- 1Department of Nuclear Medicine, The Second Hospital of Hebei Medical University, Shijiazhuang, China
- 2Department of Electronic Science and Technology, School of Electronic and Information Engineering, Beijing Jiaotong University, Beijing, China
Background: The value of semiquantitative resting myocardial perfusion imaging (MPI) in coronary artery disease (CAD) is limited. At present, quantitative MPI can be performed by a new cadmium zinc tellurium single-photon emission computed tomography (CZT-SPECT) scan. The quantitative index of resting myocardial blood flow (MBF) has received little attention, and its manifestations and clinical value in the presence of unstable coronary blood flow have not been clarified.
Purpose: In patients with ST-segment elevation myocardial infarction (STEMI), whether resting MBF can provide additional value of blood flow than semi-quantitative resting MPI is not sure. We also explored the influencing factors of resting MBF.
Methods: This was a retrospective clinical study. We included 75 patients with STEMI in the subacute phase who underwent resting MPI and dynamic scans after reperfusion therapy. General patient information, STEMI-related data, MPI, gated MPI (G-MPI), and resting MBF data were collected and recorded. According to the clinically provided culprit vessels, the resting MBF was divided into ischemic MBF and non-ischemic MBF. The paired Wilcoxon signed-rank test was used for resting MBF. The receiver operating characteristic (ROC) curves were used to determine the optimal threshold for ischemia, and multiple linear regression analysis was used to analyze the influencing factors of resting MBF.
Results: There was a statistically significant difference between the ischemic MBF and non-ischemic MBF [0.59 (0.47–0.72) vs. 0.76 (0.64–0.93), p < 0.0001]. The ROC curve analysis revealed that resting MBF could identify ischemia to a certain extent, with a cutoff value of 0.5975, area under the curve (AUC) = 0.666, sensitivity = 55.8%, and specificity = 68.7%. Male sex and summed rest score (SRS) were influencing factors for resting MBF.
Conclusion: To a certain extent, resting MBF can suggest residual ischemia after reperfusion therapy in patients with STEMI. There was a negative correlation between male sex, SRS, and ischemic MBF. A lower resting MBF may be associated with more severe myocardial ischemia.
Introduction
ST-segment elevation myocardial infarction (STEMI) is an acute severe aspect of coronary artery disease (CAD), commonly occurring as an acute myocardial infarction (AMI) (1, 2). Notably, myocardial perfusion imaging (MPI) can assess myocardial ischemia due to myocardial infarction. With the development of imaging technology and the clinical application of cyclotrons, MPI has entered the era of absolute quantification (3, 4). Moreover, recent studies have supported the consistency of the quantitative parameters between cadmium zinc tellurium single-photon emission computed tomography (CZT-SPECT) and positron emission tomography (PET) (4, 5). Quantitative indicators of myocardial blood flow (MBF) and myocardial flow reserve (MFR) reflect whole coronary perfusion and provide additional value for patients with CAD, especially for those with balanced ischemia and ischemia and no obstractive coronary artery disease (INOCA) (6, 7).
In patients with STEMI, timely reperfusion therapy is beneficial for salvaging ischemic myocardium (8). Consequently, most patients cannot complete MPI during the acute stage. Semiquantitative MPI, as a non-invasive, true response to myocardial perfusion, has been widely applied to subacute and long-term AMI and has been confirmed to have prognostic value (9–12). Moreover, some studies have utilized MBF and MFR to perform a hemodynamic analysis and prognostic prediction for patients after treatment for AMI (13, 14).
Our study investigated using safer and simpler quantitative resting MPI to evaluate patients with early STEMI. We attempted to analyze whether resting MBF made sense in early STEMI and focused on whether there was a difference between ischemia-related MBF and non-ischemia-related MBF. Furthermore, we explored the optimal threshold for ischemia and analyzed the connection between clinical characteristics and resting MBF.
Materials and methods
Study design and patients
This was a single-center, retrospective study with a small sample size. We collected data from the Second Hospital of Hebei Medical University between May 2021 and April 2023.
The inclusion criteria for patients were as follows: (1) had primary STEMI, which was diagnosed by experienced cardiologists according to guidelines (1); and (2) underwent reperfusion treatment, including pharmacological thrombolysis therapy and percutaneous coronary intervention (PCI) on infarction-related vessels. Cardiologists determined culprit vessels through patients' symptoms, electrocardiogram, and coronary angiography; (3) all participants completed quantitative resting MPI within 14 days after reperfusion treatment. The exclusion criteria were as follows: (1) patients with reinfarction; (2) patients who did not receive reperfusion treatment in the acute stage; (3) patients whose clinical information was missing, such as resting heart rate and blood pressure; (4) patients did not undergo a resting dynamic scan; and (5) patients whose imaging did not meet the clinical diagnosis.
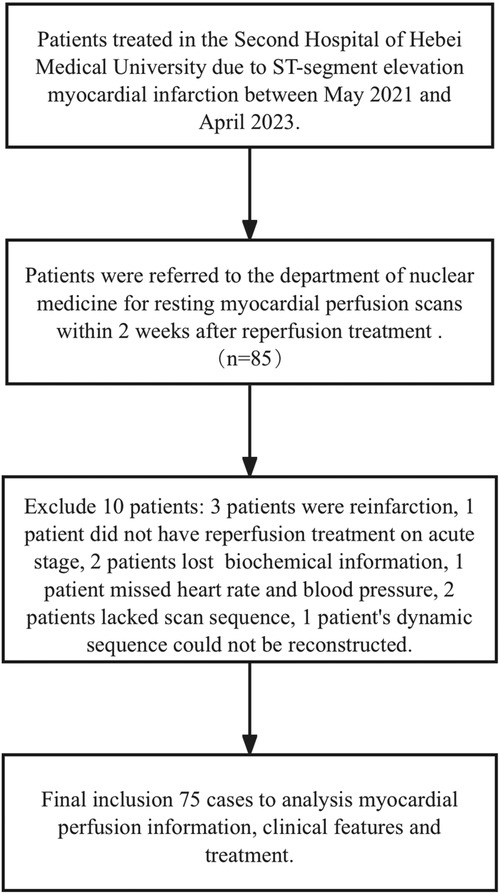
All patients undergoing MPI provided informed consent. Ultimately, 75 patients were included and 10 were excluded (Figure 1). Among those excluded, three patients had reinfarction, one did not receive reperfusion treatment in the acute stage, two lost biochemical information, one was missing heart rate and blood pressure, two lacked a scan sequence, and one dynamic sequence could not be reconstructed.
CZT-SPECT acquisition
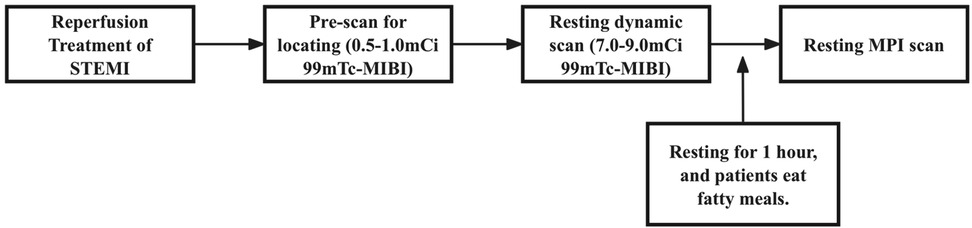
Resting dynamic and perfusion scanning was performed on D-SPECT (Spectrum Dynamics, Caesarea, Israel). D-SPECT is a commercially available dedicated cardiac CZT-SPECT system with nine cadmium zinc telluride detection columns. The acquisition procedures and reconstruction parameters were the same as in previous studies (15). The specific imaging process is shown in Figure 2.
Patients were fasted before the examination and a fatty meal was prepared. Technicians conducted quality checks on imaging devices before imaging, while nurses established intravenous access for patients. Patients were made aware of the need to lie on the examination bed, and nuclear medicine physicians measured their blood pressure and heart rate 2–3 times while the patients were calm.
A prescan injection of 0.5–1.0 mCi 99mTc-sestamibi (99mTc-MIBI) was administered for cardiac localization, followed by a 7.0–9.0 mCi 2 mL bolus injected at 1 mL/s using a high-pressure syringe pump (ACIST Medical Systems). A 6-min CZT SPECT dynamic imaging acquisition was started at the same time as the bolus injection. Patients then rested for an interval of 1 h, consuming a fatty meal to promote hepatobiliary clearance and reduce infracardiac activity. After 1 h, the tracer was stably distributed on the myocardium, and the resting MPI was performed in both the supine and upright positions.
Image reconstruction and image postprocessing
Both resting dynamic and perfusion sequences were checked for quality and clinical diagnostic requirements before they were reconstructed. Qualified scan sequences were reconstructed, and MBF was measured using QGS-QPS postprocessing commercial software (Cedars-Sinai Medical Center, Los Angeles, CA, USA). Before obtaining SPECT parameters, the endocardial and epicardial contouring and number of boluses were assessed. Dynamic images were corrected by motion correction (MC) and rate pressure product (RPP) correction to obtain the resting MBF. If the product of the resting heart rate and resting systolic blood pressure was greater than 10,000, the resting MBF equaled the product of heart rate and blood pressure divided by 10,000. Quantitative blood flow values were also calculated using the Renkin-Crone formula (16). Postprocessing software was also used to analyze the perfusion images to obtain semiquantitative perfusion parameters and G-MPI results.
The left ventricular bullseye was divided into 17 segments according to the American Heart Association (AHA) (17), each segment with an independent resting MBF. The resting MBF is divided into ischemic MBF and non-ischemic MBF. The ischemic area is dominated by the culprit vessels, and the non-ischemic area is governed by the non-culprit vessels. The segments dominated by the three branches of the coronary artery are prescribed by the AHA. The ischemic MBF is equal to the average resting MBF of each segment of the culprit vascular domination, and the non-ischemic MBF is equal to average resting MBF of each segment of the non-culprit vascular domination. All resting MBFs were measured independently by two physicians, each with at least 10 years of experience in nuclear medicine.
Clinical feature data collection
All patients with STEMI were treated with reperfusion therapy and in-hospital medication according to guidelines (1, 18). All general and clinical information came from the hospital's medical records system and standard questionnaires from the Department of Nuclear Medicine. This information included sex, age, risk factors, onset and treatment of STEMI, biochemical indicators during hospitalization, electrocardiogram, CAG, PCI surgical records, and discharge diagnosis. Due to the precision of the cTnI detection method, the peak cTnI was classified into five grades: 0.04–25.00 = grade 1; 25.00–50.00 = grade 2; 50.00–75.00 = grade 3; 75.00–100.00 = grade 4; and >100.00 = grade 5.
Statistical analyses
Continuous variables were shown as mean ± SD or median (interquartile range). Categorical variables were expressed as percentages. We also used the intraclass correlation coefficient (ICC) to test the consistency of the resting MBF. The resting MBF was the average of the two. The Wilcoxon signed-rank test of two paired samples was used for ischemic MBF and non-ischemic MBF. The resting MBF was analyzed using the receiver operating characteristic (ROC) curve, and the Youden index was calculated to obtain the cutoff value. Multiple linear regression was performed to examine the influence of ischemic, non-ischemic MBF. SPSS (version 26.0) and Prism (version 10.0) software were used. A p-value <0.05 was considered to indicate statistical significance or relevance.
Results
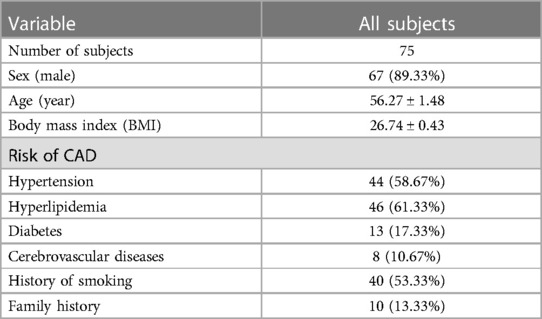
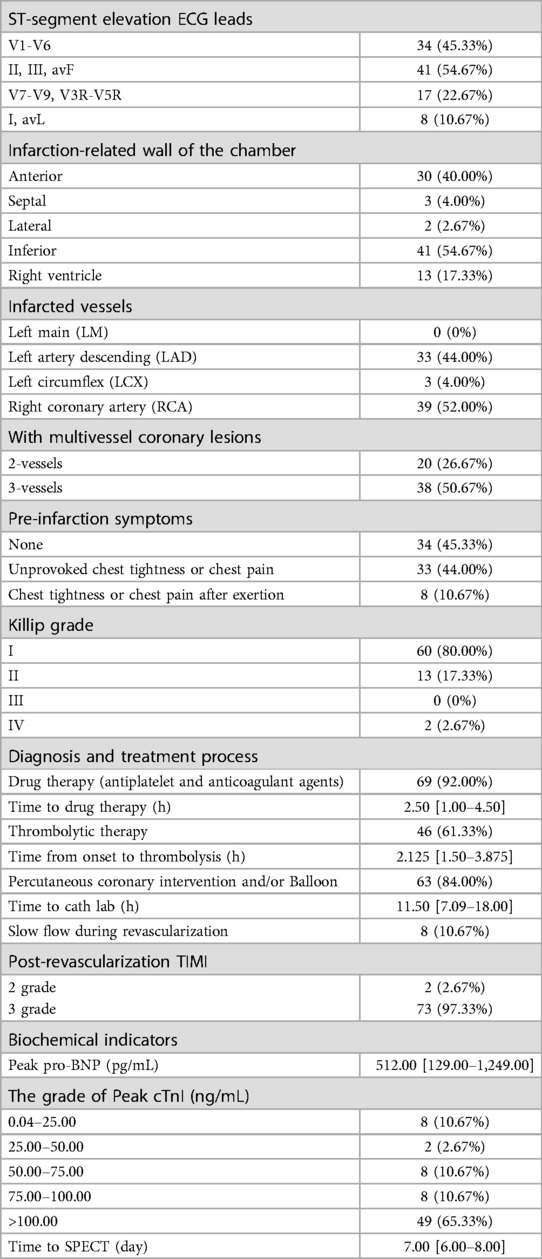
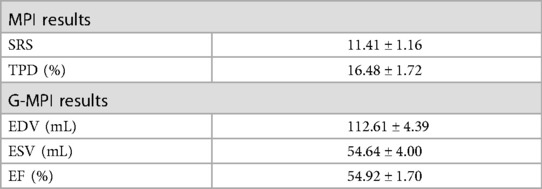
In total, 75 patients were finally included, and the mean time from revascularization to CZT-SPECT was 7.1 days. The patients' sex, age, body mass index, and risk factors were collected (Table 1). The information about STEMI is summarized in Table 2, including electrocardiogram, CAG, thrombolysis in myocardial infarction (TIMI), flow, clinical features, treatment history, Killip grading, and biochemical indices. The summed rest score (SRS), total perfusion defect (TPD), end-diastolic volume (EDV), end-systolic volume (ESV), and left ventricular ejection fraction (LVEF) data are shown in Table 3.
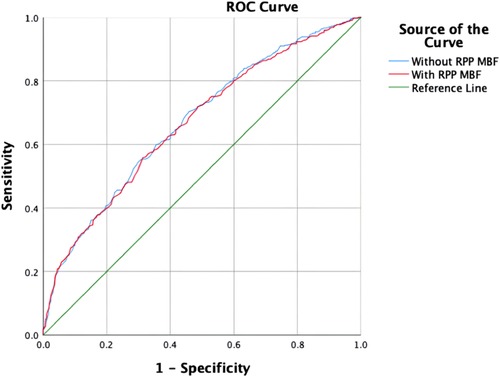
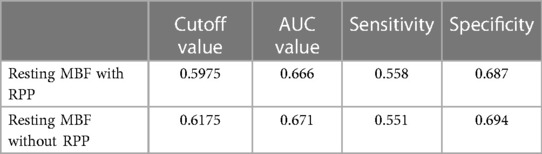
The blinded resting MBF of the ICC was good (ICC = 0.795). As shown in Figure 3 and Table 4, the resting MBF differed significantly (p < 0.0001) in ischemia- and non–ischemia-related areas. The ischemic MBF was lower than the non-ischemic MBF: the resting MBF with RPP was 0.59 (0.47–0.72) vs. 0.76 (0.64–0.93), and the resting MBF without RPP was 0.63 (0.48–0.74) vs. 0.76 (0.65–0.97). The ROC curve analysis suggested (Figure 4, Table 5) that the AUC was 0.666 for RPP (sensitivity = 55.8%, specificity = 68.7%) and the cutoff value was 0.5975; the AUC was 0.671 without RPP (sensitivity = 55.1%, specificity = 69.4%) and the cut-off value was 0.6175.
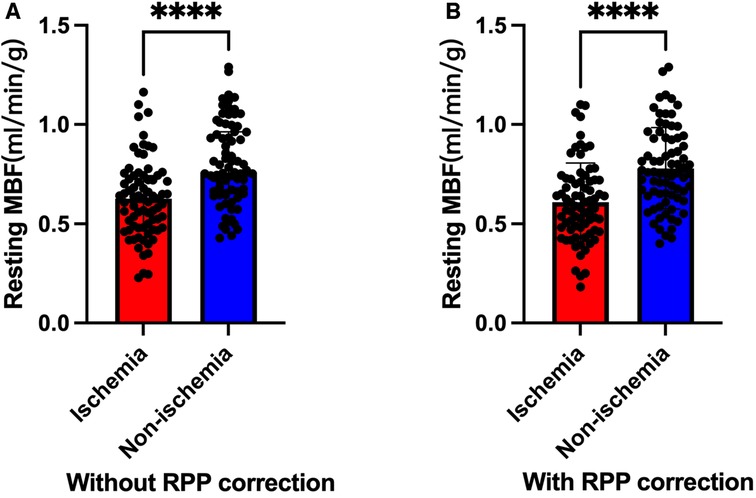
Figure 3. Ischemic MBF vs. non-ischemic MBF (****: P < 0.0001). (A) Without RPP correction. (B) With RPP correction.
On multiple linear regression (Table 6), the ischemic MBF was inversely correlated with male sex and SRS (with RPP: β = −0.336, p = 0.009; β = −1.240, p = 0.019, respectively; without RPP: β = −0.312, p = 0.017; β = −1.148, p = 0.034, respectively). The non-ischemic MBF was not associated with any factor.
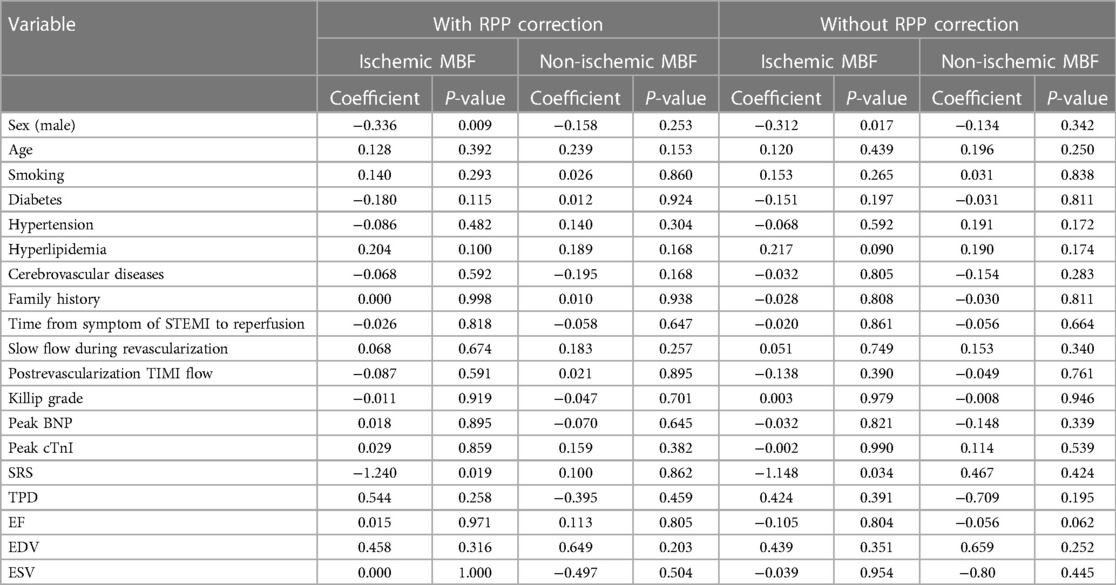
Table 6. Multivariate analysis of the correlation between patients’ characteristics and resting MBF.
Discussion
Time is important for saving ischemic myocardium, and most patients with STEMI cannot complete MPI in the acute phase and stress test shortly after their surgery. Due to the self-regulation of the coronary arteries, the resting MBF has a large range of changes in homeostasis and its application value is limited (19). Thus, our study explored the value of resting MBF in patients with STEMI.
Ischemia- and non–ischemia-related resting MBF
In our study, there was a difference between ischemic MBF and non-ischemic MBF after reperfusion treatment for patients with STEMI. We found that ischemic MBF was lower than non-ischemic MBF. In addition, the resting MBF in ischemic areas after reperfusion therapy was still low. Thus, we thought that STEMI reperfusion therapy solves the problem of epicardial blood vessel patency. However, why is the blood flow low in the area dominated by culprit vessels after treatment? This may be due to the recovery of coronary blood flow after treatment for STEMI, which requires a certain amount of time, as well as the impairment of the epicardial vessels, coronary microcirculation, and myocardium.
After STEMI reperfusion therapy, the culprit vessels already demonstrate patency. However, patients with STEMI have an instrumental injury to the epicardial vessels due to catheters, balloons, and stents, which may injure endothelial cells, causing the microthrombus to fall off, thus decreasing myocardial perfusion (20).
On the one hand, the decrease in resting MBF may be related to impaired microcirculation. Microembolization washout after thrombolysis treatment and transmural myocardial infarction may cause structural damage, leading to vascular obstruction (21). Moreover, interstitial edema, inflammatory cell infiltration, and platelet aggregation can also damage microcirculation patency (21). The effects of functional damage may occur after PCI, resulting in transient vascular contraction in epicardial vessels and microcirculation (22, 23). On the other hand, patients may have microcirculation injuries before STEMI, and most patients present with hypertension, smoking, hyperlipidemia, and diabetes, which may harm microcirculation (24, 25). Furthermore, microvascular structures may sustain further severe injury during subacute AMI through leakage of the red blood cells in blood vessels and leakage into the intercellular space of the myocardial body, forming intramyocardial hemorrhage (IMH) (26).
AMI mediates ischemia, and reperfusion causes edema of the myocardium, resulting in temporary impairment of cell function (27). A swollen myocardium and adjacent vessel compression may also affect blood flow patency. On the other hand, swelling may decrease tracer uptake, which further leads to the underestimation of resting MBF. In contrast, because of prolonged ischemia, some myocardium may undergo necrosis (26). Thus, there may be no or significantly reduced blood flow to the local myocardium.
Conversely, non-ischemic MBF is greater than ischemic MBF, possibly because the distal myocardium has a compensatory effect, resulting in increased resting MBF (28). However, this view needs to be confirmed by long-term imaging follow-up in the later stage.
Resting MPI and resting MBF identity in the ischemic area
Most studies have paid less attention to the clinical significance of resting MPI in ischemic disease (29). However, only resting MPI combined with clinical features enables the diagnosis of obstructive CAD (30). In clinical application, patients with acute coronary syndrome (ACS) in the acute period cannot undergo stressing MPI (31). For STEMI, cardiologists use electrocardiogram and coronary angiography to locate the ischemic area. In our research, we applied resting MBF to identify ischemia. According to the ROC analysis, the AUCs were 0.666 and 0.671 with or without RPP correction. To some extent, MBF is able to identify residual ischemia in patients with STEMI.
The influencing factors of resting MBF
In our study, the SRS and male sex were inversely related to the ischemic MBF.
The SRS indicates the degree of ischemia and infarct (32) and is often correlated with fixed ischemia. Previously, cardiologists and nuclear physicians mainly relied on SRS, TPD, and gated parameters to analyze the severity of myocardial infarction, prognostic information, and treatment decisions (9–12). Current CZT-SPECT systems enable the measurement of MBF, which is also proportional with regional myocardial perfusion (3, 33). SRS is derived from semiquantitative and visual analysis, which has limitations in multivessel diseases or balanceable ischemia (6). However, MBF, as a quantitative parameter, excludes the influence of human factors and is semiquantitative. It may be more sensitive for assessing and identifying ischemic areas than semiquantitative parameters (Figure 5). In our study, the higher the SRS, the lower the resting MBF. To some extent, ischemia-related MBF can reflect the severity of myocardial infarction.
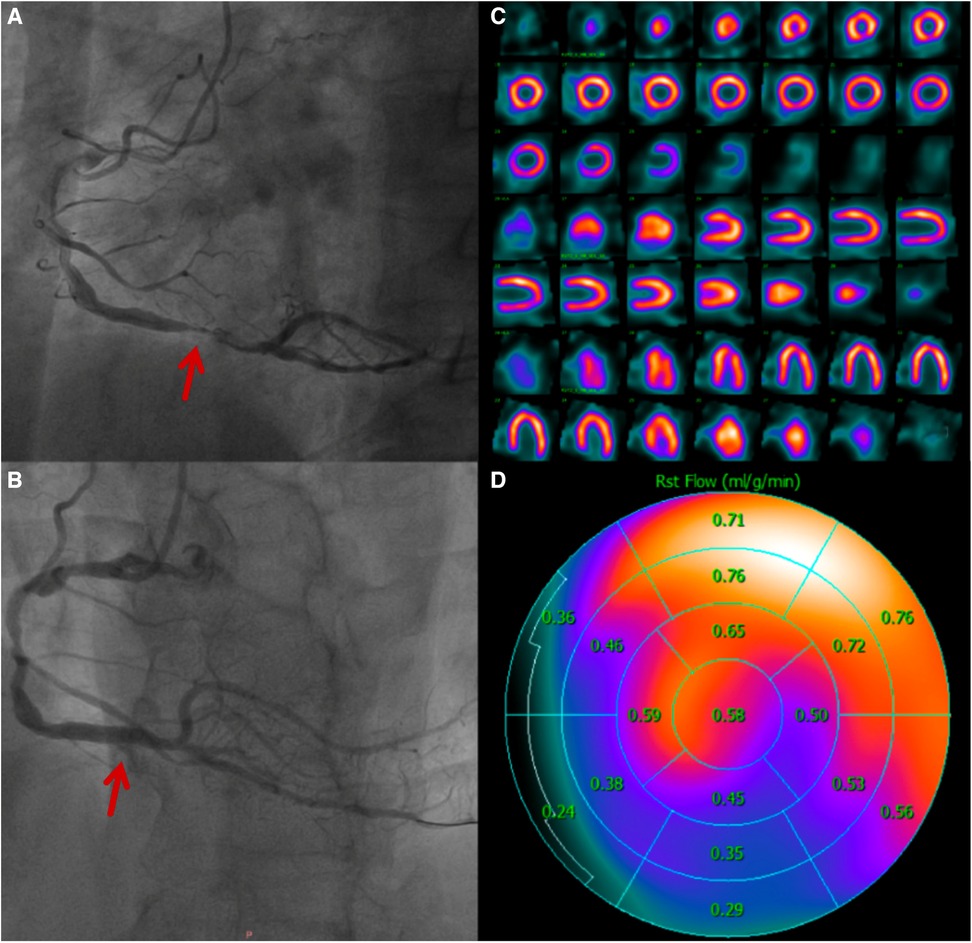
Figure 5. A 60-year-old man who was diagnosed with acute inferior ST-elevation myocardial infarction. 1.5 h after the onset of the disease, drug thrombolytic therapy was administered, the patient's clinical symptoms were alleviated, and his ST segment decreased by more than 50%. He entered the cath lab 11 h after the onset of illness. (A) CAG showed that the main lesion was located in the distal RCA posterior anterior trigeminal tubular, and approximately 95% of the stenosis and thrombosis was visible. (B) CAG after PCI, balloons placement and medication treatment. (C) After 4 days of treatment, there was normal in resting MPI. (D) Resting MBF showing that the RCA blood supply area was significantly lower than that in other regions.
Generally, male individuals have a greater risk of cardiovascular disease than female individuals (34, 35). Our study had the same characteristics as the general population distribution; male prevalence was greater, and female patients comprised only 9.33% of the study population. In a previous study, there was no significant difference in infarct size and cardiac impairment between women and men with AMI (36). However, the male sex was found to be an identical risk factor for coronary reduced flow and coronary microvascular disease (CMD) after myocardial infarction with thrombolysis (37). In our multiple linear analysis, the male sex had an inverse relationship with ischemic MBF.
RPP influence
RPP and resting MBF are directly proportional, and currently available postprocessing software generally recommends RPP correction when measuring MFR. Moreover, some studies have also suggested that MFR without RPP correction also has prognostic value (38). In our study, only resting MBF was involved. There were statistically significant differences in ischemic MBF and non-ischemic MBF, both before and after RPP correction. In addition, there were small differences in the AUC and cutoff values before and after the correction. Furthermore, an excessively high heart rate and systolic blood pressure may overestimate resting MBF (15, 39), and we believe that RPP correction is optional for resting MPI only.
Other factors
Resting MBF may also be affected by the degree of response to reperfusion treatment and individual differences.
Limitations
The present study has some limitations. First, our assessment of myocardial infarction was performed after treatment, not before. Second, the study size was small. In the future, we can combine multiple medical centers to include more samples for research. Third, the long-term prognostic value of resting MBF was not discussed in this study, and we will follow patients and explore the prognostic value of resting MBF in patients with STEMI. Fourth, our study did not assess MFR, mainly because of the high risk of stress after STEMI. Finally, this study involved only the resting MBF and did not compare to the indices of microvascular resistance (IMR) (40, 41) and invasive resting flow (42). In the future, we will include a subcohort of patients with uncomplicated STEMI for MFR measurements. If possible, we will conduct comparisons of non-invasive resting flow and invasive resting flow to further investigate hemodynamics.
Conclusion
In our small single-center study, resting MBF was able to test residual ischemia in patients with STEMI after reperfusion treatment and to identify ischemic area to an extent. Male sex and SRS had inverse corrections with ischemic MBF. A lower resting MBF may be associated with more severe myocardial ischemia. Further validation is expected in the future with a long-term follow-up.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The Ethics Committee of the Second Hospital of Hebei Medical University approved the protocol and all participants provided informed consent. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MY: Writing – review & editing, Writing – original draft, Visualization, Validation, Methodology, Formal Analysis, Data curation. HS: Writing – review & editing, Writing – original draft, Supervision, Methodology, Investigation, Funding acquisition, Formal Analysis. XG: Writing – review & editing, Supervision, Investigation, Data curation. LH: Writing – review & editing, Supervision, Methodology, Formal Analysis. SH: Writing – review & editing, Visualization, Validation, Software, Formal Analysis. HZ: Writing – review & editing, Supervision, Investigation, Formal Analysis.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article.
Hebei Provincial Department of Science and Technology, Hebei Provincial Central Guidance Local Science and Technology Development Fund Project (236Z7704G).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. (2018) 39(2):119–77. doi: 10.1093/eurheartj/ehx393
2. Song J, Murugiah K, Hu S, Gao Y, Li X, Krumholz HM, et al. Incidence, predictors, and prognostic impact of recurrent acute myocardial infarction in China. Heart (Br Cardiac Soc). (2020) 107(4):313–8. doi: 10.1136/heartjnl-2020-317165
3. Ben-Haim S, Murthy VL, Breault C, Allie R, Sitek A, Roth N, et al. Quantification of myocardial perfusion reserve using dynamic SPECT imaging in humans: a feasibility study. J Nucl Med. (2013) 54(6):873–9. doi: 10.2967/jnumed.112.109652
4. Agostini D, Roule V, Nganoa C, Roth N, Baavour R, Parienti JJ, et al. First validation of myocardial flow reserve assessed by dynamic 99mTc-sestamibi CZT-SPECT camera: head to head comparison with 15O-water PET and fractional flow reserve in patients with suspected coronary artery disease. The WATERDAY study. Eur J Nucl Med Mol Imaging. (2018) 45(7):1079–90. doi: 10.1007/s00259-018-3958-7
5. de Souza ACDAH, Harms HJ, Martell L, Bibbo C, Harrington M, Sullivan K, et al. Accuracy and reproducibility of myocardial blood flow quantification by single photon emission computed tomography imaging in patients with known or suspected coronary artery disease. Circ Cardiovasc Imaging. (2022) 15(6):e013987. doi: 10.1161/CIRCIMAGING.122.013987
6. Shiraishi S, Tsuda N, Sakamoto F, Ogasawara K, Tomiguchi S, Tsujita K, et al. Clinical usefulness of quantification of myocardial blood flow and flow reserve using CZT-SPECT for detecting coronary artery disease in patients with normal stress perfusion imaging. J Cardiol. (2020) 75(4):400–9. doi: 10.1016/j.jjcc.2019.09.006
7. Zhang H, Caobelli F, Che W, Huang Y, Zhang Y, Fan X, et al. The prognostic value of CZT SPECT myocardial blood flow (MBF) quantification in patients with ischemia and no obstructive coronary artery disease (INOCA): a pilot study. Eur J Nucl Med Mol Imaging. (2023) 50(7):1940–53. doi: 10.1007/s00259-023-06125-3
8. De Luca G, Parodi G, Sciagrà R, Venditti F, Bellandi B, Vergara R, et al. Time-to-treatment and infarct size in STEMI patients undergoing primary angioplasty. Int J Cardiol. (2013) 167(4):1508–13. doi: 10.1016/j.ijcard.2012.04.078
9. Smit JM, Hermans MP, Dimitriu-Leen AC, van Rosendael AR, Dibbets-Schneider P, de Geus-Oei LF, et al. Long-term prognostic value of single-photon emission computed tomography myocardial perfusion imaging after primary PCI for STEMI. Eur Heart J Cardiovasc Imaging. (2018) 19(11):1287–93. doi: 10.1093/ehjci/jex332
10. Zampella E, Mannarino T, Gaudieri V, D'Antonio A, Giallauria F, Assante R, et al. Effect of changes in perfusion defect size during serial stress myocardial perfusion imaging on cardiovascular outcomes in patients treated with primary percutaneous coronary intervention after myocardial infarction. J Nucl Cardiol. (2022) 29(5):2624–32. doi: 10.1007/s12350-021-02770-z
11. Calabretta R, Castello A, Linguanti F, Tutino F, Ciaccio A, Giglioli C, et al. Prediction of functional recovery after primary PCI using the estimate of myocardial salvage in gated SPECT early after acute myocardial infarction. Eur J Nucl Med Mol Imaging. (2018) 45(4):530–7. doi: 10.1007/s00259-017-3891-1
12. Cho SG, Jabin Z, Park KS, Kim J, Kang SR, Kwon SY, et al. Clinical values of left ventricular mechanical dyssynchrony assessment by gated myocardial perfusion SPECT in patients with acute myocardial infarction and multivessel disease. Eur J Nucl Med Mol Imaging. (2017) 44(2):259–66. doi: 10.1007/s00259-016-3542-y
13. Ghotbi AA, Hasbak P, Nepper-Christensen L, Lønborg J, Atharovski K, Christensen T, et al. Early risk stratification using Rubidium-82 positron emission tomography in STEMI patients. J Nucl Cardiol. (2019) 26(2):471–82. doi: 10.1007/s12350-017-0993-x
14. Teunissen PF, Timmer SA, Danad I, de Waard GA, van de Ven PM, Raijmakers PG, et al. Coronary vasomotor function in infarcted and remote myocardium after primary percutaneous coronary intervention. Heart (Br Cardiac Soc). (2015) 101(19):1577–83. doi: 10.1136/heartjnl-2015-307825
15. Yan M, Shang H, Hao L, Guo X, Zheng H, Li H, et al. A preliminary study of dobutamine myocardial flow reserve on 99mTc-Sestamibi CZT-SPECT. Ann Nucl Med. (2023) 37(6):349–59. doi: 10.1007/s12149-023-01829-w
16. Leppo JA, Meerdink DJ. Comparison of the myocardial uptake of a technetium-labeled isonitrile analogue and thallium. Circ Res. (1989) 65(3):632–9. doi: 10.1161/01.res.65.3.632
17. Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the American heart association. Circulation. (2002) 105(4):539–42. doi: 10.1161/hc0402.102975
18. Cimmino G, Gallinoro E, Di Serafino L, De Luca N, Cirillo P. Antiplatelet therapy in acute coronary syndromes. Lights and shadows of platelet function tests to guide the best therapeutic approach. Curr Vasc Pharmacol. (2020) 18(3):262–72. doi: 10.2174/1570161117666190513105859
19. Johnson NP, Gould KL, De Bruyne B. Autoregulation of coronary blood supply in response to demand: JACC review topic of the week. J Am Coll Cardiol. (2021) 77(18):2335–45. doi: 10.1016/j.jacc.2021.03.293
20. Tucker B, Vaidya K, Cochran BJ, Patel S. Inflammation during percutaneous coronary intervention-prognostic value, mechanisms and therapeutic targets. Cells. (2021) 10(6):1391. doi: 10.3390/cells10061391
21. Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. (2007) 356(8):830–40. doi: 10.1056/NEJMra061889
22. Gregorini L, Marco J, Palombo C, Kozàkovà M, Anguissola GB, Cassagneau B, et al. Postischemic left ventricular dysfunction is abolished by alpha-adrenergic blocking agents. J Am Coll Cardiol. (1998) 31(5):992–1001. doi: 10.1016/s0735-1097(98)00055-2
23. el-Tamimi H, Davies GJ, Sritara P, Hackett D, Crea F, Maseri A. Inappropriate constriction of small coronary vessels as a possible cause of a positive exercise test early after successful coronary angioplasty. Circulation. (1991) 84(6):2307–12. doi: 10.1161/01.cir.84.6.2307
24. Gurrola-Luna H, Rojas-Sernaque JK, Barajas Paulin AJ, Carvajal-Juarez I, Bermudez-Gonzalez JL, Rivera-Bravo B, et al. Comorbidities associated with reduced myocardial flow reserve in non-obstructive disease. Eur Heart J. (2021) 42(Suppl 1):ehab724.0258. doi: 10.1093/eurheartj/ehab724.0258
25. Banai A, Lupu L, Borohovitz A, Levi E, Banai S, Konigstein M. Microvascular dysfunction in patients with angina and non-obstructive coronary artery disease—preliminary data from a single center registry. Eur Heart J. (2021) 42(Suppl 1):ehab724.2095. doi: 10.1093/eurheartj/ehab724.2095
26. Beijnink CWH, van der Hoeven NW, Konijnenberg LSF, Kim RJ, Bekkers SCAM, Kloner RA, et al. Cardiac MRI to visualize myocardial damage after ST-segment elevation myocardial infarction: a review of its histologic validation. Radiology. (2021) 301(1):4–18. doi: 10.1148/radiol.2021204265
27. Bragadeesh T, Jayaweera AR, Pascotto M, Micari A, Le DE, Kramer CM, et al. Post-ischaemic myocardial dysfunction (stunning) results from myofibrillar oedema. Heart (Br Cardiac Soc). (2008) 94(2):166–71. doi: 10.1136/hrt.2006.102434
28. Thim T, van der Hoeven NW, Musto C, Nijveldt R, Götberg M, Engstrøm T, et al. Evaluation and management of nonculprit lesions in STEMI. JACC Cardiovasc Interv. (2020) 13(10):1145–54. doi: 10.1016/j.jcin.2020.02.030
29. Freitag MT, Bremerich J, Wild D, Haaf P, Zellweger MJ, Caobelli F. Quantitative myocardial perfusion 82Rb-PET assessed by hybrid PET/coronary-CT: normal values and diagnostic performance. J Nucl Cardiol. (2022) 29(2):464–73. doi: 10.1007/s12350-020-02264-4
30. Liu B, Yu W, Wang J, Shao X, Zhang F, Zhou M, et al. A model combining rest-only ECG-gated SPECT myocardial perfusion imaging and cardiovascular risk factors can effectively predict obstructive coronary artery disease. BMC Cardiovasc Disord. (2022) 22(1):268. doi: 10.1186/s12872-022-02712-8
31. Henzlova MJ, Duvall WL, Einstein AJ, Travin MI, Verberne HJ. ASNC Imaging guidelines for SPECT nuclear cardiology procedures: stress, protocols, and tracers. J Nucl Cardiol. (2016) 23(3):606–39. doi: 10.1007/s12350-015-0387-x
32. Motwani M, Berman DS, Germano G, Slomka P. Automated quantitative nuclear cardiology methods. Cardiol Clin. (2016) 34(1):47–57. doi: 10.1016/j.ccl.2015.08.003
33. Zavadovsky KV, Mochula AV, Boshchenko AA, Vrublevsky AV, Baev AE, Krylov AL, et al. Absolute myocardial blood flows derived by dynamic CZT scan vs invasive fractional flow reserve: correlation and accuracy. J Nucl Cardiol. (2021) 28(1):249–59. doi: 10.1007/s12350-019-01678-z
34. Chang SS, Lin SY, Lai JN, Chen KW, Lu CR, Chang KC, et al. Sex differences in long-term cardiovascular outcomes among patients with acute myocardial infarction: a population-based retrospective cohort study. Int J Clin Pract. (2021) 75(5):e14066. doi: 10.1111/ijcp.14066
35. Dreyer RP, Dharmarajan K, Kennedy KF, Jones PG, Vaccarino V, Murugiah K, et al. Sex differences in 1-year all-cause rehospitalization in patients after acute myocardial infarction: a prospective observational study. Circulation. (2017) 135(6):521–31. doi: 10.1161/CIRCULATIONAHA.116.024993
36. Kosmidou I, Redfors B, Selker HP, Thiele H, Patel MR, Udelson JE, et al. Infarct size, left ventricular function, and prognosis in women compared to men after primary percutaneous coronary intervention in ST-segment elevation myocardial infarction: results from an individual patient-level pooled analysis of 10 randomized trials. Eur Heart J. (2017) 38(21):1656–63. doi: 10.1093/eurheartj/ehx159
37. Li M, Su H, Jiang M, Zuo Z, Su Z, Hao L, et al. Predictive value of thrombolysis in myocardial infarction frame count for coronary microvascular dysfunction evaluated with an angiography-derived index of microcirculatory resistance in patients with coronary slow flow. Quant Imaging Med Surg. (2022) 12(10):4942–52. doi: 10.21037/qims-22-224
38. Ahmed AI, Saad JM, Alahdab F, Han Y, Alfawara M, Nabi F, et al. Prognostic value of PET derived coronary flow reserve: should we correct for resting perfusion product? Eur Heart J. (2022) 43(Suppl 2):ehac544.311. doi: 10.1093/eurheartj/ehac544.311
39. Jagathesan R, Barnes E, Rosen SD, Foale RA, Camici PG. Comparison of myocardial blood flow and coronary flow reserve during dobutamine and adenosine stress: implications for pharmacologic stress testing in coronary artery disease. J Nucl Cardiol. (2006) 13(3):324–32. doi: 10.1016/j.nuclcard.2006.03.017
40. De Maria GL, Alkhalil M, Wolfrum M, Fahrni G, Borlotti A, Gaughran L, et al. Index of microcirculatory resistance as a tool to characterize microvascular obstruction and to predict infarct size regression in patients with STEMI undergoing primary PCI. JACC Cardiovasc Imaging. (2019) 12(5):837–48. doi: 10.1016/j.jcmg.2018.02.018
41. Kodeboina M, Nagumo S, Munhoz D, Sonck J, Mileva N, Gallinoro E, et al. Simplified assessment of the Index of microvascular resistance. J Interv Cardiol. (2021) 2021:9971874. doi: 10.1155/2021/9971874
Keywords: myocardial perfusion imaging (MPI), myocardial blood flow (MBF), STEMI (myocardial infarction), post-reperfusion therapy, CZT-SPECT
Citation: Yan M, Shang H, Guo X, Hao L, Hou S and Zheng H (2024) The diagnostic role of resting myocardial blood flow in STEMI patients after revascularization. Front. Cardiovasc. Med. 11:1364772. doi: 10.3389/fcvm.2024.1364772
Received: 3 January 2024; Accepted: 27 February 2024;
Published: 20 March 2024.
Edited by:
Jie Zheng, Washington University in St. Louis, United States© 2024 Yan, Shang, Guo, Hao, Hou and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua Shang Mjc3MDAxMDdAaGVibXUuZWR1LmNu
Abbreviations STEMI, ST-segment elevation myocardial infarction; MPI, myocardial perfusion imaging; CAD, coronary artery disease; CZT-SPECT, cadmium zinc telluride single photon emission computed tomography; MBF, myocardial blood flow; CAG, coronary angiography; G-MPI, gated myocardial perfusion imaging; RPP, rate pressure product; AHA, American Heart Association; ROC, receiver operating characteristic; AUC, area under the curve; SRS, summed rest score; TPD, total perfusion defect; AMI, acute myocardial infarction; PET, positron emission tomography; MFR, myocardial flow reserve; INOCA, ischemia and no obstractive coronary artery disease; PCI, percutaneous coronary intervention; 99mTc –MIBI, 99mTc-sestamibi; MC, motion correction; ICC, intraclass correlation consistency; EDV, end-diastolic volume; ESV, end-systolic volume; LVEF, left ventricular ejection fraction; ACS, acute coronary syndrome; CMD, coronary microvascular disease; LM, left main; LAD, left artery descending; LCX, left circumflex; RCA, right coronary artery; BMI, body mass index; TIMI, thrombolysis in myocardial infarction; IMR, index of microvascular resistance.
 Ming Yan1
Ming Yan1 Hua Shang
Hua Shang Hongming Zheng
Hongming Zheng