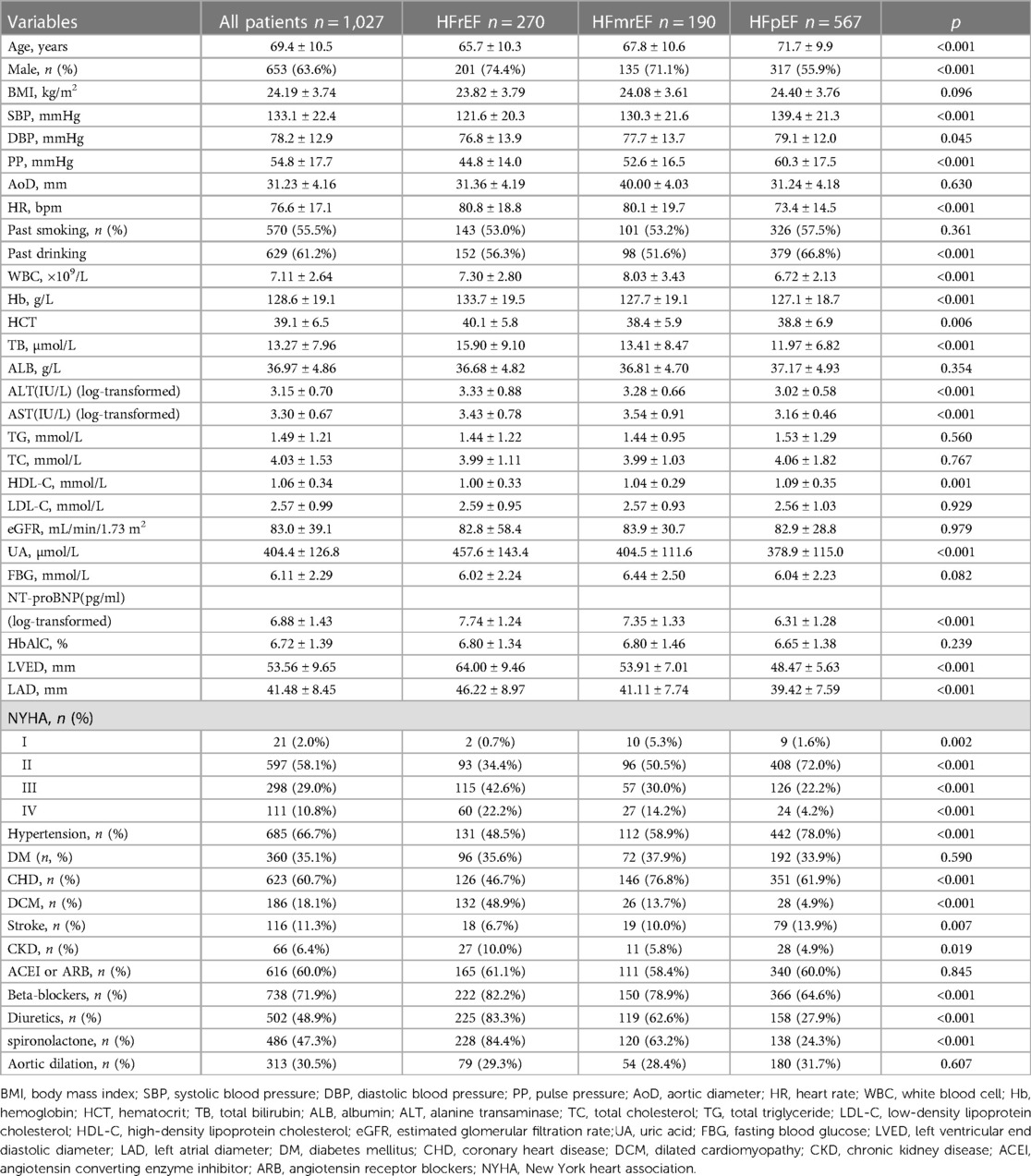
A total of 1,027 medical records of patients with HF were evaluated, including 270 (26.3%) HFrEF, 190 (18.5%) HFmrEF, and 567 (55.2%) HFpEF patients. The mean age of all patients was 69.4 ± 10.5 years, and 63.6% were males. The mean SBP and DBP were 133.1 ± 22.4 and 78.2 ± 12.9 mmHg, respectively, resulting in a mean PP of 54.8 ± 17.7 mmHg. Echocardiographic data were available for all patients; the AoD levels averaged 31.23 ± 4.16 mm. Of the 1,027 patients, 685 (66.7%) had a history of systemic hypertension, 360 (35.1%) had type 2 diabetes, and 623 patients (60.7%) had CHD. Among the HFrEF patients, 131 (48.5%) had a history of hypertension and 96 (35.6%) had type-2 diabetes. PP and AoD were 44.8 ± 14.0 mmHg and 31.36 ± 4.19 mm, 52.6 ± 16.5 mmHg and 40.00 ± 4.03 mm, and 60.3 ± 17.5 mmHg and 31.24 ± 4.18 mm in HFrEF, HFmrEF and HFpEF patients, respectively (Table 1).
3.2 Correlation between PP and AoD in patients with HFrEF
PP and AoD were found to increase with age in patients with HF (Figure 1, Supplementary Figure S1). Therefore, we further explored the association between PP and AoD in patients with HFrEF, HFmrEF, and HFpEF. The results showed that PP was positively associated with AoD in the middle-aged and the elderly with HFrEF (r = 0.380, p < 0.01), but not in patients with HFmrEF (r = 0, p = 0.99) or HFpEF (r = 0.01, p = 0.9) (Figure 2).
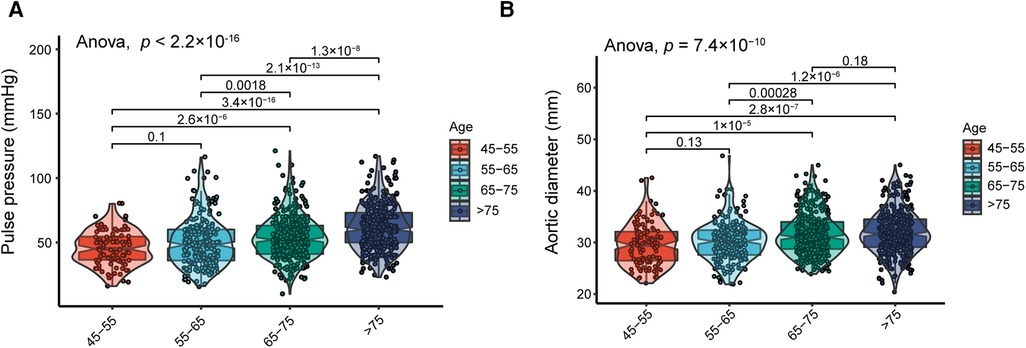
Figure 1. (A) Distribution of PP in different age groups; PP increases with age. (B) Distribution of AoD in different age groups; AoD increases with advanced age.
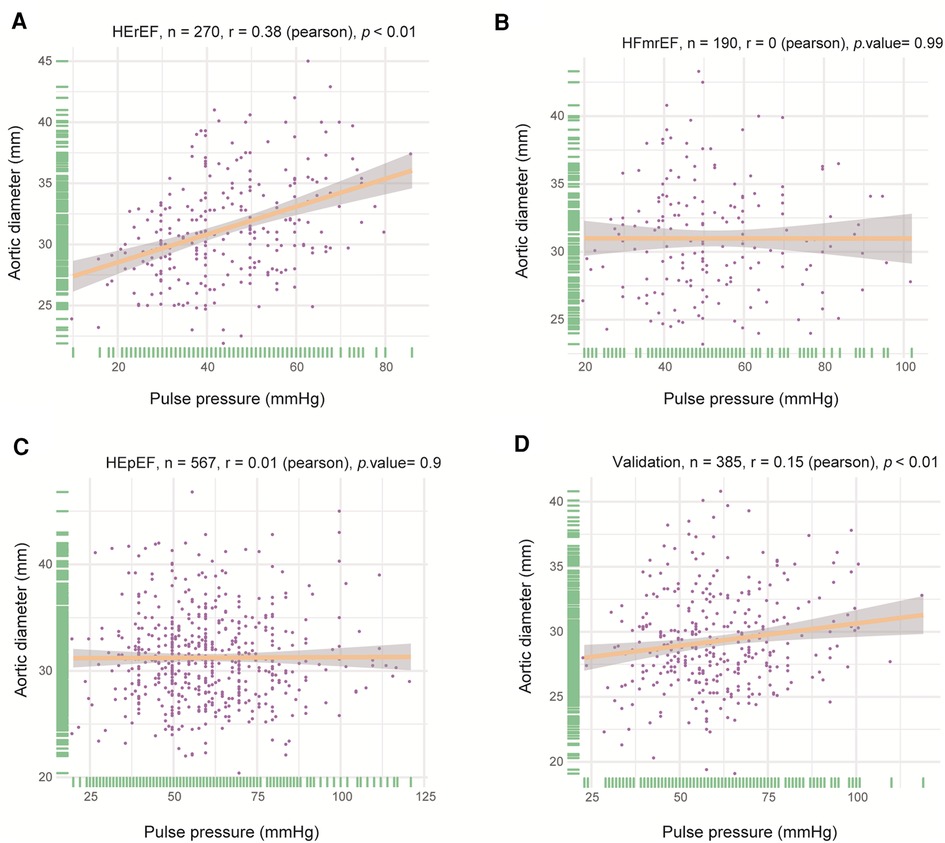
Figure 2. Correlation between PP and AoD in different types of HF. (A) PP was positively associated with AoD in HFrEF (r = 0.380, p < 0.01). (B) There was no significant correlation between PP and AoD in HFmrEF. (C) There was no significant correlation between PP and AoD in HFpEF. (D) PP was positively associated with AoD in validation patients with HFrEF (r = 0.15, p < 0.01).
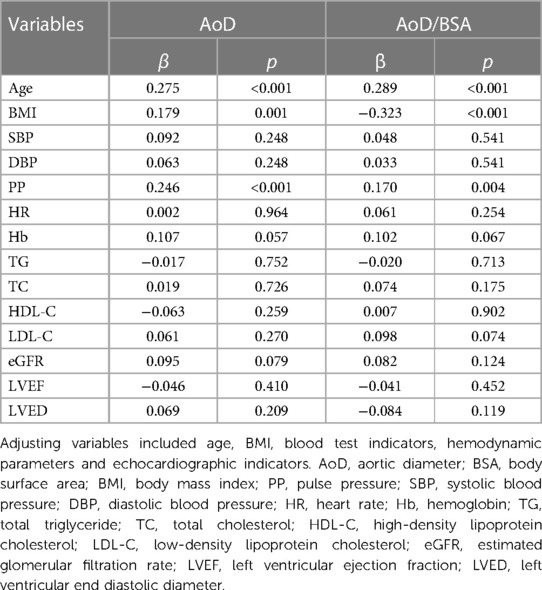
The independent association of AoD/BSA with other variables was assessed using multiple linear regression analysis. We observed that PP was independently associated with AoD/BSA (β = 0.170, p < 0.004), adjusting for age, BMI, SBP, DBP, HR, hemoglobin level, triglyceride (TG), total cholesterol(TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), eGFR, LVEF, left ventricular end diastolic diameter (LVED. The independent determinants of AoD/BSA in the fully adjusted models included PP, age, BMI, and ALB (Table 2).
To assess the non-linear association between PP and AoD, a restricted cubic spline analysis was performed. The results indicated that there was no non-linear association between PP and AoD in patients with HFrEF (Figure 3).
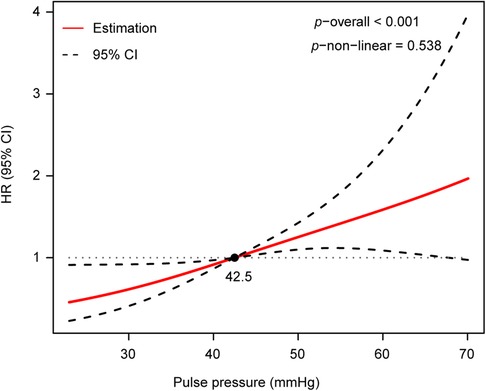
Figure 3. There was no non-linear association between PP and AoD assessed by restricted cubic spline analysis in patients with HFrEF (p−non−linear = 0.538, p−overall < 0.001).
3.3 Identification of independent predictors of AoD in patients with HFrEF
The clinical characteristics of the 270 patients with HFrEF based on the quartiles of PP levels are shown in Table 1. The mean age of the patients was 65.7 ± 10.3 years, 201 (74.4%) were males, and 69 (25.6%) were females. The average PP level was 44.8 ± 14.0 mmHg in patients with HFrEF; the lowest quartile (Q1) was 30.8 ± 6.0 mmHg, the second quartile (Q2) was 44.9 ± 4.1 mmHg, third quartile (Q3) was 58.3 ± 3.5 mmHg, and the highest quartile (Q4) was 70.8 ± 5.3 mmHg. The patients in the higher quartile levels of PP were older: 62.6 ± 9.3 (Q1), 66.0 ± 11.0 (Q2), 70.0 ± 9.2 (Q3), and 72.5 ± 9.6 (Q4) years (p < 0.001). With ascending quartile levels of PP, a trend of increasing AoD was observed: 30.07 ± 3.57 (Q1), 32.05 ± 4.54 (Q2), 32.22 ± 4.12 (Q3), and 34.68 ± 4.06 mm (Q4) (p < 0.001). The SBP was higher in Q4 (151.0 ± 12.3 mmHg) than in Q1 (108.6 ± 15.8 mmHg) (p < 0.016). The prevalence of hypertension (17 [73.9%] vs. 43 [33.3%]) and CHD (18 [78.3%] vs. 46 [35.7%]) was higher in Q4 than in Q1 (p < 0.016). No significant differences were found in DBP, blood lipids, percentage of diabetes, and use of medications between groups (Table 3).

Table 3. Comparison of clinical characteristics of patients with hFrEF in the quartile of PP levels.
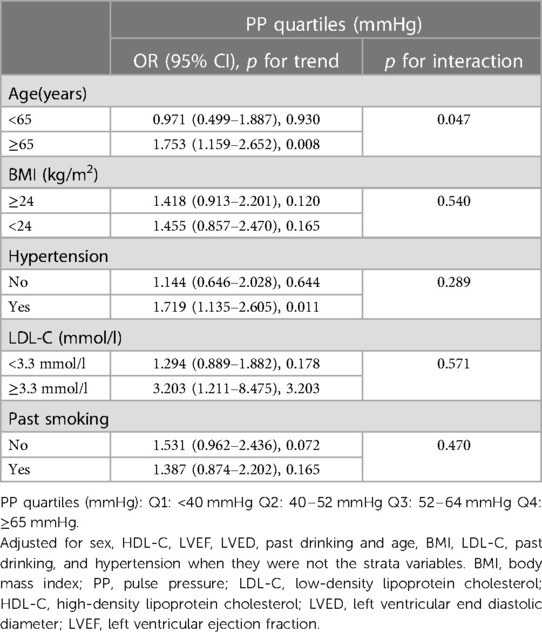
Multiple regression analysis was employed to assess the association between PP levels and aortic root dilation in three models. In the univariate logistic regression analysis, with Q1 set as the reference, the PP levels in Q4 were associated with an increased odds ratio (OR) for aortic root dilation [OR = 5.685 95% confidence interval (CI): 1.941–16.646, p for trend = 0.015]. After adjusting for age, sex, BSA, smoking, hypertension, we observed that the PP levels in Q4 were associated with an increased OR for aortic root dilation (OR = 4.897, 95% CI: 1.629–14.717, p for trend = 0.043) as compared with the PP levels in Q1. Moreover, when adjusting for complications and hematological markers simultaneously, those in Q4 had a higher risk of aortic root dilation than those in Q1 (Q4: 6.612, 95% CI: 1.838–23.783 vs. Q1: 2.411, 95% CI: 1.037–5.607; p for trend = 0.049) (Table 4). Subsequently, stratified analysis was performed to explore further the association between PP and AoD in different population settings, including age, BMI, hypertension, LDL-C, and smoking. An interaction test was performed to assess any significant dependence of the effect modifier on this association. We observed a significant modification of the association between PP levels and AoD (p for interaction = 0.047). A significant association between PP levels and AoD was only observed in patients ≥ 65 years old (OR = 1.753, 95% CI: 1.159–2.652, p for trend = 0.008), but not in patients < 65 years old (p for trend = 0.930). No significant interaction was observed in BMI, LDL-C, smoking status, and hypertension (p for interaction = 0.540, 0.571, 0.470, and 0.289, respectively), indicating that the magnitude of the relationship was the same for different population settings concerning these variables (Table 5).
3.4 Validation dataset for relationship of PP and AoD
Based on the discovery data, we validated the relationship between PP and AoD in another dataset. The information for the validation data is shown in Supplementary Table S1. Then, we further validated the association between PP and AoD in patients with HFrEF. The results showed that PP was positively associated with AoD in patients with HFrEF (r = 0.15, p < 0.01; Figure 2D). The correlations were further assessed by linear regression analysis. We observed that PP was independently associated with AoD (β = 0.205, p < 0.001), adjusting for age, BMI, blood test indicators, hemodynamic parameters and echocardiographic indicators (Supplementary Table S2).
4 Discussion
In our study, age was lowest in HFrEF group compared to the other kinds of heart failure. Although the management of HFrEF has seen significant scientific breakthrough in recent decades, HFrEF is a major public health concern with substantial morbidity and mortality. Disease morbidity and mortality remain high, with a 5-year survival rate of 25% after hospitalization for HFrEF (32). Among the three groups of heart failure types, the LVED of HFrEF was larger than that of the other two groups, suggesting that HErEF was more likely to undergo ventricular remodeling. Deleterious LV remodeling, including increases in end-diastolic and end-systolic volume and reduction in LVEF, is pathognomonic of HFrEF, and the extent of adverse remodeling correlates with risk of hospitalization and death. LV volumes and contractility worsen progressively over time (33). This also suggests that early intervention should be initiated for the treatment of HFrEF. We found that the average level of PP was 44.8 ± 14.0 mmHg in patients with HFrEF, 52.6 ± 16.5 mmHg in patients with HFmrEF, and 60.3 ± 17.5 mmHg in patients with HFpEF in our study. These findings are similar to those of a previous study showing the lowest average level of PP in the HFrEF group (34). This phenomenon may be related to the wide use of angiotensin converting enzyme inhibitors/angiotensin receptor blockers, beta-blockers, and spironolactone in patients with HFrEF, which are known to lower SBP and PP (35). Meanwhile, we found that the systolic blood pressure in the HFpEF group was the highest and their heart rate was the lowest compared to other groups. In contrast, the aortic root diameter was not significantly different between HF classification. Studies (36, 37) showed that the lower SBP being a dose-dependent marker of impaired left ventricular contractility. Advanced heart failure is usually associated with low systolic blood pressure (SBP). Studies (38, 39) have been reported that increased resting heart rate is a risk factor for cardiovascular mortality in cardiovascular diseases. In heart failure patients, increased heart rate has been correlated with adverse outcomes, independently of traditional risk factors. Accordingly, heart rate reduction has been identified as an effective therapy for patients with HF. The aortic root diameter is affected by a variety of factors, including age, gender, height, weight, body surface area (BSA), body mass index (BMI), and diseases such as hypertension, valvular heart disease, congenital heart disease, cardiomyopathy and ischemic cardiomyopathy. In HFrEF group, the aortic root diameter will increase due to cardiac remodeling. However, the aortic root diameter also increases with age. In this study, the age in HFrEF group was smaller, so AoD was significantly different between HF classification because age plays a key role in it. Moreover, we found that PP and AoD increased with age in patients with HF. The aorta has a significant influence on left ventricular afterload and hemodynamics. Age-related enlargement of AoD is a characteristic of arterial stiffness that can influence cardiovascular disease (15, 22, 23). PP, an indicator of pulsatile flow ejected by the heart, is mainly influenced by LVEF and arterial stiffness (40). It has been reported that high PP is usually related to increased SBP and systolic hypertension accelerates arterial stiffness, thereby resulting in CVD (4, 41–43). Epidemiologic studies have shown that PP is predictive of the incidence of HF in the elderly population (44). Several epidemiological studies have examined the relationship between the proximal aorta and major adverse cardiovascular events. For example, the Cardiovascular Health Study (CHS) (45) and the study by Cuspidi et al. (46) showed that increased AoD was associated with a higher incidence of cardiovascular events. However, the relationship between PP and AoD remains controversial. Cuspidi et al. (24) found a positive association between PP and AoD (r = 0.10, p = 0.004) in a total of 3,366 treated and untreated patients with essential hypertension. In a community-based cohort (n = 3,108), Daisuke et al. (22) observed that participants in the highest AoD quintile (35.2 ± 2.8 mm) had higher PP than those in the lowest quintile (30.8 ± 2.8 mm). Recently, a study in Korea demonstrated a significant positive association between invasively measured aPP and AoD/BSA (47). In contrast, there was an independent inverse relationship between AoD and PP in the Framingham cohort study (23) and in a study by Agmon et al. (25) The authors considered that the inverse association between AoD and PP might be due to reduced wave reflection as a result of higher aortic compliance in those with larger AoD and considered invasive aortic root PP data as a for possible explanation for this negative association (48). In this study, Pearson correlation analysis showed that PP was positively correlated with AoD in patients with HFrEF. Furthermore, the probability of increased aortic dilation was associated with higher PP quartiles, and a higher risk of aortic dilation was observed in patients with PP ≥ 65 mmHg, which is inconsistent with previously reported results. A possible reason for this discrepancy in the findings between this study and those of the Framingham study is related to the different populations examined. This study focused on the middle-aged and the elderly with HF. In addition, we observed a significant interaction between PP levels and age, indicating that PP and age may have an additive effect on AoD. It is well known that aging leads to arterial stiffness, which makes the arterial wall more susceptible to the harmful effects of PP (49). This phenomenon may explain the positive association of PP levels with AoD observed only in patients ≥ 65 years with HFrEF.
To our knowledge, the present study is the first to show that an increased PP is significantly associated with AoD in patients with HFrEF, but not in patients with HFmrEF or HFpEF. One reason might be that AoD can be influenced by age, height, weight, left ventricular structure (15), and lower the levels of LVED in patients with HFmrEF or HFpEF than that in patients with HFrEF. Appropriate management may be an effective strategy for lowering the risk of aortic dilation in elderly patients with HFrEF. There were no specific interventions focused on PP currently. Therefore, blood pressure management may be beneficial in reducing the risk of aortic dilation in the elderly with HFrEF. To further verify the reliability of our conclusions, we collected the dataset in another hospital to find consistent results from different hospital populations. Finally, the results improved the conclusion reliability.
Certain limitations of this study should be noted. First, this was a limited cross-sectional study of Chinese middle-aged and elderly populations; hence, the results cannot prove a causal relationship between PP and AoD in patients with HFrEF. The generalizability of our findings to the general population or other ethnic groups may be limited. Therefore, prospective studies with larger sample sizes are required to further explore causality. Second, we could not completely account for residual confounders because of unmeasured or unknown variables. For example, medications such as antihypertensive and antidiabetic drugs may affect AoD (50, 51). Additionally, the detailed molecular mechanisms supporting our findings remain elusive; therefore, laboratory-based experiments are required to gain insight into the association between PP and AoD. Finally, due to our lack of measurements, there is no central pulse pressure data. This is a retrospective study, and it is true that the central hemodynamic study data is more revealing and convincing. In fact, the population in our study is mainly about heart failure patients, and the central hemodynamic test is an invasive examination, which is not beneficial to the patients with heart failure, and only a few research institutions can carry out it in clinical practice. The results we obtained can attract the attention of qualified researchers for further validation, so that they can be generalized with simple clinical indicators. Indeed, there is a physiological difference between central artery pressure and peripheral artery pressure, that is, the physiological amplification of PP (52). It is well known that the degree of pulse pressure amplification is strongly associated with total mortality and major cardiovascular events (26).
5 Conclusions
Our findings indicated that PP level was independently and positively associated with AoD, especially in older patients with HFrEF, but not in patients with HFmrEF and HFpEF. Arterial stiffening or vascular aging may play a certain role in the older HFrEF patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Medical Faculty of Fujian Medical University Union Hospital Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LC: Writing – original draft, Writing – review & editing. WX: Conceptualization, Software, Writing – review & editing. XH: Data curation, Methodology, Writing – review & editing. HH: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article.
The study was sponsored by national key R&D program of China (2020YFC2008000) and national and Fujian province’s key clinical specialty discipline construction programs, China (2013544, 2012149).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1366282/full#supplementary-material
References
1. Desai AS, Lam CSP, McMurray JJV, Redfield MM. How to manage heart failure with preserved ejection fraction: practical guidance for clinicians. JACC Heart Fail. (2023) 11(6):619–36. doi: 10.1016/j.jchf.2023.03.011
2. Borlaug BA, Sharma K, Shah SJ, Ho JE. Heart failure with preserved ejection fraction: JACC scientific statement. J Am Coll Cardiol. (2023) 81(18):1810–34. doi: 10.1016/j.jacc.2023.01.049
3. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: executive summary: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. J Am Coll Cardiol. (2022) 79(17):1757–80. doi: 10.1016/j.jacc.2021.12.011
4. Sarnecki J, Obrycki Ł, Feber J, Chełstowska S, Jurkiewicz E, Litwin M. Isolated systolic hypertension is associated with increased left ventricular mass Index and aortic stiffness in adolescents: a cardiac magnetic resonance study. J Hypertens. (2022) 40(5):985–95. doi: 10.1097/hjh.0000000000003101
5. Qiu W, Xiao X, Cai A, Gao Z, Li L. Pulse pressure and all-cause mortality in ischaemic heart failure patients: a prospective cohort study. Ann Med. (2022) 54(1):2701–9. doi: 10.1080/07853890.2022.2128208
6. Regnault V, Lagrange J, Pizard A, Safar ME, Fay R, Pitt B, et al. Opposite predictive value of pulse pressure and aortic pulse wave velocity on heart failure with reduced left ventricular ejection fraction: insights from an eplerenone post-acute myocardial infarction heart failure efficacy and survival study (ephesus) substudy. Hypertension. (2014) 63(1):105–11. doi: 10.1161/hypertensionaha.113.02046
7. Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the strong heart study. Hypertension. (2007) 50(1):197–203. doi: 10.1161/hypertensionaha.107.089078
8. Voors AA, Petrie CJ, Petrie MC, Charlesworth A, Hillege HL, Zijlstra F, et al. Low pulse pressure is independently related to elevated natriuretic peptides and increased mortality in advanced chronic heart failure. Eur Heart J. (2005) 26(17):1759–64. doi: 10.1093/eurheartj/ehi270
9. Aronson D, Burger AJ. Relation between pulse pressure and survival in patients with decompensated heart failure. Am J Cardiol. (2004) 93(6):785–8. doi: 10.1016/j.amjcard.2003.12.011
10. Wei FF, Wu Y, Xue R, Liu X, He X, Dong B, et al. Clinical significance of mean and pulse pressure in patients with heart failure with preserved ejection fraction. Hypertension. (2021) 79(1):241–50. doi: 10.1161/hypertensionaha.121.17782
11. Mulé G, Nardi E, Morreale M, D'Amico S, Foraci AC, Nardi C, et al. Relationship between aortic root size and glomerular filtration rate in hypertensive patients. J Hypertens. (2016) 34(3):495–504; discussion 5. doi: 10.1097/HJH.0000000000000819
12. Xie W, Ke Y, You Q, Li J, Chen L, Li D, et al. Single-cell rna sequencing and assay for transposase-accessible chromatin using sequencing reveals cellular and molecular dynamics of aortic aging in mice. Arterioscler Thromb Vasc Biol. (2021) 42(2):156–71. doi: 10.1161/atvbaha.121.316883
13. Ke Y, Li D, Zhao M, Liu C, Liu J, Zeng A, et al. Gut flora-dependent metabolite trimethylamine-N-oxide accelerates endothelial cell senescence and vascular aging through oxidative stress. Free Radical Biol Med. (2018) 116:88–100. doi: 10.1016/j.freeradbiomed.2018.01.007
14. Masugata H, Senda S, Murao K, Okuyama H, Inukai M, Hosomi N, et al. Aortic root dilatation as a marker of subclinical left ventricular diastolic dysfunction in patients with cardiovascular risk factors. J Int Med Res. (2011) 39(1):64–70. doi: 10.1177/147323001103900108
15. Canciello G, Mancusi C, Losi MA, Izzo R, Trimarco B, de Simone G, et al. Aortic root dilatation is associated with incident cardiovascular events in a population of treated hypertensive patients: the campania salute network. Am J Hypertens. (2018) 31(12):1317–23. doi: 10.1093/ajh/hpy113
16. Hill MA, Jaisser F, Sowers JR. Role of the vascular endothelial sodium channel activation in the genesis of pathologically increased cardiovascular stiffness. Cardiovasc Res. (2020) 118(1):130–40. doi: 10.1093/cvr/cvaa326
17. Chirinos JA, Segers P. Noninvasive evaluation of left ventricular afterload: part 2: arterial pressure-flow and pressure-volume relations in humans. Hypertension. (2010) 56(4):563–70. doi: 10.1161/hypertensionaha.110.157339
18. Zuo X, Liu L, Liu K, Zhang X, Ye R, Yang C, et al. Proximal aorta dilatation in hypertension. J Hypertens. (2023) 41(10):1511–20. doi: 10.1097/hjh.0000000000003518
19. Sun Z. Aging, arterial stiffness, and hypertension. Hypertension. (2015) 65(2):252–6. doi: 10.1161/hypertensionaha.114.03617
20. Lam CS, Gona P, Larson MG, Aragam J, Lee DS, Mitchell GF, et al. Aortic root remodeling and risk of heart failure in the framingham heart study. JACC Heart Faile. (2013) 1(1):79–83. doi: 10.1016/j.jchf.2012.10.003
21. Rueda-Ochoa OL, Bons LR, Zhu F, Rohde S, El Ghoul K, Budde RPJ, et al. Thoracic aortic diameter and cardiovascular events and mortality among women and men. Radiology. (2022) 304(1):208–15. doi: 10.1148/radiol.210861
22. Kamimura D, Suzuki T, Musani SK, Hall ME, Samdarshi TE, Correa A, et al. Increased proximal aortic diameter is associated with risk of cardiovascular events and all-cause mortality in blacks the Jackson heart study. J Am Heart Assoc. (2017) 6(6):e005005. doi: 10.1161/jaha.116.005005
23. Lam CS, Xanthakis V, Sullivan LM, Lieb W, Aragam J, Redfield MM, et al. Aortic root remodeling over the adult life course: longitudinal data from the framingham heart study. Circulation. (2010) 122(9):884–90. doi: 10.1161/circulationaha.110.937839
24. Cuspidi C, Meani S, Fusi V, Valerio C, Sala C, Zanchetti A. Prevalence and correlates of aortic root dilatation in patients with essential hypertension: relationship with cardiac and extracardiac target organ damage. J Hypertens. (2006) 24(3):573–80. doi: 10.1097/01.hjh.0000209992.48928.1f
25. Agmon Y, Khandheria BK, Meissner I, Schwartz GL, Sicks JD, Fought AJ, et al. Is aortic dilatation an atherosclerosis-related process? Clinical, laboratory, and transesophageal echocardiographic correlates of thoracic aortic dimensions in the population with implications for thoracic aortic aneurysm formation. J Am Coll Cardiol. (2003) 42(6):1076–83. doi: 10.1016/s0735-1097(03)00922-7
26. Benetos A, Thomas F, Joly L, Blacher J, Pannier B, Labat C, et al. Pulse pressure amplification a mechanical biomarker of cardiovascular risk. J Am Coll Cardiol. (2010) 55(10):1032–7. doi: 10.1016/j.jacc.2009.09.061
27. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
28. Mitchell C, Rahko PS, Blauwet LA, Canaday B, Finstuen JA, Foster MC, et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American society of echocardiography. J Am Soc Echocardiogr. (2019) 32(1):1–64. doi: 10.1016/j.echo.2018.06.004
29. Qian J, Cai M, Gao J, Tang S, Xu L, Critchley JA. Trends in smoking and quitting in China from 1993 to 2003: national health service survey data. Bull W H O. (2010) 88(10):769–76. doi: 10.2471/blt.09.064709
30. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. (2018) 39(33):3021–104. doi: 10.1093/eurheartj/ehy339
31. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC) developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. (2016) 37(27):2129–200. doi: 10.1093/eurheartj/ehw128
32. Murphy SP, Ibrahim NE, Januzzi JL. Heart failure with reduced ejection fraction: a review. JAMA. (2020) 324(5):488–504. doi: 10.1001/jama.2020.10262
33. Lee MMY, Brooksbank KJM, Wetherall K, Mangion K, Roditi G, Campbell RT, et al. Effect of empagliflozin on left ventricular volumes in patients with type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction (SUGAR-DM-HF). Circulation. (2021) 143(6):516–25. doi: 10.1161/circulationaha.120.052186
34. Alem MM, Alshehri AM. Inter-relationships between left ventricular mass, geometry and arterial stiffness. J Int Med Res. (2020) 48(4):300060520903623. doi: 10.1177/0300060520903623
35. Bakris G, Ali W, Parati G. ACC/AHA versus ESC/ESH on hypertension guidelines: JACC guideline comparison. J Am Coll Cardiol. (2019) 73(23):3018–26. doi: 10.1016/j.jacc.2019.03.507
36. Ather S, Chan W, Chillar A, Aguilar D, Pritchett AM, Ramasubbu K, et al. Association of systolic blood pressure with mortality in patients with heart failure with reduced ejection fraction: a Complex relationship. Am Heart J. (2011) 161(3):567–73. doi: 10.1016/j.ahj.2010.12.009
37. Faselis C, Lam PH, Zile MR, Bhyan P, Tsimploulis A, Arundel C, et al. Systolic blood pressure and outcomes in older patients with HFPEF and hypertension. Am J Med. (2021) 134(4):e252–e63. doi: 10.1016/j.amjmed.2020.08.030
38. Böhm M, Swedberg K, Komajda M, Borer JS, Ford I, Dubost-Brama A, et al. Heart rate as a risk factor in chronic heart failure (shift): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet. (2010) 376(9744):886–94. doi: 10.1016/s0140-6736(10)61259-7
39. Böhm M, Reil JC, Deedwania P, Kim JB, Borer JS. Resting heart rate: risk indicator and emerging risk factor in cardiovascular disease. Am J Med. (2015) 128(3):219–28. doi: 10.1016/j.amjmed.2014.09.016
40. Angeli F, Reboldi G, Verdecchia P. Heart failure, pulse pressure and heart rate: refining risk stratification. Int J Cardiol. (2018) 271:206–8. doi: 10.1016/j.ijcard.2018.07.072
41. Haley JE, Woodly SA, Daniels SR, Falkner B, Ferguson MA, Flynn JT, et al. Association of blood pressure-related increase in vascular stiffness on other measures of target organ damage in youth. Hypertension. (2022) 79(9):2042–50. doi: 10.1161/hypertensionaha.121.18765
42. Seryan A, Martin M, Hamimatunnisa J, Annette P, Margit H, Karl-Heinz L. Cardiovascular mortality risk in young adults with isolated systolic hypertension: findings from population-based monica/kora cohort study. J Hum Hypertens. (2022) 36(12):1059–65. doi: 10.1038/s41371-021-00619-z
43. Liu R, Li D, Yang Y, Hu Y, Wu S, Tian Y. Systolic blood pressure trajectories and the progression of arterial stiffness in Chinese adults. Int J Environ Res Public Health. (2022) 19(16):10046. doi: 10.3390/ijerph191610046
44. Naka KK, Ikonomidis I. Brachial pulse pressure in heart failure: simple to measure but complex to interpret. Eur Heart J. (2019) 40(26):e8–e10. doi: 10.1093/eurheartj/ehv005
45. Gardin JM, Arnold AM, Polak J, Jackson S, Smith V, Gottdiener J. Usefulness of aortic root dimension in persons > or=65 years of age in predicting heart failure, stroke, cardiovascular mortality, all-cause mortality and acute myocardial infarction (from the cardiovascular health study). Am J Cardiol. (2006) 97(2):270–5. doi: 10.1016/j.amjcard.2005.08.039
46. Cuspidi C, Facchetti R, Bombelli M, Re A, Cairoa M, Sala C, et al. Aortic root diameter and risk of cardiovascular events in a general population: data from the pamela study. J Hypertens. (2014) 32(9):1879–87. doi: 10.1097/hjh.0000000000000264
47. Kim HL, Joh HS, Lim WH, Seo JB, Kim SH, Zo JH, et al. Association between invasively measured central aortic pulse pressure and diameter of ascending aorta. Sci Rep. (2023) 13(1):21152. doi: 10.1038/s41598-023-48597-1
48. Tosello F, Guala A, D'Ascenzo F, Bollati M, Leone D, Sabia L, et al. Central pulse pressure is inversely associated with proximal aortic remodelling. J Hypertens. (2021) 39(5):919–25. doi: 10.1097/hjh.0000000000002730
49. Avolio AP, Kuznetsova T, Heyndrickx GR, Kerkhof PLM, Li JK. Arterial flow, pulse pressure and pulse wave velocity in men and women at Various ages. Adv Exp Med Biol. (2018) 1065:153–68. doi: 10.1007/978-3-319-77932-4_10
50. Janic M, Lunder M, Sabovic M. Arterial stiffness and cardiovascular therapy. Biomed Res Int. (2014) 2014:621437. doi: 10.1155/2014/621437
51. Lunder M, Janic M, Sabovic M. Treating arterial ageing in patients with diabetes: from mechanisms to effective drugs. Int J Mol Sci. (2021) 22(6):2796. doi: 10.3390/ijms22062796
Keywords: pulse pressure, aortic root diameter, heart failure, arterial stiffness, elderly
Citation: Chen L, Xie W, Hong X and Hong H (2024) Association of pulse pressure and aortic root diameter in elderly Chinese patients with chronic heart failure. Front. Cardiovasc. Med. 11:1366282. doi: 10.3389/fcvm.2024.1366282
Received: 6 January 2024; Accepted: 20 February 2024;
Published: 1 March 2024.
Edited by:
Filippo Valbusa, Sacro Cuore Don Calabria Hospital (IRCCS), ItalyReviewed by:
Audrey Adji, Victor Chang Cardiac Research Institute, AustraliaLi-Da Wu, Nanjing Medical University, China
© 2024 Chen, Xie, Hong and Hong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huashan Hong aG9uZ2hzQGZqbXUuZWR1LmNu
†These authors have contributed equally to this work
Abbreviations PP: pulse pressure; AoD: aortic root diameter; CHF: chronic heart failure; HFrEF: heart failure with reduced ejection fraction; HFmrEF: heart failure with mid-range ejection fraction; HFpEF: heart failure with preserved ejection fraction; BMI, body mass index; BSA, body surface area; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; WBC, white blood cell; Hb, hemoglobin; HCT, hematocrit; TB, total bilirubin; ALB, albumin; ALT, alanine transaminase; TC, total cholesterol; TG, total triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate;UA, uric acid; FBG, fasting blood glucose; LVED, left ventricular end diastolic diameter; LAD, left atrial diameter; LVEF, left ventricular ejection fraction; DM, diabetes mellitus; CHD, coronary heart disease; DCM, dilated cardiomyopathy; CKD, chronic kidney disease; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blockers; NYHA, New York heart association.
‡ORCID Huashan Hong orcid.org/0000-0001-8332-0066
 Lu Chen†
Lu Chen† Huashan Hong
Huashan Hong